Abstract
Physical activity is an important element of type 1 diabetes management, and hypoglycemia is a known risk. There are few data on strategies adolescents use to mitigate this risk. We surveyed 66 adolescents with type 1 diabetes who were 12–18 years of age about blood glucose monitoring, carbohydrate intake, and insulin management before, during, and after exercise. The adolescents completed the International Physical Activity Questionnaire–Short Form and the Children’s Hypoglycemia Fear Survey. We found that adolescents with type 1 diabetes do not generally follow guidelines about glucose monitoring or about food and insulin adjustment around exercise. More targeted education and interventions are needed to improve adolescents’ uptake of recommended behaviors and improve outcomes.
Exercise is an important component of managing type 1 diabetes. It has many important cardiovascular health benefits for children and adolescents with type 1 diabetes, including weight control and reduction of triglyceride and total cholesterol levels (1). Physical activity also improves bone health (2) and sense of well-being (3). Recent meta-analyses suggest that increases in physical activity can also be associated with A1C reductions (1,4).
The American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), and many other organizations strongly encourage physical activity for children with type 1 diabetes (5,6). The ADA recommends that youth with type 1 diabetes follow the same recommendations given for healthy children, including engaging in at least 60 min of moderate- or vigorous-intensity physical activity per day (5).
Rates of physical activity for American youth are very low. Only about one-fourth of healthy adolescents get at least 60 minutes of physical activity per day (7). Most children and adolescents with type 1 diabetes also do not meet physical activity recommendations, and some reports suggest that those with type 1 diabetes engage in less physical activity than their peers (1,8,9). A recent study by Czenczek-Lewandowska et al. (10) found that this was true regardless of the method of insulin delivery used. There are significant barriers to physical activity in adolescents with type 1 diabetes, including fear of hypoglycemia (11) and hyperglycemia (5).
It is estimated that 10–20% of hypoglycemic episodes in pediatric patients with type 1 diabetes are associated with exercise (12). The most commonly identified reason for severe hypoglycemia is exercise, and most severe episodes occur at night, frequently when the patient is sleeping (11). Intensity and duration of exercise influences risk of hypoglycemia (6). McMahon et al. (13) found that glucose requirements after afternoon exercise increase both shortly after exercise and then again 7–11 hours later. The latter time point puts individuals at risk for nocturnal hypoglycemia. Of interest, these researchers did not find this same biphasic pattern if exercise is done at noon (14), suggesting that changing the timing of exercise can be a way to ameliorate the risk of hypoglycemia.
Recommendations for ways to decrease exercise-related hypoglycemia risk are available. ADA and ISPAD recommendations for diabetes care around exercise start with checking blood glucose at least before, during, and after exercise (5,6). Additional blood glucose checks might be warranted in different situations (e.g., if performing significantly strenuous exercise) (5,6). Furthermore, management includes considering insulin regimen changes and/or increased carbohydrate intake (5,6). To decrease risk of hypoglycemia, both insulin pump users and those with a multiple daily injection (MDI) insulin regimen can decrease bolus insulin that is administered within a few hours of exercise, basing the percentage reduction on exercise type, intensity, and duration (5,6). In addition, basal insulin delivered via insulin pump can be reduced before exercise (lowering the basal infusion rate or disconnecting the pump) for periods of anticipated prolonged exercise. To decrease the risk of subsequent delayed nocturnal hypoglycemia, overnight basal insulin may also be decreased, for those using either an insulin pump or an MDI insulin regimen (5,11). Food intake can also be increased or additional carbohydrates can be eaten during prolonged activity (5,6). The ISPAD guidelines also recommend eating meals with both carbohydrate and protein content after exercise, to take advantage of the high insulin sensitivity resulting from exercise (6).
Although, as detailed above, there are various guidelines regarding how adolescents with type 1 diabetes should manage their insulin and carbohydrate intake in relation to exercise, there are few data about what behaviors adolescents actually carry out around exercise. A recent study by Roberts et al. (15) looked at behavior around physical activity in youths with type 1 diabetes who were 10–18 years of age, used an insulin pump, and exercised at least once per week. They found that, although the majority of participants did make an insulin adjustment at some point surrounding exercise, only 10% made an adjustment overnight, and they concluded that many youths were not adjusting their insulin regimen adequately for exercise.
We wanted to understand the behavior of adolescents using either an MDI regimen or an insulin pump and to try to identify factors that correlate with their behavior. It is important to first understand adolescents’ current management strategies to plan new approaches to increase the time youths with type 1 diabetes spend exercising safely, which, as noted above, is an important component of diabetes management. We surveyed adolescents with type 1 diabetes about their insulin management, their physical activity, and the adaptive strategies they use to manage their diabetes around exercise. We also looked at whether their level of activity and fear of hypoglycemia influenced their insulin management practices in any way.
Research Design and Methods
Participants and Study Design
We conducted a cross-sectional study in our academic medical center diabetes clinical practice. Adolescents between 12 and 18 years of age with type 1 diabetes were recruited in two clinic sites during routine visits. There were multiple physician and nurse practitioner clinical pediatric care providers at both sites. The Indiana University Institutional Review Board approved the study, and parents and adolescents provided informed consent and assent, respectively.
Demographic data collected included sex, date of birth, and date of diagnosis. Participants’ height, weight, BMI, and A1C were abstracted from the medical record. BMI was compared with the Centers for Disease Control and Prevention (CDC) 2000 reference standards to determine z scores and percentiles according to age and sex (16). Participants completed three surveys during their visit, as outlined below. Most adolescents completed all questions, and the percentages reported are based on responses received.
Level of Activity
The International Physical Activity Questionnaire (IPAQ) short form, validated in people 15–69 years of age, was used to assess activity (17). The IPAQ consists of seven questions regarding physical activity in the past 7 days. It asks about type of activity (vigorous/moderate/walking) and length of time spent in that kind of activity. The answers are then converted into MET-minutes/week.
There are three possible categorical scores (levels of physical activity), including low, moderate, and high. These correspond to meeting at least one of two criteria, which include either engaging in a certain level of intensity for a specified amount of time or a certain number of MET-minutes/week. These scores do not specifically correlate to the CDC recommendations of 60 minutes/day of activity.
Behavior Surveys
Two surveys (one for adolescents using an MDI regimen and one for those using an insulin pump) were developed for this study to assess adolescent insulin and carbohydrate management around exercise. Survey questions focused on habits related to blood glucose monitoring around exercise, carbohydrate intake around exercise, and insulin changes before, during, and after exercise. Many of the questions used a Likert scale ranging from 0 (never) to 4 (almost always). If a participant answered the question with more than one answer, such as for “typical time of day that you exercise,” then each answer was counted.
Fear of Hypoglycemia
We assessed fear of hypoglycemia and behaviors related to that fear using the Children’s Hypoglycemia Fear Survey (HFS) developed by Cox et al. (18). The survey consists of 10 questions about behavior related to trying to reduce hypoglycemia and 15 questions related to worries that children can have about their blood glucose level being low. Each question is rated on a 5-point Likert scale ranging from 0 (never) to 4 (almost always). Both the Behavior Subscale Scores and the Worry Subscale Scores are summed, and each is divided by the total number of questions in each category to get a mean behavior (HFS-B) score and a mean worry (HFS-W) score, with a maximum mean score of 4 in each category. The higher the score, the greater the person’s fear of hypoglycemia.
Statistical Analysis
Data analyses were conducted using SPSS, v. 23, software.
Results
Sixty-six adolescents with a mean age of 14.5 years (range 12.0–18.8 years) of whom 64% were male were enrolled. Participants had an average duration of diabetes of 6 years, and just fewer than half of them were using an insulin pump (Table 1). The mean ages of those on an MDI regimen and those using an insulin pump were similar, although diabetes duration was longer in those using a pump (8.4 vs. 4.0 years). Mean A1C was 8.6 and 9.0% for those on an MDI regimen and those using an insulin pump, respectively. Eighty-two percent of participants had an A1C above the ADA goal of <7.5% (19). Mean BMI was similar between groups; about one-third of all participants were overweight or obese. Forty-three percent of those on an MDI regimen were overweight or obese, whereas 29% of those using an insulin pump were overweight or obese. A1C was not correlated to BMI z score (P = 0.76).
TABLE 1.
Demographic Characteristics of Participants
| All Participants (n = 66) | Participants Using MDI Regimen (n = 35) | Participants Using an Insulin Pump (n = 31) | |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 14.5 ± 1.8 | 14.3 ± 1.7 | 14.7 ± 2.0 |
| Range | 12–18.8 | 12.0–18.8 | 12–18.7 |
| Male sex, n (%) | 42.0 (64) | 24 (69) | 18.0 (58) |
| BMI, kg/m2 | |||
| Mean ± SD | 23.0 ± 4.6 | 23.5 ± 4.8 | 22.4 ± 4.5 |
| Range | 15.3–38.9 | 15.3–38.9 | 16.1–30.7 |
| z score, mean ± SD | 0.7 ± 0.9 | 0.8 ± 1.0 | 0.6 ± 0.9 |
| Weight status, n (%) | |||
| Normal/underweight | 42 (64) | 20 (57) | 22 (71) |
| Overweight/obese* | 24 (36) | 15 (43) | 9 (29) |
| Duration of diabetes, years | |||
| Mean ± SD | 6.0 ± 4.1 | 4.0 ± 3.2 | 8.4 ± 3.7 |
| Range | 0.2–15.7 | 0.2–13 | 1.8–15.7 |
| A1C, % | |||
| Mean ± SD | 8.8 ± 1.5 | 8.6 ± 1.6 | 9.0 ± 1.4 |
| Range | 6.0–11.6 | 6.0–11.6 | 6.6–11.6 |
| Mean ± SD excluding recently diagnosed† | 9.0 ± 1.4 | 8.9 ± 1.4 | 9.0 ± 1.4 |
| Percentage with A1C ≥7.5% | 81.8 | 74.3 | 90.3 |
| IPAQ, n (%)‡ | |||
| Low | 6 (11.1) | 5 (16.7) | 1 (4.2) |
| Moderate | 16 (29.6) | 8 (26.7) | 8 (33.3) |
| High | 32 (59.3) | 17 (56.7) | 15 (62.5) |
| HFS-B | |||
| Mean ± SD | 1.9 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.5 |
| n§ | 63 | 33 | 30 |
| HFS-W | |||
| Mean ± SD | 1.0 ± 0.6 | 0.9 ± 0.5 | 1.1 ± 0.6 |
| n§ | 65 | 34 | 31 |
Based on American Academy of Pediatrics recommendations: BMI 85th–95th percentile is overweight; BMI >95th percentile is obese.
Excluding participants who have been diagnosed within 7 months of their visit date.
Twelve participants did not fill out enough information for an IPAQ score; a total of 54 (30 of 35 participants from the MDI group and 24 of 31 participants from the insulin pump group) had an IPAQ score.
Number of participants who responded.
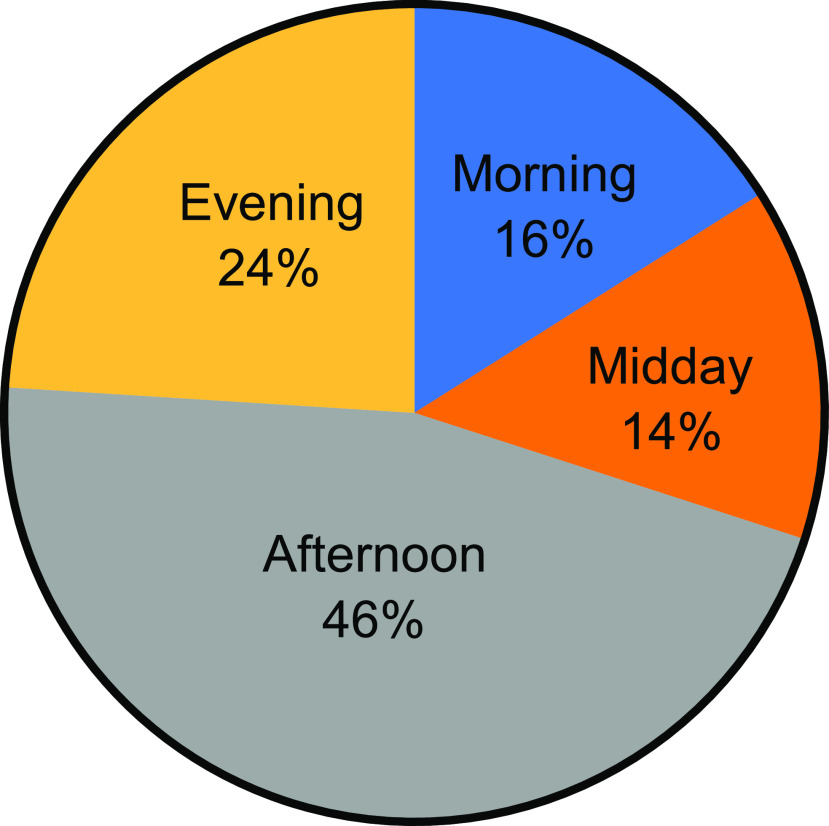
The majority of participants (89%) had an IPAQ score reflecting moderate/high levels of physical activity. Most exercised in the afternoon or evening (Figure 1). There was no correlation between level of activity and A1C (P = 0.19) or BMI z score (P = 0.4).
FIGURE 1.
Time of day adolescents reported that they engaged in physical activity.
The mean HFS-B score for all participants was 1.9 ± 0.6 (range 0.4–2.9) and did not differ between those on an MDI regimen and those using an insulin pump (P = 0.55). The mean HFS-W score for all participants was 1.0 ± 0.6 (range 0.2–2.7) and also did not differ between those on an MDI regimen and those using an insulin pump (P = 0.19).
Blood Glucose Monitoring for All Participants
Only 33 and 47% of participants reported checking their blood glucose almost always before and after exercise, respectively (Table 2). Forty-eight percent of participants reported checking their blood glucose during exercise, 20% reported rarely or never checking their blood glucose before exercise, and 14% reported rarely or never checking after exercise. These behaviors did not differ between the insulin injection group and pump group.
TABLE 2.
Carbohydrate Intake and Blood Glucose Monitoring for All Participants
| Blood Glucose Monitoring | Ingesting Carbohydrate | ||||
|---|---|---|---|---|---|
| Before Exercise | After Exercise | Before Exercise | During Exercise | After Exercise | |
| Never | 5 (7.6) | 3 (4.7) | 10 (15.2) | 9 (13.8) | 5 (7.7) |
| Rarely | 8 (12.1) | 6 (9.4) | 6 (9.1) | 10 (15.4) | 10 (15.4) |
| Every once in a while | 10 (15.2) | 9 (14.1) | 14 (21.2) | 14 (21.5) | 5 (7.7) |
| Sometimes | 21 (31.8) | 16 (25.0) | 26 (39.4) | 20 (30.8) | 33 (50.8) |
| Almost always | 22 (33.3) | 30 (46.9) | 10 (15.2) | 12 (18.5) | 12 (18.5) |
| Total | 66 (100) | 64 (100)* | 66 (100) | 65 (100)* | 65 (100)* |
Data are n (%). *Total of those answering, excluding participants who did not answer the question.
Carbohydrate Adjustments for All Participants
Only 15, 18, and 18% of all adolescents surveyed reported almost always eating or drinking carbohydrates before, during, and after exercise, respectively (Table 2). Proportions reporting rarely or never eating or drinking carbohydrates before, during, and after exercise were 24, 29, and 23%, respectively. No one in the pump group reported “almost always” eating a carbohydrate prior to exercise.
Insulin Adjustments
For Adolescents on an MDI Regimen
Seventy-two percent of adolescents using an MDI regimen reported that they did not make any insulin adjustments before exercise. The remainder reduced the amount of short-acting insulin given for the meal before exercise. Seventy-nine percent did not make any adjustments after exercise, whereas 18% reduced the short-acting insulin given for the meal after exercise, and 3% (one person) reported making a different type of insulin adjustment. No one reported reducing the amount of long-acting insulin given before bedtime.
Adolescents who had higher IPAQ scores were not more likely to adjust their insulin before exercise (P = 0.68) but were more likely to adjust it after exercise (P = 0.05). Adolescents who adjusted their insulin before exercise had greater HFS-B scores (2.21 vs. 1.73, P = 0.04) but no difference in HFS-W scores (1.03 vs. 0.86, P = 0.45). Those who adjusted their insulin after exercise had higher HFS-B scores (2.55 vs. 1.72, P = 0.001) and higher HFS-W scores (1.28 vs. 0.81, P = 0.04). Those with longer diabetes duration were more likely to adjust insulin before exercise (P = 0.04).
For Adolescents Using an Insulin Pump
Forty-seven percent of insulin pump users reported making no insulin pump adjustments before exercise, 47% reported either taking off their pump or stopping the pump, and 6% reported reducing the insulin bolus given for the meal before exercise. Forty-five percent reported never adjusting their insulin pump during exercise, 27.5% reported rarely adjusting it, and 27.5% reported that they sometimes adjusted it. No one reported changing their overnight basal infusion rate.
There was no correlation between level of activity (IPAQ score) and insulin regimen changes before exercise (P >0.46). There was also no correlation between fear of hypoglycemia measurements (HFS-B or HFS-W score) and insulin regimen adjustments before exercise (P >0.6 for both). Those with longer disease durations were less likely to adjust insulin before exercise (P = 0.007).
Adaptation Factor
Recommendations focus on adolescents either increasing carbohydrate intake or making insulin changes as adaptations for exercise to prevent or treat hypoglycemia. To see how many adolescents followed these recommendations, we developed an adaptation based on the following criteria. Adolescents reporting that they either almost always ate carbohydrates and/or changed their insulin dosing before or after exercise had a positive adaptation factor related to before or after exercise. Positive adaptation factors are summarized in Table 3.
TABLE 3.
Adaptation Factor
| Total* (n = 63) | Participants Using MDI Regimen | Participants Using an Insulin Pump | |
|---|---|---|---|
| Positive adaptation factor, n (%) | |||
| Before exercise | 33 (52) | 17 (52) [of 33] | 16 (53) [of 30] |
| After exercise | 18 (29) | 12 (35) [of 34] | 6 (21) [of 29] |
Total of those answering, excluding three participants who did not answer the questions.
Before Exercise
In the MDI group, there was a mix between those who reported almost always eating carbohydrates and those who reported making changes to their insulin regimen, with two people reporting that they did both. In the insulin pump group, no one reported that they almost always ate carbohydrate, so all 16 in this group who had a positive adaptation factor got it by virtue of changing their insulin before exercise.
After Exercise
In the MDI group, there was a mix between those who reported that they almost always ate carbohydrates and those who reported making changes to their insulin regimen, with one person reporting that he or she did both. In the insulin pump group, no one reported changing their basal insulin setting before bedtime, so all six people who had a positive adaptation factor got it by virtue of eating carbohydrates.
Overall, insulin pump users and those with an MDI regimen were equally likely to report adapting their diabetes care. Adolescents were more likely to exhibit adaptive behaviors before versus after exercise, although this was only statistically significant among the pump users (P = 0.02).
Discussion
Our study found that adolescents with type 1 diabetes followed in our academic medical center diabetes practice reported relatively high levels of physical activity. It should be noted that IPAQ surveys categorize level of activity based on total activity per week, whereas the ADA recommends 60 minutes/day of physical activity (5).
This study suggests that many adolescents do not change their insulin dosing in preparation for or in response to exercise. Those who use insulin pumps were more likely to change their insulin than those using an MDI regimen, but still about half of participants who used a pump did not make any insulin changes, and no one reported using temporary basal rates before exercise or before bedtime.
Contrary to our expectations before the study, those with higher reported levels of activity were not more likely to adjust their insulin before exercise. However, adolescents on an MDI regimen who had high activity levels did report making changes after exercise. It should be noted that, of the total number of participants who used an MDI regimen, the percentage who adjusted their insulin before exercise was small, and the percentage of those who made insulin adjustments after exercise was even smaller.
Fear of hypoglycemia is a known possible barrier to physical activity (5,11). However, there was no correlation in the insulin pump group between higher HFS scores and insulin adjustment. In the MDI group, those with higher HFS scores were more likely to adjust their insulin after exercise; for before-exercise adjustments, there was only a correlation with higher HFS-B scores, not with higher HFS-W scores.
Many adolescents do not change their carbohydrate intake in preparation for or in response to exercise. Also, the majority of adolescents surveyed do not follow recommendations about blood glucose monitoring around exercise.
Our study adds to the limited available data on strategies adolescents with type 1 diabetes use to minimize potential hypoglycemia around exercise. Compared with the study by Roberts et al. (15) of youths using insulin pumps, our cohort had a mix of pump and MDI users with a higher mean A1C, somewhat shorter mean duration of diabetes, and smaller percentage of adolescents using continuous glucose monitoring (CGM). Roberts et al. reported that most participants using a CGM device found it to be helpful with glucose management around physical activity (15,20). Differences in these cohorts likely partially explain why we found lower rates of reported insulin adjustments. However, our study results support the overall conclusion of Roberts et al. that more work needs to be done to enhance adolescent engagement with changing diabetes management behaviors in relation to exercise. Based on our study findings, those using an MDI regimen may be an especially important group to target.
This study adds to the literature by analyzing both MDI and pump users, looking at both carbohydrate intake and insulin adjustments, and using the IPAQ and HFS to analyze the potential impact of level of activity and fear of hypoglycemia on behavior.
There were a few limitations to this study, including that it used self-reported surveys, which could introduce recall bias. The data are also from one institution, which can limit applicability. However, surveys were given at two different clinic locations with different providers. Having approximately equal numbers of participants in the MDI and pump groups and similar demographics between the two groups were strengths of this study.
Various other possible explanations for this lack of guideline adherence were not examined in this study. For example, with the significant amount of education that patients receive, exercise guidelines are likely to be a lower priority. Also, as adolescents take greater control of their insulin management, they may need education refreshers. We had a limited number of adolescents who used a CGM device in our cohort, so we were unable to analyze this subgroup separately. However, as CGM becomes more prevalent, along with hybrid closed-loop and predictive low glucose suspend systems, a further direction of this research would be to look at these patients’ behavior as a separate cohort in comparison with those not using these technologies. An additional direction we would like to explore would be to look at behaviors adolescents potentially use to mitigate exercise-induced hyperglycemia.
Notwithstanding the limitations noted above, our results suggest that better-targeted education and interventions are needed to improve adolescents’ blood glucose management behaviors around exercise. These data provide clinical insights that can be helpful in practice. Given that neither the amount of activity nor fear of hypoglycemia were significant predictors of engagement in adaptive strategies, especially in pump users, additional factors need to be identified to help predict which adolescents will be more likely to make recommended behavior or treatment adaptations around exercise and determine how best to intervene to encourage more adolescents to follow such recommendations. Additional research is therefore needed.
Article Information
Acknowledgments
The authors acknowledge Linda Gonder-Frederick, PhD, and her colleagues at the Department of Psychiatry and Neurobehavioral Sciences Behavioral Medicine Center at the University of Virginia Health System for permitting us to use their Fear of Hypoglycemia survey.
Funding
This work was supported by National Institutes of Health grant 5T32DK065549-13, awarded to A.N.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
A.N. planned the study design; collected, evaluated, and interpreted data; and wrote the manuscript. S.W. and M.R. collected data and reviewed and edited the manuscript. A.Y. evaluated data and edited the manuscript. L.A.D. planned the study design, evaluated and interpreted data, reviewed and edited the manuscript, and supervised the project. L.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Previous Presentation
Portions of this work were presented in abstract form at the 2016 Pediatric Endocrine Society meeting in Baltimore, MD.
References
- 1.Quirk H, Blake H, Tennyson R, Randell TL, Glazebrook C. Physical activity interventions in children and young people with type 1 diabetes mellitus: a systematic review with meta-analysis. Diabet Med 2014;31:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggio AB, Rizzoli RR, Marchand LM, Ferrari S, Beghetti M, Farpour-Lambert NJ. Physical activity increases bone mineral density in children with type 1 diabetes. Med Sci Sports Exerc 2012;44:1206–1211 [DOI] [PubMed] [Google Scholar]
- 3.Aman J, Skinner TC, de Beaufort CE, Swift PG, Aanstoot HJ, Cameron F; Hvidoere Study Group on Childhood Diabetes . Associations between physical activity, sedentary behavior, and glycemic control in a large cohort of adolescents with type 1 diabetes: the Hvidoere Study Group on Childhood Diabetes. Pediatr Diabetes 2009;10:234–239 [DOI] [PubMed] [Google Scholar]
- 4.MacMillan F, Kirk A, Mutrie N, Matthews L, Robertson K, Saunders DH. A systematic review of physical activity and sedentary behavior intervention studies in youth with type 1 diabetes: study characteristics, intervention design, and efficacy. Pediatr Diabetes 2014;15:175–189 [DOI] [PubMed] [Google Scholar]
- 5.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adolfsson P, Riddell MC, Taplin CE, et al. ISPAD clinical practice consensus guidelines 2018: exercise in children and adolescents with diabetes. Pediatr Diabetes 2018;19(Suppl. 27):205–226 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Physical activity. Available from https://www.cdc.gov/physicalactivity/data/facts.htm. Accessed 21 May 2019
- 8.Pivovarov JA, Taplin CE, Riddell MC. Current perspectives on physical activity and exercise for youth with diabetes. Pediatr Diabetes 2015;16:242–255 [DOI] [PubMed] [Google Scholar]
- 9.Valerio G, Spagnuolo MI, Lombardi F, Spadaro R, Siano M, Franzese A. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis 2007;17:376–382 [DOI] [PubMed] [Google Scholar]
- 10.Czenczek-Lewandowska E, Leszczak J, Baran J, et al. Levels of physical activity in children and adolescents with type 1 diabetes in relation to the healthy comparators and to the method of insulin therapy used. Int J Environ Res Public Health 2019;16:E3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo-Scharer R. Preventing post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr 2010;157:784–8.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverstein J, Klingensmith G, Copeland K, et al.; American Diabetes Association . Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 [DOI] [PubMed] [Google Scholar]
- 13.McMahon SK, Ferreira LD, Ratnam N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab 2007;92:963–968 [DOI] [PubMed] [Google Scholar]
- 14.Davey RJ, Howe W, Paramalingam N, et al. The effect of midday moderate-intensity exercise on postexercise hypoglycemia risk in individuals with type 1 diabetes. J Clin Endocrinol Metab 2013;98:2908–2914 [DOI] [PubMed] [Google Scholar]
- 15.Roberts AJ, Yi-Frazier JP, Aitken KE, Mitrovich CA, Pascual MF, Taplin CE. Do youth with type 1 diabetes exercise safely? A focus on patient practices and glycemic outcomes. Pediatr Diabetes 2017;18:367–375 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Growth charts. Available from https://www.cdc.gov/growthcharts. Accessed 21 May 2019
- 17.International Physical Activity Questionnaire Home page. Available from www.ipaq.ki.se. Accessed 1 August 2014.
- 18.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association 12. Children and adolescents: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S126–S136 [DOI] [PubMed] [Google Scholar]
- 20.Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 2011;13:819–825 [DOI] [PubMed] [Google Scholar]