Abstract
Objectives:
The global incidence of HIV infection is not significantly decreasing, especially in sub-Saharan African countries. Though there is availability and accessibility of free HIV services, people are not being diagnosed early for HIV, and hence HIV-related mortality remains significantly high. We formulate a mathematical model for the spread of HIV using non linear ordinary differential equations in order to investigate the impact of late diagnosis of HIV on the spread of HIV.
Results:
The results suggest the need to encourage early initiation into HIV treatment as well as promoting HIV self-testing programs that enable more undiagnosed people to know their HIV status in order to curtail the continued spread of HIV.
Keywords: HIV, ART treatment, Basic reproduction number, Stability analysis, Numerical simulations
Introduction
Antiretroviral therapy (ART) has successfully transformed human immunodeficiency virus (HIV) infection from a fatal to a manageable chronic disease [1]. Nonetheless, there remains critical factors to be addressed along with the roll out of effective ART regimens in order to eradicate HIV. We seek to investigate the impact of late diagnosis on the transmission dynamics of HIV. Mathematical modeling of HIV dynamics is quite advanced, see for instance the following works on HIV and the references therein [2–9].
We extend a more recent HIV/AIDS mathematical model developed by Omondi et al. [8] to investigate the impact of late diagnosis on the spread and control of HIV. In their work, Omondi et al. [8] proposed a five state deterministic compartmental model for the time evolution of population states to study the trend of HIV infection in Kenya. The model was premised on dividing the infected classes according to CD4+ T cell counts in the blood. For more information about the description of parameters and model analysis, readers are referred to Omondi et al. [8].
The paper is arranged as follows; in "Main text" section, we formulate and establish the basic properties of the model. The model is analysed for stability in this section. In "Results and discussion" section, we carry out some numerical simulations. Parameter estimation and numerical results are also presented in this section. The paper is concluded in "Conclusions" section.
Main text
The model
We propose a five state compartmental model for HIV that takes into account untimely initiation of HIV positive individuals into ART. The human population comprises classes; S(t), , , and . The class S(t) represents the population at high risk of HIV infection. Upon acquiring HIV infection, susceptible individuals move to infection class which is divided into two stages according to CD4+ T cell count in the blood. The infectives class comprise of individuals with CD4+ T cell count /μL. Individuals in class are assumed to be having a lower viral load and hence are considered to be the new infections. Individuals in class progress to the second stage of infection at a rate given by . This class consists of individuals with CD4+ T cell count in the range /μL. Individuals in this stage are assumed to be having high viral load. Individuals in class are initiated into ART treatment at a rate given by . In this paper, we develop a mathematical model that takes into account the effect of late initiation into ART treatment of HIV positive patients. We define initiation of HIV positive individuals in stage into ART treatment by the expression
| 1 |
Here, represent the maximum treatment uptake per unit of time for individuals in class and r measures the extent of the effect of late initiation into ART treatment. Firstly, observe that for small , . Secondly, observe that for large , . Finally, when , we obtain , which is the case considered in Omondi et al. [8]. Individuals in class move to the class through a deteriorative process at a rate given by whereas individuals in class move to the class through an ameliorative process at a rate given by . In this model, we exclude the class of full blown AIDS patients as these are usually hospitalised and/or sexually inactive and hence their contribution to new HIV infections is negligible [8]. The total human population is thus given by
Susceptible humans are recruited into the system through births or immigration at a constant rate . Susceptible individuals acquire new HIV infections at a rate given by
| 2 |
where , , and denote the HIV transmission rates between susceptible individuals and infectious individuals. We assume that individuals in each compartment are indistinguishable and there is homogeneous mixing. Individuals in classes and experience disease related death at rates given respectively by and . The natural death rate of the general population is represented by . The differential equations for the model are given as follows;
| 3 |
with the initial conditions:
where we assume that all the model parameters are positive.
Analysis of the model
Positivity of solutions
The following theorem (Theorem 1) entails that all the state variables remain non-negative and the solutions of system (3) with positive initial conditions will remain positive for all .
Theorem 1
Given that the initial conditions of system (3) are, , , and. There existswhich solve system (3).
For more details on the proof of Theorem 1, we refer the reader to [8].
Invariant region
The feasible region for system (3) is given by
| 4 |
Results to verify that the region is positively invariant with respect to system (3) can be obtained as given in [8].
Disease-free equilibrium and the basic reproduction number
The model has a disease-free equilibrium given by
a scenario depicting a disease-free state in the community or society. The basic reproduction number of the model, is defined herein as the average number of people infected by each HIV infected individual during his/her infectious period in a population of completely susceptible individuals. The determination of is done using the next generation matrix approach [10]. It works out that, the basic reproduction number of system (3) is given by:
| 5 |
Here, the four sub-reproduction numbers , , and represent the contributions of individuals in compartments , , and on the spread of HIV infection respectively. We can clearly note that is non-negative as which implies that .
Local stability of the disease-free steady state
The following theorem follows from van den Driessche and Watmough [10] (Theorem 2).
Theorem 2
The disease-free equilibrium pointof model system equations (3) is locally asymptotically stable ifand is unstable if.
Endemic equilibrium
The endemic equilibrium denoted by satisfies
| 6 |
From the first, third, fourth and fifth equation of (6), we have expressed in terms of as follows
| 7 |
Substituting expressions (7) into the second equation of (6) leads to the following fourth order polynomial equation
| 8 |
Solving (8) gives which corresponds to the disease-free equilibrium or
| 9 |
where the coefficients , are given in (10).
| 10 |
We can clearly note that, and . We now determine the number of possible positive real zeros of the polynomial (10) using the Descartes Rule of Signs. The possibilities can be presented as shown below. Here, the number of possible positive real zeros is denoted by .
| 0 | 1 | 2 | 1 | 2 | 3 | 2 | 1 | |
Backward bifurcation
Theorem 4.1 proven in Castillo-Chavez and Song [11] will be useful. We show that system (3) undergoes a backward bifurcation. Let us make the following change of variables:
, so that . We now use the vector notation . Then, system (3) can be written in the form
, where
| 11 |
We now define
| 12 |
with signifying that the chance of acquiring HIV infection upon contact with individuals in class or upon contact with individuals in classes , and is the same, signifying a reduced chance of acquiring HIV infection upon contact with individuals in classes , and as compared to individuals in class , signifies an increased rate of acquiring HIV infection upon contact with individuals in classes , and as compared to individuals in class .
Let be the bifurcation parameter, corresponds to
| 13 |
The Jacobian matrix of model system (3) at when is given by
where , , and are defined as before.
Model system (11), with has a simple eigenvalue, hence the center manifold theory can be used to analyse the dynamics of model system (3) near . It can be shown that , has a right eigenvector given by , where
Here, we note that and . Further, the left eigenvector of , associated with the zero eigenvalue at is given by , where
Here, take note that , accordingly as and , accordingly as . Also, and .
The computations of a and b are necessary in order to apply Theorem 4.1 in Castillo-Chavez and Song [11]. For system (11), the associated non-zero partial derivatives of F at the disease-free equilibrium are given in (14).
| 14 |
It thus follows that
where
Note that if , then and if then . Lastly,
We thus have the following result
Theorem 3
If, then system (3) has a backward bifurcation at. Otherwise, ifthe endemic equilibrium is locally asymptotically stable forbut close to one.
We show the existence of a backward bifurcation through numerical example by creating bifurcation diagram around (Fig. 1). To draw a bifurcation curve (the graph of as a function of ), we fix the following parameters for illustrative purposes: .
Fig. 1.
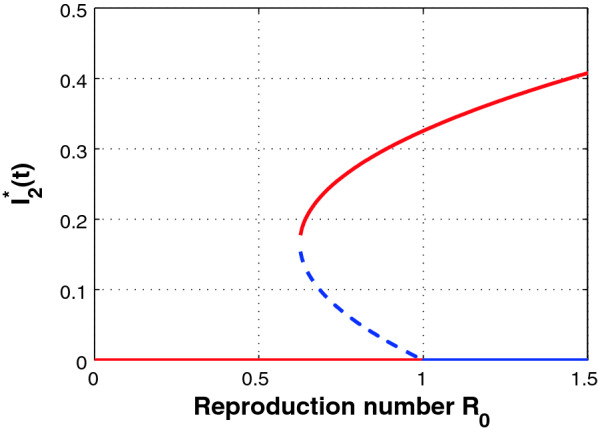
The figure showing a backward bifurcation. The solid lines denote stable states and the dotted lines denote unstable states
Remark
Epidemiologically, when a model exhibits backward bifurcation, this entails that it is not enough to only reduce the basic reproductive number to less than one in order to eliminate the disease.
Results and discussion
Numerical simulations
We carry out numerical simulations to support our theoretical findings.
Estimation of parameters
Parameter values used for numerical simulations are given in Table 1.
Table 1.
Parameter values used in numerical simulations
| Parameter | Definition | Range | Value | Source |
|---|---|---|---|---|
| Contact for individuals in S with those in | 0-1 | 0.912 | [8] | |
| Contact for individuals in S with those in | 0-1 | 0.894 | [8] | |
| Contact for individuals in S with those in | 0.095 | [8] | ||
| Contact for individuals in S with those in | 0.091 | [8] | ||
| Progression from to | 0.084 | [8] | ||
| Progression from to | 0.1 | Assumed | ||
| Progression from to | 1.0 | [8] | ||
| Progression from to | 0.096 | [8] | ||
| Progression from to | 0.112 | [8] | ||
| r | Effect of late initiation into ART | 0.45 | Assumed | |
| Disease related death of individuals in | 0.089 | [7] | ||
| Disease related death of individuals in | 0.095 | [7] | ||
| Recruitment rate into S | 0.0239 | [12–14] | ||
| Natural death rate | 0.0172 | [14] |
Numerical results
Figure 2 illustrates the effect of varying the parameter r on the prevalence of HIV. We note that increasing the parameter r results in an increase in the prevalence of HIV. In particular, increasing r from 0.1 up to 1.0 increases the prevalence rate of HIV with a level of approximately . This is a reflection that late diagnosis of HIV contributes to an increase in HIV infections. Thus, more effort should be directed towards encouraging individuals to get tested for HIV and ensuring those who are positive are timely initiated into ART treatment.
Fig. 2.
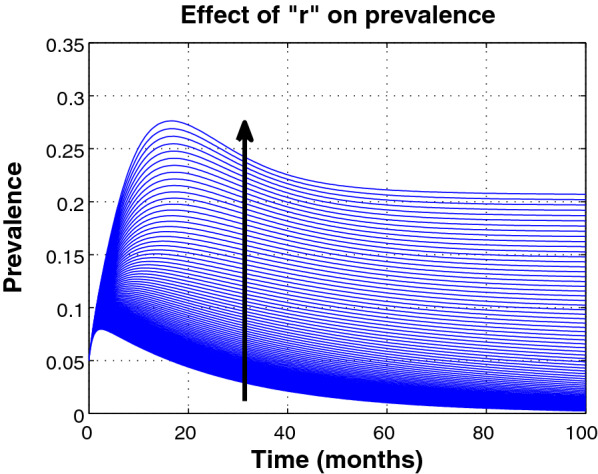
Effect of varying parameter r on the prevalence of HIV, starting from 0.1 up to 1.0 with a step size of 0.01
Conclusions
A mathematical model that describes the dynamics of HIV/AIDS has been formulated using nonlinear ordinary differential equations. The model takes into account the impact of late diagnosis on HIV/AIDS transmission dynamics. Initiation into ART treatment of individuals with a CD4+ T cell count in the range 200–350\μ L has been described by the function (1). The model developed in this paper fits well with settings in most underdeveloped countries where stigma of HIV remains prevalent. Inclusion of the treatment function (1) increases the realism of the model developed by [8] and leads to some interesting dynamical aspects such as the occurrence of backward bifurcation.
In this study, it has been shown that the classical —threshold is not the key to control the spread of HIV infection within a population. In fact HIV infection may persist in the population even with subthreshold values of . Our results suggest that considerable effort should be directed towards encouraging early initiation into ART in order to reduce HIV prevalence. For instance, strategies such as the implementation of HIV self-testing programs would be of great help in the fight against HIV.
Limitations
Like in any model development, the model is not without limitations. The model can be extended to include the contribution of pre-exposure prophylaxis (PrEP) and other control measures not considered in the work.
Acknowledgements
The author acknowledges, with thanks, the support of the Department of Mathematics, University of Zimbabwe for the production of this manuscript.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- HIV
Human immunodeficiency virus
- ART
Antiretroviral therapy
Authors’ contributions
JM participated in the fomulation, analysis and drafting of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Estimation of parameters have been stated throughout the body of the paper and included in the reference section. The graphs were produced using the MATLAB software that is available from https://www.mathworks.com/products/matlab.html.
Ethics approval and consent to participate
No ethical approval was required for this project as this is secondary research.
Consent to publish
Not applicable.
Competing interests
The author declares there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baryarama F, Mugisha J, Luboobi L. A mathematical model for the dynamics of HIV/AIDS with gradual behaviour change. Comput Math Methods Med. 2006;7(1):15–26. doi: 10.1080/10273660600906960. [DOI] [PubMed] [Google Scholar]
- 3.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model as an example. Int Stat Inst. 1994;62:229–243. doi: 10.2307/1403510. [DOI] [Google Scholar]
- 4.Mukandavire Z, Nyabadza F, Chiyaka C, Hove-Musekwa SD. Analysis of an HIV/AIDS model with public-health information campaigns and individual withdrawal. J Biol Syst. 2010;18:1–19. [Google Scholar]
- 5.Okango E, Mwambi H, Ngesa O. Spatial modeling of HIV and HSV-2 among women in Kenya with spatially varying coefficients. BMC Pub Health. 2016;16(1):355–368. doi: 10.1186/s12889-016-3022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okongo M, Kirimi J, Murwayi A, Muriithi D. Mathematical analysis of a comprehensive HIV/ AIDS model: treatment versus vaccination. Appl Math Sci. 2013;7(54):2687–2707. [Google Scholar]
- 7.Okosun K, Makinde O, Takaidza I. Impact of optimal control on the treatment of HIV/AIDS and screening of unaware infectives. Appl Math Model. 2013;37(6):3802–3820. doi: 10.1016/j.apm.2012.08.004. [DOI] [Google Scholar]
- 8.Omondi EO, Mbogo RW, Luboobi LS. Modelling the trend of HIV transmission and treatment in Kenya. Int J Appl Comput Math. 2018;4:123. doi: 10.1007/s40819-018-0558-y. [DOI] [Google Scholar]
- 9.Wodarz D, Nowak MA. Mathematical models of HIV pathogenesis and treatment. BioEssays. 2002;24(12):1178–1187. doi: 10.1002/bies.10196. [DOI] [PubMed] [Google Scholar]
- 10.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for the compartmental models of disease transmission. Math Biosci. 2002;180:29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 11.Castillo-Chavez C, Song B. Dynamical models of tuberculosis and their applications. Mathe Biosci Eng. 2004;1(2):361–404. doi: 10.3934/mbe.2004.1.361. [DOI] [PubMed] [Google Scholar]
- 12.KNBS: Kenya National Bureau of Statistics. 2018. https://www.knbs.or.ke/.
- 13.KNBS2: Kenya 1900. Population Pyramids of the World from 1950 to 2100. 2018. https://www.populationpyramid.net/kenya/1990/.
- 14.WB: World Bank Data. Birth rate, crude (per 1000 people). 2018. https://data.worldbank.org/indicator/SP.DYN.CBRT.IN.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Estimation of parameters have been stated throughout the body of the paper and included in the reference section. The graphs were produced using the MATLAB software that is available from https://www.mathworks.com/products/matlab.html.


