Abstract
Background
Unrestricted use of pesticides in agriculture is likely to increase insecticide resistance in mosquito vectors. Unfortunately, strategies for managing insecticide resistance in agriculture and public health sectors lack integration. This study explored the types and usage of agricultural pesticides, and awareness and management practices among retailers and farmers in Ulanga and Kilombero districts in south-eastern Tanzania, where Anopheles mosquitoes are resistant to pyrethroids.
Methods
An exploratory sequential mixed-methods approach was employed. First, a survey to characterize pesticide stocks was conducted in agricultural and veterinary (agrovet) retail stores. Interviews to assess general knowledge and practices regarding agricultural pesticides were performed with 17 retailers and 30 farmers, followed by a survey involving 427 farmers. Concurrently, field observations were done to validate the results.
Results
Lambda-cyhalothrin, cypermethrin (both pyrethroids) and imidacloprids (neonicotinoids) were the most common agricultural insecticides sold to farmers. The herbicide glyphosate (amino-phosphonates) (59.0%), and the fungicides dithiocarbamate and acylalanine (54.5%), and organochlorine (27.3%) were also readily available in the agrovet shops and widely used by farmers. Although both retailers and farmers had at least primary-level education and recognized pesticides by their trade names, they lacked knowledge on pest control or proper usage of these pesticides. Most of the farmers (54.4%, n = 316) relied on instructions from pesticides dealers. Overall, 93.7% (400) farmers practised pesticides mixing in their farms, often in close proximity to water sources. One-third of the farmers disposed of their pesticide leftovers (30.0%, n = 128) and most farmers discarded empty pesticide containers into rivers or nearby bushes (55.7%, n = 238).
Conclusion
Similarities of active ingredients used in agriculture and malaria vector control, poor pesticide management practices and low-levels of awareness among farmers and pesticides retailers might enhance the selection of insecticide resistance in malaria vectors. This study emphasizes the need for improving awareness among retailers and farmers on proper usage and management of pesticides. The study also highlights the need for an integrated approach, including coordinated education on pesticide use, to improve the overall management of insecticide resistance in both agricultural and public health sectors.
Keywords: Malaria Vector, Agricultural practices, Lambda-cyhalothrin, Chlorpyrifos, Chlorothalonil, Imidacloprid, Glyphosate, Pesticides knowledge, Insecticide resistance, Malaria
Background
The control of malaria and other vector-borne diseases relies primarily on insecticide-based interventions, such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [1, 2]. The effectiveness of these interventions is being compromised by the increased geographical spread of insecticide in the targeted mosquito populations [3, 4]. Insecticide-resistance by mosquito populations to the limited number of insecticides approved for vector control has been implicated as the key driver of persistent malaria transmission [5, 6].
Insecticide resistance in malaria vectors is predominantly attributed to exposure of mosquitoes to public health insecticides [3, 4]. However, agricultural pesticides also exert strong selection pressures, thus contributing to resistance in vector species [7–14]. This is because of similarities in chemicals used, applications of these chemicals simultaneously, and their indiscriminate use in agriculture [15]. This phenomenon was observed in West Africa where Anopheles gambiae sensu lato (s.l.) populations sampled from farmlands characterized by high agriculture pesticide usage showed higher levels of resistance to insecticides compared to populations sampled in areas with limited or no agricultural pesticide usage [11–13, 16]. Similarly, in Sudan agricultural usage of organophosphate and carbamates was linked to insecticide resistance in Anopheles arabiensis [17]. Aquatic exposures of mosquito larvae to sub-lethal doses of pesticides, herbicides and other pollutants have also been linked to higher tolerance to insecticides in malaria vectors [9, 18–20]. Furthermore, Chouaïbou et al. found that over 90% of the insecticides used by vegetable and rice farmers in the southern part of Côte d’Ivoire were pyrethroids similar to those approved for vector control [21].
In many malaria endemic countries, agriculture is the main economic activity. To improve crop yields in these regions there is the rampant use of pesticides, fungicides and herbicides [22–24]. For example, in Tanzania, approximately 81% of pesticides are deployed in both agricultural and veterinary sectors [25]. Concurrently, pyrethroid impregnated LLINs are also widely used against disease vectors in these regions.
The World Health Organization (WHO) Global Malaria Programme has developed a global action plan for insecticide resistance management in malaria vectors to preserve the effectiveness of LLINs and IRS [26]. The principal recommended resistance management approaches, mostly adopted from agriculture include: (i) annual rotation of insecticides with different modes of action; (ii) combination of pyrethroid-based LLINs and IRS with non-pyrethroids; (iii) mosaic spraying of two different insecticide classes in different geographical locations; and (iv) mixtures of different classes of insecticides into a single product [26]. However, resistance management policies have yet to be integrated into agricultural and disease control programmes. As a result, the programmes do not account for the collective contributions by both public health and agricultural sectors to the spread of insecticide resistance.
The purpose of this study was to explore agricultural pesticides, pesticide usage practices, awareness, and management practices among retailers and farming communities from a rural malaria endemic area in south-eastern Tanzania, where mosquito vectors are resistant to public health insecticides [27, 28]. The findings are expected to guide practical recommendations for collaboration between agriculture and public health sectors in insecticide resistance management in mosquito vectors and disease control.
Methods
Study area
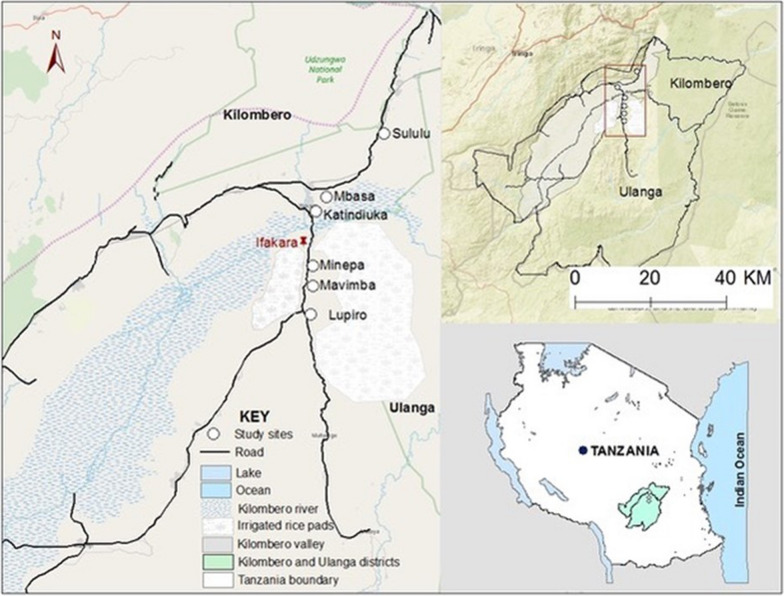
The study was conducted in six wards, in Kilombero and Ulanga districts, south-eastern Tanzania (altitude ~ 300 m; annual precipitation: 1200–1800 mm; temperatures: 20–32 °C), purposefully selected to represent different agro-ecological areas (Fig. 1). Rice farming is the main economic activity of the area [29]. Vegetable and fruit cultivation is also quite common. Farmers here widely use synthetic pesticides and chemical fertilisers. During the dry season, rice production is maintained by irrigation (locally known as “Ngapa”) rendering the area continuously favourable for mosquitoes [30]. Malaria burden remains significant, with the heaviest burden experienced in children below 5 years [31, 32]. Anopheles funestus sensu stricto (s.s.) and An. arabiensis are the predominant malaria vectors [27, 28]. Additionally, non-malaria vectors, such as Culex and Mansonia, constitute biting nuisances [33, 34]. Though pyrethroid-based LLINs are the main malaria intervention [35], mosquito populations are resistant to pyrethroids, bendiocarb (carbamates), and DDT [27, 28, 34].
Fig. 1.
Map of south-eastern Tanzania showing the study wards in the districts of Kilombero and Ulanga in the Kilombero Valley
Study design and data collection
An exploratory sequential mixed-methods study design was employed. In-depth interviews were done for collecting qualitative data and structured questionnaires were used to collect quantitative data (Additional file 1). Both data collection tools were prepared in English, translated and used in Kiswahili the local native language. The questionnaires were pre-tested on a few participants (who were not otherwise enrolled in the actual study) to ensure clarity before the actual study. Direct observations were made and photographs taken of the pesticides in the stores to identify their active ingredients, and handling practices. In the farms prior observations were validated on pesticides usage and handling practices. Data collection was conducted between February 2017 and November 2017.
Exploration of awareness and perceptions of pesticides use, storage and disposal
In-depth interviews were conducted with agricultural and veterinary (agrovet) retail stores (n = 17) and with famers in the six wards (n = 30). With the retailers, the interviews aimed to explore awareness of pesticide prescription and handling practices. Interview guides explored the retailers’ awareness and perceptions of (i) types of agricultural pesticides, knowledge of pesticides sold at their shops; and (ii) source of knowledge on using the pesticides, pesticides preferences, frequency of purchases and seasonal use of the pesticides/frequency of applications. With the community members, the interviews explored awareness and perceptions regarding different agricultural pesticides, use and storage methods, and challenges faced. Direct observations of agricultural practices in the farms, including handling and disposal practices of the pesticides were also done. Initial findings from these qualitative studies informed subsequent quantitative studies. All interviews were audio-recorded and field notes taken by the data collector.
Assessment of knowledge and practices regarding pesticide use
A cross-sectional survey using an electronic questionnaire form in an Open Data Kit (ODK) [36] was conducted with 427 randomly selected farmers from the six wards. The questionnaire assessed the farmers’ awareness and practices of agricultural pesticides use, storage and disposal. Findings from the qualitative and quantitative study and direct observations were triangulated.
Assessment of types and classes of agricultural pesticides
Direct observations of the agricultural pesticides were done at all of the 17 agrovet retail stores. Information collected included pesticide types, classes and active ingredients.
Analysis of qualitative and quantitative data
Audio recorded interviews with the retailers of agricultural pesticides and farmers were transcribed verbatim and translated to English. The transcripts were imported into MAXQDA software for coding and analysis [37]. Systematic review and analysis of key issues, concepts, and repeated themes were done following framework analysis steps as described by Gale and colleagues [38]. For the data from farmers, a weaving approach was used, in which both quantitative and qualitative components were presented together [39]. Quantitative findings from the survey were presented, and further explanations drawn from the in-depth interviews. Selected participant’s narratives from each theme are presented.
Quantitative data generated through surveys from agrovet stores were analysed descriptively, using Stata version 15 (Stata Cooperation; College Station, TX, USA). Pictures of all of the insecticides were individually reviewed and active chemical ingredients recorded to summarize their frequencies by insecticide class.
Results
Characteristics of pesticide retailers and farmers
More than half (58.8%, n = 10) of the agrovet stores were in Kilombero district, while the remaining 41.2% (n = 7) were in Ulanga district. Two-thirds of participants (65.2%, n = 11) were females with age ranging between 18 and 43 years.
Table 1 summarizes the demographic characteristics of the farmers who participated in the survey. Males comprised of 51.5% (n = 220) and females 48.4% (n = 207). Most farmers practised both small-scale subsistence farming 51.3%, (n = 219) and large-scale cultivation 48.5% (n = 207) for food and business, and had worked on their farms for at least 5 years 89.2% (n = 381). The main farm crops farmed were rice, maize, different types of vegetables and fruits.
Table 1.
Socio-demographic characteristics of farmers involved in the survey
| Variable | Category | Percentage (n) |
|---|---|---|
| Gender | Males | 51.5% (220) |
| Females | 48.5% (207) | |
| Age (years) | 18–30 | 16.9% (72) |
| 31–40 | 31.1% (133) | |
| 41–50 | 28.3% (121) | |
| 51–60 | 17.6% (75) | |
| > 60 | 6.1% (26) | |
| Education attainment | Primary school | 85.2% (364) |
| Secondary school | 9.6% (41) | |
| College/university | 0.7% (3) | |
| Professional training | 0.5% (2) | |
| No formal training | 4.0% (17) | |
| Main economic activitiesa | Small-scale subsistence farming activities | 51.3% (219) |
| Large-scale farming for food and business | 48.5% (207) | |
| Livestock keeping | 9.8% (12) | |
| Small-scale business | 41.7% (178) | |
| Large-scale business | 2.8% (3) | |
| Private employment | 0.7% (2) | |
| Others | 0.5% (42) |
aFarmers with more than one sources of income, multiple responses
Types and classes of agricultural pesticides
The agricultural pesticides (Additional file 1), chemical classes and the active ingredients observed in the agrovet stores are summarized in Table 2. Most of the agricultural pesticides (87.5%, n = 91) were approved plant protection substances under full registration category (6.7%, n = 7) or had restricted registration or provisional registration according to Tanzania regulations [40, 41]. A small proportion (2.9%, n = 3) were unregistered. Insecticides accounted for (59.6%, n = 62) of the pesticides, followed by herbicides (27.9%, n = 29) and fungicides (10.6%, n = 11). The highest proportion of agricultural insecticides surveyed were organophosphates (34%), followed by pyrethroids (30%). Herbicides from the amino-phosphonates class were the most popular (59%). The two main fungicide classes were dithiocarbamate (54.5%) and acylalanine organochlorine (27.3%), widely used by most vegetable growers (Table 2). The insecticide formulations were emulsifiable concentrate (EC) (63%), while (66%) herbicides, and (64%) fungicides were formulated as soluble (liquid) concentrate (SL) and wettable powders (WP), respectively (Additional file 2).
Table 2.
Common active ingredients found in the agricultural pesticides in the study locality
| Pesticide type | Active ingredient (s) | N | % | Chemical class |
|---|---|---|---|---|
| Insecticides (N = 62) | Abamectin | 4 | 6.5 | Macrocyclic lactones |
| Alphacypermethrin | 3 | 4.8 | Pyrethroids | |
| Carbaryl and permethrin | 1 | 1.6 | Carbamates and pyrethroids | |
| Carbofuran | 1 | 1.6 | N-methyl carbamate Ib | |
| Carbaryl and lambda-cyhalothrin | 2 | 3.2 | Carbamates and pyrethroids | |
| Chlorpyrifos | 5 | 8.1 | Organophosphates | |
| Cypermethrin | 1 | 1.6 | Pyrethroids | |
| Cypermethrin and chlorpyrifos | 1 | 1.6 | Pyrethroids and organophosphates | |
| Cypermethrin and imidacloprid | 3 | 4.8 | Pyrethroids and neonicotinoids | |
| Deltamethrin | 1 | 1.6 | Pyrethroids | |
| Diazinon | 2 | 3.2 | Organophosphates | |
| Dichlorvos | 3 | 4.8 | Organophosphates | |
| Dimethoate | 1 | 1.6 | Organophosphates | |
| Fenitrothion and deltamethrin | 3 | 4.8 | Organophosphates and pyrethroids | |
| Fipronil | 1 | 1.6 | Phenylpyrazole | |
| Imidacloprid | 3 | 4.8 | Neonicotinoids | |
| Imidacloprid and beta-cyfluthrin | 2 | 3.2 | Neonicotinoids and pyrethroids | |
| Lambda-cyhalothrin | 11 | 17.7 | Pyrethroids | |
| Lambda-cyhalothrin and acetamiprid | 1 | 1.6 | Neonicotinoids and pyrethroids | |
| Malathion | 1 | 1.6 | Organophosphates | |
| Permethrin | 1 | 1.6 | Pyrethroids | |
| Pirimiphos-methyl | 2 | 3.2 | Organophosphates | |
| Pirimiphos-methyl and permethrin | 3 | 4.8 | Organophosphates and pyrethroids | |
| Pirimiphos-methyl and thiamethoxam | 1 | 1.6 | Organophosphates and neonicotinoids | |
| Profenofos | 5 | 8.1 | Organophosphates | |
| Herbicide (N = 29) | Bispyribac sodium | 1 | 3.5 | Bispyribac sodium |
| S-metolachlor and atrazine | 1 | 3.5 | Triazines | |
| Amine salt | 4 | 13.8 | Aryloxyacides | |
| Atrazine | 1 | 3.5 | Dinitroanilines | |
| Glyphosate | 17 | 58.6 | Amino-phosphonates | |
| Paraquat | 4 | 13.8 | Pyridines | |
| Triclopyr | 1 | 3.5 | Pyridines | |
| Fungicide (N = 11) | Monopotassium and dipotassium phosphonates | 1 | 9.1 | Phosphonic acid |
| Chlorothalonil | 3 | 27.3 | Organochlorine | |
| Mancozeb | 1 | 9.1 | Dithiocarbamate | |
| Mancozeb and cymoxanil | 1 | 9.1 | Acylalanine and dithiocarbamate | |
| Metalaxyl and mancozeb | 5 | 45.5 | Dithiocarbamate and acylalanine | |
| Insecticide + fungicide (N = 2) | Imidacloprid, metalaxyl and carbendazim | 2 | 100 | Neonicotinoids, acylalanine and benzimidazole |
Most insecticides had a single active ingredient (72.6%, n = 45), while fewer were mixed products with two different active ingredients at different doses (27.4%, n = 17), as shown in Tables 2 and 3. The most common pyrethroid was lambda-cyhalothrin, while chlorpyrifos and profenofos were the predominant organophosphates (Table 2). Most of the insecticides are non-systemic broad-spectrum insecticides with contact and stomach actions against crop pests. Over half of the herbicides (59%) were based on glyphosate that were frequently used by most of the rice farmers (76.8%). The principle active ingredients in most fungicide were metalaxyl and mancozeb (45%) and chlorothalonil (27%) (Table 2). Table 3 summarizes some of the commonly used pesticide products with more than one active ingredients. A wide range of insecticide classes and active ingredients used in crop protection had similar target sites and modes of action with the limited public health insecticides (Table 4).
Table 3.
Example of pesticide products with more than one active ingredient (as obtained from the factory)
| WHO class/family | Brand name | Active ingredient(s) |
|---|---|---|
| Organophosphates and pyrethroids | Simba powder 113DP | 10 g/kg of fenitrothion and 1.3 g/kg of deltamethrin |
| Duduba 450EC | 350 g/l of chlorpyrifos and 100 g/l of cypermethrin | |
| Mupa dust | 1.0% of fenitrothion and 0.13% of deltamethrin | |
| Stocal super dust | 16 g/kg of pirimiphos-methyl and 3 g/kg of permethrin | |
| Shumba super dust | 1% of fenitrothion and 0.13% of deltamethrin | |
| Actellic Gold Dust | 16 g/kg of pirimiphos-methyl and 3.6 g/kg of thiamethoxam | |
| Haigram 90 dusting powder (DP) | 6 g/kg of pirimiphos-methyl and 3 g/kg of permethrin | |
| Actellic super dust | 16 g/kg of pirimiphos-methyl and 3 g/kg of permethrin | |
| Pyrethroids and neonicotinoids | Amekan C344 SE | 144 g/l of cypermethrin and 200 g/l of imidacloprid |
| Rapid-attack 344SE | 144 g/l of cypermethrin and 200 g/l of imidacloprid | |
| Blast 60 EC | 3% g/l lambda-cyhalothrin and 3% g/l of acetamiprid | |
| Buffalo 450OD | 2.5% of beta- cyfluthrin and 7.5% of imidacloprid | |
| Thunder Oil Dispersion (OD) 145 | 45 g/l of beta-cyfluthrin and 100 g/l of imidacloprid | |
| Farmguard 344SE | 144 g/l of cypermethrin and 200 g/l of imidacloprid | |
| Carbamates and pyrethroids | Bakiller | 5% w/w of carbaryl and 0.1% w/w of lambda cyhalothrin |
| Akheri powder | 5% w/w carbaryl and 0.1% w/w lambda-cyhalothrin | |
| Ultravin® Dudu dust | 5% w/w of carbaryl, 1% w/w of permethrin and 94% w/w of inert carriers | |
| Neonicotinoids, acylalanine and benzimidazole | Seed plus 20 wettable soluble (WS) | 10% imidacloprid, 5% metalaxyl and 5% carbendazim WS |
Table 4.
Similarities between agricultural and public health insecticide classes and reported resistance mechanisms in disease vectors
| Class of insecticide | Trade name (active ingredient (s) | Primary site/mode of action in an insect/vector | Agricultural use | Public health use | Known resistance and resistance mechanism in disease vectors |
|---|---|---|---|---|---|
| Pyrethroids | Karate 5 EC (lambda-cyhalothrin) | Voltage-gated sodium channels/neurotoxic | Control of bollworms and aphids in vegetables and cotton [42] | Disease and vector control (IRS and LLINs) [43, 44] |
Knock-down mutation [45] Metabolic resistance [46] Cuticle thickening [47] |
| Organophosphates | Dasba 40 EC (chloropyrifos) | Acetylcholinesterase (AChE) inhibitors | Insecticide against insect pests in fruits, beans, tomatoes, cotton, coffee and green vegetables [48] | Disease and vector control (IRS and LLINs) [49] | Metabolic resistance [50] |
| Neonicotinoids | Amekan C344 SE (200 g/l of imidacloprid and 144 g/l of cypermethrin) | Nicotinic acetylcholine receptors (n AChRs) | Systemic insecticides with contact and stomach action against sucking and chewing pests on cotton, vegetables and flowers [51]. | Prequalified vector and disease control products [52, 53] | Metabolic resistance and target-sites [54, 55] |
| Carbamates | Farmerzeb 80 WP (80% WP of mancozeb) | Acetylcholinesterase (AChE) inhibitors | A broad spectrum protectant and preventive fungicide for the control of fungal diseases on vegetables | Disease and vector control (IRS and LLINs) [56] | Metabolic resistance [57, 58] |
Awareness and perceptions of pesticide use among agrovet store retailers
Most retailers stated that their customers were mostly rice farmers or horticulture farmers, particularly those relying on the irrigation system. The frequency of purchasing particular pesticides depended on the season. A majority of retailers reported to have no formal training on the pesticides they were selling, and poor knowledge on the type of crop pests, disease and relevant pesticides to be used for each. They were only able to recommend the use (dilution and frequency of application) based on experiences, or based on recommendations from the store owners and pesticide suppliers:
“I have been selling pesticides for a long time. I started to work in Ifakara town shops. Also, the owner of the shop understands pesticides, and she does assist with information whenever needed” (male retailer).
A majority of the retailers also reported giving instructions to their customers on pesticide usage, dosage and application time. However, upon examining the pesticide labels, the dosage suggested by the retailers was sometimes higher or lower than those recommended by the manufacturers on the product label. The handling of pesticides was commonly practised without protective measures. However, the retailers also occasionally provided information on use of protective measures such as wearing long-sleeve shirts and boots during preparation and spraying of pesticides:
“Most of my customers do not know the dosage of chemicals to use. I tell them that quantity of chemicals depends on the size of the farm, amount and type weeds, and particular for insecticides it depends on the pest problem, if they ask me I always ask them how big their problem is, then I tell them to add 250 mls of Agroround (480 g/l of glyphosate) to a 15 L bucket” (female retailer).
A total of 18 (17.5%) pesticides were commercially found repacked into small quantities in small unlabelled bottles. Decanted pesticide products were mainly targeting average income farmers who were able to afford small amounts.
Crop calendar and pesticide usage practices
Most of the farmers reported cultivating more than one type of crop. Overall, 64.8% (421) of the farmers grew cereal crops, predominantly rice and maize, 25.8% (168) cultivated vegetables and fruits, such as spinach, cabbages and watermelon, 5.2% (34) cultivated legumes such as beans and 3.2% (27) grew other crops, such as cashew nuts and peanuts. Most farmers owned 1 to 20 ha of land. In the wet season, rice farmers prepared their land in November and December, planted in January and harvested in May or June. For the dry season (assisted by irrigation) they prepared farms starting in May, planted in June and harvested in October [29]. The irrigated farming practices used short-duration rice seeds, maturing in 4 months, while the non-irrigation farming method that depends on rainfall during wet season used long-duration rice seeds that mature within 5–6 months. The irrigated rice agro-ecosystem was reported to be prone to pest infestations, and hence, required regular insecticide applications. The farming methods also corresponded to the application patterns of various pesticides:
“Normally in the rain season there are few pests and can easily be destroyed by rainwater. From my experience, the rice seed cultivated in rainy season is not vulnerable to pests, thus different from the swamp rice farming that relies on irrigation, without pesticides application you will not have good produces” (female farmer).
Knowledge and practices of farmers regarding pesticides and pesticide application
The majority of farmers (89.3%, n = 381) had no awareness of pesticides. Most farmers (54.4%, n = 316) sprayed doses of pesticides based on instructions received from the pesticide dealers, while (18.2%, n = 106) relied on personal experiences or direct observations based on the estimation of farm sizes and incidence of pests and weeds. Only (15.5%, n = 90) farmers reported that they read product labels, and only if written in the local language, Kiswahili. The rest of the farmers (11.5%, n = 67) relied on experts, such as agricultural officers or other knowledgeable sources of information about pesticide usage:
“I always get instructions from the seller of the pesticides at the agrovet shop, but sometimes I read from the leaflet on the pesticide bottle only those written in Swahili” (female farmer).
Only 27% of farmers believed it was necessary to use recommended pesticide doses as stipulated by the manufacturer for each pesticide, though there is no evidence that they followed those instructions. On the other hand, 62.1% perceived the right pesticide dosage as any amount enough to kill all the pests in the farm. Mixing of the pesticides was mostly done in a Knapsack® Sprayer tank, traditionally recognized as “Solo”. Overall, 400 farmers (93.7%) performed pesticide dilutions and mixing at the farms, nearby water sources, such as irrigation canals or rivers (Fig. 2). Most of the pesticides come with the measuring equipment, but farmers typically used empty soda bottles/syringe pipe to measure liquid pesticides. Pesticide dose rates also varied among farmers (Table 5).
Fig. 2.

Pesticides mixing, application and disposal practices among farmers observed in rice paddies, in the study area
Table 5.
Example of pesticide spray dosages as reported by farmers compared to the recommended dosage on the product label
| Pesticide class | Trade name | Active ingredient (s) | Class of the pesticide | Knapsack spray dilution by farmers ml/l, g/l of water | Recommended knapsack dilution rate ml/l, g/l of water | Recommended dose (ml/ha) | Target crop |
|---|---|---|---|---|---|---|---|
| Insecticide | Karate 5EC | 50 gm/l of lambda-cyhalothrin | Pyrethroids | 15–40 ml/20 l | 12 ml/20 l | 300–400 ml/ha | Rice, maize, vegetables, fruits, green pepper, watermelon, beans green peas and tomatoes |
| Amekan C344 SE | 144 g/l of cypermethrin and 200 g/l of imidacloprid | Pyrethroids and Neonicotinoids | 30 ml/20 l | 8–10 ml/15 l | 500 ml/ha | Tomatoes, watermelon, okra, potatoes, rice, spinach, maize, green pepper and cabbages | |
| Duduba 450EC | 100 g/l of cypermethrin and 350 g/l of chlorpyrifos | Pyrethroids and organophosphates | 30–50 ml/20 l | 10 ml/20 l | 400 ml/ha | Rice, cucumber, tomatoes, green pepper, cereals crops and fruits | |
| Buffalo 100OD | 75 g/l of imidacloprid and 25 g/l of beta-cyfluthrin | Neonicotinoids and pyrethroids | 35–60 ml/20 l | 10 ml/20 l | 500 ml/ha | Tomatoes, maize, green peas potatoes, green pepper, beans and onions | |
| Ninja 5EC | 50 g/l of lambda-cyhalothrin | Pyrethroids | 25 ml/15 l | 40–60 ml/20 l | 150–400 ml/ha | Rice, fruits, green peas vegetables and maize | |
| KungFu 5EC | 50 gm/l of lambda-cyhalothrin | Pyrethroids | 15–40 ml/20 l | 12 ml/20 l | 300–400 ml/ha | Tomatoes, watermelon, cucumber, rice, onions, vegetables, fruits and green pepper | |
| Suracron 720 EC/720/Profecron 720 EC | 720 g/l of profenofos | Organophosphates | 200–350 ml/20 l | 20–40 ml/15 l | 500–800 ml/ha | Cabbage and tomatoes, okra, eggplant, cucumber and watermelon | |
| Nogozone 60 EC | 600 g/l diazinon | Organophosphates | 20–40 ml/20 l | 5–30 ml/15 l | 150–700 ml/ha | Watermelon and cucumber | |
| Herbicide | 2,4 d Amine | 720 g/l of 2, 4 d-dimethyl amine salt | Aryloxyacides | 150–300 ml/16 l | 200 ml/20 l | 2000 ml/ha | Rice and maize |
| Roundup | 360 g/l of glyphosate | Amino-phosphonates | 300–350 ml/15 l | 200–300 ml/20 l | 2000–3000 ml/ha | Rice and maize | |
| Parapaz 200 SL | 200 g/l of paraquat dichloride | Pyridines | 300–350 ml/15 l | 100–200 ml/20 l | 800–1600 ml/ha | Maize, rice, sugarcane and tomatoes | |
| Fungicide | Farmerzeb 800 WP | 800 g/kg of mancozeb | Dithiocarbamate | 60 g/15 l | 40–60 g/20 l | 1000––3000 g/ha | Tomatoes, African eggplant, green pepper and potatoes |
| Linkonil 500 SC | 500 g/l of chlorothalonil | Organochlorine fungicide | 20–50 ml/20 l | 46 ml/20 l | 1000–3500 ml/ha | Tomatoes, okra, eggplant, watermelon and cucumber | |
| Victory 72 WP | 640 g/kg of mancozeb and 80 g/kg of metalaxyl | Dithiocarbamate and acylalanine | 60–80 g/20 l | 50 g/20 l | 2000–2500 g/ha | Tomatoes, okra, and potatoes, cucumber, watermelon and cabbage |
Frequency and spraying patterns of pesticides
Most rice farmers reported re-applying insecticides at least twice every week, or anytime there were pests to achieve maximum control (Table 6). Other farmers reported preemptively re-spraying their farms to prevent pests coming from unsprayed neighbouring farms. Farmers also frequently sprayed herbicides to prevent or delay weeds:
“Since most of the insecticides are not as effective as they used to be, for instance, I have to re-apply Karate (lambda-cyhalothrin) two times after every week. I think it is time the effectiveness of the insecticide has depleted and cannot kill or repel pests anymore. Sometimes, I re-apply more often because there are a lot of insect-pests coming from neighbouring farms, especially those where spraying was delayed” (male farmer).
Table 6.
Farmers’ responses about insecticide spray frequency
| Application frequency | No. of farmers | Percentage (%) |
|---|---|---|
| Twice every week | 120 | 28.1 |
| Once every 2 weeks | 61 | 14.3 |
| 2–4 times per growing season | 71 | 16.6 |
| Any time I find pests in the farm | 111 | 26.0 |
| I do not remember | 64 | 15.0 |
Insecticides and fungicides were mostly used during the dry season for irrigated rice cultivation and vegetable farming. Most of the non-selective, systemic, post-emergence herbicides such as Roundup (glyphosate) were, however, sprayed before farming and planting of rice seeds, shortly before rains start during farm preparation. The selective herbicides such as 2,4-d Amine (2,4-d amine salt) were commonly used during weeding to control soft weeds in rice farms:
“I spray Kung-fu (lambda-cyhalothrin) in the dry and wet season but mostly in the dry season because this is the period there are a lot of pests. In the wet season, there are few or no pests because of rainfall. Pest does not survive when there is a lot of water, unlike in dry season” (female farmer).
Challenges faced by the farmers regarding the usage of pesticides
Farmers reported multiple challenges when using pesticides. Half of the farmers (51.3%) claimed to have experienced adverse health events, such as skin irritation or coughing after spraying pesticides. The most common challenge and concern reported by about two-third of the farmers (64.6%) was that pesticides lost their killing efficiency against weeds and pests as they have had pests rebound after pesticides application. About 7.7% of the farmers suspected some pesticides are counterfeit, and 3.3% had experienced some pesticides being more diluted than expected. Switching to different classes of insecticide or mixing pesticides was a common practice (75.6% of the farmers):
“You will find in few days sometimes even the following day after spraying there are still some pests in the farms. I surveyed and tried to spray different pesticides other than the ones I’m used to. I realized rapid attack (a mixture of cypermethrin and imidacloprid) and Amekan (a mixture of cypermethrin and imidacloprid) are far better and effective insecticides than Duduba (a mixture of cypermethrin and chlorpyrifos) alone against most of the pests affecting vegetables, watermelons and rice” (male farmer).
Use of pesticide mixtures
Tank mixing of more than one pesticide with the same or different active ingredients before spraying was commonly practised (Table 7), which was also observed at the farms, despite being against label instructions. Sometimes pesticides were combined with fertilisers before application following retailers’ recommendations (Table 7). The popular pesticide mixtures were: (i) two herbicides (38.7%); (ii) two insecticides (16.1%); (iii) one fertilizer and one insecticide (16.1%); (iv) one insecticide and one fungicide (12.9%); and (v) one herbicide and one insecticide (9.7%), and other mixtures (6.5%). Most farmers (86.4%) perceived cocktail sprays are more efficient than when sprayed as a single product. They also perceived that mixing two or more pesticides into a single spray solution simplified work and saved time. For example, a cocktail of KungFu (lambda-cyhalothrin) and Duduba (cypermethrin, chlorpyrifos) was used on fruits and vegetables such as watermelon, tomatoes, cabbages, okra and spinach.
Table 7.
Pesticide combination practices by farmers at the study sites
| Pesticides cocktail | Type of pesticides | Pesticide class |
|---|---|---|
| KungFu and Duduba | Two insecticides | Two pyrethroids and one organophosphate |
| 2,4-D and Roundup | Two herbicides | One aryloxyacetic and one amino-phosphonates |
| Booster + Supercron | One fertiliser and One insecticide | Nitrogen, phosphorous, potassium and trace elements and one organophosphate |
| Karate and KungFu | Two insecticides | Two pyrethroids |
| Rapid attack and Amekan | Two insecticides | Two (pyrethroids and neonicotinoids) |
| Echlonil and Karate | One fungicide and one insecticide | One organochlorine fungicide and one pyrethroid |
| Rapid attack and Farmerzeb | One insecticide and one fungicide | One (pyrethroids and neonicotinoids) and one dithiocarbamate |
Handling and disposal practices of left-over pesticides and pesticide containers
Most farmers practised unsafe handling and disposal of pesticides. About half of the farmers (51.8%, n = 221) reported storing pesticide leftovers in their homes for either re-spraying rebounding pests or use in the next farming season. One-third (n = 128) dumped out leftover pesticides into either rivers or nearby bushes. A small minority reported burying the left-over pesticides underground (6/427) or using the pesticides to kill domestic insects such as cockroaches and houseflies in their houses (2/427). Regarding disposal of containers, the majority of farmers (55.7%, n = 238) reported that they discarded empty pesticide containers into either running water in the rivers or bushes on the farms, while approximately one-fifth (22.0%) considered burning the empty pesticides bottles. Some (18.5%) of the farmers, however, buried the containers in the ground, and a small minority (3.7%) reported washing and re-using the empty bottles for either repacking pesticides or other domestic activities.
Discussion
Agricultural pesticides can drive selection pressure for resistance in wild mosquito vector populations breeding in agro-ecosystems [7–14], thus threatening the effectiveness of public health interventions, such as LLINs and IRS. The WHO global action plan for insecticide resistance management in malaria vectors recommends several strategies for preventing the spread of resistance, while sustaining the effectiveness of vector control interventions [26]. However, there is a lack of harmonization and integration with agricultural pesticides usage practices [8].
The current study found multiple formulations of synthetic agricultural pesticides sold at agrovet stores in the districts of Ulanga and Kilombero in south-eastern Tanzania. More than 90% of the farmers interviewed reported using either pyrethroids, organophosphates, neonicotinoids, carbamates, organochlorines or product mixtures with at least two of these classes. The active ingredients include alpha-cypermethrin, carbaryl, chlorpyrifos, chlorothalonil, cymoxanil, cypermethrin, deltamethrin, diazinon, dichlorvos, fenitrothion, imidacloprid, lambda-cyhalothrin, malathion, mancozeb, permethrin, pirimiphos-methyl, and profenofos. These insecticide groups for crop protection attack the same target sites and have similar modes of action as public health insecticides [59–61]. Most of the insecticide compounds found in use exhibit a broad spectrum of activity, indiscriminately killing even beneficial insects. These broad-spectrum insecticides are likely to be used more frequently than narrow-spectrum insecticides, thus exerting resistance selection pressure even on non-target insects, such as mosquitoes [62]. Other studies have reported extensive use of similar pesticide compounds by farmers for crop protection against pests and diseases in malaria-endemic regions [42]. For example, Philbert et al. found 48 pesticide formulations used by farmers in northern Tanzania, where malaria is endemic [63].
There are several similarities in insecticide active ingredients used in agriculture and those in public health in Tanzania. Nets impregnated with pyrethroids, mostly deltamethrin and permethrin, are widely used for malaria prevention [35]. Both lambda-cyhalothrin and bendiocarb were recently used for IRS, but have now been replaced with pirimiphos-methyl on Zanzibar Island and in some districts in north-western Tanzania [64]. Neonicotinoid-based interventions have also been tested and could be used [53]. Alpha-cypermethrin, which was found in most agricultural pesticides, is coated on Interceptor ® nets, which have been under evaluation for malaria control [65]. Beyond the basic chemical similarities, public health and agricultural pesticides also share modes of actions. For example, the voltage-gated sodium channels are targeted by pyrethroids and organochlorines, while acetylcholinesterase is targeted by both organophosphates and carbamates [59, 60].
This study also revealed the presence of candidate compounds, chlorpyrifos emulsifiable concentrate (EC) and imidacloprid for both pest control on the farms and cereal preservation under storage. Chlorpyrifos, an organophosphate, was earlier recommended by the WHO Pesticide Evaluation Scheme (WHOPES) for the control of juvenile mosquitoes [66] and has been evaluated for net impregnation against mosquitoes [49]. Additionally, imidacloprid (neonicotinoids) a nicotinic acetylcholine receptor stimulator, is also being considered as an alternative or in combinations with the commonly used pyrethroids [53].
Selection pressures are experienced when mosquitoes in their aquatic stages are exposed in their breeding habitats, where most farming activities are taking place [7]. In turn, this might cause insecticide tolerance, as part of defence mechanisms that lead to insecticide resistance to a subsequent new generation of emerged adult mosquitoes [8, 10, 11, 13]. Metabolic resistance is one of the principal mechanisms in mosquitoes [67], and has been linked to the massive use of pesticides in irrigated rice plantations that enhanced the over-production of detoxifications enzymes [68]. The over-expression of metabolic genes included four CYP6P3 and one CYP325 cytochrome P450s, two delta class GSTs, one peroxiredoxin and two cuticular pre-cursor genes in adults An. gambiae s.s. collected from different breeding habitats in Benin and Nigeria was reported to be influenced by the presence of xenobiotics and agricultural pesticides in their agro-ecological sites [14, 69]. The detoxification genes and cuticular precursor genes were linked to pyrethroid resistance and reduction of insecticide penetration, respectively [69]. A study performed by Nkya et al. found that frequent exposure of An. gambiae larvae to agricultural pollutants influenced an over-expression of multiple genes responsible for the selection of target-site mutation resistance, cuticle resistance, metabolic-based resistance and nervous and synaptic-transmission based resistance in adult mosquitoes [8, 10]. Similarly, bioassays revealed that a high level of pyrethroid resistance in An. gambiae s.l. was associated with DDT and pyrethroid residues from cotton-growing farms in West Africa [16].
Glyphosate was the most common active ingredients found in most of the herbicides. However, there were also herbicides containing 2,4-dichloro phenoxy acetic acid, S-metolachlor, atrazine, paraquat and 2,4 d-amine as active ingredients. Though herbicides are generally non-toxic to insects, many of them, and also several xenobiotics, could cause metabolic stress with the potential of modifying the insecticide detoxification systems in insects, hence causing insecticide tolerance and eventual resistance [18, 20]. In one study, Aedes aegypti larvae exposed to glyphosate were significantly tolerant to permethrin, due to the stimulation of multiple detoxification genes, including P450s and GSTs [18].
Even though most of the agricultural pesticides found were on the list of pesticides approved in Tanzania [40, 41], there were several versions deemed of less quality but with the same brand stamp as those found in the market. These findings are in line with Shao and colleagues, who reported the magnitude of counterfeit agro-inputs in Tanzania to be as high as 46.8%, that could pose a serious risk to the ecosystem [70]. In a similar study, repacking and decanting of pesticide products in un-labelled containers was done by a quarter of pesticide dealers in six study towns in Tanzania [71]. Farmers who participated in the current study reported having experienced reduced efficacy of some pesticides, hence sprayed their crops repeatedly or at a higher quantity. Previous reports have shown the reduced effectiveness of lambda-cyhalothrin against two species of rice stem borers, mainly Chilo species and Sesania calamistis in irrigated lowland rice ecosystems in the same study area [72].
Most of the retailers of agricultural pesticides and farmers lacked formal knowledge of the proper usage of pesticides, including pesticide dosages. The majority had never been trained on agricultural pesticide usage and had a lack of knowledge of crop pest biology and disease. The retailers prescribed informal instructions to the farmers on how to apply and at what amount agricultural pesticides are required based on their experiences. The findings agree with a recent study by Lekei et al., which found that most of the retailers of pesticides in Tanzania are not qualified to provide professional instructions to the end-users [71]. Similarly, most of the farmers were not knowledgeable on crop pests and diseases, pesticide usage and management of agricultural pesticides, instead relying on information received from the retailers and personal work experience. Pesticide dilution rates were confused with application dosages and in most cases were used in larger volumes than the recommended dosage. These findings are in line with reports from southern Côte d’Ivoire, where less than half of the 208 vegetable and rice farmers who participated in a study adhered to the recommended pesticide dosage [21].
In the current study, pesticides application patterns and frequencies were observed and informed mostly by experience or perception and only to a limited extent by professional advice. Previous studies conducted in Tanzania revealed an increase in pesticide applications per season as a common practice in most farmers [73]. While the use of agricultural pesticides was influenced by the farming calendar, insecticides and fungicides were heavily used in the dry season by farmers practising irrigated rice cultivation and vegetables. Though no clear association was found on how the farming calendar influences resistance, studies in rural southern Tanzania have demonstrated clear seasonal and spatial variations in phenotypic resistance to public health pesticides in both Anopheles and Culex mosquito vectors, with the most resistant mosquito populations in dry seasons in areas where irrigated rice cultivations are concentrated [31, 32]. The seasonal use of agricultural pesticides might provide an opportunity for vector control programmes to partner with agriculturalists in designing a coordinated resistance management plan.
Combining two or more pesticides or with fertiliser in a spray tank was routinely practiced among farmers, mainly to enhance efficacy and to save application time (Table 7). This practice has been reported in Tanzania [63] and elsewhere [74]. Usually, different pesticide formulations are incompatible and mixing them could induce toxicity of the plant and likely influence resistance selection pressure in crop pests and even in disease vectors [21, 63].
Unsafe storage and disposal practices of left-over agricultural pesticides were reported and observed during the cross-sectional survey. Left-over pesticides were hanged on the roof or kept under the beds. Some farmers kept left-overs for the next season. However, small quantities of pesticide left-overs (i.e. generally less than a litre) were considered unwanted and were disposed either in the farms or washed off in the running water. One participant from Lupiro sprayed the left-over pesticides on the walls and the roof of the house or discarded it in the pit latrine to abate mosquitoes. The farmers also practiced unsafe disposal of empty pesticide containers. Poor storage and disposal practices of agricultural pesticides have also been reported elsewhere [75], which might pollute the ecosystem, contaminate breeding sites of mosquitoes and influence selection pressure for insecticides resistance.
This study recommends coordinated efforts between public health and agricultural sectors to prevent or delay insecticide resistance in disease vectors, while preserving the effectiveness of agricultural pesticides. The main challenge in managing insecticide resistance is not the unavailability of appropriate methods, but ensuring their adoption by farmers and pest control operators. Hence, raising awareness among pesticide retailers and farming community of the links between agricultural pesticide usage practices and insecticide resistance development in mosquitoes is urgently needed, through regular field engagement educational activities and participatory workshops and dialogues. An integrated pest and vector management (IPVM) approach could be adopted through farmer field school’s empowerment programme, in the current and future mosquito vector insecticide resistance management strategies. The adoption of principles for IPVM provides opportunities to bridge the gap between agriculture and public health. Farmers could, therefore, make rational decisions on good agricultural practices, while minimising the use of pesticides by adopting other potential pest management options that include cultural and physical control, biocontrol and the use of biopesticides.
Study limitations
This study did not quantify the effect of agricultural pesticides in the selection of insecticide resistance in malaria vectors. Hence, there was no direct measure of association between agricultural pesticides exposure and resistance selection in malaria vectors. The study instead relied on an inventory of agricultural pesticides as well as the knowledge and practices among farmers and pesticides dealers. This research was nested in a larger study that investigated possible drivers of residual malaria transmissions [76], including insecticide resistance and resistance mechanisms in malaria vectors [27, 28], in communities where insecticidal nets are widely used, and pesticides are heavily applied in agriculture.
Conclusions
The similarity of active ingredients in agricultural insecticides and insecticides for malaria vector control, coupled with a lack of awareness among pesticide dealers and users, might accelerate the intensity and spread of resistance in malaria vectors, thereby compromising the effectiveness of insecticide-based interventions, such as LLINs and IRS. This study emphasizes the need for improving awareness among retailers and farmers on proper usage and management of agricultural pesticides. To ensure the judicious use of pesticides and preserve the effectiveness of public health insecticides, while improving crop yields, there is a pressing need for coordinated efforts between public health and agricultural sectors in the selection, timing of application and management of pesticides. One way of achieving this goal is to initiate coordinated education programmes in elementary farmer field schools on appropriate pesticide usage in both public health and agriculture sectors. Future studies should quantify pesticide residues from the soil and water, as to better estimate the magnitude of mosquito exposures to agricultural pesticides and the impact with a view to considering integrating agricultural practices for sustainable insecticide resistance management strategies in mosquito vector populations.
Supplementary information
Additional file 1. The data collection tools including the questionnaire and interview guide used in the study.
Additional file 2. Various pesticide classes and formulations found in the agrovet market and used by the farmers in the surveyed area.
Acknowledgements
We are grateful to the retailers of agrovet shops at Ifakara town in the Kilombero district and at Mavimba, Minepa and Lupiro wards in the Ulanga district for permitting us to conduct this study at their stores. We greatly appreciated the cooperation received from the farmers during the interviews. Noelia Pama and Tumpe Mwandiyana are gratefully acknowledged for their assistance during the interviews and Alex Limwagu for preparing a study area map. We thank the editor and anonymous reviewers for their careful reading of our work and providing valuable comments and suggestions that further improved the quality of this paper.
Authors’ contributions
NSM and FOO conceived the study. NSM, MT, GM and FOO contributed to study design and development of data collection tools. MT, JU, VN and FOO reviewed the data collection tools. NSM and SAM conducted interviews with the support of field technicians. NSM led data analysis and interpretation. NSM drafted the manuscript. MT, GM, SAM, MF, JU, VN and FOO critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The study was financially supported by a Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine (Grant No.: WT102350/Z/13/Z) and the World Health Organization’s Tropical Disease Research (TDR) group (Reference No.: 2015/590235-0) awarded to the senior author FOO. NSM is a recipient of a Swiss Government Excellence Scholarship via the Federal Commission for Scholarships for Foreign Students FCS (ESKAS) (Reference No: 2017.0786). The funders had no role in the design of the study, data collection, analysis and interpretation, and in the writing of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its additional files).
Ethics approval and consent to participate
Written informed consent was sought from the retailers of agrovet stores and farmers upon their agreement to be involved in the study. Ethical review and approval were granted by the Institutional Review Board (IRB) of the Ifakara Health Institute (IHI) (reference no. IHI/IRB/NO: 35-2015) and the Medical Research Coordinating Committee at the National Institute for Medical Research (NIMR) in Tanzania (Reference No. NIMR/HQ/R.8a/Vol.IX/2162).
Consent for publication
The permission to publish this work was obtained from the Director of Research Information, Technology and Communication from NIMR in Tanzania (reference no.: NIMR/HQ/P.12 VOL XXX/). Farmers provided consents for the photos to be taken and used for research dissemination.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-020-03331-4.
References
- 1.WHO . World malaria report. Geneva: World Health Organization; 2019. [Google Scholar]
- 2.WHO . Global vector control response 2017–2030: a strategic approach to tackle vector-borne diseases. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Global report on insecticide resistance in malaria vectors: 2010–2016. Geneva: World Health Organization; 2018. [Google Scholar]
- 5.Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15:225. doi: 10.1186/s12936-016-1280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nkya TE, Mosha FW, Magesa SM, Kisinza WN. Increased tolerance of Anopheles gambiae s.s. to chemical insecticides after exposure to agrochemical mixture. Tanzan J Health Res. 2014;16:329–332. doi: 10.4314/thrb.v16i4.10. [DOI] [PubMed] [Google Scholar]
- 8.Nkya TE, Akhouayri I, Poupardin R, Batengana B, Mosha F, Magesa S, et al. Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: a case study in Tanzania. Malar J. 2014;13:28. doi: 10.1186/1475-2875-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nkya TE, Akhouayri I, Kisinza W, David J-P. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43:407–416. doi: 10.1016/j.ibmb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Nkya T, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, et al. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasit Vectors. 2014;7:480. doi: 10.1186/s13071-014-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadouleton AW, Asidi A, Djouaka RF, Braïma J, Agossou CD, Akogbeto MC. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103. doi: 10.1186/1475-2875-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabate A, Baldet T, Chandre F, Akoobeto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- 13.Akogbeto M, Djouaka R, Noukpo H. [Use of agricultural insecticides in Benin] (in French) Bull Soc Pathol Exot. 2005;98:400–405. [PubMed] [Google Scholar]
- 14.Abdullahi AI, Yusuf YD. Response of Anopheles gambiae detoxification enzymes to levels of physico-chemical environmental factors from northwest Nigeria. Bayero J Pure Appl Sci. 2015;7:93–104. [Google Scholar]
- 15.Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107. doi: 10.1186/s12936-016-1162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadouleton A, Martin T, Padonou G, Chandre F, Asidi A, Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae population in Northern Benin. Parasit Vectors. 2011;4:60. doi: 10.1186/1756-3305-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abuelmaali SA, Elaagip AH, Basheer MA, Frah EA, Ahmed FT, Elhaj HF, et al. Impacts of agricultural practices on insecticide resistance in the malaria vector Anopheles arabiensis in Khartoum State, Sudan. PLoS ONE. 2013;8:e80549. doi: 10.1371/journal.pone.0080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riaz MA, Poupardin R, Reynaud S, Strode C, Ranson H, David J-P. Impact of glyphosate and benzo [a] pyrene on the tolerance of mosquito larvae to chemical insecticides. Role of detoxification genes in response to xenobiotics. Aquat Toxicol. 2009;93:61–69. doi: 10.1016/j.aquatox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Poupardin R, Reynaud S, Strode C, Ranson H, Vontas J, David J-P. Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: impact on larval tolerance to chemical insecticides. Insect Biochem Mol Biol. 2008;38:540–551. doi: 10.1016/j.ibmb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 20.David J-P, Coissac E, Melodelima C, Poupardin R, Riaz MA, Chandor-Proust A, et al. Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology. BMC Genomics. 2010;11:216. doi: 10.1186/1471-2164-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chouaïbou MS, Fodjo BK, Fokou G, Allassane OF, Koudou BG, David J-P, et al. Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Côte d’Ivoire. Malar J. 2016;15:426. doi: 10.1186/s12936-016-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diao X, Hazell PB, Resnick D, Thurlow J. The role of agriculture in development: implications for sub-Saharan Africa. Intl Food Policy Res Inst. Research Report 153, 2007.
- 23.Staatz JM, Dembele NN. Agriculture for development in sub-Saharan Africa. Background Paper for the World Development Report. Washington DC: World Bank; 2008. [Google Scholar]
- 24.Kishimba M, Henry L, Mwevura H, Mmochi A, Mihale M, Hellar H. The status of pesticide pollution in Tanzania. Talanta. 2004;64:48–53. doi: 10.1016/j.talanta.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 25.Rajabu J, Tarimo M, Hangali T. Health effects, trends and knowledge on pesticide use in Tanzania. Int J Innov Res Sci Eng Technol. 2017;4:100–122. [Google Scholar]
- 26.WHO . Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization; 2012. [Google Scholar]
- 27.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS ONE. 2017;12:e0177807. doi: 10.1371/journal.pone.0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matowo NS, Munhenga G, Tanner M, Coetzee M, Feringa WF, Ngowo HS, et al. Fine-scale spatial and temporal heterogeneities in insecticide resistance profiles of the malaria vector, Anopheles arabiensis in rural south-eastern Tanzania. Wellcome Open Res. 2017;2:96. doi: 10.12688/wellcomeopenres.12617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato F. Development of a major rice cultivation area in the Kilombero valley, Tanzania. African Study Monographs Supplement. 2007;36:3–18. [Google Scholar]
- 30.Swai JK, Finda MF, Madumla EP, Lingamba GF, Moshi IR, Rafiq MY, et al. Studies on mosquito biting risk among migratory rice farmers in rural south-eastern Tanzania and development of a portable mosquito-proof hut. Malar J. 2016;15:564. doi: 10.1186/s12936-016-1616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner M, de Savigny D, Mayombana C, Hatz C, Burnier E, Tayari S, et al. Morbidity and mortality at Kilombero Tanzania 1982–88. In: Feachem RG, Jamison DT, et al., editors. Disease and mortality in Sub-Saharan Africa. Oxford: Oxford University Press; 1982. pp. 286–305. [Google Scholar]
- 32.Schellenberg D, Menendez C, Kahigwa E, Font F, Galindo C, Acosta C, et al. African children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for death. Am J Trop Med Hyg. 1999;61:431–438. doi: 10.4269/ajtmh.1999.61.431. [DOI] [PubMed] [Google Scholar]
- 33.Ogoma SB, Lweitoijera DW, Ngonyani H, Furer B, Russell TL, Mukabana WR, et al. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl Trop Dis. 2010;4:e773. doi: 10.1371/journal.pntd.0000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matowo NS, Abbasi S, Munhenga G, Tanner M, Mapua SA, Oullo D, et al. Fine-scale spatial and temporal variations in insecticide resistance in Culex pipiens complex mosquitoes in rural south-eastern Tanzania. Parasit Vectors. 2019;12:413. doi: 10.1186/s13071-019-3676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renggli S, Mandike R, Kramer K, Patrick F, Brown NJ, McElroy PD, et al. Design, implementation and evaluation of a national campaign to deliver 18 million free long-lasting insecticidal nets to uncovered sleeping spaces in Tanzania. Malar J. 2013;12:85. doi: 10.1186/1475-2875-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G. Open data kit: tools to build information services for developing regions. In: Proceedings of the 4th ACM/IEEE international conference on information and communication technologies and development. 2010; p. 1–12.
- 37.Kuckartz U. MAXQDA: qualitative data analysis. Berlin: VERBI software; 2007. [Google Scholar]
- 38.Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs-principles and practices. Health Serv Res. 2013;48:2134–2156. doi: 10.1111/1475-6773.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.TPRI. Registered plant protection substances for use in the United Republic of Tanzania. Registrar of pesticides, Tropical Pesticides Research Institute, 2018.
- 41.Dar es Salaam: Ministry of Agriculture FSaC: United Republic of Tanzania: Plant Protection Regulations, 1999.
- 42.Zidan N, El-Naggar JB, Aref SA, El-Dewy ME. Field evaluation of different pesticides against cotton bollworms and sucking insects and their side effects. J Am Sci. 2012;8:128–136. [Google Scholar]
- 43.Mutagahywa J, Ijumba JN, Pratap HB, Molteni F, Mugarula FE, Magesa SM, et al. The impact of different sprayable surfaces on the effectiveness of indoor residual spraying using a micro encapsulated formulation of lambda-cyhalothrin against Anopheles gambiae s.s. Parasit Vectors. 2015;8:203. doi: 10.1186/s13071-015-0795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tungu PK, Malima R, Mosha FW, Lyimo I, Maxwell C, Kaur H, et al. Evaluation of ICON Maxx, a long-lasting treatment kit for mosquito nets: experimental hut trials against anopheline mosquitoes in Tanzania. Malar J. 2015;14:225. doi: 10.1186/s12936-015-0742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranson H, Jensen B, Vulule J, Wang X, Hemingway J, Collins F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Biochem Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 46.Mulamba C, Riveron JM, Ibrahim SS, Irving H, Barnes KG, Mukwaya LG, et al. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS ONE. 2014;9:e110058. doi: 10.1371/journal.pone.0110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood O, Hanrahan S, Coetzee M, Koekemoer L, Brooke B. Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit Vectors. 2010;3:67. doi: 10.1186/1756-3305-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan S, Zhang F, Deng K, Yu C, Liu S, Zhao P, et al. Spinach or amaranth contains highest residue of metalaxyl, fluazifop-p-butyl, chlorpyrifos, and lambda-cyhalothrin on six leaf vegetables upon open field application. J Agric Food Chem. 2013;61:2039–2044. doi: 10.1021/jf304710u. [DOI] [PubMed] [Google Scholar]
- 49.N’Guessan R, Boko P, Odjo A, Chabi J, Akogbeto M, Rowland M. Control of pyrethroid and DDT-resistant Anopheles gambiae by application of indoor residual spraying or mosquito nets treated with a long-lasting organophosphate insecticide, chlorpyrifos-methyl. Malar J. 2010;9:44. doi: 10.1186/1475-2875-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandre F, Darriet F, Doannio JM, Rivière F, Pasteur N, Guillet P. Distribution of organophosphate and carbamate resistance in Culex pipiens quinquefasciatus (Diptera: Culicidae) in West Africa. J Med Entomol. 1997;34:664–671. doi: 10.1093/jmedent/34.6.664. [DOI] [PubMed] [Google Scholar]
- 51.Jeschke P, Nauen R, Schindler M, Elbert A. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem. 2011;59:2897–2908. doi: 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- 52.WHO . Prequalification vector control: prequalified lists of vector control products. Geneva: World Health Organization; 2019. [Google Scholar]
- 53.Agossa FR, Padonou GG, Koukpo CZ, Zola-Sahossi J, Azondekon R, Akuoko OK, et al. Efficacy of a novel mode of action of an indoor residual spraying product, SumiShield® 50WG against susceptible and resistant populations of Anopheles gambiae s.l. in Benin, West Africa. Parasit Vectors. 2018;11:293. doi: 10.1186/s13071-018-2869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mouhamadou CS, de Souza SS, Fodjo BK, Zoh MG, Bli NK, Koudou BG. Evidence of insecticide resistance selection in wild Anopheles coluzzii mosquitoes due to agricultural pesticide use. Infect Dis Poverty. 2019;8:64. doi: 10.1186/s40249-019-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crossthwaite AJ, Rendine S, Stenta M, Slater R. Target-site resistance to neonicotinoids. J Chem Biol. 2014;7:125–128. doi: 10.1007/s12154-014-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Protopopoff N, Wright A, West PA, Tigererwa R, Mosha FW, Kisinza W, et al. Combination of insecticide treated nets and indoor residual spraying in northern Tanzania provides additional reduction in vector population density and malaria transmission rates compared to insecticide treated nets alone: a randomised control trial. PLoS ONE. 2015;10:e0142671. doi: 10.1371/journal.pone.0142671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Protopopoff N, Matowo J, Malima R, Kavishe R, Kaaya R, Wright A, et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar J. 2013;12:149. doi: 10.1186/1475-2875-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corbel V, Hougard J-M, Guessan RN, Chandre F. Evidence for selection of insecticide resistance due to insensitive acetylcholinesterase by carbamate-treated nets in Anopheles gambiae s.s. (Diptera: Culicidae) from Côte d’Ivoire. J Med Entomol. 2003;40:985–988. doi: 10.1603/0022-2585-40.6.985. [DOI] [PubMed] [Google Scholar]
- 59.WHO . Recommended insecticides for indoor residual spraying against malaria vectors. Geneva: World Health Organization; 2015. [Google Scholar]
- 60.WHO . Recommended insecticide products for treatment of mosquito nets for malaria vector control. Geneva: World Health Organization; 2014. [Google Scholar]
- 61.Sparks TC, Nauen R, IRAC Mode of action classification and insecticide resistance management. Pestic Biochem Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 62.FAO . International code of conduct on the distribution and use of pesticides: guidelines on prevention and management of pesticide resistance. Rome: Food and Agriculture Organization of the United Nations; 2012. [Google Scholar]
- 63.Philbert A, Lyantagaye SL, Nkwengulila G. Farmers’ pesticide usage practices in the malaria endemic region of north-western Tanzania: implications to the control of malaria vectors. BMC Public Health. 2019;19:1456. doi: 10.1186/s12889-019-7767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.PMI. Presidents Malaria Initiative, Malaria Operational Plan: Tanzania. 2015. USAID, 2015.
- 65.WHO. Report of the 10th WHOPES Working Group meeting Review of Spinosad 0.5% GR and 12% SC, Lambda-Cyhalothrin 10% CS, KO TAB 1-2-3 Interceptor. 11–14 December 2006. WHO/CDS/NTD/WHOPES; 2007.
- 66.WHOPES . WHOPES-recommended compounds and formulations for control of mosquito larvae. Geneva: World Health Organization; 2013. [Google Scholar]
- 67.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 68.Matowo J, Kulkarni MA, Mosha FW, Oxborough RM, Kitau JA, Tenu F, et al. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. Malar J. 2010;9:193. doi: 10.1186/1475-2875-9-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Djouaka RF, Bakare AA, Coulibaly ON, Akogbeto MC, Ranson H, Hemingway J, et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao D, Edward S. Combating fake agro-inputs products in Tanzania using mobile phones. Int J Comput Appl. 2014;97:21–25. [Google Scholar]
- 71.Lekei EE, Ngowi AV, London L. Pesticide retailers’ knowledge and handling practices in selected towns of Tanzania. J Environ Health. 2014;13:79. doi: 10.1186/1476-069X-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.January B, Rwegasira M, Tefera T. Lepidopteran stem borer species abundance and associated damages on irrigated Kilombero low land rice ecosystem in Tanzania. J Entomol. 2018;15:28–35. [Google Scholar]
- 73.Ngowi A, Mbise T, Ijani A, London L, Ajayi O. Pesticides use by smallholder farmers in vegetable production in northern Tanzania. Crop Protection (Guildford, Surrey). 2007;26:1617. doi: 10.1016/j.cropro.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mengistie BT, Mol AP, Oosterveer P. Pesticide use practices among smallholder vegetable farmers in Ethiopian Central Rift Valley. Environ Dev Sustain. 2017;19:301–324. [Google Scholar]
- 75.Lekei EE, Ngowi AV, London L. Farmers’ knowledge, practices and injuries associated with pesticide exposure in rural farming villages in Tanzania. BMC Public Health. 2014;14:389. doi: 10.1186/1471-2458-14-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14:e0217414. doi: 10.1371/journal.pone.0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The data collection tools including the questionnaire and interview guide used in the study.
Additional file 2. Various pesticide classes and formulations found in the agrovet market and used by the farmers in the surveyed area.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its additional files).