Abstract
OBJECTIVE
To explore gallbladder- and biliary tract–related events reported for the liraglutide and placebo groups in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial.
RESEARCH DESIGN AND METHODS
LEADER was an international, randomized, double-blind, controlled cardiovascular (CV) outcomes trial. Participants with type 2 diabetes at high risk for CV events (n = 9,340) were randomized 1:1 to receive either liraglutide (≤1.8 mg daily; n = 4,668) or placebo (n = 4,672), with both groups also receiving standard care (treatment period: 3.5–5 years). Acute gallstone disease was a medical event of special interest. This post hoc analysis categorized captured events of acute gallbladder or biliary disease into four groups: uncomplicated gallbladder stones, complicated gallbladder stones, cholecystitis, and biliary obstruction. Time to first event by treatment group was analyzed using Cox regression.
RESULTS
There was an increased risk of acute gallbladder or biliary disease with liraglutide versus placebo (n = 141 of 4,668 vs. n = 88 of 4,672 patients, respectively; hazard ratio [HR] 1.60; 95% CI 1.23, 2.09; P < 0.001). Similar trends were observed for each of the four categories of gallbladder- or biliary tract–related events. Cholecystectomy was performed more frequently in liraglutide-treated patients (HR 1.56; 95% CI 1.10, 2.20; P = 0.013) but for similar proportions of the patients who experienced gallbladder- or biliary tract–related events (57% with liraglutide vs. 59% with placebo).
CONCLUSIONS
Although LEADER was not specifically designed to assess acute gallbladder or biliary disease, the trial showed an increased risk of gallbladder- or biliary tract–related events with liraglutide versus placebo, which appeared to be consistent across four categories of these events. Further studies should investigate the relevant mechanisms.
Introduction
Patients with type 2 diabetes are at approximately twofold greater risk of biliary disease than those without diabetes (1). Research into the mechanisms underlying gallstone disease is ongoing, but these mechanisms are thought to be linked to insulin resistance, obesity, the metabolic syndrome, and type 2 diabetes (2).
Treatment of type 2 diabetes with glucagon-like peptide 1 receptor agonists (GLP-1RAs) has also been associated with increased risk of gallbladder events in clinical trials, including higher rates of cholelithiasis, versus comparators (3–5). Possible heterogeneity in the risk of gallbladder events across the GLP-1RA class is reflected by the current medication labels, which do not include these events as adverse drug reactions for all GLP-1RAs (6,7). Cholelithiasis is a frequently observed side effect of weight-lowering therapies (8). For example, in people with obesity, the prevalence of new gallstones can exceed 10% after 8–16 weeks of a low-calorie diet and exceed 30% after gastric bypass surgery (8). Use of GLP-1RAs for the treatment of type 2 diabetes is associated with weight loss (9,10). Therefore, weight loss should not be disregarded when considering findings of increased gallbladder-related adverse events (AEs) (11). However, other mechanisms may be involved (4,11). For example, it has also been suggested that GLP-1RAs may have an inhibitory effect on gallbladder motility (4,12).
A higher incidence of gallbladder-related AEs was reported with liraglutide 3.0 mg versus placebo in the clinical development program of this GLP-1RA for weight management (13–15), although no imbalances in gallbladder-related AEs were observed between liraglutide 1.8 mg and comparators in the type 2 diabetes clinical development program (16–22).
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) cardiovascular (CV) outcomes trial (CVOT) of liraglutide included patients with type 2 diabetes at high CV risk (23). The trial showed significantly decreased risks of the primary composite major adverse CV events outcome (time to first occurrence of CV death, nonfatal myocardial infarction, or nonfatal stroke), CV death, and all-cause death with liraglutide versus placebo (23). Rates of nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure were nonsignificantly lower with liraglutide versus placebo, while coronary revascularization and hospitalization for unstable angina pectoris were each experienced by similar proportions of patients in the two treatment groups (23). Liraglutide was associated with significant reductions from baseline in glycated hemoglobin, weight, and systolic blood pressure versus placebo and an increase in diastolic blood pressure and heart rate at 36 months (23).
In LEADER, the proportion of patients with acute gallstone disease was higher with liraglutide than with placebo (in 145 [3.1%] and 90 [1.9%] patients, respectively; P < 0.001) (23). This finding was driven by greater proportions of patients experiencing cholelithiasis (68 patients [1.5%] vs. 50 [1.1%]) and acute cholecystitis (36 patients [0.8%] vs. 21 [0.4%]) with liraglutide compared with placebo (23). However, to date, data regarding the subtypes of gallstone disease observed, and their implications (e.g., cholecystectomy), have not been fully described.
The aim of the present post hoc analysis was to explore the clinical features of gallbladder- and biliary tract–related events reported for the liraglutide and placebo groups in LEADER. We also examined the relationship among liraglutide, the risk of gallbladder- or biliary tract–related events, and body weight loss.
Research Design and Methods
Study Design and Oversight
Methods relating to the LEADER trial, including the trial protocol, have previously been published (23,24). Briefly, 410 sites from 32 countries participated in this randomized, double-blind, placebo-controlled trial. The trial included patients with type 2 diabetes at high risk for CV events (aged ≥50 years with either established CV disease or chronic kidney disease or aged ≥60 years with ≥1 CV risk factor). Participants were randomized 1:1 to receive either 1.8 mg s.c. liraglutide daily (or maximum tolerated dose of 0.6–1.8 mg daily) or matching placebo—both in addition to standard-of-care therapy. The treatment period was 3.5–5 years, with a 30-day follow-up period. The primary outcome was time to first occurrence of CV death, nonfatal (including silent) myocardial infarction, or nonfatal stroke. The trial was approved by institutional review boards, and all patients provided written informed consent (23).
A selective approach to safety data collection was used (25), and only events meeting the definition of a serious AE or medical event of special interest (MESI) were systematically collected and reported. Acute gallstone disease, defined as biliary colic or acute cholecystitis, was prespecified as a MESI. These events, both serious and nonserious, were required to be reported. As with other prespecified MESIs, information related to acute gallstone disease events was collected using a designated form. Events were captured for analysis based on prespecified standard search terms from the Medical Dictionary for Regulatory Activities (MedDRA), version 18.0 (standard MedDRA queries [SMQs]): functional, inflammatory, and gallstone-related biliary disorders (SMQ); infectious biliary disorders (SMQ).
In this post hoc analysis, baseline characteristics of patients with events of acute gallbladder or biliary disease were evaluated. A blinded post hoc review, performed by the sponsor and first author, grouped the events of acute gallbladder or biliary disease by nature and severity into more clinically meaningful categories. This review included assessment of clinical features; imaging findings of cholecystitis, cholelithiasis, or bile duct stones; pain; clinical signs of cholestasis; interventions including cholecystectomy or endoscopic retrograde cholangiopancreatography (ERCP); hospitalizations; fever; treatment with antibiotics; and reason for investigation. Events were classified into four categories: uncomplicated gallbladder stones, complicated gallbladder stones, cholecystitis with/without/unknown gallstones, and biliary obstruction (Table 1).
Table 1.
Categorization of events according to the algorithm
| Category | Criteria |
|---|---|
| Uncomplicated gallbladder stones | - Events with imaging results supportive of gallbladder stones and not supportive of cholecystitis or bile duct stones. |
| - Absence of pain or clinical signs of cholestasis. | |
| - Event not leading to cholecystectomy or ERCP at a later stage. | |
| Complicated gallbladder stones | - Events with imaging results suggestive of gallbladder stones and not supportive of cholecystitis or bile duct stones AND |
| - Presence of typical pain AND/OR leading to cholecystectomy or ERCP. | |
| Cholecystitis with/without gallbladder stones | - Events with imaging results suggestive of cholecystitis AND not supportive of bile duct stones. |
| - Presence of typical pain AND/OR leading to cholecystectomy or ERCP. | |
| Biliary obstruction | - Events with imaging results suggestive of bile duct stones. |
| Additional clinical review | In addition to the above strict criteria, events grouped as “complicated gallbladder stones” and that had copresence of either fever and/or clinical signs of cholestasis were reviewed and potentially regrouped. Events with presence of clinical signs of cholestasis but with imaging results showing no presence of bile duct stones were reviewed and grouped into one of the four categories. |
Events for which sufficient information was reported were classified using an algorithm (as defined in Table 1). Events with insufficient information for algorithm-based classification were further reviewed and classified manually by the sponsor and first author into the four categories based on all available data and clinical assessment of the event as a whole. This analysis mainly relied on the clinical diagnoses provided by the investigators, even if there was limited additive information. Events categorized by this review were termed “non–algorithm-based events.”
Analyses were performed using the pooled categorized events (both algorithm-based and non–algorithm-based events). Events captured by the MedDRA search but found during case review to be unrelated to biliary disease were excluded from subsequent analyses. It should be noted that a single patient could have had several events during the trial and therefore could be represented in different categories.
Body weight was ascertained at baseline, 6 and 12 months after randomization, and then every 12 months until end of treatment.
Statistical Analyses
Time to first event by treatment (liraglutide vs. placebo) was analyzed using Cox regression analysis, with treatment as a fixed factor, and visualized using Kaplan-Meier curves. Separate analyses were conducted for each of the gallbladder- or biliary tract–related event categories. Patients without an event of interest were censored at time of death or time of last follow-up. No adjustment for multiple testing was performed.
Additional analyses were performed that explored the relationship among treatment, the risk of gallbladder- or biliary tract–related events, and body weight loss. The additional time-dependent analyses used Cox regression, with treatment as a fixed factor and change from baseline in body weight as a time-dependent covariate, adjusted for body weight at baseline. Change in body weight was calculated for each applicable visit that was prior to an event or censoring.
Data and Resource Availability
The subject-level analysis data sets for the research presented in this article are available from the corresponding author upon reasonable request.
Results
In LEADER, 9,340 patients were randomized to either liraglutide (n = 4,668) or placebo (n = 4,672) and followed for a median of 3.8 years. The disposition of trial participants has previously been published (23).
A schematic overview of the review and categorization of gallbladder- and biliary tract–related events is available in Supplementary Fig. 1. In total, 275 events were identified in 235 patients. Seven events were excluded from the analyses after review, as these were assessed to be unrelated to biliary disease (Supplementary Fig. 1 and Supplementary Table 1). Of the remaining 268 events, 201 events in 181 patients were identified by the algorithm-based classification and 67 events in 52 patients by the non–algorithm-based classification (Supplementary Fig. 1). For the purposes of these analyses, we focused on the pooled algorithm-based and non–algorithm-based events, since algorithm-based and clinical diagnosis–related classification led to broadly similar proportions for each category (Supplementary Fig. 1). Categories of gallbladder and biliary disease by algorithm-based and non–algorithm-based events are shown in Supplementary Table 2.
In this exploratory, post hoc analysis, there was an increased risk of gallbladder- or biliary tract–related events with liraglutide versus placebo (n = 141 vs. n = 88 patients, respectively; hazard ratio [HR] 1.60; 95% CI 1.23, 2.09; P < 0.001) (Fig. 1A). Baseline characteristics of the 229 patients with gallbladder- or biliary tract–related events were similar between the treatment groups and comparable with the total study population (Table 2). Among patients with gallbladder- or biliary tract–related events, fewer in the liraglutide group had a history of cholecystitis at screening than in the placebo group, but this observation was based on low patient numbers (Table 2). More patients treated with liraglutide than those treated with placebo had uncomplicated gallbladder stones (n = 16 and n = 5 for liraglutide and placebo), complicated gallbladder stones (n = 52 and n = 40), cholecystitis (n = 51 and n = 33), and biliary obstruction (n = 25 and n = 16) (Table 3 and Fig. 1B–E). In separate evaluations as algorithm-based and non–algorithm-based events, a similar pattern of results (more events with liraglutide across most categories) was observed (Supplementary Table 2).
Figure 1.
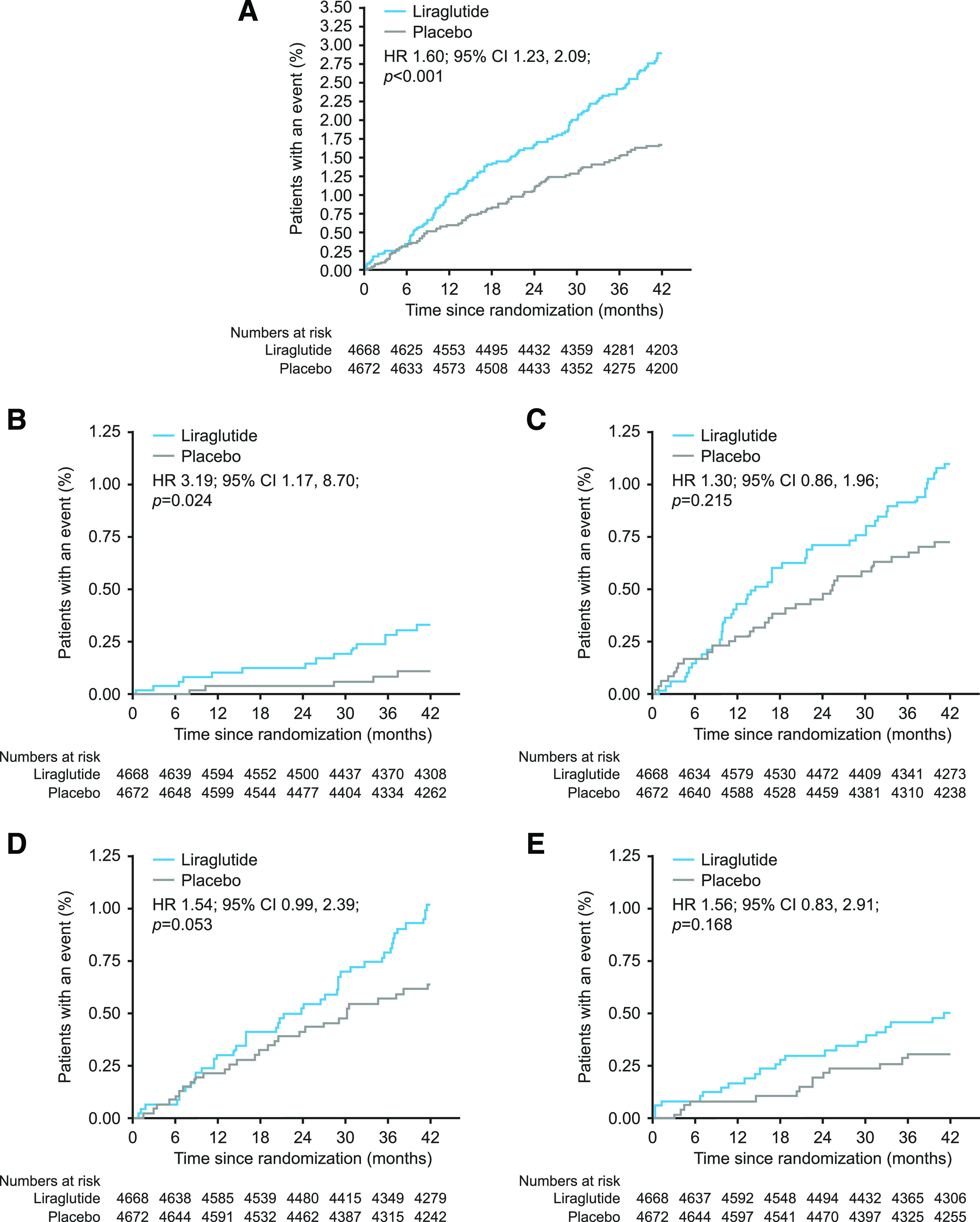
Time to first gallbladder or biliary disease event in patients from all four gallbladder- or biliary tract–related event categories (A), patients with uncomplicated gallbladder stones (B), patients with complicated gallbladder stones (C), patients with cholecystitis (D), and patients with biliary obstruction (E). The two-sided P values are from the testing of the hypothesis of no difference between treatments. The parameter has been analyzed using a Cox regression model with treatment as a fixed factor. No adjustment for multiple testing was performed. The x-axes are truncated at 42 months because a smaller number of patients had an observation time beyond the minimum treatment period of 42 months.
Table 2.
Demographics at baseline or screening and clinical characteristics
| Patients with gallbladder- or biliary tract–related events |
Patients without gallbladder- or biliary tract–related events |
Total patients in LEADER | |||
|---|---|---|---|---|---|
| Treated with liraglutide | Treated with placebo | Treated with liraglutide | Treated with placebo | ||
| n | 141 | 88 | 4,527 | 4,584 | 9,340 |
| Age (years) | 65.4 (7.9) | 65.0 (7.4) | 64.2 (7.2) | 64.4 (7.2) | 64.3 (7.2) |
| Male, n (%) | 86 (61.0) | 53 (60.2) | 2,925 (64.6) | 2,939 (64.1) | 6,003 (64.3) |
| BMI (kg/m2) | 32.8 (5.9) | 32.9 (6.9) | 32.5 (6.3) | 32.5 (6.3) | 32.5 (6.3) |
| Weight (kg) | 93.2 (21.1) | 90.9 (22.1) | 91.9 (21.2) | 91.6 (20.7) | 91.7 (21.0) |
| HbA1c (%) | 8.6 (1.5) | 9.0 (1.7) | 8.7 (1.6) | 8.7 (1.5) | 8.7 (1.5) |
| HbA1c (mmol/mol) | 70.3 (16.2) | 75.1 (18.3) | 72.0 (17.0) | 71.0 (16.3) | 71.5 (16.7) |
| Diabetes duration (years) | 12.8 (8.3) | 12.0 (7.7) | 12.8 (7.9) | 12.9 (8.1) | 12.8 (8.0) |
| Pancreatitis and gallbladder history, n (%) | |||||
| Pancreatitis | 5 (3.5) | 5 (5.7) | 141 (3.1) | 113 (2.5) | 264 (2.8) |
| Gallstone disease | 20 (14.2) | 14 (15.9) | 549 (12.1) | 520 (11.3) | 1,103 (11.8) |
| Cholecystitis | 4 (2.8) | 11 (12.5) | 339 (7.5) | 313 (6.8) | 667 (7.1) |
| Lipids (mmol/L) | |||||
| HDL cholesterol | 1.2 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) |
| LDL cholesterol | 2.4 (0.9) | 2.4 (1.0) | 2.3 (0.9) | 2.3 (0.9) | 2.3 (0.9) |
| Total cholesterol | 4.5 (1.2) | 4.5 (1.1) | 4.4 (1.2) | 4.4 (1.2) | 4.4 (1.2) |
| Triglycerides | 2.1 (1.3) | 2.4 (2.6) | 2.1 (1.5) | 2.0 (1.7) | 2.1 (1.6) |
| Biochemistry | |||||
| Total bilirubin (μmol/L) | 7.9 (4.5) | 7.8 (4.1) | 7.8 (4.1) | 7.8 (4.3) | 7.8 (4.2) |
| ALT (units/L) | 25.9 (14.7) | 25.9 (15.4) | 27.0 (16.6) | 26.5 (15.7) | 26.8 (16.1) |
| Weight change from baseline at end of treatment (kg) | −5.3 (10.1) | −3.3 (8.8) | −3.0 (7.1) | −0.6 (6.6) | — |
Values are mean (SD) unless otherwise stated. Pancreatitis and gallbladder history data were obtained at screening. ALT, alanine aminotransferase; HbA1c, glycated hemoglobin; n, number of patients.
Table 3.
Categories of gallbladder or biliary disease complications (pooled algorithm-based and non–algorithm-based events)
| Category of gallbladder or biliary complication | Liraglutide, n = 4,668 |
Placebo, n = 4,672 |
Total, n = 9,340 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | E | N | % | E | N | % | E | |
| All events | 141 | 3.02 | 155 | 88 | 1.88 | 113 | 229 | 2.45 | 268 |
| Uncomplicated gallbladder stones | 16 | 0.34 | 17 | 5 | 0.11 | 7 | 21 | 0.22 | 24 |
| Complicated gallbladder stones | 52 | 1.11 | 54 | 40 | 0.86 | 42 | 92 | 0.99 | 96 |
| Leading to cholecystectomy | 31 | 0.66 | 32 | 22 | 0.47 | 22 | 53 | 0.57 | 54 |
| Cholecystitis | 51 | 1.09 | 56 | 33 | 0.71 | 35 | 84 | 0.90 | 91 |
| Without gallbladder stones | 19 | 0.41 | 21 | 8 | 0.17 | 8 | 27 | 0.29 | 29 |
| With gallbladder stones | 30 | 0.64 | 33 | 22 | 0.47 | 24 | 52 | 0.56 | 57 |
| Unknown stone status | 2 | 0.04 | 2 | 3 | 0.06 | 3 | 5 | 0.05 | 5 |
| Leading to cholecystectomy | 39 | 0.84 | 43 | 26 | 0.56 | 28 | 65 | 0.70 | 71 |
| Biliary obstruction | 25 | 0.54 | 28 | 16 | 0.34 | 29 | 41 | 0.44 | 57 |
| Bile duct stones | 22 | 0.47 | 24 | 13 | 0.28 | 18 | 35 | 0.37 | 42 |
| Bile duct stenosis | 3 | 0.06 | 4 | 3 | 0.06 | 11 | 6 | 0.06 | 15 |
| Intervention | |||||||||
| ERCP | 9 | 0.19 | 11 | 6 | 0.13 | 18 | 15 | 0.16 | 29 |
| Papillotomy | 4 | 0.09 | 5 | 6 | 0.13 | 10 | 10 | 0.11 | 15 |
| Stenting (biliary prosthesis) | 1 | 0.02 | 2 | 2 | 0.04 | 11 | 3 | 0.03 | 13 |
| Cholecystectomy | 12 | 0.26 | 13 | 8 | 0.17 | 9 | 20 | 0.21 | 22 |
Seven events and six patients were excluded, since the seven events were assessed to be unrelated to biliary disease. The seven excluded events occurred in seven patients, but, as one of the patients also had another gallbladder- or biliary tract–related event, this patient was included in the analyses for the other event. For some patients, cholecystectomy was reported for two separate events with the same start date. Therefore, more cholecystectomy events than patients with cholecystectomy are listed for some categories of gallbladder or biliary disease. E, number of events; n, number of patients; N, number of patients with an event; %, percentage of patients.
Cholecystectomy was more common in patients treated with liraglutide versus placebo (n = 81 [1.74%] and n = 52 [1.11%], respectively; HR 1.56; 95% CI 1.10, 2.20; P = 0.013). However, among patients who had a gallbladder- or biliary tract–related event during the trial, the proportion undergoing cholecystectomy was similar with liraglutide versus placebo: 57% vs. 59% (n = 81 of 141 patients who experienced gallbladder- or biliary tract–related events in the liraglutide group vs. n = 52 of 88 patients who experienced gallbladder- or biliary tract–related events in the placebo group).
The average weight loss in the trial was greater in patients experiencing a gallbladder- or biliary tract–related event with liraglutide (−5.3 kg) or placebo (−3.3 kg) compared with those patients not experiencing an event (−3.0 kg and −0.6 kg for liraglutide and placebo, respectively) (Table 2). Additional exploratory analyses, using Cox regression adjusted for body weight at baseline, with change in body weight as a time-dependent covariate and assuming a crude linear relationship with risk of gallbladder- or biliary tract–related events, showed a slightly attenuated effect of liraglutide versus placebo on the risk of a gallbladder- or biliary tract–related event (HR 1.47; 95% CI 1.12, 1.92) compared with the main analysis (HR 1.60; 95% CI 1.23, 2.09). Qualitatively similar results were obtained from a mediation analysis assessing the role of weight loss in gallbladder events with liraglutide treatment (data not shown). The time-dependent Cox regression analyses also indicated that a weight loss of 1 kg during the trial was associated with an ∼4% increase of the risk of a gallbladder- or biliary tract–related event.
Conclusions
In LEADER, the overall risk of acute gallstone disease was increased with liraglutide versus placebo (23). In this post hoc analysis of LEADER data, which explored gallbladder- and biliary tract–related events after clinical review, the increase in the overall risk of gallbladder- or biliary tract–related events remained (Fig. 1A). The observation appeared to be consistent across the four categories of gallbladder- or biliary tract–related events (uncomplicated gallbladder stones, complicated gallbladder stones, cholecystitis with/without gallbladder stones, and biliary obstruction).
From a clinical perspective, the severity and implications of the gallbladder- and biliary tract–related events (e.g., cholecystectomy) are of interest. The four categories of gallbladder- and biliary tract–related events used in this analysis represent, to some extent, different severities across the spectrum of gallbladder- and biliary tract–related diseases. The estimated effect sizes (HRs) for the different categories of gallbladder- or biliary tract–related events all showed an imbalance with liraglutide versus placebo, favoring placebo. We also showed that cholecystectomy was performed more frequently for liraglutide-treated than placebo-treated patients but for similar proportions of patients who experienced gallbladder- or biliary tract–related events during the trial. The latter, nonrandomized comparison suggests that gallbladder- and biliary tract–related events may have had similar severity and implications with liraglutide and placebo, on average, based on the relative need for cholecystectomy. However, this comparison should be interpreted with caution, as patients who experienced gallbladder- or biliary tract–related events in the liraglutide or placebo groups may not have been comparable across these treatment groups. It should also be noted that cholecystectomy was more common in patients treated with liraglutide versus placebo overall, probably because more gallbladder- and biliary tract–related events occurred in liraglutide-treated individuals. These results are consistent with findings from a trial of liraglutide 3.0 mg conducted in people without type 2 diabetes, in which more gallbladder-related events were observed in liraglutide-treated participants compared with placebo-treated participants, but there were similar proportions of elective cholecystectomies among those with cholelithiasis or cholecystitis (15).
Gallbladder events are the most common cause of acute pancreatitis, accounting for ∼45% of cases (26). While numbers of relevant events were low, the LEADER trial demonstrated that fewer patients treated with liraglutide experienced acute pancreatitis compared with those treated with placebo (n = 18 and n = 23 for liraglutide and placebo, respectively) (23,27). This observation suggests that the increase in gallbladder- and biliary tract–related events observed with liraglutide versus placebo did not result in an increased occurrence of acute pancreatitis events in the LEADER trial.
CVOTs of exenatide once weekly and lixisenatide reported that more patients experienced cholecystitis/cholelithiasis events (n = 178 [2.4%] for exenatide and n = 146 [2.0%] for placebo) or hepatobiliary serious AEs (n = 36 [1.2%] for lixisenatide and n = 28 [0.9%] for placebo), respectively, with GLP-1RA treatment (28,29). Moreover, a numeric imbalance in hepatobiliary disorders was observed with albiglutide versus placebo in the Harmony Outcomes trial (n = 51 patients [1%] for albiglutide and n = 41 patients [1%] for placebo; relative risk 1.24; 95% CI 0.83, 1.87) (30). In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN 6), a CVOT with eligibility criteria similar to those for LEADER, gallbladder disorders occurred in similar numbers of patients treated with subcutaneous semaglutide and placebo (n = 58 vs. n = 61, respectively) (31). Interestingly, this observation was made despite an overall higher mean body weight loss with the active drug relative to placebo in SUSTAIN 6 at 2 years (2.9 kg for 0.5 mg semaglutide and 4.3 kg for 1.0 mg semaglutide) (31) compared with LEADER at 3 years (in which weight loss with liraglutide relative to placebo was 2.3 kg) (23). The relatively short duration of follow-up in the SUSTAIN 6 trial (2.1 years) should be noted, however (31). While no imbalance in gallbladder disorders was observed with semaglutide versus placebo, it remains possible that an imbalance may have emerged after a longer duration of follow-up. Possible heterogeneity in the relationship between gallbladder events and different GLP-1RAs is reflected by the current medication labels, which do not list these events as adverse drug reactions for all GLP-1RAs (6,7). However, more evidence is needed regarding the relative effects of individual GLP-1RAs on gallbladder events and the underlying mechanisms.
The increased risk of gallbladder- and biliary tract–related events reported in the present post hoc analysis could potentially be due to an investigation bias related to the emergence of gastrointestinal symptoms with liraglutide (based on phase IIIa trials, nausea and diarrhea are observed in ≥1 of 10 patients) (32). If true, the increased risk would be expected to result from a greater number of uncomplicated (asymptomatic) cases, particularly early in the course of therapy (2–4 months), when gastrointestinal AEs are more common with liraglutide. A greater number of uncomplicated events was indeed observed with liraglutide, and a between-group difference in the proportion of patients with these events emerged shortly after randomization (Fig. 1B). However, these observations were based on low numbers of patients in both treatment groups. Furthermore, the imbalance favoring placebo was not confined to uncomplicated events but, rather, was observed across all four clinical categories of gallbladder- or biliary tract–related events, and gallbladder events accrued at a relatively constant rate throughout the trial.
Weight loss in patients undergoing low-calorie diets or gastric bypass surgery is associated with an increased risk of gallstones (8). GLP-1RA treatment is also associated with weight loss (5,9). Liraglutide 1.8 mg daily reduces energy intake in patients with type 2 diabetes versus placebo, without significantly altering macronutrient distribution, which may contribute to liraglutide-induced weight loss (33). Rapid weight loss mobilizes cholesterol from the liver and adipose tissue, and in the short term, fasting increases the cholesterol saturation of gallbladder bile (34). In the longer term, fasting causes gallbladder stasis, which can lead to sludge and gallstone formation (34). Therefore, reduced energy intake and weight loss associated with liraglutide could represent potential mechanisms to explain, in part, the effects of liraglutide on gallbladder- or biliary tract–related events (11,33). Other medications associated with weight loss and increased frequencies of gallbladder- or biliary tract–related events versus placebo include orlistat (cholelithiasis: 2.9% vs. 1.8% with placebo) and naltrexone/bupropion (cholecystitis reported in <2% of patients but with an incidence at least twice that of placebo) (35,36). However, this is not a uniform finding in studies examining treatments associated with weight loss. For example, weight loss has been observed with the sodium–glucose cotransporter 2 inhibitor dapagliflozin versus placebo, but serious AEs of cholelithiasis and acute cholecystitis were reported in similar proportions of patients in the two treatment groups (37).
In the current analysis, we have shown that patients with a gallbladder- or biliary tract–related event experienced more weight loss in the trial than those without an event. However, the relationship among GLP-1RA treatment, weight loss, and gallbladder-related events is not fully clear, as exemplified by differences in observations for subcutaneous semaglutide in SUSTAIN 6 compared with liraglutide in LEADER, as described above (23,31). In one of the SCALE weight-management trials for liraglutide 3.0 mg in individuals with overweight or obesity and without type 2 diabetes, more participants experienced gallbladder-related events with liraglutide than with placebo (n = 61 [2.5%] vs. n = 12 [1.0%], respectively), and weight loss among these participants was higher than the mean weight loss in the total trial cohort (15). However, while the majority of the weight loss was observed during the first ∼40 weeks of treatment, the incidence of gallbladder-related AEs remained relatively constant over the full 160 weeks (14). The increased incidence of gallbladder-related AEs with liraglutide 3.0 mg could therefore be due to both weight loss–dependent and weight loss–independent effects. The results we report in the current analysis, whereby adjusting for baseline body weight and change in body weight only slightly attenuated the effect of liraglutide on the risk of a gallbladder- or biliary tract–related event, predominantly support weight-independent effects and only to a smaller extent indicate weight-dependent effects.
Another potential mechanism suggested is an inhibitory effect on gallbladder motility (4,12). Impaired gallbladder mobility can lead to sludge and gallstone formation and has been described as a contributing factor in the development of gallstones with other medications, e.g., the somatostatin analog octreotide (34). GLP-1 infusion suppresses the secretion of cholecystokinin after a meal, which reduces gallbladder contractility (38). Exenatide, lixisenatide, and albiglutide have been shown to reduce cholecystokinin-induced gallbladder ejection fraction, compared with placebo, in healthy individuals (39–41). Liraglutide has no effects on fasting gallbladder volumes or the maximum postprandial gallbladder ejection fraction but does appear to delay the time to maximum contraction of the gallbladder (12,42). However, the clinical significance of these findings is yet to be elucidated (12). Differential effects of GLP-1RAs on gallbladder motility have not been examined.
Overall, the mechanism for the increased occurrence of gallbladder stones with liraglutide remains unclear, but it may involve altered bile acid production and secretion, decreased gallbladder emptying, weight loss, or a combination of all of these (5,43). A further, recently proposed explanation for the gallbladder events observed in GLP-1RA clinical trials is GLP-1RA–induced prolongation of gallbladder refilling (44,45).
This post hoc analysis has a number of limitations. First, the LEADER trial was not specifically designed to assess the risk of gallbladder- and biliary tract–related events with liraglutide. Second, the finding that cholecystectomy was performed for similar proportions of liraglutide-treated and placebo-treated patients who experienced gallbladder- or biliary tract–related events should be interpreted with caution due to potential differences between these patients. Third, performing multiple comparisons within a study can increase the probability of obtaining a false-positive result (46), and no adjustment for multiple statistical comparisons was made in this analysis. Fourth, LEADER was conducted in a population of patients at high risk of CV disease, which may limit the generalizability of these findings to the wider population of patients with type 2 diabetes. Additionally, with respect to gallstone disease event reporting, only acute events were required to be reported in the trial, so the number of gallbladder stones was likely underestimated. In other studies of populations with diabetes, the reported prevalence of gallstones, at 18%, was much higher than reported here for LEADER (<2.45%) (47,48) (Table 3). Further studies are required to confirm the increase in gallbladder- or biliary tract–related events and cholecystectomy with liraglutide and to investigate the relevant mechanisms.
Supplementary Material
Article Information
Acknowledgments. The authors thank Søren Rasmussen (Novo Nordisk A/S) for statistical support and review of and input into the manuscript. Medical writing and editing assistance were provided by Kate Booth and Izabel James, of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk A/S. Additional medical writing support was provided by Laura Elson on behalf of Watermeadow Medical, funded by Novo Nordisk A/S. The authors thank the LEADER trial participants, investigators (see Supplementary Data), and site staff and the leadership, employees, and contractors of the sponsor who were involved in the conduct of the trial.
Funding. J.B.B. is supported by a grant from the National Institutes of Health (UL1TR002489).
Duality of Interest. This study was sponsored by Novo Nordisk. M.A.N. reports fees for serving on advisory boards from AstraZeneca, Berlin-Chemie, Eli Lilly & Co., Fractyl, Hanmi, Merck Sharp & Dohme, Novo Nordisk, and Intarcia Therapeutics/Servier; lecture fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Eli Lilly and Co., Medscape, Merck Sharp & Dohme, and Novo Nordisk; travel support from Berlin-Chemie, Eli Lilly and Co., Merck Sharp & Dohme, Novo Nordisk, and Intarcia Therapeutics/Servier; and grant support (to his institution) from AstraZeneca, Eli Lilly & Co., GlaxoSmithKline, Intarcia Therapeutics/Servier, Merck Sharp & Dohme, Novartis, and Novo Nordisk. M.L.M.G., E.K., and H.A.S. are employees of Novo Nordisk. M.L.M.G. and E.K. are shareholders of Novo Nordisk. J.B.B.’s contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly and Co., MannKind, NovaTarg, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen and he has received grant support from Novo Nordisk, Sanofi, and vTv Therapeutics. J.B.B. is a consultant to Neurimmune AG and holds stock options in Mellitus Health, PhaseBio, and Stability Health. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.N., M.L.M.G., E.K., H.A.S., and J.B.B. participated in analysis or interpretation of data, revised the manuscript for important intellectual content, approved the final version, and accept accountability for all aspects of the work. M.A.N. and J.B.B. were involved in the development, conduct, and oversight of the protocol. M.A.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 54th Annual Meeting of the European Association for the Study of Diabetes (EASD), Berlin, Germany, 1–5 October 2018, and at the 3rd EASD Incretin Study Group Meeting, Bochum, Germany, 24–26 January 2019.
Footnotes
Clinical trial reg. no. NCT01179048, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0415/-/DC1.
A complete list of the members of the LEADER Publication Committee and of the LEADER Trial Investigators is included in the Supplementary Data online.
References
- 1.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009;32:834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol 2018;34:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faillie JL, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of bile duct and gallbladder diseases with the use of incretin-based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med 2016;176:1474–1481 [DOI] [PubMed] [Google Scholar]
- 4.Pizzimenti V, Giandalia A, Cucinotta D, et al. Incretin-based therapy and acute cholecystitis: a review of case reports and EudraVigilance spontaneous adverse drug reaction reporting database. J Clin Pharm Ther 2016;41:116–118 [DOI] [PubMed] [Google Scholar]
- 5.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab 2017;19:1233–1241 [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency. Medicines [Internet], 2019. Available from https://www.ema.europa.eu/en/medicines. Accessed 22 May 2019
- 7.U.S. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products [Internet], 2019. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 22 May 2019
- 8.Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol 2000;12:1347–1352 [DOI] [PubMed] [Google Scholar]
- 9.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtney H, Nayar R, Rajeswaran C, Jandhyala R. Long-term management of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Diabetes Metab Syndr Obes 2017;10:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalra S. Follow the LEADER-liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results trial. Diabetes Ther 2016;7:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nexøe-Larsen CC, Sørensen PH, Hausner H, et al. Effects of liraglutide on gallbladder emptying: a randomized, placebo-controlled trial in adults with overweight or obesity. Diabetes Obes Metab 2018;20:2557–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxenda (liraglutide). Highlights of prescribing information [Internet], 2018. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206321s007lbl.pdf. Accessed 22 May 2019
- 14.le Roux CW, Astrup A, Fujioka K, et al.; SCALE Obesity Prediabetes NN8022-1839 Study Group . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–1409 [DOI] [PubMed] [Google Scholar]
- 15.Pi-Sunyer X, Astrup A, Fujioka K, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22 [DOI] [PubMed] [Google Scholar]
- 16.Marre M, Shaw J, Brändle M, et al.; LEAD-1 SU Study Group . Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauck M, Frid A, Hermansen K, et al.; LEAD-2 Study Group . Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garber A, Henry R, Ratner R, et al.; LEAD-3 (Mono) Study Group . Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 19.Zinman B, Gerich J, Buse JB, et al.; LEAD-4 Study Investigators . Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell-Jones D, Vaag A, Schmitz O, et al.; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group . Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buse JB, Rosenstock J, Sesti G, et al.; LEAD-6 Study Group . Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 22.Pratley R, Nauck M, Bailey T, et al.; 1860-LIRA-DPP-4 Study Group . One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 2011;65:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marso SP, Poulter NR, Nissen SE, et al. Design of the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial. Am Heart J 2013;166:823–830.e5 [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Determining the extent of safety data collection needed in late-stage premarket and postapproval clinical investigations: guidance for industry [Internet], 2016. Available from https://www.fda.gov/media/82664/download. Accessed 22 May 2019
- 26.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med 1994;330:1198–1210 [DOI] [PubMed] [Google Scholar]
- 27.Steinberg WM, Buse JB, Ghorbani MLM, Ørsted DD, Nauck MA; LEADER Steering Committee; LEADER Trial Investigators . Amylase, lipase, and acute pancreatitis in people with type 2 diabetes treated with liraglutide: results from the LEADER randomized trial. Diabetes Care 2017;40:966–972 [DOI] [PubMed] [Google Scholar]
- 28.Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 30.Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes Committees and Investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 31.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 32.Novo Nordisk A/S. Victoza (liraglutide) Summary of Product Characteristics [Internet], 2018. Available from https://www.ema.europa.eu/en/documents/product-information/victoza-epar-product-information_en.pdf. Accessed 22 May 2019
- 33.Flint A, Kapitza C, Zdravkovic M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes Obes Metab 2013;15:958–962 [DOI] [PubMed] [Google Scholar]
- 34.Njeze GE. Gallstones. Niger J Surg 2013;19:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xenical (orlistat). Highlights of prescribing information [Internet], 2015. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020766s035lbl.pdf. Accessed 22 May 2019
- 36.Contrave (naltrexone HCl and bupropion HCl). Highlights of prescribing information [Internet], 2018. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/200063s013lbl.pdf. Accessed 22 May 2019
- 37.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 38.Rehfeld JF, Knop FK, Asmar A, Madsbad S, Holst JJ, Asmar M. Cholecystokinin secretion is suppressed by glucagon-like peptide-1: clue to the mechanism of the adverse gallbladder events of GLP-1-derived drugs. Scand J Gastroenterol 2018;53:1429–1432 [DOI] [PubMed] [Google Scholar]
- 39.Keller J, Trautmann ME, Haber H, et al. Effect of exenatide on cholecystokinin-induced gallbladder emptying in fasting healthy subjects. Regul Pept 2012;179:77–83 [DOI] [PubMed] [Google Scholar]
- 40.Shaddinger BC, Young MA, Billiard J, Collins DA, Hussaini A, Nino A. Effect of albiglutide on cholecystokinin-induced gallbladder emptying in healthy individuals: a randomized crossover study. J Clin Pharmacol 2017;57:1322–1329 [DOI] [PubMed] [Google Scholar]
- 41.Committee for Medicinal Products for Human Use. Assessment report: lyxumia [Internet], 2012. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002445/WC500140449.pdf. Accessed 22 May 2019
- 42.Smits MM, Tonneijck L, Muskiet MH, et al. Biliary effects of liraglutide and sitagliptin, a 12-week randomized placebo-controlled trial in type 2 diabetes patients. Diabetes Obes Metab 2016;18:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smits MM, van Raalte DH, Tonneijck L, Muskiet MH, Kramer MH, Cahen DL. GLP-1 based therapies: clinical implications for gastroenterologists. Gut 2016;65:702–711 [DOI] [PubMed] [Google Scholar]
- 44.Gether IM, Nexøe-Larsen C, Knop FK. New avenues in the regulation of gallbladder motility-implications for the use of glucagon-like peptide-derived drugs. J Clin Endocrinol Metab 2019;104:2463–2472 [DOI] [PubMed] [Google Scholar]
- 45.Gether IM, Nexoe-Larsen C, Sonne DP, Knop PK. Effects of glucagon-like peptides on gallbladder motility. Ugeskr Laeger 2018;180:V05180386 [in Danish] [PubMed] [Google Scholar]
- 46.Althouse AD. Adjust for multiple comparisons? It’s not that simple. Ann Thorac Surg 2016;101:1644–1645 [DOI] [PubMed] [Google Scholar]
- 47.Agunloye AM, Adebakin AM, Adeleye JO, Ogunseyinde AO. Ultrasound prevalence of gallstone disease in diabetic patients at Ibadan, Nigeria. Niger J Clin Pract 2013;16:71–75 [DOI] [PubMed] [Google Scholar]
- 48.Sodhi JS, Zargar SA, Khateeb S, et al. Prevalence of gallstone disease in patients with type 2 diabetes and the risk factors in North Indian population: a case control study. Indian J Gastroenterol 2014;33:507–511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


