Abstract
In this study, the effects of ozone addition on the cool flame and NTC (negative temperature coefficient) regions of stoichiometric C3H8/O2 mixtures are computationally studied through the explosion limit profiles. The results show that with minute quantities of ozone addition (the mole fraction of ozone is 0.1%), the cool flame area is enlarged to the low-temperature region. Further increases in the mole fraction of ozone gradually weaken the NTC behavior, and a monotonic explosion limit is eventually achieved. The sensitivity analysis of the main reactions involving ozone reveals that the explosion limit is mainly controlled by the ozone unimolecular decomposition reaction O3 (+M) = O2 + O (+M). However, as its reverse reaction is a third-body reaction, this reaction will lose its effect on the explosion limit in the high-pressure region. On the contrary, the reaction O3 + HO2 = OH + O2 + O2 has a significant effect on the explosion limit in the high-pressure and low-temperature region, as the concentration of HO2 increases through the rapid third-body reaction H + O2 + M = HO2 + M.
1. Introduction
Propane is considered as a combustion archetype as it exhibits all the key features of larger n-alkanes. These long-chain hydrocarbons exhibit certain experimental combustion characteristics that are consistent with both the explosion limit curves and practical considerations. The first is that they exhibit negative temperature coefficients of the reaction rate.1 Newitt and Thornes found that the spontaneous ignition temperature of the propane–oxygen mixtures showed a negative temperature coefficient (NTC) behavior.2 A characteristic negative coefficient behavior was also observed when the ignition delay times for the propane/air mixtures were plotted as a function of temperature.3 The temperature range at which NTC was observed for propane depends on the pressure and mixture composition, which was usually located at low temperatures.3,4 The latest research showed that the NTC behavior of propane occurred between 600 and 650 K.5 Generally, there was no NTC behavior when the temperature was higher than 875 K.6−8
The second is that they exhibit cool flames. The cool flames are governed by the low-temperature chemistry via oxygen addition to fuel radicals and subsequent isomerization and radical branching reactions.9 It was found that the existence of the cool flame can dramatically extend the lean and rich flammability limits of the conventional high-temperature flame.10,11 Furthermore, a cool flame and two-stage ignition were observed for the propane/oxygen mixtures in microgravity conditions.12 Moreover, the cool flame may be related to a two-stage ignition phenomenon. The first-stage ignition was marked by a temperature increase of 10–100 K followed by a hot ignition stage.13,14
As ozone has a significant effect on cool flame, it has been used in the ignition timing control of gasoline compression ignition engines15,16 and HCCI engines.17 In addition, the cool flames of butane isomers,18 dimethyl ether,19n-decane,20 and n-heptane21 activated by ozone were also widely investigated. Furthermore, the cool flames could be stabilized through the ozone addition in flat laminar flame.22 Previous research showed that the O radical was released through the decomposition of O3 prior to the reaction zone, which reacts with fuel to generate OH radicals. This reaction path is critical to activate the low-temperature chemistry through the chain-branching reactions and enables the observation of cool flames.20,23,24 Further research showed that since O3 can significantly promote the low-temperature reactivity of the fuel, the reactivity of the cool flame is basically independent of the size of n-alkane molecules.25
The objectives of the present work are the following. First, because the cool flame is very important in the actual combustion process, it is necessary to investigate the specific temperature and pressure ranges of the cool flame for larger hydrocarbons. In this case, propane is selected as a representative of larger hydrocarbons as it has similar combustion characteristics to larger hydrocarbons. Second, since O3 is often used to promote and enhance the stability of cool flame, the effect of O3 addition on the cool flame explosion region should be studied. Third, because the S-shaped explosion limit curve has been recognized for propane/oxygen systems, we will scrutinize whether the reactivity of the cool flame will be significantly promoted with the addition of ozone in the system, thereby reforming the explosive limit characteristics. Finally, the heat release rates will be determined and sensitivity analyses will be conducted to find out the most important elementary reactions that control the explosion limit of the propane/oxygen/ozone systems.
2. Computational Methods
The SENKIN code was used to compute the explosion limit of the C3H8/O2/O3 mixtures.26 Five C3 submechanisms are taken from the recently published research. The comprehensive iso-octane model (KAUST, 153 species, and 929 reactions),27 2-butene (AramcoMech 2.0, 151 species, and 943 reactions) model,28 Green’s propane model,13 DTU propane model,29 and LLNL n-heptane model30 are used for the mechanism validation. The ten-step O3 submechanism developed by Zhao and Ju31 was adopted to account for the ozone chemistry, and it was added to each of the C3 oxidation mechanisms.
In this work, the simulation was carried out by assuming an adiabatic system with a constant volume to study the spontaneous ignition problems. Furthermore, six radial wall termination reactions were added to the mechanism. Since the reaction vessel used in the Newitt and Thornes experiment is 28 cm long and 5.5 cm in diameter, the reaction rate was calculated with the real surface/volume ratio instead of the spherical chamber assumption in the previous research.32
The carriers were destroyed at the wall according to the following reactions
with the equivalent reaction rate constants per unit volume, kH, kO, kOH, kHO2, kH2O2,
and kHCO, given by
| 1 |
where v̅ = (8kBT/πm)1/2 is the average velocity of the thermal motion of the carriers at temperature T, m is the molar mass, and kB is the Boltzmann constant. The sticking coefficient ε measures the destruction efficiency in the collision with the wall, and usually ε ≈ 10–5–10–2 for glass and quartz. As the reaction vessel used in the Newitt and Thornes experiment is made of transparent silica, the sticking coefficient is taken as 10–3 in this study. Further calculations indicate that the sticking coefficient has moderate effects on the first explosion limits; however, only a slight effect is shown for the second and third explosion limits.
The calculations were all conducted under the stoichiometric conditions (except for the model validation with the experimental data) with different mole fractions of ozone, which is defined as [O3]/([O2] + [O3]). The explosion and nonexplosion conditions were searched within the temperature range of 350–2500 K and pressure range of 3.5–109 Pa. An explosion criterion of a 50 K increase of the initial temperature within 10 s was employed in this study. This criterion is selected to ensure that the temperature change is small and the residence time is long enough compared with the characteristic time and temperature scales in the practical applications. Another criterion like an average pressure rise rate of 3%/ms was used for the determination of cool flame ignition.33 The explosion limit is the temperature vs pressure boundaries between the explosion and nonexplosion conditions.
3. Results and Discussion
3.1. Validation and Calculation of the Explosion Limit
To begin with, the calculated explosion limits of the equimolecular propane/oxygen mixtures were compared with the experimental data of Newitt and Thornes34 to verify the chemistry kinetic models to be used, as shown in Figure 1. As the experimental data were acquired in a much longer time, most of them were between 0.5 and 2.5 min; the residence time in the calculation was set as 1.5 min to be consistent with the experiment. Because the explosion criterion in the experiment is based on the chemiluminescence of formaldehyde, there may be some deviations between the experimental explosion limit and the calculation results. It should be noticed that the residence time of all of the other calculations is 10 s. As the hot flame limit is also included in the experimental data, the calculated hot flame limits are also provided in Figure 1 for comparison, where the explosion criterion of the temperature is changed to 400 K. The explosion limit calculated with the 50 K temperature increase criterion is named as the cool flame explosion limit. Generally, cool flame explosion limits calculated with the AramcoMech 2.0, KAUST, Green, and DTU models all show the NTC behaviors as the experimental data. However, the NTC behavior of the cool flame explosion limit is not that obvious when using the LLNL oxidation model. Furthermore, the NTC behaviors of the cool flame and hot flame explosion limits lie in the much lower pressure and higher temperature regions when using the Green and DTU models compared with the experimental data. In addition, the cool flame and hot flame explosion limits calculated by the AramcoMech 2.0 and KAUST models almost coincide with each other. Moreover, the NTC behaviors are in the same temperature and pressure ranges as the experimental data. As a better agreement with the experimental data is found using the AramcoMech 2.0 mechanism and the KAUST model compared with the DTU, LLNL, and Green models, the AramcoMech 2.0 mechanism is used in the following calculations.
Figure 1.
Comparison of the calculated and experimental cool flame and hot flame explosion limits of equimolecular C3H8/O2 mixtures.
3.2. Effect of Ozone Addition on the Explosion Limit
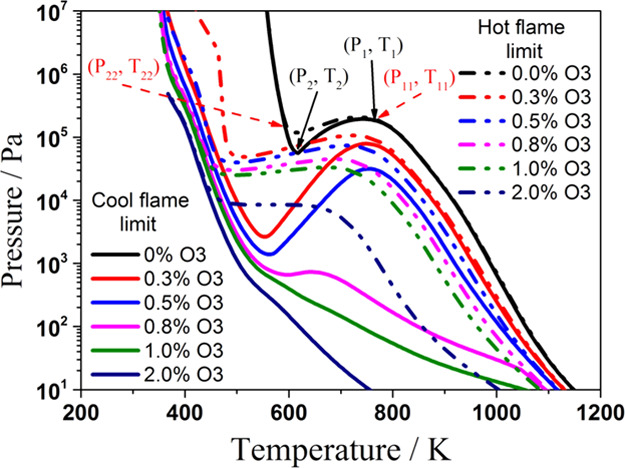
The cool flame and hot flame explosion limits of the stoichiometric C3H8/O3/O2 mixtures with the addition of ozone are shown in Figure 2. The S-shaped cool flame explosion limit curve gradually transits to a monotonic curve with the addition of O3 in the C3H8/O3/O2 mixture.
Figure 2.
Effect of ozone addition on the cool flame and hot flame limits of stoichiometric C3H8/O2/O3 mixtures. (a) 400 K temperature increase criterion and (b) maximum 850 K temperature criterion.
It is shown in Figure 2 that when minute quantities of ozone were added in the system, the cool flame explosion limit of the C3H8/O3/O2 mixture is shifted to the low-temperature region, especially in the NTC region. The area of the cool flame region is expanded to the lower-pressure and lower-temperature region, which means the cool flame can be significantly promoted with the addition of O3. Moreover, when the mole fraction of ozone is less than or equal to 0.8%, the explosion limit curve remains S-shaped, and the NTC response still exists in the low-temperature region, which is bounded by two turning points (P1, T1) and (P2, T2). With the addition of O3, the turning point of (P2, T2) moves to the low-temperature and low-pressure region. In contrast, the pressure of the turning point (P1, T1) is gradually reduced and the temperature of the turning point (P1, T1) is almost unchanged with the addition of ozone. However, with a further increase of the mole fraction of O3 to more than 0.8%, the pressure of the turning point (P1, T1) is lower than that of the turning point (P2, T2). In this case, the NTC response no longer exists. As shown in Figure 2, when the mole fraction of ozone is larger than or equal to 1.0%, a monotonic explosion limit is achieved. With a further increase of the mole fraction of ozone, the explosion limit of C3H8/O3/O2 mixtures is totally moved to the low-temperature region. In this case, the low-temperature reactivity of the fuel is significantly promoted by O3 so that the S-shaped explosion limit characteristics of the larger n-alkanes disappear.
For the hot flame explosion limits, Figure 2a,b shows two results calculated by different explosion criteria. One uses a 400 K increase of the initial temperature within 10 s, as shown in Figure 2a, and the other uses the maximum temperature reach of 850 K within 10 s, as shown in Figure 2b. There are also two turning points (P11, T11) and (P22, T22) for both of the hot flame explosion limits. For the C3H8/O2 mixture, point (P1, T1) and point (P11, T11) are roughly coinciding with each other, and together with point (P2, T2) and point (P22, T22), they are forming the cool flame peninsula. With the addition of O3 in the mixture, the turning point (P22, T22) is almost always attached to the explosion limit of the cool flame. However, the cool flame turning point (P1, T1) is gradually moving away from the hot flame turning point (P11, T11). That is, the turning point (P1, T1) and turning point (P11, T11) are separated from each other. Furthermore, the hot flame explosion limit curve always keeps NTC features when the mole fractions of ozone are less than or equal to 1.0%. However, the hot flame explosion limit loses its nonmonotonic behavior for the cases with ozone mole fractions larger than or equal to 2.0%. The difference between these two hot flame explosion criteria at 700–800 K is due to the initial temperature becoming too close to the 850 K criterion.
The temperature and pressure profiles of three cases with 0.5% O3 under different explosion statuses are compared in Figure 3a,b. Along the isotherm line of 600 K, the initial pressures of the three cases are chosen as 1000, 10 000, and 100 000 Pa for the nonexplosion, cool flame, and hot flame conditions, respectively. The temperature and pressure curves of the hot flame cases have the typical characteristics of two-stage ignition, as shown in Figure 3a,b. Furthermore, the pressure and temperature curves show only a single-stage rise in the cool flame region. For nonexplosion conditions, the temperature and pressure curves are only slightly increased, as shown in Figure 3a,b.
Figure 3.
Comparison of the temperatures (a) and pressures (b) under the nonexplosion, cool flame, and hot flame conditions.
The comparisons of the mole fractions of propane, ozone, and formaldehyde during the oxidation process under different conditions are shown in Figure 4. For the nonexplosion condition, the mole fractions of propane and ozone decrease slowly, while the mole fractions of formaldehyde increase slowly. For the cool flame condition, ozone is almost consumed completely in the first half second. Meanwhile, there is an obvious consumption of propane, which consumes about 10% of the fuel. Moreover, the mole fraction of propane remains almost unchanged after 0.5 s. At the same time, the mole fraction of formaldehyde shows a sharp increase and reaches its peak value at around 0.5 s. For the hot flame case, propane shows a two-stage consumption process, and ozone is almost completely depleted in the first stage of the reaction. A large amount of formaldehyde is generated in the first-stage reaction, while due to the consumption of formaldehyde in the following reaction process, the mole fraction of formaldehyde decreases gradually. At the beginning of the hot flame stage, the mole fraction of formaldehyde increases sharply. However, because of the high temperature in the second-stage ignition, formaldehyde completely oxidizes immediately.
Figure 4.
Mole fractions of the main species under the nonexplosion, cool flame, and hot flame conditions.
The main reactions with the largest absolute peak heat release rate for the cool flame and hot flame cases are compared in Figure 5. For the hot flame case, only the main reactions in the first-stage ignition are given in Figure 5b. For both of the cases, the reactions in the low-temperature reaction path of propane comprise most of the main exothermic reactions, including the H-abstraction reaction of propane by OH radicals, the oxygen addition to normalpropyl (n-C3H7) and isopropyl (i-C3H7), and the decomposition reaction of the propylperoxyl radicals. Moreover, the H2O2 production reaction through the reaction of HO2 with HO2 also appears in both cases. It should be noticed that the peak heat release rate of the ozone decomposition reaction is higher than that of the three reactions involving CH3 and CH3O in the cool flame case, as shown in Figure 5a. However, the peak heat release rates of the three reactions involving CH3 and CH3O are obviously higher than that of the ozone decomposition reaction for the hot flame case, as shown in Figure 5b.
Figure 5.
Heat release rates of the main reactions under the cool flame (a) and hot flame (b) conditions.
3.3. Key Reactions Analysis with O3 Addition
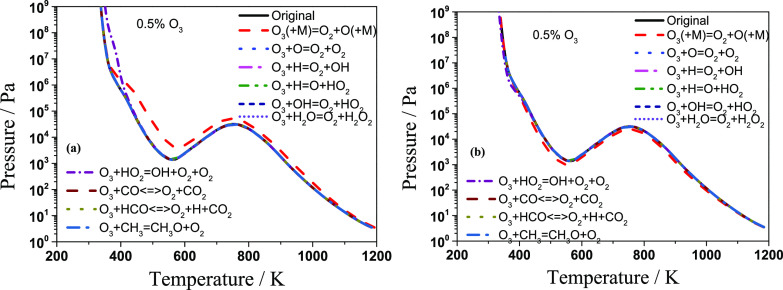
To find the dominant O3-involved elementary reaction affecting the explosion limit of the C3H8/O3/O2 mixtures, the chemical reaction rate perturbation method was used to study the sensitivity of each elementary reaction on the explosion limit. More specifically, in each calculation, a 90% decrease or a corresponding increase of 90% of the original reaction rate was conducted individually for the ten O3-involved reactions; then, the explosion limits calculated by the modified mechanism were compared with the explosion limit calculated by the original mechanism. The reaction that can lead to the most significant deviation of the explosion limit from the original explosion limit is the reaction with the largest sensitivity. The explosion limits calculated by the perturbed and original mechanisms are compared in Figure 6a,b. It was found that only two reactions have a significant effect on the explosion limit of the C3H8/O3/O2 mixtures.
Figure 6.
Sensitivity of the main reactions involving ozone: (a) 90% decrease of the original rate and (b) corresponding increase of 90% of the reaction rate.
The most sensitive reaction to the overall trend of the explosion limit is the O radical generation reaction through the decomposition of O3. Since this unimolecular reaction has a first-order with low activation energy, it will proceed faster than the other radical involved in the higher-order reaction.35 The produced O radical will react with propane to generate propyl and OH radicals. Then, propane will react with OH and O radicals through the hydrogen-abstraction reactions. Consequently, the overall reaction rate is greatly improved. However, when the ozone was not present in the mixtures, the reaction will be initiated by the reaction of propane with O2 through the H-abstraction reaction. Therefore, the initial reactivity of the system is lower.
When the temperature is lower than the bottom temperature in the cool flame region, the explosion pressure increases monotonically with a decrease in temperature. In addition, the effect of the third-body reaction increases gradually with an increase in pressure. When the pressure increases to a certain extent, the effect of the reverse reaction of O3 + (M) = O2 + O + (M) is comparable with its forward reaction. Therefore, the effect of the ozone unimolecular decomposition reaction on the explosion limit will gradually decrease until it disappears with an increase in pressure. However, the increased pressure will promote the HO2 generation rate through the rapid third-body reaction H + O2 + M = HO2 + M.32 This makes the reaction O3 + HO2 = OH + O2 + O2 to have a significant effect on the explosion limit in the high-pressure and low-temperature region, as the concentration of HO2 is greatly increased. Otherwise, the consumption rate of HO2 is dominantly controlled by 2HO2 = H2O2 + O2 and the radical wall destruction reaction of HO2 in the higher-pressure regime where the temperature is low.36
The effect of pressure on the forward and backward reaction rates of the ozone decomposition reaction, O3 (+M) = O2 + O (+M), has been investigated. The ratio of these two reaction rates is given in Figure 7. At the very beginning of the reaction process, this rate ratio is very large, which is larger than 1015. This is because the concentration of the O radical is almost zero during the initial stage, which results in a very small backward reaction rate. As the reaction is initiated, the concentration of the O radical is increased, which leads to a sharp reduction in the reaction rate ratio. After that, the reaction rate ratio gradually reduces to 1.0, which means that the forward and backward reactions reach an equilibrium state. However, for the nonexplosion case (low-pressure condition), the ratio stays around 3.0 × 104, which is because O3 is not completely consumed. It should be noticed that the ratio decreases with an increase in pressure, which means the effect of the reverse third-body reaction increases.
Figure 7.
Forward and backward reaction ratios of the ozone decomposition reaction.
To further investigate the effect of ozone addition on the explosion limit of the C3H8/O3/O2 mixtures, the reaction pathway analysis was conducted to identify the key reaction path of the mixtures with different ozone fractions (0.0 and 0.5%). The initial temperature and pressure were chosen as 600 K and 100 000 Pa, respectively. Only the reaction paths with the relative (to the total) rate of production values for each species larger than 5% are retained in the figure. As shown in Figure 8a, when there is no ozone in the mixture, propane is mainly consumed through the H-abstraction reaction by OH radicals. Two isomers normalpropyl (n-C3H7) and isopropyl (i-C3H7) are generated, which account for 46.4 and 47.6% of the propane consumption rate, respectively. Then, isopropyl reacts through the following route: IC3H7 → IC3H7O2 → IC3H7O2H → IC3H7O → CH3 → CH3O2 → CH3O2H → CH3O. The normalpropyl reaction route is NC3H7 → NC3H7O2 → C3H6OOH → C3H6OOHO2 → C3ket → CH2CHO → O2CH2CHO → HO2CH2CO → CO. When ozone is added to the mixture, two extra propane consumption paths appear, which are the H-abstraction reactions by O radicals, as shown in Figure 8b. They account for 21.6 and 12.3% of the propane consumption rate. Furthermore, the propane consumption rates through the OH radicals are reduced to 31.1 and 31.8%, respectively. It should be noticed that another OH radical can be generated through the H-abstraction reaction by the O radical, which can significantly increase the reactivity of the mixture. The reaction path of the generated normalpropyl and isopropyl is quite similar to that of the mixture without ozone.
Figure 8.
Effect of ozone addition on the propane oxidation pathway: (a) stoichiometric C3H8/O2 mixtures and (b) stoichiometric C3H8/O2/O3 mixture with 0.5% O3. Initial condition: 600 K, 100 000 Pa. Pathway output condition: 650 K.
4. Conclusions
In this paper, the effect of ozone addition on explosion limit profiles of the C3H8/O2 mixtures is studied systematically. The result shows that when minute quantities of ozone were added in the system, less than or equal to 0.8% of the mole fraction of ozone for stoichiometric mixtures, the explosion limit is extended to the low-temperature region, especially in the high-pressure region. In addition, the explosion limit profile remains S-shaped. However, when the mole fraction of ozone is further increased from 0.8 to 1.0%, the NTC response no longer exists. When the mole fraction of ozone is larger than or equal to 1.0%, a monotonic explosion limit appears. The sensitivity analysis of the main reactions involving ozone reveals that the most sensitive reaction is the O radical generation reaction through the decomposition of O3. However, the effect of the ozone unimolecular decomposition reaction on the explosion limit will gradually decrease until it disappears with an increase in pressure. In addition, the reaction O3 + HO2 = OH + O2 + O2 has a significant effect on the explosion limit in the high-pressure and low-temperature region. The dominant pathway of the oxygen radical from the low temperature is the chain-branching reaction of the oxygen radical with propane.
Acknowledgments
This study is supported by the International Science and Technology Cooperation of China (No. 2019YFE0100200) and the National Natural Science Foundation of China (No. 51406007).
The authors declare no competing financial interest.
References
- Guo J.; Peng W.; Zhang S.; Lei J.; Jing J.; Xiao R.; Tang S. Comprehensive Comparison of the Combustion Behavior for Low-Temperature Combustion of n-Nonane. ACS Omega 2020, 5, 4924–4936. 10.1021/acsomega.9b03786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newitt D. M.; Thornes L. S. The Oxidation of Propane. Part I. The Products of the Slow Oxidation at Atmospheric and at Reduced Pressures. J. Chem. Soc. 1937, 0, 1656–1665. 10.1039/jr9370001656. [DOI] [Google Scholar]
- Gallagher S. M.; Curran H. J.; Metcalfe W. K.; Healy D.; Simmie J. M.; Bourque G. A rapid compression machine study of the oxidation of propane in the negative temperature coefficient regime. Combust. Flame 2008, 153, 316–333. 10.1016/j.combustflame.2007.09.004. [DOI] [Google Scholar]
- Dames E. E.; Rosen A. S.; Weber B. W.; Gao C. W.; Sung C.-J.; Green W. H. A detailed combined experimental and theoretical study on dimethyl ether/propane blended oxidation. Combust. Flame. 2016, 168, 310–330. 10.1016/j.combustflame.2016.02.021. [DOI] [Google Scholar]
- Hamid H.; Jakob M. C.; Lawrence B. H.; Stephen J. K.; Peter G. High-pressure oxidation of propane. Proc. Combust. Inst. 2019, 37, 461–468. 10.1016/j.proci.2018.07.009. [DOI] [Google Scholar]
- Herzler J.; Jerig L.; Roth P. Shock-tube study of the ignition of propane at intermediate temperatures and high pressures. Combust. Sci. Technol. 2004, 176, 1627–1637. 10.1080/00102200490487201. [DOI] [Google Scholar]
- Cadman P.; Thomas G. O.; Butler P. The auto-ignition of propane at intermediate temperatures and high pressures. Phys. Chem. Chem. Phys. 2000, 2, 5411–5419. 10.1039/b003665j. [DOI] [Google Scholar]
- Lam K.-Y.; Hong Z.; Davidson D. F.; Hanson R. K. Shock tube ignition delay time measurements in propane/O2/argon mixtures at near-constant-volume conditions. Proc. Combust. Inst. 2011, 33, 251–258. 10.1016/j.proci.2010.06.131. [DOI] [Google Scholar]
- Yu R.; Liu J.; Ma B. The dependence of NTC behavior on the equivalence ratio and nitrogen fraction in cool flame region. Fuel 2020, 271, 117623 10.1016/j.fuel.2020.117623. [DOI] [Google Scholar]
- Liang W.; Law C. K. Extended flammability limits of n-heptane/air mixtures with cool flames. Combust. Flame 2017, 185, 75–81. 10.1016/j.combustflame.2017.06.015. [DOI] [Google Scholar]
- Ju Y. On the propagation limits and speeds of premixed cool flames at elevated pressures. Combust. Flame 2017, 178, 61–69. 10.1016/j.combustflame.2017.01.006. [DOI] [Google Scholar]
- Fairlie R.; Griffiths J. F.; Hughes K. J.; Pearlman H. Cool flames in space: experimental and numerical studies of propane combustion. Proc. Combust. Inst. 2005, 30, 1057–1064. 10.1016/j.proci.2004.08.081. [DOI] [Google Scholar]
- Merchant S. S.; Goldsmith C. F.; Vandeputte A. G.; Burke M. P.; Klippenstein S. J.; Green W. H. Understanding low-temperature first-stage ignition delay: Propane. Combust. Flame. 2015, 162, 3658–3673. 10.1016/j.combustflame.2015.07.005. [DOI] [Google Scholar]
- Bai S.; Davis M. J.; Sivaramakrishnan R.; Skodje R. T. A chemical pathway perspective on the kinetics of low-temperature ignition of propane. Combust. Flame 2019, 202, 154–178. 10.1016/j.combustflame.2019.01.006. [DOI] [Google Scholar]
- Kobashi Y.; Wang Y.; Shibata G.; Ogawa H.; Naganuma K. Ignition control in a gasoline compression ignition engine with ozone addition combined with a two-stage direct-injection strategy. Fuel 2019, 249, 154–160. 10.1016/j.fuel.2019.03.101. [DOI] [Google Scholar]
- Pinazzi P. M.; Foucher F. Influence of injection parameters, ozone seeding and residual NO on a Gasoline Compression Ignition (GCI) engine at low load. Proc. Combust. Inst. 2017, 36, 3659–3668. 10.1016/j.proci.2016.06.075. [DOI] [Google Scholar]
- Foucher F.; Higelin P.; Mounaïm-Rousselle C.; Dagaut P. Influence of ozone on the combustion of n-heptane in a HCCI engine. Proc. Combust. Inst. 2013, 34, 3005–3012. 10.1016/j.proci.2012.05.042. [DOI] [Google Scholar]
- Alfazazi A.; Al-Omier A.; Secco A.; Selim H.; Ju Y.; Sarathy S. M. Cool diffusion flames of butane isomers activated by ozone in the counter flow. Combust. Flame 2018, 191, 175–186. 10.1016/j.combustflame.2017.12.034. [DOI] [Google Scholar]
- Hajilou M.; Ombrello T.; Won S. H.; Belmont E. Experimental and numerical characterization of freely propagating ozone-activated dimethyl ether cool flames. Combust. Flame 2017, 176, 326–333. 10.1016/j.combustflame.2016.11.005. [DOI] [Google Scholar]
- Alam F. E.; Won S. H.; Dryer F. L.; Farouk T. I. Ozone assisted cool flame combustion of sub-millimeter sized n-alkane droplets at atmospheric and higher pressure. Combust. Flame 2018, 195, 220–231. 10.1016/j.combustflame.2018.01.015. [DOI] [Google Scholar]
- Won S. H.; Jiang B.; Diévart P.; Sohn C. H.; Ju Y. Self-sustaining n-heptane cool diffusion flames activated by ozone. Proc. Combust. Inst. 2015, 35, 881–888. 10.1016/j.proci.2014.05.021. [DOI] [Google Scholar]
- Hajilou M.; Belmont E. Characterization of ozone-enhanced propane cool flames at sub-atmospheric pressures. Combust. Flame 2018, 196, 416–423. 10.1016/j.combustflame.2018.07.001. [DOI] [Google Scholar]
- Sohn C. H.; Son J. W.; Won S. H.; Ju Y. Computational studies of diffusion cool flame structures of n-heptane with/without ozone sensitization with a reduced chemistry. J. Mech. Sci. and Technol. 2015, 29, 1297–1305. 10.1007/s12206-015-0245-4. [DOI] [Google Scholar]
- Keum S.; Idicheria C. A.; Najt P. M.; Kuo T.-W. A skeletal iso-octane reaction mechanism for low temperature plasma ignition with ozone surrogate. Proc. Combust. Inst. 2017, 36, 4129–4136. 10.1016/j.proci.2016.08.035. [DOI] [Google Scholar]
- Sun W.; Gao X.; Wu B.; Ombrello T. The effect of ozone addition on combustion: Kinetics and dynamics. Prog. Energy Combust. Sci. 2019, 73, 1–25. 10.1016/j.pecs.2019.02.002. [DOI] [Google Scholar]
- Lutz A. E.; Kee R. J.; Miller J. A.. SENKIN: a FORTRAN Program for Predicting Homogeneous Gas Phase Chemical Kinetics with Sensitivity Analysis, Sandia National Laboratories Report SAND, 1988; pp 87–8248.
- Atef N.; Kukkadapu G.; Mohamed S. Y.; Rashidi M. A.; Banyon C.; Mehl M.; Heufer K. A.; Nasir E. F.; Alfazazi A.; Das A. K.; Westbrook C. K.; Pitz W. J.; Lu T.; Farooq A.; Sung C.-J.; Curran H. J.; Sarathy S. M. A comprehensive iso-octane combustion model with improved thermochemistry and chemical kinetics. Combust. Flame 2017, 178, 111–134. 10.1016/j.combustflame.2016.12.029. [DOI] [Google Scholar]
- Li Y.; Zhou C. W.; Somers K. P.; Zhang K.; Curran H. J. The Oxidation of 2-Butene: A High Pressure Ignition Delay, Kinetic Modeling Study and Reactivity Comparison with Isobutene and 1-Butene. Proc. Combust. Inst. 2017, 36, 403–411. 10.1016/j.proci.2016.05.052. [DOI] [Google Scholar]
- Hashemi H.; Christensen J. M.; Harding L. B.; Klippenstein S. J.; Glarborg P. High-pressure oxidation of propane. Proc. Combust. Inst. 2019, 37, 461–468. 10.1016/j.proci.2018.07.009. [DOI] [Google Scholar]
- Seiser R.; Pitsch H.; Seshadri K.; Pitz W. J.; Curran H. J. Extinction and autoignition of n-Heptane in counter flow configuration. Proc. Combust. Inst. 2000, 28, 2029–2037. 10.1016/S0082-0784(00)80610-4. [DOI] [Google Scholar]
- Zhao H.; Yang X.; Ju Y. Kinetic studies of ozone assisted low temperature oxidation of dimethyl ether in a flow reactor using molecular-beam mass spectrometry. Combust. Flame 2016, 173, 187–194. 10.1016/j.combustflame.2016.08.008. [DOI] [Google Scholar]
- Liu J.; Wang J.; Zhang N.; Zhao H. On the explosion limit of syngas with CO2 and H2O additions. Int. J. Hydrogen Energy 2018, 43, 3317–3329. 10.1016/j.ijhydene.2017.12.176. [DOI] [Google Scholar]
- Guo J.; Peng W.; Zhang S.; Lei J.; Jing J.; Xiao R.; Tang S. Comprehensive Comparison of the Combustion Behavior for Low-Temperature Combustion of n-Nonane. ACS Omega 2020, 5, 4924–4936. 10.1021/acsomega.9b03786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newitt D. M.; Thornes L. S. The oxidation of propane. Part III. The kinetics of the oxidation. J. Chem. Soc. 1937, 350, 1669–1676. 10.1039/jr9370001669. [DOI] [Google Scholar]
- Liang W.; Wang Y.; Law C. K. Role of ozone doping in the explosion limits of hydrogen-oxygen mixtures: Multiplicity and catalyticity. Combust. Flame 2019, 205, 7–10. 10.1016/j.combustflame.2019.03.038. [DOI] [Google Scholar]
- Sánchez A. L.; Fernández-Tarrazo E.; Williams F. A. The chemistry involved in the third explosion. Combust. Flame 2014, 161, 111–117. 10.1016/j.combustflame.2013.07.013. [DOI] [Google Scholar]