Abstract
Recently, Rho GTPases substrates include Rac (Rac1 and Rac2) and Cdc42 that have been reported to exert multiple cellular functions in osteoclasts, the most prominent of which includes regulating the dynamic actin cytoskeleton rearrangements. In addition, natural products and their molecular frameworks have a long tradition as valuable starting points for medicinal chemistry and drug discovery. Although currently, there are reports about the natural product, which could play a therapeutic role in bone loss diseases (osteoporosis and osteolysis) through the regulation of Rac1/2 and Cdc42 during osteoclasts cytoskeletal structuring. There have been several excellent studies for exploring the therapeutic potentials of various natural products for their role in inhibiting cancer cells migration and function via regulating the Rac1/2 and Cdc42. Herein in this review, we try to focus on recent advancement studies for extensively understanding the role of Rho GTPases substrates Rac1, Rac2 and Cdc42 in osteoclastogenesis, as well as therapeutic potentials of natural medicinal products for their properties on the regulation of Rac1, and/or Rac2 and Cdc42, which is in order to inspire drug discovery in regulating osteoclastogenesis.
Keywords: bone, Cdc42, natural compounds, Rac1, Rac, Rho GTPase
Introduction
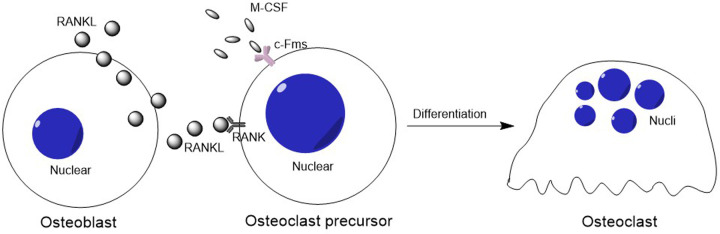
Osteoclastogenesis has been defined as a multistep processes of osteoclast differentiation [1], including several osteoclastic cellular biological functions; such as: migration, cellular contact, cellular fusion and cellular response extracellular factors [2]. Documented studies demonstrated that osteoclastogenesis initially mediated by two critical cytokines, the macrophage colony stimulating factor-1 (M-CSF) and the receptor activator of nuclear factor-κB ligand (RANKL) [3]. M-CSF binds to its receptor (cFms) present in osteoclast precursors, which stimulates their proliferation and inhibits their apoptosis; while, RANKL interacts with its receptor RANK in osteoclast precursor cells, and osteoclastognesis is induced [4] (Figure 1).
Figure 1. The schematic of osteoclastogenesis.
The cytokines M-CSF and RANKL (from osteoblasts) bind to its receptors cFms and RANKL present in osteoclast precursors, respectively. Then the M-CSF stimulates osteoclast precursors proliferation and inhibits their apoptosis. Besides that, RANKL interacts with its receptor RANK in osteoclast precursor cells, then osteoclastognesis is induced.
However, at the late stage of osteoclastogenesis, osteoclastic polarization characterized the final maturation of bone resorptive osteoclasts. Notably, during the bone resorption process, osteoclastic polarization involves rearrangement of the actin cytoskeleton, in which a filamentous (F)-actin ring that comprises dense continuous zones of highly dynamic podosomes are formed and consequently an area of membrane that develops into the ruffled border is isolated [5,6].
Cytoskeletal rearrangement during osteoclastogenesis
It is worthy to note that during the cytoskeletal rearrangement in the osteoclastogenesis, podosome is the most prominent cytoskeletal structure for the degradation of mineralized bone matrix and associates with the mobility of osteoclasts [7]. In fact, podosome is not the exclusive organelle in osteoclast, which also includes endothelial cells, and cells from the monocytic lineage such as: dendritic cells (DCs) and macrophages [8]. Regardless, the presentation of podosomes in various cells, podosomes patterning plays a crucial and unique role in the support osteoclast final maturation [8]. As early as individual podosome forms within an osteoclast, they are collectively and sequentially organized into different patterns along the life of the same cell. However, these patterns evolve along with osteoclastogenesis from monocytes/macrophages to osteoclast precursors, further to the bone resorptive matured osteoclast. In the early stage of osteoclastogenesis, podosome pattern from apparently random groups of ‘clusters’ to circle pattern ‘rings’ in the middle-term stage [9]. Eventually, in the late stage of osteoclastogenesis, podosome patterns into much massive circular structures, i.e., either ‘sealing zone like structures’ (SZL, also known as ‘belts’) or ‘sealing zones’ (SZ) [10].
Rac isoforms (Rac1 and Rac2) in regulation of cytoskeletal arrangement during osteoclastogenesis
It has been reported that Rac1 and Rac2 are critical GTPases for osteoclast formation and maturation. In fact, Rac1 and Rac2, are intimately associated with the organization of the different types of cellular cytoskeleton, such as: osteoclasts, DCs and macrophages. Notably, these two isoforms are also involved in the osteoclastic adhesive function formation and subsequent bone resorption [11,12]. However, the specific role of Rac1 and Rac2 in osteoclastogenesis is still unknown. For example, osteoclasts contain NADPH diaphorase activity [13,14], and free radicals which both could influence bone resorption, however, Rac1 and Rac2 are also essential components of NADPH oxidase [15–18], the enzyme responsible for generating free radicals. Besides that, a study has also demonstrated that Rac1 and Rac2 could regulate the generation of reactive oxygen species (ROS) [19] and actin remodeling participating in the osteoclastogenesis regulation. Recent study has found that both Rac1 and Rac2 are required for normal RANKL-induced osteoclast differentiation, but Rac1 deletion results in a more profound reduction in osteoclast formation in vitro because of its regulatory role in pre‐osteoclast M‐CSF mediated chemotaxis and actin assembly and RANKL‐mediated ROS generation [20]. These results speculated that Rac1 and Rac2 might function in osteoclastic organelle actin dynamics regulating, such as: actin filament ends and podosomes. In fact, Rac1 and Rac2 proteins have overlapping roles in podosome assembly and SZL formation by localizing Arp2/3 at podosome sites during osteoclastogenesis [7,21–27]. Osteoclasts generated from the Rac1 and Rac2 double knockout mouse are devoid of podosomes and SZ, which finally showed impaired bone resorption capacities [24,28–32]. Notably, however, these defects are observed only if Rac1 and Rac2 deletion occurs at the early osteoclast precursor stage, which means the Rac1- and Rac2-deficient osteoclasts lack the capabilities of actin cytoskeletal formation.
The role of Cdc42 in regulation of the podosome of osteoclast
Cdc42 is another Rho family small GTPase [33]. As a downstream signaling of RANKL, Cdc42 might interact with the Crib domain of the adaptor Par3 [34,35], Par6 and atypical PKC (aPKC) [36–38], which forms a quaternary complex to cascade the upper signaling transduction from RANKL and RANK binding, further stimulating the osteoclastogenesis. However, unlike Rac1 and Rac2 the definition role of Cdc42 in osteoclastogenesis is much clearly associated with its actin regulative effects, i.e. the podosome regulation. Recent studies using mice with increased Cdc42 activation due to knockout of its negative regulator Cdc42GAP have shown increased SZ formation and bone resorption, compared with wildtype cells [27,39].
Cdc42 stands as a central player in the regulation of podosome dynamics as it orchestrates podosome actin polymerization, which from the monomeric globular (G)-actin into filamentous (F)-actin, through its canonical effector, Wiscott–Aldrich Syndrome protein (WASp) [40–42]. WASp depletion in macrophages leads to a virtual absence of podosomes and a defective chemotactic response under a gradient of M-CSF. Cdc42 binds directly to WASp, a multidomain adapter protein regulating transmission of signals to the actin cytoskeleton. This binding, together with phosphorylation of WASp on tyrosine, induces a dramatic conformational change [40,41,43]. The hydrophobic core is disrupted, releasing the VCA (Verprolin Homology domain-cofilin homology domain–acidic region) domain and enabling its interaction with the Arp2/3 complex, thereby promoting actin nucleation [44–46].
Natural products targeting the regulation of Rac1 and Rac2, and Cdc42
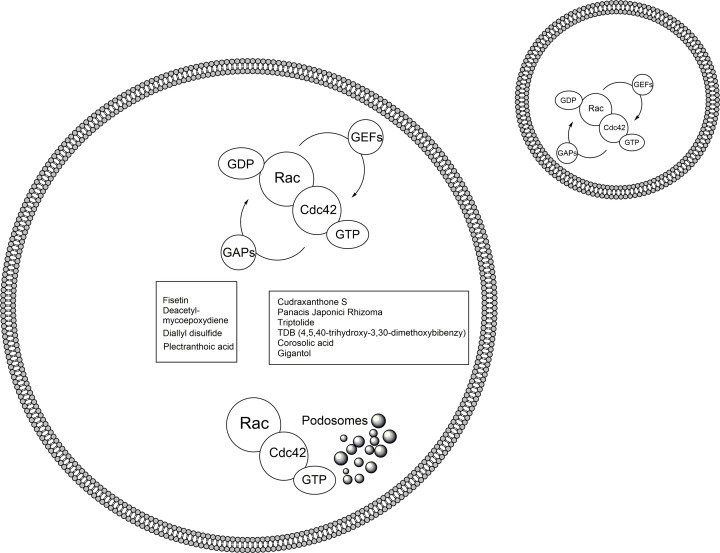
Natural products and their molecular frameworks have a long tradition as valuable starting points for medicinal chemistry and drug discovery. Recently, there has been a revitalization of interest in the inclusion of these chemotypes in compound collections for screening and achieving selective target modulation. Although currently, there have been no reports on the natural product, which could play a therapeutic role in bone loss diseases (osteoporosis and osteolysis) through the regulation of Rac1/2 and Cdc42 during osteoclasts cytoskeletal structuring. There have been several excellent studies exploring the therapeutic potentials of various natural products in regulating cancer cells migration and function (Table 1). Here we collected several natural products with a focus on recent advances in their properties on the regulation of Rac1 and/or Rac2 and Cdc42, and related signaling molecules, in order to inspire drug discovery in regulating osteoclastogenesis (Figure 2).
Table 1. The source, structure, cells or animal models and mechanisms of ten natural compounds.
| Compound name | Source | Structure | Cell lines used for in vitro studies | Animal models | Dose | Mechanisms | Studies |
|---|---|---|---|---|---|---|---|
| Fisetin | polyphenolic molecule of flavonoids | 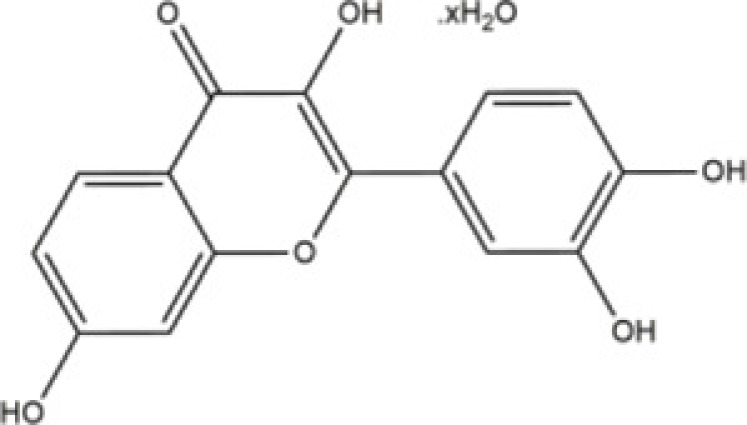 |
Neuron | Wistar rats | 30 mg/kg | Rac1/Cdc42 | Jacob [50] |
| Deacetyl- mycoepoxydiene | Phomopsis sp., of costal mangrove | 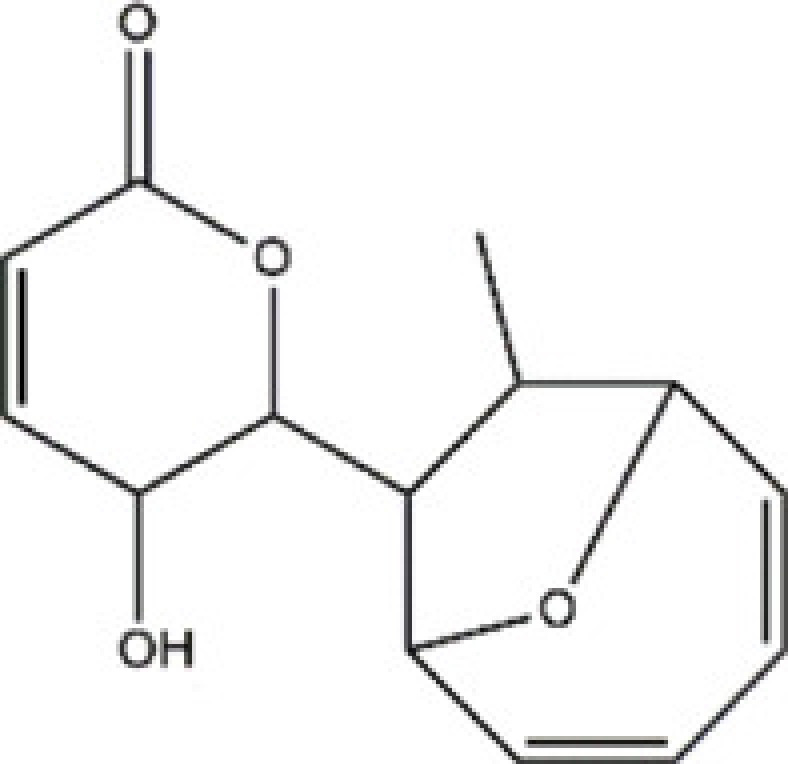 |
Human breast cancer MCF-7 cells | BABL/c mice | 5, 10, 20 mg/kg | Rac1 | Zhao [59] |
| Diallyl disulfide | garlic | 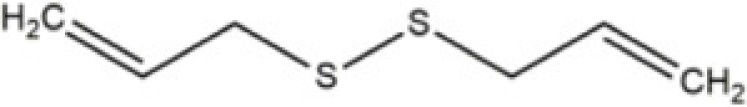 |
Human gastric cancer MGC803 cell line | BALB/c nude mice | 100mg/kg | Rac1 | Su [55] |
| Plectranthoic acid | Ficus microcarpa | 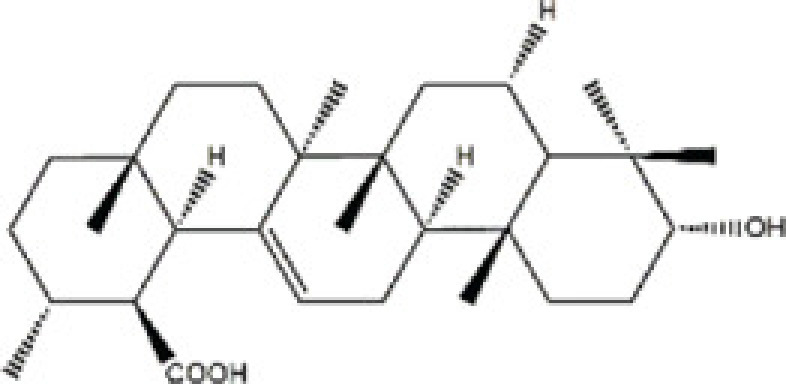 |
Prostate cancer cell lines (DU145, PC3, NA22, NB26) | N/A | N/A | Rac1 | Akhtar [61] |
| Cudraxanthone S | Cudrania cochinchinensis | 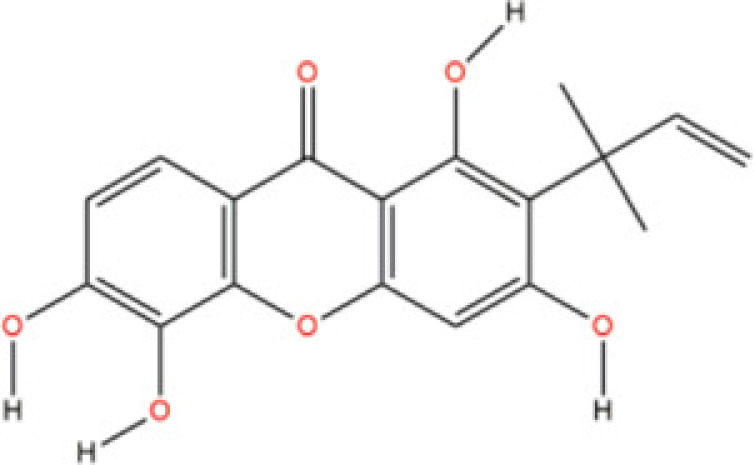 |
N/A | N/A | N/A | Cdc42 | Gopal [65] |
| Panacis Japonici Rhizoma | Panax japonicus C. A. Meyer | 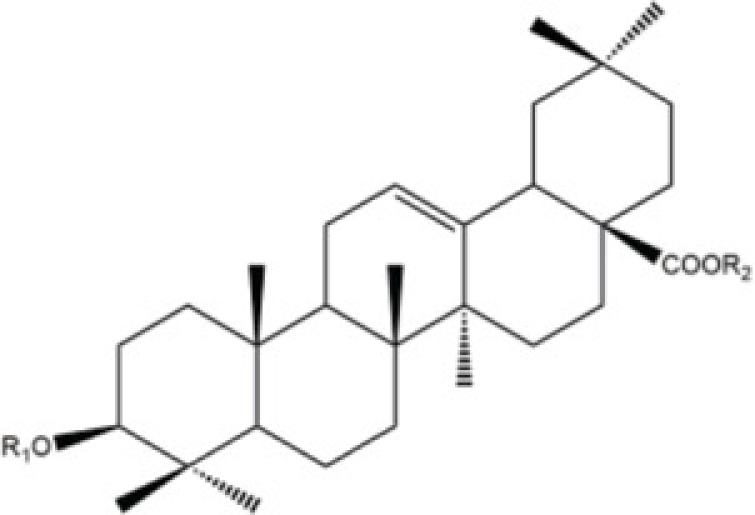 |
A2780 cell line | N/A | N/A | Cdc42 | Chen [66] |
| Triptolide | Triterygium wilfordii Hook. f. | 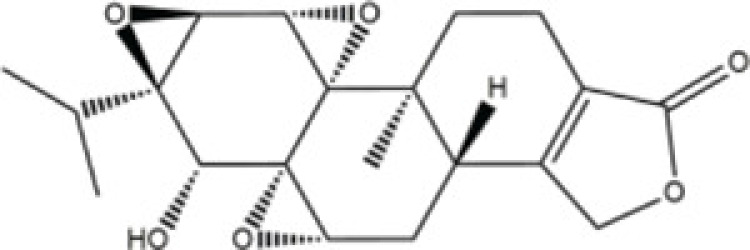 |
Sprague–Dawley (SD) rats | Sprague–Dawley (SD) rats | 100 mg/kg | Rac1, Cdc42 | Wang [51] |
| TDB (4,5,40-trihydroxy-3, 30- dimethoxybibenzy) | Dendrobium ellipsophyllum Tang and Wang | 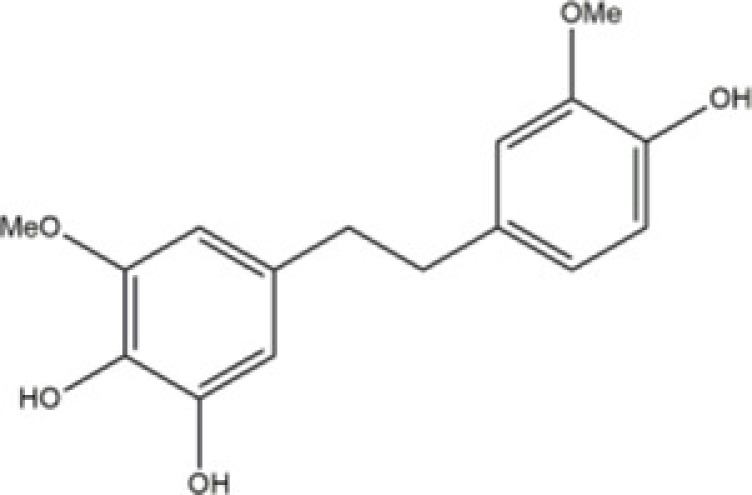 |
Human lung cancer H292 cells | N/A | N/A | Rac1/Cdc42 | Chaotham [74] |
| Corosolic acid | Actinidia chinensis, | 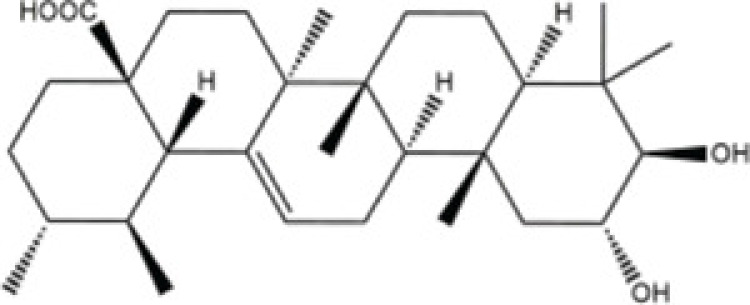 |
Hepatocellular carcinoma cell lines (Huh7, HepG2 and Hep3B) | NOD/SCID mice | 5 mg/kg | Cdc42 | Ku [77] |
| Gigantol | Thai orchid, Dendrobium draconis | 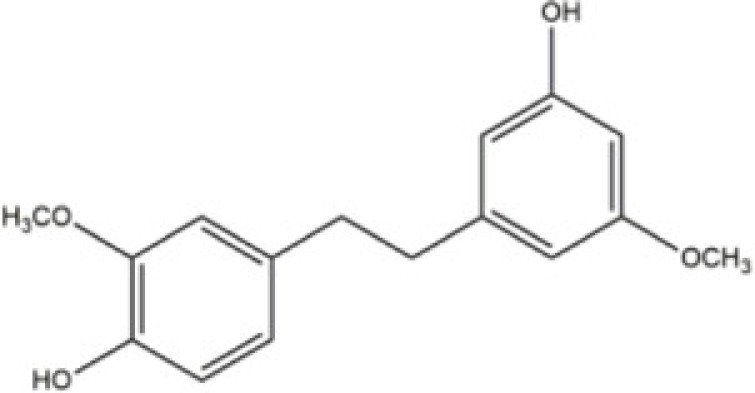 |
Human lung carcinoma cells NCI-H460 and NCI-H292 | N/A | N/A | Cdc42 | Charoenrungruang [78] |
Figure 2. The schematic of molecular mechanisms of Rho GTPases Rac and Cdc42, and relevant therapeutic natural compounds.
During the osteoclastogenesis, after RANKL and RANK binding, the intracellular Rac1, Rac2 and Cdc42 are via GTP associate with podosomes regulation. However, these regulation effects might inhibited by various compounds (Left panel: inhibitory compounds for Rac1 and Rac2; Right panel: inhibitory compounds for Cdc42).
Rac1 and Rac2 regulative natural products
Fisetin (3,3′,4′,7-Tetrahydroxyflavone), is natural product that could be found in vegetables and fruits [47]. Fisetin has been well established and possesses antioxidant [48] and anti-neurodegenerative progression [49]. Most recently, Jacob et al. [50] have reported Fisetin showed a significant protective effect on developmental Methyl mercury neurotoxicity in the F1 generation of MeHg exposed rats. In that, Methyl mercury is a teratogenic and neurodevelopmental toxicant in the environment. Whereas MeHg could affect several biological pathways critical for brain development. Most recently, authors present study validated the effect of Fisetin on developmental MeHg exposure induced alterations in mitochondrial apoptotic pathway and Rho GTPase mRNA expressions in hippocampus of F1 generation rats. Their extensive study showed that Fisetin against gestational MeHg exposure induced changes in expression of ERK/Caspase 3 genes of apoptosis signaling pathway and Rho A/Rac1/Cdc42 genes of Rho GTPase signaling pathway in hippocampus of F1 generation weaning Wistar rats.
Deacetyl-mycoepoxydiene (DA-MED) is a 248 molecular weight compound that has been isolated from the endophytic fungus, Phomopsis sp., of costal mangrove plants and has been shown to be a secondary metabolite with a rare oxygen-bridged cyclopentadiene skeleton [51]. This compound has cytotoxic activities toward various cell lines, including A549, HCC-S102 and HepG2 cells with IC50 values ranging from 1.05 to 1.95 mg/ml. Recently, Xie et al. [52] have reported that DA-MED treatment drives Rac1 activation and promotes robust production of ROS, activating mitochondrial permeability transition and the intrinsic apoptotic pathway. Knockdown of Rac1 decreases ROS production in DA-MED-treated cells, resulting in a concomitant decrease in DA-MED-induced apoptosis. DA-MED-activated Rac1 induces autophagy by inhibiting mammalian target of rapamycin, leading to anti-apoptotic and anti-metastatic effects. Therefore, the present study provides novel insight into the complex cytotoxic and pro-survival mechanisms associated with a potent Rac1 agonist and suggests that further development of more potent Rac1 agonists could be an effective strategy for future non-small cell lung cancer treatments.
Diallyl disulfide (DADS), one of the sulfur compounds derived from garlic, exhibits biological activity via modulating molecules and signaling pathways in various cell physiologies [53–57]. These properties suggesting that DADS could be used as a potential therapeutic compound for the treatment or prevention of various diseases. Moreover, study has demonstrated that transforming growth factor-β1 (TGF-β1) could promote epithelial–mesenchymal transition (EMT), invasion and proliferation through the activation of Rac1 and β-catenin signaling pathways. Therefore, Su et al. [55] have conducted a study for investigating the effects of DADS on TGF-β1-induced EMT and cellular invasion. Primarily, they found TGF-β1 treatment augmented EMT and invasion, concomitantly with increased expression of Rac1 and β-catenin. However, the DADS treatment could decrease the activities of Rac1 and β-catenin. DADS, TGF-β1 receptor inhibitor as well as Rac1 inhibitor antagonized the up-regulation of the TGF-β1-induced expression of these genes, abolishing the enhanced effects of TGF-β1 on EMT and invasion. These data indicated that the blockage of TGF-β1/Rac1 signaling by DADS may be responsible for the suppression of EMT and cellular invasion.
Mulberry (Morus alba L.) is a common fruit in temperate, subtropical and tropical areas, and contains abundant polyphenols and anthocyanin components [58,59]. Study showed the anthocyanins from the mulberry could inhibit the B16-F1 cell linage invasion [60]. The underlying molecular mechanisms is anthocyanins partly suppressed the Ras/PI3K signaling pathway. In addition, mulberry polyphenol extract (MPE) is rich in polyphenols that have antioxidant, anti-inflammatory, anti-aging, anti-obesity and anti-tumor effects. Considering the biological effects of anthocyanins, further study performed by Yu et al. [58] investigated that MPE on treating vascular smooth muscle cellular migration and proliferation. Their results showed that MPE could suppress the expression of FAK, Src, PI3K, Akt, c-Raf, and inhibit the signaling axis of FAK/Src/PI3K in cell. Besides that, their study also showed that MPE decrease the expression of small Rac1 and Cdc42 to affect F-actin cytoskeleton rearrangement.
As aforementioned, cytoskeletal structure rearrangement grant various cellular functions in various cell linages, such as: podosome patterning in osteoclasts and EMT transition. This has led to a surge in the efforts for identification of safer and more effective compounds which can modulate these cellular behaviors. Plectranthoic acid (PA), a natural compound isolated from the extracts of Ficus microcarpa, has been reported to possess the capability to induce cell cycle arrest and apoptosis in prostate cancer cells [61,62]. Recently, Akhtar et al. [61] extensively studied the PA biological effects on suppressing the cellular migration. Through the proteomic analysis, authors identified that Rac1 is the major cadherin signaling protein modulated with PA treatment.
Cdc42 regulative natural products
Cudrania cochinchinensis (Moraceae) has been reported for its potent biological activites such as: anti-inflammation [63] and neuroprotective effects [64]. Whereas, the compound Cudraxanthone-S derived from C. cochinchinensis was studied for its pharmacokinetics and binding potential in treating the fungal infection of Candida albicans, which could cause several lethal infections in immune-suppressed patients and recently emerged as drug-resistant pathogens worldwide [65]. Authors found that Cudraxanthone-S had exhibited ability on regulating the Cdc42 in MAPK signaling pathway.
Panacis Japonici Rhizoma (PJR), derived from dry rhizome of Panax japonicus C. A. Meyer (Araliaceae), distributes in the southwest of China [66–68]. As a widely used focal medicine, the PJR manifested similar clinical merits in anti-tussive and anti-inflammatory diseases [69,70]. Recently, Chen et al. [71] have demonstrated that PJR could suppress the HEY and A2780 cells migration and invasion by decreasing the Cdc42 and Rac activities.
Triptolide (TP), derived from the medicinal plant Triterygium wilfordii Hook. f. (TWHF), is a diterpene triepoxide with variety of biological and pharmacological activities [72]. Wang et al. [73] has studied the cytoskeletal structuring effects of TP on Sertoli cells, which play a critical role during spermatogenesis. Their study results demonstrate that TP can regulating the Sertoli cellular cytoskeleton structuring via inhibiting the expression of Cdc42.
The compound (4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl (TDB)) extract from Dendrobium ellipsophyllum Tang and Wang, has been demonstrated to have antimetastatic activity through the sensitization of detachment-induced cell death [74,75]. Study from Chaotham et al. [76] showing that TDB reduced such cell migration and invasion by decreasing migration-regulating proteins, including integrins αv, α4, β1, β3 and β5, as well as downstream signaling proteins, such as activated focal adhesion kinase (pFAK), activated Rac1 and Cdc42. As the presence of cellular protrusion, called filopodia, has been indicated as a hallmark of migrating cells, we showed that the reduction in the mentioned proteins correlated well with the disappearance of filopodia. In summary, the present study demonstrates the promising activity of TDB and its mechanism in the inhibition of lung cancer cell migration, which might be useful for encouraging the development of this compound for anti-metastatic approaches.
Inhibition of VEGFR2 activity has been proposed as an important strategy for the clinical treatment of hepatocellular carcinoma (HCC). Corosolic acid (CA), which exists in the root of Actinidia chinensis, as having a significant anti-cancer effect on HCC cells by decreasing the tumor cellular migration. Ku et al. [77] have extensively studied the effects of CA on its cellular regulating effects found that CA inhibits VEGFR2 kinase activity by directly interacting with the ATP-binding pocket. Moreover, they found CA could decrease the VEGFR2/Src/FAK/Cdc42 axis, subsequently decreasing F-actin formation and migratory activity in vitro.
Gigantol is a bibenzyl compound derived from the Thai orchid, Dendrobium draconis. It exhibits significant cytotoxic activity against several cancer cell lines. Study conducted by Charoenrungruang et al. [78] demonstrates that gigantol suppresses the migratory cellular behavior via decreasing Cdc42, thereby suppressing filopodia formation. The inhibitory activity of Gigantol on lung cancer cellular migration suggests that this compound may be suitable for further development for the treatment of osteoclastogenesis by regulating the osteoclastic cytoskeletal structuring.
Conclusion
Characterized by the unique property, osteoclasts have been extensively studied for their differentiation and cellular functions during the bone homeostasis and pathological process, which makes them as a critical target for therapy in the bone loss diseases, such as: osteoporosis and osteolysis. Given that the low production costs and the increasing evidence of the ability to target the cellular activities and signaling cascades relevant to various diseases, naturally occurring compounds have received extensive attention as potential therapeutic osteoclastogenesis. Our current review has outlined some naturally occurring compounds, which have shown merit in terms of regulating macrophage polarization. However, given that current natural compounds have the Rac and Cdc42 regulatory effects on cancer cell line, the specific mechanisms and therapeutic effects on osteoclastognesis remain incompletely understood. Clearly, more in-depth characterization of osteoclast cytoskeleton rearrangement and relevant therapeutic compounds should be conducted to identify the best possible strategies.
Abbreviations
- Arp2/3
actin-related proteins-2/3
- CA
corosolic acid
- DADS
diallyl disulfide
- DA-MED
deacetyl-mycoepoxydiene
- DC
dendritic cell
- EMT
epithelial–mesenchymal transition
- HCC
hepatocellular carcinoma
- MPE
mulberry polyphenol extract
- M-CSF
macrophage colony stimulating factor-1
- PA
plectranthoic acid
- PJR
Panacis Japonici Rhizoma
- PKC
protein kinase C
- RANKL
receptor activator of nuclear factor-κB ligand
- ROS
reactive oxygen species
- SZ
sealing zone
- SZL
sealing zone like structure
- TDB
4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl
- TGF-β1
transforming growth factor-β1
- TP
triptolide
- VEGFR
vascular endothelial growth factor receptor
- WASp
Wiscott–Aldrich Syndrome protein
Contributor Information
Baorong He, Email: baoronghespine@163.com.
Lingbo Kong, Email: lingbokong@163.com.
Data Availability
All data and materials are included in the manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the China Postdoctoral Science Foundation, P.R. China [grant number 2018T111085]; the Shaanxi Provincial Science and Technology Department, Science and Technology Achievement Transfer and Promotion Plan Award Achievement Transformation Project, P.R. China [grant number 2018HJCG-08]; the Xi’an Science and Technology Bureau, Social Development Public Project [grant number 2017115 SF/YX009].
Author Contribution
Yuan Liu, Yusheng Dou, Baorong He, Lingbo Kong: conception and design, drawing and interpretation of data; drafted the manuscript and revised it critically for important intellectual content; final approval of the version to be published. Liang Yan, Xiaobin Yang: acquisition of data (manuscript reviewing), analysis and interpretation of data. Wanli Smith: design, revision of the manuscript critically for important intellectual content. All authors had gave the final approval of the version to be published.
Consent for Publication
The manuscript is approved by all authors for publication.
References
- 1.Feng W., Xia W., Ye Q. and Wu W. (2014) Osteoclastogenesis and osteoimmunology. Front. Biosci. (Landmark Ed.) 19, 758–767 10.2741/4242 [DOI] [PubMed] [Google Scholar]
- 2.Kukita T., Kukita A., Watanabe T. and Iijima T. (2001) Osteoclast differentiation antigen, distinct from receptor activator of nuclear factor kappa B, is involved in osteoclastogenesis under calcitonin-regulated conditions. J. Endocrinol. 170, 175–183 10.1677/joe.0.1700175 [DOI] [PubMed] [Google Scholar]
- 3.Baud’huin M., Lamoureux F., Duplomb L., Redini F. and Heymann D. (2007) RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell. Mol. Life Sci. 64, 2334–2350 10.1007/s00018-007-7104-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotti T.N., Dharmapatni A.A., Alias E. and Haynes D.R. (2015) Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J. Immunol. Res. 2015, 281287 10.1155/2015/281287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furlan F., Galbiati C., Jorgensen N.R., Jensen J.E., Mrak E., Rubinacci A. et al. (2007) Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. J. Bone Miner. Res. 22, 1387–1396 10.1359/jbmr.070516 [DOI] [PubMed] [Google Scholar]
- 6.Roscher A., Hasegawa T., Dohnke S., Ocana-Morgner C., Amizuka N., Jessberger R. et al. (2016) The F-actin modulator SWAP-70 controls podosome patterning in osteoclasts. Bone Rep. 5, 214–221 10.1016/j.bonr.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgess D., Machuca-Gayet I., Blangy A. and Jurdic P. (2014) Podosome organization drives osteoclast-mediated bone resorption. Cell Adh. Migr. 8, 191–204 10.4161/cam.27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S., Planus E., Georgess D., Place C., Wang X., Albiges-Rizo C. et al. (2011) Podosome rings generate forces that drive saltatory osteoclast migration. Mol. Biol. Cell 22, 3120–3126 10.1091/mbc.e11-01-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luxenburg C., Addadi L. and Geiger B. (2006) The molecular dynamics of osteoclast adhesions. Eur. J. Cell Biol. 85, 203–211 10.1016/j.ejcb.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Jurdic P., Saltel F., Chabadel A. and Destaing O. (2006) Podosome and sealing zone: specificity of the osteoclast model. Eur. J. Cell Biol. 85, 195–202 10.1016/j.ejcb.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Croke M., Ross F.P., Korhonen M., Williams D.A., Zou W. and Teitelbaum S.L. (2011) Rac deletion in osteoclasts causes severe osteopetrosis. J. Cell Sci. 124, 3811–3821 10.1242/jcs.086280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzouk S., Lieberherr M. and Cournot G. (1999) Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur. J. Cell Biol. 78, 249–255 10.1016/S0171-9335(99)80058-2 [DOI] [PubMed] [Google Scholar]
- 13.Darden A.G., Ries W.L., Wolf W.C., Rodriguiz R.M. and Key L.L. Jr (1996) Osteoclastic superoxide production and bone resorption: stimulation and inhibition by modulators of NADPH oxidase. J. Bone Miner. Res. 11, 671–675 10.1002/jbmr.5650110515 [DOI] [PubMed] [Google Scholar]
- 14.Goettsch C., Babelova A., Trummer O., Erben R.G., Rauner M., Rammelt S. et al. (2013) NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Invest. 123, 4731–4738 10.1172/JCI67603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bokoch G.M. (2005) Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 15, 163–171 10.1016/j.tcb.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Kwong C.H., Adams A.G. and Leto T.L. (1995) Characterization of the effector-specifying domain of Rac involved in NADPH oxidase activation. J. Biol. Chem. 270, 19868–19872 10.1074/jbc.270.34.19868 [DOI] [PubMed] [Google Scholar]
- 17.Lacy P., Mahmudi-Azer S., Bablitz B., Gilchrist M., Fitzharris P., Cheng D. et al. (1999) Expression and translocation of Rac2 in eosinophils during superoxide generation. Immunology 98, 244–252 10.1046/j.1365-2567.1999.00873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X., Carnevale K.A. and Cathcart M.K. (2003) Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J. Biol. Chem. 278, 40788–40792 10.1074/jbc.M302208200 [DOI] [PubMed] [Google Scholar]
- 19.Lee N.K., Choi Y.G., Baik J.Y., Han S.Y., Jeong D.W., Bae Y.S. et al. (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106, 852–859 10.1182/blood-2004-09-3662 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Lebowitz D., Sun C., Thang H., Grynpas M.D. and Glogauer M. (2008) Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J. Bone Miner. Res. 23, 260–270 10.1359/jbmr.071013 [DOI] [PubMed] [Google Scholar]
- 21.Shin B., Kupferman J., Schmidt E., Polleux F., Delany A.M. and Lee S.K. (2020) Rac1 inhibition via Srgap2 restrains inflammatory osteoclastogenesis and limits the Clastokine, SLIT3. J. Bone Miner. Res. 35, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guimbal S., Morel A., Guerit D., Chardon M., Blangy A. and Vives V. (2019) Dock5 is a new regulator of microtubule dynamic instability in osteoclasts. Biol. Cell 111, 271–283 10.1111/boc.201900014 [DOI] [PubMed] [Google Scholar]
- 23.Soares M.P.R., Silva D.P., Uehara I.A., Ramos E.S. Jr, Alabarse P.V.G., Fukada S.Y. et al. (2019) The use of apocynin inhibits osteoclastogenesis. Cell Biol. Int. 43, 466–475 10.1002/cbin.11110 [DOI] [PubMed] [Google Scholar]
- 24.Xu S., Zhang Y., Wang J., Li K., Tan K., Liang K. et al. (2018) TSC1 regulates osteoclast podosome organization and bone resorption through mTORC1 and Rac1/Cdc42. Cell Death Differ. 25, 1549–1566 10.1038/s41418-017-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Wang Z., Ma C., Wei Z., Chen K., Wang C. et al. (2020) Dracorhodin perchlorate inhibits osteoclastogenesis through repressing RANKL-stimulated NFATc1 activity. J. Cell. Mol. Med. 24, 3303–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasca A., Astleford K., Lederman A., Jensen E.D., Lee B.S., Gopalakrishnan R. et al. (2017) Regulation of osteoclast differentiation by Myosin X. Sci. Rep. 7, 7603 10.1038/s41598-017-07855-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itzstein C., Coxon F.P. and Rogers M.J. (2011) The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2, 117–130 10.4161/sgtp.2.3.16453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerasimcik N., Westerberg L.S. and Severinson E. (2018) Methods to study the role of Cdc42, Rac1, and Rac2 in B-cell cytoskeletal responses. Methods Mol. Biol. 1821, 235–246 10.1007/978-1-4939-8612-5_16 [DOI] [PubMed] [Google Scholar]
- 29.Kotelevets L., Walker F., Mamadou G., Lehy T., Jordan P. and Chastre E. (2018) The Rac1 splice form Rac1b favors mouse colonic mucosa regeneration and contributes to intestinal cancer progression. Oncogene 37, 6054–6068 10.1038/s41388-018-0389-7 [DOI] [PubMed] [Google Scholar]
- 30.Lin J., Lee D., Choi Y. and Lee S.Y. (2015) The scaffold protein RACK1 mediates the RANKL-dependent activation of p38 MAPK in osteoclast precursors. Sci. Signal. 8, ra54 10.1126/scisignal.2005867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L., Li J., Zhang L., Zhang F., Zhang R., Chen X. et al. (2015) Cofilin phosphorylation is elevated after F-actin disassembly induced by Rac1 depletion. Biofactors 41, 352–359 10.1002/biof.1235 [DOI] [PubMed] [Google Scholar]
- 32.Joshi S., Singh A.R., Zulcic M., Bao L., Messer K., Ideker T. et al. (2014) Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS ONE 9, e95893 10.1371/journal.pone.0095893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S., Prigmore E., Govind S., Veryard C., Kozma R., Wientjes F.B. et al. (1998) Cryptic Rac-binding and p21(Cdc42Hs/Rac)-activated kinase phosphorylation sites of NADPH oxidase component p67(phox). J. Biol. Chem. 273, 15693–15701 10.1074/jbc.273.25.15693 [DOI] [PubMed] [Google Scholar]
- 34.Manser E., Chong C., Zhao Z.S., Leung T., Michael G., Hall C. et al. (1995) Molecular cloning of a new member of the p21-Cdc42/Rac-activated kinase (PAK) family. J. Biol. Chem. 270, 25070–25078 10.1074/jbc.270.42.25070 [DOI] [PubMed] [Google Scholar]
- 35.Meriane M., Mary S., Comunale F., Vignal E., Fort P. and Gauthier-Rouviere C. (2000) Cdc42Hs and Rac1 GTPases induce the collapse of the vimentin intermediate filament network. J. Biol. Chem. 275, 33046–33052 10.1074/jbc.M001566200 [DOI] [PubMed] [Google Scholar]
- 36.Ito Y., Teitelbaum S.L., Zou W., Zheng Y., Johnson J.F., Chappel J. et al. (2010) Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J. Clin. Invest. 120, 1981–1993 10.1172/JCI39650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H., Choi H.K., Shin J.H., Kim K.H., Huh J.Y., Lee S.A. et al. (2009) Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J. Clin. Invest. 119, 813–825 10.1172/JCI36809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mediero A., Perez-Aso M. and Cronstein B.N. (2014) Activation of EPAC1/2 is essential for osteoclast formation by modulating NFkappaB nuclear translocation and actin cytoskeleton rearrangements. FASEB J. 28, 4901–4913 10.1096/fj.14-255703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touaitahuata H., Blangy A. and Vives V. (2014) Modulation of osteoclast differentiation and bone resorption by Rho GTPases. Small GTPases 5, e28119 10.4161/sgtp.28119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amato C., Thomason P.A., Davidson A.J., Swaminathan K., Ismail S., Machesky L.M. et al. (2019) WASP restricts active rac to maintain cells’ front-rear polarization. Curr. Biol. 29, 4169.e4164–4182.e4164 10.1016/j.cub.2019.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonfim-Melo A., Ferreira E.R. and Mortara R.A. (2018) Rac1/WAVE2 and Cdc42/N-WASP participation in actin-dependent host cell invasion by extracellular amastigotes of Trypanosoma cruzi. Front. Microbiol. 9, 360 10.3389/fmicb.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tetley G.J.N., Szeto A., Fountain A.J., Mott H.R. and Owen D. (2018) Bond swapping from a charge cloud allows flexible coordination of upstream signals through WASP: multiple regulatory roles for the WASP basic region. J. Biol. Chem. 293, 15136–15151 10.1074/jbc.RA118.003290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menotti M., Ambrogio C., Cheong T.C., Pighi C., Mota I., Cassel S.H. et al. (2019) Wiskott-Aldrich syndrome protein (WASP) is a tumor suppressor in T cell lymphoma. Nat. Med. 25, 130–140 10.1038/s41591-018-0262-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carabeo R.A., Dooley C.A., Grieshaber S.S. and Hackstadt T. (2007) Rac interacts with Abi-1 and WAVE2 to promote an Arp2/3-dependent actin recruitment during chlamydial invasion. Cell. Microbiol. 9, 2278–2288 10.1111/j.1462-5822.2007.00958.x [DOI] [PubMed] [Google Scholar]
- 45.Egile C., Loisel T.P., Laurent V., Li R., Pantaloni D., Sansonetti P.J. et al. (1999) Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146, 1319–1332 10.1083/jcb.146.6.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinoza-Sanchez S., Metskas L.A., Chou S.Z., Rhoades E. and Pollard T.D. (2018) Conformational changes in Arp2/3 complex induced by ATP, WASp-VCA, and actin filaments. Proc. Natl. Acad. Sci. U.S.A. 115, E8642–E8651 10.1073/pnas.1717594115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hytti M., Piippo N., Korhonen E., Honkakoski P., Kaarniranta K. and Kauppinen A. (2015) Fisetin and luteolin protect human retinal pigment epithelial cells from oxidative stress-induced cell death and regulate inflammation. Sci. Rep. 5, 17645 10.1038/srep17645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha R., Srivastava S., Joshi A., Joshi U.J. and Govil G. (2014) In-vitro anti-proliferative and anti-oxidant activity of galangin, fisetin and quercetin: role of localization and intermolecular interaction in model membrane. Eur. J. Med. Chem. 79, 102–109 10.1016/j.ejmech.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 49.Singh S., Singh A.K., Garg G. and Rizvi S.I. (2018) Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci. 193, 171–179 10.1016/j.lfs.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 50.Jacob S. and Thangarajan S. (2018) Fisetin impedes developmental methylmercury neurotoxicity via downregulating apoptotic signalling pathway and upregulating Rho GTPase signalling pathway in hippocampus of F1 generation rats. Int. J. Dev. Neurosci. 69, 88–96 10.1016/j.ijdevneu.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 51.Wang M., Zhang W., Xu W., Shen Y. and Du L. (2016) Optimization of genome shuffling for high-yield production of the antitumor deacetylmycoepoxydiene in an endophytic fungus of mangrove plants. Appl. Microbiol. Biotechnol. 100, 7491–7498 10.1007/s00253-016-7457-0 [DOI] [PubMed] [Google Scholar]
- 52.Xie W., Zhang W., Sun M., Lu C. and Shen Y. (2018) Deacetylmycoepoxydiene is an agonist of Rac1, and simultaneously induces autophagy and apoptosis. Appl. Microbiol. Biotechnol. 102, 5965–5975 10.1007/s00253-018-9058-6 [DOI] [PubMed] [Google Scholar]
- 53.Almatroodi S.A., Alsahli M.A., Almatroudi A. and Rahmani A.H. (2019) Garlic and its active compounds: a potential candidate in the prevention of cancer by modulating various cell signalling pathways. Anticancer Agents Med. Chem. 19, 1314–1324 10.2174/1871520619666190409100955 [DOI] [PubMed] [Google Scholar]
- 54.Cobb-Abdullah A., Lyles L.R. II, Odewumi C.O., Latinwo L.M., Badisa V.L. and Abazinge M. (2019) Diallyl disulfide attenuation effect on transcriptome in rat liver cells against cadmium chloride toxicity. Environ. Toxicol. 34, 950–957 10.1002/tox.22766 [DOI] [PubMed] [Google Scholar]
- 55.Su B., Su J., Zeng Y., Ding E., Liu F., Tan T. et al. (2018) Diallyl disulfide inhibits TGFbeta1induced upregulation of Rac1 and betacatenin in epithelialmesenchymal transition and tumor growth of gastric cancer. Oncol. Rep. 39, 2797–2806 [DOI] [PubMed] [Google Scholar]
- 56.Xia L., Lin J., Su J., Oyang L., Wang H., Tan S. et al. (2019) Diallyl disulfide inhibits colon cancer metastasis by suppressing Rac1-mediated epithelial-mesenchymal transition. Onco Targets Ther. 12, 5713–5728 10.2147/OTT.S208738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y., Su J., Shi L., Liao Q. and Su Q. (2013) DADS downregulates the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling pathway, inhibiting cell migration and invasion. Oncol. Rep. 29, 605–612 10.3892/or.2012.2168 [DOI] [PubMed] [Google Scholar]
- 58.Yu M.H., Yang T.Y., Ho H.H., Huang H.P., Chan K.C. and Wang C.J. (2018) Mulberry polyphenol extract inhibits FAK/Src/PI3K complex and related signaling to regulate the migration in A7r5 cells. J. Agric. Food Chem. 66, 3860–3869 10.1021/acs.jafc.8b00958 [DOI] [PubMed] [Google Scholar]
- 59.Zhao X., Yang R., Bi Y., Bilal M., Kuang Z., Iqbal H.M.N. et al. (2019) Effects of dietary supplementation with mulberry (Morus alba L.) leaf polysaccharides on immune parameters of weanling pigs. Animals (Basel) 10, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang H.P., Shih Y.W., Chang Y.C., Hung C.N. and Wang C.J. (2008) Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J. Agric. Food Chem. 56, 9286–9293 10.1021/jf8013102 [DOI] [PubMed] [Google Scholar]
- 61.Akhtar N., Syed D.N., Khan M.I., Adhami V.M., Mirza B. and Mukhtar H. (2016) The pentacyclic triterpenoid, plectranthoic acid, a novel activator of AMPK induces apoptotic death in prostate cancer cells. Oncotarget 7, 3819–3831 10.18632/oncotarget.6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avila-Carrasco L., Majano P., Sanchez-Tomero J.A., Selgas R., Lopez-Cabrera M., Aguilera A. et al. (2019) Natural plants compounds as modulators of epithelial-to-mesenchymal transition. Front. Pharmacol. 10, 715 10.3389/fphar.2019.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bueno-Silva B., Alencar S.M., Koo H., Ikegaki M., Silva G.V., Napimoga M.H. et al. (2013) Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 61, 4546–4550 10.1021/jf305468f [DOI] [PubMed] [Google Scholar]
- 64.Hiep N.T., Kwon J., Kim D.W., Hwang B.Y., Lee H.J., Mar W. et al. (2015) Isoflavones with neuroprotective activities from fruits of Cudrania tricuspidata. Phytochemistry 111, 141–148 10.1016/j.phytochem.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 65.Gopal D., Muddebihalkar A.G., Skariyachan S., C A.U., Kaveramma P., Praveen U. et al. (2019) Mitogen activated protein kinase-1 and cell division control protein-42 are putative targets for the binding of novel natural lead molecules: a therapeutic intervention against Candida albicans. J. Biomol. Struct. Dyn. 29, 1–16 10.1080/07391102.2019.1682053 [DOI] [PubMed] [Google Scholar]
- 66.Chen C., Wu W., Xu X., Zhang L., Liu Y. and Wang K. (2014) Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 105, 308–316 10.1016/j.carbpol.2014.01.089 [DOI] [PubMed] [Google Scholar]
- 67.Huang Z. and Zhang L. (2009) Chemical structures of water-soluble polysaccharides from Rhizoma Panacis Japonici. Carbohydr. Res. 344, 1136–1140 10.1016/j.carres.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 68.Qi D., Yang X., Chen J., Li F., Shi X., Zhang C. et al. (2013) Determination of chikusetsusaponin V and chikusetsusaponin IV in rat plasma by liquid chromatography-mass spectrometry and its application to a preliminary pharmacokinetic study. Biomed. Chromatogr. 27, 1568–1573 10.1002/bmc.2961 [DOI] [PubMed] [Google Scholar]
- 69.Iijima R., Watanabe T., Ishiuchi K., Matsumoto T., Watanabe J. and Makino T. (2018) Interactions between crude drug extracts used in Japanese traditional Kampo medicines and organic anion-transporting polypeptide 2B1. J. Ethnopharmacol. 214, 153–159 10.1016/j.jep.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 70.Sasaki Y., Komatsu K. and Nagumo S. (2008) Rapid detection of Panax ginseng by loop-mediated isothermal amplification and its application to authentication of Ginseng. Biol. Pharm. Bull. 31, 1806–1808 10.1248/bpb.31.1806 [DOI] [PubMed] [Google Scholar]
- 71.Chen X., Wu Q.S., Meng F.C., Tang Z.H., Chen X., Lin L.G. et al. (2016) Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest, triggers apoptosis and inhibits migration and invasion in ovarian cancer cells. Phytomedicine 23, 1555–1565 10.1016/j.phymed.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 72.Yuan K., Li X., Lu Q., Zhu Q., Jiang H., Wang T. et al. (2019) Application and mechanisms of triptolide in the treatment of inflammatory diseases-a review. Front. Pharmacol. 10, 1469 10.3389/fphar.2019.01469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X., Zhao F., Lv Z.M., Shi W.Q., Zhang L.Y. and Yan M. (2016) Triptolide disrupts the actin-based Sertoli-germ cells adherens junctions by inhibiting Rho GTPases expression. Toxicol. Appl. Pharmacol. 310, 32–40 10.1016/j.taap.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 74.Chaotham C., Pongrakhananon V., Sritularak B. and Chanvorachote P. (2014) A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 34, 1931–1938 [PubMed] [Google Scholar]
- 75.Sritularak B., Duangrak N. and Likhitwitayawuid K. (2011) A new bibenzyl from Dendrobium secundum. Z. Naturforsch C. J. Biosci. 66, 205–208 10.1515/znc-2011-5-602 [DOI] [PubMed] [Google Scholar]
- 76.Chaotham C. and Chanvorachote P. (2015) A bibenzyl from Dendrobium ellipsophyllum inhibits migration in lung cancer cells. J. Nat. Med. 69, 565–574 10.1007/s11418-015-0925-5 [DOI] [PubMed] [Google Scholar]
- 77.Ku C.Y., Wang Y.R., Lin H.Y., Lu S.C. and Lin J.Y. (2015) Corosolic acid inhibits hepatocellular carcinoma cell migration by targeting the VEGFR2/Src/FAK pathway. PLoS ONE 10, e0126725 10.1371/journal.pone.0126725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Charoenrungruang S., Chanvorachote P., Sritularak B. and Pongrakhananon V. (2014) Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J. Nat. Prod. 77, 1359–1366 10.1021/np500015v [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are included in the manuscript.