Abstract
Human copper transporter 1 (hCtr1) is the main transporter of copper which has been involved as an essential cofactor in biological processes and mechanisms of action for cisplatin and its analogues. Although expression of hCtr1 is present in all tissues that require copper, several studies have showed that levels of expression are highly variable between normal and neoplastic tissues. We evaluated the potential diagnostic of the 64CuCl2-PET/CT in patients with wild type non-small cell lung cancer (NSCLC). Eleven patients were included. Baseline 18F-FDG-PET/CT and 64CuCl2-PET/CT performed before to initiate treatment with platinum-based chemotherapy. 18F-FDG-PET/CT detected a total of 68 lesions in different corporal sites: lung (24), regional lymph node (30), distant non-bone metastases (17) and bone metastases (14). Of total, 73% demonstrated high focal uptake of 64CuCl2-PET/CT: 36% in primary tumor and 27% in lymph-nodes metastases. The detection-rates (DRs) was lower with 64CuCl2 PET/CT compared to 18F-FDG-PET/CT, however, these was not statistically significant (P = 0.108). A complete match was found in 2 patients. All patients were treated with platinum-based chemotherapy. According to RECIST 1.1 and PERCIST 1.0 criteria, most patients with highest uptake 64CuCl2-PET/CT presented partial response (mean 3 cycles) corroborated with 18F-FDG PET/CT. On the other hand, patients with very low uptake or faint uptake have progressive disease (3/16 patients). To our knowledge, this is the first study with 64CuCl2-PET/CT in-human in patients with NSCLC chemo-naïve. Our results may represent that 64CuCl2-PET/CT had a good ability for detect lesions. In addition, the 64CuCl2 uptake is based on the expression of Ctr1 transporters seeking to differentiate between those patients who may benefit from platinum-based therapy. More studies are necessary for confirm these findings.
Keywords: Non-small cell lung cancer, 64CuCl2 PET/CT, platinum, human copper transporter
Introduction
Lung cancer is the leading cause of death among women and men in worldwide. Non-small cell lung cancer (NSCLC) is the main histologic sub-group, representing 80-85% of the lung cancers [1]. According to data from GLOBOCAN [2], 7811 incident lung cancer cases occur in Mexico every year. In terms of mortality, a total of 6733 deaths due to lung cancer were estimated in Mexico, making this the highest-mortality neoplastic disease in Mexico (PMID: 31558229, 31276353).
Platinum based chemotherapy, have been recognized as a gold standard treatment for patients with lung cancer without oncogenic driver detected. Their antineoplastic activity was discovered by Barnett Rosenberg at the 60’s and cisplatin was the first platinum drug approved by the FDA at 1978 [3]. The combination of two drugs, a platinum and a non-platinum agent was of standard of care a long time, before of combination with immunotherapy, in patients with lung cancer, having a response rate of 25-30% in advanced diseases and a median overall survival of 9-11 months [4].
The mechanisms of the molecular efficacy of platinum agents are based on the presence of platinum membrane transporters analogs of multidrug resistance protein 1 (MDR1). Initially it was thought that the passive diffusion was incorporated at the SARs trough the necessity of neutrality of charge, nevertheless, in the last years, the active transport by the Copper transporters 1 and 2 (Ctr1, Ctr2) has been implied as an important access route for platinum drugs to the cell, however this matter hasn’t been solved without ambiguity and new data is continually emerging [5,6].
It’s been confirmed that in human cell the cisplatin makes a rapid degradation of the membrane Ctr1, having as a result the diminishing in the affluence of cisplatin and the resistance to the drug so that many patients relapse and develop treatment resistance [7].
Nowadays we know cisplatin acts at multiple cellular targets that represent different signal transduction paths, and more recently the Human Copper transporter 1 (hCtr1) has been demonstrated as the main transporter of copper, as well as for cisplatin and its analogues. Although the hCtr1 expression is omnipresent because all the tissues require copper, the studies have shown that the levels of the expression of these transporters were highly variable between the normal and neoplastic tissues, depending on the control at the transcriptional and post-translational levels. The studies have also suggested that two flow transporters of copper (Cu) ATP7A y ATP7B regulate the flow of cisplatin into the cell [8-11].
The use of radioactive copper chloride has been evaluated in diseases such as melanoma, prostate cancer, hepatocarcinoma and breast cancer given their overexpression of hCtr1. Initially, two stable isotopes of Cu, 63Cu and 65Cu, had been proven to be very effective in providing information related to copper absorption, bioavailability, and excretion patterns and their primary advantages as the stable isotopes of copper were that they could be used as tracers with no exposure to radioactivity and that they do not decay, however, these two stable isotopes are relatively abundant which is not ideal for metabolic studies because the experimental 63Cu or 65Cu intake cannot be separated in collected samples from what is consumed through meals and from endogenous mineral copper. Thus, two copper radioisotopes, 64Cu (t 1/2 = 12.8 h) and 67Cu (t 1/2 = 58.5 h) have been used in metabolic research. 64Cu enters inside the cells using the enzyme Ctr1 and it is distributed in different organelles (Golgi complex, mitochondria, etc). Then, it enters inside the nucleus, inside deoxyribonucleic acid (DNA), using the enzyme Human antioxidant protein 1 (ATOX1). In normal cells 64Cu remains in the cytoplasm while in tumor cells it enters the nucleus. In this condition, 64Cu is able to produce the theranostic effect, without toxicity to normal cells. The radioisotope 64Cu has the advantage of a relatively long half-life shorter than that of 67Cu and can be conveniently produced by a biomedical cyclotron, being commercially available in a ready-to-use chemical form [12,13].
Flurodeoxyglucose positron emission tomography (18F-FDG PET/CT) plays a main role in staging, determining prognosis and treatment of NSCLC, where, according to the eighth edition of the TNM staging system, it is the standard of care [14]. It has been found to have a sensitivity, accuracy and positive predictive value in detecting primary malignancy of 94%, 94% and 100%, respectively, being able to change treatment planning in 34% of the patients. Meanwhile, it does not provide information about platinum-resistance [15]. Our objective is to evaluate the diagnostic value of PET/CT with 64CuCl2 in patients with non-small cell lung cancer without mutations. This retrospective evaluation represents the first clinical use of the 64CuCl2 for PET imaging performed at cancer center in Mexico.
Material and methods
This is a retrospective clinical study, conducted at nuclear medicine and molecular imaging department from the National Cancer Institute in Mexico City. All PET and PET/CT examinations were performed in compliance with 1964 declaration of Helsinki, and the responsible regulatory agencies in Mexico. All patients received 64CuCl2 under the “extensive use program” clause of the Mexico. Written informed consent was obtained from each subject.
Synthesis of [64Cu]CuCl2
The development of Cu chelators is considered a very important challenge because there are many Cu-chelating proteins in vivo. High specific activity 64Cu in the chemical form of Cu dichloride was produced at the Unidad Radiofarmacia-Ciclotrón, Facultad de Medicina, UNAM, via the 64Ni(p,n) 64Cu reaction with 11-MeV protons, by methods previously reported by Avila-Rodriguez et al. [16]. After radiochemical purification, the copper fraction was evaporated to dryness and recovered with 5 ml of physiological saline solution and sterilized by passing it through a 0.22-μm syringe filter (Millex-GV). The pH of the reconstituted solution of 64CuCl2 in physiological saline was around 5.5, with a typical apparent molar activity in the range of 150-700 GBq/μmol as determined by titration of 64CuCl2 with the chelator 2-S-(4-Aminobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic-acid (p-NH2-Bn-NOTA). Radionuclidic purity at the time of injection was > 99% as determined by gamma spectroscopy in a HPGe detector.
Patients
Between September 2016 and January 2017 a total of 11 patients from the National Cancer Institute in Mexico City of at least 18 years old with histologically confirmed NSCLC without oncogenic driver detected and chemo naïve, measurable disease per RECIST criteria and an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 were mandatory.
Imaging protocol
Imaging was performed with a Biograph mCT 20 PET/CT (Siemens Medical Solutions, USA) at the nuclear medicine and molecular Imaging department in Instituto Nacional de Cancerologia. After intravenous injection of 64CuCl2 a mean of 250 MBq (4 MBq per kg bodyweight, range 230-290 MBq) WB emission scans were acquired at 24 h post-injection. Low-dose CT (from skull to mid-thigh) correction was performed for both attenuation correction and topographic localization. The CT parameters used for acquisition were 140 kV, 80 mA, and 0.5 s per rotation, with a pitch of 6:1 and a slice thickness of 5 mm. After completion of the CT scan, PET data were acquired for 3 min per bed position. Emission data were corrected for randoms, dead time, scatter, and attenuation and were reconstructed iteratively by an ordered-subsets expectation maximization algorithm (4 iterations, 8 subsets) followed by a post-reconstruction smoothing gaussian filter (5 mm in full width at one-half maximum).
Imaging analysis
PET/CT images in all standard planes were reviewed by use of the dedicated software (syngo by SIEMENS). Images were analyzed visually and quantitatively by two nuclear medicine physicians with more than 5 years of experience (κ = 0.85). Maximum standardized uptake values (SUVmax) were obtained by drawing circular regions of interest, which were automatically adapted (40% isocontour) to a 3D VOI using syngo software.
Figure 1 exhibited a normal biodistribution in a healthy patient any focal, non-physiologic uptake higher than that of the surrounding background level was considered pathologic.
Figure 1.
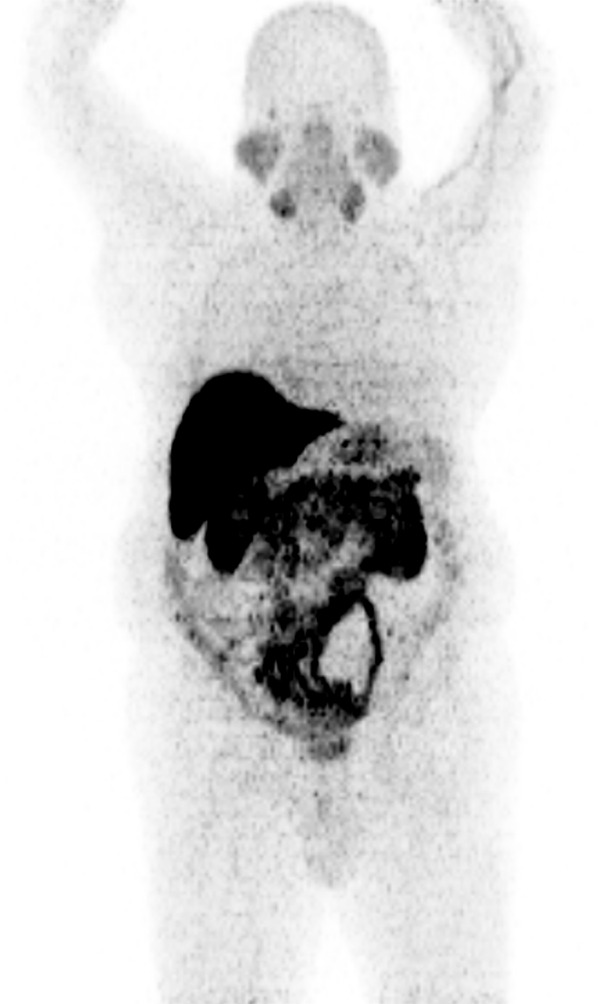
Maximal intensity projection (MIP) in a healthy volunteer with 64CuCl2 PET/CT. The normal biodistribution tissues were salivary glands, liver, pancreas, kidneys and bowel.
18F-FDG PET/CT and 64CuCl2 PET/CT studies were interpreted visually and semiquantitatively using the SUVmax values, on a patient-by-patient and lesion-by-lesion basis. In patient-based analysis, detection rate (DR) was defined as the ability to detect at least one pathologic finding in each patient.
Standard of reference
The primary endpoint was calculate and compare the DRs of the 64CuCl2 PET/CT with the standard imaging 18F-FDG PET/CT, the DR of 18F-FDG PET/CT was correlated with standard reference with pathology or morphologic findings; this confirmed the presence of disease. For lymph-node and distant metastases, we used a follow-up based on 18F-FDG PET/CT findings, and reduction of CEA (carcinoembryonic antigen) after therapy. A median follow-up time was 6 months (range 4-14 months).
Statistical methods
This study is a proof of concept which could provide some confirmation of the site of disease. DRs were calculated as the ratio between the number of positive lesions visualized by 64CuCl2 PET/CT and the total number of lesions visualized by 18F-FDG PET/CT. The McNemar test was used to compare DRs between both radiotracers on the same patients. Continuous variables were summarized as arithmetic means or medians, with SDs or percentiles for descriptive purposes, and categorical variables are presented as frequencies and percentages. A P < 0.05 was considered statistically significant. Statistical analyses were conducted with software SPSS (version 22).
Results
From September 2016 through January 2017 a total of 11 patients with NSCL were reviewed for eligibility. Baseline characteristics are resumed in Table 1. The median age was 59 years (49-69). Of total of population, 5 were men (45%) and 6 women (55%). The time between 18F-FDG-PET/CT and 18F-FDG-PET/CT was 7 ± 4 days.
Table 1.
Patient characteristics, n = 11
| Characteristics | N |
|---|---|
| Age median (range) | 59 (49-69) |
| Sex | |
| Female | 6 |
| Male | 5 |
| Clinical stage (n, %) | |
| IIIB | 1 (%) |
| IIIC | 3 (%) |
| IVA | 3 (%) |
| IVB | 4 (%) |
| NSCLC subtype | |
| ADC (n, %) | 7 (%) |
| SCC (n, %) | 4 (%) |
| Differentiation | |
| Poor | 6 (%) |
| Moderate | 5 (%) |
Abbreviations: NSCLC = non-small-cell lung cancer; ADC = adenocarcinoma; SCC = squamous cell carcinoma.
Overall, 8 patients (73%), demonstrated high focal uptake of 64CuCl2 on PET/CT images. Four of them with higher uptake in the primary tumor. Three patients (27%) showed tracer uptake in lymph nodes metastases, of which 2 patients do not demonstrated metastatic sites when were compared with previous 18F-FDG PET/CT or exhibit mismatch lesions (Figure 2). In 2 patients very low uptake was observed in the primary tumor in lung; and 3 patients very faint focal uptake was noted in previous non-pathologic bone fractures which was a nonspecific inflammatory process.
Figure 2.
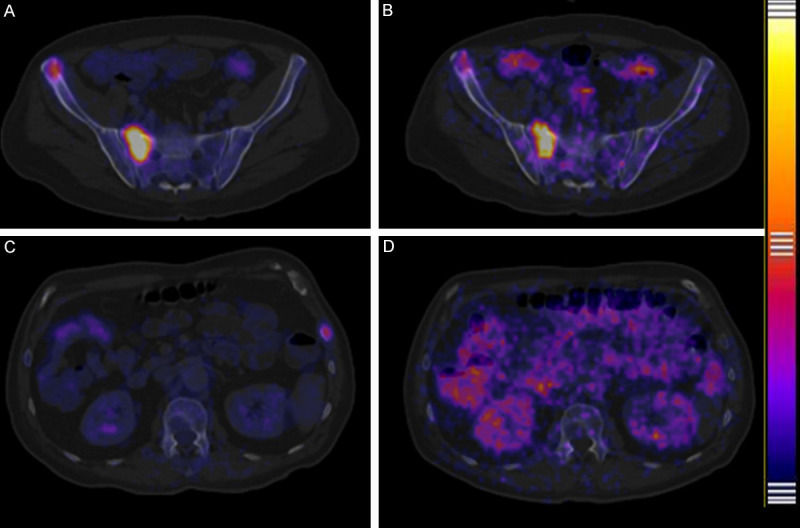
Comparison bone metastases between 18F-FDG PET/CT and 64CuCl2 PET/CT fused images. Bone metastases lesion in sacrum and right iliac crest evaluate with 18F-FDG PET/CT (A) and the same patient evaluate with 64CuCl2 PET/CT (B) a complete match was found, obviously 18F-FDG PET/CT show better target/background than 64CuCl2 PET/CT. In another patient bone metastases in 8° left costal arch was visualized with 18F-FDG PET/CT (C), but the same lesion was not visualized with 64CuCl2 PET/CT (D) a mismatch was found.
To determine the DR of two radiotracers in detecting lesions, we performed a lesion-based analysis; the results are summarized in Table 2. A total 68 lesions were detected by 18F-FDG PET/CT, 24 of which were in the lung, 30 regional lymph-node metastases, 17 distant non-bone metastases (including non-regional lymph-node metastases) and 14 bone metastases (Figure 3). 64CuCl2 PET/CT showed significantly lower DRs than did 18F-FDG PET/CT. A complete match was found with a previous 18F-FDG PET/CT study in 2 patients (Figure 4). No significant difference in bone metastases DR was observed between 18F-FDG PET/CT and 64CuCl2 PET/CT. The difference between the DR of 64CuCl2 PET/CT and that of 18F-FDG PET/CT was not statistically significant (P = 0.108).
Table 2.
Lesion-based analysis
| NSCLC subtype | Site lesion | 18F-FDG | 64CuCl2 | P* |
|---|---|---|---|---|
| ADC | Local | 6/6 | 5/6 | 0.02 |
| Regional lymph-node | 4/4 | 2/4 | ||
| Distant non-bone metastases | 2/2 | 1/2 | ||
| Bone metastases | 4/4 | 3/4 | ||
| SCC | Local | 5/5 | 3/5 | 0.05 |
| Regional lymph-node | 7/7 | 3/7 | ||
| Distant non-bone metastases | 4/4 | 2/4 | ||
| Bone metastases | 3/3 | 3/3 |
McNemar test vs. 64CuCl PET/CT.
Figure 3.
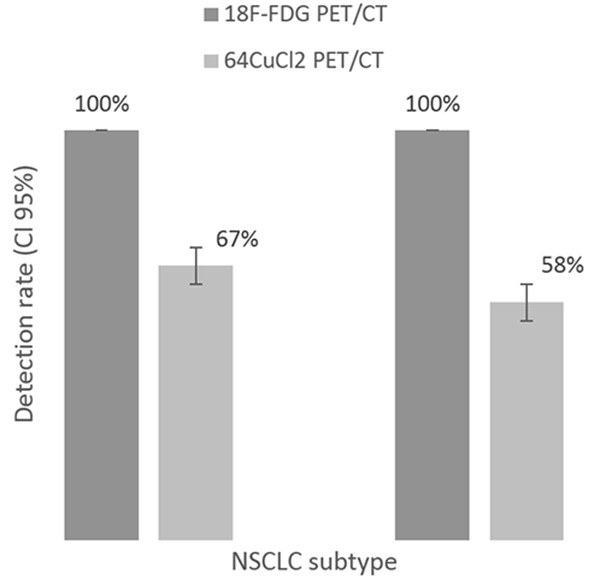
Lesion-based analysis. Comparison of 64CuCl2 PET/CT vs 18F-FDG PET/CT according to NSCLC subtype, left column corresponding to adenocarcinoma and right column to squamous cell carcinoma. DR was calculated was correlated with standard reference with pathology or morphologic findings.
Figure 4.
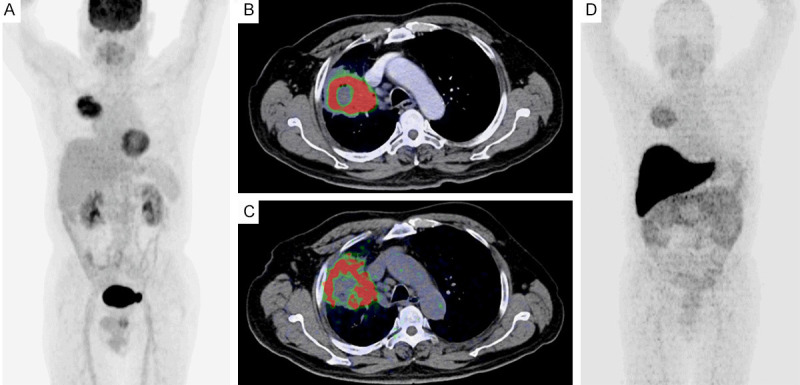
Comparison primary tumor between 18F-FDG PET/CT and 64CuCl2 PET/CT. Maximal intensity projection (MIP) evaluate with 18F-FDG PET/CT showed a right lung tumor (A) and fused image observed more metabolic in the peripheral lesion (B); in the same patient a fused and MIP image with 64CuCl2 PET/CT showed the same lesion with the similarity pattern (C and D) a complete match was found.
Implication on clinical management
All patients were initially treated with platinum based chemotherapy, the four patients who showed the highest uptake in the primary tumor with 64CuCl2 PET/CT, presented partial response according to Response Evaluation Criteria In Solid Tumors (RECIST 1.1) and PET Response Criteria in Solid Tumors (PERCIST 1.0) after 3-4 cycles, mean 3 cycles.
In patients with very low uptake or faint uptake progressive disease was seen in three patients (27%), and stable disease was presented in the rest of patients, therapeutic management was changed these patients. No complete response was observed.
Discussion
Although some guidelines list many platinum regimens, the best regimens have not yet clarified. Either cisplatin, carboplatin, plus bevacizumab or taxanes, showed good overall survival these regimens as acceptable first-choice regimens [17].
Our study represented the first to retrospectively evaluation in Latin-American population with NSCLC with both radiotracers 18F-FDG and 64CuCl2 previous to receive the best platinum regimens for chemo-naïve patients.
Many preclinical studies of 64CuCl2 have been conducted with various types of cancer. Peng F et al., showed when 64CuCl2 was used as a tracer in human prostate cancer xenografts, PET imaging detected high levels of hCtr1 after 24 hours, yet not after 1 hour; they demonstrated that after 24 hours, uptake of 64CuCl2 by tumor cells that did not express hCtr1 was significantly reduced compared to the uptake of 64CuCl2 by control tumor cells that did express hCtr1 [18].
Jørgensen et al., evaluate the capacity of 64CuCl2 to diagnose five different human cancer cells in xenograft mice models, including glioblastoma, colorectal cancer, head and neck cancer, ovarian cancer, and neuroendocrine lung carcinoid. 1 h and 22 h post injection of 64CuCl2. One hour after the injection of 64CuCl2, very high uptake of the radionuclide was detected in the liver. However, 21 hours later, a marked decrease in 64CuCl2 uptake was observed. They conclude that No relationship was found between tumor uptake of 64CuCl2 and gene expression of Ctr1 [19].
Avila-Rodriguez et al., reported the first-in-human study on biodistribution and radiation dosimetry of 64CuCl2 in six healthy volunteers, showed that the liver has the highest uptake, followed by intestine and pancreas while the urinary excretion is very poor [16].
Grubmüller B et al., evaluated the diagnostic potential of 64Cu-labeled prostate-specific membrane antigen (PSMA) ligand (PSMA-617) PET compared with 18F-choline PET/CT or 68Ga-PSMA PET/CT in patients with prostate cancer in 29 patients with high suspicion of recurrent disease or for possible surgical or PSMA therapy planning from two nuclear medicine centers, they demonstrated in 23 of 29 patients, at least one focus of pathological tracer uptake suggestive of locally disease or recurrent with excellent contrast as early as 1 hour after intravenous administration of 64Cu-PSMA PET/CT with high detection rates even at low prostate-specific antigen (PSA) levels [20].
Kim E et al. describe maybe the most important paper about this approach, they observed in 30 tissue blocks of NSCLC and platinum concentrations who underwent neoadjuvant platinum-based chemotherapy, that tissue platinum concentration significantly correlated with tumor response in patients who received neoadjuvant platinum-based chemotherapy with statistical significance meanwhile Ctr1 was differentially expressed in NSCLC tumors, a fraction of patients with undetectable Ctr1 expression in their tumors had reduced platinum concentrations and tumor response compared to those with any level of Ctr1 expression booth results was statistical significance [21].
The main advantage of using this radiotracer is the half-life (t 1/2 = 12.8 h), which allowed the study to be carried out with a good detection rate at 24 h after the administration of a standard dose of 64CuCl2. In our study lesion-based analysis, the DR of 18F-FDG PET/CT was significantly higher than that of 64CuCl2 PET/CT. This difference stems from the low DR of 64CuCl2 PET/CT uptake is based on the expression of Ctr1 transporters seeking to differentiate between those patients who may benefit from platinum-based therapy.
Our study has some important limitations. Does not directly provide data for best platinum regimens because our objective was observed DR compared with standard PET/CT imaging based in a proof-concept. In addition we do not evaluated the Ctr1 status because technique and material limitations; moreover, however, we observed that the partial response of four patients was in those who presented higher 64CuCl2 uptake, compared to those who showed lower uptake, and that these findings were statistically significant according to lesion-based analysis.
Conclusion
This study reports the first in-human application of a 64CuCl2 PET/CT, in the primary staging of patient’s with NSCLC chemo-naïve. The results of our study may represent that 64CuCl2 PET/CT had a good ability for detect lesions because DR of 64CuCl2 PET/CT and that of 18F-FDG PET/CT was not statistically significant; in addition, the 64CuCl2 uptake is based on the expression of Ctr1 transporters seeking to differentiate between those patients who may benefit from platinum-based therapy. More studies are necessary for confirm these findings.
Acknowledgements
The authors would like to thank Miguel Angel Avila-Rodriguez, PhD for provide 64CuCl2 doses; and Mario Romero-Piña, for acquisition 64CuCl2 PET/CT. The institutional review board of our institute approved this prospective study, and the requirement to obtain informed consent was waived.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward CA. Cancer statistics, 2010. Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2018 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2018. Available from: http://globocan.iarc.fr, accessed on 20/May/2019. [Google Scholar]
- 3.Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–6. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 4.Non-small cell lung cancer: NCCN clinical practice guidelines in oncology. v.2. 2019. [Google Scholar]
- 5.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–94. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivy KD, Kaplan JH. A re-evaluation of the role of hCTR1, the human high-affinity copper transporter, in platinum-drug entry into human cells. Mol Pharmacol. 2013;83:1237–46. doi: 10.1124/mol.113.085068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 9.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, Liu HS, Su WC. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–34. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 12.Merli M, Patriarca M, Loudianos G, Valente C, Riggio O, De Felice G, Petrucci F, Caroli S, Attili AF. Use of the stable isotope 65Cu test for the screening of Wilson’s disease in a family with two affected members. Ital J Gastroenterol Hepatol. 1998;30:270–5. [PubMed] [Google Scholar]
- 13.Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem. 2009;284:717–721. doi: 10.1074/jbc.R800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandathil A, Kay FU, Butt YM, Wachsmann JW, Subramaniam RM. Role of FDG PET/CT in the eighth edition of TNM staging of non-small cell lung cancer. Radiographics. 2018;38:2134–2149. doi: 10.1148/rg.2018180060. [DOI] [PubMed] [Google Scholar]
- 15.Budak E, Çok G, Akgün A. The contribution of fluorine 18F-FDG PET/CT to lung cancer diagnosis, staging and treatment planning. Mol Imaging Radionucl Ther. 2018;27:73–80. doi: 10.4274/mirt.53315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manrique-Arias JC, Martinez-Hernandez R, García-Pérez FO, Jalilian AR, Martinez-Rodriguez E, Romero-Piña ME, Diaz-Ruiz A. Biodistribution and radiation dosimetry of [64Cu]copper dichloride: first-in-human study in healthy volunteers. EJNMMI Res. 2017;7:98. doi: 10.1186/s13550-017-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horita N, Nagashima A, Nakashima K, Shibata Y, Ito K, Goto A, Yamanaka T, Kaneko T. The best platinum regimens for chemo-naive incurable non-small cell lung cancer: network meta-analysis. Sci Rep. 2017;7:13185. doi: 10.1038/s41598-017-13724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng F, Lu X, Janisse J, Muzik O, Shields AF. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2 . J Nucl Med. 2006;47:1649–1652. [PubMed] [Google Scholar]
- 19.Jørgensen JT, Persson M, Madsen J, Kjær A. High tumor uptake of 64Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl Med Biol. 2013;40:345–350. doi: 10.1016/j.nucmedbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Grubmüller B, Baum RP, Capasso E, Singh A, Ahmadi Y, Knoll P, Floth A, Righi S, Zandieh S, Meleddu C, Shariat SF, Klingler HC, Mirzaei S. 64Cu-PSMA-617 PET/CT imaging of prostate adenocarcinoma: first in-human studies. Cancer Biother Radiopharm. 2016;31:277–286. doi: 10.1089/cbr.2015.1964. [DOI] [PubMed] [Google Scholar]
- 21.Kim ES, Tang X, Peterson DR, Kilari D, Chow CW, Fujimoto J, Kalhor N, Swisher SG, Stewart DJ, Wistuba II, Siddik ZH. Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer. 2014;85:88–93. doi: 10.1016/j.lungcan.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


