Abstract
Hepatitis B virus (HBV) cannot be eliminated completely from infected hepatocytes because of the presence of intrahepatic covalently closed circular DNA (cccDNA). As chronic hepatitis B (CHB) can progress to cirrhosis and hepatocellular carcinoma (HCC), it is important to manage CHB to prevent HCC development in high-risk patients with high viral replicative activity or advanced fibrosis. Serum biomarkers are noninvasive and valuable for the management of CHB. Hepatitis B core-related antigen (HBcrAg) correlates with serum HBV DNA and intrahepatic cccDNA. In CHB patients with undetectable serum HBV DNA or loss of HBsAg, HBcrAg still can be detected and the decrease in HBcrAg levels is significantly associated with hopeful outcomes. Therefore, HBcrAg can predict HCC occurrence or recurrence. Measurement of the Mac-2 binding protein glycosylation isomer (M2BPGi) has been introduced for the evaluation of liver fibrosis. Because elevated M2BPGi in CHB is related to liver fibrosis and the prediction of HCC development, monitoring its progression is essential. Because alpha fetoprotein (AFP) has insufficient sensitivity and specificity for early-stage HCC, a combination of AFP plus protein induced by vitamin K absence factor II, or AFP plus Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein might improve the diagnosis of HCC development. Additionally, Dickkopf-1 and circulating immunoglobulin G antibodies are the novel markers to diagnose HCC or assess HCC prognosis. This review provides an overview of novel HBV biomarkers used for the management of intrahepatic viral replicative activity, liver fibrosis, and HCC development.
Keywords: Hepatitis B core-related antigen, Mac 2-binding protein glycan isomer, Alpha-fetoprotein, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein, Protein induced by vitamin K absence or antagonist-II
INTRODUCTION
Hepatitis B virus (HBV) is a common cause of acute and chronic liver disease. The World Health Organization estimates that in 2015, 257 million people suffered from chronic hepatitis B (CHB), defined as hepatitis B surface antigen (HBsAg)-positive [1]. The majority of new HBV infections occur in highly endemic areas such as China, Southeast Asia, and sub-Saharan Africa [2]. HBV has been the most common cause of hepatocellular carcinoma (HCC), being responsible for >50% of cases worldwide [3]. Compared with uninfected patients, the relative risk of HCC development increases 15–20-fold in patients with HBV infection [4]. Meanwhile, Ho et al. [5] reported the clinical presentations of HCC have significantly changed over the past 12 years. In their report, hepatitis B and C virus-associated HCC became less common, and more patients were diagnosed at early cancer stage [5].
Unfortunately, although nucleos(t)ide analogues (NAs) or interferon (IFN) can efficiently suppress HBV replication, these are not curative treatments [6]. These drugs do not directly target covalently closed circular DNA (cccDNA), the key molecule responsible for intrahepatic viral persistence. cccDNA is a stable, extra-chromosomal transcriptional template for all HBV mRNAs such as pregenomic RNA [7-9]. The amount and transcriptional activity of cccDNA in hepatocytes is important for CHB progression and clinical outcomes [10]. The current aim of anti-HBV treatment is primarily to suppress complications associated with progressive inflammation and fibrosis, i.e., liver failure and decompensated cirrhosis [11,12].
HCC diagnosis and surveillance are mostly based on the detection of tumor markers and imaging techniques [11-13]. There is still a requirement for more reliable, non-invasive, and cost-effective biomarkers for CHB management. Especially, a significant number of CHB patients with non-cirrhotic liver develop HCC [14]. Current guidelines advise 6-monthly abdominal ultrasound surveillance for HCC in advanced fibrosis or cirrhotic CHB patients and in noncirrhotic patients depending on ethnic background and age [15,16]. Therefore, identifying biomarkers to better predict or diagnose HCC remains an important clinical and research priority.
In this review, we introduce novel biomarkers with great potential for CHB management and prognostic evaluation. The first is a surrogate marker of intrahepatic HBV replication, hepatitis B corerelated antigen (HBcrAg), which has revealed a good correlation with cccDNA [17]. The second, Mac-2-binding protein glycosylation isomer (M2BPGi), is a liver fibrosis marker that might predict HCC development [18]. Finally, the third is a tumor marker, alpha-fetoprotein (AFP), a protein induced by vitamin K absence or antagonist-II (PIVKA-II), Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3), Dickkopf-1 (DKK-1) and circulating immunoglobulin G (IgG) antibodies. We will focus on the clinical utility of these markers as predictors of HBV-related HCC development (Fig. 1).
Figure 1.
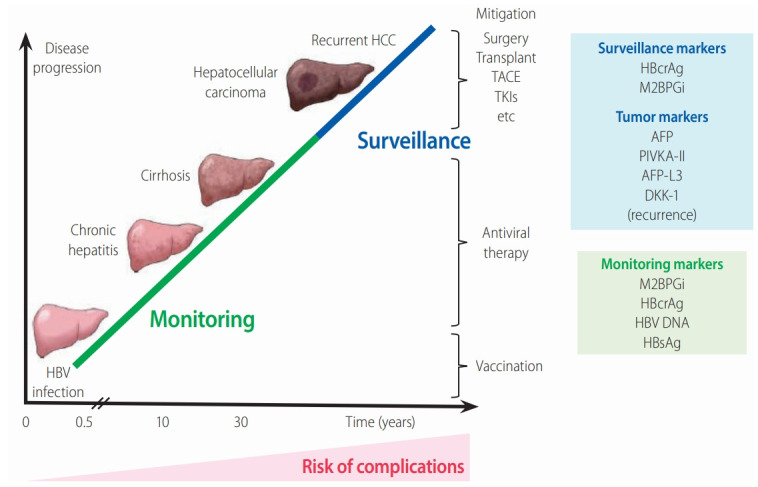
The relationship between chronic HBV infection and liver disease progression. The figure shows the clinical stages involved in the natural history of CHB. The serum biomarkers HBcrAg and M2BPGi provide valuable predictive data for the effective management of CHB. It is important to monitor patients at high risk and to treat them early to prevent liver complications, cirrhosis, and HCC development. AFP, PIVKA-II, and AFP-L3 are HCCspecific tumor markers summarized in this review. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; TKIs, tyrosine kinase inhibitors; AFP, alpha fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; AFP-L3, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein; DKK-1, Dickkopf-1; HBcrAg, hepatitis B core-related antigen; M2BPGi, Mac-2 binding protein glycan isomer; HBsAg, hepatitis B surface antigen.
MONITORING OF BIOMARKERS RELATED TO HBV INFECTION
With recent developments in molecular research methods, several biomarkers associated with the natural history of CHB and effectiveness of antiviral therapy have been identified [17,19]. Conventional serological biomarkers include serum HBV DNA levels and HBsAg titers, both of which predict the risk of cirrhosis and HCC [20,21]. Quantitation of HBsAg has been used as a predictive marker of liver disease, spontaneous HBsAg seroclearance, cirrhosis, and HCC development, when complementary to the measurement of HBV DNA [22-25]. However, cirrhosis and HCC can still occur in patients with undetectable HBV DNA [26] and HBsAg seroclearance [27,28]. Additionally, essential information of innovative and effective biomarkers including prediction of spontaneous or treatment-induced hepatitis B envelope antigen (HBeAg) seroconversion, and responses before/after cessation of NA treatment are still required.
SURROGATE MARKER OF INTRAHEPATIC HBV REPLICATION: HBcrAg
We recently described the clinical application of a new effective biomarker, HBcrAg, including the association of HBcrAg with other biomarkers, its competency for the prediction of clinical outcomes, and its role as a predictor of treatment [10] and HCC occurrence and recurrence [29]. Here, we introduce the unique features of HBcrAg and focus on its application for CHB management.
Components of HBcrAg (Fig. 2)
Figure 2.
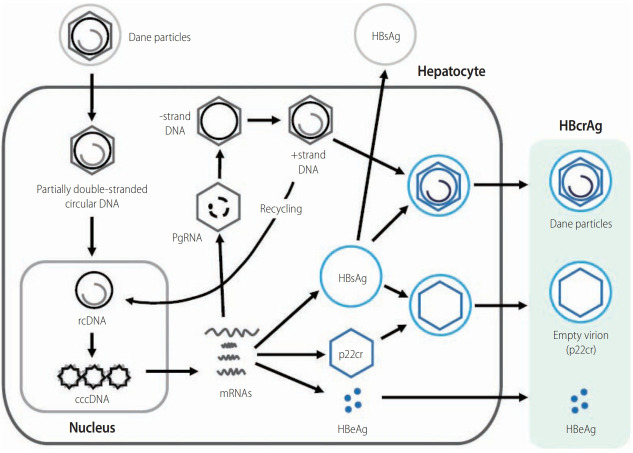
The lifecycle of HBV and HBcrAg. The schematic shows the steps involved in the lifecycle of HBV. Importantly, it shows the sources of various HBV-related molecules used routinely for diagnosis, clinical monitoring, and prognosis of HBV infection. These include the core-related antigens (p22cr, HBe, and core antigens), HBsAg, and HBV DNA measured in serum. cccDNA, although measurable in serum in association with liver injury, is mainly measured from liver biopsy samples. HBsAg, hepatitis B surface antigen; cccDNA, covalently closed circular DNA; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus.
HBcrAg contains three products encoded by the precore/core gene. HBeAg is a circulating peptide derived from the precore protein by proteolysis and secreted from hepatocytes [7,30]. HBcAg is a component of the virion and forms the nucleocapsid surrounding the viral DNA. p22cr is a 22-kDa precore protein present in HBV DNA-negative empty Dane-like particles [31]. All three proteins share an identical 149 amino acid sequence [31,32]. HBcAg, p22cr, and HBeAg can all be measured as HBcrAg by serological testing [33,34].
Development of an HBcrAg assay
The first report of HBcrAg in 2002 concerned the development of a sensitive enzyme immunoassay specific for HBcAg and HBeAg [35]. Kimura et al. [35] designated the precore/core gene products, comprised of HBcAg and HBeAg, as HBcrAg. This assay detects HBcAg and HBeAg, even in anti-HBc or anti-HBe positive specimens. Because the level of HBcrAg reflects the serum HBV DNA level, this test can be used supplementary to HBV DNA to monitor CHB patients [35].
Further progress was achieved with the development of a chemiluminescence enzyme immunoassay for the detection of HBcrAg. The clinical performance of this assay was evaluated in CHB patients and the HBcrAg concentration correlated positively with HBV DNA concentration (P<0.001) over a 100,000-fold range [33]. The accuracy of HBV load measurement obtained by the HBcrAg assay was not affected by the absence of HBeAg in the sera or the presence of precore mutations in the HBV genome. More detailed information on the latest applications of HBcrAg measurement were recently reviewed [36]. Current quantitative HBcrAg assays have a lower limit of detection of 100 U/mL [37], but the current recommended cut-off point is 1,000 (3 log) U/mL. Compared with “prototype HBcrAg assays”, which measured only HBc, recently developed systems using p22cr (empty particle) and HBeAg [36] are automatic, and more sensitive and rapid by deactivating anti-HBc and anti-HBe in the test samples [10].
Japan was first to recommend HBcrAg in clinical guidelines for CHB management followed by the greater Asian region and then Europe [11,38,39].
Recent clinical assessments of HBcrAg
The novel biomarker, HBcrAg, has been used to support monitoring of CHB and the prediction of clinical outcomes. In this section, we describe briefly the recently reported clinical applications of HBcrAg (Table 1).
Table 1.
Clinical applications of HBcrAg in CHB patients
| Category | Finding | HBcrAg level (log U/mL) and point | Reference |
|---|---|---|---|
| Natural history | HBeAg seroconversion | <4.92 log U/mL during the clinical course | [37] |
| HBsAg seroclearance | Undetectable (79%), 2.7 log U/mL (median of 21%) during the clinical course | [37,48] | |
| cccDNA activity | Lower amounts of intrahepatic cccDNA and lower cccDNA activity | <3 log U/mL | [38] |
| Identification of inactive carriers with a high accuracy (any HBV genotype) | HBcrAg ≤3 log U/mL plus HBV DNA ≤2,000 IU/mL | [39] | |
| Anti-HBV treatment | HBeAg seroconversion by PEG-IFN at 12 weeks | >8 log U/mL (no response) at the beginning of therapy | [49] |
| HBeAg seroconversion by PEG-IFN plus NA for 4 weeks followed by PEG-IFN for 20 weeks | >4.5 log U/mL (no response) at the beginning of therapy | [140] | |
| No LAM resistance | <4.6 log U/mL at 6 months of treatment | [141] | |
| Virological relapse within 1 year of NA cessation | >3.7 log U/mL at NA cessation | [56] | |
| Virological relapse regardless of undetectable HBV DNA for at least 6 months | 3.2-3.7 log U/mL at NA (LAM or ETV) cessation | [53,54] | |
| HCC occurrence/recurrence | At high risk for HCC with intermediate viral load (HBV DNA 2,000-19,999 U/mL) | ≥4.0 log U/mL | [65] |
| Cumulative incidence of HCC during NA treatment | ≥3.4 log U/mL at the time of HBV DNA disappearance | [56] | |
| HCC development during NA treatment | Detectable HBcrAg during NA treatment | [63] | |
| Long-term effect of NA treatment on HCC progression | Higher serum levels of HBcrAg and BCP mutations were associated with progression to HCC, independent of NA therapy | [62] | |
| Evaluation of HCC occurrence | HBcrAg >3.0 log U/mL and HBsAg >3.0 log IU/mL (cut-off values) | [67] | |
| Incidence of HCC for treatment-experienced patients | >4.67 log U/mL at pre-treatment, >3.89 log U/mL at post-treatment | [66] | |
| HCC development during NA treatment | Detectable HBcrAg during NA treatment | [63] | |
| Incidence of HCC for treatment-naïve patients | >2.9 log U/mL during follow-up period | [61,64] | |
| HCC recurrence within 2 years | >4.8 log U/mL at time of HCC diagnosis | [61,64] | |
| HBV reactivation | HBV reactivation by high-risk immunosuppressive therapy within 2 years | Detectable HBcrAg at baseline | [142] |
| HBV reinfection | High levels of post-liver transplantation cccDNA | >4 log U/mL before liver transplantation | [143] |
HBcrAg, hepatitis B core-related antigen; CHB, chronic hepatitis B; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; cccDNA, covalently closed circular DNA; HBV, hepatitis B virus; PEG-IFN, pegylated interferon; NA, nucleos(t)ide analogue; LAM, lamivudine; ETV, entecavir; HCC, hepatocellular carcinoma; BCP, basal core promoter.
Serum HBcrAg levels are closely associated with intrahepatic cccDNA levels, as well as serum HBV DNA [38,40]. Riveiro-Barciela et al. [39] reported that HBsAg levels <3 log IU/mL were only valuable for detecting genotype D HBV inactive carriers. A single HBcrAg measurement ≤3 log U/mL plus HBV DNA ≤2,000 IU/mL was highly accurate in detecting HBV inactive carriers, regardless of their HBV genotype [39]. Testoni et al. [38] recently confirmed serum HBcrAg levels were significantly higher in HBeAg-positive patients than in HBeAg-negative patients without antiviral treatment and that they correlated with serum HBV DNA, intrahepatic HBV DNA, pgRNA and cccDNA levels, in addition to transcriptional activity. Patients negative for HBcrAg (<3 log U/mL) had lower amounts of intrahepatic cccDNA and lower cccDNA activity than HBcrAg-positive patients [38]. Hasegawa et al. [41] created a helpful prediction model for intrahepatic cccDNA levels in CHB patients. The method was named the fasting blood sugar (FBS)-cres score based on the variables used (FBS, HBcrAg, HBeAg, and HBsAg). The FBS-cres score is calculated by the following equation: 3.1686 – (0.0148 × FBS) + (0.1982 × HBcrAg) + (0.0008168 × HBeAg) + (0.1761 × log10 (HBsAg)). For example, in the training cohort, a significant correlation was shown between HBcrAg and cccDNA levels (P<0.0001, r=0.67), whereas the FBS-cres score was more closely correlated to cccDNA level (P<0.0001, r=0.81) [41].
In resource-limited areas such as Africa, HBV DNA quantification assays have limited availability and are expensive. Shimakawa et al. [42] evaluated the prospect of HBcrAg to identify Gambian patients qualified for treatment, using a new experimental algorithm that does not include HBV DNA. An easy treatment algorithm using HBcrAg without HBV DNA showed high area under the receiver operating characteristics (AUROC) (0.91; 95% confidence interval [CI], 0.88–0.95]), with a sensitivity of 96.6% and specificity of 85.8%. The measurement of HBcrAg, which is 5–10 times less expensive per test than the quantification of HBV DNA, might replace HBV DNA testing [42].
Change in HBcrAg and other HBV markers under NA therapy
A reduction in serum HBV DNA does not correlate with a decrease of intrahepatic cccDNA in patients receiving anti-HBV therapy. In a study of 43 patients treated with NA (median, 126 months) who are positive or negative for HBeAg, although 51% still had detectable intrahepatic cccDNA [43]. Similar findings were reported in 24 patients positive for HBeAg treated with sequential therapy of pegylated interferon (PEG-IFN) and adefovir (ADV) followed by ADV monotherapy—46% and 66% patients had undetectable serum HBV DNA and detectable intrahepatic cccDNA, respectively [44].
The difference between serum HBcrAg and HBV DNA can be explained by the action of NA on reverse transcription and the subsequent prevention of HBV DNA replication, while HBcrAg production remains persistently. The reduction of HBcrAg demonstrated a good correlation with the magnitude of change in intrahepatic cccDNA levels [45,46]. In contrast to serum HBV DNA, the reduction of HBcrAg was slower during NA treatment, with an increase in the ratio of serum HBcrAg:HBV DNA after 3 months of lamivudine [33]. Furthermore, in patients receiving NA treatment with undetectable serum HBV DNA, 78% had persistent HBcrAg [46]. Even in patients with documented HBsAg seroclearance, 21% had detectable serum HBcrAg, in contrast to detectable serum HBV DNA in only 2.1% [47,48].
Serum HBcrAg levels at baseline and changes while on anti-HBV therapy may also predict suitable indicators in CHB patients. Because HBV DNA is not detectable in most patients receiving NAs, HBcrAg will be used as a surrogate marker. For patients positive for HBeAg treated with PEG-IFN, a high baseline HBcrAg level >8 log U/mL conferred a >94.4% negative predictive value (NPV) for achieving HBeAg seroconversion and suppression of HBV DNA at 12 weeks (n=46) [49]. Furthermore, HBcrAg levels changed by treatment might predict clinical outcomes. In 58 patients positive for HBeAg treated with PEG-IFN, HBcrAg levels at week 12 predicted HBeAg seroconversion at 24 weeks after finishing treatment with an AUROC of 0.896 [50]. For patients treated with NA therapy (n=39), HBcrAg levels were lower in patients with NA-induced HBeAg seroconversion compared with patients who remained HBeAg-positive [45].
As an advanced technique, a combination of HBcrAg and HBsAg assays may be used to identify patients who are unlikely to achieve treatment end-points, i.e., HBeAg seroconversion [51]. Wang et al. [51] identified predictors of seroconversion using serum quantitative HBsAg and HBcrAg, in HBeAg-positive patients. Data and samples were obtained from 118 HBeAg-positive adults with HBV genotypes A–G treated with NA. Approximately 36.4% of patients achieved HBeAg seroconversion after NA treatment (median, 39 months). Regarding the treatment kinetics of HBV DNA, HBsAg and HBcrAg differed between patients with and without HBeAg seroconversion [51].
Additionally, baseline HBcrAg level is an independent predictor of long-term HBcrAg below the limit of detection [52]. Wang et al. [52] investigated the long-term kinetics of serum HBcrAg and its correlation with serum HBsAg in CHB patients positive or negative for HBeAg with NA therapy over 8 years. At 8 years from the start of NA treatment, 21.3% of patients achieved a serum HBcrAg level of <3 log10U/mL, and only baseline HBcrAg was an independent predictor [52].
HBcrAg in the prediction of NA cessation point
Once seroconversion to anti-HBe occurs, the reduction of HBcrAg and HBsAg followed by HBsAg loss is a major goal of current treatment efforts in CHB [11]. In HBeAg-negative CHB patients, the goal of antiviral therapy is a sustained off-treatment response and HBsAg clearance [49]. However, because it is difficult to obtain HBsAg clearance during NA therapy, the reduction of HBsAg and HBcrAg might be useful when judging the efficacy of NA therapy.
Therefore, HBcrAg might be more useful in the HBeAg-negative phase. With reference to the first published papers [53,54], the Japan Society of Hepatology (JSH) Guidelines for the Management of HBV Infection [13] described the criteria for the cessation of NA therapy (see Table 15) [13]. The three laboratory criteria for the cessation of NA therapy are: 1) at least 2 years administration of NAs; 2) undetectable serum HBV DNA levels (using real-time polymerase chain reaction); and 3) negative serum HBeAg at time of treatment cessation. When these criteria are met, the risk of relapse can be determined from HBsAg and HBcrAg levels at the time of cessation of therapy.
A decrease in HBcrAg was observed under NA therapy, and the pattern of reduction may provide predictive information on the risk of post-treatment HBV reactivation [55]. A serum HBcrAg level >3.7 log U/mL at NA cessation predicted virological relapse within 1 year of NA cessation [56]. Therefore, serum HBcrAg as a surrogate marker, may have a better influence on patients who are planning NA cessation.
Regarding entecavir (ETV) or tenofovir (TDF), the HBcrAg level at NA cessation is an independent relapse predictor, as well as HBsAg, age, alanine aminotransferase (ALT), and TDF use. Hsu et al. [57] enrolled 135 CHB patients who had stopped ETV or TDF after accomplishing viral reduction for a median of 25.2 months. All patients stopped NA with negative HBeAg and undetectable HBV DNA. During the follow-up period (median, 25.9 months), clinical relapse and HBsAg loss occurred in 66 and eight patients, respectively, with a 5-year cumulative incidence of 56.1% and 8.8%, respectively. A SCALE-B score was calculated using the equation 35 × HBsAg (log IU/mL) + 20 × HBcrAg (log U/mL) + 2 × age (years) + ALT (U/L) + 40 for TDF use. The concordance rates for clinical relapse were 0.87, 0.88, 0.87, 0.85, and 0.90 at 1, 2, 3, 4, and 5 years, respectively. Moreover, complete HBsAg loss occurred in low-risk patients predicted by the score [57].
HBcrAg in the prediction of HCC occurrence and recurrence
To predict which patients will develop advanced liver disease including HCC during NA treatment is difficult [58]. High levels of HBV DNA were correlated with increased risk of cirrhosis and HCC development [21]. Low or undetectable HBV viral load decreased the risk of HCC development but did not prevent it completely [59-61]. Several HBV markers are related to HCC development in CHB patients including serum HBcrAg level (Table 1) [62-64].
HBcrAg was superior to HBV DNA in terms of predictive power for HCC development in treatment-naïve CHB patients [64]. During follow-up (median, 10.7 years), 78 of 1,031 (7.6%) CHB patients without NA treatment developed HCC. HBcrAg >2.9 log U/mL (hazard ratio [HR], 5.05; 95% CI, 2.40–10.63) and basal core promoter (BCP) mutations (HR, 28.85; 95% CI, 4.00–208.20) were independently associated with HCC occurrence [64]. In another study, Tseng et al. [65] reported HBcrAg level was an independent risk factor of HCC in CHB patients with intermediate viral load (HBV DNA from 2,000–19,999 IU/mL). HBcrAg level of 4.0 log U/mL identified patients with an intermediate viral load who were at high risk for HCC [65].
For treatment-experienced patients, NA reduced, but did not eradicate, the risk of HCC occurrence [59-60]. Ando et al. [59] reported that the cumulative incidence of HCC at 1, 3, and 5 years was 0.0%, 13.6%, and 17.7%, respectively in patients with serum HBcrAg levels ≥3.4 log U/mL at the time of HBV DNA disappearance and 0.0%, 0.0%, and 2.4%, respectively in patients with serum HBcrAg levels <3.4 log U/mL (P=0.005). HBcrAg among 109 CHB patients receiving NA treatment for at least 2 years was an independent risk factor for HCC development (HR, 3.53) [63]. In addition, post-treatment HBcrAg >3.89 log U/mL predicted HCC with an odds ratio of 3.27. Considering only non-cirrhotic patients, a cutoff of >3.90 log U/mL predicted HCC with an odds ratio of 5.95 [66]. To investigate the long-term effect of NA treatment on HCC progression, Kumada et al. [62] compared CHB patients who received NA therapy with those who did not. They concluded that higher HBcrAg levels and BCP mutations were associated with HCC progression, independent of NA therapy [62].
A recent report showed that a combination of HBsAg and HBcrAg values was an effective biomarker for evaluating HCC occurrence in CHB patients [67]. When cut-off values of HBsAg and HBcrAg were defined as 3.0 log U/mL and 3.0 log U/mL, respectively, patients with HCC history were frequently found in the low HBsAg (P=0.002) and high HBcrAg (P<0.001) groups. When HBsAg and HBcrAg were combined, HCC history was most frequent in the subset with low HBsAg and high HBcrAg, among HBeAg-negative patients, despite NA therapy.
HBcrAg level before surgery might be a potential marker to stratify post-surgical surveillance approaches and to identify patients with a high risk of HCC recurrence. A recent study also confirmed the predictive value of HBcrAg for HCC recurrence after curative surgery. In a study of 55 patients, a serum HBcrAg level >4.8 log U/mL at the time of HCC diagnosis gave an HR of 8.96 for subsequent HCC recurrence within 2 years [61]. Finally, the HCC recurrence-free survival rates were significantly lower in HCC patients with high intrahepatic cccDNA and serum HBcrAg levels than those with low cccDNA/HBcrAg levels (P= 0.035 and P=0.003, respectively) [68].
LIVER FIBROSIS MARKER: M2BPGi
Innovative glycoproteomic studies revealed that fibrosis leads to a specific modification of the glycosylation and sugar chain structure on the Mac-2 binding protein (M2BP) [69,70]. Based on these observations, M2BPGi has been developed as a novel surrogate serum glycoprotein-based biomarker (glycobiomarker) that reflects fibrosis stage. Here, we describe the improvement and latest assessment of M2BPGi, as a surrogate marker of HBV-related liver fibrosis and as a predictor of HBV-related HCC occurrence.
M2BPGi and its features
In 2013, M2BPGi (also called Wisteria floribunda agglutinin [WFA]-positive M2BP [WFA+ -M2BP]) was introduced as a novel, noninvasive, rapidly assayed serological glycobiomarker for the evaluation of liver fibrosis [69]. Fibrosis results in specific modification of the glycosylation and sugar chain structure of M2BP, whereby levels of these modified M2BP proteins correlate significantly with the progression of fibrosis [69,70].
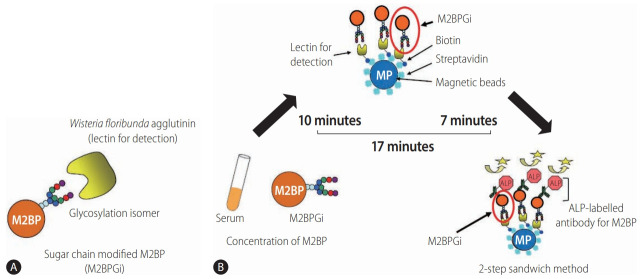
Quantification assays of serum M2BP with fibrosis-specific modified sugar chain structures, i.e., M2BPGi, were developed, evaluated, and approved for clinical use in Japan [69-71]. M2BPGi is detected using a specific lectin called WFA that recognizes the Nacetylgalactosamine residue of N-glycans and O-glycans on M2BP (Fig. 3A) [71]. An automated and high-throughput assay measures M2BPGi levels in 10 µL serum samples in 17 minutes (Fig. 3B) [69]. The M2BPGi assay (Sysmex Corp., Hyogo, Japan) has a reported cut-off index (C.O.I.) range of 0.1–20 and samples with less than 1.0 C.O.I. are considered negative.
Figure 3.
Detection of WFA+-M2BP [139]. (A) Structure of WFA+-M2BP. WFA+-M2BP, which was recently shown to be a liver fibrosis glycobiomarker with a unique fibrosis-related glycoalteration, has multibranching and sialylated N-glycans. WFA lectin binds specifically to the glycosylation isomer. (B) Quantification of serum WFA+-M2BP. WFA+-M2BP quantification is measured based on a lectin-antibody sandwich immunoassay using a fully-automatic immunoanalyzer HISCL-2000i (Sysmex Corp., Hyogo, Japan). The lectin-binding WFA+-M2BP recognizes ALP-labelled antibody in a chemiluminescence enzyme immunoassay (CLEIA). Every reaction is accustomed to the platform during the automatic assay, which is finished after 17 minutes. M2BP, Mac2 binding protein; M2BPGi, Mac-2-binding protein glycosylation isomer; ALP, alkaline phosphatase; WFA+-M2BP, Wisteria floribunda agglutinin-positive Mac-2 binding protein.
M2BPGi for HCC prediction in CHB
M2BPGi is predominantly used to evaluate liver fibrosis in CHC patients [72]. However, the diagnostic value of M2BPGi to assess liver fibrosis was demonstrated in patients with viral hepatitis [69], CHB [73], non-alcoholic fatty liver disease [74], and biliary atresia [75], as well as CHC [76]. In addition, M2BPGi levels are prognostic of the risk of HCC development in CHB [77,78], CHC [76] or non-alcoholic fatty liver disease [79]. Furthermore, post-sustained virological response M2BPGi is a predictive factor for the development of HCC in patients with no previous HCC history and those treated with DAAs for hepatitis C virus (HCV) infection [80]. In this section, we summarize studies showing M2BPGi has good performance for the assessment of liver fibrosis and HCC prediction in CHB.
M2BPGi level predicted HCC development in CHB patients [78]. Kim et al. [78] reported that M2BPGi level was an independent predictor of HCC development (adjusted HR, 1.143; 95% CI, 1.139– 1.829), together with male gender and diabetes (all P<0.05) in multivariate analysis. In patients without cirrhosis (n=1,087), M2BPGi levels ≥1.8 were associated with a higher risk of HCC development (P<0.001), whereas M2BPGi levels ≥1.8 tended to be associated with a higher risk of HCC development in patients with cirrhosis (n=236) (P=0.073) [78].
Conversely, M2BPGi accurately identified CHB patients with a low risk of HCC. Mak et al. [81] evaluated M2BPGi for HCC prediction in 207 non-treated HBeAg-negative patients with recognized HBeAg seroconversion. Using a cut-off of 0.68 C.O.I., the baseline M2BPGi showed good performance (area under the curve [AUC], 0.88; sensitivity, 90%; specificity, 80%). In these HBeAg-negative patients, the NPV was very high (99.3%), while the positive predictive value was low (25.8%) [81].
Additionally, M2BPGi was more effective than AFP for predicting HCC occurrence and was an independent predictor of HCC [82]. Jun et al. [18] enrolled 947 treatment-naive patients mono-infected with HBV or HCV and without HCC at baseline. The median M2BPGi was significantly higher among patients with cirrhosis (2.67 vs. 0.80; P<0.001) and those who developed HCC (3.22 vs. 1.16; P<0.001). M2BPGi outperformed AFP for patients with CHB (0.84 vs. 0.75; P=0.02).
In CHB patients receiving NA treatment, M2BPGi level before treatment and 48 weeks after the start of NA treatment is an indicator of HCC occurrence. Hsu et al. [83] reported the baseline level can be factored into the risk prediction of HCC in NA-treated cirrhosis patients. Baseline M2BPGi level was associated with HCC risk (HR, 1.07 per C.O.I.; 95% CI, 1.01–1.14) in cirrhosis patients, whereas year 1 or 2 levels were not independently predictive [83]. Shinkai et al. [84] reported serum M2BPGi levels ≥1.215 C.O.I. at 48 weeks were associated with HCC development (HR, 5.73; P≤0.001).
In patients with undetectable HBV DNA during NA therapy, higher pre-treatment M2BPGi level was associated with increased risk of HCC development [83,85]. Cheung et al. [85] compared 57 NAtreated patients with undetectable HBV DNA and HCC development with 57 controls. There was a significant difference in median levels of pre-treatment M2BPGi between HCC and control groups (0.67 vs. 0.41 C.O.I.; P<0.001). Among patients with cirrhosis, the median level of M2BPGi was higher in the HCC group than the control group (0.74 vs. 0.47 C.O.I.; P=0.014). Among patients without cirrhosis, the median level of M2BPGi of the HCC group was also higher (0.48 vs. 0.28 C.O.I.; P=0.002). With a cutoff value of 0.69 C.O.I., the AUROC of pre-treatment M2BPGi to predict HCC development for the whole cohort was 0.70. With cut-off values of 0.69 and 0.34 C.O.I. for patients with and without cirrhosis, AUROCs that predicted HCC were 0.67 and 0.77, respectively [85].
In patients with HBV-related HCC, Kim et al. [86] showed that the level of M2BPGi is an independent predictive factor of HBV-related HCC recurrence after curative resection. Also, Heo et al. [87] evaluated its accuracy in assessing liver fibrosis and in predicting the risk of developing HCC in patients with CHB. They concluded the level of M2BPGi significantly reflected degree/extent of liver fibrosis and independently predicted the risk of developing HCC in patients with CHB [87].
Therefore, M2BPGi might be suitable for assessing fibrosis level and prediction of HCC in CHB patients. Clinical applications of M2BPGi in patients with chronic liver disease are summarized in Table 2.
Table 2.
Clinical applications of M2BPGi in CHB patients
| Category | Finding | M2BPGi level (C.O.I.) and point | Reference |
|---|---|---|---|
| Liver fibrosis | Significant fibrosis (≥F2) in CHB | ≥1.06 C.O.I. (AUC, 0.753) | [73] |
| HCC occurrence/recurrence | Higher risk of HCC development in CHB patients | ≥1.8 C.O.I. for patients without cirrhosis (P<0.001) | [78] |
| ≥1.8 C.O.I. for patients with cirrhosis (P=0.073) | |||
| Low risk of HCC in HBeAg-negative patients | ≤0.68 C.O.I. at baseline | [81] | |
| Risk for HCC development in CHB patients with cirrhosis treated with NAs | M2BPGi-based score* ≥652.5 at baseline | [83] | |
| Risk for HCC development in CHB patients treated with NAs | ≥1.215 C.O.I. at 48 weeks | [84] | |
| HCC development in CHB patients | ≥0.69 C.O.I. at baseline | [85] |
M2BPGi, Mac-2 binding protein glycosylation isomer; CHB, chronic hepatitis B; C.O.I., cut-off index; AUC, area under the curve; HCC, hepatocellular carcinoma; HBeAg, hepatitis B envelope antigen; NAs, nucleos(t)ide analogues.
M2BPGi-based score, 8 × age (years) + 7 × baseline M2BPGi (COI) + 10 × body mass index (kg/m2). The score was calculable in 171 CHB patients with cirrhosis with a median of 652.5 (IQR, 581.3, 709.4) point.
TUMOR MARKERS: AFP, AFP-L3, PIVKA-II, DKK-1 AND CIRCULATING IgG ANTIBODIES
Measuring serum levels of tumor biomarkers for HCC is essential for CHB management. AFP, AFP-L3, and PIVKA-II are HCC-specific tumor markers [88]. Although tumor marker levels are not included in diagnostic criteria for HCC, screening recommendations in the guidelines of the American Association for the Study of Liver Diseases [89], or the European Association for the Study of the Liver [90], they provide valuable supportive information for diagnosing HCC.
Levels of AFP, AFP-L3, and PIVKA-II usually increase as HCC develops, i.e., with increased size and number of HCC lesions and progression to portal vein invasion [88]. Other studies reported increased tumor marker levels suggested a high degree of HCC malignancy regardless of morphological progression [91]. Therefore, increased levels of these tumor markers indicates an unfavorable prognosis after initial diagnosis [88].
An increased level of DKK-1 occurs in a wide variety of cancers, including multiple myeloma, prostate cancer and HCC [92,93]. Circulating anti-CD25 IgG antibodies may have prognostic rather than early diagnostic values for HCC [94]. Circulating IgG antibody to p16 protein might be a useful biomarker for HCC prognosis assessment rather than for early diagnosis of HCC [95].
In this section, we introduce three tumor markers, AFP, AFP-L3, and PIVKA-II, as indicators of HCC development and predictors of patient outcome. In addition, we provide information of DKK-1 and circulating IgG antibodies as novel markers of HCC.
AFP and AFP-L3
AFP was first discovered in the serum of HCC patients in 1964 and has since been the primary diagnostic biomarker for HCC [96]. It is normally produced at a low concentration by liver, yolk sac, and gastrointestinal tract during fetal and neonatal development [97]. High levels of serum AFP correlated with the presence and development of HCC and it is used as a diagnostic and prognostic factor [98].
Despite this, there are multiple problems using AFP as a diagnostic marker, such as elevation in non-HCC diseases including cirrhosis, hepatitis, cholangiocarcinoma, testicular germ cell tumor, and metastatic colon cancer [98]. Because of its low diagnostic accuracy, with sensitivities ranging from 18–60% and specificity of 85–90%, AFP has recently been excluded from American Association for Study of Liver Diseases (AASLD) HCC surveillance guidelines [99]. Indeed, only 60–80% of HCC have elevated AFP levels, leaving a large margin for false-negatives and missed diagnoses [100]. Therefore, AFP alone is not recommended as the main screening test for HCC [98]. To significantly improve the diagnostic accuracy of HCC, additional biomarkers are needed to complement AFP [100].
AFP exists as three glycoforms, each with different binding competence to lectin Lens culinaris agglutinin. AFP-L1 is a nonbinding fraction, AFP-L2 is a weak binding fraction, and AFP-L3 is a binding fraction [100]. AFP-L1 is increased in chronic hepatitis and liver cirrhosis, whereas AFP-L3 is specifically increased in HCC [100]. Because AFP-L3 has a high specificity of 92.0–99.4% for HCC and a low sensitivity of 18.8–37.0% [101-103], it is considered a more specific biomarker for HCC [104]. Moreover, because AFP-L3 is typically not detected when AFP levels are <20 ng/mL, AFP-L3 is not relevant for the diagnosis of HCC in individuals with a total AFP concentration <20 ng/mL. Thus, the sensitivity for AFP-L3 appears to be adversely affected by the total AFP concentration [100,105]. Furthermore, AFP and AFP-L3 levels were detected in blood 6months prior to a diagnosis of HCC [106].
Recent improvements in highly sensitive analytical methods have enhanced the sensitivity of the AFP-L3 immunoassay, referred to as “highly sensitive AFP-L3” (hs-AFP-L3) [107]. Toyoda et al. [107] examined patients with benign liver disease (n=44) including chronic hepatitis, cirrhosis, and HCC (n=54) and determined the performance of hs-AFP-L3. The sensitivity and specificity of hsAFP-L3 were 84.9% and 88.6%, respectively [107]. Additionally, postoperative AFP-L3% was a prognostic factor of HCC recurrence after curative treatment and detected small tumors and early stage HCC [108,109]. These results indicate hs-AFP-L3% might be a valuable biomarker for detecting early-stage HCC.
PIVKA-II
PIVKA-II, known as des-γ-carboxyprothrombin, is an abnormal prothrombin molecule that is increased in HCC [100]. During malignant transformation in hepatocytes, the vitamin K-dependent carboxylase system becomes damaged [110]: a deficiency in post-translational carboxylation leads to the production of PIVKA-II [111]. During this process, PIVKA-II loses its normal prothrombin function but may promote malignant proliferation in HCC. One benefit of PIVKA-II is that it is less likely to be elevated in non-HCC liver diseases than AFP [98]. In a screening test for HCC, when compared to cases of cirrhosis and chronic hepatitis, PIVKA-II yielded a sensitivity of 72.7% and a specificity of 90.0%, which was comparable to AFP [110].
Recently, the performance of AFP was compared with PIVKA-II for the diagnosis of early-stage HCC in France [112]. This study included 43 cirrhosis patients and 85 HCC patients including 32 patients with early HCC. PIVKA-II (threshold value, 42 mAU/mL) performed better than AFP (threshold value, 5.5 ng/mL) for earlystage HCC diagnosis (AUC, 0.81; 95% CI, 0.697–0.924 vs. AUC, 0.582; 95% CI, 0.443–0.722), and a PIVKA-II level >90 mAU/mL was an independent predictor of microvascular invasion (HR, 3.5; 95% CI, 1.08–11.8; P=0.043), a major prognostic factor in HCC [112].
As described above, PIVKA-II is a potential serum biomarker for the early diagnosis of HCC. Furthermore, Chen et al. [113] reported a meta-analysis where PIVKA-II had a better accuracy than AFP for the detection of HCC in CHB, regardless of tumor size, patient ethnic background (American, European, Asian, or African), or etiology of HCC (HBV-related or mixed).
Most importantly, serum PIVKA-II level is a suitable parameter for the development of portal venous invasion [114]. Koike et al. [114] enrolled 227 HCC patients who did not show portal venous invasion and who received percutaneous ethanol injection therapy and/or microwave coagulation therapy at the time of their first hospital admission. After their HCC was treated, the patients were followed for a mean of 19 months. Of the 227 patients, 24 (11%) later developed portal venous invasion, and the PIVKA-II level at the time of initial diagnosis of HCC had a significant correlation with the later development of portal venous invasion [114]. Yamashita et al. [115] reported that even in cases with small HCC (≤2 cm), patients with a high PIVKA-II level (>100 mAU/mL) were at risk for microinvasion. Therefore, in such patients, hepatic resection with a wide tumor margin should be recommended [115].
Moreover, Lee et al. [116] investigated whether pre- or post-ablation serum AFP and PIVKA-II levels predicted prognosis in patients with curative radiofrequency ablation (RFA) for HBV-related HCC. They retrospectively analyzed 412 patients with HBV-related single HCC treated with percutaneous RFA. AFP and PIVKA-II levels were measured before and 1 month after treatment. Among the tumor markers, post-ablation PIVKA-II was an independent prognostic factor for overall and recurrence-free survival (HR, 3.438; 95% CI, 1.331–8.877; P=0.011 and HR, 4.934; 95% CI, 2.761–8.816; P<0.001, respectively). They concluded that postablation serum PIVKA-II is a useful biomarker for predicting survival and recurrence after curative RFA in patients with HBV-related HCC [116].
Combination of AFP and PIVKA-II
Because PIVKA-II is more specific to HCC and has a reduced tendency to be elevated in other chronic liver diseases, a combination of AFP and PIVKA-II had a higher efficiency for the diagnosis of early HCC in cirrhosis patients. Lok et al. [117] reported a combination of AFP and PIVKA-II had increased sensitivity (65% to 87%) compared with AFP alone, but decreased specificity (84% to 69%). For screening, this increased sensitivity is obviously valuable [117]. Song et al. reported that in 120 patients with HCC, PIVKAII alone was inferior to the combination of AFP and PIVKA-II because of its lower sensitivity (53.3% vs. 78.3%) [118]. A recent metaanalysis including 11 studies by Caviglia et al. [119] also described that the use of AFP and PIVKA-II may improve the effectiveness of surveillance among patients at risk for HCC development. The weighted summary AUC (sAUC) of PIVKA-II and AFP for the discrimination between patients with HCC and those without was 0.791 (0.746–0.837) and 0.767 (0.732–0.803), respectively. The combination of PIVKA-II and AFP resulted in a sAUC of 0.859 (0.837–0.882), suggesting that the performance for HCC detection of PIVKA-II andAFP was significantly superior to each biomarker used alone (ΔsAUC, 0.068; P=0.032 and ΔsAUC, 0.092; P<0.001, respectively) [119].
Previous studies suggested specific biomarkers for HCC diagnosis, of which the combination of AFP and PIVKA-II appeared to be the best. Chen et al. [120] selected five representative biomarkers (AFP, AFP-L3, PIVKA-II, squamous cell carcinoma antigen, and centromere protein F autoantibody) with diagnostic potential for detecting HCC. Serum levels of the five biomarkers were simultaneously measured in a large sample set (n=846) including patients with HCC, cirrhosis, chronic HBV infection, and healthy controls.120 Overall, for two-marker combinations, a prediction algorithm including AFP and PIVKA-II had the best diagnostic value. The apparent AUC (without correction) of the two-marker algorithm in detecting early-stage HCC was 0.86 (95% CI, 0.81–0.90). Moreover, in another report of the diagnosis of HCC associated with alcoholic and non-alcoholic fatty liver disease, AFP and PIVKA-II appeared to be the best combination of biomarkers [121], suggesting that PIVKA-II might be a complementary biomarker for the diagnosis of HCC and to improve the identification of patients with AFP-negative HCC. Meanwhile, as the recent report, combined microRNA-122 (miR-122), AFP plus PIVKA-II had an adjusted HR for HCC development of 10.63 [122]. AUCs were 0.675 for miR-122, 0.791 for AFP and 0.846 for PIVKA-II, respectively, while their combination improved the discrimination power between cirrhosis and HCC (AUC, 0.918) [122].
DKK-1
DKK-1 is a glycoprotein which is a secretory antagonist of the Wnt/beta-catenin signaling pathway [123]. Although its detailed function is not completely understood, the increase of DKK-1 expression occurs in a wide variety of cancers, including multiple myeloma, prostate cancer and HCC [92].
Several studies have shown that higher DKK-1 levels in HCC patients than in healthy individuals [95,124-126] or than in patients with cirrhosis without HCC [126-128]. Shen et al. [126] reported serum DKK-1 and AFP levels in a cohort of 1,284 patients. DKK-1 was more useful for detection of AFP-negative HCC, while combining DKK-1 with AFP enhanced the detection rate of early-stage HCC to help in detection in HCCs, which do not produce AFP [126]. Another report from South Korea showed that the combination of AFP and DKK-1 was just slightly better than AFP alone for the detection of early-stage HCC (AUC, 0.693 vs. 0.691) in HCC patients with mainly HBV-infection (n=208) [129].
In addition, a meta-analysis published in 2014 containing four studies [130] and other recently published study [128] showed that higher levels of DKK-1 expression in patients with HCC were associated with the lower survival rate.
Circulating IgG antibodies
In recent studies, Wang et al. [94] found that serum IgG antibodies against linear peptide antigens derived from p16 protein, interleukin 2 receptor α-subunit (also called CD25) and forkhead/wingedhelix transcription factor box P3 (FOXP3) were significantly changed in the patients with HCC. Therefore, circulating IgG antibodies for these target molecules may be either diagnostic or prognostic values for solid tumors [131]. Serum levels of IgG antibodies for p16a, CD25a and FOXP3 were significantly higher in HCC patients than control subjects [131].
Further analysis showed that increased levels of plasma IgG for these three peptide antigens were mainly shown in patients with the intermediate and late-stage HCC. This study has confirmed that serum IgG antibodies to p16, CD25 and FOXP3 are significantly increased in HCC patients, especially in late-stage HCC. These autoantibodies may be useful biomarkers for assessment of HCC prognosis [131].
DISCUSSION
In this review, we described the characteristics and clinical applications of novel biomarkers. HBcrAg is appropriate for evaluating the amount of intrahepatic cccDNA in CHB patients. M2BPGi can assess the severity of liver fibrosis and the risk of HCC development in CHB patients. Regarding tumor markers including AFP, PIVKA-II, and AFP-L3, DKK-1 and circulating IgG antibodies, using only one had a restricted detection rate for HCC, but the combination by two showed improved detection rates.
Here, we discuss the future prospects of HBcrAg, M2BPGi, and tumor markers. First, the amount of HBcrAg is associated with that of HBV DNA in all CHB disease states. Even in patients who achieve a “functional cure” with undetectable serum HBV DNA and HBsAg, severe complications including HBV reactivation and HCC occurrence may be reported. Because some patients achieving a “functional cure” still have detectable serum HBcrAg, prospective studies comparing the long-term outcome between HBcrAg-positive and HBcrAg-negative patients are required. To date, most reports have been published from East Asia. For HBcrAg to be used more in clinical practice, large cohort studies should be completed in other areas, especially the United States and Europe.
To confirm a functional cure of HBV, higher sensitivity assays for HBsAg and HBcrAg are required. Recently, a new HBcrAg assay with approximately 10-fold higher sensitivity is being developed [29]. Furthermore, based on a fully-automated pretreatment technique before HBcrAg measurement, new highly sensitive HBcrAg measurements should be used to monitor HBeAg-negative patients.
Second, M2BPGi has been used as a valuable biomarker to evaluate liver fibrosis, especially in CHC patients. Although past studies have documented the accuracy of M2BPGi assay in patients with CHC, few have addressed CHB [73,78,87,132,133].
The M2BPGi cut-off value for predicting cirrhosis or HCC varies, depending on disease etiology. Ichikawa et al. [134] reported that M2BPGi ≥0.71 was a risk factor for HCC development in CHB patients. As described above, M2BPGi levels ≥1.215 48 weeks after starting NA treatment [84] were associated with HCC. Taken together, the M2BPGi levels of CHB patients seem to be lower than those of CHC patients. Conversely, in CHC independent of liver fibrosis, whether the M2BPGi level is increased is still controversial. Only one study has evaluated M2BPGi as a predictor of HCC in non‐Asian patients and patients outside East Asia [18]. Further studies should evaluate M2BPGi as an HCC biomarker in broader patient populations, including non‐Asians and those with severe liver fibrosis.
Third, although the diagnosis of HCC remains difficult, especially in the early stage, the early and accurate diagnosis of HCC is vital to improve CHB patient outcomes. Current information suggests no single biomarker is likely to have optimal sensitivity and specificity for the detection of HCC, particularly at the early stages of development. Many studies reported combinations of biomarkers improved the detection of early stage HCC. Additional studies are needed to evaluate further the effectiveness of combined biomarkers for HCC diagnosis.
Recent studies reported a combination of these three tumor markers had very high sensitivity and specificity for diagnosing HCC without the use of imaging studies [135], and improved the sensitivity and specificity of early HCC diagnosis [136]. In the JSH-HCC guidelines, simultaneous measurement of AFP, AFP-L3, and PIVKA-II with ultrasound examination is recommended for HCC surveillance of high-risk populations [16].
When considering HCC biomarker applications, there must be a careful and an accurate consideration as to the various metabolic profiles of variable ethnic groups [137]. Nguyen et al. [138] reported clear ethnic differences in the diagnostic value of AFP. Patients with HCV-related HCC in Asia, Europe, and Central and South America had a normal AFP level of 18%, whereas African-American patients had normal level of 43%. Furthermore, there was difference between the underlying etiologies of liver disease, where HCV-related HCC was more strongly associated with elevated AFP compared with HBV-related HCC [138]. Further studies are needed to evaluate PIVKA-II as an HCC biomarker in broader patient populations, including non‐Asians and those with mild liver fibrosis. In summary, etiological, dietary, genetic, and environmental factors that differ between populations suggest the need for validation studies in various regions to establish better diagnostic and screening tools.
CONCLUSIONS
HBcrAg and M2BPGi are useful novel biomarkers for the management of CHB including predicting HCC occurrence. AFP, AFPL3, PIVKA-II, DKK-1 and circulating IgG antibodies are HCC-specific tumor markers. Combinations of these biomarkers might have better prospects for application in clinical practice. Further global studies are required to increase the application of these useful biomarkers for many aspects of CHB clinical practice.
Acknowledgments
This work was supported by a grant-in-aid from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development (AMED JP19fk0310101) and the Ministry of Education, Culture, Sports, Science, and Technology (19H03640).
Abbreviations
- AASLD
American Association for Study of Liver Diseases
- ADV
adefovir
- AFP-L3
Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein
- AFP
alpha fetoprotein
- ALT
alanine aminotransferase
- AUC
area under the curve
- AUROC
area under the receiver operating characteristics
- BCP
basal core promoter
- C.O.I.
cut-off index
- cccDNA
covalently closed circular DNA
- CHB
chronic hepatitis B
- CI
confidence interval
- DKK-1
Dickkopf-1
- ETV
entecavir
- FBS
fasting blood sugar
- FOXP
factor box P3
- HBcrAg
hepatitis B core-related antigen
- HBeAg
hepatitis B envelope antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- hs-AFP-L3
highly sensitive AFP-L3
- IFN
interferon
- IgG
immunoglobulin G
- JSH
Japan Society of Hepatology
- M2BP
Mac-2 binding protein
- M2BPGi
Mac-2- binding protein glycosylation isomer
- miR-122
microRNA-122
- NA
nucleos(t) ide analogue
- NPV
negative predictive value
- PEG-IFN
pegylated interferon
- PIVKA-II
protein induced by vitamin K absence factor II
- RFA
radiofrequency ablation
- sAUC
weighted summary AUC
- TDF
tenofovir
- WFA
Wisteria floribunda agglutinin
- WFA+ -M2BP
Wisteria floribunda agglutinin-positive Mac-2 binding protein
Footnotes
Authors’ contribution
Conceptualization, T.I. and Y.T.; Writing and Original Draft Preparation, T.I.; Writing, Review and Editing, Y.T.
Conflicts of Interest: Takako Inoue is currently supported by a research grant from Gilead Sciences and MSD.K.K. Yasuhito Tanaka is currently conducting research sponsored by Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Fujirebio, Inc., and Gilead Sciences. Lecture fees are follows: Fujirebio, Inc. and Gilead Sciences.
REFERENCES
- 1.World Health Organization (WHO) Hepatitis B factsheet. WHO web site, < https://www.who.int/news-room/fact-sheets/detail/hepatitis-b>. Accessed 31 Mar 2020.
- 2.Inoue T, Tanaka Y. Hepatitis B virus and its sexually transmitted infection - an update. Microb Cell. 2016;3:420–437. doi: 10.15698/mic2016.09.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.Ho SY, Liu PH, Hsu CY, Hsia CY, Huang YH, Lei HJ, et al. Evolution of etiology, presentation, management and prognostic tool in hepatocellular carcinoma. Sci Rep. 2020;10:3925. doi: 10.1038/s41598-020-61028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology. 2017;66:1296–1313. doi: 10.1002/hep.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glebe D, Bremer CM. The molecular virology of hepatitis B virus. Semin Liver Dis. 2013;33:103–112. doi: 10.1055/s-0033-1345717. [DOI] [PubMed] [Google Scholar]
- 8.Lopatin U. Drugs in the pipeline for HBV. Clin Liver Dis. 2019;23:535–555. doi: 10.1016/j.cld.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Gane EJ. Future anti-HBV strategies. Liver Int. 2017;37 Suppl 1:40–44. doi: 10.1111/liv.13304. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Tanaka Y. The role of hepatitis B core-related antigen. Genes (Basel) 2019;10:357. doi: 10.3390/genes10050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44 Suppl S1:1–58. doi: 10.1111/hepr.12269. [DOI] [PubMed] [Google Scholar]
- 14.Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 15.Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25:245–263. doi: 10.3350/cmh.2018.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 17.Lin CL, Kao JH. Perspectives and control of hepatitis B virus infection in Taiwan. J Formos Med Assoc. 2015;114:901–909. doi: 10.1016/j.jfma.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Jun T, Hsu YC, Ogawa S, Huang YT, Yeh ML, Tseng CH, et al. Mac-2 binding protein glycosylation isomer as a hepatocellular carcinoma marker in patients with chronic hepatitis B or C infection. Hepatol Commun. 2019;3:493–503. doi: 10.1002/hep4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang HC, Kao JH. Looking into the crystal ball: biomarkers for outcomes of HBV infection. Hepatol Int. 2016;10:99–101. doi: 10.1007/s12072-015-9698-x. [DOI] [PubMed] [Google Scholar]
- 20.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 22.Kawanaka M, Nishino K, Nakamura J, Oka T, Urata N, Goto D, et al. Quantitative levels of hepatitis B virus DNA and surface antigen and the risk of hepatocellular carcinoma in patients with hepatitis B receiving long-term nucleos(t)ide analogue therapy. Liver Cancer. 2014;3:41–52. doi: 10.1159/000343857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuaypen N, Posuwan N, Chittmittraprap S, Hirankarn N, Treeprasertsuk S, Tanaka Y, et al. Predictive role of serum HBsAg and HBcrAg kinetics in patients with HBeAg-negative chronic hepatitis B receiving pegylated interferon-based therapy. Clin Microbiol Infect. 2018;24:306. doi: 10.1016/j.cmi.2017.07.016. e7-306.e13. [DOI] [PubMed] [Google Scholar]
- 24.Brouwer WP, Chan HL, Brunetto MR, Martinot-Peignoux M, Arends P, Cornberg M, et al. Repeated measurements of hepatitis B surface antigen identify carriers of inactive HBV during long-term follow-up. Clin Gastroenterol Hepatol. 2016;14:1481–1489. doi: 10.1016/j.cgh.2016.01.019. e5. [DOI] [PubMed] [Google Scholar]
- 25.Yeo YH, Ho HJ, Yang HI, Tseng TC, Hosaka T, Trinh HN, et al. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV Infection: a systematic review and meta-analysis. Gastroenterology. 2019;156:635–646. doi: 10.1053/j.gastro.2018.10.027. e9. [DOI] [PubMed] [Google Scholar]
- 26.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Wang XW, Chen L, Hu P, Ren H, Hu HD. Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 2016;43:1253–1261. doi: 10.1111/apt.13634. [DOI] [PubMed] [Google Scholar]
- 28.Kim GA, Lee HC, Kim MJ, Ha Y, Park EJ, An J, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol. 2015;62:1092–1099. doi: 10.1016/j.jhep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Baudi I, Inoue T, Tanaka Y. Novel biomarkers of hepatitis B and hepatocellular carcinoma: clinical significance of HBcrAg and M2BPGi. Int J Mol Sci. 2020;21:949. doi: 10.3390/ijms21030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locarnini S, Zoulim F. Molecular genetics of HBV infection. Antivir Ther. 2010;15 Suppl 3:3–14. doi: 10.3851/IMP1619. [DOI] [PubMed] [Google Scholar]
- 31.Kimura T, Ohno N, Terada N, Rokuhara A, Matsumoto A, Yagi S, et al. Hepatitis B virus DNA-negative dane particles lack core protein but contain a 22-kDa precore protein without C-terminal argininerich domain. J Biol Chem. 2005;280:21713–21719. doi: 10.1074/jbc.M501564200. [DOI] [PubMed] [Google Scholar]
- 32.Hadziyannis E, Laras A. Viral biomarkers in chronic HBeAg negative HBV infection. Genes (Basel) 2018;9:469. doi: 10.3390/genes9100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rokuhara A, Tanaka E, Matsumoto A, Kimura T, Yamaura T, Orii K, et al. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J Viral Hepat. 2003;10:324–330. doi: 10.1046/j.1365-2893.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 34.Maasoumy B, Wiegand SB, Jaroszewicz J, Bremer B, Lehmann P, Deterding K, et al. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clin Microbiol Infect. 2015;21:606. doi: 10.1016/j.cmi.2015.02.010. e1-e10. [DOI] [PubMed] [Google Scholar]
- 35.Kimura T, Rokuhara A, Sakamoto Y, Yagi S, Tanaka E, Kiyosawa K, et al. Sensitive enzyme immunoassay for hepatitis B virus corerelated antigens and their correlation to virus load. J Clin Microbiol. 2002;40:439–445. doi: 10.1128/JCM.40.2.439-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47:43–54. doi: 10.1111/apt.14376. [DOI] [PubMed] [Google Scholar]
- 37.Seto WK, Wong DK, Fung J, Huang FY, Liu KS, Lai CL, et al. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clin Microbiol Infect. 2014;20:1173–1180. doi: 10.1111/1469-0691.12739. [DOI] [PubMed] [Google Scholar]
- 38.Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615–625. doi: 10.1016/j.jhep.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Riveiro-Barciela M, Bes M, Rodríguez-Frías F, Tabernero D, Ruiz A, Casillas R, et al. Serum hepatitis B core-related antigen is more accurate than hepatitis B surface antigen to identify inactive carriers, regardless of hepatitis B virus genotype. Clin Microbiol Infect. 2017;23:860–867. doi: 10.1016/j.cmi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Liu YY, Liang XS. Progression and status of antiviral monitoring in patients with chronic hepatitis B: from HBsAg to HBV RNA. World J Hepatol. 2018;10:603–611. doi: 10.4254/wjh.v10.i9.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa K, Nishikawa H, Enomoto H, Iwata Y, Sakai Y, Ikeda N, et al. Proposed model for the prediction of intrahepatic covalently closed circular DNA level in patients with chronic hepatitis B. Hepatol Res. 2019;49:271–283. doi: 10.1111/hepr.13280. [DOI] [PubMed] [Google Scholar]
- 42.Shimakawa Y, Ndow G, Njie R, Njai HF, Takahashi K, Akbar SMF, et al. Hepatitis B core-related antigen: an alternative to hepatitis B virus DNA to assess treatment eligibility in Africa. Clin Infect Dis. 2020;70:1442–1452. doi: 10.1093/cid/ciz412. [DOI] [PubMed] [Google Scholar]
- 43.Lai CL, Wong D, Ip P, Kopaniszen M, Seto WK, Fung J, et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol. 2017;66:275–281. doi: 10.1016/j.jhep.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 44.Lutgehetmann M, Volzt T, Quaas A, Zankel M, Fischer C, Dandri M, et al. Sequential combination therapy leads to biochemical and histological improvement despite low ongoing intrahepatic hepatitis B virus replication. Antivir Ther. 2008;13:57–66. [PubMed] [Google Scholar]
- 45.Wong DK, Tanaka Y, Lai CL, Mizokami M, Fung J, Yuen MF. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol. 2007;45:3942–3947. doi: 10.1128/JCM.00366-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong DK, Seto WK, Cheung KS, Chong CK, Huang FY, Fung J, et al. Hepatitis B virus core-related antigen as a surrogate marker for covalently closed circular DNA. Liver Int. 2017;37:995–1001. doi: 10.1111/liv.13346. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki F, Miyakoshi H, Kobayashi M, Kumada H. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J Med Virol. 2009;81:27–33. doi: 10.1002/jmv.21339. [DOI] [PubMed] [Google Scholar]
- 48.Seto WK, Tanaka Y, Wong DK, Lai CL, Shinkai N, Yuen JC, et al. Evidence of serologic activity in chronic hepatitis B after surface antigen (HBsAg) seroclearance documented by conventional HBsAg assay. Hepatol Int. 2012;7:98–105. doi: 10.1007/s12072-012-9354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuaypen N, Posuwan N, Payungporn S, Tanaka Y, Shinkai N, Poovorawan Y, et al. Serum hepatitis B core-related antigen as a treatment predictor of pegylated interferon in patients with HBeAg-positive chronic hepatitis B. Liver Int. 2016;36:827–836. doi: 10.1111/liv.13046. [DOI] [PubMed] [Google Scholar]
- 50.Ma H, Yang RF, Li XH, Jin Q, Wei L. HBcrAg identifies patients failing to achieve HBeAg seroconversion treated with pegylated interferon alfa-2b. Chin Med J (Engl) 2016;129:2212–2219. doi: 10.4103/0366-6999.189904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang B, Carey I, Bruce M, Montague S, Dusheiko G, Agarwal K. HBsAg and HBcrAg as predictors of HBeAg seroconversion in HBeAg-positive patients treated with nucleos(t)ide analogues. J Viral Hepat. 2018;25:886–893. doi: 10.1111/jvh.12889. [DOI] [PubMed] [Google Scholar]
- 52.Wang ML, Deng R, Chen EQ, Tao CM, Liao J, Zhou TY, et al. Performance of serum HBcrAg in chronic hepatitis B patients with 8-year nucleos(t)ide analogs therapy. Clin Res Hepatol Gastroenterol. 2019;43:301–309. doi: 10.1016/j.clinre.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto A, Tanaka E, Minami M, Okanoue T, Yatsuhashi H, Nagaoka S, et al. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol Res. 2007;37:661–666. doi: 10.1111/j.1872-034X.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 54.Shinkai N, Tanaka Y, Orito E, Ito K, Ohno T, Hirashima N, et al. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol Res. 2006;36:272–276. doi: 10.1016/j.hepres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Caviglia GP, Abate ML, Noviello D, Olivero A, Rosso C, Troshina G, et al. Hepatitis B core-related antigen kinetics in chronic hepatitis B virus genotype D-infected patients treated with nucleos(t)ide analogues or pegylated-interferon-α. Hepatol Res. 2017;47:747–754. doi: 10.1111/hepr.12811. [DOI] [PubMed] [Google Scholar]
- 56.Jung KS, Park JY, Chon YE, Kim HS, Kang W, Kim BK, et al. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J Gastroenterol. 2016;51:830–839. doi: 10.1007/s00535-015-1153-1. [DOI] [PubMed] [Google Scholar]
- 57.Hsu YC, Nguyen MH, Mo LR, Wu MS, Yang TH, Chen CC, et al. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment Pharmacol Ther. 2019;49:107–115. doi: 10.1111/apt.15058. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki Y, Maekawa S, Komatsu N, Sato M, Tatsumi A, Miura M, et al. HBV preS deletion mapping using deep sequencing demonstrates a unique association with viral markers. PLoS One. 2019;14:e0212559. doi: 10.1371/journal.pone.0212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ando Y, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, et al. Cumulative incidence and risk factors for the development of hepatocellular carcinoma in patients with chronic hepatitis B who achieved sustained disappearance of viremia by nucleos(t)ide analog treatment. Hepatol Res. 2018;48:E240–E251. doi: 10.1111/hepr.12976. [DOI] [PubMed] [Google Scholar]
- 60.Wong GL, Wong VW. Risk prediction of hepatitis B virus-related hepatocellular carcinoma in the era of antiviral therapy. World J Gastroenterol. 2013;19:6515–6522. doi: 10.3748/wjg.v19.i39.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosaka T, Suzuki F, Kobayashi M, Fujiyama S, Kawamura Y, Sezaki H, et al. Impact of hepatitis B core-related antigen on the incidence of hepatocellular carcinoma in patients treated with nucleos(t)ide analogues. Aliment Pharmacol Ther. 2019;49:457–471. doi: 10.1111/apt.15108. [DOI] [PubMed] [Google Scholar]
- 62.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–433. doi: 10.1016/j.jhep.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 63.Honda M, Shirasaki T, Terashima T, Kawaguchi K, Nakamura M, Oishi N, et al. Hepatitis B virus (HBV) core-related antigen during nucleos(t)ide analog therapy is related to intra-hepatic HBV replication and development of hepatocellular carcinoma. J Infect Dis. 2016;213:1096–1106. doi: 10.1093/infdis/jiv572. [DOI] [PubMed] [Google Scholar]
- 64.Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, et al. HBcrAg predicts hepatocellular carcinoma development: an analysis using time-dependent receiver operating characteristics. J Hepatol. 2016;65:48–56. doi: 10.1016/j.jhep.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Tseng TC, Liu CJ, Hsu CY, Hong CM, Su TH, Yang WT, et al. High level of hepatitis B core-related antigen associated with increased risk of hepatocellular carcinoma in patients with chronic HBV infection of intermediate viral load. Gastroenterology. 2019;157:1518–1529. doi: 10.1053/j.gastro.2019.08.028. e3. [DOI] [PubMed] [Google Scholar]
- 66.Cheung KS, Seto WK, Wong DK, Lai CL, Yuen MF. Relationship between HBsAg, HBcrAg and hepatocellular carcinoma in patients with undetectable HBV DNA under nucleos(t)ide therapy. J Viral Hepat. 2017;24:654–661. doi: 10.1111/jvh.12688. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki Y, Maekawa S, Komatsu N, Sato M, Tatsumi A, Miura M, et al. Hepatitis B virus (HBV)-infected patients with low hepatitis B surface antigen and high hepatitis B core-related antigen titers have a high risk of HBV-related hepatocellular carcinoma. Hepatol Res. 2019;49:51–63. doi: 10.1111/hepr.13277. [DOI] [PubMed] [Google Scholar]
- 68.Chen S, Jia J, Gao Y, Li H, Fang M, Feng H, et al. Clinical evaluation of hepatitis B core-related antigen in chronic hepatitis B and hepatocellular carcinoma patients. Clin Chim Acta. 2018;486:237–244. doi: 10.1016/j.cca.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 69.Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narimatsu H. Development of M2BPGi: a novel fibrosis serum glyco-biomarker for chronic hepatitis/cirrhosis diagnostics. Expert Rev Proteomics. 2015;12:683–693. doi: 10.1586/14789450.2015.1084874. [DOI] [PubMed] [Google Scholar]
- 71.Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76–84. doi: 10.1007/s00535-014-0946-y. [DOI] [PubMed] [Google Scholar]
- 72.Sato S, Genda T, Ichida T, Amano N, Sato S, Murata A, et al. Prediction of hepatocellular carcinoma development after hepatitis C virus eradication using serum Wisteria floribunda agglutininpositive Mac-2-binding protein. Int J Mol Sci. 2016;17:2143. doi: 10.3390/ijms17122143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou X, Zhu MY, Yu DM, Li W, Zhang DH, Lu FJ, et al. Serum WFA+-M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2017;37:35–44. doi: 10.1111/liv.13188. [DOI] [PubMed] [Google Scholar]
- 74.Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50:776–784. doi: 10.1007/s00535-014-1007-2. [DOI] [PubMed] [Google Scholar]
- 75.Yamada N, Sanada Y, Tashiro M, Hirata Y, Okada N, Ihara Y, et al. Serum Mac-2 binding protein glycosylation isomer predicts grade F4 liver fibrosis in patients with biliary atresia. J Gastroenterol. 2017;52:245–252. doi: 10.1007/s00535-016-1235-8. [DOI] [PubMed] [Google Scholar]
- 76.Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Elevated serum levels of Wisteria floribunda agglutininpositive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563–1570. doi: 10.1002/hep.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chuaypen N, Chittmittraprap S, Pinjaroen N, Sirichindakul B, Poovorawan Y, Tanaka Y, et al. Serum Wisteria floribunda agglutininpositive Mac-2 binding protein level as a diagnostic marker of hepatitis B virus-related hepatocellular carcinoma. Hepatol Res. 2018;48:872–881. doi: 10.1111/hepr.13187. [DOI] [PubMed] [Google Scholar]
- 78.Kim SU, Heo JY, Kim BK, Park JY, Kim DY, Han KH, et al. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts the risk of HBV-related liver cancer development. Liver Int. 2017;37:879–887. doi: 10.1111/liv.13341. [DOI] [PubMed] [Google Scholar]
- 79.Kawanaka M, Tomiyama Y, Hyogo H, Koda M, Shima T, Tobita H, et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts the development of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatol Res. 2018;48:521–528. doi: 10.1111/hepr.13054. [DOI] [PubMed] [Google Scholar]
- 80.Yasui Y, Kurosaki M, Komiyama Y, Takada H, Tamaki N, Watakabe K, et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus. Hepatol Res. 2018;48:1131–1139. doi: 10.1111/hepr.13233. [DOI] [PubMed] [Google Scholar]
- 81.Mak LY, To WP, Wong DK, Fung J, Liu F, Seto WK, et al. Serum Mac2 binding protein glycosylation isomer level predicts hepatocellular carcinoma development in E-negative chronic hepatitis B patients. World J Gastroenterol. 2019;25:1398–1408. doi: 10.3748/wjg.v25.i11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Hu HH, Lee MH, Korenaga M, Jen CL, Batrla-Utermann R, et al. Serum levels of M2BPGi as short-term predictors of hepatocellular carcinoma in untreated chronic hepatitis B patients. Sci Rep. 2017;7:14352. doi: 10.1038/s41598-017-14747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsu YC, Jun T, Huang YT, Yeh ML, Lee CL, Ogawa S, et al. Serum M2BPGi level and risk of hepatocellular carcinoma after oral antiviral therapy in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48:1128–1137. doi: 10.1111/apt.15006. [DOI] [PubMed] [Google Scholar]
- 84.Shinkai N, Nojima M, Iio E, Matsunami K, Toyoda H, Murakami S, et al. High levels of serum Mac-2-binding protein glycosylation isomer (M2BPGi) predict the development of hepatocellular carcinoma in hepatitis B patients treated with nucleot(s)ide analogues. J Gastroenterol. 2018;53:883–889. doi: 10.1007/s00535-017-1424-0. [DOI] [PubMed] [Google Scholar]
- 85.Cheung KS, Seto WK, Wong DK, Mak LY, Lai CL, Yuen MF. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget. 2017;8:47507–47517. doi: 10.18632/oncotarget.17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim HS, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein level predicts recurrence of hepatitis B virus-related hepatocellular carcinoma after curative resection. Clin Mol Hepatol. 2020;26:33–44. doi: 10.3350/cmh.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heo JY, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, et al. Use of Wisteria floribunda agglutinin-positive human Mac-2 binding protein in assessing risk of hepatocellular carcinoma due to hepatitis B virus. Medicine (Baltimore) 2016;95:e3328. doi: 10.1097/MD.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 2015;4:126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heimbach JK. Overview of the updated AASLD guidelines for the management of HCC. Gastroenterol Hepatol (N Y) 2017;13:751–753. [PMC free article] [PubMed] [Google Scholar]
- 90.Aghemo A. Update on HCC management and review of the new EASL guidelines. Gastroenterol Hepatol (N Y) 2018;14:384–386. [PMC free article] [PubMed] [Google Scholar]
- 91.Yamamoto K, Imamura H, Matsuyama Y, Hasegawa K, Beck Y, Sugawara Y, et al. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:2795–2804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 92.Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 93.Song PP, Xia JF, Inagaki Y, Hasegawa K, Sakamoto Y, Kokudo N, et al. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2016;22:262–274. doi: 10.3748/wjg.v22.i1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Xu Y, Zhao H, Zhang X. Change of circulating antibodies against CD25-derived peptide antigen in hepatocellular carcinoma. J Cancer Res Ther. 2017;13:813–816. doi: 10.4103/jcrt.JCRT_823_17. [DOI] [PubMed] [Google Scholar]
- 95.Xu Y, Gu L, Wang J, Wang Z, Zhang P, Zhang X. Detection of circulating antibodies to p16 protein-derived peptides in hepatocellular carcinoma. Lab Med. 2020 Mar 20; doi: 10.1093/labmed/lmaa006. doi: 10.1093/labmed/lmaa006. [DOI] [PubMed] [Google Scholar]
- 96.Tatarinov IuS. Detection of embryo-specific alpha-globulin in the blood serum of a patient with primary liver cancer. Vopr Med Khim. 1964;10:90–91. [PubMed] [Google Scholar]
- 97.Linson EA, Hanauer SB. More than a tumor marker...A potential role for alpha-feto protein in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1271–1276. doi: 10.1093/ibd/izy394. [DOI] [PubMed] [Google Scholar]
- 98.Ayoub WS, Steggerda J, Yang JD, Kuo A, Sundaram V, Lu SC. Current status of hepatocellular carcinoma detection: screening strategies and novel biomarkers. Ther Adv Med Oncol. 2019;11:1758835919869120. doi: 10.1177/1758835919869120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reichl P, Mikulits W. Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: an update for clinicians (review) Oncol Rep. 2016;36:613–625. doi: 10.3892/or.2016.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573–10583. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kudo M. Alpha-fetoprotein-L3: useful or useless for hepatocellular carcinoma? Liver Cancer. 2013;2:151–152. doi: 10.1159/000343847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104–113. doi: 10.1016/j.cgh.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 103.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis. 2006;26:385–390. doi: 10.1055/s-2006-951606. [DOI] [PubMed] [Google Scholar]
- 105.Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313:15–19. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- 106.Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69:1983–1994. doi: 10.1002/hep.30233. [DOI] [PubMed] [Google Scholar]
- 107.Toyoda H, Kumada T, Tada T. Highly sensitive Lens culinaris agglutinin-reactive α-fetoprotein: a new tool for the management of hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:61–65. doi: 10.1159/000333263. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi M, Hosaka T, Ikeda K, Seko Y, Kawamura Y, Sezaki H, et al. Highly sensitive AFP-L3% assay is useful for predicting recurrence of hepatocellular carcinoma after curative treatment preand postoperatively. Hepatol Res. 2011;41:1036–1045. doi: 10.1111/j.1872-034X.2011.00858.x. [DOI] [PubMed] [Google Scholar]
- 109.Setsu T, Tsuchiya A, Watanabe T, Nagoya T, Ikarashi S, Hayashi K, et al. Early detection of hepatocellular carcinoma recurrence using the highly sensitive fucosylated fraction of alpha-fetoprotein. Case Rep Gastroenterol. 2017;11:142–147. doi: 10.1159/000462969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carr BI, Kanke F, Wise M, Satomura S. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gammacarboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci. 2007;52:776–782. doi: 10.1007/s10620-006-9541-2. [DOI] [PubMed] [Google Scholar]
- 111.Naraki T, Kohno N, Saito H, Fujimoto Y, Ohhira M, Morita T, et al. Gamma-carboxyglutamic acid content of hepatocellular carcinoma-associated des-gamma-carboxy prothrombin. Biochim Biophys Acta. 2002;1586:287–298. doi: 10.1016/s0925-4439(01)00107-7. [DOI] [PubMed] [Google Scholar]
- 112.Pote N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848–854. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Chen J, Wu G, Li Y. Evaluation of serum des-gamma-carboxy prothrombin for the diagnosis of hepatitis B virus-related hepatocellular carcinoma: a meta-analysis. Dis Markers. 2018;2018:8906023. doi: 10.1155/2018/8906023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, et al. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–569. doi: 10.1002/1097-0142(20010201)91:3<561::aid-cncr1035>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 115.Yamashita Y, Tsuijita E, Takeishi K, Fujiwara M, Kira S, Mori M, et al. Predictors for microinvasion of small hepatocellular carcinoma ≤ 2 cm. Ann Surg Oncol. 2012;19:2027–2034. doi: 10.1245/s10434-011-2195-0. [DOI] [PubMed] [Google Scholar]
- 116.Lee S, Rhim H, Kim YS, Kang TW, Song KD. Post-ablation desgamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int. 2016;36:580–587. doi: 10.1111/liv.12991. [DOI] [PubMed] [Google Scholar]
- 117.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song P, Gao J, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, et al. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and China. Liver Cancer. 2013;2:31–39. doi: 10.1159/000346220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caviglia GP, Ribaldone DG, Abate ML, Ciancio A, Pellicano R, Smedile A, et al. Performance of protein induced by vitamin K absence or antagonist-II assessed by chemiluminescence enzyme immunoassay for hepatocellular carcinoma detection: a metaanalysis. Scand J Gastroenterol. 2018;53:734–740. doi: 10.1080/00365521.2018.1459824. [DOI] [PubMed] [Google Scholar]
- 120.Chen H, Zhang Y, Li S, Li N, Chen Y, Zhang B, et al. Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res. 2018;10:1947–1958. doi: 10.2147/CMAR.S167036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, et al. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caviglia GP, Abate ML, Gaia S, Petrini E, Bosco C, Olivero A, et al. Risk of hepatocellular carcinoma in HBV cirrhotic patients assessed by the combination of miR-122, AFP and PIVKA-II. Panminerva Med. 2017;59:283–289. doi: 10.23736/S0031-0808.17.03353-5. [DOI] [PubMed] [Google Scholar]
- 123.Sassa T, Kumada T, Nakano S, Uematsu T. Clinical utility of simultaneous measurement of serum high-sensitivity des-gammacarboxy prothrombin and Lens culinaris agglutinin A-reactive alpha-fetoprotein in patients with small hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1999;11:1387–1392. doi: 10.1097/00042737-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 124.Erdal H, Gül Utku Ö, Karatay E, Çelik B, Elbeg Ş, Doğan İ. Combination of DKK1 and AFP improves diagnostic accuracy of hepatocellular carcinoma compared with either marker alone. Turk J Gastroenterol. 2016;27:375–381. doi: 10.5152/tjg.2016.15523. [DOI] [PubMed] [Google Scholar]
- 125.Ge T, Shen Q, Wang N, Zhang Y, Ge Z, Chu W, et al. Diagnostic values of alpha-fetoprotein, dickkopf-1, and osteopontin for hepatocellular carcinoma. Med Oncol. 2015;32:59. doi: 10.1007/s12032-014-0367-z. [DOI] [PubMed] [Google Scholar]
- 126.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 127.Fouad YM, Mohamed HI, Kamal EM, Rasek MA. Clinical significance and diagnostic value of serum dickkopf-1 in patients with hepatocellular carcinoma. Scand J Gastroenterol. 2016;51:1133–1137. doi: 10.3109/00365521.2016.1172337. [DOI] [PubMed] [Google Scholar]
- 128.Sakabe T, Azumi J, Umekita Y, Toriguchi K, Hatano E, Hirooka Y, et al. Expression of cancer stem cell-associated DKK1 mRNA serves as prognostic marker for hepatocellular carcinoma. Anticancer Res. 2017;37:4881–4888. doi: 10.21873/anticanres.11897. [DOI] [PubMed] [Google Scholar]
- 129.Jang ES, Jeong SH, Kim JW, Choi YS, Leissner P, Brechot C. Diagnostic performance of alpha-fetoprotein, protein induced by vitamin K absence, osteopontin, dickkopf-1 and its combinations for hepatocellular carcinoma. PLoS One. 2016;11:e0151069. doi: 10.1371/journal.pone.0151069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y, Tang W, Xie L, Wang J, Deng Y, Peng Q, et al. Prognostic significance of dickkopf-1 overexpression in solid tumors: a metaanalysis. Tumour Biol. 2014;35:3145–3154. doi: 10.1007/s13277-013-1411-x. [DOI] [PubMed] [Google Scholar]
- 131.Wang J, Xu Y, Wang Y, Zhang X, Zhang G. Further study of circulating antibodies to P16, CD25 and FOXP3 in hepatocellular carcinoma. Onco Targets Ther. 2019;12:10487–10493. doi: 10.2147/OTT.S226404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, et al. The gamma-glutamyl-transpeptidase to platelet ratio does not show advantages than APRI and Fib-4 in diagnosing significant fibrosis and cirrhosis in patients with chronic hepatitis B: a retrospective cohort study in China. Medicine (Baltimore) 2016;95:e3372. doi: 10.1097/MD.0000000000003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nishikawa H, Enomoto H, Iwata Y, Kishino K, Shimono Y, Hasegawa K, et al. Serum Wisteria floribunda agglutinin-positive Mac2-binding protein for patients with chronic hepatitis B and C: a comparative study. J Viral Hepat. 2016;23:977–984. doi: 10.1111/jvh.12575. [DOI] [PubMed] [Google Scholar]
- 134.Ichikawa Y, Joshita S, Umemura T, Shobugawa Y, Usami Y, Shibata S, et al. Serum Wisteria floribunda agglutinin-positive human Mac2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol Res. 2017;47:226–233. doi: 10.1111/hepr.12712. [DOI] [PubMed] [Google Scholar]
- 135.Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5811. doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Balaceanu LA. Biomarkers vs imaging in the early detection of hepatocellular carcinoma and prognosis. World J Clin Cases. 2019;7:1367–1382. doi: 10.12998/wjcc.v7.i12.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim JU, Shariff MI, Crossey MM, Gomez-Romero M, Holmes E, Cox IJ, et al. Hepatocellular carcinoma: Review of disease and tumor biomarkers. World J Hepatol. 2016;8:471–484. doi: 10.4254/wjh.v8.i10.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–417. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 139.inoue t, tanaka y. today’s new laboratory tests-how to select and use (3) liver disease weekly japan medical news. 2015;4771:40–45. [Google Scholar]
- 140.Matsumoto A, Yatsuhashi H, Nagaoka S, Suzuki Y, Hosaka T, Tsuge M, et al. Factors associated with the effect of interferon-α sequential therapy in order to discontinue nucleoside/nucleotide analog treatment in patients with chronic hepatitis B. Hepatol Res. 2015;45:1195–1202. doi: 10.1111/hepr.12488. [DOI] [PubMed] [Google Scholar]
- 141.Tanaka E, Matsumoto A, Suzuki F, Kobayashi M, Mizokami M, Tanaka Y, et al. Measurement of hepatitis B virus core-related antigen is valuable for identifying patients who are at low risk of lamivudine resistance. Liver Int. 2006;26:90–96. doi: 10.1111/j.1478-3231.2005.01200.x. [DOI] [PubMed] [Google Scholar]
- 142.Seto WK, Wong DK, Chan TS, Hwang YY, Fung J, Liu KS, et al. Association of hepatitis B core-related antigen with hepatitis B virus reactivation in occult viral carriers undergoing high-risk immunosuppressive therapy. Am J Gastroenterol. 2016;111:1788–1795. doi: 10.1038/ajg.2016.436. [DOI] [PubMed] [Google Scholar]
- 143.Yasunaka T, Takaki A, Yagi T, Iwasaki Y, Sadamori H, Koike K, et al. Serum hepatitis B virus DNA before liver transplantation correlates with HBV reinfection rate even under successful low-dose hepatitis B immunoglobulin prophylaxis. Hepatol Int. 2011;5:918–926. doi: 10.1007/s12072-011-9265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]