Abstract
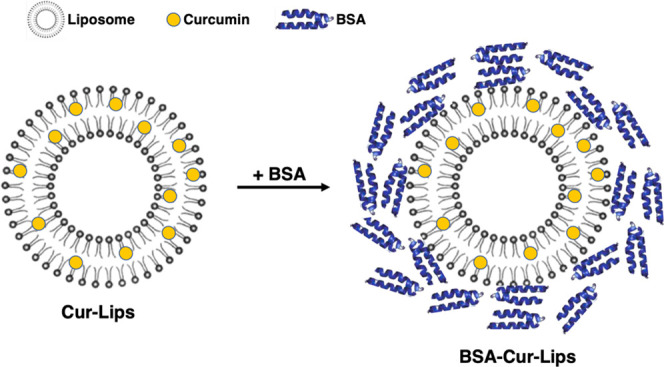
Although the bioavailability and stability of curcumin can be greatly improved by liposomes encapsulation, its application is still limited due to the short circulating time. In this present work, we aim to construct a long-circulating delivery system of liposomal curcumin (Cur-Lips) by coating bovine serum albumin (BSA), namely, BSA-coated liposomal curcumin (BSA-Cur-Lips). The effects of coating albumin on the physicochemical properties of Cur-Lips were investigated. It was found that BSA-Cur-Lips was more spherical, more homogeneous in size, and significantly larger than Cur-Lips. Combining sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Coomassie bright blue staining, and X-ray photoelectron spectroscopy analysis (XPS), we confirmed that albumin molecules were stably located on the surface of BSA-Cur-Lips. In addition, the impacts of the coating albumin on the Cur-Lips release and phagocytosis by mouse macrophages Raw264.7 in vitro were investigated. We found that no significant initial burst drug release effect was observed for both Cur-Lips and BSA-Cur-Lips and the presence of albumin can enhance the liposome structure stability and slow down the release of Cur. More importantly, the macrophage phagocytosis of Cur-Lips was significantly reduced after coating albumin. In conclusion, coating albumin is a promising approach for developing a long-circulating delivery system of liposomal curcumin, and its properties including low phagocytosis, slow drug release, enhanced stability, and nontoxicity give this system great prospects for practical use.
Introduction
Curcumin is a natural polyphenolic compound extracted from the rhizome of the herb Curcuma longa and has many pharmacological effects, such as antioxidation, anti-inflammatory, antibacterial, antiviral, antifungal, and antitumor.1−5 However, curcumin is difficult to dissolve in water, and its biological utilization is low. In addition, curcumin is stabilized in a solution with a pH < 6.5, and the degradation rate is significantly accelerated as the pH value of the solution increased. These properties of curcumin seriously limit its clinical application.6−9 As we know, liposomes have shown great potentials in drug delivery due to its drug-loading capacity, avoiding immune response, biodegradability, and biocompatibility.10−12 Liposomes are composed of a hydrophobic lipid bilayer and a hydrophilic inner aqueous chamber; lipophilic drugs can distribute in lipid bilayers in high percentages.13−15 Therefore, the liposomes as the carrier of curcumin have obvious advantages and several recent studies have confirmed this.16−20 Our previous study also found that the water-solubility and stability of curcumin were significantly improved after encapsulating it into liposomes.21
However, the application of liposomal curcumin is still limited due to their rapid clearance from the systematic circulation.22,23 Liposomes will be quickly absorbed by the reticuloendothelial system and rapidly cleared from blood circulation after they enter the body.24,25 It has been demonstrated that liposomes will be coated by a series of plasma proteins immediately upon their entry in the blood. These plasma proteins include immunoglobulin (IgG) and complement which play important roles in opsonization. Their adsorption onto the liposomes will lead to the uptake of the liposomes by the macrophages associated with their corresponding receptors expressed on the phagocyte surface.26−28 Hence, coated by a series of plasma proteins is one substantial cause for liposomes being quickly absorbed by the reticuloendothelial system and rapidly cleared from blood circulation. Therefore, how to reduce the adsorption of plasma proteins especially IgG and complement to liposomes is the key to prolong the circulation time of liposomes. In the past decade, among the technologies for inhibiting proteins adsorption, the method to modify the surface of the liposomes with hydrophilic molecules such as poly(ethylene glycol) (PEG) has been the most commonly used.29−32 Progress is made; however, the results vary in different reports.33−35
In recent years, there have been more and more studies on the liposomes–plasma protein interaction, but unfortunately, no one has proposed how to use this effect to solve the problem.24,36 As we know, albumin is one of the most abundant proteins in plasma, which can transport and detoxify, and has a plasma half-life of more than 15 days. It is also widely used as a drug carrier due to its ready availability, biocompatibility and long circulation time.37−39 Most importantly, albumin is a component in the plasma protein corona absorbed by liposomes.40 Therefore, it is possible to extend the plasma half-life of liposomes by adsorbing albumin onto the surface of the liposomes. However, many other plasma proteins have stronger adsorption to liposomes, and albumin is at a disadvantage in competitive adsorption. We have done a lot of work on this, and our previous studies found that preformation of an albumin corona around the nanoparticles can reduce the macrophages phagocytosis of nanoparticles and prolong their circulating time.41,42
Therefore, in the present study, albumin and liposomal curcumin were fully incubated in vitro to form a complex by the spontaneous adsorption of plasma proteins on liposomes. We expected without the competing adsorption of other plasma proteins that the surface of liposomal curcumin can form a stable albumin protective layer. It is hoped that coating albumin can reduce the phagocytosis by macrophages and construct a long-circulating delivery system of liposomal curcumin.
Results and Discussion
Characterization of Liposomal Curcumin (Cur-Lips) and BSA-Coated Liposomal Curcumin (BSA-Cur-Lips)
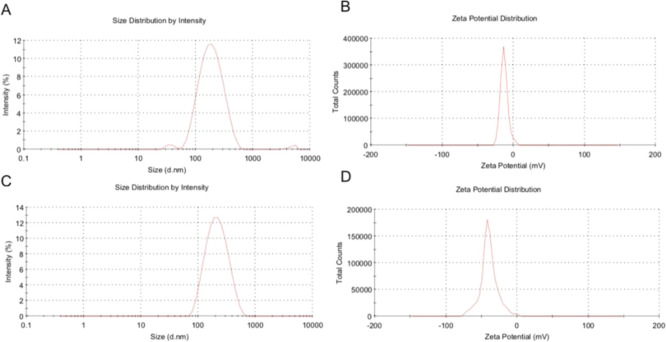
Particle size, ζ-potential, and encapsulation efficiency are important parameters for quality control of liposomes. The particle size and ζ-potential of Cur-Lips and BSA-Cur-Lips determined by dynamic light scattering (DLS) or electrophoretic light scattering are shown in Table 1, and the typical particle size and ζ-potential diagram are shown in Figure 1.
Table 1. Size and ζ-Potential of Cur-Lips and BSA-Cur-Lipsa.
| samples | size (nm) | PDI | ζ-potential (mV) |
|---|---|---|---|
| Cur-Lips | 165.9 ± 3.6 | 0.221 ± 0.030 | –14.0 ± 0.4 |
| BSA- Cur-Lips | 189.1 ± 2.7b | 0.173 ± 0.024b | –39.1 ± 0.5c |
Data are presented as mean ± standard deviation (s.d.) (n = 3). Significant difference between Cur-Lips and BSA-Cur-Lips,.
P < 0.05.
P < 0.01.
Figure 1.
Typical size and ζ-potential distribution graphs. (A, B) Typical particle size and ζ-potential distribution diagrams of Cur-Lips, respectively. (C, D) Typical particle size and ζ-potential distribution of BSA-Cur-Lips, respectively.
As can be seen from Table 1, the mean particle size of BSA-Cur-Lips is about 189 nm, significantly larger than that of Cur-Lips (P < 0.05). Studies have shown that the biodistribution as well as imaging data indicate that the larger the liposomes, the higher their splenic uptake. The remarkably high and rapid splenic uptake of the 220 nm liposomes is most likely due to physical filtration rather than phagocytosis by spleen macrophages.43 Therefore, in this study, the particle size of both Cur-Lips and BSA-Cur-Lips were controlled within 200 nm. Particle size polydispersity index (PDI) is an important index for evaluating liposomes. The PDI of BSA-Cur-Lips is significantly lower than Cur-Lips (P < 0.05), indicating that compared with Cur-Lips, the particle size distribution in BSA-Cur-Lips is more uniform; that is, the formation of the complex improves the particle size distribution.
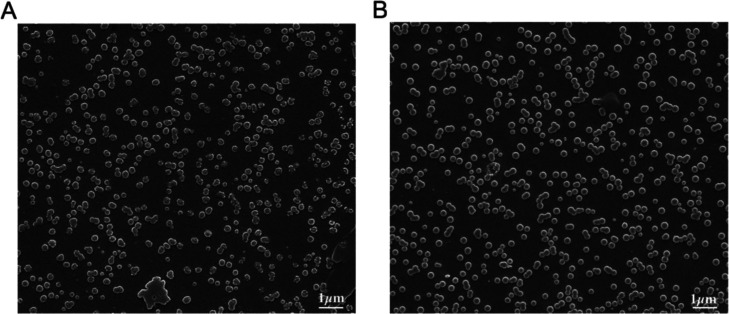
The morphology of Cur-Lips and BSA-Cur-Lips was observed by a scanning electron microscope (SEM). As shown in Figure 2, BSA-Cur-Lips are spherical, more homogeneous in size, and significantly larger than Cur-Lips. These results are fully consistent with the PDI values and the size distribution graphs measured by dynamic light scattering technology and electrophoretic light scattering technology. However, interestingly, the ζ-potential of BSA-Cur-Lips is significantly lower than that of Cur-Lips (P < 0.01). As we know, albumin is negatively charged, therefore, it is assumed that albumin adsorbed on the surface of Cur-Lips increases its particle size and reduces its ζ-potential.
Figure 2.
SEM images. (A) Cur-Lips and (B) BSA-Cur-Lips. Scale bar: 1 μm.
In this work, the encapsulation efficiencies of Cur-Lips and BSA-Cur-Lips are 80.61 ± 1.17 and 80.17 ± 2.72%, respectively. There is no statistical difference between the two, indicating that both Cur-Lips and BSA-Cur-Lips prepared in this experiment have good drug-carrying capacity, and the presence of albumin does not affect the encapsulation efficiency of Cur-Lips.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Coomassie Bright Blue Staining
SDS-PAGE is an important and convenient qualitative method to identify the type of protein adsorbed on the nanomaterials. To verify that the increased size and reduced ζ-potential were attributable to the adsorbed bovine serum albumin (BSA), 12% SDS-PAGE and Coomassie bright blue staining were performed.
As shown in Figure 3, there is no signal for Cur-Lips (band 2), while BSA-Cur-Lips (band 3) shows an obvious signal at the same molecular weight area, with standard BSA (band 1). These results prove the existence of BSA in BSA-Cur-Lips.
Figure 3.
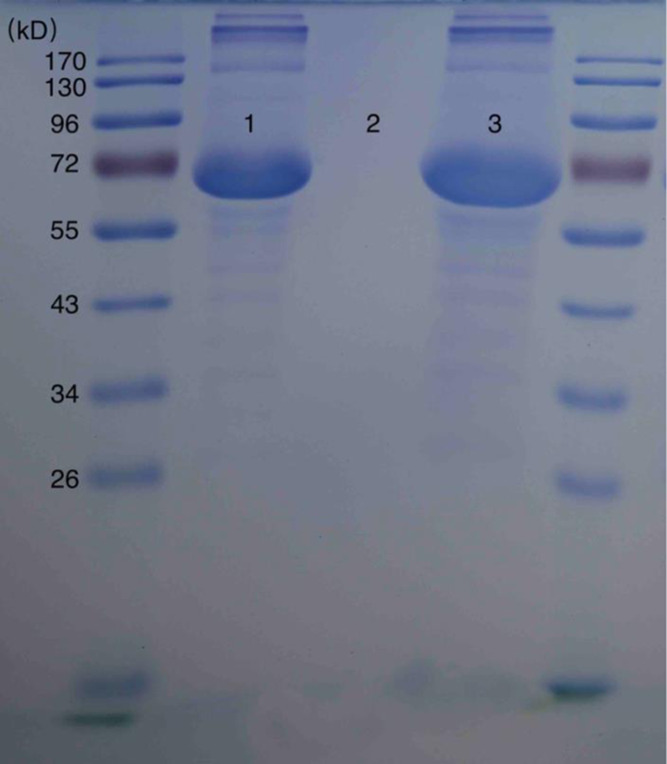
Twelve percent SDS-PAGE and Coommas bright blue staining. Band (1): BSA standard; band (2): Cur-Lips; and band (3): BSA-Cur-Lips.
X-ray Photoelectron Spectroscopy (XPS) Analysis
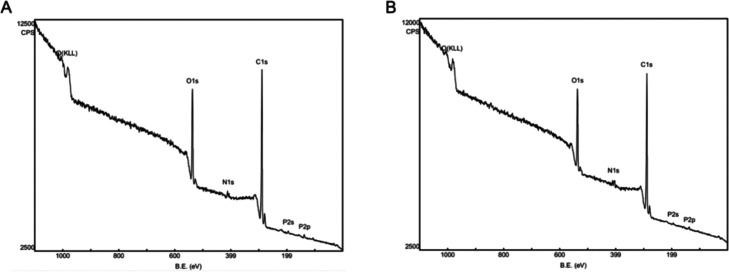
SDS-PAGE can only confirm the existence of BSA, whereas XPS can help us confirm the location of BSA due to its ability of surface chemical elements analysis. To further verify the existence and location of BSA in BSA-Cur-Lips, XPS was applied and the surface contents of elements C, N, O, and P were analyzed.
As shown in Figure 4, signals for elements C, N, O, and P can be found in both Cur-Lips and BSA-Cur-Lips, but their intensities vary. The detailed elements analysis is shown in Table 2. We can see that as the contents of elements N and O increase, the contents of elements C and P decrease after the formation of BSA-Cur-Lips, which is consistent with the element intensities shown in Figure 4. Because albumin contains a large amount of elements N and O, it is assumed that such changes undoubtedly resulted from BSA. Therefore, these results indicate BSA is stably located on the surface of BSA-Cur-Lips. It is assumed that the adsorption of albumin is largely due to the extremely high surface energy of Cur-Lips. In addition, the hydrogen bond formation, hydrophobic effect, and van der Waals forces may also be involved. In many studies, the automated HPLC-Chip technology coupled to a high-resolution mass spectrometer is employed for protein corona characterization.24 These methods are effective but relatively complicated to implement. As far as we know, few studies have utilized XPS technology to confirm and characterize the composition of the albumin corona of liposomes, our research shows that it is an effective and simple method.
Figure 4.
XPS graphs of surface element analysis. (A) Cur-Lips and (B) BSA-Cur-Lips.
Table 2. XPS Analysis of Surface Elements of Cur-Lips and BSA-Cur-Lipsa.
| elements
content (%) |
||||
|---|---|---|---|---|
| samples | C | N | O | P |
| Cur-Lips | 76.71 | 2.48 | 19.57 | 1.24 |
| BSA-Cur-Lips | 74.52b | 4.82b | 19.73 | 1.14 |
Significant difference between Cur-Lips and BSA-Cur-Lips.
P < 0.05.
In Vitro Release Study
Drug release in the liposome delivery system is an important evaluation index. The presence of albumin may influence the release profile of BSA-Cur-Lips compared with Cur-Lips.
As shown in Figure 5, the cumulatively released amounts of BSA-Cur-Lips are significantly lower than that of Cur-Lips at all time points. This result indicates that the release of curcumin from BSA-Cur-Lips becomes substantially slower in the presence of the BSA. As we know, there are three ways for drug release from liposomes: (a) the drug molecules adsorbed on the surface of liposomes are released via desorption upon contacting with release medium, (b) the encapsulated drugs are released by diffusion through the liposomes skeleton, or/and (c) following the degradation or disintegration of liposomes.44 It is assumed that in this study the adsorption of BSA onto the Cur-Lips surface can stabilize the particle skeleton to some extent. The enhanced stability can reduce the collapse of liposomes. In addition, the coated BSA acts as a physical barrier, can limit the permeation of curcumin into the release medium, and, in turn, slow down the release rate.
Figure 5.
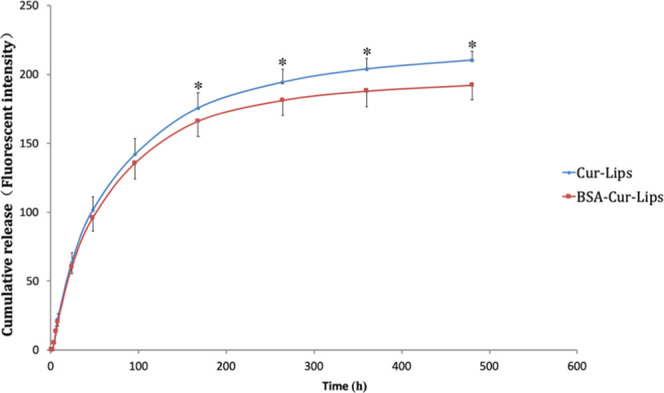
In vitro release profiles of Cur-Lips and BSA-Cur-Lips in PBS containing 0.5% (m/v) of the Tween-80 (pH 7.4). Data presented as the mean ± SD (n = 3). *P < 0.05.
Initial burst drug release is a very common phenomenon in many liposomes delivery systems and becoming a significant problem for efficient drug delivery. In the present study, we can see that no significant initial burst drug release effect is observed for both Cur-Lips and BSA-Cur-Lips. This is very difficult and important for liposomal curcumin.
In Vitro Phagocytosis by Raw264.7 Cells
The recognition and phagocytosis by macrophages are an important way for the body to remove foreign substances. Liposomal curcumin entry into the body is also cleared by the phagocytosis of macrophages. It will be ineffective for the long circulation of the drug delivery system if a large number of particles are easily recognized and phagocytosed by macrophages after administration, no matter how stable they are.45 Therefore, reducing the phagocytosis rate has a substantial impact on increasing the circulation time of Cur-Lips. In this study, the phagocytosis of Cur-Lips and BSA-Cur-Lips was investigated by mouse macrophages Raw264.7 cells.
As shown in Figure 6, the fluorescence signals of curcumin in cells are concentration-dependent in both Cur-Lips and BSA-Cur-Lips groups; however, compared to those in Cur-Lips, the fluorescence signals in BSA-Cur-Lips groups are weaker at all concentrations. The detailed amounts of curcumin phagocytosed by macrophages are shown in Figure 7, which are consistent with the immunofluorescence images given above. The phagocytosis of Cur-Lips and BSA-Cur-Lips by macrophages is concentration-dependent; at the same concentration, the phagocytosis of BSA-Cur-Lips is lower than that of Cur-Lips, and the difference is statistically significant (P < 0.05). These results indicate that after coating BSA, the phagocytosis of Cur-Lips is significantly reduced. We speculate that the presence of albumin protective layer reduces the adsorption of complement and immunoglobulin to Cur-Lips, reduces its optonogenesis, and thus reduces the phagocytosis of macrophages. This is a critical property for long-circulating liposomes delivery system.
Figure 6.
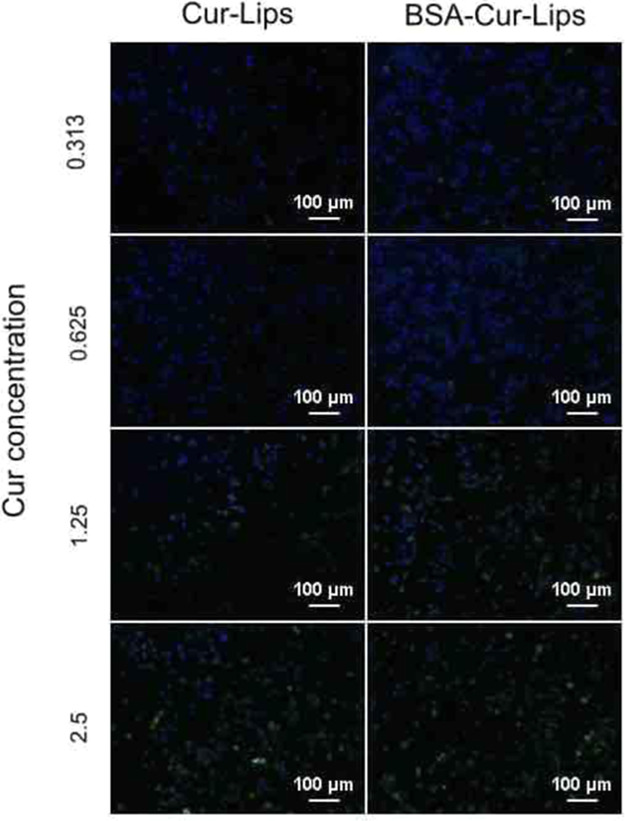
Fluorescent micrographs of mouse macrophages Raw264.7 exposed to different concentrations of Cur-Lips and BSA-Cur-Lips. Scale bar: 100 μm.
Figure 7.
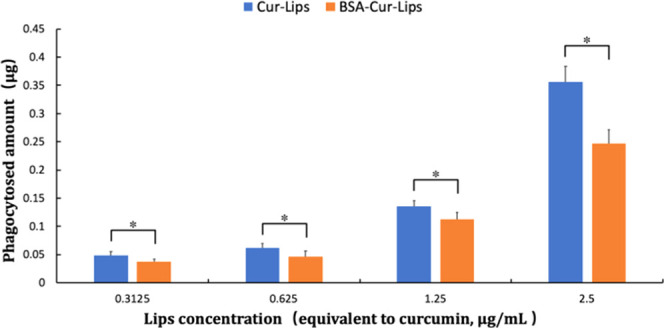
Phagocytosis amounts of Cur-Lips and BSA-Cur-Lips with different concentrations after incubation with mouse macrophages Raw264.7. Data are presented as mean ± s.d. (n = 3). *P < 0.05.
At present, it has been recognized that the adsorption of plasma proteins by liposomes is an important reason for the rapid removal of liposomes.27 Many strategies have been used to reduce the adsorption of plasma proteins by liposomes, in which the method to modify the surface of the liposomes with hydrophilic molecules such as poly(ethylene glycol) (PEG) has been the most commonly used. However, there are still some problems caused by this chemical modification, such as the stability and toxicity of PEGylated liposomes, the high production cost and the accelerated blood clearance after repeated injection, etc.33−35,46 Inspired by this, searching for a material that can overcome these disadvantages and possesses the similar effects to PEGylation may be an approach to solve this issue. In addition, in the last few decades, the interaction between liposomes and plasma proteins has attracted more and more attention and many related papers can be retrieved,24,36,47 but few of them focus on how to utilize such interaction to optimize the liposomes-based drug delivery systems. Studies have shown that the adsorption of plasma proteins by liposomes is spontaneous and inevitable;25 therefore, we probably can use this to search for the material that can replace the PEG. In the plasma protein corona absorbed by liposomes, albumin is an important component, but it is at a disadvantage in competitive adsorption.48 As we know, albumin is an approved material for pharmaceutical use due to its ready availability, biocompatibility, and long circulation time.37 Therefore, in this study, we choose the albumin to interact with liposomal curcumin and form a pure protein corona in advance. Our results indicate that this is an effective method to reduce the adsorption of plasma proteins by liposomes and its properties including low phagocytosis, slow drug release, enhanced stability, and nontoxicity give this system great prospects for practical use.
Conclusions
In the present work, spontaneous adsorption of plasma proteins by liposomes was utilized to fully incubate albumin and Cur-Lips in vitro to form BSA-Cur-Lips. It is found that without the competitive adsorption of other plasma proteins, a stable albumin protective layer is formed on the surface of Cur-Lips. We have confirmed the formation of the BSA corona around Cur-Lips surfaces via size increases, SDS-PAGE, and X-ray photoelectron spectroscopy. Albumin can enhance liposome structure stability, act as a physical barrier, and slow down the release of Cur-Lips in vitro. More importantly, the phagocytosis by macrophages is significantly reduced after coating with albumin. Therefore, preformation of a BSA corona is a promising approach for developing a long-circulating delivery system of liposomal curcumin. Although further efforts investigating the in vivo behavior of the liposomal curcumin are needed to support its great applications for long-circulating delivery, its properties including low phagocytosis, slow drug release, enhanced stability, and nontoxicity provide it great potential to achieve that goal. This study provides a new idea for the construction of a long-circulating delivery system of liposomal curcumin, which has an important clinical significance.
Experimental Section
Materials
Phospholipids (soybean lecithin for injection use, the content of phosphatidylcholine higher than 70%) were obtained from Shanghai Tai-Wei Pharmaceutical Co., Ltd., (Shanghai, China). Cholesterol, bovine serum albumin-V, and sodium deoxycholate were purchased from Ameresco (Solon, OH). Curcumin was purchased from Sigma (St. Louis, MO). Poloxamer 188 (F68) was obtained from BASF (China) Co., Ltd., (Shanghai, China). Coomassie bright blue was purchased from Sigma (St. Louis, MO). Chloroform was obtained from Kelon Co., Ltd., (Chengdu, China). Methanol was obtained from Comio reagent Co. Ltd. (Tianjin, China). All other chemical reagents used in this study were of analytical grade or better.
Preparation of Liposomal Curcumin (Cur-Lips)
The Cur-Lips were prepared using a modified ethanol injection method according to our previous work.21 Briefly, curcumin, phospholipid, and cholesterol were dissolved in ethanol as the organic phase and phosphate buffer solution (PBS, 0.001 mol/L, pH 7.4) with F68 was dissolved as the aqueous phase. The organic phase was slowly injected into the aqueous phase under magnetic agitation, and then the organic solution was removed by rotating evaporation for 30 min in a 37 °C water bath using a rotary evaporator under dark conditions. The obtained suspension was first used to remove the free curcumin by low-speed centrifugation (3000 rpm for 5 min), then the supernatant was discarded by high-speed centrifugation (16 000 rpm for 10 min), and the precipitate was resuspended in PBS (pH 7.4) to yield Cur-Lips. In this experiment, the mass ratio of phospholipid to cholesterol was 5:1, the mass fraction of F68 was 1%, the ratio of organic phase to water phase was 10:1, the final concentration of phospholipid was 10 mg/mL, and the final concentration of curcumin was 0.4 mg/mL.
Preparation of Albumin-Coated Liposomal Curcumin (BSA-Cur-Lips)
The BSA-Cur-Lips were prepared according to our previous reports.41,42 Briefly, the prepared Cur-Lips and 20 mg/mL albumin solution were evenly mixed and incubated for 2 h at room temperature, and the precipitation was collected by centrifugation (16 000 rpm for 10 min). After fully cleaning and removing the unsolidly adsorbed albumin, double-steamed water was added into the precipitation to obtain BSA-Cur-Lips.
Characterization of Cur-Lips and BSA-Cur-Lips
The particle size of Cur-Lips and BSA-Cur-Lips were examined by dynamic light scattering (DLS) technology. The particle size was presented by intensity distribution and the polydispersity index (PDI) was used to evaluate the uniformity of the size distribution; ζ-potential was determined by electrophoretic laser scattering (ELS) technology using Zetasizer Nano ZS90 (Malvern Instruments Ltd., Malvern, U.K.) at 25 °C. The morphology of Cur-Lips and BSA-Cur-Lips was observed by scanning electron microscope (SEM, INSPECT F, FEI, Netherlands). Briefly, one drop of the properly diluted Cur-Lips or BSA-Cur-Lips suspension was placed on a clean glass sheet, followed by air-drying. The samples were coated with gold and then observed by SEM.
Encapsulation Efficiency (EE) Determination
The encapsulation efficiencies of Cur-Lips and BSA-Cur-Lips were determined by a high-speed centrifugation method. An appropriate amount of Cur-Lips or BSA-Cur-Lips was centrifuged at a low speed (3000 rpm for 5 min) to precipitate nondissolved curcumin; then, the supernatant was subjected to high-speed centrifugation (16 000 rpm for 10 min) to separate Cur-Lips or BSA-Cur-Lips from the tiny dissolved curcumin. After centrifugation, the supernatant (1–10 mL) was diluted to the scale with anhydrous ethanol and filtered through a 0.22 μm organic filter membrane. The content of free curcumin was determined by fluorescence spectrophotometry (excitation: 458 nm; emission: 548 nm) in the supernatant and presented as F1, i.e., in addition, 1 mL of Cur-Lips or BSA-Cur-Lips was added to a 10 mL measuring bottle, anhydrous ethanol was added to the scale, and then the mixture was filtered through the 0.22 μm organic filter membrane. The content of free curcumin was determined by fluorescence spectrophotometry in the filtrate and presented as F0, i.e., the encapsulation efficiency (EE) of the curcumin was calculated using the following equation: EE% = (F0 – F1)/F0 × 100%.
SDS-PAGE and Coomassie Bright Blue Staining
To confirm BSA in the prepared BSA-Cur-Lips, 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie bright blue staining were carried out for Cur-Lips and BSA-Cur-Lips. Briefly, the freshly prepared Cur-Lips or BSA-Cur-Lips solution was separated by centrifugation (16 000 rpm for 5 min) and washed three times to get rid of the free BSA. Then, 12% SDS-PAGE gel electrophoresis was used for Cur-Lips and BSA-Cur-Lips, and finally the strips were stained by Coomassie bright blue.
X-ray Photoelectron Spectroscopy (XPS) Analysis
To estimate the location of BSA in the prepared BSA-Cur-Lips, surface element analysis was carried out for Cur-Lips and BSA-Cur-Lips by X-ray photoelectron spectroscopy analysis. Briefly, the freshly prepared Cur-Lips and BSA-Cur-Lips were condensed by filtering through a 0.22 μm microporous membrane, respectively. After washing with a large quantity of water, the free BSA was removed and Cur-Lips or BSA-Cur-Lips was gathered on the filter membrane. After natural drying, the chemical elements carbon (C), nitrogen (N), oxygen (O), and phosphorus (P) in these samples were analyzed using X-ray photoelectron spectroscopy.
In Vitro Release Study
The in vitro release property of Cur-Lips and BSA-Cur-Lips was investigated using the dynamic dialysis method.44 Briefly, 0.8 mL of Cur-Lips or BSA-Cur-Lips (final concentration of curcumin was 2 mg/mL) was added into dialysis bags with an interception molecular weight of 8000 Da (curcumin was allowed to pass only). After tightly bundling at two ends, the sample-loaded dialysis bags were soaked in 8 mL of release medium (PBS containing 0.5% (m/v) of the Tween-80, pH 7.4) and placed on a horizontal shaker (100 rpm, 37 ± 1 °C). The release medium was collected at fixed time intervals (0.5, 1, 2, 4, 6, 8, 24, 48, 96, 168, 264, 360, 480 h) and replaced with 8 mL of fresh release medium. The collected samples were stored at −80 °C. After the collection of all samples, the samples were centrifuged at 12 000 rpm for 3 min, and the contents of curcumin were analyzed by fluorescence spectrophotometry (excitation 458 nm; emission 548 nm).
In Vitro Phagocytosis by Raw264.7 Cells
Raw264.7 cells were cultured with RPMI-1640 containing 10% fetal bovine serum (FBS) under standard conditions (37 °C, 5% CO2), and 50 000 cells/mL cell suspensions were prepared after digestion with trypsin. One milliliter of cell suspension was mixed with 0.5 mL diluted into a certain concentration of Cur-Lips or BSA-Cur-Lips and incubated at 37 °C in a centrifugation tube (the final concentration of curcumin in the resultant mixture was 0.3125, 0.625, 1.25, or 2.5 μg/mL). After incubation at 37 °C for 1 h, the free Cur-Lips or BSA-Cur-Lips was isolated by centrifugation (3000 rpm for 3 min), and the curcumin content in the supernatant was measured by fluorescent spectrophotometry after proper dilution (excitation 458 nm; emission 548 nm). The amount of curcumin phagocytosis by macrophages was calculated by the total amount of curcumin minus the amount of free curcumin. In addition, the cells were resuspended, and the fluorescent signals of macrophage phagocytosis of Cur-Lips and BSA-Cur-Lips were observed under a fluorescence microscope.
Statistical Analysis
All assays in this study were repeated at least thrice. All of the data are presented as mean ± s.d. (standard deviation). The t-test was used for comparison between the two groups, and one-way analysis of variance was used to analyze differences among groups, which was considered to be statistically significant when the P-value was less than 0.05.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (81500826).
The authors declare no competing financial interest.
References
- Ghalandarlaki N.; Alizadeh A. M.; Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. BioMed Res. Int. 2014, 2014, 394264 10.1155/2014/394264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komal K.; Chaudhary S.; Yadav P.; Parmanik R.; Singh M. The Therapeutic and Preventive Efficacy of Curcumin and Its Derivatives in Esophageal Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 1329–1337. 10.31557/APJCP.2019.20.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Zhong C.; Wang Q.; Chen W.; Yuan Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antiviral Res. 2019, 167, 98–103. 10.1016/j.antiviral.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandadi M.; Sahebkar A. Curcumin: An Effective Inhibitor of Interleukin-6. Curr. Pharm. Des. 2017, 23, 921–931. 10.2174/1381612822666161006151605. [DOI] [PubMed] [Google Scholar]
- Rai B.; Kaur J.; Catalina M. Anti-oxidation actions of curcumin in two forms of bed rest: oxidative stress serum and salivary markers. Asian Pac. J. Trop. Med. 2010, 3, 651–654. 10.1016/S1995-7645(10)60157-5. [DOI] [Google Scholar]
- Arozal W.; Ramadanty W. T.; Louisa M.; Satyana R. P. U.; Hartono G.; Fatrin S.; Purbadi S.; Estuningtyas A.; Instiaty I. Pharmacokinetic Profile of Curcumin and Nanocurcumin in Plasma, Ovary, and Other Tissues. Drug Res. 2019, 69, 559–564. 10.1055/a-0863-4355. [DOI] [PubMed] [Google Scholar]
- Mirzaei H.; Shakeri A.; Rashidi B.; Jalili A.; Banikazemi Z.; Sahebkar A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112. 10.1016/j.biopha.2016.11.098. [DOI] [PubMed] [Google Scholar]
- Aqil F.; Munagala R.; Jeyabalan J.; Agrawal A. K.; Gupta R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017, 19, 1691–1702. 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- Wei X.; Senanayake T. H.; Bohling A.; Vinogradov S. V. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: synthesis, pharmacokinetics, and tumor growth inhibition. Mol. Pharm. 2014, 11, 3112–3122. 10.1021/mp500290f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crommelin D. J. A.; van Hoogevest P.; Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what?. J. Controlled Release 2020, 318, 256–263. 10.1016/j.jconrel.2019.12.023. [DOI] [PubMed] [Google Scholar]
- El-Hammadi M. M.; Arias J. L. An update on liposomes in drug delivery: a patent review (2014-2018). Expert Opin. Ther. Pat. 2019, 29, 891–907. 10.1080/13543776.2019.1679767. [DOI] [PubMed] [Google Scholar]
- Joshi S.; Bawage S.; Tiwari P.; Kirby D.; Perrie Y.; Dennis V.; Singh S. R. Liposomes: a promising carrier for respiratory syncytial virus therapeutics. Expert Opin. Drug Delivery 2019, 16, 969–980. 10.1080/17425247.2019.1652268. [DOI] [PubMed] [Google Scholar]
- He H.; Lu Y.; Qi J. P.; Zhu Q. G.; Chen Z. J.; Wu W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Lu W. L.; Gu W.; Lu S. S.; Chen Z. P.; Cai B. C.; Yang X. X. Drug-in-cyclodextrin-inliposomes: a promising delivery system for hydrophobic drugs. Expert Opin. Drug Delivery 2014, 11, 565–577. 10.1517/17425247.2014.884557. [DOI] [PubMed] [Google Scholar]
- Signorell R. D.; Luciani P.; Brambilla D.; Leroux J. C. Pharmacokinetics of lipid-drug conjugates loaded into liposomes. Eur. J. Pharm. Biopharm. 2018, 128, 188–199. 10.1016/j.ejpb.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Li L.; Braiteh F. S.; Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 2005, 104, 1322–1331. 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- Basak S. K.; Zinabadi A.; Wu A. W.; Venkatesan N.; Duarte V. M.; Kang J. J.; Dalgard C. L.; Srivastava M.; Sarkar F. H.; Wang M. B.; Srivatsan E. S. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget 2015, 6, 18504–18517. 10.18632/oncotarget.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T.; Wei Y. M.; Lee R. J.; Zhao L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter M.; Wittmann M.; Koll-Weber M.; Suss R. The suitability of liposomes for the delivery of hydrophobic drugs - A case study with curcumin. Eur. J. Pharm. Biopharm. 2019, 140, 20–28. 10.1016/j.ejpb.2019.04.013. [DOI] [PubMed] [Google Scholar]
- Bulboacă A. E.; Bolboaca S. D.; Bulboaca A. C.; Porfire A. S.; Tefas L. R.; Suciu S. M.; Dogaru G.; Stanescu I. C. Liposomal Curcumin Enhances the Effect of Naproxen in a Rat Model of Migraine. Med. Sci. Monit. 2019, 25, 5087–5097. 10.12659/MSM.915607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Q.; Zhu J. F.; Wang X. B.; Ba K. Improving the Stability of Liposomal Curcumin by Adjusting the Inner Aqueous Chamber pH of Liposomes. ACS Omega 2020, 5, 1120–1126. 10.1021/acsomega.9b03293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.; Wu Z. H.; McClements D. J.; Zou L. Q.; Peng S. F.; Zhou W.; Liu W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf., B 2019, 183, 110460 10.1016/j.colsurfb.2019.110460. [DOI] [PubMed] [Google Scholar]
- Fernández-Romero A. M.; Maestrelli F.; Mura P. A.; Rabasco A. M.; Gonzalez-Rodriguez M. L. Novel Findings about Double-Loaded Curcumin-in-HP beta cyclodextrin-in Liposomes: Effects on the Lipid Bilayer and Drug Release. Pharmaceutics 2018, 10, 256 10.3390/pharmaceutics10040256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriotti A. L.; Caracciolo G.; Caruso G.; Cavaliere C.; Pozzi D.; Samperi R.; Laganà A. Analysis of plasma protein adsorption onto DC-Chol-DOPE cationic liposomes by HPLC-CHIP coupled to a Q-TOF mass spectrometer. Anal. Bioanal. Chem. 2010, 398, 2895–2903. 10.1007/s00216-010-4104-y. [DOI] [PubMed] [Google Scholar]
- Diederichs J. E. Plasma protein adsorption patterns on liposomes: establishment of analytical procedure. Electrophoresis 1996, 17, 607–611. 10.1002/elps.1150170332. [DOI] [PubMed] [Google Scholar]
- Sercombe L.; Veerati T.; Moheimani F.; Wu S. Y.; Sood A. K.; Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo G. Liposome-protein corona in a physiological environment: Challenges and opportunities for targeted delivery of nanomedicines. Nanomedicine 2015, 11, 543–557. 10.1016/j.nano.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Amici A.; Caracciolo G.; Digiacomo L.; Gambini V.; Marchini C.; Tilio M.; Capriotti A. L.; Colapicchioni V.; Matassa R.; Familiari G.; Palchetti S.; Pozzi D.; Mahmoudi M.; Lagana A. In vivo protein corona patterns of lipid nanoparticles. RSC Adv. 2017, 7, 1137–1145. 10.1039/C6RA25493D. [DOI] [Google Scholar]
- Wang Y. Y.; Yang Y. N.; Yu Y. B.; Li J. Y.; Pan W. S.; Yang X. G.; Zhang Z. R.; Jiang S.; Yang X. B.; Wang X. B. Transferrin Modified Dioscin Loaded PEGylated Liposomes: Characterization and In Vitro Antitumor Effect. J. Nanosci. Nanotechnol. 2020, 20, 1321–1331. 10.1166/jnn.2020.16955. [DOI] [PubMed] [Google Scholar]
- Wang H.; Zheng M.; Gao J. Y.; Wang J.; Zhang Q.; Fawcett J. P.; He Y.; Gu J. K. Uptake and release profiles of PEGylated liposomal doxorubicin nanoparticles: A comprehensive picture based on separate determination of encapsulated and total drug concentrations in tissues of tumor-bearing mice. Talanta 2020, 208, 120358 10.1016/j.talanta.2019.120358. [DOI] [PubMed] [Google Scholar]
- Dave V.; Gupta A.; Singh P.; Tak K.; Sharma S. PEGylated Lipova E120 liposomes loaded with celecoxib: in-vitro characterization and enhanced in-vivo anti-inflammatory effects in rat models. J. Biosci. 2019, 44, 94 10.1007/s12038-019-9919-x. [DOI] [PubMed] [Google Scholar]
- Sesarman A.; Tefas L.; Sylvester B.; Licarete E.; Rauca V.; Luput L.; Patras L.; Porav S.; Banciu M.; Porfire A. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Delivery Transl. Res. 2019, 9, 260–272. 10.1007/s13346-018-00598-8. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Ichihara M.; Wang X. Y.; Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J. Controlled Release 2006, 115, 243–250. 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mohamed M.; Abu Lila A. S.; Shimizu T.; Alaaeldin E.; Hussein A.; Sarhan H. A.; Szebeni J.; Ishida T. PEGylated liposomes: immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. 10.1080/14686996.2019.1627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Lila A. S.; Nawata K.; Shimizu T.; Ishida T.; Kiwada H. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. Int. J. Pharm. 2013, 456, 235–242. 10.1016/j.ijpharm.2013.07.059. [DOI] [PubMed] [Google Scholar]
- La Barbera G.; Capriotti A. L.; Caracciolo G.; Cavaliere C.; Cerrato A.; Montone C. M.; Piovesana S.; Pozzi D.; Quagliarini E.; Lagana A. A comprehensive analysis of liposomal biomolecular corona upon human plasma incubation: The evolution towards the lipid corona. Talanta 2020, 209, 120487 10.1016/j.talanta.2019.120487. [DOI] [PubMed] [Google Scholar]
- Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Controlled Release 2008, 132, 171–183. 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Li X.; Zhu X. Y.; Sun J.; Qiu Q. Q.; Huang W. L.; Qian H. Phenylbutazone, a New Long-Acting Agent that can Improve the Peptide Pharmacokinetic Based on Serum Albumin as a Drug Carrier. Chem. Biol. Drug Des. 2016, 87, 936–945. 10.1111/cbdd.12726. [DOI] [PubMed] [Google Scholar]
- Park K. Albumin: A versatile carrier for drug delivery. J. Controlled Release 2012, 157, 3. 10.1016/j.jconrel.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Cedervall T.; Lynch I.; Foy M.; Berggad T.; Donnelly S. C.; Cagney G.; Linse S.; Dawson K. A. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem., Int. Ed. 2007, 46, 5754–5756. 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- Peng Q.; Zhang S.; Yang Q.; Zhang T.; Wei X. Q.; Jiang L.; Zhang C. L.; Chen Q. M.; Zhang Z. R.; Lin Y. F. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 2013, 34, 8521–8530. 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- Peng Q.; Wei X. Q.; Yang Q.; Zhang S.; Zhang T.; Shao X. R.; Cai X. X.; Zhang Z. R.; Lin Y. F. Enhanced biostability of nanoparticles based drug delivery system by albumin corona. Nanomedicine 2015, 10, 205–214. 10.2217/nnm.14.86. [DOI] [PubMed] [Google Scholar]
- Boerman O. C.; Oyen W. J.; van Bloois L.; Koenders E. B.; van der Meer J. W.; Corstens F. H.; Storm G. Optimization of technetium-99m-labeled PEG liposomes to image focal infection: effects of particlesize and circulation time. J. Nucl. Med. 1997, 38, 489–493. [PubMed] [Google Scholar]
- Peng Q.; Zhang Z. R.; Gong T.; Chen G. Q.; Sun X. A Rapid- Acting, Long-Acting Insulin Formulation Based on a Phospholipid Complex Loaded PHBHHx Nanoparticles. Biomaterials 2012, 33, 1583–1588. 10.1016/j.biomaterials.2011.10.072. [DOI] [PubMed] [Google Scholar]
- Lesniak A.; Salvati A.; Santos-Martinez M. J.; Radomski M. W.; Dawson K. A.; A°berg C. Nanoparticle Adhesion to the Cell Membrane and Its Effect on Nanoparticle Uptake Efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. 10.1021/ja309812z. [DOI] [PubMed] [Google Scholar]
- Laverman P.; Boerman O. C.; Oyen W. J. G.; Corstens F. H. M.; Storm G. In vivo applications of PEG liposomes: unexpected observations. Crit. Rev. Ther. Drug Carrier Syst. 2001, 18, 16 10.1615/CritRevTherDrugCarrierSyst.v18.i6.40. [DOI] [PubMed] [Google Scholar]
- Walkey C. D.; Chan W. C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. 10.1039/C1CS15233E. [DOI] [PubMed] [Google Scholar]
- Ruh H.; Kuhl B.; Brenner-Weiss G.; Hopf C.; Diabate S.; Weiss C. Identification of serum proteins bound to industrial nanomaterials. Toxicol. Lett. 2012, 208, 41–50. 10.1016/j.toxlet.2011.09.009. [DOI] [PubMed] [Google Scholar]