Abstract
Cochlear hair cells (HCs) are the mechanoreceptors of the auditory system, and because these cells cannot be spontaneously regenerated in adult mammals, hearing loss due to HC damage is permanent. However, cochleae of neonatal mice harbor some progenitor cells that retain limited ability to give rise to new HCs in vivo. Here we review the regulatory factors, signaling pathways, and epigenetic factors that have been reported to play roles in HC regeneration in the neonatal mammalian cochlea.
Keywords: Cochlea, inner ear progenitor, hair cell regeneration, transcription factor, signaling pathway
Introduction
Sensorineural hearing loss, one of the most common health problems around the world, is mainly caused by cochlear hair cell (HC) damage or loss [1]. In non-mammalian vertebrates, such as birds and fish, HCs can be spontaneously regenerated from supporting cells (SCs) after damage [2-4]. However, HCs in the adult mammalian cochlea cannot be spontaneously regenerated, and only neonatal cochlear HCs have a limited capacity for regeneration [5,6]. Damaged mammalian vestibular organs can also generate new HCs from SCs in limited numbers [7-9]. It has been reported that progenitor cells can be isolated from the auditory and vestibular organs of the inner ear and can form spheres and self-renew in vitro [10-13]. HCs are regenerated through two mechanisms. In mitotic regeneration, inner ear progenitors re-enter the cell cycle, divide mitotically, and then differentiate into new HCs. In direct trans-differentiation, inner ear progenitors switch cell fate and directly differentiate into new HCs [14-16]. We will focus in this review on the mechanisms through which transcription factors, regulatory factors and signaling pathways regulate HC regeneration.
Inner ear progenitors in the neonatal cochlea
In recent years, researchers have found that the SCs of the cochlea have certain ability for proliferation and differentiation, and as described above, these cells can first divide and then differentiate into HCs or they can trans-differentiate directly into HCs [10,17]. White et al. isolated P27+ transgenic neonatal mouse cochlear SCs and tested the ability of the cell cycle re-entry and HC regeneration [10]. The presence of both BrdU+ and BrdU- regenerated HCs indicated that SCs can generate new HCs through both direct differentiation and mitotic pathways [10,18].
Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5), a Wnt signaling downstream target gene, has been reported to be a progenitor/stem cell marker in many other tissues [19,20]. Chai et al. and Shi et al. both reported that cochlear Lgr5+ cells, a subset of SCs including inner pillar cells, inner border cells, third-row Deiters’ cells, and the lateral greater epithelial ridge (Figure 1), are the inner ear progenitors in the neonatal mouse cochlea [21,22]. These Lgr5+ progenitors have been shown to regenerate HCs in the neonatal cochlea both in vivo and in vitro, and Wnt signaling induction either by Wnt agonists or in β-catenin overexpression transgenic mice promotes the proliferation of Lgr5+ progenitors and HC regeneration [21,23].
Figure 1.
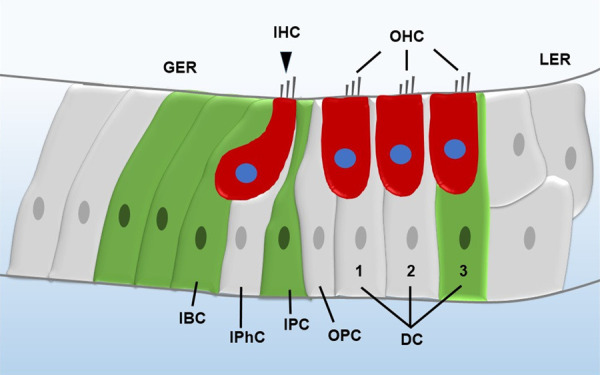
Illustration of the mammalian cochlea. The red cells are HCs, and the green cells are Lgr5+ progenitors. IHC, inner hair cell; OHC, outer hair cell; GER, greater epithelial ridge; LER, lesser epithelial ridge; DC, Deiters’ cell; OPC, outer pillar cell; IPC, inner pillar cell; IPhC, inner phalangeal cell; IBC, inner border cell.
In another study, Jan et al. used reporter mice for Axin2 gene, which is a downstream negative feedback gene of the Wnt signaling pathway [24], and showed in both in vitro cell culture and in vivo animal experiments that Axin2+ tympanic border cells have similar characteristics as cochlear progenitors. These cells can proliferate into cell colonies and can be differentiated into SCs and HCs. Moreover, the ability of these Axin2+ cells to proliferate and differentiate can be induced by Wnt agonists and suppressed by Wnt inhibitors, similar with Lgr5+ progenitors. Therefore, it is suggested that Axin2+ cells might also be a potential source of progenitors for treating hearing disorders.
Recently, two other genes have been reported to be novel inner ear progenitor markers. The first is Lgr6, which is also a Wnt-signaling downstream target gene. Lgr6+ cells, which only include inner pillar cells in the neonatal mouse cochlea, are a subpopulation of Lgr5+ progenitors, and Lgr6+ cells can generate Myosin7a+ HCs in vitro in a similar manner as Lgr5+ progenitors [25]. The same number of isolated Lgr6+ cells generates significantly more Myosin7a+ HCs compared to Lgr5+ progenitors, while Lgr5+ progenitors form more cell spheres than Lgr6+ cells in vitro [26], which suggests that Lgr6+ cells have greater ability for differentiation and lesser ability for proliferation compared to Lgr5+ progenitors. Another reported inner ear progenitor marker is Frizzled9, which is a Wnt receptor gene. Frizzled9 is expressed in inner phalangeal cells, inner border cells, and third-row Deiters’ cells in neonatal cochlea, and Frizzled9+ cells could regenerate HCs both in vivo and in vitro. Moreover, Frizzled9+ cells have a similar capacity for proliferation, differentiation, and HC generation as Lgr5+ progenitors [27].
In summary, the discovery of inner ear progenitors has provided a new approach for cell transplantation therapy. As mentioned above, there are two mechanisms for HC regeneration. One is trans-differentiation in which the inner ear progenitors switch cell fate to become HCs, and the other is mitotic regeneration in which inner ear progenitors proliferate and then differentiate into new HCs. Many transcription factors and signaling pathways are reported to be involved in the development of the inner ear, and several factors have been shown to be involved in HC regeneration in the neonatal mouse cochlea, including Atoh1, p27Kip1, pRb, Foxg1, and the Wnt, Notch, Hedgehog, and Ephrin signaling pathways (Figure 2).
Figure 2.
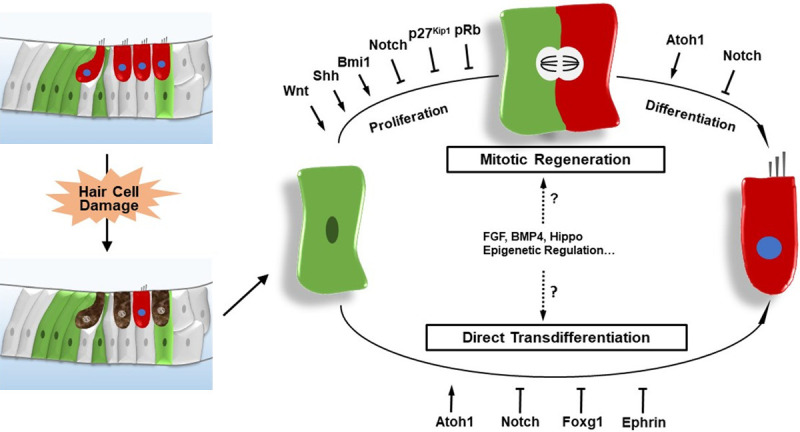
The regulation of HC regeneration in the neonatal mammalian cochlea after HC damage. HCs are regenerated through mitotic regeneration-in which progenitors re-enter the cell cycle, mitotically divide, and then differentiate into new HCs-or through direct trans-differentiation in which progenitors switch cell fate and directly differentiate into new HCs.
HC regeneration: transcription factors and regulatory factors
Atho1 (also called Math1) is a helix-loop-helix transcription factor that is essential for HC differentiation. The expression of Atoh1 is visible from embryonic day 14.5 in the cochlea. Deletion of the Atoh1 gene leads to the failure of HC formation, while its overexpression induces ectopic HCs [28,29]. Atoh1 also plays important roles later during inner ear development in HC survival and maturation [30,31]. In neonatal mice, Atoh1 is also important by promoting HC regeneration, and ectopic activation of Atoh1 induces new HCs generation in young postnatal mice [32,33]. Moreover, in the young adult deafened guinea pig model, forced expression of Atoh1 induces HC regeneration and decreases the hearing threshold [34]. However, only a subset of these cells is able to give rise to new HCs, and they do so only at early postnatal stages.
Cyclin-dependent kinase inhibitors (CKIs) are divides into two families, the Cip/Kip family and the Ink4 family, which play roles in governing cell cycle transitions and maintaining postmitotic state of numerous cell types [35,36]. p19Ink4d (Cdkn2d) and p21Cip1 (Cdkn1a) have been shown to be required in maintenance of the postmitotic state of HCs [37,38]. p27Kip1 (Cdkn1b), begins to be expressed in prosensory cells during the embryonic development of the mammalian cochlea, and it persists at high levels in SCs of the mature organ of Corti [39,40]. Deletion of the p27Kip1 gene in the mouse cochlea results in continuous cell proliferation in the postnatal and adult mouse cochlea and to the appearance of supernumerary HCs and SCs [39,41]. Deletion of p27Kip1 in SCs of the neonatal cochlea leads to the proliferation of pillar cells without cell fate conversion [42-44], which suggests that other factors are required to induce the differentiation of SCs into HCs.
pRb is a retinoblastoma protein encoded by the retinoblastoma gene Rb1 and plays important roles in cell cycle exit, differentiation, and survival [45,46]. And it has been shown that deletion of Rb1 gene leads to the cell-cycle re-entry of both embryonic and postnatal mammalian HCs [47-49]. In neonatal mice, inactivation of pRb in SCs results in cell cycle re-entry of both pillar and Deiters’ cells and an increase in the number of pillar cells. The nuclei of Rb-/- mitotic pillar and Deiters’ cells were observed to migrate toward the HC layer and these cells divide near the epithelial surface, similar to the SCs in the regenerating avian cochlea. However, there are no newly regenerated HCs, and SC death followed by HC loss occurs [50].
Foxg1 (formerly called BF-1), one of the forkhead box family proteins, is involved in morphogenesis, cell fate determination, and proliferation in many tissues, especially in the brain [51-55]. Foxg1 knockout mice die in the perinatal period and show shortened cochleae with multiple extra rows of HCs and SCs along with vestibular defects [56,57]. It was recently reported that conditional knockdown of Foxg1 in SCs and progenitors in neonatal mice induces their direct trans-differentiation, but not their proliferation, and subsequently leads to extra HCs [58].
HC regeneration: signaling pathways
During cochlear development, the canonical Wnt/β-catenin signaling pathway regulates cell proliferation, cell fate decision, and HC differentiation, and Wnt signaling activation induces inner ear progenitor proliferation and HC regeneration in both mammalian and non-mammalian vertebrates [59,60]. The inhibition of Wnt signaling in the embryonic mouse cochlea by small molecule inhibitors or in transgenic mice reduces the proliferation of prosensory cells [61]. Conversely, Wnt signaling activation promotes the prosensory domain formation and increases the number of HCs [62]. As mentioned above, Lgr5 and Lgr6, the Wnt signaling downstream targets, are expressed in embryonic and neonatal inner ear progenitors [22,25]. And these progenitors can act as inner ear progenitors both in vivo and in vitro due to their ability of self-renew, proliferation, and differentiation into HCs [21,23,63,64]. In neonatal cochlea, both Wnt agonists treatment and β-catenin overexpression promote the proliferative capacity of Lgr5+ progenitors and subsequent HC formation, whereas Wnt antagonists treatment reduce the proliferation and HC regeneration ability of Lgr5+ progenitors [23,62,65]. Wnt activation also causes the Axin2+ tympanic border cells to proliferate and differentiate into HCs and SCs in newborn mice [24]. The combined expression of β-catenin and Atoh1 in Lgr5+ cells increases the HC regeneration capacity of the postnatal cochlea by ten-fold, and these newly regenerated HCs can survive until adulthood [66]. However, the combined expression of β-catenin and Atoh1 cannot induce HC regeneration in the adult mammalian cochlea.
Because Notch signaling pathway plays important roles in HC differentiation during inner ear development, many researchers have examined its roles in HC regeneration in postnatal cochlea. In both the zebrafish lateral line and mature avian basilar papilla, inhibition of Notch signaling increases HC regeneration through SC mitotic division and direct trans-differentiation. In contrast, Notch activation maintains SCs in a quiescent state, thereby inhibiting regeneration of HCs [67,68]. In the mammalian postnatal cochlea, the Notch inhibition by γ-secretase inhibitor upregulates Atoh1 expression and results in the trans-differentiation of adjacent SCs into HCs [69,70]. Li et al. reported a direct interaction between the Notch and Wnt signaling pathways, that Notch inhibition induces mitotically generated HCs in mammalian cochleae via activating the Wnt pathway [71]. In addition, Notch and Wnt co-regulation promotes SC proliferation and HC regeneration in both the cochlea and utricle in neonatal mice [72,73]. A particularly exciting finding is that a genetic reprogramming process involving β-catenin activation, Notch1 deletion, and Atoh1 overexpression is established and can promote extensive SC proliferation followed by mitotic HC regeneration [74].
Hedgehog signaling is important for the formation of the dorsoventral axis of the inner ear, and plays important roles in the prosensory domain formation [75], the progenitor proliferation, and HC differentiation during inner ear development [76]. The cell fate of progenitors, whether differentiate into vestibular cells or auditory cells, is depend on the balance between Wnt and Hedgehog signaling [77,78]. A few studies have reported the roles of Hedgehog signaling in mammalian HC regeneration. Hedgehog signaling induces SC proliferation and HC regeneration in the postnatal rat cochlea after neomycin treatment [79], and Sonic Hedgehog recombinant protein effectively promotes in vitro sphere formation, proliferation, and differentiation of Lgr5+ progenitors isolated from the neonatal cochlea. Hedgehog signaling was also proved to induce SC proliferation and HC regeneration in neomycin damaged cochlea by using transgenic R26-SmoM2 mice which constitutively activate Hedgehog signaling in the SCs leads to [80].
Ephrins and their receptors Ephs also play role in HC regeneration. EphA4 receptor is expressed in HCs, while Ephrin-B2 is present in SCs, and this complementary pattern of expression is necessary for the establishment of the compartment boundary between HCs and SCs [81]. Jean Defourny et al. demonstrated that mammalian HCs can be directly generated from SCs by inhibition of ephrin-B2 signaling. Using either ephrin-B2 conditional knockout mice, shRNA-mediated gene silencing, or soluble inhibitors, they found that downregulation of ephrin-B2 signaling at late embryonic stages after HC production, results in translocation of SC into HC layers and subsequent cell fate switch from SC to HC [81]. Interestingly, throughout inner ear development, Ephrin-B2 and Notch are expressed in similar SC types [82]. Moreover, Ephrin-B2, whose expression is induced by Notch signaling, is reported to be a direct Notch signaling downstream target [83]; therefo-re, Ephrin-B2 might be required following Notch lateral inhibition in order to segregate the SCs from adjacent HCs.
HC regeneration: epigenetic regulation
Epigenetic factors have recently emerged as important regulators in both inner ear development and in HC regeneration. In the neuromasts of developing zebrafish larva, inhibition of the histone-modifying enzyme lysine-specific demethylase 1 (LSD1) disrupts cell proliferation, induces apoptosis, and reduces the numbers of sensory HCs and SCs [84]. And epigenetic regulation of Atoh1 was reported to guide HC development in the developing mouse cochlea [10]. Inhibition of histone acetyltransferase activity reduces H3K9 acetylation at the Atoh1 locus and therefore prevents Atoh1 mRNA increase and subsequent HC differentiation. Interestingly, the H3K4me3/H3K27me3 bivalent chromatin structure, observed in progenitors, persists at the Atoh1 locus in perinatal SCs [10], suggesting the important roles of such structures in HC regeneration.
Histone deacetylase (HDAC) inhibitor treatment of HC-damaged chicken utricles reduces proliferation of SCs, but does not affect HC regeneration [63]. Similarly, inhibition of HDAC activity in HC-damaged zebrafish larvae also reduces SC proliferation and subsequent HC regeneration [23]. Bmi1, a Polycomb group protein and a component of the Polycomb repressive complex 1, maintains the proliferative capacity of SCs by sustaining high levels of Wnt signaling in the neonatal mouse cochlea. In neonatal Bmi1-deficient cochleae, SCs fail to re-enter the cell cycle in response to HC damage, and the in vitro sphere-forming ability of Bmi1-deficient cochlear progenitors is also reduced [11].
Future perspectives
Although HC regeneration can be induced by many factors and signaling pathways in the neonatal mammalian cochlea, HCs cannot be regenerated in the adult mammalian cochlea and current technologies are still quite far from restoring hearing functions in the HC-damaged mammalian cochlea. Thus, further research is needed to find ways to induce HC regeneration in both the neonatal and adult mammalian cochlea.
First, more pathways and important factors, including those that might regulate the proliferation and differentiation of stem cells and progenitors, such as FGF, BMP4, and Hippo signaling pathway, should be explored in the study of HC regeneration. The FGF signaling pathway has been shown to be important in inner ear development and to be related to the otic placode induction and the otic vesicle development [85-87]. Deletion of the FGF receptor 1 (Fgfr1) gene in the inner ear results in decrease of the number of proliferative prosensory cells and subsequent decrease of the numbers of HCs and SCs [88,89]. The roles of the FGF signaling pathway in HC regeneration has been explored in the utricles of chickens and the lateral lines of zebrafish [90-93]. Many reports has shown that BMP4 plays important roles in mammalian and non-mammalian inner ear development [94-100], and it is recently reported that BMP4 can also antagonize HC regeneration in the avian auditory epithelium [101]. The Hippo/Yap signaling pathway plays important roles in development, homeostasis, and regeneration in many tissues and cancer cells [102-106], and it has been reported that Hippo/Yap controls proliferation and differentiation of lung and plays key roles in regeneration and fibrogenesis after kidney injury. In zebrafish lateral line, Yap1 plays important roles in HC differentiation. Knockdown of Yap1 in developing zebrafish affects development of the lateral line system and recapitulates the Prox1a deficiency in mechanosensory cells of neuromast [107]. All of the above factors and signaling pathways can be used as good candidates for further HC regeneration study in the mammalian inner ear. As mentioned above, many epigenetic regulators such as LSD1, histone modifications, and HDAC inhibitors, which have been studied in inner ear development and HC regeneration in non-mammalian organisms, are also very good candidates for studying HC regeneration in the mammalian inner ear.
Second, the interactions of multiple pathways in cell proliferation and HC differentiation should be explored. As mentioned above, some research has studied the cross talk between two or more signaling pathways and factors [72-74], but these studies are far from regenerating HCs and repairing inner ear damage in adult mammals.
And lastly, the maturation and survival of newly generated HCs and HC regeneration in adult mammals still remains a challenge. Bradley Walters et al. found that combining p27Kip1 deletion with ectopic Atoh1 expression surmounts age-related decline of HC regeneration from SCs, leading to conversion of SCs to HCs in mature mouse cochleae and after noise damage [108]. Moreover, co-activation of GATA3 or Pou4f3 and Atoh1 promoted conversion of SCs to HCs in adult mice and activation of Pou4F3 alone also converted mature SCs to HCs in vivo [108]. In another recent report, Yilai Shu et al. reported that transient co-activation of cell cycle activator Myc and inner ear progenitor gene Notch1 induces proliferation of diverse adult cochlear sensory epithelial cell types, and enables adult SCs to respond to transcription factor Atoh1 and efficiently trans-differentiate into HC-like cells [109]. Although it is excited to see these two recent reports that HC could now be regenerated from SCs in adult mice by genes and signaling regulation, the regeneration efficiency and the maturation of regenerated HCs remains still a problem. More efforts, such as other genes and signaling co-regulation, apoptosis inhibition and maturation induction of newly regenerated HCs, should be made in the future.
In summary, much effort has been put into exploring the mechanisms of HC regeneration in the mammalian inner ear, and many factors and signaling pathways have been shown to play important roles in the neonatal cochlea. However, these studies are still far from regenerating HCs and repairing HC damage in adult mammals, which is the ultimate research objective in this field.
Acknowledgements
This work was supported by grants from the Major State Basic Research Development Program of China (2017YFA01039000), the Strategic Priority Research Program of the Chinese Academy of Science (XDA16010303), the National Natural Science Foundation of China (Nos. 81970892, 81970882), the Jiangsu Province Natural Science Foundation (BK20190062, BE2019711), Boehringer Ingelheim Pharma GmbH, the Fundamental Research Funds for the Central Universities (2242020R40137), the Excellence Project of Southeast University, the Open Research Fund of the State Key Laboratory of Genetic Engineering, Fudan University (No. SKLGE1809) and Shenzhen Fundamental Research Program (JCYJ20190814093401920).
Disclosure of conflict of interest
None.
References
- 1.Wagner EL, Shin JB. Mechanisms of hair cell damage and repair. Trends Neurosci. 2019;42:414–424. doi: 10.1016/j.tins.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corwin JT, Oberholtzer JC. Fish n’ chicks: model recipes for hair-cell regeneration? Neuron. 1997;19:951–954. doi: 10.1016/s0896-6273(00)80386-4. [DOI] [PubMed] [Google Scholar]
- 3.Tucci DL, Rubel EW. Physiologic status of regenerated hair cells in the avian inner ear following aminoglycoside ototoxicity. Otolaryngol Head Neck Surg. 1990;103:443–450. doi: 10.1177/019459989010300317. [DOI] [PubMed] [Google Scholar]
- 4.Cotanche DA, Dopyera CE. Hair cell and supporting cell response to acoustic trauma in the chick cochlea. Hear Res. 1990;46:29–40. doi: 10.1016/0378-5955(90)90137-e. [DOI] [PubMed] [Google Scholar]
- 5.Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 6.Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 8.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 9.Burns JC, Stone JS. Development and regeneration of vestibular hair cells in mammals. Semin Cell Dev Biol. 2017;65:96–105. doi: 10.1016/j.semcdb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 12.Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol. 2009;493:141–162. doi: 10.1007/978-1-59745-523-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco B, Malgrange B. Concise review: regeneration in mammalian cochlea hair cells: help from supporting cells transdifferentiation. Stem Cells. 2017;35:551–556. doi: 10.1002/stem.2554. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Shu Y, Tang M, Li H. Mammalian cochlear hair cell regeneration and ribbon synapse reformation. Neural Plast. 2016;2016:2523458. doi: 10.1155/2016/2523458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: similarities and differences. Development. 2015;142:1561–1571. doi: 10.1242/dev.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, Sinkkonen W, Cheng AG, Oshima K, Heller S. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011;1:26. doi: 10.1038/srep00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savoy-Burke G, Gilels FA, Pan W, Pratt D, Que J, Gan L, White PM, Kiernan AE. Activated notch causes deafness by promoting a supporting cell phenotype in developing auditory hair cells. PLoS One. 2014;9:e108160. doi: 10.1371/journal.pone.0108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 20.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 21.Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, Cheng AG. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011;12:455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, Zuo J, Cheng AG. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan TA, Chai R, Sayyid ZN, van Amerongen R, Xia A, Wang T, Sinkkonen ST, Zeng YA, Levin JR, Heller S, Nusse R, Cheng AG. Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development. 2013;140:1196–1206. doi: 10.1242/dev.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Chen Y, Ni W, Guo L, Lu X, Liu L, Li W, Sun S, Wang L, Li H. Dynamic expression of Lgr6 in the developing and mature mouse cochlea. Front Cell Neurosci. 2015;9:165. doi: 10.3389/fncel.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Guo L, Lu X, Cheng C, Sun S, Li W, Zhao L, Lai C, Zhang S, Yu C, Tang M, Chen Y, Chai R, Li H. Characterization of Lgr6+ cells as an enriched population of hair cell progenitors compared to Lgr5+ cells for hair cell generation in the neonatal mouse cochlea. Front Mol Neurosci. 2018;11:147. doi: 10.3389/fnmol.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Liu D, Dong Y, Zhang Z, Zhang Y, Zhou H, Guo L, Qi J, Qiang R, Tang M, Gao X, Zhao C, Chen X, Qian X, Chai R. Frizzled-9+ supporting cells are progenitors for the generation of hair cells in the postnatal mouse cochlea. Front Mol Neurosci. 2019;12:184. doi: 10.3389/fnmol.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chonko KT, Jahan I, Stone J, Wright MC, Fujiyama T, Hoshino M, Fritzsch B, Maricich SM. Atoh1 directs hair cell differentiation and survival in the late embryonic mouse inner ear. Dev Biol. 2013;381:401–410. doi: 10.1016/j.ydbio.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J Neurosci. 2013;33:10110–10122. doi: 10.1523/JNEUROSCI.5606-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32:6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32:6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham JJ, Roussel MF. Cyclin-dependent kinase inhibitors in the development of the central nervous system. Cell Growth Differ. 2001;12:387–396. [PubMed] [Google Scholar]
- 36.Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene. 2000;247:1–15. doi: 10.1016/s0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 37.Laine H, Doetzlhofer A, Mantela J, Ylikoski J, Laiho M, Roussel MF, Segil N, Pirvola U. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci. 2007;27:1434–1444. doi: 10.1523/JNEUROSCI.4956-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- 39.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 40.Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(Suppl 220):1–44. [PubMed] [Google Scholar]
- 41.Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, Zhang L, Mellado Lagarde MM, Valentine MB, Yu Y, Cox BC, Zuo J. Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J Neurosci. 2012;32:10530–10540. doi: 10.1523/JNEUROSCI.0686-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maass JC, Berndt FA, Canovas J, Kukuljan M. p27Kip1 knockdown induces proliferation in the organ of Corti in culture after efficient shRNA lentiviral transduction. J Assoc Res Otolaryngol. 2013;14:495–508. doi: 10.1007/s10162-013-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. p27(Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10:1237–1248. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 46.Lipinski MM, Jacks T. The retinoblastoma gene family in differentiation and development. Oncogene. 1999;18:7873–7882. doi: 10.1038/sj.onc.1203244. [DOI] [PubMed] [Google Scholar]
- 47.Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, Vetter DE, Chen ZY. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci U S A. 2006;103:7345–7350. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 49.Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Weber T, Yamashita T, Liu Z, Valentine MB, Cox BC, Zuo J. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J Neurosci. 2010;30:5927–5936. doi: 10.1523/JNEUROSCI.5989-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian C, Gong Y, Yang Y, Shen W, Wang K, Liu J, Xu B, Zhao J, Zhao C. Foxg1 has an essential role in postnatal development of the dentate gyrus. J Neurosci. 2012;32:2931–2949. doi: 10.1523/JNEUROSCI.5240-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 53.Huh S, Hatini V, Marcus RC, Li SC, Lai E. Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev Biol. 1999;211:53–63. doi: 10.1006/dbio.1999.9303. [DOI] [PubMed] [Google Scholar]
- 54.Adesina AM, Veo BL, Courteau G, Mehta V, Wu X, Pang K, Liu Z, Li XN, Peters L. FOXG1 expression shows correlation with neuronal differentiation in cerebellar development, aggressive phenotype in medulloblastomas, and survival in a xenograft model of medulloblastoma. Hum Pathol. 2015;46:1859–1871. doi: 10.1016/j.humpath.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Pratt T, Tian NM, Simpson TI, Mason JO, Price DJ. The winged helix transcription factor Foxg1 facilitates retinal ganglion cell axon crossing of the ventral midline in the mouse. Development. 2004;131:3773–3784. doi: 10.1242/dev.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang CH, Simeone A, Lai E, Wu DK. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev Dyn. 2009;238:2725–2734. doi: 10.1002/dvdy.22111. [DOI] [PubMed] [Google Scholar]
- 58.Zhang S, Zhang Y, Dong Y, Guo L, Zhang Z, Shao B, Qi J, Zhou H, Zhu W, Yan X, Hong G, Zhang L, Zhang X, Tang M, Zhao C, Gao X, Chai R. Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea. Cell Mol Life Sci. 2020;77:1401–1419. doi: 10.1007/s00018-019-03291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero-Carvajal A, Navajas Acedo J, Jiang L, Kozlovskaja-Gumbriene A, Alexander R, Li H, Piotrowski T. Regeneration of sensory hair cells requires localized interactions between the notch and wnt pathways. Dev Cell. 2015;34:267–282. doi: 10.1016/j.devcel.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacques BE, Montgomery WH 4th, Uribe PM, Yatteau A, Asuncion JD, Resendiz G, Matsui JI, Dabdoub A. The role of Wnt/beta-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev Neurobiol. 2014;74:438–456. doi: 10.1002/dneu.22134. [DOI] [PubMed] [Google Scholar]
- 61.Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis AK, Kelley MW, Dabdoub A. A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi F, Hu L, Jacques BE, Mulvaney JF, Dabdoub A, Edge AS. β-catenin is required for hair-cell differentiation in the cochlea. J Neurosci. 2014;34:6470–6479. doi: 10.1523/JNEUROSCI.4305-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang T, Chai R, Kim GS, Pham N, Jansson L, Nguyen DH, Kuo B, May LA, Zuo J, Cunningham LL, Cheng AG. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun. 2015;6:6613. doi: 10.1038/ncomms7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, Chalasani K, Steigelman KA, Fang J, Rubel EW, Cheng AG, Zuo J. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141:816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci U S A. 2013;110:13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. In vivo cochlear hair cell generation and survival by coactivation of beta-catenin and Atoh1. J Neurosci. 2015;35:10786–10798. doi: 10.1523/JNEUROSCI.0967-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS One. 2013;8:e73276. doi: 10.1371/journal.pone.0073276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY, Li H. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A. 2015;112:166–171. doi: 10.1073/pnas.1415901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J, Li W, Lin C, Chen Y, Cheng C, Sun S, Tang M, Chai R, Li H. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci Rep. 2016;6:29418. doi: 10.1038/srep29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni W, Zeng S, Li W, Chen Y, Zhang S, Tang M, Sun S, Chai R, Li H. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget. 2016;7:66754–66768. doi: 10.18632/oncotarget.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ni W, Lin C, Guo L, Wu J, Chen Y, Chai R, Li W, Li H. Extensive supporting cell proliferation and mitotic hair cell generation by in vivo genetic reprogramming in the neonatal mouse cochlea. J Neurosci. 2016;36:8734–8745. doi: 10.1523/JNEUROSCI.0060-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zarei S, Zarei K, Fritzsch B, Elliott KL. Sonic hedgehog antagonists reduce size and alter patterning of the frog inner ear. Dev Neurobiol. 2017;77:1385–1400. doi: 10.1002/dneu.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown AS, Epstein DJ. Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development. 2011;138:3967–3976. doi: 10.1242/dev.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu N, Chen Y, Wang Z, Chen G, Lin Q, Chen ZY, Li H. Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein. Biochem Biophys Res Commun. 2013;430:700–705. doi: 10.1016/j.bbrc.2012.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y, Lu X, Guo L, Ni W, Zhang Y, Zhao L, Wu L, Sun S, Zhang S, Tang M, Li W, Chai R, Li H. Hedgehog signaling promotes the proliferation and subsequent hair cell formation of progenitor cells in the neonatal mouse cochlea. Front Mol Neurosci. 2017;10:426. doi: 10.3389/fnmol.2017.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Defourny J, Mateo Sanchez S, Schoonaert L, Robberecht W, Davy A, Nguyen L, Malgrange B. Cochlear supporting cell transdifferentiation and integration into hair cell layers by inhibition of ephrin-B2 signalling. Nat Commun. 2015;6:7017. doi: 10.1038/ncomms8017. [DOI] [PubMed] [Google Scholar]
- 82.Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 83.D’Amato G, Luxan G, de la Pompa JL. Notch signalling in ventricular chamber development and cardiomyopathy. FEBS J. 2016;283:4223–4237. doi: 10.1111/febs.13773. [DOI] [PubMed] [Google Scholar]
- 84.Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schimmang T. Expression and functions of FGF ligands during early otic development. Int J Dev Biol. 2007;51:473–481. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- 87.Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- 88.Ono K, Kita T, Sato S, O’Neill P, Mak SS, Paschaki M, Ito M, Gotoh N, Kawakami K, Sasai Y, Ladher RK. FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 2014;10:e1004118. doi: 10.1371/journal.pgen.1004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 90.Ku YC, Renaud NA, Veile RA, Helms C, Voelker CC, Warchol ME, Lovett M. The transcriptome of utricle hair cell regeneration in the avian inner ear. J Neurosci. 2014;34:3523–3535. doi: 10.1523/JNEUROSCI.2606-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bermingham-McDonogh O, Stone JS, Reh TA, Rubel EW. FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev Biol. 2001;238:247–259. doi: 10.1006/dbio.2001.0412. [DOI] [PubMed] [Google Scholar]
- 92.Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014;111:E1383–1392. doi: 10.1073/pnas.1402898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pirvola U, Cao Y, Oellig C, Suoqiang Z, Pettersson RF, Ylikoski J. The site of action of neuronal acidic fibroblast growth factor is the organ of Corti of the rat cochlea. Proc Natl Acad Sci U S A. 1995;92:9269–9273. doi: 10.1073/pnas.92.20.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerlach LM, Hutson MR, Germiller JA, Nguyen-Luu D, Victor JC, Barald KF. Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development. 2000;127:45–54. doi: 10.1242/dev.127.1.45. [DOI] [PubMed] [Google Scholar]
- 95.Blauwkamp MN, Beyer LA, Kabara L, Takemura K, Buck T, King WM, Dolan DF, Barald KF, Raphael Y, Koenig RJ. The role of bone morphogenetic protein 4 in inner ear development and function. Hear Res. 2007;225:71–79. doi: 10.1016/j.heares.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vervoort R, Ceulemans H, Van Aerschot L, D’Hooge R, David G. Genetic modification of the inner ear lateral semicircular canal phenotype of the Bmp4 haplo-insufficient mouse. Biochem Biophys Res Commun. 2010;394:780–785. doi: 10.1016/j.bbrc.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 97.Liu W, Oh SH, Kang Yk Y, Li G, Doan TM, Little M, Li L, Ahn K, Crenshaw EB 3rd, Frenz DA. Bone morphogenetic protein 4 (BMP4): a regulator of capsule chondrogenesis in the developing mouse inner ear. Dev Dyn. 2003;226:427–438. doi: 10.1002/dvdy.10258. [DOI] [PubMed] [Google Scholar]
- 98.Waqas M, Sun S, Xuan C, Fang Q, Zhang X, Islam IU, Qi J, Zhang S, Gao X, Tang M, Shi H, Li H, Chai R. Bone morphogenetic protein 4 promotes the survival and preserves the structure of flow-sorted Bhlhb5+ cochlear spiral ganglion neurons in vitro. Sci Rep. 2017;7:3506. doi: 10.1038/s41598-017-03810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, Heller S. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol. 2005;5:16. doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lewis RM, Keller JJ, Wan L, Stone JS. Bone morphogenetic protein 4 antagonizes hair cell regeneration in the avian auditory epithelium. Hear Res. 2018;364:1–11. doi: 10.1016/j.heares.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13:324–337. doi: 10.1038/nrgastro.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu V, Plouffe SW, Guan KL. The Hippo pathway in organ development, homeostasis, and regeneration. Curr Opin Cell Biol. 2017;49:99–107. doi: 10.1016/j.ceb.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zygulska AL, Krzemieniecki K, Pierzchalski P. Hippo pathway - brief overview of its relevance in cancer. J Physiol Pharmacol. 2017;68:311–335. [PubMed] [Google Scholar]
- 105.Mueller KA, Glajch KE, Huizenga MN, Wilson RA, Granucci EJ, Dios AM, Tousley AR, Iuliano M, Weisman E, LaQuaglia MJ, DiFiglia M, Kegel-Gleason K, Vakili K, Sadri-Vakili G. hippo signaling pathway dysregulation in human huntington’s disease brain and neuronal stem cells. Sci Rep. 2018;8:11355. doi: 10.1038/s41598-018-29319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poon CL, Mitchell KA, Kondo S, Cheng LY, Harvey KF. The hippo pathway regulates neuroblasts and brain size in drosophila melanogaster. Curr Biol. 2016;26:1034–1042. doi: 10.1016/j.cub.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Loh SL, Teh C, Muller J, Guccione E, Hong W, Korzh V. Zebrafish yap1 plays a role in differentiation of hair cells in posterior lateral line. Sci Rep. 2014;4:4289. doi: 10.1038/srep04289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, Zuo J. In vivo interplay between p27(Kip1), GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice. Cell Rep. 2017;19:307–320. doi: 10.1016/j.celrep.2017.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang S, Zhang Y, Dong Y, Guo L, Zhang Z, Shao B, Qi J, Zhou H, Zhu W, Yan X, Hong G, Zhang L, Zhang X, Tang M, Zhao C, Gao X, Chai R. Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea. Cell Mol Life Sci. 2020;77:1401–1419. doi: 10.1007/s00018-019-03291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


