Abstract
The ability to locate and identify molecular interactions in cells has significant importance for understanding protein function and molecular biology. Functionalized metallic nanoparticles have been used as probes for protein tracking and drug delivery due to their ability to carry therapeutic agents and readily functionalized surfaces. In this work, we present a super-resolution surface-enhanced Raman scattering (SERS) approach for imaging and tracking membrane receptors interacting with peptide functionalized gold nanostars (AuNS). The αvβ3 integrin receptors in colon cancer cells are successfully targeted and imaged using AuNS with the high affinity amino acid sequence arginine-glycine-aspartic acid-phenylalanine-cysteine (RGDFC) attached. The RGDFC peptide interaction with the integrin receptor provides a bright and fluctuating SERS signal that can be analyzed with localization microscopy algorithms. Additionally, the observed SERS spectrum is used to confirm protein-peptide interaction. Experiments with functionalized and bare AuNS illustrate specific and non-specific binding events. Specific binding is monitored with a localization precision of ~6 nm. The observed spatial resolution is associated with tight binding, which was confirmed by the slower diffusion coefficient measured from 4.4 × 10−11 cm2/s for the AuNS-RGDFC compared to 7.8 × 10−10 cm2/s for the bare AuNS. Super-resolution SERS images at different focal planes show evidence of internalized particles and suggest insights into protein orientation on the surface of cells. Our work demonstrates super-resolution SERS imaging to probe membrane receptor interactions in cells, providing chemical information and spatial resolution with potential for diverse applications in life science and biomedicine.
Keywords: super-resolution microscopy, single-molecule SERS, membrane receptors, gold nanostars, label-free, Raman imaging
Graphical Abstract
Protein receptors1,2 located in the membrane barrier between cells and their environment facilitate communication between extracellular medium and intracellular components. When external molecules or ligands bind to one of these receptors, it can trigger changes within the cells. Activating and blocking protein receptors are common strategies pursued in drug development1-4. While there are significant strides being made in biological engineering and pharmacotherapy toward discovering new drug targets and appropriate ligands for drug delivery and disease treatments, the development of techniques to track and locate these (bio)molecules, essential to understanding drugs interaction and delivery, remains very challenging5.
A suite of advanced techniques have emerged to provide high resolution biological images such as cryo-electron microscopy (cryo-EM)6 and X-ray crystallography.7 Additionally fluorescence-based methods like resonance energy transfer (FRET)8 and DNA points accumulation for imaging in nanoscale topography (DNA-PAINT)9 have been demonstrated with optical techniques. These approaches provide valuable information about biochemical interactions and enable scientists to track and locate (bio)molecules with sub-diffraction limited resolution. While these techniques have had success, they rely on time-consuming sample preparation steps, require significant technical expertise, and/or often rely on labels that have autofluorescence and photobleaching issues. Surface-enhanced Raman scattering (SERS) is another approach that has been used to image biomolecules in cells10,11. SERS labels and probes have been extensively used as an important bioimaging strategy due to their stable signals over time, reduced sample background (autofluorescence) and, narrow spectral bands that facilitate multiplexing compared to common fluorophores10,12. However, SERS is high-sensitivity technique that can also be used as a label-free method to provide a molecular fingerprint and also obtain spatial information13-18. In addition to providing chemical information about the cellular components, SERS is minimally invasive and has the versatility to work in adverse environments, e.g. in vitro and in vivo19-24, which have led to the emergence of SERS as a widely applied and important analytical tool for biomedical applications25.
The ανβ3 integrin is a cell surface glycoprotein receptor that is known to bind with high specificity to short amino acid sequences, such as Arginine-glycine-aspartic acid (RGD) containing peptides. ανβ3 integrin is widely targeted in cancer treatment because if its increased expression in cancer cells1-4. Recently, our group reported that the SERS response from a gold nanostar (AuNS) functionalized with the peptide RGD-phenylalanine-cysteine (RGDFC) interacting with the ανβ3 receptor could be monitored by the distinct Raman signal observed and that the peptide on the AuNS inhibited the irreversible adsorption of proteins and biomolecules (corona formation), which is believed to be a key to successful in vitro and in vivo targeting26,27. The Raman signal enhancements in SERS arise from the interaction of a few molecules (or a single-molecule) located in the highly localized electromagnetic field at the nanoparticle surface that arises from the excitation of localized surface plasmon resonances (LSPR).13,28,29 The molecule enhancements are known to arise from localized areas of high intensity, commonly called “hotspots” on the plasmonic nanostructures30,31. Previously, our group has shown that the signal obtained from tip-enhanced Raman spectroscopy (TERS)32,33 and SERS26,27 of the AuNS-RGDFC particle can be used to differentiate the binding of the RGDFC peptide to different receptors in cell membranes. In our prior TERS work, 25-50 nm spatial resolution was achieved over a 1-2 μm square region. In contrast, SERS can map an entire cell, approximately a 10 × 10 μm square area; however, the spatial resolution was limited by the 1 μm step size and the diffraction limit for the focal spot (~400 nm), which impedes determining the precise location or internalization of the nanoparticle within different compartments of the cell. Nonetheless, the spectrum observed in both the SERS and TERS experiments generated the same chemical signatures associated with the binding interaction. The intensity of the SERS signals obtained from the AuNS-RGDFC interaction is substantially bright to suggest it can be detected and analyzed, for example, using localization microscopy techniques for improved spatial resolution wide-field imaging34.
Super-resolution SERS imaging can provides very fast acquisitions, on the order of microseconds (μs) to milliseconds (ms), and with nanometer spatial resolution even using wide-field illumination from a narrow linewidth laser source34-37. Similar to photo-activated localization microscopy (PALM)38,39 or stochastic optical reconstruction microscopy (STORM)40, in super-resolution SERS-STORM35-37,41,42, sequential images (frames) are recorded using a 2-dimensional (2D) array, such as a charge-coupled device (CCD) or complementary metal-oxide–semiconductor (CMOS) camera, where the spatial resolution is the convolution of the point spread function (p.s.f.) and the pixel size of this camera. However, rather than the “on-off” blinking and switching behavior of single fluorophores40,43 in fluorescence microscopy, the natural time-dependence of the SERS signal in single-molecule regime13,14,16-18, SERS intensity fluctuations (SIFs)42,44 can provide a temporally fluctuating signal appropriate for localization algorithms. The origin of those SIFs is not fully understood, but has been associated with the “on-off” diffusion of molecules visiting the hotspots in solution31 or surface reconstruction of atomic features that confine light into so-called “picocavities” in air18,42. The reconstruction of these picocavities is believed to occur on sub-millisecond time scales at room temperature18,42,45. Therefore, the signal variance from frame to frame contains information about the molecule probing the hotspot solved in space. The p.s.f of the SIFs can be fit by a traditional 2D Gaussian function and, therefore, the position (or centroid) of the emission can be determined within the diffracted-limited spot to a precision on the order of nanometers18,34,46. This means information from the near field is accessed in the far-field, in our case, from a wide-field illumination (λ = 659 nm). Thus far, there are only a few applications of super-resolution SERS imaging for biological samples: macrophage cells47, bacteria41 and DNA fragments36,37. Most of these reports were performed on structured surfaces, and we are unaware of any reports taking advantage of nanoparticle-based SERS as labels or probes to achieve super-resolution images.
In this paper, we demonstrate in vitro super-resolution SERS imaging and tracking of nanoparticle-membrane receptor interactions in intact SW620 colon cancer cells. The functionalized nanoparticles interact via modification of the surface with an RGDFC peptide that binds specifically to the αvβ3 integrin receptor. The SERS spectrum obtained further confirms the interaction of RGDFC-AuNS bound to αvβ3 integrin receptor. Using the temporal fluctuations of the Raman signal in the image, a localization precision of ≤6 nm is achieved with the RGDFC-AuNS. The diffusion coefficient determined experimentally suggests the AuNS-RGDFC are strongly bound. Our results suggest that SIFs are coming from the interaction and conformation of the AuNS-RGDFC-αvβ3 receptor at the membrane surface. By taking images at different focal planes, a Z-stack provides a 3D perspective of AuNS position and suggests internalization of the nanoparticles within the cells.
EXPERIMENTAL SECTION
Chemicals.
Tetrachloroauric (III) acid (HAuCl4), cetyltrimethylammonium chloride (CTAC), sodium borohydride (NaBH4), silver nitrate (AgNO3), hydrochloric acid (HCl), and ascorbic acid (C6H8O6) were all purchased from Sigma Aldrich (USA). Milli-Q water was used. Cyclic(-Arg-Gly-Asp-D-Phe-Cys) was purchased from Peptide international. PBS, FBS, and RPMI-1640 medium were purchased from Thermo Fischer Scientific (USA).
Nanofabrication of silver nanoislands (AgI).
Briefly, the coverslips (1 inch2) were previous cleaned as previously described48 and then placed in the thermal evaporator for deposition of the silver layer. A thin film of 15 nm of silver nanoislands (AgI) was deposited over the cleaned coverslips.
Synthesis and Characterization of AuNS.
The AuNS synthesis procedure described by Sloan-Dennison27 was utilized in this study. Rather than using HPLC water, Milli-Q water (18.2 MΩ cm) was used in our synthesis. AuNS were characterized via UV-vis, DLS, Nanosight, and zeta potential measurements.
AuNS functionalization with RGDFC.
Cyclic RGDFC (200 μM) was added to AuNS with an LSPR of 657 (1 mL) and left to shake overnight. The sample was centrifuged to remove excess RGDFC. RGDFC-AuNS were resuspended in 100 μL of water and kept in the fridge until used.
Cell culture.
Human SW620 colon cancer cells (ATCC, Manassas, Va.) were passaged at approximately 80% confluency in RPMI-1640 medium supplemented with 10% FBS. The cells were cultured in a humidified atmosphere containing 5% CO2 with a temperature of 37°C.
Cell incubation with AuNS-RGDFC.
Cells and RPMI-1640 medium were transferred to a 35 mm diameter culture flask (2 mL) with AgI coverslip. The culture flask was left to incubate for two days, allowing the cells time to adhere to the AgI coverslip. 200 μL of bare AuNS-RGDFC was added to the culture flask and was left to incubate for two hours. The cell media was then removed and the adherent cells on the AgI coverslip were washed with sterile PBS (1 mL) three times. Next, they were fixed by immersing the AgI coverslip in 4% formaldehyde for 15 minutes. The AgI coverslip was then removed from formaldehyde, washed with PBS and H2O, and left to dry.
Super resolution SERS Imaging.
The sample was imaged using our homebuilt wide-field Raman microscope, which combines an upright Olympus BX microscope with a CMOS camera (ORCA-Flash 4.0 V3, Hamamatsu) for high speed image acquisition. In a typical experiment imaging a fixed cell in air, the excitation laser consisted of a 659 nm single longitudinal mode laser with a power density ~0.2 kW/cm2. The laser provided wide-field illumination onto the sample, the Raman scattering was collected using a 100 × objective (NA = 0.9) in air, filtered using an edge filter (660 nm), and images were recorded at 100 ms per frame. In addition to recording wide-field imaging, our microscope can divert the collected Raman scattering to an f/2 spectrometer equipped with a CCD detector (Andor) to record the Raman spectrum. The spectral resolution was estimated as ~15 cm−1, limited by the diameter of the optical fiber used to transmit the collected Raman signal into the spectrograph.
RESULTS AND DISCUSSION
Super-Resolution SERS Experiment.
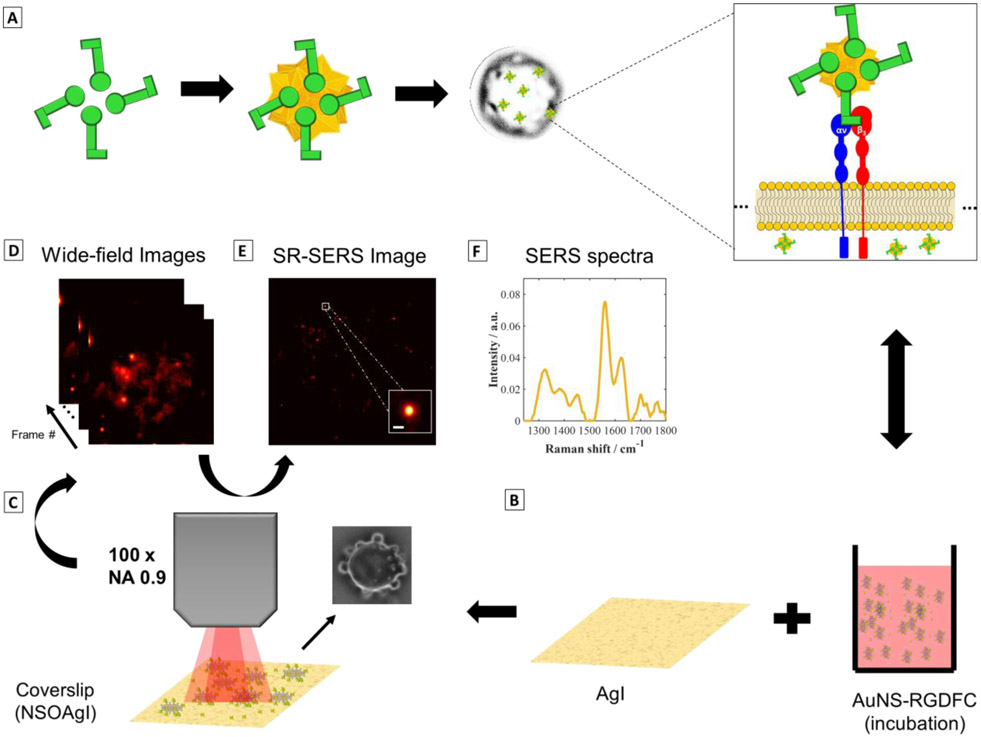
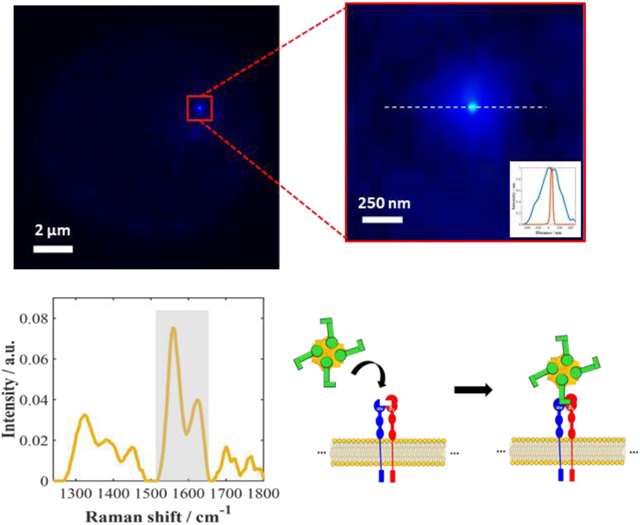
Figure 1 illustrates the approach used in this work to perform super-resolution SERS imaging of functionalized nanostars interacting with integrin receptors. Gold nanostars (AuNs) covalently modified with the RGDFC peptide (AuNS-RGDFC) through Au-S bonding with the cysteine residue were used as SERS probes (Figure 1A). After peptide functionalization on the AuNS (See supporting information for more experimental details), AuNS-RGDFC were then incubated with SW620 colon cancer cells, Figure 1A. The SW620 cells were incubated with AuNS-RGDFC in RPMI-1640 medium® over a semi- reflective/transparent silver nanoisland film (AgI, thickness of 15 nm) (Figure 1B). The AuNS were added to the media, and after 2 hours, allowing for the AuNS to interact with cell receptors, the cells were fixed with glutaldehyde for imaging. The thin AgI film substrate provided increased contrast compared to a plain coverslip (Figure S2). Kneipp et al.49 have demonstrated that the SERS signal from cells on a Au nanoisland film is quite intense, which helps differentiate between signals from the bottom of the cell and nanoparticles interacting at the top or within the cells.
Figure 1. Super-resolution SERS imaging of AuNS-RGDFC in cancer cells.
(A) RGDFC-functionalized AuNS were used as label free SERS probes. AuNS had an average size of 110±2nm and the LSPR peak max was at 657 nm (See supporting information, Figure S1 and Table S1). RGDFC functionalized AuNS were incubated with SW620 colon cancer cells in RPMI media to enable the AuNS-RGDFC to bind with αυβ3 integrin receptor as previous demonstrated. (B) The cells were fixed on Ag island covered coverslips. (C) Wide-field SERS images were collected using a 659 nm laser, with a power density of ~0.2 kW/cm2, and a 100× objective (NA 0.9) and 100 ms per frame. The inset image shows a bright-field image of a cell observed in this experiment. (D) A series of images recorded by collecting the Stokes scattering use an edge filter to reject the Rayleigh scattered light. (E) Super-resolution algorithms are applied to the wide field SERS images (separated in frames) to increase the localization of the AuNS probes interacting with the cell. The data shown was processed using ThunderSTORM50, with the following parameters adjusted for our camera: 65 nm per pixel, 1.6 photoelectrons per count, and camera base level of 50. The inset shows the improvement in the resolution when compared with the images in (D). The inset scale bar is 100 nm. (F) A typical Raman spectrum collected in this experiment is shown. The spectrum is obtained by directing the collected light through an optical fiber and then to a spectrograph and CCD. The integration time for the spectrum shown was 30 s.
The sample was imaged using our homebuilt wide-field Raman microscope for high speed image acquisition (Figure 1C). The laser was defocused to provide wide-field illumination onto the cells, and the Raman scattering was recorded on the CMOS array using an edge filter to collect only the SERS stokes signal (images and spectrum from 200 – 1800 cm−1). Our system allows us to illuminate a large area (diameter of illuminated area ≈ 50 μm), which is larger than the size of a single cell of approximately 10 × 10 μm. The wide-field images were processed to achieve super-resolution SERS images (Figure 1E). In addition to recording wide-field images, the observed Raman spectrum was recorded (Figure 1F).
Super-resolution SERS images from colon cancer cells using AuNS-RGDFC.
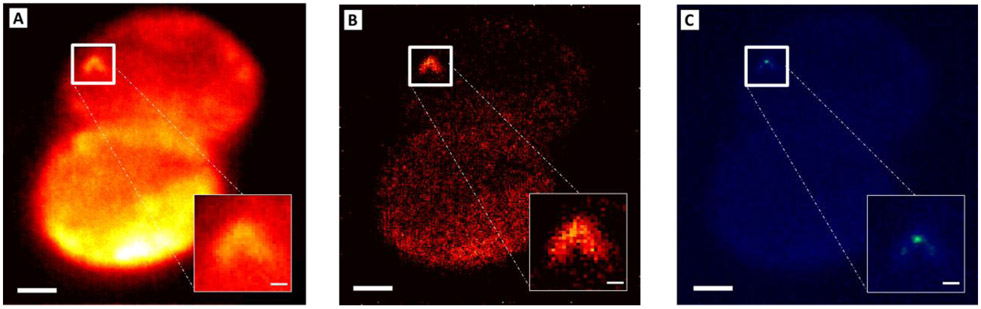
Figure 2 shows results obtained from two SW620 cells incubated with AuNS-RGDFC. In the data, a persistent background is observed from the fixed cells. The background may arise from autofluorescence of other cellular components. In addition to this background, there are bright and intense fluctuations observed at discrete locations in different frames of the recorded images, as indicated in Figure 2B. The fluctuating signals are attributed to SIFs arising from the previously reported intense SERS associated with the interaction of RGDFC-AuNS binding to integrin receptors on the cell membrane.26,27,32,33. The time dependent SIFs are used to differentiate the AuNS probes from the cellular background (Figure S3, see supplementary video 1). For example, one of these fluctuating bright spots is observed in frame #126 (Figure 2B, indicated by the green arrow), is not observed earlier in frame #01. The “on-off” SIFs is characteristic of single-molecule SERS experiments17,18,42 observed in Figure 2C (the black arrows indicate the “on” intensities above a previously established user defined threshold of ~17). We can therefore use super-resolution SERS-STORM18,28,34,35,37,41 methodology to localize these fluctuating signals.
Figure 2. Super-resolution SERS results for fixed cells incubated with AuNS-RGDFC recorded at 10 Hz.
(A) Bright-field image of two SW620 colon cells. (B) Individual frames, #01 and #126, and the average wide-field SERS image (n = 460 frames). The green arrow in frame #126 highlights a particularly active cluster. (C) Analysis of the active cluster noted by the green arrow generates an integrated SERS intensity trajectory for the cluster (25x26 pixels). The red line in (C) shows the threshold used to eliminate the noise and low-level background fluctuations. The black arrows indicate the “on” SIFs. (D) The resulting averaged super-resolution SERS image for Figure 2B is shown. Analysis of the zoom image indicates the active cluster is composed of three or more particles on the cell (See Figure S4 in SI) The blur in the image is partially attributed to motion of the AuNS from frame to frame. (E) The SERS spectra are shown collected from fixed cells incubated with AuNS-RGDFC (yellow) and bare AuNS (blue), as well as AuNS-RGDFC on glass (red). The scale bars are 2 μm for (C) and (D), and 150 nm for the insets.
Figure 2D shows the resulting super-resolution SERS image obtained from 460 frames. Analysis of the fluctuating spot noted in Figure 2D suggests at least three scattering centers were found, which are not discernable in the diffraction limited image (See Figure S4). Given the spacing of the centers, greater than 100 nm, we believe these signals arise from different AuNS probes. While the entire cell is illuminated by the laser (Figure 2B, C), non-fluctuating signals do not get included in the super-resolved image (Figure 2D). Pixels with signal fluctuations below a set threshold are treated as noise, are not fit, and therefore not included in the super-resolved image. A video illustrating the ThunderSTORM fitting for each frame (#1 to 1000) is shown in Supplementary Video S2. The crosses in the video illustrate the centroids determined for each fluctuation, attributed to individual nanoparticles, in time.18,28,34,35,37,41
SERS spectra were collected to verify the origin of the SIFs, Figure 2E. The SERS spectra were collected with laser power at the sample of ~4 mW and an integration time of 30 s. Our previous results indicate the αvβ3 receptor can be selectively detected and discriminated from the other cell membrane parts using the observed Raman signal26,27,32,33. To validate the origin of the SIFs, SERS spectra were recorded from three samples: 1) bare AuNS incubated with cells (blue spectrum), 2) AuNS-RGDFC incubated in cells (yellow spectrum), and 3) AuNS-RGDFC on glass (red spectrum). The SERS spectra show distinct differences, the SERS spectrum from the AuNS-RGDFC and cells (yellow spectrum) show strong bands around 1559 and 1625 cm−1, which are absent in the other spectra (blue and red spectra). Those bands are assigned to tryptophan and phenylalanine5, 6, 36, 37, and are consistent with our previous reports of AuNS-RGDFC bound to αvβ3 receptor in cells. These results suggest that this super-resolution SERS imaging approach can be used to selectively monitor the AuNS-RGDFC with nanometer precision.
The chosen threshold in ThunderSTORM is an important parameter in the analysis that can improve the resolution and avoid a false positive identification50,51; however in the presence of high background it can be difficult to avoid false positives and possibly miss real signals. The threshold used here was 17 (Figure 2C). This threshold is somewhat arbitrary and indicates that proper background correction is needed.
A comprehensive analysis of the ThunderSTORM output was carried out, as shown in Figure S5. The frame by frame ThunderSTORM output demonstrated that the bright spot and the heterogeneous background were both recovered in resulting super-resolution SERS image (Figure S5A&B). We suspect two possible origins for the observed background: scattering from the out of focus nanoparticles, and scattering/fluorescence from the other cell components that transmit through the filter and reach the detector. In either case, the signal can be comparable to the conventional SERS spectra recorded by a traditional Raman spectrometer, such as a scattering from the AuNS-RGDFC-integrin interaction, and requires proper correction18,42.
We have incorporated two approaches to correct for the non-uniform background observed in our images. The first approach utilizes the single-molecule accurate localization by local background subtraction (SMALL-LABS)52 algorithm reported by Biteen and co-workers. Briefly, the SMALL-LABS algorithm estimates the “true” background from “off-frames”, where the molecule of interest is not emitting, or “off”, to detect the “on”, or emitting molecules, in the image sequence. By estimating the mean background from the “off-frames”, the background corrected image of each emitter is obtained by subtracting the off-frames background from each raw image. The super-resolution algorithm is then applied, and the detected signals are returned (green and pink) and false signals are also identified (red). Figure S5C shows the results obtained from applying the SMALL-LABS algorithm to the data in Figure 2. Most of the features in Figure 2D are identified as artifacts; however, the bright spot in #126 frame was inconsistently identified in the data (Figure S5C). We hypothesize that the absolute difference in signal intensity for SERS imaging is less than is typical for fluorescence imaging, where the SMALL-LABS algorithm was designed.
We also explored the extreme value-based emitter recovery (EVER)53,54 algorithm for background correction. In the EVER algorithm53,54, the emitters (or nanoparticles) and the background change at different frequencies and are fit by separate Poisson distributions. When the intensity fluctuations of the emitter are similar to the background, it can become difficult to differentiate them from the background. However, since the median of the intensities of the emitter and the background are found as extremes values in our data, a linear function can describe the contribution of the background in the observed signal. Once established, the “true” background can be easily separated from the foreground emitters/nanoparticles. Figure 3 shows the importance of the background correction to achieve a reliable super-resolution SERS image in this work. Figure 3B shows the average SERS wide-field image recovered after using the EVER algorithm. It is clear that the background is significantly reduced, and the previously noted bright spot is quite pronounced (Figures 3A&B). ThunderSTORM50 analysis was then performed to detect the nanoparticles after correcting the background with EVER (Figure S5D). Figure S5D shows that nanoparticle was correctly detected, and no false positives were observed. Moreover, the resulting super-resolution SERS image in Figure S5E confirms that only the region surrounding the bright spot was recovered rather than the background previously observed (Figure S5B). Figure 3C shows a merged image plot of the average, EVER background corrected SERS image in blue and super-resolution image in green, highlighting the gain in resolution. The advantage of properly corrected the background in the super-resolution image demonstrated here is clear. This result demonstrates an advantage of our method in comparison to fluorescence-based super-resolution microscopy approaches. When the density of a labeled protein is high, it is challenging to precisely identify a single fluorophore inside the diffract-limited spot, which requires a multiemitter fitting55,56. While here the entire cell is illuminated by the laser excitation, only a few regions exhibit SIFs from enhanced scattering, which is consistent with detection of the AuNS-RGDFC binding to αvβ3 receptor26,27,32,33. The spurious signals, not originating from nanoparticle binding are removed after the background subtraction. Figure S6 shows similar results for a single cell, where three isolated nanoparticles were detected.
Figure 3. Impact of background on super-resolution analysis.
(A) Average wide-field SERS image (n = 460 frames). A strong background also is observed. (B) The EVER algorithm is used to generate a background corrected image, where it is possible to visualize the enhancement of the probed area and background reduction. (C) Merged imaged of the EVER-corrected background (in blue) and super-resolution SERS image (green) shows the gain in resolution after the SERS fitting. The zoomed in- images show the active cluster is composed of a bright central emitter and possibly three other particles (C), which are not evident in the diffracted-limited spot in (A) and (B). The scale bars are 2 μm for (A), (B) and (C), and 400 nm for the insets.
To further evaluate our methodology, images were recorded at a rate of 100 Hz, or 10 ms per frame (Figure S7). We found that even with faster image acquisition, it was possible to image an entire single cell with a good S/N ratio and detect signals characteristic of single nanoparticles binding to the integrin receptor (Figure S7B). The full-width at half maximum (FWHM) of the super-resolution image was determined to be 50 nm (Figure S7C, D). By comparison, bare AuNS (Figure S8) exhibit a poorer resolution (FWHM = 140 nm) even with longer signal accumulation (rate = 10 Hz). The poorer localization suggests that bare AuNS are not successfully targeted to a specific receptor on the membrane, and therefore may retain some translational mobility. Some of the average super-resolution images (Figure S8) show a certain degree of asymmetry or elongation, which may suggest a nanoparticle dimer46,57 is detected. This may indicate in the absence of the protein-ligand interaction, the scattered Raman signal is only strong enough to be detected from aggregates.
Super-resolution SERS nanoparticle tracking: AuNS-RGDFC vs bare AuNS.
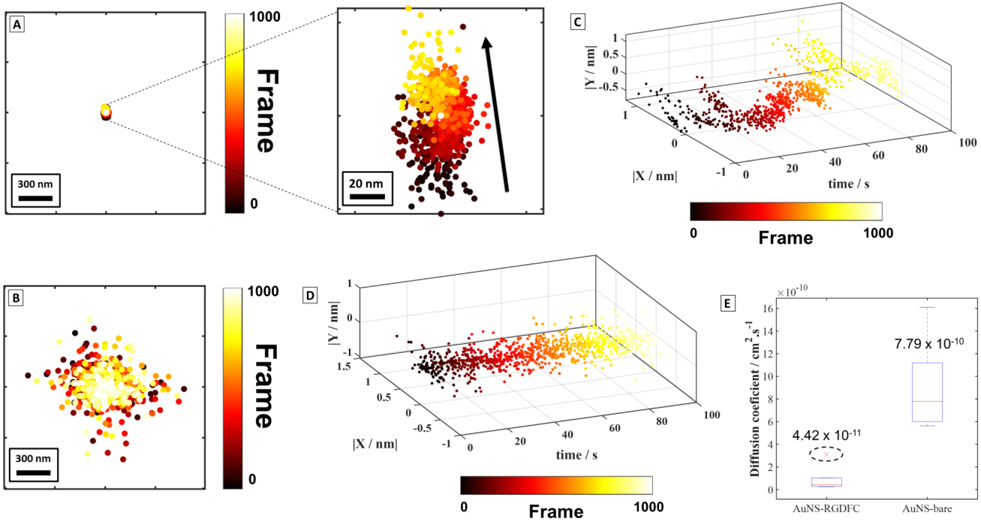
The results demonstrated in the previous section indicate that we can locate and track the AuNS-RGDFC in cells with a higher localization precision than bare AuNS. This suggest that SERS images can use to pinpoint and identify single nanoparticles binding to receptors.18,35,42 Figure 4 shows a comparison of individual functionalized (AuNS-RGDFC) and non-functionalized (bare AuNS) nanoparticles detected in cells. Figure 4A shows the centroids resulting from the 2D Gaussian fit to the emission of a very bright AuNS-RGDFC nanoparticle and Figure 4B shows the result for a bare AuNS. We estimated the lateral resolution using the standard deviation of centroids as a function of time, consistent with prior reports16, 23, 25, 26, 28, 35. Again, the cells incubated with AuNS-RGDFC shows a superior precision within ~6 nm (Figure 4A) versus ~123 nm for the bare AuNS (Figure 4B). This change in localization precision is remarkable and it may result from strong binding between the RGDFC peptide and the integrin receptor shown in the scheme in Figure 1A. Furthermore, this precision is smaller than AuNS probe, which suggests we are only collecting SERS photons originating from the AuNS-RGDFC interacting with the αvβ3 receptor system. This was further confirmed by estimating the uncertainty of a Gaussian fit to be ~1.4 nm (sigma = 14 nm (precision), minimum photon intensity, N = 296; standard deviation of the background in photons, b = 3.77; and the pixel size of the EM-CCD/CMOS camera in nanometers, a = 65 nm) for the AuNS-RGDFC.58 Prior work shows the AuNS-RGDFC bound to the αvβ3 integrin receptor gives a significant and specific enhanced Raman response26,27,34,59. These results corroborate the previous observations using TERS32,33 and later by a diffracted-limited illumination using SERS.26,27 The increased localization is believed to derive specifically from RGDFC-integrin interaction on the AuNS. While incubating cells with bare AuNS has been shown to have a SERS signal, the enhanced bands observed in the SERS spectrum are heterogeneous and non-specific, suggesting random and weak interactions with the membrane of cells and emission from the entire AuNS. The quality of the fits further supports this analysis, as the residuals of the centroids plotted in Figure S9 and S10 indicate a single particle is fit in each frame.
Figure 4. Super-resolution SERS trajectories solved in time for individual nanoparticles.
SIFs trajectory over time for an individual (A) AuNS-RGDFC (localization precision = 6 nm) and (B) bare AuNS (localization precision = 123 nm) plotted in the same scale. The data for the bare AuNS (B) are spread over a larger area than the AuNS-RGDFC (A). The black arrow in the zoomed-in figure shows a vector (r = 68 nm) indicating the net direction of the movement of the centroids in time for the AuNS-RGDFC. No preferential direction is observed for bare AuNS in (B). (C) 3-dimensional (3D) plot shows the stochastic changes in the centroid trajectory over time for a different AuNS-RGDFC interacting in a cell (localization precision = 7 nm). (D) 3D plot for showing that the centroid trajectory is constant for the bare AuNS. The axis in (C) and (D) were normalized between −1 and 1 for comparison. (E) Diffusion coefficient estimated for AuNS-RGDFC and bare AuNS (n = 6 nanoparticles). The dashed circle indicates an outlier detected.
Further examination of centroid movement shown Figure 4A (zoomed-in), indicates preferential motion along the y-axis is observed in relation to movement along the x-axis. The black displacement vector (r = 68 nm) annotated on the zoomed-in image indicates that the centroid position of the nanoparticle is changing in time from frame #0 to #1000 (black to yellow color). Figure 4C shows this preferential translation from a second experiment visualized using a 3-dimensional (3D) plot (∣X / nm∣, ∣Y / nm∣ and time / s). In Figures 4C, D, we have normalized the displacement data to look for this preferential translation. This behavior is observed repeatedly for different AuNS-RGDFC. It is important to note that the unfunctionalized AuNS shows no preferential motion and in either direction over the same periods of time (Figure 4D).
The centroid displacement can be used to calculate the diffusion coefficient for the nanoparticles interacting with the cells (See Equation 2 SI). Figure 4E shows a boxplot built using the values in Table S2 for individual nanoparticles with AuNS-RGDFC and bare AuNS. The diffusion coefficient obtained for AuNS-RGDFC was 4.4 × 10−11 cm2/s (n = 6) against 7.8 × 10−10 cm2/s (n = 6) for bare AuNS (Figure 4E). The slower diffusion coefficient for AuNS-RGDFC is consistent with nanoparticles bound to the membrane receptor, and is consistent with other results reported in literature33,60. Interesting, by increasing the laser power from 2 mW to 7 mW at the sample, the measured diffusion coefficient increases one order of magnitude from 4.4 × 10−11 cm2/s to 1.1 × 10−10 cm2/s (See Figure S11 for more details). The calculated Stokes-Einstein diffusion coefficient is 2.2 ×10−8 cm2/s for a 110 nm particle in water at 298K, clearly showing reduced mobility in our samples. The ~3× increase in the magnitude of diffusion coefficient would correspond to a temperature increase to ~900 K, or more likely, an increase in mobility suggesting a weakening of the binding interaction with the surface. The diffusions coefficients for the AuNS-RGDFC are still slower than the expected bulk diffusion, which further indicates that AuNS-RGDFC are interacting with the membrane surface.
Previous literature reports demonstrate a shift in the SERS centroid over time due to changes in the near field scattering properties of the nanoparticle.34,35 These phenomena are dependent on the shape, size, interparticle spacing, and polarization angle of illumination. In our experiments, we are monitoring individual nanostars with near uniform excitation illumination. In contrast to the previous work where a change in the centroid could be mapped onto the structure of the nanoparticles, in our experiments the particle has the ability to move and/or reorient in space and in relation to the electric field polarization. Consequently, the changes in SERS intensities can also originate from the AuNS-RGDFC-αvβ3 conformation, which could be manifest as blur in the spots recovered in the previous section and the large FWHM observed before (Compare Figure S7, S8).
Figure S12A, B shows a revised plot of Figure 4A correlating the signal intensities with their positions and in time. The centroids are color coded with respect to the intensity thresholds indicated (Figure S12A, B). While low SERS intensities spread out around the center, while high SERS intensities localized in the middle of the pattern (Figure S12A, B). The intensity data indicates the highest intensities are localized to a very small areas (≤ 6 nm), which is similar to other reports18,42. Figure S12C examines the time scales of the observed fluctuations. One explanation is there are changes in the interaction, and thus enhanced vibrational modes, between the AuNS-RGDFC and αvβ3 receptor61-64. The AuNS-RGDFC- αvβ3 conformation may be tilting and altering the alignment with respect to the illumination polarization (Figure S12D). This is confirmed by the 2D plot of SERS spectra shown in Figure S13, where characteristic SIFs were observed for the Raman bands at 1559 cm−1 (tryptophan) and 1625 cm−1 (phenylalanine) (Figure 2E). The asymmetric structure of the AuNS is expected to have a polarization dependence with respect to the electric field and the orientation of the branches, consequently, a change in orientation could alter the intensity of the centroid of the nanoparticle over time (Figures 4A, C). The fact that the high intensity fluctuations and distribution appear to occur in the same physical space and are very localized supports the idea of a changing conformation relative to the polarization of the electric field as demonstrated before in single-nanoparticle SERS experiments42. This change can be easily visualized by the frequencies of SIFs in Figure S12C, where most of the SIFs rate presented were ≤25 Hz (white threshold), or ≥40 ms. This result is reasonable compared to the time scale of changes in protein conformation65-67. This interpretation is also consistent with previous literature on integrin conformation61-63 and thermal activation by the laser illumination. This observation suggests the protein conformation is active, as shown also in Figure S12C, however, it is turned-off after a few seconds due to the interconversion process61-63. Figure S12D proposes a mechanism by which the orientation of the AuNS-RGDFC-αvβ3 conformation could change and give rise to fluctuations in the SIFs observed here in this work.
Super-resolution SERS imaging in the Z-direction.
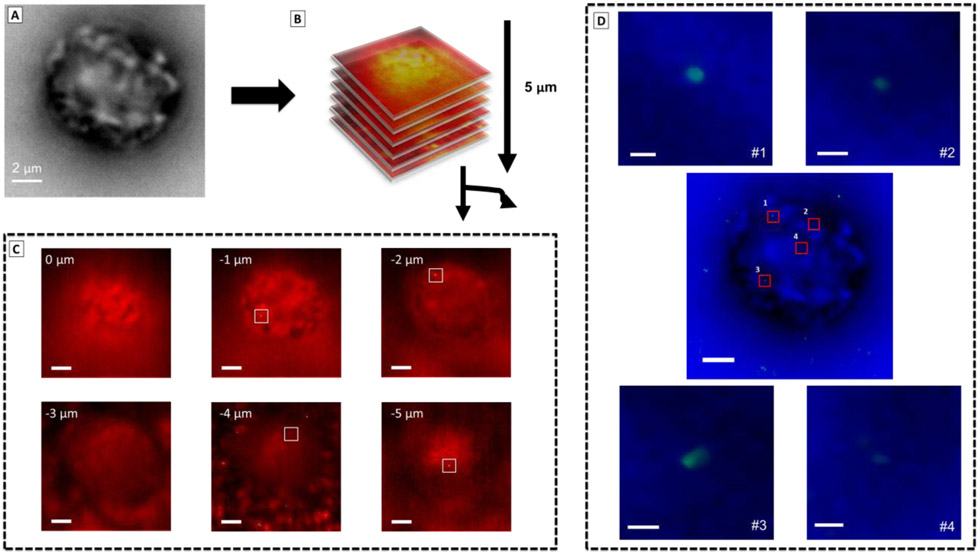
Using the localization precision demonstrated, we sought to address the question of whether nanoparticles stay on the cell surface or are internalized after binding to the receptors.68,69 To answer this question, we collected wide-field SERS images in series while changing the focal plane every 1000 frames by 1 μm steps in the Z-direction to create a 3D-image stack through a full cell (Figure 5). Figure 5C shows the merged plots of wide-field SERS images (in red) after the EVER background correction and super-resolution SERS nanoparticle (in green) detected by ThunderSTORM50 for different Z-slices. Four nanoparticles were detected on the cell in the highlighted areas in the −1, −2, −4 and −5 μm slices (Figure 5C). While the first nanoparticle was detected on top of the cell in the position −1 μm, the particles in the −2, −4 and −5 μm slices suggest possible internalization within the cells (Figure 5C). Although our spatial resolution is limited to −1 μm steps, these results suggest that different nanoparticles are being probed and detected from different x, y and z positions in the cell. An interesting fact in the super-resolution Z-stack SERS image in Figure S14 is that the nanoparticles on top (−1 μm) and at the bottom (−5 μm) of the cell present a crescent like shape pattern, which was not observed in the nanoparticle within the cell (−2 μm and −4 μm). This result is consistent with an oriented probe due to the curvature of the cell membrane, as the cell shape is not uniform.57,70-72 The size of the crescent features is consistent with a single nanostar, and may indicated binding to two or more branches of the AuNS. These initial results suggest further improvements in z-imaging may provide additional insight into the conformation of the AuNS-peptide probes interacting with cells. In summary, the results showed here suggest that our method is not only suitable for locating and tracking binding events on the membrane of the cells but also for internalization of the nanoparticles and possible caveats about the probed biomolecule’s orientation.
Figure 5. Z-stack super-resolution image for fixed cells incubated with AuNS-RGDFC.
(A) Bright-field image of a single cell. (B) Z-stack mapping were carried out with steps of 1 μm for every 1000 frames totalizing 5 μm, where 0 μm position is the top and −5 μm is the bottom of the cell. (C) Merged image of background corrected image (in red) and super-resolution SERS images (in green) for each Z-stack slices, suggesting the internalization of some nanoparticles. The scale bars are 2 μm. (D) Merged image of bright field image of the cell (in blue) and super-resolution SERS images (in green). Zoomed-in image are the selected are in the cell and shows nanoparticles found in independent locations in x,y in the cell, where with the crescent shaped p.s.f. More details in supporting information and main text. The scale bars are 100 nm (#1) and 150 nm (#2 to 4).
CONCLUSIONS
A super-resolution SERS imaging method for monitoring integrin receptors in cells is demonstrated. AuNS-RGDFC binding to ανβ3 receptor on the membrane of the cell was visualized with a precision of up to ~ 6 nm, and the identity of the binding was further confirmed by the SERS spectrum. The slower diffusion coefficient for AuNS-RGDFC demonstrated that the nanoparticles were strongly bound on membrane receptor in comparison to bare AuNS. The nanoparticles show some translational diffusion over the surface, and possible orientation changes that suggest protein-conformation changes on the time scale of ≥40 ms. Z-stack images provide a 3D correlation between the super-resolution SERS images and evidence that some of the AuNS-RGDFC were internalized within the cells. Additionally, the p.s.f of some nanoparticles evinced a crescent shape on top of the cells, suggesting the orientation or possible clustering of AuNS-RGDFC-ανβ3 can also be elucidated. This method provides non-invasive, fast, and chemically specific high spatial resolution cellular imaging, with potential implications for new applications in life science and biomedical analysis. The ability to image receptor binding events with high precision and also obtain vibrational spectra of the chemical interactions on nanometer length scales provides researchers a new approach to understand important biochemical interactions and mechanism. This is an important advance toward understanding, for example, biochemical signaling and drug delivery interactions in biological samples.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health, National Institute of General Medical Sciences award RO1 GM109988 and the Ohio State University. The authors thank Brain Scarpitti and Makenzie Bevins for assistance with the samples used in this work, and Katherine Willets for helpful discussions regarding super-resolution SERS imaging.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications Website including: Results of the nanostar characterization; Quality control results: comparison between the cell fixation in coverslip and AgI; background characterization; Quality of the multiple fitting for each frame of the AuNS-RGDFC cluster; Comprehensive characterization of the background and comparison of the ThunderSTORM fitting with and without background correction; Results of ThunderSTORM fitting for another single cell and in fast acquisition (100 ms); Results of ThunderSTORM fitting for AuNS bare; Quality of Gaussian fit of a single AuNS-RGDFC and AuNS bare SIFs; Temperature experiment for AuNS-RGDFC; Proposed mechanism for AuNS-RGDFC SERS intensity fluctuations; Results of time series 2D SERS spectra; Z-stack results of a single cell. Videos of raw data and super-resolution fitting analysis. Results of the diffusion coefficient for AuNS-RGDFC and AuNS bare.
The authors declare no competing financial interest.
REFERENCES
- (1).The International Transporter, C.; Giacomini KM; Huang S-M; Tweedie DJ; Benet LZ; Brouwer KLR; Chu X; Dahlin A; Evers R; Fischer V; Hillgren KM; Hoffmaster KA; Ishikawa T; Keppler D; Kim RB; Lee CA; Niemi M; Polli JW; Sugiyama Y; Swaan PW, et al. Membrane transporters in drug development. Nat. Rev. Drug Discov 2010, 9, 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Yin H; Flynn AD Drugging Membrane Protein Interactions. Annu. Rev. Biomed. Eng 2016, 18, 51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Sawada K; Ohyagi-Hara C; Kimura T; Morishige K.-i. Integrin Inhibitors as a Therapeutic Agent for Ovarian Cancer. J. Oncol. Pract 2012, 2012, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kobayashi M; Sawada K; Kimura T Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers 2017, 9, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pujals S; Albertazzi L Super-resolution Microscopy for Nanomedicine Research. ACS Nano 2019, 13, 9707–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bai X.-c.; McMullan G; Scheres SHW How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci 2015, 40, 49–57. [DOI] [PubMed] [Google Scholar]
- (7).Miao J; Ishikawa T; Robinson IK; Murnane MM Beyond crystallography: Diffractive imaging using coherent x-ray light sources. Science 2015, 348, 530–535. [DOI] [PubMed] [Google Scholar]
- (8).Sekar RB; Periasamy A Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol 2003, 160, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sharonov A; Hochstrasser RM Wide-Field Subdiffraction Imaging by Accumulated Binding of Diffusing Probes. Proc. Natl. Acad. Sci. U.S.A 2006, 103, 18911–18916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fabris L SERS Tags: The Next Promising Tool for Personalized Cancer Detection? ChemNanoMat 2016, 2, 249–258. [Google Scholar]
- (11).Kneipp J Interrogating Cells, Tissues, and Live Animals with New Generations of Surface-Enhanced Raman Scattering Probes and Labels. ACS Nano 2017, 11, 1136–1141. [DOI] [PubMed] [Google Scholar]
- (12).Lenzi E; Jimenez de Aberasturi D; Liz-Marzán LM Surface-Enhanced Raman Scattering Tags for Three-Dimensional Bioimaging and Biomarker Detection. ACS Sensors 2019, 4, 1126–1137. [DOI] [PubMed] [Google Scholar]
- (13).Nie S; Emory SR Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [DOI] [PubMed] [Google Scholar]
- (14).Kneipp K; Wang Y; Kneipp H; Perelman LT; Itzkan I; Dasari RR; Feld MS Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett 1997, 78, 1667–1670. [Google Scholar]
- (15).Etchegoin PG; Le Ru EC A perspective on single molecule SERS: current status and future challenges. Phys. Chem. Chem. Phys 2008, 10, 6079–6089. [DOI] [PubMed] [Google Scholar]
- (16).Dieringer JA; Lettan RB; Scheidt KA; Van Duyne RP A Frequency Domain Existence Proof of Single-Molecule Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc 2007, 129, 16249–16256. [DOI] [PubMed] [Google Scholar]
- (17).de Albuquerque CDL; Sobral-Filho RG; Poppi RJ; Brolo AG Digital Protocol for Chemical Analysis at Ultralow Concentrations by Surface-Enhanced Raman Scattering. Anal. Chem 2018, 90, 1248–1254. [DOI] [PubMed] [Google Scholar]
- (18).Lindquist NC; de Albuquerque CDL; Sobral-Filho RG; Paci I; Brolo AG High-speed imaging of surface-enhanced Raman scattering fluctuations from individual nanoparticles. Nat. Nanotechnol 2019, 14, 981–987. [DOI] [PubMed] [Google Scholar]
- (19).Antonio KA; Schultz ZD Advances in Biomedical Raman Microscopy. Anal. Chem 2014, 86, 30–46. [DOI] [PubMed] [Google Scholar]
- (20).Lane LA; Qian X; Nie S SERS Nanoparticles in Medicine: From Label-Free Detection to Spectroscopic Tagging. Chem. Rev 2015, 115, 10489–10529. [DOI] [PubMed] [Google Scholar]
- (21).Kircher MF; de la Zerda A; Jokerst JV; Zavaleta CL; Kempen PJ; Mittra E; Pitter K; Huang R; Campos C; Habte F; Sinclair R; Brennan CW; Mellinghoff IK; Holland EC; Gambhir SS A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med 2012, 18, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Harmsen S; Huang R; Wall MA; Karabeber H; Samii JM; Spaliviero M; White JR; Monette S; O’Connor R; Pitter KL; Sastra SA; Saborowski M; Holland EC; Singer S; Olive KP; Lowe SW; Blasberg RG; Kircher MF Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci. Transl. Med 2015, 7, 271ra277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Matschulat A; Drescher D; Kneipp J Surface-Enhanced Raman Scattering Hybrid Nanoprobe Multiplexing and Imaging in Biological Systems. ACS Nano 2010, 4, 3259–3269. [DOI] [PubMed] [Google Scholar]
- (24).Kneipp K; Kneipp H; Kneipp J Surface-Enhanced Raman Scattering in Local Optical Fields of Silver and Gold NanoaggregatesFrom Single-Molecule Raman Spectroscopy to Ultrasensitive Probing in Live Cells. Acc. Chem. Res 2006, 39, 443–450. [DOI] [PubMed] [Google Scholar]
- (25).Langer J; Jimenez de Aberasturi D; Aizpurua J; Alvarez-Puebla RA; Auguié B; Baumberg JJ; Bazan GC; Bell SEJ; Boisen A; Brolo AG; Choo J; Cialla-May D; Deckert V; Fabris L; Faulds K; García de Abajo FJ; Goodacre R; Graham D; Haes AJ; Haynes CL, et al. Present and Future of Surface Enhanced Raman Scattering. ACS Nano 2020, 28–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sloan-Dennison S; Bevins MR; Scarpitti BT; Sauvé VK; Schultz ZD Protein corona-resistant SERS tags for live cell detection of integrin receptors. Analyst 2019, 5538–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sloan-Dennison S; Schultz ZD Label-free plasmonic nanostar probes to illuminate in vitro membrane receptor recognition. Chem. Sci 2019, 10, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Willets KA; Duyne RPV Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem 2007, 58, 267–297. [DOI] [PubMed] [Google Scholar]
- (29).Fang Y; Seong N-H; Dlott DD Measurement of the Distribution of Site Enhancements in Surface-Enhanced Raman Scattering. Science 2008, 321, 388–392. [DOI] [PubMed] [Google Scholar]
- (30).Hao E; Schatz GC Electromagnetic fields around silver nanoparticles and dimers. The Journal of Chemical Physics 2003, 120, 357–366. [DOI] [PubMed] [Google Scholar]
- (31).Ru ECL; Etchegoin PG Single-Molecule Surface-Enhanced Raman Spectroscopy. Annu. Rev. Phys. Chem 2012, 63, 65–87. [DOI] [PubMed] [Google Scholar]
- (32).Xiao L; Wang H; Schultz ZD Selective Detection of RGD-Integrin Binding in Cancer Cells Using Tip Enhanced Raman Scattering Microscopy. Anal. Chem 2016, 88, 6547–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Xiao L; Bailey KA; Wang H; Schultz ZD Probing Membrane Receptor–Ligand Specificity with Surface- and Tip- Enhanced Raman Scattering. Anal. Chem 2017, 89, 9091–9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Stranahan SM; Willets KA Super-resolution Optical Imaging of Single-Molecule SERS Hot Spots. Nano Lett. 2010, 10, 3777–3784. [DOI] [PubMed] [Google Scholar]
- (35).Willets KA Super-resolution imaging of SERS hot spots. Chem. Soc. Rev 2014, 43, 3854–3864. [DOI] [PubMed] [Google Scholar]
- (36).Ertsgaard CT; McKoskey RM; Rich IS; Lindquist NC Dynamic Placement of Plasmonic Hotspots for Super-resolution Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 10941–10946. [DOI] [PubMed] [Google Scholar]
- (37).Olson AP; Ertsgaard CT; Elliott SN; Lindquist NC Super-Resolution Chemical Imaging with Plasmonic Substrates. ACS Photonics 2016, 3, 329–336. [Google Scholar]
- (38).Hess ST; Girirajan TPK; Mason MD Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J 2006, 91, 4258–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Betzig E; Patterson GH; Sougrat R; Lindwasser OW; Olenych S; Bonifacino JS; Davidson MW; Lippincott-Schwartz J; Hess HF Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [DOI] [PubMed] [Google Scholar]
- (40).Rust MJ; Bates M; Zhuang X Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Olson AP; Spies KB; Browning AC; Soneral PAG; Lindquist NC Chemically imaging bacteria with super-resolution SERS on ultra-thin silver substrates. Sci Rep 2017, 7, 9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).de Albuquerque CDL; Hokanson K; Thorud S; Sobral-Filho RG; Lindquist NC; Brolo AG Dynamic Imaging of Multiple SERS Hotspots on Single Nanoparticles. ACS Photonics 2020, 7, 434–443. [Google Scholar]
- (43).Dickson RM; Cubitt AB; Tsien RY; Moerner WE On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature 1997, 388, 355–358. [DOI] [PubMed] [Google Scholar]
- (44).dos Santos DP; Temperini MLA; Brolo AG Intensity Fluctuations in Single-Molecule Surface-Enhanced Raman Scattering. Acc. Chem. Res 2019, 52, 456–464. [DOI] [PubMed] [Google Scholar]
- (45).Carnegie C; Griffiths J; de Nijs B; Readman C; Chikkaraddy R; Deacon WM; Zhang Y; Szabó I; Rosta E; Aizpurua J; Baumberg JJ Room-Temperature Optical Picocavities below 1 nm3 Accessing Single-Atom Geometries. J. Phys. Chem. Lett 2018, 9, 7146–7151. [DOI] [PubMed] [Google Scholar]
- (46).Titus EJ; Weber ML; Stranahan SM; Willets KA Super-Resolution SERS Imaging beyond the Single-Molecule Limit: An Isotope-Edited Approach. Nano Lett. 2012, 12, 5103–5110. [DOI] [PubMed] [Google Scholar]
- (47).Ando J; Fujita K; Smith NI; Kawata S Dynamic SERS Imaging of Cellular Transport Pathways with Endocytosed Gold Nanoparticles. Nano Lett. 2011, 11, 5344–5348. [DOI] [PubMed] [Google Scholar]
- (48).Nelson DA; Schultz ZD Impact of Plasmon-Induced Optically Rectified Electric Fields on Second Harmonic Generation. J. Phys. Chem. C 2019, 123, 20639–20648. [Google Scholar]
- (49).Milewska A; Zivanovic V; Merk V; Arnalds UB; Sigurjónsson ÓE; Kneipp J; Leosson K Gold nanoisland substrates for SERS characterization of cultured cells. Biomed. Opt. Express 2019, 10, 6172–6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Ovesný M; Křížek P; Borkovec J; Švindrych Z; Hagen GM ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics 2014, 30, 2389–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Křížek P; Raška I; Hagen GM Minimizing detection errors in single molecule localization microscopy. Opt. Express 2011, 19, 3226–3235. [DOI] [PubMed] [Google Scholar]
- (52).Isaacoff BP; Li Y; Lee SA; Biteen JS SMALL-LABS: Measuring Single-Molecule Intensity and Position in Obscuring Backgrounds. Biophys. J 2019, 116, 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Ma H; Jiang W; Xu J; Liu Y Enhanced super-resolution microscopy by extreme value based emitter recovery. bioRxiv 2018, 295261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ma H; Xu J; Liu Y WindSTORM: Robust online image processing for high-throughput nanoscopy. Science Advances 2019, 5, eaaw0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Manley S; Gillette JM; Patterson GH; Shroff H; Hess HF; Betzig E; Lippincott-Schwartz J High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 2008, 5, 155–157. [DOI] [PubMed] [Google Scholar]
- (56).Yu Y; Li M; Yu Y Tracking Single Molecules in Biomembranes: Is Seeing Always Believing? ACS Nano 2019, 13, 10860–10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Stranahan SM; Titus EJ; Willets KA SERS Orientational Imaging of Silver Nanoparticle Dimers. J. Phys. Chem. Lett 2011, 2, 2711–2715. [Google Scholar]
- (58).Mortensen KI; Churchman LS; Spudich JA; Flyvbjerg H Optimized localization analysis for single-molecule tracking and super-resolution microscopy. Nat. Methods 2010, 7, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wertz E; Isaacoff BP; Flynn JD; Biteen JS Single-Molecule Super-Resolution Microscopy Reveals How Light Couples to a Plasmonic Nanoantenna on the Nanometer Scale. Nano Lett. 2015, 15, 2662–2670. [DOI] [PubMed] [Google Scholar]
- (60).Liao Y-H; Lin C-H; Cheng C-Y; Wong WC; Juo J-Y; Hsieh C-L Monovalent and Oriented Labeling of Gold Nanoprobes for the High-Resolution Tracking of a Single-Membrane Molecule. ACS Nano 2019, 13, 10918–10928. [DOI] [PubMed] [Google Scholar]
- (61).Wei Y; Czekay R-P; Robillard L; Kugler MC; Zhang F; Kim KK; Xiong J.-p.; Humphries MJ; Chapman HA Regulation of α5β1 integrin conformation and function by urokinase receptor binding. J. Cell Biol 2005, 168, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Askari JA; Buckley PA; Mould AP; Humphries MJ Linking integrin conformation to function. J. Cell Sci 2009, 122, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Campbell ID; Humphries MJ Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Lee J; Crampton KT; Tallarida N; Apkarian VA Visualizing vibrational normal modes of a single molecule with atomically confined light. Nature 2019, 568, 78–82. [DOI] [PubMed] [Google Scholar]
- (65).Yang H; Luo G; Karnchanaphanurach P; Louie T-M; Rech I; Cova S; Xun L; Xie XS Protein Conformational Dynamics Probed by Single-Molecule Electron Transfer. Science 2003, 302, 262–266. [DOI] [PubMed] [Google Scholar]
- (66).Michalet X; Weiss S; Jäger M Single-Molecule Fluorescence Studies of Protein Folding and Conformational Dynamics. Chem. Rev 2006, 106, 1785–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Henzler-Wildman K; Kern D Dynamic personalities of proteins. Nature 2007, 450, 964. [DOI] [PubMed] [Google Scholar]
- (68).Cho K; Wang X; Nie S; Chen Z; Shin DM Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res 2008, 14, 1310–1316. [DOI] [PubMed] [Google Scholar]
- (69).Behzadi S; Serpooshan V; Tao W; Hamaly MA; Alkawareek MY; Dreaden EC; Brown D; Alkilany AM; Farokhzad OC; Mahmoudi M Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev 2017, 46, 4218–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Stone MB; Shelby SA; Veatch SL Super-Resolution Microscopy: Shedding Light on the Cellular Plasma Membrane. Chem. Rev 2017, 117, 7457–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Bartko AP; Dickson RM Imaging Three-Dimensional Single Molecule Orientations. J. Phys. Chem. B 1999, 103, 11237–11241. [Google Scholar]
- (72).Patra D; Gregor I; Enderlein J Image Analysis of Defocused Single-Molecule Images for Three-Dimensional Molecule Orientation Studies. J. Phys. Chem. A 2004, 108, 6836–6841. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.