Abstract
Background
Serum creatinine (Cr) and cystatin C (CysC) can both be used to estimate glomerular filtration rate (eGFRCr and eGFRCysC). However, certain conditions may cause discrepancies between eGFR trends from Cr and CysC, and these remain undetermined in patients with chronic kidney disease (CKD).
Methods
A total of 1069 patients from the Korean CKD cohort (KNOW-CKD), which enrolls pre-dialytic CKD patients, whose Cr and CysC had been followed for more than 4 years were included in the sample. We performed trajectory analysis using latent class mixed modeling and identified members of the discrepancy group when patient trends between eGFRCr and eGFRCysC differed. Multivariate logistic analyses with Firth’s penalized likelihood regression models were performed to identify conditions related to the discrepancy.
Results
Trajectory patterns of eGFRCr were classified into three groups: two groups with stable eGFRCr (stable with high eGFRCr and stable with low eGFRCr) and one group with decreasing eGFRCr. Trajectory analysis of eGFRCysC also showed similar patterns, comprising two groups with stable eGFRCysC and one group with decreasing eGFRCysC. Patients in the discrepancy group (decreasing eGFRCr but stable & low eGFRCysC; n = 55) were younger and had greater proteinuria values than the agreement group (stable & low eGFRCr and eGFRCysC; n = 706), differences that remained consistent irrespective of the measurement period (4 or 5 years).
Conclusions
In the present study, we identify conditions related to discrepant trends of eGFRCr and eGFRCysC. Clinicians should remain aware of such potential discrepancies when tracing both Cr and CysC.
Keywords: Chronic kidney disease, Creatinine, Cystatin C, Estimated glomerular filtration rate, Trajectory pattern
Background
Accurate measurements of glomerular filtration rate (GFR) are important in nephrology. Because actual GFR is difficult to measure and expensive when used for screening, GFR is often estimated using serum creatinine (eGFRCr). However, serum creatinine (Cr) is affected by non-GFR determinants such as muscle mass, body size, diet, and nutritional status [1]. Recently, cystatin C (CysC), which is a 13.3 kDa protein serine protease inhibitor produced by all nucleated cells, was proposed as a marker for estimating GFR [2, 3]. Because CysC is less influenced by muscle mass than other measures, eGFR with CysC (eGFRCysC) may reflect GFR more accurately than eGFR with Cr (eGFRCr) in patients with muscle wasting, chronic disease, and limb amputation [1]. The Kidney Disease Improving Global Outcomes guidelines for the evaluation of chronic kidney disease (CKD) recommends using eGFRCr as an initial assessment of renal function, and eGFRCysC as a confirmation of CKD in certain circumstances when eGFRCr is less accurate, with an evidence level of 2B. eGFRCysC may be also used in adult patients with eGFRCr of 45–59 ml/min/1.73 m2 who do not have markers of kidney damage, with an evidence level of 2C [4]. Nevertheless, the utility of eGFRCysC and conditions under which eGFRCysC differs from eGFRCr are unknown.
Intra-individual dynamic change in laboratory measurements provides better prognostic information than cross-sectional data alone [5]. In this respect, trajectory analysis has been applied to evaluate clinical parameters such as blood pressure [6], disability and functional decline [7, 8], and body mass index [9]. Variability in renal function is commonly observed in clinical settings [5]. Previously, trajectory analysis of eGFR demonstrated that CKD patients with catastrophic declining patterns had high rates of co-morbidities and mortality [10, 11].. However, the trajectory patterns of eGFRCysC have not been evaluated. The KNOW-CKD (KoreaN cohort Study for Outcomes in patients With Chronic Kidney Disease), a representative Korean CKD cohort, had traced values of eGFRCysC, and we identified certain patients had a discrepancy trend between eGFRCr and eGFRCysC. To identify conditions related to discrepancies, we traced the patterns of both types of eGFR results. To enhance accuracy, both Cr and CysC were measured using calibrations traceable to the international standard reference material.
Methods
Study population
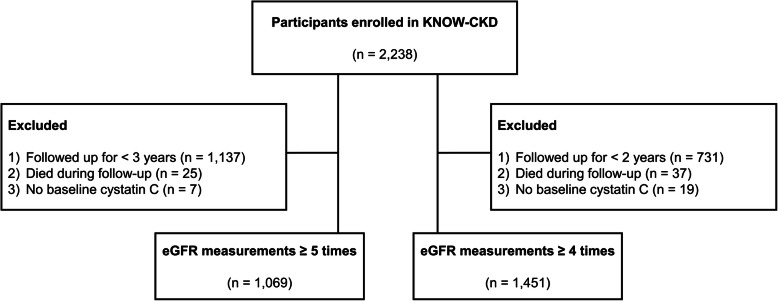
Study subjects were selected among participants in the KNOW-CKD, which is a representative prospective Korean pre-dialytic CKD cohort that began enrolling patients in 2011, wherein kidney transplant recipients were not included. The detailed design and method of the KNOW-CKD were described previously [12]. Briefly, a total of 2238 participants were enrolled in the KNOW-CKD study. Both serum Cr and CysC were measured at baseline, 6 months and 1 year after enrollment, and thereafter once per year. Patients who measured both eGFRCr and eGFRCysC ≥ 5 times from baseline were included. Patients who died during the follow-up period (n = 25) and those without baseline CysC (n = 7) were excluded. Consequently, 1069 patients were analyzed in the present study. For sensitivity analysis, we defined another group that included patients for whom clinicians measured both eGFRCr and eGFRCysC ≥ 4 times (Fig. 1).
Fig. 1.
Flow diagram of the study. eGFR, estimated glomerular filtration rate; Cr, creatinine; CysC, cystatin C
Variable measurements
Data for all of the covariates were collected at the time of enrollment including age, sex, comorbidities (diabetes, hypertension, age-adjusted Charlson comorbidity index), body mass index, body surface area, waist and hip circumference, systolic and diastolic pressures, and laboratory findings including white blood cell count, hemoglobin, platelet count, blood urea nitrogen, uric acid, calcium, phosphorus, alkaline phosphatase, total bilirubin, total cholesterol, low density lipoprotein, high density lipoprotein, triglyceride, fasting glucose, albumin, spot urine protein/creatinine ratio (uPCR), and spot urine albumin/creatinine ratio (uACR).
Blood and random voided urine (if possible, second urine in the morning) were collected. All of the samples were measured at a central laboratory (Lab Genomics, Gyeonggi-do, South Korea). Serum Cr was measured by the Jaffe rate blank method using alkaline picrate in a central laboratory and an assay traceable to isotope dilution mass spectrometry (IDMS) (ADVIA® Chemistry Creatinine 2, Siemens, Germany). Serum CysC was measured with a latex-particle enhanced immunoturbidimetric assay (ADVIA® Chemistry Cystatin C Reagents, Siemens, Germany) with calibration traceable to international reference material [13, 14]. The eGFR was estimated by serum Cr or/and CysC using the CKD Epidemiology Collaboration (CKD-EPI) equation [15]. Because of ethical issues and data protection regulations, data that support the findings of the present study cannot be made publicly available.
Statistical analysis
All statistical analyses were carried out using R (version 3.5.2; The R Foundation for Statistical Computing, Vienna, Austria). Continuous and categorical variables were presented as means±standard deviation and proportions, respectively. We used one-way analysis of variance and the χ2 test for comparisons of continuous variables and categorical variables, respectively. For trajectory analysis, we applied latent class mixed modeling (lcmm R package) and the R code is provided in the Supplemental materials. We calculated the entropy, Akaike’s information criteria and Bayesian information criteria for goodness-of-fit statistics and these were described in the Supplemental materials. Subsequently, we defined the discrepancy group as having decreasing eGFRCr but stable eGFRCysC and the agreement group as having both stable eGFRCr and eGFRCysC.
To identify factors related to discrepant trends, Firth’s penalized likelihood ratio method was used to account for rare events because of potential bias to the maximum likelihood estimator [16–18]. To identify independent conditions related to discrepant trends, univariate and multivariate logistic regression models with backward elimination method were applied. Adjusted variables in multivariate analysis conducted with or without the stepwise conditional method included age, sex, eGFR calculated with Cr and CysC (eGFRCrCysC) that represented a renal function, and variables that had P-values < 0.1 in univariate analysis. Statistical significance was set as P < 0.05 using two-tailed tests.
Ethics statement
The study protocol was approved by the Institutional Review Board at each participating clinical center [Seoul National University Hospital (1104–089-359), Seoul National University Bundang Hospital (B-1106/129–008), Yonsei University Severance Hospital (4–2011-0163), Kangbuk Samsung Medical Center (2011–01-076), Seoul St. Mary’s Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105–01), Chonnam National University Hospital (CNUH-2011-092), and Busan Paik Hospital (11–091)]. Written informed consent was obtained from each patient. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Results
Baseline characteristics
Baseline characteristics of total enrolled participants were described in Table 1. The mean age of these patients was 53.2 ± 12.1 years and 646 (60.4%) were male. Patients with diabetes and hypertension comprised 283 (26.5%) and 1031 (96.4%) patients, respectively. Mean values for eGFRCr, eGFRCysC, and eGFRCrCysC were 58.5 ± 28.9 mL/min/1.73 m2, 58.4 ± 31.2 mL/min/1.73 m2, and 58.0 ± 30.6 mL/ min/1.73 m2, respectively. Median values for uPCR and uACR were 0.4 g/g (0.1–1.0 g/g) and 272.4 mg/g (49.7–705.1 mg/g), respectively. The numbers of patients with uACR 3000 mg/g and uPCR > 3 g/g were 30 (2.8%) and 60 (5.6%), respectively.
Table 1.
Baseline characteristics of study participants
| Variables | Total (n = 1069) |
|---|---|
| Age (years) | 53.2 ± 12.1 |
| Male (%) | 60.4 |
| Age-adjusted Charlson comorbidity index (%) | 3.9 ± 1.8 |
| Low (≤3) | 58.7 |
| Moderate (4–5) | 27.2 |
| High (6–7) | 12.3 |
| Very high (≥8) | 1.8 |
| Diabetes mellitus (%) | 26.5 |
| Hypertension (%) | 96.4 |
| Systolic blood pressure (mmHg) | 126.1 ± 14.6 |
| Diastolic blood pressure (mmHg) | 76.4 ± 10.3 |
| Body mass index (kg/m2) | 24.5 ± 3.4 |
| Body surface area (m2) | 1.7 ± 0.2 |
| Systolic blood pressure (mmHg) | 126.1 ± 14.0 |
| Diastolic blood pressure (mmHg) | 76.4 ± 10.3 |
| Cause of chronic kidney disease (%) | |
| Diabetic nephropathy | 15.4 |
| Non-diabetic nephropathy | 84.6 |
| eGFR (ml/min/1.73 m2) | |
| eGFRCr | 58.5 ± 28.9 |
| eGFRCysC | 58.4 ± 31.2 |
| eGFRCrCysC | 58.0 ± 30.6 |
| Laboratory findings | |
| Hemoglobin (g/dL) | 13.21 ± 1.85 |
| Blood urea nitrogen (mg/dL) | |
| Uric acid (mg/dL) | 6.90 ± 1.86 |
| Phosphorus (mg/dL) | |
| Total bilirubin (mg/dL) | |
| Albumin (g/dL) | 4.26 ± 0.35 |
| uPCR (mean, interquartile range) | 0.4 (0.1–1.0) |
| < 0.3 g/g (%) | 45.0 |
| 0.3–0.9 g/g (%) | 30.6 |
| 1.0–3.0 g/g (%) | 18.8 |
| ≥ 3 g/g (%) | 5.6 |
| uACR (mean, interquartile range) | 272.4 (49.7–705.1) |
| < 30 mg/g (%) | 19.2 |
| 30–299 mg/g (%) | 33.4 |
| ≥ 300 mg/g (%) | 47.4 |
| ESRD event (%) | 69.0 |
eGFR Estimated glomerular filtration rate; Cr Creatinine; CysC Cystatin C; uPCR Urine protein/creatinine ratio; uACR Urine albumin creatinine ratio
Trajectory patterns of eGFRCr and eGFRCysC
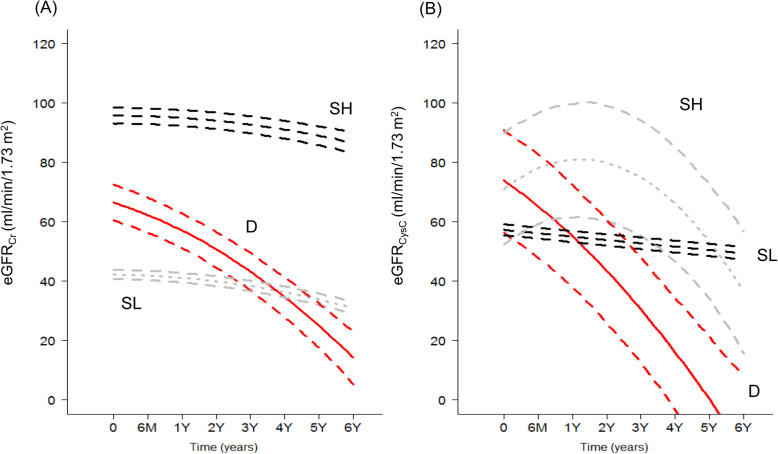
The relationship between baseline eGFRCr and eGFRCysC is shown as a Bland-Altman plot (Supplemental Figure 1). The mean value of difference was 0.148, and standard deviation was 11.327. The correlation coefficient (r) between eGFRCr and eGFRCysC was 0.93. We identified three distinct trajectory patterns for eGFRCr (Fig. 2): two groups with stable eGFRCr (stable with high eGFRCr [SH] and stable with low eGFRCr [SL]) and one group with decreasing eGFRCr (D). Trajectories of eGFRCysC also showed similar patterns, with two groups with stable eGFRCysC (SH and SL) and one group with decreasing eGFRCysC (D). Baseline characteristics according to the group of eGFRCr and eGFRCysC were described in Supplemental Table 1. Particularly, 69% of the ESRD events were occurred in the decreasing eGFRCr (D) group. We conducted cross-tabulation using these groups (Fig. 3). Most patients (97.6%; n = 1043) were classified into the SL group in eGFRCysC, followed by the SH (1.31%, n = 14) and D (1.12%, n = 12) groups. There were small numbers of patients in the D and SH groups with eGFRCysC.
Fig. 2.
Trajectory patterns of eGFRCr and eGFRCysC. eGFR, estimated glomerular filtration rate; Cr, creatinine; CysC, cystatin C; SH, stable and high eGFR group; SL, stable and low eGFR group; D, decreasing eGFR group
Fig. 3.
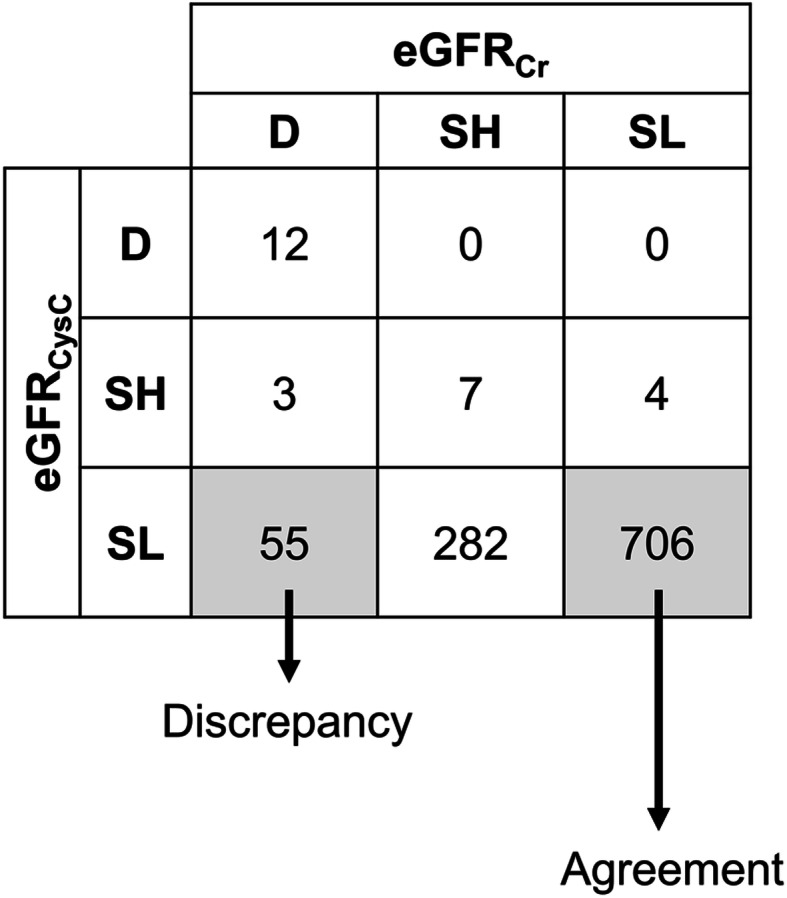
Cross-table between trends of eGFRCr and eGFRCysC. eGFR, estimated glomerular filtration rate; Cr, creatinine; CysC, cystatin C; SH, stable and high eGFR group; SL, stable and low eGFR group; D, decreasing eGFR group
Conditions related to discrepant trends between eGFRCr and eGFRCysC
Table 2 summarizes baseline characteristics according to discrepant trends. The patients in the agreement group were older than those in the discrepancy group. There were no differences in underlying disease, including diabetes and hypertension, or body mass index. Body surface area was greater in the discrepancy group than in the agreement group. Proteinuria values represented by uPCR and uACR and baseline renal function evaluated by eGFRCr, eGFRCysC, and eGFRCrCysC were higher in the discrepancy group than in the agreement group.
Table 2.
Baseline characteristics according to discrepancy between the trends of eGFRCr and eGFRCysC
| Discrepancy (n = 55) |
Agreement (n = 706) |
P | |
|---|---|---|---|
| Age (years) | 44.8 ± 10.3 | 56.8 ± 10.7 | < 0.001 |
| Male (%) | 61.8 | 62.2 | 0.957 |
| Age-adjusted Charlson comorbidity index (%) | 2.4 ± 1.7 | 4.0 ± 1.7 | < 0.001 |
| Low (≤3) | 74.5 | 42.9 | |
| Moderate (4–5) | 21.8 | 36.5 | |
| High (6–7) | 3.6 | 18.1 | |
| Very high (≥8) | 0 | 2.4 | |
| Diabetes (%) | 27.3 | 31.4 | 0.622 |
| Hypertension (%) | 98.2 | 98.9 | 1.000 |
| Systolic blood pressure (mmHg) | 128.0 ± 13.0 | 125.9 ± 14.7 | 0.306 |
| Diastolic blood pressure (mmHg) | 78.4 ± 10.7 | 75.7 ± 10.1 | 0.062 |
| Body mass index (kg/m2) | 24.4 ± 4.0 | 24.7 ± 3.3 | 0.514 |
| Body surface area (m2) | 1.8 ± 0.2 | 1.7 ± 0.2 | 0.036 |
| Systolic blood pressure (mmHg) | 128.0 ± 13.0 | 125.9 ± 14.7 | 0.306 |
| Diastolic blood pressure (mmHg) | 78.4 ± 10.7 | 75.7 ± 10.1 | 0.062 |
| Cause of chronic kidney disease (%) | 0.924 | ||
| Non-diabetic nephropathy | 81.8 | 80.3 | |
| Diabetic nephropathy | 18.2 | 19.7 | |
| eGFR (ml/min/1.73 m2) | |||
| eGFRCr | 66.4 ± 16.6 | 41.9 ± 15.2 | < 0.001 |
| eGFRCysC | 58.2 ± 19.72 | 41.6 ± 17.9 | < 0.001 |
| eGFRCrCysC | 61.0 ± 18.3 | 40.8 ± 16.1 | < 0.001 |
| Laboratory findings | |||
| Hemoglobin (g/dL) | 13.4 ± 1.7 | 12.9 ± 1.9 | 0.029 |
| Blood urea nitrogen (mg/dL) | 20.8 ± 5.8 | 20.2 ± 8.6 | < 0.001 |
| Uric acid (mg/dL) | 6.8 ± 1.7 | 7.4 ± 1.7 | 0.020 |
| Phosphorus (mg/dL) | 3.5 ± 0.5 | 3.6 ± 0.6 | 0.041 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.087 |
| Albumin (g/dL) | 4.2 ± 0.3 | 4.2 ± 0.3 | 0.967 |
| uPCR (mean, interquartile range) | 0.5 (0.2–1.5) | 0.4 (0.1–1.0) | 0.054 |
| < 0.3 g/g (%) | 30.9 | 41.3 | 0.011 |
| 0.3–0.9 g/g (%) | 27.3 | 33.1 | |
| 1.0–3.0 g/g (%) | 27.3 | 20.6 | |
| ≥ 3.0 g/g (%) | 14.5 | 5.0 | |
| uACR (mean, interquartile range g) | 427 (120–1206) | 295 (72–744) | 0.040 |
| < 30 mg/g (%) | 7.3 | 14.5 | 0.202 |
| 30–299 mg/g (%) | 32.7 | 36.1 | |
| ≥ 300 mg/g (%) | 60.0 | 49.4 | |
eGFR Estimated glomerular filtration rate; Cr Creatinine; CysC Cystatin C; uPCR Urine protein/creatinine ratio; uACR Urine albumin creatinine ratio
When the discrepancy group was set as the dependent variable, younger age and proteinuria were selected as predictors of discrepancies between trends of eGFRCr and eGFRCysC. When the backward elimination method was applied (model 2 in Table 3), age and proteinuria remained significant for predicting the discrepancy of trends. These results remained consistent in the subgroup analyses according to the age and proteinuria (Supplementary Tables 4, 5).
Table 3.
Analysis to identify conditions related to discrepant trends of eGFRCr and eGFRCysC
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P |
| Age | 0.92 (0.89–0.95) | < 0.001 | 0.92 (0.89–0.95) | < 0.001 |
| Male | 1.60 (0.61–4.26) | 0.343 | ||
| Age-adjusted CCI | ||||
| Low (≤3) | Reference | |||
| Moderate (4–5) | 1.30 (0.53–3.12) | 0.563 | ||
| High (6–7) | 1.66 (0.28–7.28) | 0.541 | ||
| Very high (≥8) | 2.48 (0.02–30.54) | 0.608 | ||
| Body surface area | 2.01 (0.18–20.83) | 0.562 | ||
| Diastolic blood pressure | 1.01 (0.98–1.04) | 0.579 | ||
| Hemoglobin | 0.90 (0.71–1.13) | 0.350 | ||
| Blood urea nitrogen | 0.99 (0.92–1.05) | 0.661 | ||
| Uric acid | 0.91 (0.74–1.12) | 0.379 | ||
| Phosphorus | 0.83 (0.42–1.62) | 0.590 | ||
| Total bilirubin | 2.00 (0.52–7.35) | 0.307 | ||
| uPCR (g/g) | ||||
| < 0.3 | Reference | Reference | ||
| 0.3–0.9 | 1.69 (0.49–5.26) | 0.392 | 1.53 (0.68–3.45) | 0.305 |
| 1.0–3.0 | 4.54 (0.95–21.44) | 0.058 | 3.32 (1.43–7.84) | 0.006 |
| ≥ 3.0 | 17.87 (3.14–102.19) | 0.001 | 12.38 (4.07–37.39) | < 0.001 |
| uACR (mg/g) | ||||
| < 30 | Reference | |||
| 30–299 | 2.53 (0.74–10.73) | 0.145 | ||
| ≥ 300 | 1.55 (0.27–10.19) | 0.630 | ||
| eGFRCrCysC | 1.07 (1.04–1.10) | < 0.001 | 1.07 (1.05–1.09) | < 0.001 |
Model 1: Adjusted for age, sex, eGFRCrCys and the variables which had P value less than 0.1 in univariate analysis
Model 2: Model 1 with backward elimination method
CCI Charlson comorbidities index; OR Odds ratio; CI Confidence interval; uPCR Urine protein/creatinine ratio; uACR Urine albumin/creatinine ratio; eGFR, estimated glomerular filtration rate; Cr Creatinine; CysC Cystatin C
Sensitivity analysis with patients for whom eGFRs were measured ≥4 times
The sensitivity analysis was conducted in patients for whom eGFRs were measured more than 4 times (n = 1451). The results for most baseline features were similar to those of the previous patient group (Supplemental Table 6). Their mean age was 53.2 ± 12.1 years old and 59.5% of enrolled patients were male. Diabetic patients accounted for 29.3%. Mean values of eGFRCr, eGFRCysC and eGFRCrCysC were 57.5 ± 29.6 mL/min/1.73 m2, 57.1 ± 31.4 mL/min/1.73 m2, and 56.9 ± 31.1 mL/ min/1.73 m2, respectively.
The trajectory patterns of eGFRCr and eGFRCysC were classified into 3 groups (Supplemental Figure 1), and there were discrepancies between trends similar to those observed in the main analysis (Supplemental Figure 2). In multivariate analysis, young patient age, proteinuria, and other variables such as male sex and large body surface area had tendencies for discrepancy compared with the counterpart groups (Supplemental Table 7).
Discussion
Information about eGFRs trends may be more helpful to predict prognosis than single measurements of eGFR. Although CysC has been used as an additional parameter to calculate GFR, eGFRCysC trends have not been evaluated and compared to those of eGFRCr. In the present study, we first compared eGFRCr and eGFRCysC trends and found that certain factors such as young age and proteinuria were related to discrepancies in trends between two eGFRs.
In the present study, we identified young age as a condition related to discrepancies between two eGFR trends, and the possible mechanisms are described as follows. There was a non-linear association between age and CysC concentration [19], and the increment rates of CysC levels were accelerated in patients aged over 50–60 years [20, 21]. Serum Cr remained relatively constant in healthy individuals between 20 and 70 years old [22]. Because there is a gap between the time point of increasing Cr and CysC, age may be a factor underlying discrepancies between eGFR trends. Additionally, when the CKD-EPI equation was developed, a large number of young patients were included from various diabetic cohorts [23], so that the proportions of younger diabetic patients differed from those in more recent studies (≤40 years, 11%; and 41–50 years, 20% in the KNOW-CKD cohort vs. ≤40 years, > 40% in the CKD-EPI-developing cohort). Such baseline differences might affect the non-GFR determinants of CysC because CysC is associated with insulin resistance, obesity, hypertension, and oxidative stress, which in turn are closely dependent on diabetes [24–26]. Inflammation could be a reason for the discrepancy between trends of eGFRCr and eGFRCysC, as inflammation is a representative determinant of CysC [27]. Although a wide ranges of inflammatory markers were not measured in the study cohort, young and old participants might have different inflammatory milieu that affects eGFRCysC trends. Because these hypotheses have not been thoroughly tested, further evaluations regarding the mechanisms underlying this phenomenon are needed.
Most filtered CysC is reabsorbed and metabolized by the proximal tubule cells [28, 29]. Previous study identified that the concentration of CysC was influenced by urine protein excretion, an influence stronger than that of Cr [30]. Similarly, several studies suggest that heavy proteinuria influenced renal handling of CysC [31, 32]. The association between urinary CysC and proteinuria was predominant in pediatric cases with nephrotic syndrome compared with controls [32]. Proteinuria itself decreases the tubular uptake of low molecular weight proteins, including CysC, primarily throughout the competition for a common transport mechanism in the preclinical model [31]. The present findings regarding the relationship between proteinuria and discrepant trends might be attributable to these factors.
Non-GFR determinants are well-known for serum Cr and CysC, respectively. A representative non-GFR determinant for Cr is muscle mass. Body mass index is a simple index for body composition but does not distinguish between excess fat, muscle, and bone mass [33, 34]. In the present study, we did not detect the independent significance of body mass index underlying the discrepancy between eGFR trends, although a dependent relationship with body surface area was detected. This difference might be because body mass index and body surface area do not reflect muscle mass. In the present study, the mean body mass index was 24.5 ± 3.4 kg/m2, which was lower than that of another CKD cohort (32.1 ± 7.9 kg/m2 in CRIC) [35]. In this respect, population-related factors also hamper the distinctive relationship between body mass index and muscle mass and thus, the effects of body mass index and body surface area might disappear in the final analysis.
The study has some limitations that deserve attention. The number of subjects was modest, although the statistical power was sufficient. Particularly, we could not compare the discrepant trends between some groups with low patient numbers. The study sample was entirely comprised of East Asians and CKD-EPI eGFR equations were not validated in the Korean population. As noted above, non-eGFRCr determinants such as muscle mass differed from those of individuals of European descent, which warrants further study to identify other significant conditions. The present findings were obtained from patients with non-dialytic CKD, and thus, the application of results to healthy individuals or the general population is limited. Standard measurements of GFR such as inulin excretion rate were not available for the study cohort, and such data would be useful to determine which trend was more accurate.
The results of the present study demonstrate discrepant conditions between trends from eGFRCr and eGFRCysC. Although further studies are needed to confirm our findings in other independent cohorts, clinicians should remain aware that discrepant conditions may occur when both Cr and CysC are used to evaluate and trace renal function. Because the present guidelines do not urge caution when determining the condition of eGFRCysC, our results may constitute the basis of future updates.
Conclusions
In conclusion, we identify conditions related to discrepant trends of eGFRCr and eGFRCysC. Clinicians should remain aware of such potential discrepancies when tracing both Cr and CysC.
Supplementary information
Acknowledgements
Not applicable.
Financial disclosure
The authors have nothing to disclose.
Conflict of interest
The authors have nothing to disclose.
Declarations
Nothing to declare.
Abbreviations
- Cr
Creatinine
- CysC
Cystatin C
- eGFR
Estimated glomerular filtration rate
- GFR
Glomerular filtration rate
- KNOW-CKD
KoreaN cohort study for outcomes in patients with chronic kidney disease
- CKD
Chronic kidney disease
- uPCR
Spot urine protein/creatinine ratio
- uACR
Spot urine albumin/creatinine ratio
- IDMS
Isotope dilution mass spectrometry
- CKD-EPI
Chronic kidney disease epidemiology collaboration
- SH
Stable with high eGFR
- SL
Stable with low eGFR
- D
Decreasing eGFR
Authors’ contributions
Study design: SSH, KHO and CA, Acquisition of Data: SKP, WC, YKO, DWC, YSK, and KHO, Data analysis: EK, SSH and JK, Writing the manuscript: EK and SSH, Review, revision and final approval: SSH and KHO. All authors have read and approved the manuscript.
Funding
This study was supported by the research program funded by the Korea Center for Disease Control and Prevention (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, and 2016E3300200). The study was supervised by the CKD Advisory Committee composed of members from the KCDC and the Korean Society of Nephrology (KSN, NCT01630486 at http://www.clinicaltrials.gov). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The dataset can be available that is within the perspective of the scientific objectives of KNOW-CKD and researchers who approved by the KNOW-CKD investigators can be accessed the data (http://www.know-ckd.org/ckd/main/main.html).
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board at each participating clinical center [Seoul National University Hospital (1104–089-359), Seoul Navtional University Bundang Hospital (B-1106/129–008), Yonsei University Severance Hospital (4–2011-0163), Kangbuk Samsung Medical Center (2011–01-076), Seoul St. Mary’s Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105–01), Chonnam National University Hospital (CNUH-2011-092), and Busan Paik Hospital (11–091)]. Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eunjeong Kang and Seung Seok Han co-first authors
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12882-020-01932-4.
References
- 1.Levey AS, Fan L, Eckfeldt JH, Inker LA. Cystatin C for glomerular filtration rate estimation: coming of age. Clin Chem. 2014;60(7):916–919. doi: 10.1373/clinchem.2014.225383. [DOI] [PubMed] [Google Scholar]
- 2.Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H. Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218(5):499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 3.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45(2):97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 4.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Balasubramanian S, McDonald JR, Scherrer JF, O'Hare AM. Greater variability in kidney function is associated with an increased risk of death. Kidney Int. 2012;82(11):1208–1214. doi: 10.1038/ki.2012.276. [DOI] [PubMed] [Google Scholar]
- 6.Tielemans SM, Geleijnse JM, Menotti A, Boshuizen HC, Soedamah-Muthu SS, Jacobs DR, Jr, Blackburn H, Kromhout D. Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota business and professional men study and the Zutphen study. J Am Heart Assoc. 2015;4(3):e001378. doi: 10.1161/JAHA.114.001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. Jama. 2003;289(18):2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara K, Honda T, Nakagawa T, Yamamoto S, Hayashi T, Mizoue T. Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci Rep. 2017;7:43521. doi: 10.1038/srep43521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Hare AM, Batten A, Burrows NR, Pavkov ME, Taylor L, Gupta I, Todd-Stenberg J, Maynard C, Rodriguez RA, Murtagh FE, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59(4):513–522. doi: 10.1053/j.ajkd.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z. Estimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortality. Am J Kidney Dis. 2016;68(2):219–228. doi: 10.1053/j.ajkd.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, Han SH, Yoo TH, Lee K, Kim YS, et al. KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. 2014;15:80. doi: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blirup-Jensen S, Grubb A, Lindstrom V, Schmidt C, Althaus H. Standardization of Cystatin C: development of primary and secondary reference preparations. Scand J Clin Lab Invest Suppl. 2008;241:67–70. doi: 10.1080/00365510802150067. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FIRTH D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 17.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 18.Heinze G. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat Med. 2006;25(24):4216–4226. doi: 10.1002/sim.2687. [DOI] [PubMed] [Google Scholar]
- 19.Odden MC, Tager IB, Gansevoort RT, Bakker SJ, Katz R, Fried LF, Newman AB, Canada RB, Harris T, Sarnak MJ, et al. Age and cystatin C in healthy adults: a collaborative study. Nephrol Dial Transplant. 2010;25(2):463–469. doi: 10.1093/ndt/gfp474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37(1):79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 21.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37(Pt 1):49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 22.Delanaye P, Cavalier E, Pottel H. Serum Creatinine: not so simple! Nephron. 2017;136(4):302–308. doi: 10.1159/000469669. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surendar J, Indulekha K, Aravindhan V, Ganesan A, Mohan V. Association of cystatin-C with metabolic syndrome in normal glucose-tolerant subjects (CURES-97) Diabetes Technol Ther. 2010;12(11):907–912. doi: 10.1089/dia.2010.0077. [DOI] [PubMed] [Google Scholar]
- 25.Servais A, Giral P, Bernard M, Bruckert E, Deray G, Isnard Bagnis C. Is serum cystatin-C a reliable marker for metabolic syndrome? Am J Med. 2008;121(5):426–432. doi: 10.1016/j.amjmed.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Demircan N, Gurel A, Armutcu F, Unalacak M, Aktunc E, Atmaca H. The evaluation of serum cystatin C, malondialdehyde, and total antioxidant status in patients with metabolic syndrome. Med Sci Monit. 2008;14(2):Cr97–C101. [PubMed] [Google Scholar]
- 27.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahran A, El-Husseini A, Shoker A. Can cystatin C replace creatinine to estimate glomerular filtration rate? A literature review. Am J Nephrol. 2007;27(2):197–205. doi: 10.1159/000100907. [DOI] [PubMed] [Google Scholar]
- 29.Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, Padrini R, Palatini P. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002;48(6 Pt 1):850–858. [PubMed] [Google Scholar]
- 30.Liu X, Foster MC, Tighiouart H, Anderson AH, Beck GJ, Contreras G, Coresh J, Eckfeldt JH, Feldman HI, Greene T, et al. Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. doi: 10.1053/j.ajkd.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thielemans N, Lauwerys R, Bernard A. Competition between albumin and low-molecular-weight proteins for renal tubular uptake in experimental nephropathies. Nephron. 1994;66(4):453–458. doi: 10.1159/000187863. [DOI] [PubMed] [Google Scholar]
- 32.Tkaczyk M, Nowicki M, Lukamowicz J. Increased cystatin C concentration in urine of nephrotic children. Pediatr Nephrol. 2004;19(11):1278–1280. doi: 10.1007/s00467-004-1566-1. [DOI] [PubMed] [Google Scholar]
- 33.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond). 29(1):2005, 1–8. [DOI] [PubMed]
- 34.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60(1):23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset can be available that is within the perspective of the scientific objectives of KNOW-CKD and researchers who approved by the KNOW-CKD investigators can be accessed the data (http://www.know-ckd.org/ckd/main/main.html).