Abstract
Islet autoimmunity has been identified as a component of both type 1 (T1D) and type 2 (T2D) diabetes, but the pathway through which islet autoimmunity develops in T1D and T2D may be different. Acknowledging the presence of islet autoimmunity in the pathophysiology of T2D, a historically nonautoimmune metabolic disease, would pave the way for important changes in classifications of and therapeutic options for T2D. In order to fully appreciate the importance of islet autoimmunity in T2D, the underlying mechanisms for immune system activation need to be explored. In this review, we focus on the potential origin of immune system activation (innate and adaptive) leading to the development of islet autoimmunity in T2D.
Introduction
Diabetes is classified into two major types: type 1 (T1D) and type 2 (T2D). Insulin deficiency underlies both T1D and T2D; historically, however, the disease etiologies have been considered to originate from different mechanisms, irrespective of the patient’s age at onset of disease. T1D is considered to be of autoimmune etiology, requiring immediate insulin replacement, whereas T2D is considered primarily to result from insufficient insulin production and insulin resistance unrelated to islet autoimmunity. However, discoveries highlighting similarities between the two pathologies and the involvement of the immune system in the pathogenesis of both T1D and T2D have challenged this simplistic categorization of diabetes (1). For example, similar genes have been implicated in disruptions of glucose metabolism and uptake in both T1D and T2D (2,3). In addition, the more severe insulin deficiency and the physiological need for immediate insulin replacement historically associated with T1D have now been identified in patients with the T2D phenotype (4). Moreover, insulin resistance, which has been regarded as a pathology primarily associated with T2D, has also been confirmed to be a component of T1D pathophysiology (5,6). These similarities suggest that the pathogeneses of T1D and T2D may not be as distinct as previously postulated.
Diabetes classification guidelines are established to promote the education of health care professionals in order to assist them in caring for patients with diabetes, prescribing therapies, and directing avenues for future research. Unfortunately, outdated classification schemes may ultimately create challenges to treating patients with diabetes. The concept of immune system involvement in obesity, insulin resistance, and T2D has recently highlighted the necessity for changes in the current diabetes classification system to include the involvement of immune responses in T2D pathophysiology. If the current classifications for T1D and T2D were expanded to include immune involvement in both types, these changes may help physicians embrace a broader range of therapeutic options for treating patients with T2D. In this review, we outline the immune system’s involvement in the development of obesity, metabolic syndrome, insulin resistance, and T2D. We also propose potential mechanisms leading to the development of islet autoimmunity in T2D and discuss how the pathophysiologies of T1D and T2D may be closer than previously appreciated.
Islet Autoimmunity in T2D
The development of T2D is associated with β-cell dysfunction, islet inflammation, systemic inflammation, insulin resistance, and often obesity. We and our colleagues identified islet-reactive T cells in patients with T2D with, and in those without, islet antibodies, which are the historical hallmark of autoimmune classification in patients with T1D (7). We also demonstrated that cellular responses to islet proteins in the peripheral blood of patients with the T2D phenotype can be used to identify patients with a more severe β-cell lesion and a larger decline in β-cell function than in those with T2D without islet-specific T-cell reactivity (8,9). Furthermore, we observed that attenuation of islet-specific T-cell responses in patients with autoimmune T2D who were treated with rosiglitazone, a thiazolidinedione, resulted in C-peptide improvement. In comparison, T2D patients treated with glyburide experienced no change in their islet-specific T cells and experienced a progressive loss of β-cell function throughout the study. (10). Our studies support the concept that islet-specific T cells are important contributors to the progressive decline of β-cell function associated with T2D progression. Sarikonda et al. (11) also identified CD4+ islet-reactive T cells in patients with T2D. They concluded that both patients with T1D and patients with T2D have islet-reactive autoimmune T cells and that these cells are involved in the pathogenesis of β-cell destruction. Butcher et al. (12) also described proinflammatory cytokines in islets with dysfunctional β cells in patients with T2D.
Further support for autoreactivity in T2D comes from studies associated with the Pima Indians. Pima Indians have been historically the “gold standard” model of nonautoimmune T2D. T2D is highly prevalent among this select group of Native Americans and has been regarded as “classic” T2D without immune system involvement. However, Chang et al. (13), using high-density protein microarrays, identified novel autoantibodies associated with insulin secretion in Pima Indians with T2D. The association of autoantibody differences between T1D and T2D may indicate different immune-stimulating antigens between the two types. The antigenic differences may reflect the different pathways activated autoreactive cells journey from the intestine to the pancreas: directly to the target organs in T1D versus indirectly through magnification in adipose tissue in T2D. These pathways are discussed further in the sections below.
Williams et al. (14) identified an HLA haplotype that is associated with increased insulin secretion, indicating a potential role for loss of self-tolerance in the development of T2D in Pima Indians. Moreover, Frankl et al. (15), using high-throughput sequencing of T-cell receptor CDR3 regions, reported differences in T-cell receptor repertoires associated with and predictive of the development of T2D. These results suggest that islet autoimmunity—both related to autoantibodies and mediated by T cells—may be important components of β-cell dysfunction in the historic “gold standard” of nonautoimmune T2D. The shift from anti-inflammatory (T helper [TH]2 cells) to inflammatory (TH1, TH17 cells) and the presence of “new” adipose tissue and endoplasmic reticulum (ER) stress proteins available to the immune system may pave the way for the development of the metabolic syndrome, autoimmune diseases, and cancer (detailed in the following sections). The increased availability of these “altered” or “new” antigens in the intestine, adipose tissue, and other organs, which are potentially undergoing ER stress, along with increasing T-cell reactivity to these “novel” proteins, may be the underlying mechanism for the antigen-spreading phenomenon identified in patients who are developing autoimmune diseases (16,17). These observations support the concept that islet autoimmune reactivity is involved with β-cell dysfunction and destruction in both T1D and T2D. In the following sections, we discuss the differential pathways and potential mechanisms involved in the development of islet autoimmune reactivity in T1D and T2D.
Roles of Systemic Inflammation in Obesity, Insulin Resistance, Autoimmunity, and Cancer
Studies indicate that in diet-induced obesity, antigen-stimulated, proinflammatory innate (macrophages, natural killer cells) and adaptive immune cells (TH1, TH17, CD4+, and CD8 T lymphocytes) infiltrate visceral adipose tissue (VAT) and replace the anti-inflammatory environment dominated by anti-inflammatory TH2 cells and T-regulatory cells (Tregs), which are associated with lean fat (18). McLaughlin et al. (19) demonstrated that proinflammatory CD4+ TH1, TH17, and CD8+ T cells populate human adipose tissue and correlate with the development of systemic inflammation and insulin resistance. They also showed that proinflammatory cells infiltrate VAT significantly more frequently and at a higher magnitude than in subcutaneous adipose tissue (SAT). The accumulation of more inflammatory cells in VAT than in SAT, as described by McLaughlin et al., may also indicate an underlying mechanism for the stronger association of accumulation in VAT with metabolic and autoimmune diseases, cancers, and cardiovascular diseases. The T-cell profiles these researchers observed in SAT and VAT correlated significantly with T-cell profiles in the peripheral blood of patients in whom the proinflammatory cells were able to migrate to various tissues, creating a systemic inflammatory state. These researchers also observed that the presence of anti-inflammatory TH2 cells in VAT, SAT, and peripheral blood was inversely correlated with systemic insulin resistance. It is important to recognize the ability of antigen-stimulated innate and adaptive immune cells from adipose and other tissues—especially those cells that have been associated with autoreactivity, such as TH17 cells—to circulate throughout the body (20). In fact, Morton et al. (21) demonstrated that bowel-derived TH17 cells, effectors of autoimmune disease, migrate from the gut to other organs and to joints and contribute to the development of autoreactive arthritis.
Accumulation of TH17 cells has been observed in VAT from insulin-resistant patients, and their numbers in the periphery positively correlate with insulin resistance in both T1D and T2D (19,22,23). These cells are also found in peripheral blood from women and children who are obese, are more abundant in VAT than SAT, are present in islet inflammation in patients with T1D and patients with T2D, and are involved in β-cell destruction in T1D (19,20,22–25). Mahmoud and Al-Ozairi (26) also observed that patients with T2D and coronary heart disease, a common complication of T2D, demonstrated significantly more CD4+ T effector cells that produce interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin (IL)-17, and significantly fewer Tregs than patients with T2D but without coronary heart disease. We observed similar findings in subjects with T2D who developed cellular islet autoimmunity and identified increases in IL-17, IFN-γ, IL-12, and proinflammatory monocytes (B.M.B.-W, J.P.P., unpublished data). The presence of the proinflammatory cells, particularly TH17 cells, provides the potential for destructive inflammation to be established in organs that are remote from the intestine or fat, resulting in metabolic complications or organ destruction and adding another commonality between T1D and T2D pathogeneses.
A role is now widely accepted for systemic circulation of the proinflammatory cytokines IL-1β, IL-17, IL-6, and TNF-α in mediating insulin resistance (27). Studies suggest that systemic upregulation of proinflammatory cytokines, resulting from the establishment of systemic inflammation while obesity develops, may be the link between cancer, obesity, autoimmunity, and chronic diseases. Proinflammatory cytokines such as IL-1β, IL-6, IFN-γ, IL-17, IL-12, IL-22, and TNF-α have enhanced tumor survival and proliferation (28). TNF-α has been shown to stimulate cancer development by enhancing DNA damage, promoting uncontrolled cellular proliferation and metastasis (29). Other cytokines such as IL-6 stimulate the expression of genes that promote angiogenesis and tumor cell proliferation, invasiveness, survival, and metastasis (30). Moreover, the downregulation of Tregs, specifically in visceral obesity, may create conditions that favor the establishment of tumors and tumor survival.
The importance of systemic inflammation and adipocyte changes in the development of T2D was recently confirmed in a prospective study of 1,014 individuals participating in the Danish arm of the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION-PRO) (31). In that study, Deichgræber et al. (31) investigated the association between the macrophage-activation marker soluble CD163, adiponectin, and C-reactive protein (CRP) and changes in glycemia, insulin resistance, and insulin secretion in individuals at high risk of T2D. They observed that increases in soluble CD163 and CRP serum concentrations were positively associated with changes in HOMA-β. Changes in serum adiponectin, however, were inversely associated with changes in 2-h glucose and HOMA-β. Deichgræber et al. concluded that systemic inflammatory mechanisms and changes in adipose tissue play a role in changing glucose homeostasis in individuals at high risk of T2D.
The driving force establishing and maintaining systemic inflammation and insulin resistance may be immune system recognition of ER stress proteins, ER chaperones, neoantigens, or all three, as a result of ER stress in organs or tissues. These neoantigens may be responsible for upregulating innate and adaptive immune responses capable of migrating to other tissues/organs, creating inflammation, recognizing similarly produced antigens, and downregulating regulatory responses (32). If migrating activated immune cells encounter similar antigens in other tissues such as the pancreas, to which they were initially activated, tissues/organs may be destroyed (autoreactivity). The importance of tissue antigens has been shown by inhibiting adipocyte death in VAT. If adipocyte death is inhibited and adipocyte antigens are not available to the immune system, insulin resistance is prevented (33).
Neoantigen Availability: Creating Self-Reactivity?
The establishment of antigen-specific immune responses in obesity-related systemic inflammation requires antigen. The development of obesity has been demonstrated to be associated with the necrotic death of adipocytes and the presentation of antigen to cells of the adaptive immune system. Wensveen et al. (34) proposed a model of obesity-induced adipose tissue inflammation suggesting that in response to a high-fat diet, adipocytes initially become hypertrophic and later hyperplastic, with an associated shift in adipokine production. The adipocytes undergo apoptosis as a result of ER stress and release antigens previously not available to the immune system. The presence of necrotic adipocytes and the upregulation of stress markers on the surface of adipocytes in obesity provide the components necessary to stimulate adaptive immune cells and further destroy adipocytes. Adipocyte death in tissues of obese mice and humans has been observed and is assumed to be one of the driving forces for recruiting antigen presenting cells and stimulation of effector cells of the adaptive immune system in obesity (35). During adipocyte necrosis, release of adipocyte self-proteins may stimulate naïve antigen-specific T cells (CD4+, CD8+, and TH17 cells) or stimulate antigen-activated immune cells arriving from the circulation. Immune cells, which may have been activated in the intestine or other inflamed tissues, may then contribute to further adipocyte destruction, magnifying the responses occurring in the fat and adding to the number of immune cells circulating throughout the body.
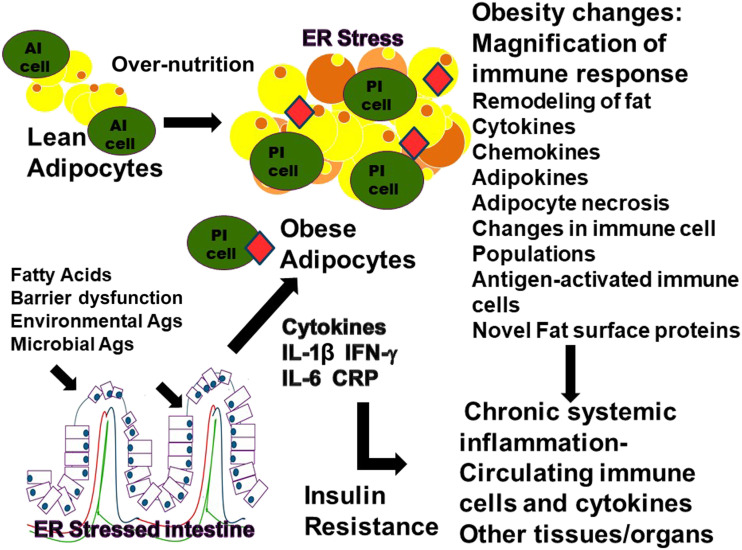
Adipose tissue macrophages (ATMs) and adipocytes have been shown to act as antigen-presenting cells, promoting the activation and proliferation of effector T cells (36). The establishment of antigen-specific upregulation of the adaptive immune system in adipose tissue is further supported by studies using macrophage-specific MHCII knockout mice (37). These mice have macrophages that are incapable of presenting antigens to effector T cells. Therefore, the mice are protected from developing insulin resistance through inhibiting effector/memory T-cell generation. T-cell receptor repertoires seem to be very homogenous within the inflammatory environment of VAT, further implying localized antigen stimulation and expansion of antigen-specific T cells (38). Thus, the evidence exists for clonal expansion of an adaptive immune response in the tissues of obese mice and humans though antigens presented by ATMs are still unidentified. Interactions between the immune cells, both innate and adaptive, within adipose tissue are believed to be an important component of the development and magnification of ongoing chronic systemic inflammation and subsequent development of metabolic disturbances and disease. Figure 1 illustrates the changes in adipose tissue during the transition from lean to obese.
Figure 1.
Obesity-induced changes in adipocytes. In an obese state, the immune composition of the adipose tissue shifts and takes on a proinflammatory (PI) phenotype, whereas in a lean state, the immune system of the adipose tissue primarily has an anti-inflammatory (AI) composition. These changes incorporate cytokines, adipokines, newly expressed proteins on the surface of the adipocytes, and ER stress proteins; apoptosis of adipocytes; and changes toward a PI immune composition. The PI immune cells may recognize the “novel” adipocyte proteins or proteins resulting from adipocyte apoptosis or ER stress. They may include immune cells that were stimulated in the intestine or other tissues and are migrating to the adipose tissue or cells that were stimulated within the adipose tissue. The antigen (Ag)-stimulated PI cells and cytokines may then enter the bloodstream and migrate to other tissues and organs.
ER Stress and Diabetes
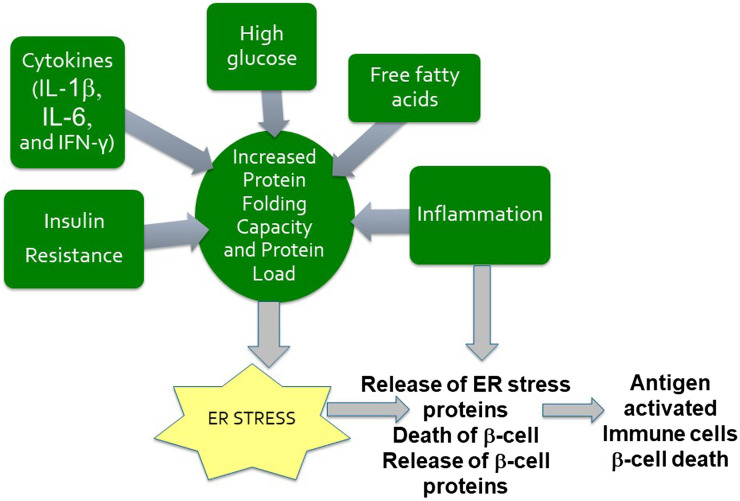
ER stress has been demonstrated to supply proteins that were previously unavailable to the immune system (39,40). These ER stress proteins, along with microbial antigens, play an important role in upregulating the proinflammatory cells (adaptive and innate) and proinflammatory cytokines in the intestine, which circulate to other organs and tissues, elevating or creating inflammatory conditions. ER stress can occur in multiple organs, such as adipose tissue and the pancreas. The ER is important for protein folding, protein maturation, and protein movement throughout a cell. When the ER becomes stressed, newly synthesized unfolded proteins, improperly folded proteins, or both accumulate. Disruptions of the ER, causing “ER stress,” result in the breakdown of and functional changes in the protein-folding machinery of the ER. The ER responds by activating the altered protein response, or “unfolded protein response” (UPR). This response functions to restore normal protein homeostasis (39,41). Professional secretory cells, such as the β-cells of the pancreas and adipocytes in obesity, have an elaborate ER. During the UPR, new chaperone and ER stress proteins are synthesized in order to aid in folding accumulated misfolded proteins. Because of the secretory functions of pancreatic β-cells, they are significantly affected by the UPR resulting from ER stress. Obesity-induced insulin resistance also adds to the ER stress applied specifically on pancreatic β-cells (42). Figure 2 illustrates some of the components associated with metabolic stress, which can act directly on the cells of the pancreas, leading to ER stress in the pancreatic β-cells and shifting the balance toward autoreactivity against β-cells.
Figure 2.
ER stress in pancreatic β-cells. Numerous factors such as insulin resistance, high glucose, free fatty acids, cytokines, and incoming inflammation may be responsible for the development of ER stress in pancreatic cells. ER stress may then be responsible for an increase in the number of abnormal posttranslational modifications or in the expression of endogenous ER proteins on the surface of pancreatic cells. This may occur to a level that is sufficient to generate autoimmune responses to these “new” surface proteins, or neoproteins. The “pro-inflammatory” antigen-stimulated immune cells arriving in the pancreas may recognize the “novel” islet proteins or the proteins resulting from β-cell apoptosis or ER stress. Antigen may activate other immune cells within the pancreas, leading to further β-cell destruction or dysfunction.
ER stress proteins have been produced in adipose tissue of obese insulin-resistant individuals (43). ER stress may thus be responsible for an increase in abnormal posttranslational modifications or the surface expression of endogenous ER proteins to a level sufficient to generate autoreactive responses to these “new” surface proteins, or neoproteins (44–47). Major ER chaperones such as glucose response proteins 78 (GRP78/BiP) and 94 (GRP94), calreticulin, and protein disulfide isomerase all exhibit cell surface forms and secreted forms that differ from the ER forms (that are not present on the surface or secreted) during ER stress. This supports the possibility that the immune system recognizes these altered “self-proteins” as autoantigens (44–47).
Immune response genes may also be potentially important for determining which cellular populations are activated or which neoproteins are presented, thus determining which autoimmune disease develops. The robustness of the immune system (much more robust in younger individuals than in older individuals) may also play a role in determining when autoimmune disease develops. Younger patients with diabetes may develop islet autoimmunity with activated immune cells coming from the intestines (see the section potential sites of initiation: intestinal immune system and gut microbiome) directly to the target organ, whereas the less robust responses in older patients with diabetes may need to be magnified by systemic inflammation and the involvement of multiple tissues (adipose). It may also explain the grouping of autoimmune diseases: The diseases group together on the basis of similar antigens being presented in multiple tissues or organs.
Islet Inflammation and Diabetes
In addition to systemic inflammation and ER stress, inflammation has been observed within the pancreatic islets. Increased numbers of immune cells and cytokines within the islets, characteristic of islet inflammation, have been reported to be an important contributor to β-cell dysfunction and apoptosis in both T1D and T2D (48–50). Islet-reactive T cells have been identified in both T1D and T2D (7–11), though the cells involved in islet inflammation may differ between T1D and T2D. Adaptive immune cells have been identified in patients with T1D, whereas cells in the islets of patients with T2D at this time seem to be primarily from the innate immune system (48). However, further studies are needed to investigate the cells involved in islet inflammation in T2D. Interestingly, in T2D, IL-22 administration, which suppresses ER stress and inflammation, has been shown to restore glucose homeostasis and insulin sensitivity (51). It would have been of interest to determine the effect of IL-22 on inflammatory cells, but unfortunately, these studies did not investigate the cellular immune responses to the novel islet proteins following IL-22 administration. The presence of islet inflammation, ER stress, and “novel” proteins in both T1D and T2D demonstrates another similarity in immune pathology between T1D and T2D.
Potential Sites of Initiation: Intestinal Immune System and Gut Microbiome
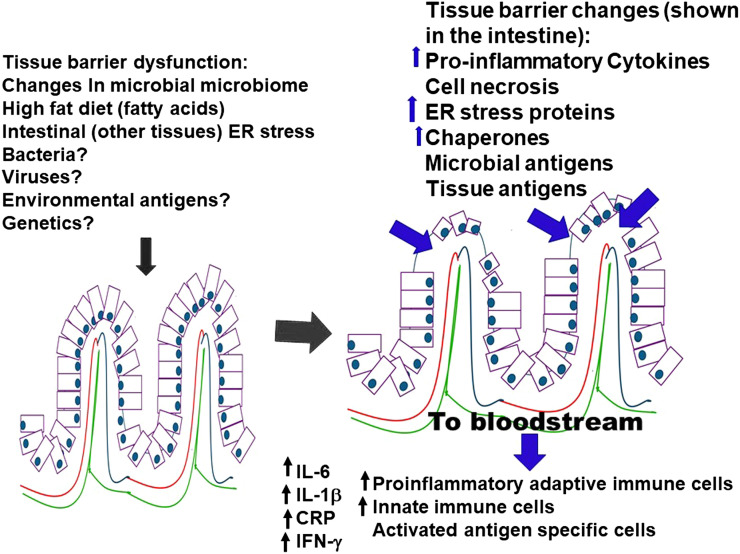
The cells of the intestinal tract and the gut microbiome, with its resident bacterial populations, are important primary sites in the body where environmental and potentially altered antigens are encountered. When certain classes of dietary antigens are consumed, such as fatty acids, the intestinal barrier may break down. Changes in intestinal tract permeability and the gut’s microbial constitution are emerging as important components in the development of ER stress, obesity, insulin resistance, systemic inflammation, and autoimmune responses (52). Fatty acids and other components of high-fat diets lead to increases in proinflammatory cytokines such as IL-1β, IFN-γ, and IL-6, increases in intestinal proinflammatory immune cells (innate and adaptive), increases in systemic endotoxemia, changes in gut bacterial populations, migration of proinflammatory cells from the gut to other tissues, and decreases in proportions of Tregs (40,52,53). ER stress in intestinal cells also seems to be an important contributor to establishing and maintaining intestinal inflammation (40,52). The increase in intestinal permeability further allows bacteria and bacterial products, such as lipopolysaccharide, to cross the intestinal barrier into the systemic circulation and into the visceral adipose tissue, enhancing the antigenic stimulation of immune cells outside of the intestinal environment. In fact, systemic endotoxemia has been proposed as a major component of the upregulation of systemic inflammation and the development of insulin resistance (54). Figure 3 illustrates some of the changes that occur in the intestinal system, which may be important for establishing innate and adaptive immune responses and for the subsequent migration of these cells and cytokines to other tissues. However, these changes in tissue barriers may not be solely a dysfunction associated with the intestinal tract. Other tissue barrier dysfunctions may occur, leading to the upregulation of proinflammatory environments, tissue inflammation, and tissue destruction.
Figure 3.
Intestinal assault and resulting changes. An intestinal assault compromises the intestinal integrity or alters the bacterial microbiome, allowing for external environmental antigens to enter the environment. These changes shift the immune composition of the intestine from an anti-inflammatory phenotype to a proinflammatory phenotype. These changes incorporate novel expression of proteins on the surface of the intestinal epithelial cells (ER stress), apoptosis of intestinal cells, and upregulation of proinflammatory cells (innate and adaptive) and cytokines (IL-6, IL-1β, TNF-α, IFN-γ, CRP). The immune cells and cytokines are released into the bloodstream. The antigen-stimulated proinflammatory immune cells and cytokines arriving in the pancreas or other organs may recognize the “novel” proteins resulting from ER stress to which they were initially stimulated.
An important issue to consider is that ER stress changes occur in the intestinal tract as a result of a high-fat diet (55). It is therefore conceivable that the neoantigens produced by the UPR stimulate activated immune systems (innate and adaptive) in the gut. The same ER antigens would then be available in other tissues, such as adipose tissue and pancreas, for the migrating activated intestinal immune cells to recognize. This autoreactivity could lead to further activation and destruction of tissues and organs, advancing the progression of autoimmune diseases. The generation of neoantigens may also lend insight into the link between obesity, autoimmune disease, and cancer. In fact, calreticulin, an ER chaperone, has been used as a component in cancer chemotherapy, with encouraging results (49). Moreover, immune recognition of neoepitopes has recently been recognized as a key component of the development of islet autoimmunity in T1D (44,56,57). Hypothesizing that the development of autoimmune disease originates in the gut may also explain the “environmental” factors associated with autoimmune disease development.
Dysbiosis, Changes in Gut Microbial Populations, and Insulin Resistance
Another key component in the pathophysiology of insulin resistance is changes in the bacterial composition of the gut microbiome, known as dysbiosis (52). Vrieze et al. (58) conducted a small study in which they transferred intestinal fecal bacterial populations from lean human donors to insulin-resistant obese subjects. The fecal transplants improved insulin sensitivity in the obese recipients. These results suggest that the microbial populations present in the lean donors may be one mode of protection against insulin resistance. Current research points to important roles for gut dysbiosis, damage of intestinal barriers, and systemic inflammation in the development of insulin resistance in both T1D and T2D (52,58,59). Wilkin (60) (who proposed the accelerator hypothesis, which states that the etiology of T1D is driven by insulin resistance and not islet autoimmunity) suggested that islet autoimmunity is a response to, rather than the cause of, β-cell stress (ER stress and β-cell apoptosis). This systemic inflammatory response may be triggered in the intestinal tract by changes in microbial populations, gut barrier permeability, environmental antigens, or ER stress proteins, and it may be magnified in the adipose or other tissues before an autoimmune attack in other organs. Therefore, the underlying mechanism responsible for insulin resistance, chronic disease, autoreactivity, and cancers appears to be the change in the balance from an anti-inflammatory to a proinflammatory systemic immune state.
Clinical Relevance and Where We Go from Here
Innate and adaptive immune responses are involved in obesity-associated systemic inflammation, insulin resistance, autoimmune diseases, cancers, and ultimately β-cell dysfunction/destruction in T1D and T2D. Acknowledgment of the important role the immune system plays in the development and progression of both T1D and T2D opens the door for the inclusion of immunotherapy in the treatment of T2D. Unfortunately, which adipose tissue antigens, ER antigens, or β-cell antigens the immune system recognizes, and which thus result in β-cell attack, are not known. In fact, T cells from both patients with T1D and patients with T2D recognize multiple islet proteins, likely reflecting antigen spreading within the autoimmune response (16,17). Therefore, until the autoantigens are defined, targeted antigen-based therapy is not possible. Based on the aforementioned studies, it is likely that the antigens involved in an autoimmune attack may not be specific to β-cells but may come from ER stress or from proteins that are upregulated in many tissues under conditions of stress. However, our studies suggest that the T-cell responses to β-cell proteins are specific in patients with diabetes. Thus, continued research toward identifying the “initiating” antigen(s) is important in order to develop future antigen-based therapies. It is also becoming apparent that therapy will most likely come from a combination of treatments addressing the overall inflammatory condition, ER stress, and the intestinal microbiome.
In a previous study (10), we were able to attenuate islet-specific T-cell responses and improve β-cell function with a peroxisome proliferator–activated receptor-γ antagonist (rosiglitazone). Prieur et al. (61) also demonstrated that treating ATMs from obese mice with rosiglitazone prevents ATM polarization toward the proinflammatory state, suggesting that rosiglitazone suppresses the establishment of obesity-associated inflammation. Both of these studies suggest that attenuating the autoreactive or inflammatory T-cell response would be beneficial to patients. At this time, rosiglitazone is no longer used for treating patients with T2D, so new anti-inflammatory therapies are needed for use in such patients.
Tauroursodeoxycholic acid (TUDCA), a taurine conjugate of ursodeoycholic acid that interferes with the mitochondrial cell death pathway, reducing ER stress and stabilizing the UPR, significantly decreased pancreatic lymphocytic infiltration, β-cell death, and development of T1D in NOD mice (62). TUDCA also increased insulin sensitivity in the liver and muscle of insulin-resistant obese subjects. TUDCA did not, however, have an effect on insulin sensitivity or ER stress in adipose tissue (63). Two current therapies used clinically in order to treat T2D, pioglitazone and metformin, improved ER stress and have been investigated in cardiovascular diseases. Unfortunately, these two drugs did not have a significant effect on the inflammatory component (64). Many T2D therapies may lack long-term efficacy because of their inability to suppress systemic inflammation, autoreactive responses, and ER stress.
Another potential therapy might be to stabilize or reconstitute the gut microbiome. The impact of a number of antidiabetes therapies on the gut microbiome is currently being investigated (65). Again, the limiting issue is the potential necessity to include therapies aimed at the gut microbiome, the resulting inflammation, and ER stress. Because ER stress may be involved in the initial events that lead to the development of metabolic syndrome and diabetes, it may be crucial to identify proteins involved in the initial stimulation of immune responses. Identifying ER stress or adipose tissue proteins that stimulate immune cells and inflammatory cytokines (IL-1β, INF-γ, IL-6) may be important to target for future therapies. Importantly, because of the concurrent appearance of multiple etiologies, combination therapies will likely be most beneficial for patients.
Summary and Conclusions
Although diabetes includes a spectrum of disease manifestations, the loss of β-cell function is central to all types of diabetes. What has recently become clearer, however, is the involvement of the immune system, both innate and adaptive, in the development of metabolic syndromes and β-cell dysfunction/destruction in T2D. Under lean conditions, the immune system downregulates inflammatory reactions and upregulates Tregs. In obesity, however, the continual upregulation of proinflammatory innate and adaptive immune reactivity establishes a systemic inflammatory state that seems to be driven by antigens, but the specific ones have not yet been identified. The establishment of a systemic inflammatory state can be regarded as a “malfunction” of immune regulation and is often highlighted by autoimmune disorders and cancers. The significance of islet inflammation and islet autoimmunity in T2D has yet to be fully appreciated, but islet-specific T-cell autoimmunity seems to increase the loss of β-cell function.
The important role of the intestinal microbial composition, tissue barrier dysfunction, and the immune system in the pathogenesis of both T1D and T2D is also becoming increasingly appreciated. Researchers may eventually understand the development of islet autoimmunity in T1D and T2D and be able to target their pathogeneses. We hope future studies will help to elucidate the triggers that initiate islet autoimmunity in both T1D and T2D and to develop or identify therapeutic interventions capable of inhibiting autoimmune reactivity and preserving β-cell function in patients with diabetes. Studies demonstrating a link between antigen recognition of ER proteins by inflammatory immune cells in the intestine, adipose tissue, pancreas, and other tissues/organs may hold the key to the underlying associations between obesity, cancer, and the development of autoimmune diseases. Figure 4 summarizes the potential pathways (direct vs. indirect) by which islet autoimmunity develops in T1D and T2D and the potential links between islet autoimmunity, insulin resistance, and cancer.
Figure 4.
ER stress in T1D and T2D pathogenesis. In patients with T1D or T2D, ER stress, tissue barrier dysfunction, or a breakdown in the integrity of the intestines or other organs may be responsible for generating “new” surface proteins, or neo-proteins, leading to antigen activation of innate and adaptive proinflammatory (PI) immune cells and cytokines. These antigen-activated PI immune cells and cytokines may then circulate in the periphery, setting up systemic inflammation. The resulting systemic inflammation creates insulin resistance and allows for the PI cells and cytokines to target other tissues and organs. The arrival of antigen-activated immune cells and circulating PI cytokines may create ER stress in susceptible β-cells of the pancreas and other organs and tissues (e.g., adipose). The development of ER stress in islet β-cells and other tissues may produce altered self-proteins, contributing to the initiation of an autoimmune response. The PI immune cells in the pancreas may recognize the “novel” islet proteins or the proteins resulting from β-cell apoptosis or ER stress. These ER proteins may be the same proteins that are released during ER stress in the intestines or other tissues (adipose tissue), adding to the potential cross-reactivity between various tissues. Alternatively, the ER stress in the β-cell may develop novel proteins that stimulate autoreactive immune cells that are specific to the ER proteins of the pancreas. Differences in genetics, the robustness of the immune responses, antigen presentation, environmental antigens, or microbial antigens may underlie the differences in autoimmune reactivity between T1D and T2D. Immune responses in older individuals may need to be magnified as a result of a less robust immune system by adding inflammation in other tissues/organs in order to shift the immune balance toward autoimmunity. Autoreactive cells and PI cytokines may also migrate to other organs, establishing autoimmunity or cancer.
Some questions will need to be addressed in the future:
What specific antigens (ER stress proteins, microbial antigens, environmental antigens, or others?) drive the changes in T-cell populations in adipose, intestinal, and other tissues from an anti-inflammatory state to a proinflammatory state as individuals progress toward obesity-related disorders and diabetes?
Are T-cell stimulatory antigens widespread among various tissues?
Are different antigens recognized as a result of the different antigen-presentation capabilities of various immune system genes?
Does the difference in the ability to recognize antigens or the antigens recognized lead to the development of different autoimmune diseases?
Do different therapy combinations targeting the intestinal tract or other barrier dysfunctions, organ damage or changes, ER stress, or systemic inflammation protect against autoimmune disease development? What are these therapies?
What changes or differences in immune cells, cytokines, intestinal barrier integrity, or intestinal microbial populations determine protection from or activation of autoimmune disease?
The increasing importance of T-cell recognition of autoantigens in the historically nonautoimmune T2D and the increasing number of similarities between T1D and T2D with regard to immune system involvement underlie the need for a new look at diabetes classifications. Changes in how researchers and physicians perceive T1D and T2D may help them pursue more immune-regulating therapies for treating historically “nonautoimmune” T2D.
Article Information
Funding. The writing of this manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant 5R01DK107560 for the Restoring Insulin Secretion Study [RISE] and 5R01DK104832 for the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study [GRADE]).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.M.B.-W. and J.P.P. prepared, reviewed, and edited the manuscript. B.M.B.-W. and J.P.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
B.M.B.-W. and J.P.P. contributed equally to this study.
References
- 1.Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia 2016;59:13–20 [DOI] [PubMed] [Google Scholar]
- 2.Hosszúfalusi N, Vatay A, Rajczy K, et al. Similar genetic features and different islet cell autoantibody pattern of latent autoimmune diabetes in adults (LADA) compared with adult-onset type 1 diabetes with rapid progression. Diabetes Care 2003;26:452–457 [DOI] [PubMed] [Google Scholar]
- 3.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–885 [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care 2006;29:2575–2579 [DOI] [PubMed] [Google Scholar]
- 5.Bingley PJ, Mahon JL, Gale EAM; European Nicotinamide Diabetes Intervention Trial Group . Insulin resistance and progression to type 1 diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetes Care 2008;31:146–150 [DOI] [PubMed] [Google Scholar]
- 6.Nokoff NJ, Rewers M, Cree Green M. The interplay of autoimmunity and insulin resistance in type 1 diabetes. Discov Med 2012;13:115–122 [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks-Worrell BM, Reichow JL, Goel A, Ismail H, Palmer JP. Identification of autoantibody-negative autoimmune type 2 diabetic patients. Diabetes Care 2011;34:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goel A, Chiu H, Felton J, Palmer JP, Brooks-Worrell B. T-cell responses to islet antigens improves detection of autoimmune diabetes and identifies patients with more severe β-cell lesions in phenotypic type 2 diabetes. Diabetes 2007;56:2110–2115 [DOI] [PubMed] [Google Scholar]
- 9.Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care 2014;37:3286–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks-Worrell BM, Palmer JP. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients. Clin Exp Immunol 2013;171:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarikonda G, Pettus J, Phatak S, et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun 2014;50:77–82 [DOI] [PubMed] [Google Scholar]
- 12.Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 2014;57:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang DC, Piaggi P, Hanson RL, et al. Use of a high-density protein microarray to identify autoantibodies in subjects with type 2 diabetes mellitus and an HLA background associated with reduced insulin secretion. PLoS One 2015;10:e0143551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams RC, Muller YL, Hanson RL, et al. HLA-DRB1 reduces the risk of type 2 diabetes mellitus by increased insulin secretion. Diabetologia 2011;54:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankl JA, Thearle MS, Desmarais C, Bogardus C, Krakoff J. T-cell receptor repertoire variation may be associated with type 2 diabetes mellitus in humans. Diabetes Metab Res Rev 2016;32:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks-Worrell BM, Starkebaum GA, Greenbaum C, Palmer JP. Peripheral blood mononuclear cells of insulin-dependent diabetic patients respond to multiple islet cell proteins. J Immunol 1996;157:5668–5674 [PubMed] [Google Scholar]
- 17.Brooks-Worrell B, Gersuk VH, Greenbaum C, Palmer JP. Intermolecular antigen spreading occurs during the preclinical period of human type 1 diabetes. J Immunol 2001;166:5265–5270 [DOI] [PubMed] [Google Scholar]
- 18.Lee B-C, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 2014;1842:446–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin T, Liu L-F, Lamendola C, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol 2014;34:2637–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamali AN, Noorbakhsh SM, Hamedifar H, et al. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol Immunol 2019;105:107–115 [DOI] [PubMed] [Google Scholar]
- 21.Morton AM, Sefik E, Upadhyay R, Weissleder R, Benoist C, Mathis D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc Natl Acad Sci U S A 2014;111:6696–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother 2018;101:287–292 [DOI] [PubMed] [Google Scholar]
- 23.Honkanen J, Nieminen JK, Gao R, et al. IL-17 immunity in human type 1 diabetes. J Immunol 2010;185:1959–1967 [DOI] [PubMed] [Google Scholar]
- 24.Zapata-Gonzalez F, Auguet T, Aragonès G, et al. Interleukin-17A gene expression in morbidly obese women. Int J Mol Sci 2015;16:17469–17481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chehimi M, Vidal H, Eljaafari A. Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J Clin Med 2017;6 pii: E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoud F, Al-Ozairi E. Inflammatory cytokines and the risk of cardiovascular complications in type 2 diabetes. Dis Markers 2013;35:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winer S, Winer DA. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol Cell Biol 2012;90:755–762 [DOI] [PubMed] [Google Scholar]
- 28.Font-Burgada J, Sun B, Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metab 2016;23:48–62 [DOI] [PubMed] [Google Scholar]
- 29.Yan B, Wang H, Rabbani ZN, et al. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res 2006;66:11565–11570 [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74 [DOI] [PubMed] [Google Scholar]
- 31.Deichgræber P, Witte DR, Møller HJ, et al. Soluble CD163, adiponectin, C-reactive protein and progression of dysglycaemia in individuals at high risk of type 2 diabetes mellitus: the ADDITION-PRO cohort. Diabetologia 2016;59:2467–2476 [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Chen F, Wang J, Zeng Z, Yang Q, Shao S. Th17 and Treg lymphocytes in obesity and type 2 diabetic patients. Clin Immunol 2018;197:77–85 [DOI] [PubMed] [Google Scholar]
- 33.Alkhouri N, Gornicka A, Berk MP, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 2010;285:3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B. The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 2015;45:2446–2456 [DOI] [PubMed] [Google Scholar]
- 35.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 36.Morris DL, Cho KW, Delproposto JL, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013;62:2762–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng T, Lyon CJ, Minze LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab 2013;17:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosomi S, Grootjans J, Tschurtschenthaler M, et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J Exp Med 2017;214:2985–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey VK, Mathur A, Kakkar P. Emerging role of unfolded protein response (UPR) mediated proteotoxic apoptosis in diabetes. Life Sci 2019;216:246–258 [DOI] [PubMed] [Google Scholar]
- 42.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 43.Boden G, Duan X, Homko C, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008;57:2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James EA, Pietropaolo M, Mamula MJ. Immune recognition of β-cells: neoepitopes as key players in the loss of tolerance. Diabetes 2018;67:1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corrigall VM, Bodman-Smith MD, Fife MS, et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol 2001;166:1492–1498 [DOI] [PubMed] [Google Scholar]
- 46.Weber CK, Haslbeck M, Englbrecht M, et al. Antibodies to the endoplasmic reticulum-resident chaperones calnexin, BiP and Grp94 in patients with rheumatoid arthritis and systemic lupus erythematosus. Rheumatology (Oxford) 2010;49:2255–2263 [DOI] [PubMed] [Google Scholar]
- 47.Obeid M, Panaretakis T, Tesniere A, et al. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res 2007;67:7941–7944 [DOI] [PubMed] [Google Scholar]
- 48.Abdulreda MH, Berggren PO. Islet inflammation in plain sight. Diabetes Obes Metab 2013;15(Suppl. 3):105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 50.Donath MY, Størling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J Mol Med (Berl) 2003;81:455–470 [DOI] [PubMed] [Google Scholar]
- 51.Hasnain SZ, Borg DJ, Harcourt BE, et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 2014;20:1417–1426 [DOI] [PubMed] [Google Scholar]
- 52.Winer DA, Winer S, Dranse HJ, Lam TKT. Immunologic impact of the intestine in metabolic disease. J Clin Invest 2017;127:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown EM, Kenny DJ, Xavier RJ. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu Rev Immunol 2019;37:599–624 [DOI] [PubMed] [Google Scholar]
- 54.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 55.Gulhane M, Murray L, Lourie R, et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep 2016;6:28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kracht MJL, Zaldumbide A, Roep BO. Neoantigens and microenvironment in type 1 diabetes: lessons from antitumor immunity. Trends Endocrinol Metab 2016;27:353–362 [DOI] [PubMed] [Google Scholar]
- 57.Marré ML, Piganelli JD. Environmental factors contribute to β-cell endoplasmic reticulum stress and neo-antigen formation in type 1 diabetes. Front Endocrinol (Lausanne) 2017;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913–916.e7 [DOI] [PubMed] [Google Scholar]
- 59.Abdellatif AM, Sarvetnick NE. Current understanding of the role of gut dysbiosis in type 1 diabetes. J Diabetes 2019;11:632–644 [DOI] [PubMed] [Google Scholar]
- 60.Wilkin TJ. The convergence of type 1 and 3.type 2 diabetes in childhood: the accelerator hypothesis. Pediatr Diabetes 2012;13:334–339 [DOI] [PubMed] [Google Scholar]
- 61.Prieur X, Mok CY, Velagapudi VR, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 2011;60:797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engin F, Yermalovich A, Nguyen T, et al. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes [published correction appears in Sci Transl Med 2013;5:214er11] Sci Transl Med 2013;5:211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 2010;59:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 2010;107:1071–1082 [DOI] [PubMed] [Google Scholar]
- 65.Xourgia E, Papazafiropoulou A, Papanas N, Melidonis A. Anti-diabetic treatment leads to changes in gut microbiome. Front Biosci (Landmark Ed) 2019;24:688–699 [DOI] [PubMed] [Google Scholar]