Abstract
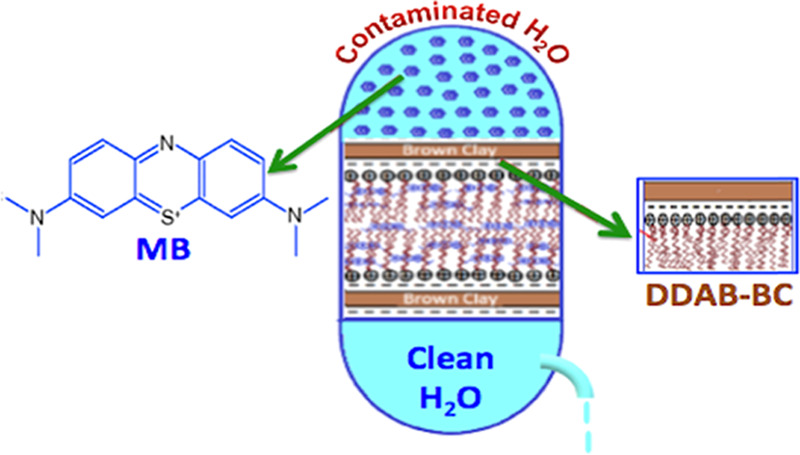
In the present investigation, brown clay (BC) was modified with didodecyldimethylammonium bromide (DDAB) to produce a sorbent (DDAB-BC), which was characterized and applied for sorption of methylene blue (MB) from aqueous media. BC was functionalized using DDAB by cation exchange of the DDAB moiety with positive ions existing inside the interlayer spaces of the BC. X-ray diffraction (XRD) studies confirmed that the d-spacing of DDAB-BC became wider (3.39 Å) than that of BC (3.33 Å). Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) were exploited to explore the functional groups and morphological structure of sorbents, respectively. The Brunauer–Emmett–Teller (BET) surface area, pore volume, and pore diameter of DDAB-BC were determined as 124.6841 m2/g, 0.316780 cm3/g, and 8.75102 nm, respectively. Batch sorption investigations were carried out to determine the optimum experimental conditions, using the one-factor one-time procedure. The sorption of MB on DDAB-BC strongly obeyed the Langmuir isotherm and agreed well with pseudo-second-order kinetics. Sorption of MB onto DDAB-BC showed maximum efficiency (∼98%) and maximum sorption capacity (∼164 mg/g) at optimal values of pertinent factors: dye concentration (100 mg/L), pH (7), and temperature (55 °C). Sorption isothermal studies predicted that removal of MB on DDAB-BC follows multilayer sorption at higher MB dye concentrations and monolayer sorption at lower MB dye concentrations.
1. Introduction
Industrial revolution and expansion of occupational activities for the needs of bulk population have increased the environmental pollution. The contaminants and pollutants discharged from various industrial units are creating a threat to the life on the earth and atmosphere. Among all of the environmental pollutions, water pollution is of extraordinary concern. Leather and textile industries are the leading dyestuff consumers in Pakistan and therefore the main sources of dyeing waste.1,2 The deterioration of aquatic environment by dye-containing effluents eventually affects aquatic fauna and flora. According to recent investigations, the researchers have reported that a total quantity of 7 × 105 tons of dyestuff is manufactured yearly and about 12–14% of which is discarded to the environment.3
Broadly, dyes are classified as anionic and cationic ones, on the basis of chemical functionality, and are toxic to a great extent and harmful to mankind and the environment. As they are having a toxic effect on the biotic component of the atmosphere, the investigators have tried their best to get rid of these pollutants. All available options have been employed to develop technologies for the abatement of water pollution caused by dyestuff. These technologies work via physicochemical,4 biological,5 ozonation,6 membrane filtration,7 advanced oxidation,8 or integrated treatment processes,4 but all of them have complete or partial limitations. Among these technologies, the process of adsorptive removal is groundbreaking, a cost-effective alternative, and easy to operate with a reliable performance.9,10 Of countless sorbents used for the mentioned purpose, the most pertinent is the activated carbon, which unfortunately is accessible at high prices and is limited in amounts. Thus, many investigators are working to discover sustainable alternative sorbents such as agriculture waste, bagasse, and rice husk. However, these biosorbents need a number of physical and chemical modifications to improve their efficiency, in addition to their limited quantities.11−13
Simultaneously, clay sorbents are becoming popular for wastewater treatment.14,15 Clay minerals possess distinct physical and chemical characteristics, which have transformed them into the ultimate choice for the sorption process due to being abundant in availability, low in cost, and classified as an environment friendly material. Therefore, clay can substitute low-performance biosorbents as well as costly activated carbon.16 Clay sorbents may be used as such or in modified form depending upon the nature of the target pollutants.17,18 Due to its geographical location, Pakistan possesses a great variety of clay minerals, and different types of clays abundantly exist in northern areas of this country, especially in the Azad Jammu and Kashmir (AJK) region.
A thorough literature survey reveals that no precise research study on the sorption potential of these clays, especially brown clay (BC), has been reported so far. Removal of organic pollutants from aqueous environments is primarily controlled by hydrophobic interactions at the interfaces of the sorbate and sorbent depending upon the carbon content of the sorbent rather than the mineral part of the clay sorbents.19,20 The modification of clay can significantly improve its surface properties, thereby improving its water-decontaminating efficiency. Surface modification with quaternary ammonium salts (i.e., surfactants) causes the inversion of the surface charge of the clays, making them cation and anion exchangers, and facilitates the sorption of organic molecules that have less affinity toward the unmodified clay surface.21
Organoclays prepared by intercalating surfactants have a higher carbon content and enhanced hydrophobicity, especially when using a cationic surfactant like didodecyldimethylammonium bromide (DDAB) for intercalation.22 In this way, cationic surfactants can exchange natural inorganic cations (sodium, calcium, potassium, and magnesium) on the clay surface, producing a carbon-enriched surface.23 Intercalation of DDAB into the clay interlayers not only changes the hydrophilic environment to hydrophobicity but also greatly increases the interlayer spacing.24,25 In addition, these organoclays are low-cost sorbents that can be used to treat most organic wastewater of the printing and dyeing unit.26 Therefore, to achieve the above goal, BC was modified by DDAB, switching to an alternative organoclay, which has potential sorption characteristics including low cost, abundant availability, nontoxicity, and eco-friendly nature as well as enhancing the sorption capacity of BC for the sorption of methylene blue (MB) dye via intercalation of DDAB on the clay interspatial spaces.
MB is a basic dye (Figure S1) extensively used for cotton and silk painting. The most common harmful effect of MB is eye burn since it is thought to be the cause of permanent injury to eyes. It is also evident to cause mental confusion, profuse sweating, vomiting, nausea, breathing problem, and methemoglobinemia.27 The present study was intended to investigate optimal values of various factors such as sorbent dose, particle size, pH, contact time, initial dye concentration, and temperature required for maximum removal of MB dye from aqueous environments. Furthermore, we have studied the sorption kinetic, equilibrium, and thermodynamics to understand equilibrium properties and the mechanism of the sorption process.
2. Results and Discussion
2.1. Screening of Different Clays for Removal of MB
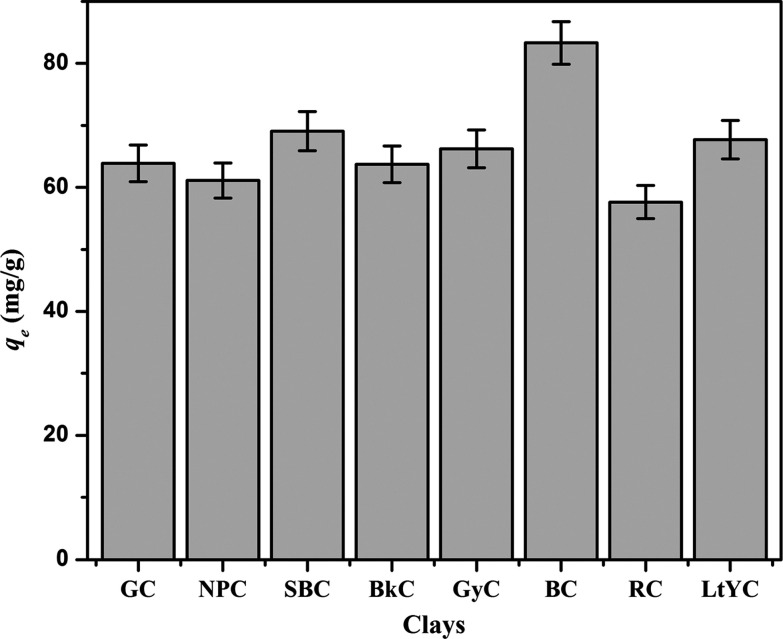
The preliminary screening experiments have been performed to select the best clay with the highest sorption capacity for the removal of MB. For this purpose, eight different clays have been selected from various regions and tested for the sorption of MB. Eight flasks, labeled with clay names, with 100 mL of 50 mg/L MB solution each containing 0.04 g of clay samples are agitated in an incubator shaker for 60 min. The sorptive removal of MB is calculated, and results are illustrated in Figure 1. The results depict that the maximum sorption capacity for MB is shown by BC with respect to all other clay samples; hence, BC is selected for modification in rest of the study. The BC is further modified with the DDAB surfactant to improve the sorption capacity of the clay, and a comparative study of raw BC and DDAB-modified BC is performed.
Figure 1.
Comparison of sorption capacities of different clays: green clay (GC), natural pink clay (NPC), sky blue clay (SBC), black clay (BkC), gray clay (GyC), brown clay (BC), red clay (RC), and light yellow clay (LtYC).
2.2. BC and DDAB-BC Characterization
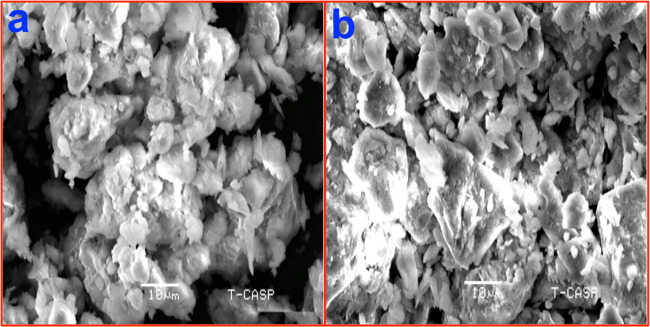
Visual assessment of scanning electron microscopy (SEM) images gives the morphology of the surface in BC and DDAB-BC sorbents and gives evidence about the textural properties of the sorbents. The SEM images of BC and DDAB-BC before MB sorption are displayed in Figure 2, and SEM images of MB-loaded BC and DDAB-BC are illustrated in Figure 3. The SEM image (Figure 2a) of the BC exhibits apparently the beads of nonmodified clay. In contrast, the DDAB-BC, synthesized by the interaction of cationic surfactant with clay mineral, is texturally more homogeneous, demonstrating that intra-particle areas have adequately been filled with the DDAB surfactant, as displayed in Figure 2b. Further, SEM micrographs of MB-loaded BC and DDAB-BC exhibit the complete filling of the sorption sites of the studied sorbents (Figure 3).
Figure 2.
SEM images of (a) BC and (b) DDAB-BC before sorption of MB.
Figure 3.
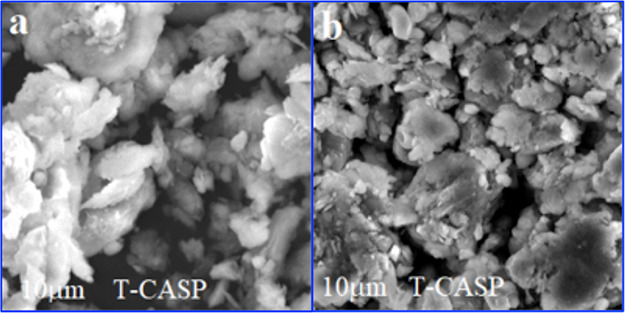
SEM images of (a) MB-loaded BC and (b) MB-loaded DDAB-BC after MB sorption.
X-ray diffraction (XRD) is an excellent technique to compare the interlayer distance of BC and DDAB-BC sorbents. XRD spectra of BC and DDAB-BC sorbents are presented in Figure 4. The values of d-spacing for the most intense peak cantered at a 2θ value of about 26.50 are 3.33 and 3.39 Å for BC and DDAB-BC, respectively. The treatment of clay with DDAB results in an increase in the d-spacing, which could be due to the higher number of carbon atoms in DDAB. The higher d-spacing facilitates sorption of larger dye or phenol molecules, and it decreases diffusion resistance during the sorption process.28,29
Figure 4.
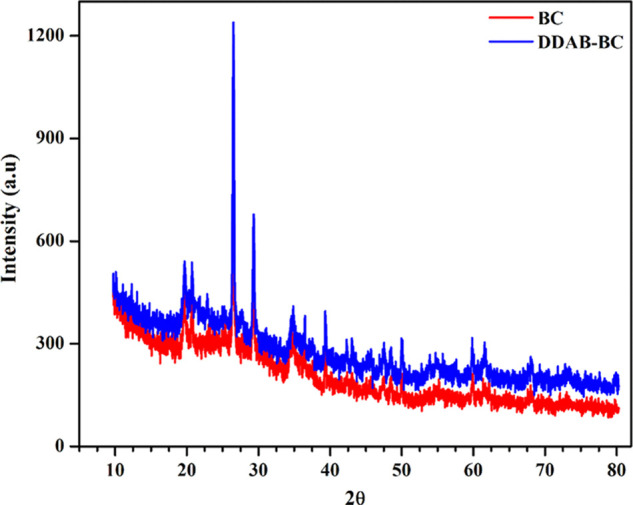
X-ray diffraction (XRD) patterns of BC and DDAB-BC.
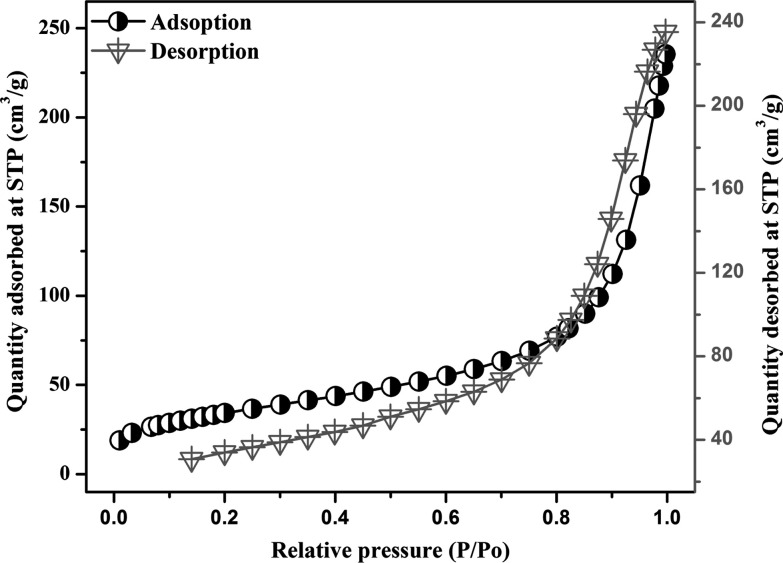
The behavior and sorption capacity of BC and DDAB-BC can be forecasted from the information about porosity and surface area. The information of sorption isotherms of BC is presented in Figure S2 and Table S1, whereas the information of sorption isotherms of DDAB-BC is presented in Figure 5 and Table S1, which specify that all samples have exhibited a much longer straight-line portion of the curve. Surface area analysis vouchsafes that the specific surface area of the DDAB-BC as determined by the Brunauer–Emmett–Teller (BET) procedure is much higher (124.6841 m2/g) than that of BC (14.5884 m2/g). The pore volume and the diameter of BC are determined to be 0.031916 cm3/g and 10.16264 nm, respectively, whereas the pore volume and the diameter of DDAB-BC are determined to be 0.316780 cm3/g and 8.75102 nm, respectively.
Figure 5.
BET isotherm plot of the DDAB-BC sorbent.
2.3. Effect of Solution pH
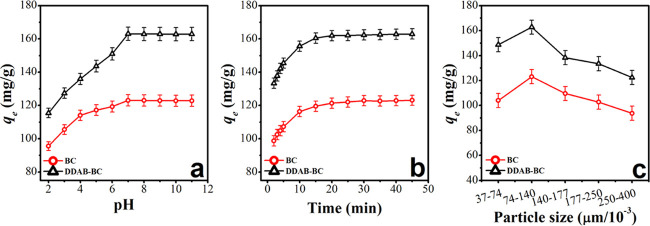
To investigate the influence of pH on MB sorption, the pH of the MB solution was varied from 2 to 11. The results of sorption behavior of MB at different pH values are illustrated in Figure 6a. The sorption studies demonstrate the enhanced sorption efficiency of MB from acidic to basic pH on both BC and DDAB-BC. An appreciable increase in dye uptake has been observed up to pH 7; thereafter, a slight decrease in the sorption of MB has been observed.
Figure 6.
Effect of pH (a), time (b), and particle size (c) on sorption of MB onto BC and DDAB-BC.
The variation in the sorption behavior of MB on BC and DDAB-BC with respect to initial pH alteration can be elucidated on the basis of point of zero charge (PZC) and molecular structure of MB. The PZC values for BC and DDAB-BC are determined to be 8.1 and 8.7, respectively (Figure S3). It is known that, as we move above pHpzc, the adsorbent particles attain a negative surface charge. MB is basic in nature and remains in an ionized form up to pH 7.4 with positive surface charge. As the pH of the solution was increased from 2 to 7, the BC and DDAB-BC surface became more and more deprotonated, providing more and more sorption sites for MB with increased sorption efficiency. At above pH 7.4, MB exists in an unionized form with neutral surface. The BC and DDAB-BC surfaces acquire a negative charge above their PZC (pHpzc 8.1 and 8.7). Therefore, it can easily be understood that electrostatic interactions along with the nonpolar interactions are responsible for the increase in dye uptake up to pH 7, and the decrease in electrostatic forces of attraction due to the neutral sorbate surface is credited for maintaining MB sorption at a steady rate above pH 7. The same trend has been supported by the literature for MB sorption.30,31 Consequently, the pH 7.0 has been reflected to be the optimum level because at this pH the maximum sorption capacity (123 ± 2.5 mg/g for BC and 163 ± 4.2 mg/g for DDAB-BC) has been observed.
2.4. Influence of Contact Time
The influence of contact time is a pertinent factor since it provides the relationship between fixed amounts of sorbent and contact time. It is utilized to estimate the sorption–desorption equilibrium contact time. The effect of time variation on the sorption capacity of BC and DDAB-BC for the removal of MB has been investigated in the range of 2–45 min as illustrated in Figure 6b. A rapid increase in the sorption capacity of both BC and DDAB-BC is noted during the first 10 min, and afterward, a very slow increase in the MB uptake is observed up to 45 min for both BC and DDAB-BC. The maximum sorption capacity (122.88 ± 2.7 mg/g for BC and 162.40 ± 3.2 mg/g for DDAB-BC) is determined at equilibrium time (30 min). The higher rates of sorption capacity of both BC and DDAB-BC at the beginning may be attributed to the presence of a large number of sorption sites at the surface of both BC and DDAB-BC, and low increase in sorption capacity is credited to the sorption sites existed in the internal regions of both BC and DDAB-BC. The enhanced sorption capacity of organoclay DDAB-BC could be due to surfactant modification, which has increased the carbon content of the sorbent.
2.5. Influence of Particle Size
A very important factor affecting the sorption behavior of MB is the size of particles of the sorbents (BC and DDAB-BC). The influence of particle size is studied by changing the particle size from 37 to 400 μm as illustrated in Figure 6c. An inverse relationship exists between the particle size of the BC and DDAB-BC and the sorption behavior of MB molecules because the surface area of sorbents increases with the decrease in particle size of the BC and DDAB-BC sorbents. A smaller particle size of the sorbent results in a higher sorption capacity of BC and DDAB-BC.32 The sorbent size of about 74 μm is found to be optimum for BC and DDAB-BC. The maximum sorption capacities for BC and DDAB-BC are 123.05 ± 2.3 and 162.72 ± 4.1 mg/g, respectively. Afterward, the reduction in particle size shows no appreciable increase in the removal of MB. These studies have provided a firm basis to believe that sorption capacity is increased up to a definite particle size, below which it remains invariable. Thus, the smallest particle size of 74 μm was utilized for further studies.
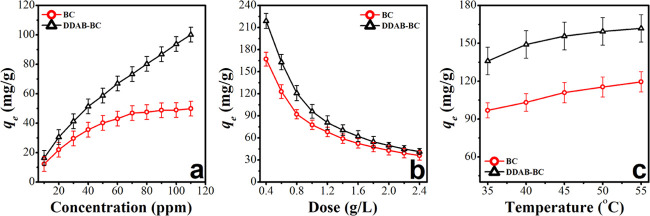
2.6. Influence of Initial Dye Concentration
The initial dye concentration is another important factor, which controls the sorption behavior of the dye. The influence of initial dye concentration has a relationship with the available sorption sites on the surface of the sorbent. The effect of MB concentration (10–110 mg/L) on the sorption capacity of BC and DDAB-BC is shown in Figure 7a. Experimental investigations reveal that on varying the sorbate MB concentration from 10 to 110 mg/L, the sorption capacity is increased from 16.25 ± 0.8 to 120.14 ± 2.9 mg/g in the case of BC and from 16.57 ± 1.2 to 160.87 ± 4.4 mg/L in the case of DDAB-BC. Similar results have been reported in the literature.33,34 The quantity of sorption sites at the surface of BC and DDAB-BC is greater, in the case of lower MB concentration, as compared to the MB molecules available; consequently, the majority of the MB molecules are adsorbed at the surface of the BC and DDAB-BC. Thus, a high percentage of MB molecules is adsorbed and high sorption efficiency is noted (results not shown) but low sorption capacity due to smaller amount of MB molecules available for both BC and DDAB-BC. Contrarily, in the case of higher concentrations of MB, the number of active sorption sites present on the surface of BC and DDAB-BC are smaller than the quantity of MB molecules available; thus, sorbate molecules have to compete among themselves for the fixed quantity of sorption sites on the surface of BC and DDAB-BC. Resultantly, the excess amounts of the sorbate molecules remain unsuccessful to be adsorbed on the surface of BC and DDAB-BC and stay free in the solution. Therefore, sorption capacity is increased while sorption efficiency (results not shown) is decreased with the increase of the concentration of MB. The study of the effect of MB concentration again shows clearly that sorption capacity is extremely higher for DDAB-BC compared to BC.
Figure 7.
Effect of MB concentration (a), sorbent dose (b), and temperature (c) on sorption of MB onto BC and DDAB-BC.
2.7. Influence of BC and DDAB-BC Dose
To study the effect of sorbent dose, the quantity of the sorbent is varied from 0.4 to 2.4 g/L, and the results are shown in Figure 7b. The results illustrate that in the case of BC the sorption potential decreases from 167.02 ± 4.7 to 36.14 ± 1.3 mg/g and sorption efficiency increases from 66.81 to 88.72% (results not shown) when the quantity of BC is increased from 0.4 to 2.4 g/L. A similar trend is also observed for DDAB-BC, that is, the sorption capacity of DDAB-BC decreases from 218.88 ± 6.2 to 41.41 ± 1.2 mg/g and the sorption efficiency increases from 87.56 to 99.38% (results not shown) on increasing the amount of DDAB-BC from 0.4 to 2.4 g/L, but further increment of the sorbent dose does not show any change in the sorption efficiency of DDAB-BC because majority of fixed number of dye molecules are adsorbed on excess number of sorption sites and negligible number of dye molecules are left. A similar trend has also been reported elsewhere.35−37 The maximum MB sorption was found at 0.6 g/L sorbent dose for both BC and DDAB-BC.
2.8. Influence of Temperature
The temperature change could derive the diffusion of MB molecules on the sorption sites present at the surface and inner porous parts of the BC and DDAB-BC. To investigate the variation of sorption behavior of BC and DDAB-BC as a function of temperature toward MB removal, the study is carried out in the temperature range from 35 °C (308 K) to 55 °C (328 K), as shown in Figure 7c. The results reveal that for BC, an increase in the sorption capacity (96.84–119.50 mg/g) is observed on increasing the temperature from 308 to 328 K. The same trend has also been observed for DDAB-BC with the only disparity that a higher sorption capacity (136.03–161.92 mg/g) is observed as a function of the change of temperature from 308 to 328 K, thus, the sorption process is endothermic. The endothermic sorption may be credited to the static interactions.38,39
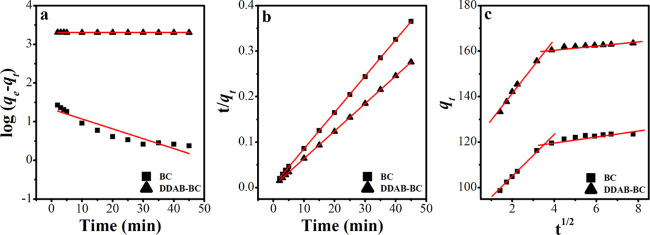
2.9. Adsorption Kinetic
Sorption kinetics studies are executed with the intention to establish the mechanism of the MB sorption process onto BC and DDAB-BC. The sorbate molecules are passed through a number of phases: first, transference of sorbate molecules to sorbent (BC and DDAB-BC) surface sites from the solution, for instance shifting of sorbate molecules to the outer surface of the sorbent particles; binding with the exterior sorption sites of the sorbent; and attachment with the sorption sites present in the interior surface parts of the porous region through diffusion.2,40 Generally, researchers apply a number of kinetics models to determine the sorption mechanism, and the most common kinetics models are pseudo-first-order and pseudo-second-order models.41−46 The mathematical forms of these models are given below in eqs 1 and 2, and graphical forms are illustrated in Figure 8.
| 1 |
| 2 |
where k1 (min–1) and k2 (g/mg.min) are the pseudo-first and pseudo-second-order rate constants, respectively. The experimental results reveal that the pseudo-first-order model, as shown in Figure 8a and Table 1 (R2 = 0.722 for BC and R2 = 0.666 for DDAB-BC), is failed to fit well as compared to the pseudo-second-order model, as presented in Figure 8b and Table 1 (R2 = 0.998 for BC and R2 = 0.999 for DDAB-BC). Further, the experimental qe values are closer to the theoretical qe values calculated from the pseudo-second order model, which also confirms the suitability of the pseudo-second-order model for MB sorption onto BC and DDAB-BC.
Figure 8.
Pseudo-first-order model (a), pseudo-second-order model (b), and intraparticle diffusion model (c) for MB sorption onto BC and DDAB-BC.
Table 1. Kinetic Parameters for Sorption of MB onto BC and DDAB-BC.
| parameters | BC | DDAB-BC |
|---|---|---|
| experimental qe (mg/g) | 122.9 | 162.4 |
| Pseudo-First-Order | ||
| theoretical qe (mg/g) | 44.65 | 73.37 |
| k1 × 10–3 (min–1) | 10.48 | 7.139 |
| R2 | 0.722 | 0.666 |
| Pseudo-Second-Order | ||
| theoretical qe (mg/g) | 125.0 | 163.9 |
| k2 × 10–3 (g/mg min) | 1.185 | 1.128 |
| R2 | 0.998 | 0.999 |
| Intraparticle Diffusion Model | ||
| region I | ||
| kip (mg/(g min1/2)) | 8.636 | 11.10 |
| Ci | 87.43 | 119.2 |
| R2 | 0.983 | 0.981 |
| region II | ||
| kip (mg/(g min1/2)) | 0.638 | 0.515 |
| Ci | 119.0 | 159.6 |
| R2 | 0.826 | 0.977 |
The prediction that the pseudo-second-order kinetics model explains more accurately the sorption of MB is further verified by the literature. Literature confirms that sorption of most of the dyes follows the pseudo-second-order kinetic model.42,47−49 The pseudo-second-order kinetic model predicts that chemical sorption is the limiting step of MB sorption and thus controls the sorption process.
Weber and Morris in 1963 investigated the direct relation between sorbate sorption and t1/2 as an alternative of contact time “t” and is mathematically represented by eq 3
| 3 |
where kip (mg/g.min1/2) and Ci represent the intraparticle diffusion rate constant and boundary layer thickness, respectively. When a graph is plotted between qt and t1/2, if it is linear and passes through the origin, then the uptake of sorbate is driven solely by intraparticle diffusion. In the study of sorption behavior of MB, two tendencies reflect that MB uptake is carried out by a combination of other mechanisms alongside intraparticle diffusion (Figure 8c). The first step illustrates that the fast MB uptake occurs at the exterior of the BC and DDAB-BC surface, and the shifting of MB molecules from the solution to the surface sorption sites of the sorbents is accomplished. The second step is the slowest step where the curve moves nearly parallel to the horizontal axis. This second step mainly controls the overall sorption of MB and establishment of equilibrium.50,51 Further, the greater values of Ci are related to the larger role of the boundary layer effect for the rate-determining step.
2.10. Adsorption Equilibrium
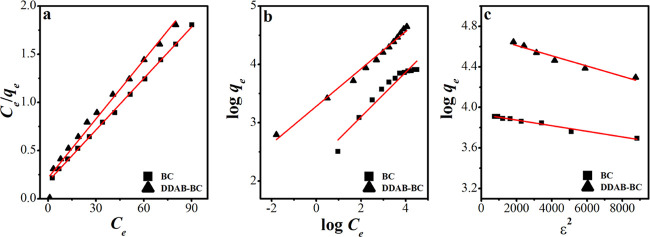
The study of the MB-loaded BC and MB-loaded DDAB-BC equilibrium system affords the interactions between BC and DDAB-BC particles and MB molecules, which provide the basis to design the sorption system.52,53 Investigations of sorption at constant temperature are utilized to optimize the sorption system and to select the scale. A variety of isothermal models such as Langmuir,54 Freundlich,49,55 and Dubinin–Raduskevich (D–R) have been applied for the sorption of MB onto BC and DDAB-BC.1,56 The mathematical expressions of the Langmuir, Freundlich, and Dubinin–Raduskevich models are represented by eqs 4–7.
| 4 |
| 5 |
| 6 |
| 7 |
where qm (mg/g) is the maximum sorption capacity in monolayer sorption. KL, KF, and n represent the Langmuir constant, Freundlich constant, and feasibility of the sorption process, respectively. ε is the Polanyi potential, and K is the activity coefficient used to find out the sorption energy E (kJ/mol). The plots of all isotherms are shown in Figure 9, and the values of parameters calculated from all isotherms are shown in Table 2. The R2 values calculated form the Langmuir, Freundlich, and Dubinin–Raduskevich models are 0.996, 0.939, and 0.803 for BC and 0.997, 0.954, and 0.833 for DDAB-BC, respectively.
Figure 9.
Langmuir model (a), Freundlich model (b), and Dubinin–Raduskevich model (c) for MB sorption onto BC and DDAB-BC.
Table 2. Adsorption Isotherm Parameters for Sorption of MB onto BC and DDAB-BC.
| parameters | BC | DDAB-BC |
|---|---|---|
| Langmuir | ||
| qm (mg/g) | 123.5 | 166.7 |
| KL (L/mg) | 0.336 | 0.899 |
| RL | 0.029 | 0.100 |
| R2 | 0.996 | 0.997 |
| Freundlich | ||
| KF (mg/g)(mg/L)1/n | 36.10 | 63.28 |
| 1/n | 0.348 | 0.372 |
| R2 | 0.939 | 0.954 |
| Dubinin–Radushkevich | ||
| qm (mg/g) | 89.26 | 113.6 |
| K × 10–6 (mol2/kJ2) | –0.100 | –0.040 |
| E (kJ/mol) | –2.236 | –3.535 |
| R2 | 0.803 | 0.833 |
The comparison of R2 values shows that the Langmuir model is best fitted and effectively describes the sorption of MB onto BC and DDAB-BC. Furthermore, the suitability of the Langmuir model is also established by the nearness of the theoretical qm (123.5 mg/g for BC and 166.7 mg/g for DDAB-BC) and experimental qe (122.9 mg/g for BC and 162.4 mg/g for DDAB-BC). The similar studies and behavior of dye sorption onto different sorbents have been reported by many researchers.42,49,57,58 Additionally, the values in the range of 1–10 for n (Freundlich constant) also predict favorable MB sorption onto BC and DDAB-BC. The Langmuir model also suggests that MB uptake from the aqueous environment follows the monolayer sorption mechanism, and it is decided using the factor RL, which is a dimensionless constant.2 The values of RL as calculated from the equation (RL = 1/1 + KLCo) are between 0 and 1 (RL = 0.029 for BC and RL = 0.100 for DDAB-BC), which show that MB uptake onto BC and DDAB-BC is favorable (Table 2).
The D–R model is rooted on the basis that although there are no homogeneous sorbent surface sites but it gives insight into the sorption energy as well as biomass porosity. The values of D–R model constants are presented in Table 2. The values of K are −10 × 10–8 and −4 × 10–8 mol2/kJ2 for BC and DDAB-BC and values of E are −2.236 and −3.535 kJ/mol for BC and DDAB-BC, respectively. The values of E show that the sorption of MB onto BC and DDAB-BC is also showing some contribution of physisorption. Similar behavior has been reported by many authors in their studies.59,60
The sorption capacity of DDAB-BC has been compared with that of other adsorbents available in the literature (Table 3), which shows that DDAB-BC evidently reveals excellent sorption performance for MB than other earlier reported adsorbents.
Table 3. Comparison of the Adsorption Capacities of Different Adsorbents for MB Previously Reported with That of DDAB-BC.
| adsorbent | adsorption capacity (mg/g) | pH | reference |
|---|---|---|---|
| Kaolin | 99.90 | 9.0 | (72) |
| heteroatom-codoped porous carbon | 100.2 | (73) | |
| cross-linked chitosan/bentonite composite | 142.9 | 11.0 | (74) |
| α-Fe2O3@yeast composite | 112.7 | 6.0 | (75) |
| H2SO4 cross-linked magnetic chitosan nanocomposite beads | 20.41 | 6.0 | (76) |
| Fe3O4 activated montmorillonite nanocomposite | 106.4 | 7.4 | (77) |
| C, N-doped MnO | 154.0 | 9.0 | (78) |
| Fe3O4-CTMAC/SEIA-Mt | 246.0 | (79) | |
| DDAB-BC | 163.0 | 7.0 | this study |
2.11. Adsorption Thermodynamics
A very important factor affecting the sorption of MB on the surface of BC and DDAB-BC is the temperature. The influence of temperature on the sorption behavior of MB onto BC and DDAB-BC is explained by determining the thermodynamic parameters such as standard enthalpy change (ΔH°), standard free energy change (ΔG°), and standard entropy change (ΔS°), using the following mathematical eqs 8 and 9.
| 8 |
| 9 |
where R is the gas constant, T is the temperature in kelvin, and the values of Ka are estimated with respect to temperature by plotting Ln qe/Ce vs qe and extra plotting qe to zero.61
The values of ΔS° and ΔH° are calculated from the intercept and slope of the straight-line plot between ln Ka and 1/T, respectively (Figure S4). The values of ΔG° were calculated using eq 8. The values of thermodynamic constants are given in Table 4. The values of ΔG° vary from −2.146 to −3.928 kJ/mol and −5.125 to −11.02 kJ/mol for BC and DDAB-BC, respectively. Thus, values of ΔG° reveal that sorption of MB onto BC and DDAB-BC is spontaneous and feasible at all temperatures. Furthermore, positive values of the ΔS° and ΔH° also show increase in randomness and endothermic nature of MB sorption onto BC and DDAB-BC, which suggests that the sorption process is thermodynamically stable. Moreover, the values of ΔG° slightly decrease at higher temperature, reflecting that the sorption process of MB onto BC and DDAB-BC is more promising at higher temperature.
Table 4. Thermodynamic Parameters for MB Sorption onto BC and DDAB-BC.
| BC |
DDAB-BC |
|||||
|---|---|---|---|---|---|---|
| temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/K mol) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/K mol) |
| 308 | –2.146 | –5.125 | ||||
| 313 | –2.592 | –6.905 | ||||
| 318 | –3.173 | 25.76 | 90.67 | –8.393 | 84.84 | 292.7 |
| 323 | –3.550 | –9.712 | ||||
| 328 | –3.928 | –11.02 | ||||
2.12. FTIR before and after Sorption of MB onto BC and DDAB-BC
Figure 10 shows the FTIR spectra of BC and DDAB-BC before (a,c) and after (b,d) MB sorption. The bands at 1041 and 915 cm–1 represent the bending vibrations of Si–O and Al–O, respectively in BC.62−64 The band at 3627.5 cm–1 is attributed to the stretching vibration of the OH group in the BC. The bands at 1467 and 1486 cm–1 are assigned to ammonium ions in the DDAB-BC showing the exchange of Na+ ions by NH4+ ions. The bands at 2922.53 and 2852.28 cm–1 represent the symmetric and antisymmetric stretching vibrations of CH2. The band at 1640.38 cm–1 represents the water present in the interlayers of the BC. Majority of the bands of the BC and DDAB-BC are analogous to each other, indicating that the original crystal of the DDAB-BC remains the same.65 The results show that the introduction of DDAB into BC has a stronger interaction with the siloxane surface than the water molecules and hydrated cations in unmodified BC. It was observed that the intensity of the band decreased with the introduction of DDAB. The observed shift of band from HOH bending to CH2 bending proves that the intercalated surfactant can replace the hydrated cation and shows the hydrophobic properties of the resulting organoclay.66−68 Due to the observed changes in the infrared spectrum, it is believed that the interlayer space of BC has been successfully embedded with DDAB.
Figure 10.
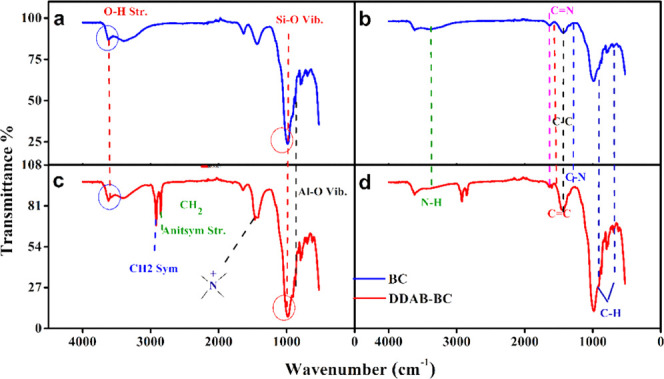
FTIR spectra of (a) BC, (b) MB-loaded BC, (c) DDAB-BC, and (d) MB-loaded DDAB-BC.
To confirm MB sorption onto BC and DDAB-BC sorbents, the FTIR analysis is also performed for MB-loaded BC and DDAB-BC samples. Figure 10b,d illustrates the FTIR spectra of MB-loaded BC and DDAB-BC, respectively. The peaks at 1600 and 1450 cm–1 represent C=C present in MB, and the peaks at 1690 and 1640 cm–1 represent the C=N stretching of MB, which confirms the successful sorption of MB onto BC and DDAB-BC.69 Further, the peaks at 1300 and 1000 cm–1 represent C-O, and peaks at 3500–3300 cm–1 represent N–H present in MB.69 The FTIR spectrum of DDAB-BC shows that as MB interacts with the organoclay, the strength of the two bands (Si–O stretching and CH stretching) increases, indicating an increased organic carbon content between the layers and stronger stretching of siloxane surfaces as well.
3. Conclusions
In this study, a local BC was modified with a cationic surfactant DDAB to improve its sorption capability to remove MB from the aqueous environment. The results thus obtained have revealed that DDAB-BC has sufficient potential due to acquired properties to be employed as a sorbent for the removal of organic pollutants from water environments. The maximum sorption of MB onto BC and DDAB-BC was found at pH 7, sorbent dose 0.6 g/L, and particle size 74 μm. The sorption equilibrium between MB and DDAB-BC was established in 30 min. Under optimized conditions, the sorption potentials of BC and DDAB-BC for the removal of MB from aqueous environments were 123.5 and 166.7 mg/g, respectively. The isothermal studies showed that MB uptake for BC and DDAB-BC followed the Langmuir isotherm model (R2 = 0.996 and 0.997) and pseudo-second-order kinetic model (R2 = 0.998 and 0.999), which fitted well to explain the sorption behavior of MB onto BC and DDAB-BC. Further, for thermodynamics, investigations showed that the sorption process of MB onto BC and DDAB-BC was spontaneous, endothermic in nature, and more favorable at higher elevated temperature. From the results obtained, it may be concluded that the modification of BC with DDAB has successfully improved the sorption properties of the clay. Further, more modification of clays with surfactants could provide new insights into the development of eco-efficient and effective sorbents for the removal of stringent organic pollutants. Hence, bulk availability of BC at a local level boosts its applicability as a cost-effective and highly economical source of cleaner production.
4. Experimental Section
4.1. Sorbent Preparation
Eight different clay samples were obtained from the areas of northern Punjab and Bhember AJK, Pakistan. The names of clays are green clay (GC), natural pink clay (NPC), sky blue clay (SBC), black clay (BkC), gray clay (GyC), brown clay (BC), red clay (RC), and light yellow clay (LtYC). All of the clays were ground into a fine powder using pestle and mortar. Further, clays were dried by keeping in an oven for a 24 h time period (at 105 °C) to evaporate the moisture and were then preserved in airtight containers. After the preliminary study, BC was found to be more efficient for the removal of MB, so it was selected for further study. BC was further refined into an amorphous powder to collect different particle sizes utilizing grading sieves. The sorbent was dried by keeping in an oven for a 24 h time period to evaporate the moisture and was then kept in airtight containers. Chemical composition of BC as examined is given in Table S2.
DDAB-BC was prepared by intercalation of the clay with cationic surfactant DDAB according to the method given in the literature.70 Briefly, 10 g of BC was suspended in 1.0 L of deionized water. The mixture was continuously agitated for a 24 h time period at ambient conditions utilizing a magnetic stirrer. The solution of DDAB tantamount to the cation-exchange capacity (CEC) of the clay was prepared, and the required quantity of DDAB was added to the BC suspension to carry out the positive ion exchange reaction. The suspension of DDAB-BC was further stirred for an interval of 12 h, and then water was removed from DDAB-BC using vacuum filtration. Residual chloride and other ions were removed from DDAB-BC by adding fresh deionized water and stirring for a 6 h time period. The process of rinsing was repeated until the absence of chloride, which was confirmed with a silver nitrate (AgNO3) solution. The acquired DDAB-BC was dried under vacuum, at 60 °C for 24 h, and ground by a pestle and mortar into a fine powder.
4.2. Sorbent Characterization
Inert BC and DDAB-BC sorbents were characterized using suitable analytical techniques. Through the BET method, exploiting N2 sorption isotherms (at 77 K), the pore diameter and specific surface area were estimated by a pore size and surface area analyzer. Exploiting the Barrett–Joyner–Halenda (BJH) route, pore distributions of BC and DDAB-BC were computed. The gases were removed from BC and DDAB-BC using a sonicator degasser prior to accomplishment of surface analyses. The morphological structures of BC and DDAB-BC samples were visually appraised from the data acquired by a JSM 5 × 910 instrument (JEOL, Japan), i.e., SEM micrographs. To determine the type of functional groups existing on BC and DDAB-BC, FTIR analysis was carried out exploiting a FTIR spectrometer (Jasco, USA). XRD spectra for BC and DDAB-BC were obtained using Cu Kα radiation (Cu anode, Kα1 wavelength 1.540598, Kα2 wavelength 1.5444426 such that the ratio of Kα2 to Kα1 was 0.5) operated at a generator voltage of 40 kV and tube current of 30 mA. The pHpzc was determined by the drift route as reported in the literature.71 Suitable concentrations (0.1 M) of both NaOH and HCl solutions were added systematically to regulate the pH.
4.3. Sorption Studies
MB was obtained from the market and was utilized in the experimental investigations without any purification treatment. The stock solution of 1000 mg/L of MB (C16H18ClN3S and M = 319.85 g/mol) was prepared by dissolving accurately measured 1.0 g of MB per 1000 mL of doubly distilled water. This stock solution was used to prepare the standard experimental solutions diluted by distilled water. A UV/vis spectrophotometer (PG Instrument T60, U.K.) was used to measure the absorbance of the MB solutions at 670 nm wavelength.
Sorption studies were conducted using the batch method to explore the influence of applied factors such as pH, sorbent dosage, temperature, initial dye concentration, and contact time on the sorption of MB onto BC and DDAB-BC sorbents. The experimental criteria such as pH (2–11), sorbent particle size (37–400 μm), MB concentration (10–110 mg/L), BC and DDAB-BC dose (0.4–2.4 g/L), temperature (35–55 °C), and contact time (2–45 min) were evaluated exploiting the one-factor one-time approach. For each single batch, 100 mL portion of the MB sorbate solution was shaken in a 250 mL titration flask with a weighed amount of BC and DDAB-BC sorbents for a definite time period at predefined conditions. The details of each run were mentioned in the relative portion of result and discussion. The optimum set of experimental conditions used in all of the experiments, unless otherwise specified, are as follows: sorbent dose (0.6 g/L), sorbate concentration (100 mg/L), contact time (30 min), pH 7, temperature (55 °C), and stirring speed (200 ppm). An orbital shaker has been utilized to equilibrate the MB-BC and MB-DDAB-BC heterogeneous systems. After equilibrating, the heterogeneous systems (MB-BC and MB-DDAB-BC) were subjected to centrifugation and filtration. The residual MB concentration was determined using a spectrophotometer by analyzing the filtrate. The residual MB concentration was used to determine the sorption capacity qe (mg/g) exploiting eq 10
| 10 |
where Ce (mg/L) is the MB concentration at equilibrium, Co (mg/L) is the initial MB concentration, w (g) is the sorbent mass, and V (L) is the MB solution volume. All of the experiments were performed in triplicate. The results were reported as mean ± SD.
The quantity of MB uptake qt (mg/g) at time “t” was acquired exploiting eq 11
| 11 |
where qt (mg/g) and Ct (mg/L) represent the amount of MB sorbed and MB concentration at any instant time “t”, respectively.
Acknowledgments
The authors express their gratitude to the Department of Chemistry, University of Gujrat, Pakistan, for the provision of lab facility. The authors acknowledge financial support from the Higher Education Commission of Pakistan through NRPU projects (20-4557/NRPU/R&D/HEC/14/481 and 6515/Punjab/NRPU/R&D/HEC/2016). The authors also appreciate funding from the Researchers Supporting Project (RSP-2020/58) from the King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01613.
Molecular structure of MB; the BET isotherm plot of BC; BET surface parameters of BC and DDAB-BC; composition of BC; the point of zero charge (pHPZC) of BC and DDAB-BC sorbents; and plots of Ln Ka vs 1/T for MB sorption onto BC and DDAB-BC (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rehman M. S. U.; Munir M.; Ashfaq M.; Rashid N.; Nazar M. F.; Danish M.; Han J.-I. Adsorption of Brilliant Green dye from aqueous solution onto red clay. Chem. Eng. J. 2013, 228, 54–62. 10.1016/j.cej.2013.04.094. [DOI] [Google Scholar]
- Safa Y.; Bhatti H. N. Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination 2011, 272, 313–322. 10.1016/j.desal.2011.01.040. [DOI] [Google Scholar]
- Auta M.; Hameed B. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem. Eng. J. 2011, 171, 502–509. 10.1016/j.cej.2011.04.017. [DOI] [Google Scholar]
- Qian F.; Sun X.; Liu Y. Removal characteristics of organics in bio-treated textile wastewater reclamation by a stepwise coagulation and intermediate GAC/O3 oxidation process. Chem. Eng. J. 2013, 214, 112–118. 10.1016/j.cej.2012.09.130. [DOI] [Google Scholar]
- Javaid M.; Saleemi A. R.; Naveed S.; Zafar M.; Ramzan N. Anaerobic treatment of desizing effluent in a mesophilic anaerobic packed bed reactor. J. Pak. Inst. Chem. Eng. 2011, 39, 61–67. [Google Scholar]
- Aziz F.; Rehman M. S.; Batool A.; Muhammad A.; Mahmood T. Pretreatment of municipal, industrial and composite wastewater by ozonation. Environ. Process Eng. 2012, 1, 1–8. [Google Scholar]
- Kurt E.; Koseoglu-Imer D. Y.; Dizge N.; Chellam S.; Koyuncu I. Pilot-scale evaluation of nanofiltration and reverse osmosis for process reuse of segregated textile dyewash wastewater. Desalination 2012, 302, 24–32. 10.1016/j.desal.2012.05.019. [DOI] [Google Scholar]
- Yasar A.; Khalil S.; Tabinda A. B.; Malik A. Comparison of cost and treatment efficiency of solar assisted advance oxidation processes for textile dye bath effluent. Korean J. Chem. Eng. 2013, 30, 131–138. 10.1007/s11814-012-0110-4. [DOI] [Google Scholar]
- Zafar M. N.; Aslam I.; Nadeem R.; Munir S.; Rana U. A.; Khan S. U. D. Characterization of chemically modified biosorbents from rice bran for biosorption of Ni(II). J. Taiwan Inst. Chem. Eng. 2015, 46, 82–88. 10.1016/j.jtice.2014.08.034. [DOI] [Google Scholar]
- Liu Y.; Wang S.; Lu Y.; Zhao Y.; Zhang Y.; Xu G.; Zhang J.; Fang Z.; Xu W.; Chen X. Loading Control of Metal–Organic Frameworks in Fe3O4@MOFs Series Composite Adsorbents for Optimizing Dye Adsorption. Ind. Eng. Chem. Res. 2019, 58, 22244–22249. 10.1021/acs.iecr.9b03501. [DOI] [Google Scholar]
- Rehman M. S. U.; Kim I.; Han J.-I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. 10.1016/j.carbpol.2012.06.078. [DOI] [PubMed] [Google Scholar]
- Rehman M. S. U.; Han J.-I. Biosorption of methylene blue from aqueous solutions by Typha angustata phytomass. Int. J. Environ. Sci. Technol. 2013, 10, 865–870. 10.1007/s13762-012-0128-5. [DOI] [Google Scholar]
- Bulgariu L.; Escudero L. B.; Bello O. S.; Iqbal M.; Nisar J.; Adegoke K. A.; Alakhras F.; Kornaros M.; Anastopoulos I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. 10.1016/j.molliq.2018.12.001. [DOI] [Google Scholar]
- Auta M.; Hameed B. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198, 219–227. 10.1016/j.cej.2012.05.075. [DOI] [Google Scholar]
- Auta M.; Hameed B. Acid modified local clay beads as effective low-cost adsorbent for dynamic adsorption of methylene blue. J. Ind. Eng. Chem. 2013, 19, 1153–1161. 10.1016/j.jiec.2012.12.012. [DOI] [Google Scholar]
- Kismir Y.; Aroguz A. Z. Adsorption characteristics of the hazardous dye Brilliant Green on Saklıkent mud. Chem. Eng. J. 2011, 172, 199–206. 10.1016/j.cej.2011.05.090. [DOI] [Google Scholar]
- Nassar M. M.; El-Geundi M. S.; Al-Wahbi A. A. Equilibrium modeling and thermodynamic parameters for adsorption of cationic dyes onto Yemen natural clay. Desalin. Water Treat. 2012, 44, 340–349. 10.1080/19443994.2012.691701. [DOI] [Google Scholar]
- Bai T.; Zhao K.; Gao Q.; Qi M.; Zhang Y.; Lu Z.; Zhao H.; Gao H.; Wei J. Kaolin/CaAlg Hydrogel Thin Membrane with Controlled Thickness, High Mechanical Strength, and Good Repetitive Adsorption Performance for Dyes. Ind. Eng. Chem. Res. 2020, 59, 4958–4967. 10.1021/acs.iecr.9b06687. [DOI] [Google Scholar]
- Yaqoob S.; Ullah F.; Mehmood S.; Mahmood T.; Ullah M.; Khattak A.; Zeb M. A. Effect of waste water treated with TiO2 nanoparticles on early seedling growth of Zea mays L. J. Water Reuse Desalin. 2018, 8, 424–431. 10.2166/wrd.2017.163. [DOI] [Google Scholar]
- Boyd S. A.; Mortland M. M.; Chiou C. T. Sorption characteristics of organic compounds on hexadecyltrimethylammonium-smectite. Soil Sci. Soc. Am. J. 1988, 52, 652–657. 10.2136/sssaj1988.03615995005200030010x. [DOI] [Google Scholar]
- Ozola R.; Krauklis A.; Burlakovs J.; Klavins M.; Vincevica-Gaile Z.; Hogland W. Surfactant-Modified Clay Sorbents for the Removal of p-nitrophenol. Clays Clay Miner. 2019, 67, 132–142. 10.1007/s42860-019-00015-2. [DOI] [Google Scholar]
- Dutta A.; Singh N. Surfactant-modified bentonite clays: preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res. 2015, 22, 3876–3885. 10.1007/s11356-014-3656-3. [DOI] [PubMed] [Google Scholar]
- Sun K.; Shi Y.; Chen H.; Wang X.; Li Z. Extending surfactant-modified 2:1 clay minerals for the uptake and removal of diclofenac from water. J. Hazard. Mater. 2017, 323, 567–574. 10.1016/j.jhazmat.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Zawrah M. F.; Khattab R. M.; Saad E. M.; Gado R. A. Effect of surfactant types and their concentration on the structural characteristics of nanoclay. Spectrochim. Acta, Part A 2014, 122, 616–623. 10.1016/j.saa.2013.11.076. [DOI] [PubMed] [Google Scholar]
- Saitoh T.; Shibayama T. Removal and degradation of β-lactam antibiotics in water using didodecyldimethylammonium bromide-modified montmorillonite organoclay. J. Hazard. Mater. 2016, 317, 677–685. 10.1016/j.jhazmat.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Ahmadishoar J.; Bahrami H. S.; Movassagh B.; Amirshahi H. S.; Arami M. Removal of Disperse Blue 56 and Disperse Red 135 dyes from aqueous dispersions by modified montmorillonite nanoclay. Chem. Ind. Chem. Eng. Q. 2017, 23, 21–29. 10.2298/CICEQ150116049A. [DOI] [Google Scholar]
- Balarak D.; Jaafari J.; Hassani G.; Mahdavi Y.; Tyagi I.; Agarwal S.; Gupta V. K. The use of low-cost adsorbent (Canola residues) for the adsorption of methylene blue from aqueous solution: Isotherm, kinetic and thermodynamic studies. Colloids Interface Sci. Commun. 2015, 7, 16–19. 10.1016/j.colcom.2015.11.004. [DOI] [Google Scholar]
- Abd El-Ghaffar M. A.; Youssef A.; El-Hakim A. A. Polyaniline nanocomposites via in situ emulsion polymerization based on montmorillonite: Preparation and characterization. Arabian J. Chem. 2015, 8, 771–779. 10.1016/j.arabjc.2014.01.001. [DOI] [Google Scholar]
- Bhattacharyya K. G.; Sharma A. Kinetics and thermodynamics of methylene blue adsorption on neem (Azadirachta indica) leaf powder. Dyes Pigm. 2005, 65, 51–59. 10.1016/j.dyepig.2004.06.016. [DOI] [Google Scholar]
- Beltrán-Suito R.; Pinedo-Flores A.; Bravo-Hualpa F.; Ramos-Muñoz J.; Sun-Kou M. d. R. Adsorption of N, N-dimethylamine from aqueous solutions by a metal organic framework, MOF–235. J. Disp. Sci. Technol. 2018, 1–8. 10.1080/01932691.2018.1489275. [DOI] [Google Scholar]
- Reddy D. D.; Ghosh R. K.; Bindu J. P.; Mahadevaswamy M.; Murthy T. Removal of methylene blue from aqueous system using tobacco stems biomass: Kinetics, mechanism and single-stage adsorber design. Environ. Prog. Sustainable Energy 2017, 36, 1005–1012. 10.1002/ep.12542. [DOI] [Google Scholar]
- Reske R.; Mistry H.; Behafarid F.; Roldan Cuenya B.; Strasser P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. 10.1021/ja500328k. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Jain R.; Mittal A.; Saleh T. A.; Nayak A.; Agarwal S.; Sikarwar S. Photo-catalytic degradation of toxic dye amaranth on TiO 2/UV in aqueous suspensions. Mater. Sci. Eng.: C 2012, 32, 12–17. 10.1016/j.msec.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Ahmad R.; Kumar R. Adsorption of amaranth dye onto alumina reinforced polystyrene. Clean Soil, Air, Water. 2011, 39, 74–82. 10.1002/clen.201000125. [DOI] [Google Scholar]
- Mane V. S.; Babu P. V. Kinetic and equilibrium studies on the removal of Congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust. J. Taiwan Inst. Chem. Eng. 2013, 44, 81–88. 10.1016/j.jtice.2012.09.013. [DOI] [Google Scholar]
- El-Bindary A. A.; El-Sonbati A. Z.; Al-Sarawy A. A.; Mohamed K. S.; Farid M. A. Adsorption and thermodynamic studies of hazardous azocoumarin dye from an aqueous solution onto low cost rice straw based carbons. J. Mol. Liq. 2014, 199, 71–78. 10.1016/j.molliq.2014.08.010. [DOI] [Google Scholar]
- El-Bindary A. A.; Hussien M. A.; Diab M. A.; Eessa A. M. Adsorption of Acid Yellow 99 by polyacrylonitrile/activated carbon composite: kinetics, thermodynamics and isotherm studies. J. Mol. Liq. 2014, 197, 236–242. 10.1016/j.molliq.2014.05.003. [DOI] [Google Scholar]
- Yao Y.; Xu F.; Chen M.; Xu Z.; Zhu Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. 10.1016/j.biortech.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Ho Y.-S.; McKay G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. 10.1016/S0923-0467(98)00076-1. [DOI] [Google Scholar]
- Mane V. S.; Babu P. V. Studies on the adsorption of Brilliant Green dye from aqueous solution onto low-cost NaOH treated saw dust. Desalination 2011, 273, 321–329. 10.1016/j.desal.2011.01.049. [DOI] [Google Scholar]
- Ho Y.; Ng J.; McKay G. Kinetics of pollutant sorption by biosorbents: review. Sep. Purif. Methods 2000, 29, 189–232. 10.1081/SPM-100100009. [DOI] [Google Scholar]
- Zafar M. N.; Amjad M.; Tabassum M.; Ahmad I.; Zubair M. SrFe2O4 nanoferrites and SrFe2O4/ground eggshell nanocomposites: Fast and efficient adsorbents for dyes removal. J. Clean. Prod. 2018, 199, 983–994. 10.1016/j.jclepro.2018.07.204. [DOI] [Google Scholar]
- Lagergren S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakad Handlngar 1898, 24, 1–39. [Google Scholar]
- Ho Y. S.; McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. 10.1016/S0032-9592(98)00112-5. [DOI] [Google Scholar]
- Zafar M. N.; Abbas I.; Nadeem R.; Sheikh M. A.; Ghauri M. A. Removal of Nickel onto Alkali Treated Rice Bran. Water, Air, Soil Pollut. 2009, 197, 361–370. 10.1007/s11270-008-9817-6. [DOI] [Google Scholar]
- Kumari S.; Khan A. A.; Chowdhury A.; Bhakta A. K.; Mekhalif Z.; Hussain S. Efficient and highly selective adsorption of cationic dyes and removal of ciprofloxacin antibiotic by surface modified nickel sulfide nanomaterials: Kinetics, isotherm and adsorption mechanism. Colloids Surf., A 2020, 586, 124264 10.1016/j.colsurfa.2019.124264. [DOI] [Google Scholar]
- Abdellaoui K.; Pavlovic I.; Bouhent M.; Benhamou A.; Barriga C. A comparative study of the amaranth azo dye adsorption/desorption from aqueous solutions by layered double hydroxides. Appl. Clay Sci. 2017, 143, 142–150. 10.1016/j.clay.2017.03.019. [DOI] [Google Scholar]
- Nasuha N.; Hameed B. Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem. Eng. J. 2011, 166, 783–786. 10.1016/j.cej.2010.11.012. [DOI] [Google Scholar]
- Zafar M. N.; Dar Q.; Nawaz F.; Zafar M. N.; Iqbal M.; Nazar M. F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. 10.1016/j.jmrt.2018.06.002. [DOI] [Google Scholar]
- Sreenivasulu B.; Sreedhar I.; Venugopal A.; Reddy B. M.; Raghavan K. V. Thermo-kinetic investigations of high temperature carbon capture using Coal-Flyash doped sorbent. Energy Fuels 2017, 10.1021/acs.energyfuels.6b02721. [DOI] [Google Scholar]
- Pan M.; Lin X.; Xie J.; Huang X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. 10.1039/C6RA26802A. [DOI] [Google Scholar]
- Kavitha D.; Namasivayam C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. 10.1016/j.biortech.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ghaedi M.; Hossainian H.; Montazerozohori M.; Shokrollahi A.; Shojaipour F.; Soylak M.; Purkait M. A novel acorn based adsorbent for the removal of brilliant green. Desalination 2011, 281, 226–233. 10.1016/j.desal.2011.07.068. [DOI] [Google Scholar]
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. 10.1021/ja02242a004. [DOI] [Google Scholar]
- Freundlich H. Adsorption in solutions. J. Phys. Chem. A 1906, 57, 384–410. [Google Scholar]
- Gardner L.; Kruk M.; Jaroniec M. Reference data for argon adsorption on graphitized and nongraphitized carbon blacks. J. Phys. Chem. B 2001, 105, 12516–12523. 10.1021/jp011745+. [DOI] [Google Scholar]
- Kipling J. J.Adsorption from Solutions of Non-Electrolytes; Academic Press, 2017. [Google Scholar]
- Li L.; Liu F.; Jing X.; Ling P.; Li A. Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel IDA-chelating resin: isotherm and kinetic modeling. Water Res. 2011, 45, 1177–1188. 10.1016/j.watres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Abia A.; Horsfall M.; Didi O. The use of chemically modified and unmodified cassava waste for the removal of Cd, Cu and Zn ions from aqueous solution. Bioresour. Technol. 2003, 90, 345–348. 10.1016/S0960-8524(03)00145-7. [DOI] [PubMed] [Google Scholar]
- Elinge C.; Itodo A.; Peni I.; Birnin-Yauri U.; Mbongo A. Assessment of heavy metals concentrations in bore-hole waters in Aliero community of Kebbi State. Adv. Appl. Sci. Res. 2011, 2, 279–282. [Google Scholar]
- Zubair M.; Jarrah N.; Manzar M. S.; Al-Harthi M.; Daud M.; Mu’azu N. D.; Haladu S. A. Adsorption of eriochrome black T from aqueous phase on MgAl-, CoAl- and NiFe- calcined layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2017, 230, 344–352. 10.1016/j.molliq.2017.01.031. [DOI] [Google Scholar]
- Zhou Q.; He H. P.; Zhu J. X.; Shen W.; Frost R. L.; Yuan P. Mechanism of p-nitrophenol adsorption from aqueous solution by HDTMA + -pillared montmorillonite—implications for water purification. J. Hazard. Mater. 2008, 154, 1025–1032. 10.1016/j.jhazmat.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou K.; Liu J.; Zeng W.; Hu Y.; Gui Z. In situ synthesis, morphology, and fundamental properties of polymer/MoS 2 nanocomposites. Compos. Sci. Technol. 2015, 107, 120–128. 10.1016/j.compscitech.2014.11.017. [DOI] [Google Scholar]
- Peng K.; Fu L.; Li X.; Ouyang J.; Yang H. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. 10.1016/j.clay.2017.01.003. [DOI] [Google Scholar]
- Yuan P.; Annabi-Bergaya F.; Tao Q.; Fan M.; Liu Z.; Zhu J.; He H.; Chen T. A combined study by XRD, FTIR, TG and HRTEM on the structure of delaminated Fe-intercalated/pillared clay. J. Colloid Interface Sci. 2008, 324, 142–149. 10.1016/j.jcis.2008.04.076. [DOI] [PubMed] [Google Scholar]
- He H.; Frost R. L.; Bostrom T.; Yuan P.; Duong L.; Yang D.; Xi Y.; Kloprogge J. T. Changes in the morphology of organoclays with HDTMA+ surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. 10.1016/j.clay.2005.10.011. [DOI] [Google Scholar]
- He H.; Yang D.; Yuan P.; Shen W.; Frost R. L. A novel organoclay with antibacterial activity prepared from montmorillonite and Chlorhexidini Acetas. J. Colloid Interface Sci. 2006, 297, 235–243. 10.1016/j.jcis.2005.10.031. [DOI] [PubMed] [Google Scholar]
- He H.; Zhou Q.; Martens W. N.; Kloprogge T. J.; Yuan P.; Xi Y.; Zhu J.; Frost R. L. Microstructure of HDTMA+-modified montmorillonite and its influence on sorption characteristics. Clays Clay Miner. 2006, 54, 689–696. 10.1346/CCMN.2006.0540604. [DOI] [Google Scholar]
- Ghodake G.; Jadhav U.; Tamboli D.; Kagalkar A.; Govindwar S. Decolorization of textile dyes and degradation of mono-azo dye amaranth by Acinetobacter calcoaceticus NCIM 2890. Indian J. Microbiol. 2011, 51, 501–508. 10.1007/s12088-011-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H.; Ma Y.; Zhu J.; Yuan P.; Qing Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl. Clay Sci. 2010, 48, 67–72. 10.1016/j.clay.2009.11.024. [DOI] [Google Scholar]
- Çakmak M.; Taşar Ş.; Selen V.; Özer D.; Özer A. Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay. J. Central South Univ. 2017, 24, 743–753. 10.1007/s11771-017-3476-y. [DOI] [Google Scholar]
- Mouni L.; Belkhiri L.; Bollinger J.-C.; Bouzaza A.; Assadi A.; Tirri A.; Dahmoune F.; Madani K.; Remini H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. 10.1016/j.clay.2017.11.034. [DOI] [Google Scholar]
- Chen B.; Yang Z.; Ma G.; Kong D.; Xiong W.; Wang J.; Zhu Y.; Xia Y. Heteroatom-doped porous carbons with enhanced carbon dioxide uptake and excellent methylene blue adsorption capacities. Microporous Mesoporous Mater. 2018, 257, 1–8. 10.1016/j.micromeso.2017.08.026. [DOI] [Google Scholar]
- Bulut Y.; Karaer H. Adsorption of Methylene Blue from Aqueous Solution by Crosslinked Chitosan/Bentonite Composite. J. Dispersion Sci. Technol. 2015, 36, 61–67. 10.1080/01932691.2014.888004. [DOI] [Google Scholar]
- Wang Y.; Zhang W.; Qin M.; Zhao M.; Zhang Y. Green one-pot preparation of α-Fe2O3@carboxyl-functionalized yeast composite with high adsorption and catalysis properties for removal of methylene blue. Surf. Interface Anal. 2018, 50, 311–320. 10.1002/sia.6370. [DOI] [Google Scholar]
- Rahmi; Ishmaturrahmi; Mustafa I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. 10.1016/j.microc.2018.09.032. [DOI] [Google Scholar]
- Chang J.; Ma J.; Ma Q.; Zhang D.; Qiao N.; Hu M.; Ma H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. 10.1016/j.clay.2015.06.038. [DOI] [Google Scholar]
- Xu Y.; Ren B.; Wang R.; Zhang L.; Jiao T.; Liu Z. Facile Preparation of Rod-like MnO Nanomixtures via Hydrothermal Approach and Highly Efficient Removal of Methylene Blue for Wastewater Treatment. Nanomaterials 2019, 9, 10 10.3390/nano9010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani S.; Zeynizadeh B.; Karami S. Removal of cationic methylene blue dye using magnetic and anionic-cationic modified montmorillonite: kinetic, isotherm and thermodynamic studies. Appl. Clay Sci. 2020, 184, 105391 10.1016/j.clay.2019.105391. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.