Disorders of consciousness are challenging to diagnose. Hermann et al. identify a new clinical sign, the habituation of auditory startle reflex, which indexes the activity of a fronto-parietal network and predicts improved consciousness at 6 months. The measure could be a new bedside sign of minimally conscious state.
Keywords: disorders of consciousness, minimally conscious state, cortically mediated state, auditory startle reflex, habituation
Abstract
Neurological examination of non-communicating patients relies on a few decisive items that enable the crucial distinction between vegetative state (VS)—also coined unresponsive wakefulness syndrome (UWS)—and minimally conscious state. Over the past 10 years, this distinction has proven its diagnostic value as well as its important prognostic value on consciousness recovery. However, clinicians are currently limited by three factors: (i) the current behavioural repertoire of minimally conscious state items is limited and restricted to a few cognitive domains in the goldstandard revised version of the Coma Recovery Scale; (ii) a proportion of ∼15–20% clinically VS/UWS patients are actually in a richer state than VS/UWS as evidenced by functional brain imaging; and (iii) the neurophysiological and cognitive interpretation of each minimally conscious state item is still unclear and debated. In the current study we demonstrate that habituation of the auditory startle reflex (hASR) tested at bedside constitutes a novel, simple and powerful behavioural sign that can accurately distinguish minimally conscious state from VS/UWS. In addition to enlarging the minimally conscious state items repertoire, and therefore decreasing the low sensitivity of current behavioural measures, we also provide an original and rigorous description of the neurophysiological basis of hASR through a combination of functional (high density EEG and 18F-fluorodeoxyglucose PET imaging) and structural (diffusion tensor imaging MRI) measures. We show that preservation of hASR is associated with the functional and structural integrity of a brain-scale fronto-parietal network, including prefrontal regions related to control of action and inhibition, and meso-parietal areas associated with minimally conscious and conscious states. Lastly, we show that hASR predicts 6-month improvement of consciousness. Taken together, our results show that hASR is a cortically-mediated behaviour, and suggest that it could be a new clinical item to clearly and accurately identify non-communicating patients who are in the minimally conscious state.
Introduction
Since 2002, neurological examination of awake but non-communicating patients has taken advantage of the Coma Recovery Scale-Revised (CRS-R), which is an extremely valuable behavioural tool allowing the distinction between a vegetative state (VS)—also termed unresponsive wakefulness syndrome (UWS)—and a minimally conscious state (MCS) (Giacino et al., 2002; Giacino and Kalmar, 2005). The CRS-R rapidly became the gold standard behavioural scale to explore disorders of consciousness because of: (i) the relatively short amount of time required to score a patient (30–45 min); (ii) its excellent inter-examiner reproducibility (Giacino et al., 2004); (iii) its ability to detect residual signs of non-reflex behaviours in 30–40% of patients who were mistaken for being in a VS/UWS (Schnakers et al., 2009); and (iv) its value to predict consciousness recovery (Luauté et al., 2010; Faugeras et al., 2018).
In a recent study, we proposed a reinterpretation of the MCS as defined by the CRS-R (Naccache, 2018a). Rather than providing any univocal evidence for a residual conscious state, the CRS-R MCS items allow clinicians to detect with certitude behaviours that recruit cortical networks, as opposed to VS/UWS items that correspond to reflexive behaviours mediated by subcortical and brainstem structures. For instance, the presence of smooth visual pursuit demonstrates the involvement of an occipito-parieto-frontal cortical network, whereas visual startle relies on a brainstem circuit. In other terms, rather than defining an elusive MCS (Bernat, 2002; Fischer and Truog, 2015), the 11 MCS items of the CRS-R enable the identification of a cortically mediated state (CMS). This new interpretation of MCS as a CMS discards the ambiguity related to the phrasing of MCS (‘What is minimal in MCS?’, ‘Are MCS patients conscious?’), and clarifies on solid grounds the importance of CRS-R for probing of consciousness in these patients: given that being conscious requires the functioning of vast cortical networks, the more a patient is MCS (or CMS), the more he/she is prone to be conscious or to recover consciousness. Note that this conception also emphasizes the necessity to use functional brain imaging tools (e.g. PET, MRI, EEG) to probe covert cognitive and cortical processes inaccessible to the CRS-R scoring. The recent fractionation of MCS in MCS− and MCS+, with the implicit idea that the latter would be closer to an unequivocal conscious state than the former (Bruno et al., 2011, 2012), and the recent concept of cognitive-motor dissociation (Schiff, 2015), a situation in which command following is demonstrated in clinically unresponsive patients using functional MRI (Owen et al., 2006; Monti et al., 2010) and/or EEG active paradigms (Cruse et al., 2012; Edlow et al., 2017; Curley et al., 2018; Claassen et al., 2019), support this novel interpretation.
In this context, the ability to probe additional residual cortically-mediated behaviours becomes a crucial goal in order to enrich the bedside clinical tool-box of caregivers who examine patients. Several years ago, we made the following observation: when probing the presence of an auditory startle reflex (ASR), the lowest item of the CRS-R auditory subscale, some patients seemed to be unable to stop blinking, while in other patients, the ASR was extinguishable. We thus designed a new clinical test to probe these two response profiles and hypothesized that ASR extinction or habituation (hASR) would require the contribution of cortical networks related to executive functions and inhibition. Indeed, several works originating from decision-making (Libet, 1985; Haggard, 2008; Schultze-Kraft et al., 2016), memory (Jacoby, 1991) and visual perception (Persaud and Cowey, 2008) pointed to the links between the ability to inhibit a behaviour and consciousness. If confirmed, this observation and its proposed neurophysiological interpretation could lead to the definition of a new basic clinical sign of MCS/CMS at bedside during behavioural examination, and could also enrich our current knowledge of the physiological mechanisms of ASR. Indeed, while the neural circuitry subtending ASR is well described, the one in charge of its habituation remains more discussed. ASR is a purely subcortical reflex whose pathways have been located in the brainstem, with an initial relay in the cochlear nuclei, followed by an intermediate relay in the brainstem reticular formation, connected to spinal cord and brainstem motor neurons, which provide the output motor response (Yeomans and Frankland, 1995). However, the neuronal mechanisms underpinning its short-term habituation are far less understood and long debated in the literature, with proponents of an ‘intrisinc’ modulation, i.e. habituation originating directly within the brainstem ASR pathway (Fox, 1979; Leaton et al., 1985), and proponents of an ‘extrinsic’ modulation by distant inhibitory projections, notably of cortical origin (Du et al., 2011).
In the present work we confirm our hypothesis and our main predictions: (i) by defining a rigorous and reproducible behavioural procedure to measure hASR at bedside; (ii) by demonstrating its value as a new powerful MCS/CMS sign; (iii) by showing that its presence is correlated with cortical activity both during an auditory task and during resting state periods, as measured with high-density EEG and 18F-fluorodeoxyglucose-PET (FDG-PET) imaging; (iv) by correlating hASR presence with a more preserved structural integrity of whole-brain white matter tracts; and (v) by showing that hASR predicts consciousness improvement at 6 months.
Materials and methods
Ethics statement
This research was approved by the local ethics committee Comité de Protection des Personnes Ile de France 1 (Paris, France) under the code ‘Recherche en soins courants’ (NEURODOC protocol, n° 2013-A01385-40). Patient’s family gave their informed consent for the participation of their relative and all investigations conformed to the Declaration of Helsinki and the French regulations.
Habituation of auditory startle response paradigm
In this study we prospectively assessed the diagnostic performances and neural underpinnings of a new behavioural sign, the ASR habituation paradigm, based on the ability of patients to inhibit the ASR when presented with repeated sounds, according to the following procedure.
Method
For each trial, present a loud noise by clapping your hands directly above the patient’s head and out of view. Clap 10 times consecutively at a frequency of ∼120 bpm (i.e. 2 Hz). Administer four trials.
Response
An auditory startle is present if eyelid flutter or blink occurs immediately following the stimulus on at least two trials. The reflex is considered inextinguishable (absence of habituation), if there is an eyelid flutter or a blink following each and every clap. Otherwise, the reflex is considered extinguishable. To ensure that an extinguishable response does not happen by chance, we required to observe the extinguishable response for at least two trials, as for the visual fixation or the visual pursuit, two others frequently observed MCS signs. It should be noted that in order to seek the reflex, one should not clap too fast (ideally two claps per second, 2 Hz), since there is a refractory period during which no blink can be obtained regardless of the hASR profile of the patient. Consequently, in case of a single suppressed blink, we advise repeating the procedure.
The hASR assessment was always performed by two raters trained in the CRS-R (one administering the test and both scoring the response). No discrepancy between raters was observed. See Supplementary Video 1 for footage of typical inextinguishable and extinguishable responses.
Participants
All participants of the study were patients suffering from brain injuries hospitalized in Paris Pitié-Salpêtrière teaching hospital and referred to our team for consciousness assessment. In addition to the studied behavioural test, patient evaluation comprised clinical assessment (neurological examination and CRS-R) and functional and structural brain imaging in the absence of contraindications (see below). CRS-R was carried out by trained clinicians and used as the reference gold standard (Giacino et al., 2018) to define a patient’s state of consciousness on the day of the test. To test the hASR diagnostic value, we only included patients exhibiting a standard ASR as defined by the first item of the CRS-R auditory subscale. Six-month outcome was gathered by phone interview of the treating physician and/or family. Responsiveness recovery was defined as reproducible response to command and consciousness recovery as accurate functional communication, following the scoring guidelines of the CRS-R.
Electroencephalogram
Local global paradigm
Quantitative high-density scalp EEG was recorded at the bedside, on the same day as the clinical assessment (CRS-R and hASR) using the ‘local-global’ auditory oddball paradigm, in order to study unconscious and conscious cognitive processing of auditory novelty by observing brain response to violation of auditory regularity at two different timescales (Bekinschtein et al., 2009) (Supplementary material).
Acquisition and preprocessing
Impedances were set below 100 kΩ before acquisitions. Recordings were made at a 250 Hz sampling frequency using a 256 electrodes HydroCel Geodesic Sensor Net (Electrical Geodesics) referenced to the vertex with impedances set below 100 kΩ prior to acquisition. EEG were preprocessed using an automated pipeline previously described (Supplementary material) (Sitt et al., 2014; Engemann et al., 2018). Only the 224 scalp electrodes were included in the two types of analyses that were performed on the EEG.
EEG markers analysis
Twenty-eight markers from spectral, connectivity, complexity and evoked domains were computed from the EEG and resumed according to their averages and fluctuations over time and space as described previously (Supplementary material) (Sitt et al., 2014; Engemann et al., 2018), yielding a total of 112 EEG features per patients. These EEG features were used to predict the state of consciousness of each patient using a linear support vector machine (SVM) classifier trained on a separate dataset of 311 recordings from 244 patients (n = 150 VS/UWS and n = 161 MCS) (Supplementary material). In addition, we analysed scalp topographies of individual markers (Supplementary material).
Event-related potentials analysis
We analysed group-level event-related potentials (ERP) elicited by the local-global paradigm according to the state of consciousness and the presence or absence of an habituation to auditory startle. Epochs were baseline corrected over the 800 ms window preceding the onset of the fifth sound and ERPs were extracted by group-level averaging of trials over regions of interest for two contrasts: the local contrast (local deviant versus local standard), which is the response to the violation of the short time-scale regularity; and the global contrast (global deviant versus global standard) for the response to violation of the long time-scale regularity. The local effect was observed over a Fz-centred region of interest (mean of channels 6, 7, 14, 15, 16, 22, 23) while the global effect was observed over a Pz-centred region of interest (mean of channels 100, 101, 110, 119, 128, 129).
We also analysed the contingent negative variation (CNV) ERP component, which corresponds to a slow negative drift beginning from the onset of the first sound to the onset of the fifth sound, indexing the expectancy of the fifth sound (Faugeras et al., 2012; Chennu et al., 2013). Epochs were baseline corrected over the 200 ms preceding the onset of the first sound and we computed the slope of the ordinary least-squares regression with patients’ average voltage over global standard trials as the dependent variable and time over the 0–600 ms period as the independent variable, both at the sensor-level and over Cz-centred region of interest (mean over channels 6, 7, 14, 15, 22, 23).
FDG-PET acquisition and preprocessing
18F-fluorodeoxyglucose-PET (FDG-PET) was acquired in the nuclear medicine department of the hospital only for patients without mechanical ventilation and free of sedation for at least 48 h. As PET was not always carried out on day of clinical assessment, only PET performed within 1 week from the test were kept. PET acquisition parameters and quantification procedure, which followed the procedure described by Stender et al. (2016), are described in the Supplementary material.
MRI acquisition and processing
Deep white matter integrity was assessed using diffusion tensor imaging (DTI) from diffusion-weighted imaging sequences (DWI) acquired in the neuroradiology department of the hospital, following a previously described methodology (Van Der Eerden et al., 2014). This procedure allowed us to quantify the deep white matter fractional anisotropy (FAdeep) and mean diffusivity (MDdeep) expressed as normalized values according to the mean values of healthy control subjects acquired with the same MRI protocol (Supplementary material). Extensive quality control of raw and processed images has been carried out. In particular, patients’ examinations for which DTI co-registration onto the MNI standard space failed due to the presence of huge deformations or the presence of massive haematomas have been removed from the analysis.
Statistical analysis
Clinical analysis
Population characteristics were describe using the mean ± standard deviation or the median (interquartile range, IQR) as appropriate for quantitative data. Group differences were tested by two-sample Student t-test or Mann-Whitney U-test, respectively. Categorical data were compared by the chi-squared (χ2) test or the Fisher’s exact test (in case of cells with n ≤ 5). No adjustment for multiple comparisons was performed for these tests. Diagnostic performance of the startle habituation test was assessed using standard classification metrics [sensitivity, specificity, predictive values, likelihood ratios, accuracy and area under the ROC curve (AUC)] with their 95% confidence intervals (95% CI) (Supplementary material). Extinguishable response was set as a positive test and MCS diagnosis based on the gold standard CRS-R as the positive reference. We then made a qualitative comparison of these performances to the performances of every other CRS-R items defining MCS state. However, since MCS diagnosis is defined by the CRS-R, these comparisons are limited and we pursued the explorations of the added value and underlying signification of hASR using measures of brain activity independent of the behaviour. Prognostic value of hASR was assessed using logistic regression with 6-month outcome (consciousness and/or responsiveness recovery) as the dependent variable and hASR alone or with the clinical state of consciousness as predictors.
EEG analysis
To investigate the specific effect of the type of ASR response on the multivariate prediction of the SVM, we ran a type II 2 × 2 ANOVA with the predicted probability from the classifier as the dependent variable and both the type of response (extinguishable or inextinguishable) and the state of consciousness (VS/UWS or MCS) as factors without including an interaction term in the main manuscript (justification of this choice and analyses including the interaction term are supplied in the Supplementary material). Our hypothesis was that by probing executive/inhibitory control cortical networks, which are not probed by the CRS-R items, the value of the hASR as a CMS sign would be the same in both VS/UWS and MCS. Two post hoc comparisons contrasting extinguishable response to inextinguishable response within each state of consciousness group were carried out by means of Student’s t-test (or Welch's test in in case of unequal variance), with a significance threshold set at P < 0.05. We did not adjust for multiple comparisons as we only focused on these two comparisons. This allowed us to investigate the added value of hASR to the CRS-R without multiplying unnecessary comparisons such as MCS-EX (extinguishable) versus VS/UWS-IN (inextinguishable) or MCS-IN versus VS-IN. We then explored the effect of startle on single marker topographies by performing the same ANOVA at each sensor. In this topographical analysis, we used a robust non-parametric cluster-based permutation procedure to control for multiple comparisons (Maris and Oostenveld, 2007), with F-values corresponding to P < 0.05 used to compute the first-step cluster mass and 10 000 random permutations of the patients’ labels to construct the null hypothesis surrogate distribution of cluster masses. Since the clustering procedure was not possible with pairs of channels for the weighted symbolic mutual information (wSMI) connectivity metric, we reported results from the ANOVA thresholded at P < 0.005 uncorrected.
We probed ERP local and global effects by contrasting deviant from standard region of interest time series using paired t-tests over all time points followed by a temporal cluster-based permutation procedure to control for multiple comparisons. Clusters of adjacent time samples with P < 0.05 over the whole time series were formed, including the 800 ms baseline, thus ensuring that significant effect, if any, would be superior to random baseline fluctuations. CNV topographies were analysed as the other markers’ topographies, with a spatial cluster-based permutation procedure. At the region of interest level, we performed a one-sample t-test against zero of the individual patient CNV slope distribution.
PET analysis
We first analysed mean metabolic activity of the highest of both hemispheres, a measure that was shown to be one of the best diagnostic markers of MCS (Stender et al., 2016), with a 2 × 2 ANOVA including state of consciousness and ASR response type as factors. We then performed voxel-wise analysis of the metabolic index using linear models with age as covariate, since glucose uptake decreases with ageing (Moeller et al., 1996; Petit-Taboué et al., 1998; Hsieh et al., 2012; Shen et al., 2012). To that end, images were smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel and masked using a grey matter MNI template. We first performed a simple contrast between the group with extinguishable reflex and the group with inextinguishable reflex, with age as a covariate. To account for the different proportions of VS/UWS and MCS patients in each group, we then added the state of consciousness as covariate in a second analysis. Significance threshold was set to P < 0.005, uncorrected, with a minimum extent of 100 voxels per cluster.
MRI analysis
The relationship between structural integrity of white matter tracts and hASR was assessed on both FAdeep and MDdeep values. These metrics have previously been associated with long-term outcome in both traumatic (Galanaud et al., 2012) and non-traumatic (Luyt et al., 2012; Velly et al., 2018) injuries. As VS/UWS patients have more severe white matter damage than MCS patients (Newcombe et al., 2010; Fernández-Espejo et al., 2011; Lant et al., 2016), we performed the 2 × 2 ANOVA and post hoc comparisons described above, in order to isolate the independent effect of the type of ASR response on the deep white matter tracts integrity. Contrary to EEG datasets, MRI and PET recordings were not always performed on the same day of the CRS-R and ASR habituation testing. Consequently, both analyses were carried out using the closest available CRS-R score and ASR habituation testing.
Software
All analyses except for the PET quantification procedure were carried out using open-source softwares R [version 3.3.2 (2016-10-31)] and Python (version 3.6.7). More precisely EEG preprocessing was done using homemade scripts, markers computations used the freely available NICE library (Engemann et al., 2018) (https://github.com/nice-tools/nice) and MNE-python (Gramfort et al., 2014) and Scikit-learn (Pedregosa et al., 2011) packages. Statistical analyses were carried out using Statsmodels and Seaborn Python packages, as well as caret (Kuhn, 2008), pROC (Robin et al., 2011) and epiR packages in R. For the PET quantification procedure, dicoms images were converted to niftis using MRIcro, images were registered on templates using Advanced Normalization Tools (ANTs version 2.0.3), then processed in MATLAB [MATLAB 9.1 (R2016b) Mathworks Inc., Natick, Massachusetts] using SPM8 toolbox (Statistical Parametric Mapping version 12, Wellcome Trust Centre for Neuroimaging, University of London).
Data availability
Patients’ main demographic and clinical data are available in Supplementary Table 2. Other data, including brain imaging data, are available upon reasonable request, but cannot be made open because of ethics protocol requirement and the sensitive nature of patient data.
Results
Habituation of auditory startle reflex as a new clinical sign of MCS
Between January 2014 and July 2019, 96 patients (48 VS/UWS and 48 MCS) were prospectively tested with the presented ASR habituation paradigm: mean age 44.2 ± 16.4 years, sex ratio 1.8. Median delay from injury was 58 (31–236) days and 49 (51%) were still mechanically ventilated. The two most frequent aetiologies were post-anoxic encephalopathy (41%) and traumatic brain injury (28%). A habituation was observed in 53 (55%) patients [extinguishable startle response (ASR-EX)], while there was no habituation in the remaining 43 (45%) patients [inextinguishable startle response (ASR-IN)]. Demographic characteristics were comparable between ASR-EX and ARS-IN patients, except for a significantly lower age in the former (40.1 ± 16.7 versus 47.4 ± 15.6 years, P = 0.0315) (Table 1). Patient-level data are described in Supplementary Table 2. It is important to note that although hearing capacity was not specifically assessed, deafness or hearing loss is very unlikely since (i) we included only patients exhibiting at least a standard ASR; (ii) no patient except one (who had an extinguishable ASR) had a previous past medical history of hearing loss; and (iii) none of the patients exhibited a CRS-R score pattern suggestive of hearing loss, that is high-level or complex behaviours in visual, motor, oromotor subscales with poor auditory subscale response.
Table 1.
Population characteristics
| Habituation of auditory startle |
||||
|---|---|---|---|---|
| All | Inextinguishable | Extinguishable | P | |
| (n = 96) | (n = 43) | (n = 53) | ||
| Demographic characteristics | ||||
| Age, years | 44.2 ± 16.4 | 40.1 ± 16.7 | 47.4 ± 15.6 | 0.0315 |
| Sex ratio, male/female | 1.8 | 2.1 | 1.7 | 0.7543 |
| Time since injury, days | 58 [31–236] | 57 [30–364] | 58 [31–181] | 0.9003 |
| Aetiology | 0.2682 | |||
| Anoxia | 39 (41%) | 20 (47%) | 19 (36%) | |
| Traumatic | 27 (28%) | 14 (33%) | 13 (24%) | |
| Vascular | 12 (12%) | 3 (7%) | 9 (17%) | |
| Other | 18 (19%) | 6 (14%) | 12 (23%) | |
| Mechanical ventilation | 49 (51%) | 23 (53%) | 26 (49%) | 0.8207 |
| ICU | 59 (61%) | 23 (53%) | 36 (68%) | 0.2171 |
| Behaviour | ||||
| CRS-R total score | 7 [5–10] | 5 [5–8] | 10 [7–12] | <0.0001 |
| Audio subscore | 1 [1–2] | 1 [1–1] | 2 [1–2] | 0.0004 |
| Visual subscore | 1 [0–3] | 0 [0–1] | 2 [1–3] | <0.0001 |
| Motor subscore | 2 [1–2] | 2 [1–2] | 2 [2–5] | 0.0003 |
| Verbal subscore | 1 [1–1] | 1 [1–1] | 1 [1–1] | 0.0028 |
| Communication subscore | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0.0573 |
| Arousal subscore | 2 [1–2] | 1 [1–2] | 2 [1–2] | 0.0422 |
| State of consciousness | 0.0002 | |||
| VS/UWS | 48 (50%) | 31 (72%) | 17 (32%) | |
| MCS | 48 (50%) | 12 (28%) | 36 (68%) | |
| Brain imaging | ||||
| EEG | ||||
| Performed | 96 (100%) | 43 (100%) | 53 (100%) | 1.0 |
| Analysable | 84 (88%) | 41 (95%) | 43 (81%) | 0.0598 |
| FDG-PET (only non-mechanically-ventilated patients) | ||||
| Performed | 40/47 (85%) | 18/20 (90%) | 22/27 (81%) | 0.6916 |
| Analysable | 35/47 (74%) | 17 (40%) | 18 (34%) | 0.7257 |
| DTI MRI | ||||
| Performed | 80 (83%) | 38 (88%) | 42 (79%) | 0.2795 |
| Analysable | 56 (58%) | 24 (56%) | 32 (60%) | 0.8081 |
Quantitative data are expressed as mean ± standard deviation or median [interquartile range] and compared through Student t-test and Mann-Whitney U-test, respectively. Categorical data are expressed as n (%) and compared through chi-squared test or Fisher’s exact test. DTI = diffusion tensor imaging MRI.
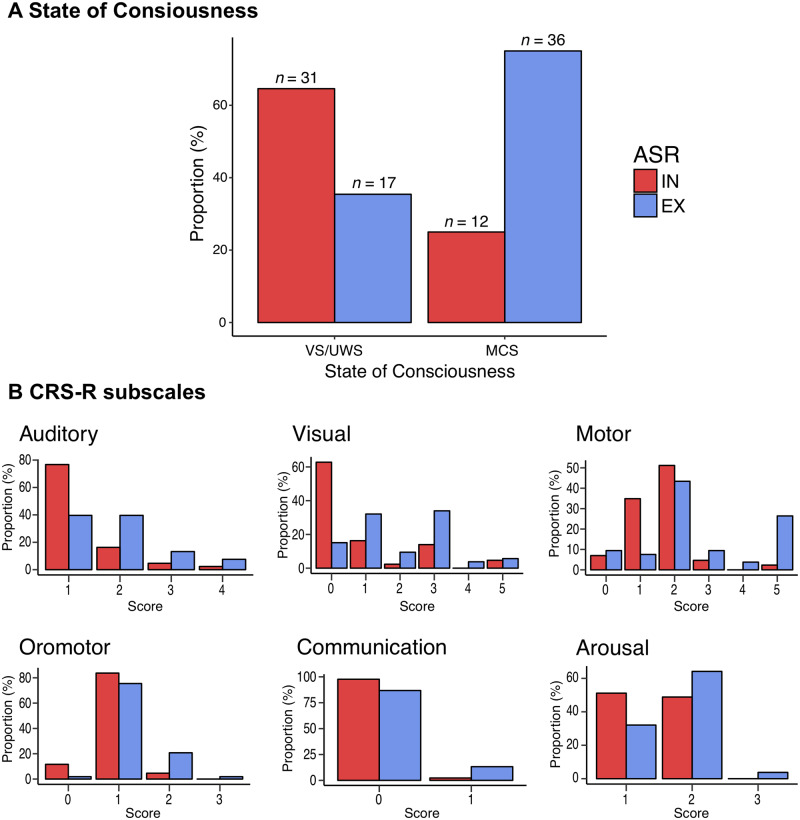
The proportion of ASR response type differed significantly according to the state of consciousness measured on the day of ASR testing: habituation was present in 36 of 48 (75%) MCS patients but only in 17 of 48 (35%) VS/UWS patients, χ2(1) = 13.6, P = 0.0002 (Fig. 1A). Likewise, patients with ASR-EX had higher scores in every CRS-R subscale except for the communication subscale (Table 1 and Fig. 1B). This association between hASR and current MCS items suggests that it could be a valuable additional clinical sign to diagnose MCS. AUC for the discrimination of MCS from VS/UWS was 0.70 (0.60–0.79), with a 75% 95% CI (60–86) sensitivity, 65% (49–78) specificity, 2.12 (1.40–3.21) positive and 0.39 (0.23–0.66) negative likelihood ratios. Diagnostic performances were comparable for acute patients (≤45 days from injury) and chronic patients (>45 days from injury) (Supplementary material). We then compared this performance to every other CRS-R item defining MCS (Table 2). The hASR performed next to the best CRS-R item, the visual pursuit, in terms of discrimination [AUC 0.70 (0.60–0.79) versus 0.75 (0.68–0.82)] and accuracy [70% (60–79) versus 75% (65–83)], with a higher sensitivity [75% (60–86) versus 50% (35–65)]. No other MCS items showed better discrimination metrics (Table 2).
Figure 1.
The hASR response and CRS-R. Proportion of the type of response to auditory startle reflex (ASR), either extinguishable (EX, in blue) or inextinguishable (IN, in red), according to the clinical state of consciousness defined by the CRS-R showing that ASR-EX is more frequent in minimally conscious (MCS) patients than in vegetative (VS/UWS) patients and vice versa, χ2(1) = 13.6, P = 0.0002 (A). Proportions of ASR response according to CRS-R scores within each subscale (B).
Table 2.
Performances of habitation to ASR versus other CRS-R MCS items
| Pr (%) | AUC [95% CI] | Sen, % [95% CI] | Sp, % [95% CI] | PPV, % [95% CI] | NPV, % [95% CI] | PLR [95% CI] | NLR [95% CI] | Acc, % [95% CI] | |
|---|---|---|---|---|---|---|---|---|---|
| Habituation of auditory startle reflex | |||||||||
| Exhaustible | 55 | 0.70 [0.60–0.79] | 75 [60–86] | 65 [49–78] | 68 [54– 80] | 72 [56–85] | 2.12 [1.40–3.21] | 0.39 [0.23–0.66] | 70 [60–79] |
| Auditory | |||||||||
| Reproducible (3) | 9 | 0.59 [0.54–0.66] | 19 [9–33] | – | – | 55 [44–66] | – | 0.81 [0.71–0.93] | 59 [49–69] |
| Systematic (4) | 5 | 0.55 [0.51–0.6] | 10 [3–23] | – | – | 53 [42–63] | – | 0.90 [0.81–0.99] | 55 [45–65] |
| Visual | |||||||||
| Fixation (2) | 6 | 0.56 [0.52–0.61] | 12 [5–25] | – | – | 53 [43–64] | – | 0.88 [0.79–0.97] | 56 [46–66] |
| Pursuit (3) | 25 | 0.75 [0.68–0.82] | 50 [35–65] | – | – | 67 [55–77] | – | 0.50 [0.38–0.66] | 75 [65–83] |
| Localization (4) | 2 | 0.52 [0.5–0.55] | 4 [1–14] | – | – | 51 [41–62] | – | 0.96 [0.90–1.02] | 52 [42–62] |
| Recognition (5) | 5 | 0.55 [0.51–0.59] | 10 [3–23] | – | – | 53 [42–63] | – | 0.90 [0.81–0.99] | 55 [45–65] |
| Motor subscale | |||||||||
| Localization (3) | 7 | 0.57 [0.53–0.62] | 15 [6–28] | – | – | 54 [43–65] | – | 0.85 [0.76–0.96] | 57 [47–67] |
| Manipulation (4) | 2 | 0.52 [0.5–0.55] | 4 [1–14] | – | – | 51 [41–62] | – | 0.96 [0.90–1.02] | 52 [42–62] |
| Automatic (5) | 16 | 0.66 [0.59–0.72] | 31 [19–46] | – | – | 59 [48–70] | – | 0.69 [0.57–0.83] | 66 [55–75] |
| Oromotor and verbal subscale | |||||||||
| Verbalization (3) | 1 | 0.51 [0.5–0.53] | 2 [0–11] | – | – | 51 [40–61] | – | 0.98 [0.94–1.02] | 51 [41–61] |
| Communication subscale | |||||||||
| Intentional (1) | 8 | 0.58 [0.53–0.64] | 17 [7–30] | – | – | 55 [44–65] | – | 0.83 [0.73–0.95] | 58 [48–68] |
Acc = accuracy; AUC = area under the ROC curve; NLR = negative likelihood ratio; NPV = negative predictive value; PLR = positive likelihood ratio; PPV = positive predictive value; Pr = prevalence; Sen = sensitivity; Sp = specificity. CRS-R MCS item scores are given in parentheses.
Note, however, that this reasoning under-evaluates the performance of hASR compared to other MCS items. Indeed, each of the MCS items included in the CRS-R have, by definition, a perfect specificity, positive predictive value and precision. Yet, it is known that CRS-R fails to identify ∼15–20% of VS/UWS patients able to show signs of higher-order cognition and/or cognitive-motor dissociation on neuroimaging both at acute or chronic stages (Owen et al., 2006; Sitt et al., 2014; Kondziella et al., 2016; Edlow et al., 2017; Claassen et al., 2019). We then turned to EEG and FDG-PET correlates of hASR in order to confirm our hypothesis of a new sign of CMS by testing if clinically VS/UWS patients with hASR were more prone to show MCS patterns of cortical activity.
Habituation of auditory startle reflex correlates with MCS-like EEG activity
Multivariate prediction of consciousness based on EEG markers
ASR habituation was tested on the same day as EEG recordings. After an automated preprocessing pipeline (see ‘Material and methods’ section and Supplementary material), 12 recordings were discarded because of failed quality control, resulting in 84/96 (88%) patients with available EEG data: 29 patients were in the VS/UWS and showed an inextinguishable ASR (VS-IN), 15 were in the VS/UWS with an extinguishable ASR (VS-EX), 12 patients were in the MCS with inextinguishable ASR (MCS-IN), and 28 patients were in the MCS with extinguishable ASR (MCS-EX) (Supplementary Table 2).
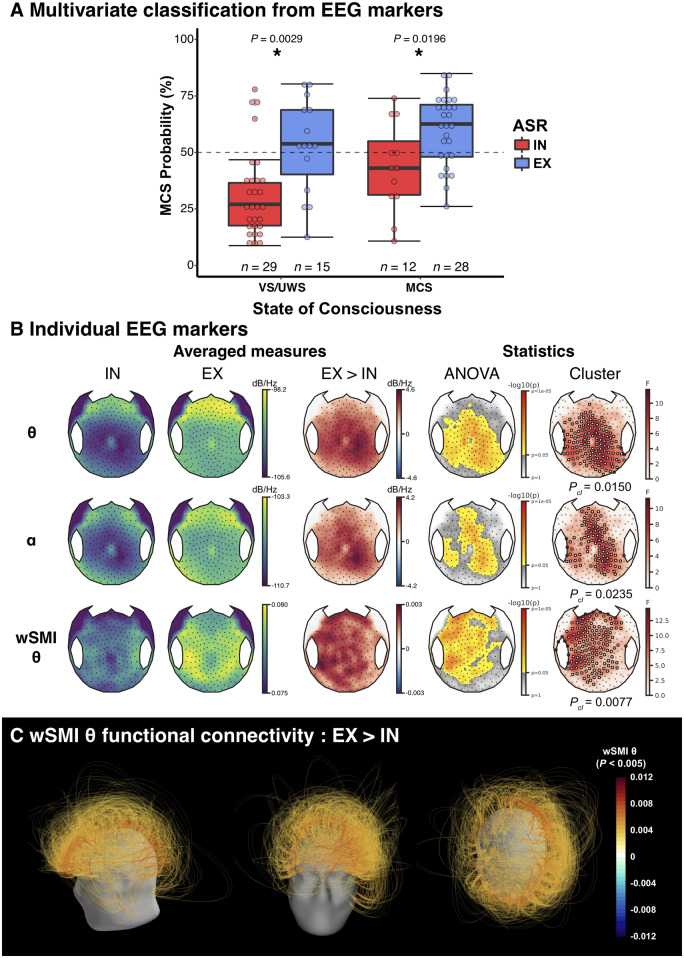
We first measured the impact of hASR on EEG-based multivariate prediction of UWS/VS-MCS status. Previously reported quantitative EEG markers of spectral power, functional connectivity, complexity and ERPs were used to classify each patient status with a linear SVM classifier trained on a separate dataset of 311 recordings [all recordings reported in Engemann et al. (2018), except those of patients included in the present study)]. We ran a 2 × 2 ANOVA with the SVM predicted probability as dependent variable and both the state of consciousness (VS/UWS or MCS) and the ASR response type (ASR-IN versus ASR-EX) as explanatory factors. As predicted, this analysis yielded a significant main effect of the state of consciousness with [F(1,81) = 4.7, P = 0.0339], but more importantly, we found an even stronger main effect of the ASR response type [F(1,81) = 19.6, P ≤ 1 × 10−4]. Post hoc testing showed that the presence of an ASR habituation resulted in higher probability of being MCS than its absence, for both clinically VS/UWS patients (52.6 ± 20.8% versus 31.4 ± 19.5%, P = 0.0029), and MCS patients (59.8 ± 15.5 versus 43.2 ± 19.8, P = 0.0196). Importantly, the proportion of clinically VS/UWS patients who were predicted to be MCS by the EEG-based classifier was significantly higher for ASR-EX patients than for ASR-IN patients [10/15 (67%) versus 4/29 (14%), Fisher's exact test P = 0.0007] (Fig. 2A). When including the clinical state × hASR response interaction term in the model, no significant interaction was found and both main effects of hASR and of clinical state remained significant (Supplementary material). Importantly, these results were essentially the same when computed on an independent 5 min resting state EEG (Supplementary material and Supplementary Fig. 1).
Figure 2.
Multivariate prediction of consciousness based on EEG markers. (A) Relationship between auditory startle reflex (ASR) habituation and the multivariate prediction of consciousness based on EEG markers. Predicted probability of being classified minimally conscious (MCS) was higher in extinguishable patients (EX) than in inextinguishable patients (IN), regardless of the clinical state of consciousness [vegetative (VS/UWS) or MCS] with a main effect of ASR habituation using a 2 × 2 analysis of variance (ANOVA), F(1,81) = 19.6, P ≤ 1 × 10−4. Post hoc testing revealed that EX patients had a significantly higher probability than IN patients in both VS and MCS populations (P < 0.05, uncorrected). (B) Scalp topographies of some univariate markers [raw theta (θ) and alpha (α) power spectral densities and weighted symbolic mutual information in the theta band (wSMI θ)] showed a significant difference between the two groups, Pcl = 0.0150, Pcl = 0.0235 and Pcl = 0.0077, respectively. A cluster-based approach was used for the statistical comparison, with the main effect of ASR response (independent of the state of consciousness) in an ANOVA as a first step statistic followed by a 10 000 permutations spatial clustering procedure. Channels included in the significant cluster are highlighted by white circles. (C) wSMI θ connectivity differences between ASR-EX and ASR-IN. Only pairs of electrodes exhibiting a significant main effect of the ASR habituation are represented (P < 0.005, uncorrected). Pcl = cluster P-value. *P < 0.05.
The same 2 × 2 ANOVA without the interaction term were performed at the sensor-level for univariate EEG markers. These analyses revealed a main effect of habituation to ASR on scalp topographies of markers previously associated with higher states of consciousness (King et al., 2013; Sitt et al., 2014; Hermann et al., 2020). Indeed, an extinguishable reflex was associated with higher raw theta power spectral density (significant cluster encompassing a large centro-posterior region, P = 0.0150) and raw alpha power spectral density (median centro-parietal cluster P = 0.0235), together with higher wSMI in the theta band values (left and mesial temporo-frontal cluster, P = 0.0077) (Fig. 2B). The hASR seemed especially linked to a higher functional connectivity to prefrontal regions (parieto- and temporo-prefrontal connectivity as well as prefronto-prefrontal connectivity) (Fig. 2C). Importantly, similar results were obtained when focusing only on the VS/UWS population (Supplementary Fig. 3). Again, no interaction was found and the main effect were the same when including the interaction term (Supplementary material).
ERPs to local and global violations of auditory regularities
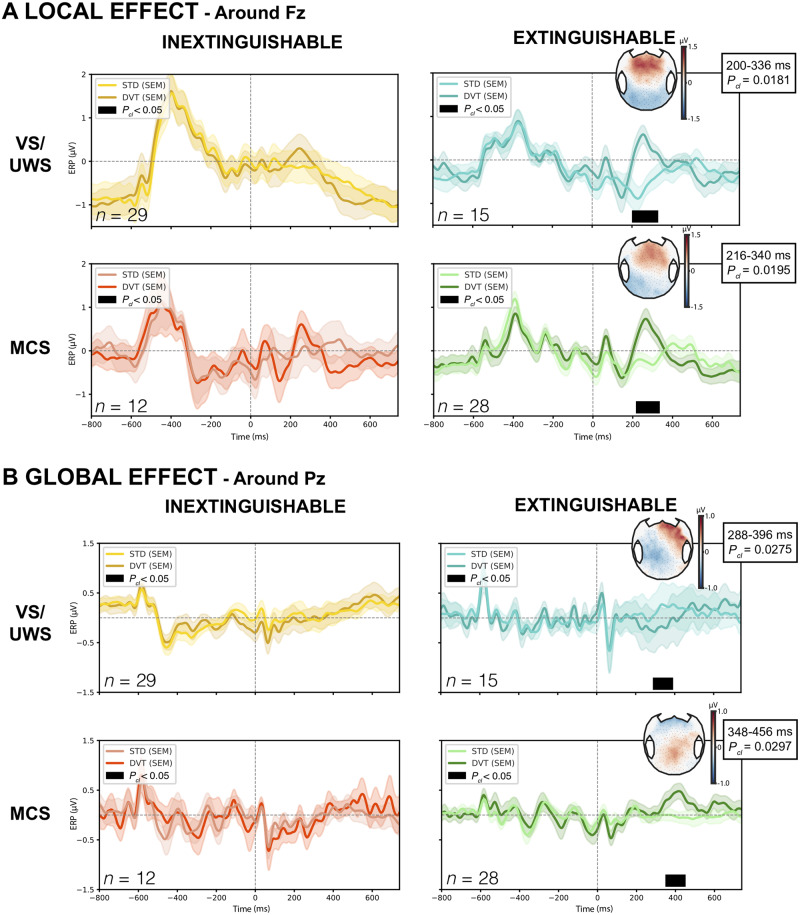
We then investigated the response to violations of auditory regularities using the ‘local-global’ paradigm. This paradigm elicits both unconscious signatures to auditory novelty processing (mismatch negativity and P3a) when auditory regularity is violated on a short timescale (local effect), and conscious signature of auditory novelty processing (P3b) when it is violated on a longer timescale (global effect). We investigated both local and global effects in each of the four following groups: VS-IN (n = 29), VS-EX (n = 15), MCS-IN (n = 12) and MCS-EX (n = 28).
While no significant local effect was found in the absence of habituation (VS-IN and MCS-IN), a local effect was found in both ASR-EX groups with very similar timing and topography reminiscent of a P3a ERP component: a significant cluster was present in the Fz region of interest during the 200–336 ms time window after the onset of the fifth sound in the VS-EX group (P = 0.0181) and during the 216–340 ms time window in the MCS-EX group (P = 0.0195) (Fig. 3A).
Figure 3.
Local-global auditory oddball paradigm. (A) Local effect ERP over Fz-centred region of interest showing a significant cluster (black) only in vegetative (VS/UWS) and minimally conscious (MCS) patients with ASR extinguishable response, Pcl = 0.0181 and Pcl = 0.0195, respectively. Scalp representations of averaged voltage values over the time period of the cluster clearly show an anterior positivity compatible with a P3a component. (B) Similarly, a global effect over Pz-centred region of interest was only found in VS/UWS and MCS patients with ASR extinguishable response, with a centro-posterior positivity suggestive of a P3b in MCS, Pcl = 0.0297 and a negative and more lateralized topography in VS/UWS, Pcl = 0.0275. Only results surviving multiple comparisons through a temporal cluster-based permutation procedure with 10 000 permutations are presented. DVT = deviant; Pcl = cluster P-value; SEM = standard error of the mean; STD = standard.
The analysis of the global effect showed a similar and consistent pattern. While no effect was found on Pz-region of interest in the two ASR-IN groups (VS-IN and MCS-IN), a significant global effect was present in the VS-EX group during the 288–396 ms time window (P = 0.0275), and in the MCS-EX group from 348–456 ms (P = 0.0297) (Fig. 3B). While the topography and time window of the effect in the MCS-EX group was suggestive of a P3b component that is reported as a signature of conscious access, the global effect found in VS-EX occurred earlier, and showed an opposite polarity.
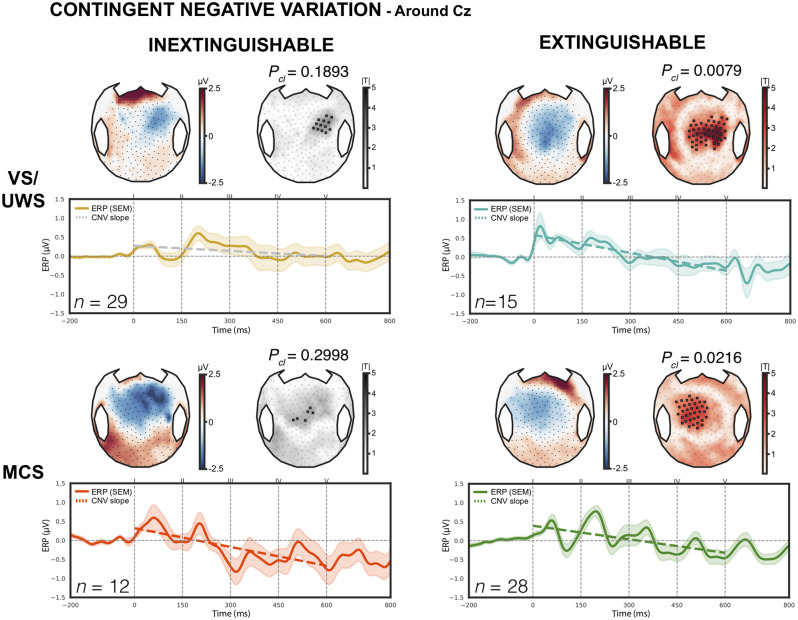
Finally, we also investigated the CNV component that we previously reported as an additional EEG marker of conscious expectation of the fifth auditory sound that conveyed the critical information both for local and global effects (Faugeras et al., 2012). A significant CNV was present in VS-EX and MCS-EX both on the Cz region of interest analysis (slope = −1.57, P = 0.0005 and slope = −1.19, P = 0.0216, respectively) and on the cluster-based topographical analysis (centro-anterior cluster, P = 0.0079 and P = 0.0216, respectively). In the MCS-IN group, a significant CNV was only found on the region of interest analysis (slope = −1.65, P = 0.0477, best cluster P = 0.2998). No CNV was detected in the VS-IN group (slope = −0.45, P = 0.4032, best cluster P = 0.1893) (Fig. 4).
Figure 4.
Contingent negative variation. CNV elicited by the first four sounds of the local-global paradigm, with both scalp topographies on top (average values and cluster-based permutation statistics) and ERPs averaged over the Cz-centred region of interest. Coloured dashed lines indicate a significant CNV slope while grey lines indicate a non-significant slope. Only vegetative (VS/UWS) and minimally conscious (MCS) patients with extinguishable response exhibited a significant CNV in both methods, Pcl = 0.0079 and Pcl = 0.0216, respectively. Only results surviving multiple comparisons through a spatial cluster-based permutation procedure with 10 000 permutations are presented. Pcl = cluster P-value; SEM = standard error of the mean.
Habituation of auditory startle reflex correlates with MCS-like cortical metabolism
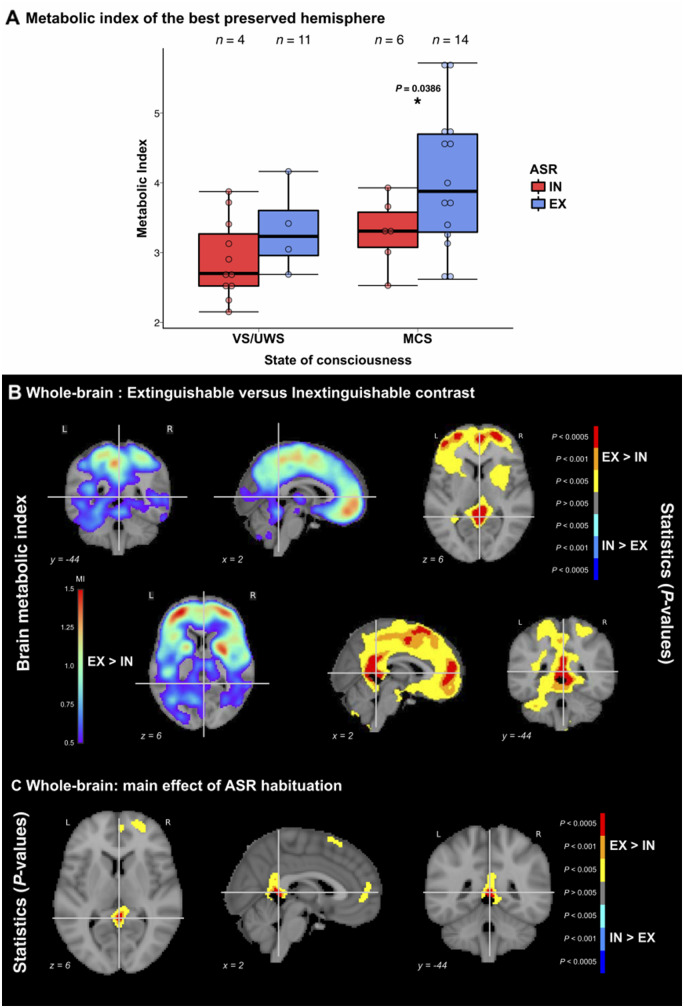
Out of 47 non-mechanically ventilated patients, 40 (85%) had undergone FDG-PET acquisition and 35 (74%) recordings were available [five were discarded because of bad quality, independently assessed by nuclear medicine physicians (M.O.H., A.K.), blind to the clinical state and ASR habituation], including 18 ASR-EX patients (four VS/UWS and 14 MCS) and 17 ASR-IN patients (11 VS/UWS and six MCS). Median delay between the clinical assessment and the PET was 1 day [interquartile range = (−1:1)], with no significant difference between the two groups (P = 0.25). Characteristics of this sample of 35 patients are described in Supplementary Table 4. As implemented by Stender et al. (2016), we computed the metabolic index of each hemisphere and kept the highest of these two values for the next analyses, as this metric was proven to be one of the most reliable to diagnose MCS. Not only the metabolic index was higher in the ASR-EX group than in the ASR-IN group (3.88 ± 0.96 versus 3.04 ± 0.56, P = 0.0035), but a significant main effect of ASR response type was present using the same ANOVA model as previously described for EEG markers [F(1,32) = 4.63, P = 0.0391]. Post hoc comparison showed higher metabolic index in MCS-EX than in MCS-IN patients (4.04 ± 0.99 versus 3.29 ± 0.49, P = 0.0386), with no significant difference between VS-IN and VS-EX groups (2.90 ± 0.57 versus 3.33 ± 0.63, P = 0.29) (Fig. 5A).
Figure 5.
FDG-PET brain metabolism according to ASR habituation response. (A) Relationship between auditory startle reflex (ASR) habituation and the metabolic index of the best preserved hemisphere showing a higher index in patients with extinguishable response (EX) as compared to patients with inextinguishable response (IN), regardless of the clinical state of consciousness [vegetative (VS/UWS) or minimally conscious (MCS)], as demonstrated by an analysis of variance (ANOVA) main effect of ASR habituation, F(1,32) = 4.63, P = 0.0391. Post hoc testing revealed that EX patients had a significantly higher probability than IN patients in the MCS population (P < 0.05, uncorrected). (B) Whole-brain voxel-based analysis of the metabolic index showing higher values in EX patients than in IN patients in parietal and medial frontal regions (left), with significant differences mainly observed in precuneus/posterior cingulate, premotor area and anterior cingulate (right). (C) ANOVA showed an independent main effect of the ASR habituation in posterior and anterior cingulate and supplementary motor area. For voxel-wise analyses, both metabolic index and P-values (thresholded at P < 0.005 uncorrected with a minimum extent of 100 voxels per cluster) are shown superimposed coronal, sagittal and axial slices of the MNI 152 T1 brain template with related y, x and MNI coordinates. L = left; R = right. *P < 0.05.
Finally, in order to probe the regional metabolic correlates of hASR, we performed a whole-brain voxel-based analysis using linear modelling between voxel-wise metabolic indices and the independent variables with age as a covariate. We first contrasted ASR-EX to ASR-IN patients. Metabolic activity was significantly higher in ASR-EX patients compared to ASR-IN patients in posterior cingulate, precuneus, premotor areas, anterior cingulate, orbito-frontal and dorso-lateral prefrontal cortices. No cluster showed a significantly higher metabolism in the ASR-IN group compared to the ASR-EX group (Fig. 5B). After inclusion of the state of consciousness as covariate, a significant independent effect of ASR response type persisted in the posterior and anterior cingulate, premotor area and anterior prefrontal cortex, although less extended than in the previous contrast (Fig. 5C). Voxel-wise analysis restricted within the VS/UWS population also showed a higher metabolism in the same regions in VS-EX patients than in VS-IN patients (Supplementary Fig. 3).
Habituation of auditory startle reflex is associated with a relative preservation of white matter integrity
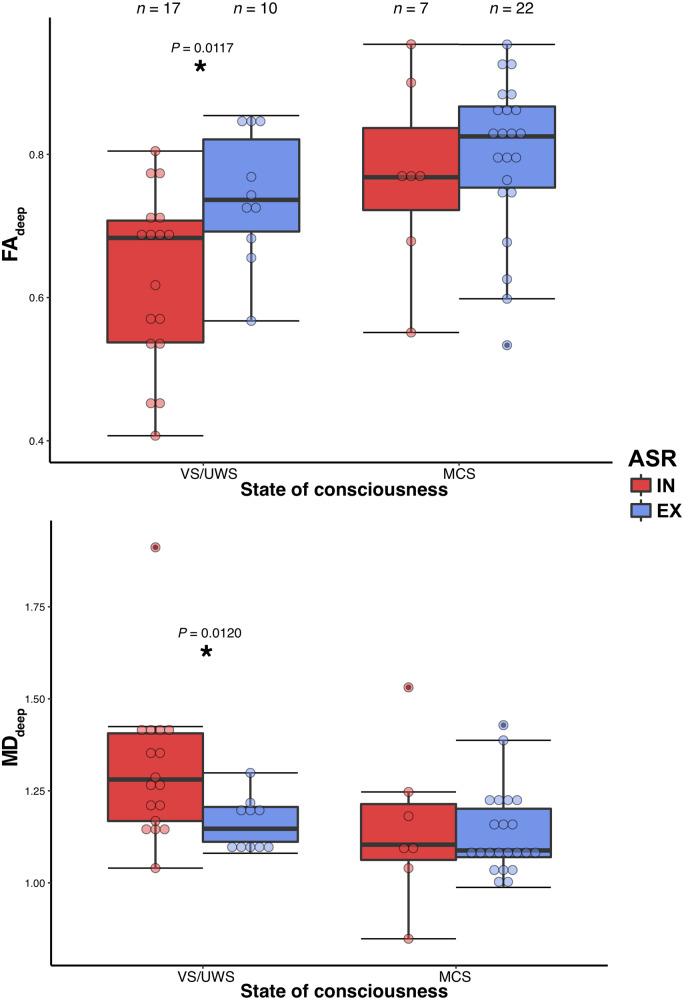
Of the 96 patients, two had a contraindication to MRI and 15 had no acquisition of DWI sequences during the MRI (10 because they had a previous recent MRI and five because of too much motion inside the scanner). Of the 79 DWI acquisitions, 23 did not pass the quality control leaving 56 patients with successful DTI processing (58%): 17 VS-IN, 10 VS-EX, seven MCS-IN and 22 MCS-EX patients (Supplementary Table 5). Using a similar ANOVA model as for the EEG multivariate classification and PET metabolic index, we found a main effect of ASR response type on the FAdeep [F(1,53) = 4.9, P = 0.0306]. Post hoc testing revealed that VS-EX patients had higher FAdeep than VS-IN patients (0.74 ± 0.09 versus 0.63 ± 0.12, P = 0.0117) but no significant difference was found between MCS-EX and MCS-IN (0.79 ± 0.13 versus 0.80 ± 0.11, P = 0.6250). The same pattern was found on MDdeep of VS patients (1.30 ± 0.19 versus 1.16 ± 0.07, P = 0.0120), despite non-significant main effect [F(1,53) = 3.4, P = 0.0693] (Fig. 6). When including the interaction term in the ANOVA model on the FAdeep as the dependent variable, both main effects remained significant and there was no interaction (Supplementary material).
Figure 6.
Deep white matter integrity according to ASR habituation response. Relationship between auditory startle reflex habituation (ASR) and the deep white matter integrity assessed by DTI imaging, showing a higher fractional anisotropy [FAdeep, left, main effect of ASR habituation, F(1,53) = 4.9, P = 0.0306] and lower mean diffusivity [MDdeep, right, F(1,53) = 3.4, P = 0.0693] in patients with extinguishable response (EX) compared to patients with inextinguishable response (IN). Post hoc testing revealed a significant effect (P < 0.05 uncorrected) only in vegetative (VS/UWS) patients. *P < 0.05.
Habituation of auditory startle reflex predicts 6-month command following in unresponsive patients
Another way to validate hASR in this situation of imperfect gold standard is to assess its prognostic value. As it is well established that MCS patients have a better outcome than VS patients, both regarding the recovery of consciousness and the functional outcome (Multi-Society Task Force on PVS, 1994; Luauté et al., 2010; Faugeras et al., 2018), the hypothesis was that ASR-EX, by probing MCS/CMS specific networks, should mimic the MCS prognostic value. Six-month outcome was available in 95/96 (99.0%) patients. We first assessed the recovery of consciousness at 6 months in all patients, as defined by the recovery of functional communication. ASR-EX was significantly associated with consciousness recovery at 6 months, odds ratio (OR) = 4.18 (1.57–12.57), P = 0.0067, with 21/52 (40.4%) ASR-EX patients who had recovered consciousness versus only 6/43 (14.0%) ASR-IN patients. However, this effect was not independent from the clinical state of consciousness in multivariate logistic regression, as MCS state [P = 0.0002, OR = 5.95 (2.51–17.68)], but not ASR-EX [P = 0.2284, OR = 2.05 (0.64–6.87)] was significantly associated with consciousness recovery at 6 months.
However, 6 months is a short-term outcome as many of our patients suffered from subacute-chronic disorders of consciousness [median time since injury 58 (31–236) days], a situation in which the expected rate of recovery is not only low but also slow, especially for VS/UWS patients and/or post-anoxic aetiology (Nakase-Richardson et al., 2012; Noé et al., 2012; Hammond et al., 2019; Bareham et al., 2019). Therefore, it is likely that we lacked power to detect the predictive value of ASR-EX on this outcome metric, which is not adapted to all patients. We thus focused on a more realistic yet still clinically relevant outcome measure depending on the patient’s ability on the day of first assessment. This metric, which we called improvement at 6 months, was defined as the recovery of command following in a patient unresponsive on the day of CRS-R and hASR testing (CRS-R auditory subscale score ≤ 2) and the recovery of consciousness for patients already responsive (CRS-R auditory subscale score > 2). The hASR significantly predicted this improvement, P = 0.0053, OR = 3.56 (1.49–9.01) with 27/52 (51.9%) of ASR-EX versus 10/43 (23.3%) ASR-IN improving at 6 months. This significant prognostic value of the hASR holds true when focusing only on the recovery of command following either in all the unresponsive patients [P = 0.0031, OR = 4.40 (1.70–12.33)], with 22/42 (52.4%) unresponsive ASR-EX versus 8/32 (20.0%) unresponsive ASR-IN), or only in the VS/UWS patients [OR = 7.91 (1.56–59.91), P = 0.0201, 6/17 (35.3%) VS/UWS-EX versus 2/31 (6.5%) VS/UWS-IN]. Lastly, as most death occurred after withdrawal of life-sustaining therapy [23/24 (95.8%)], it is important to note that we found the same prognostic value of hASR after the exclusion of patients who died following such decisions (Supplementary material).
Discussion
In the present study we proposed, tested and validated a new sign of MCS: the habituation of ASR. Our hypothesis was based on the CMS framework, and predicted that habituation of the subcortical ASR would reflect the anatomo-functional preservation of a large-scale cortical network, recruiting in particular prefrontal regions, implied in the control of action and in behavioural inhibition.
Cortical origin of ASR habituation
By correlating hASR with higher resting state cortical PET metabolism (in particular in fronto-parietal regions) and with richer EEG brain activity in ASR-EX than in ASR-IN patients independently of the state of consciousness (i.e. independent of the VS/UWS versus MCS status), our findings strongly support our hypothesis of a cortical origin of this form of behavioural inhibition. Indeed, the regional PET metabolic activity signature of preserved hASR encompassed multiple brain-scale cortical networks including the salience and default mode (DMN) networks. The former includes pre-supplementary motor areas, anterior cingulate and right insula cortices and has been reliably shown to be associated to the inhibition of multiple cognitive and motor processes, such as stopping an action in response to unexpected events (Sharp et al., 2010). Structural integrity of these regions was also found to be essential for an efficient modulation of DMN activity during inhibitory control (Bonnelle et al., 2012), and disruption of their functional connectivity secondary to traumatic brain injury has also been linked to impaired performance in another motor inhibition behaviour during a stop signal task (Sharp et al., 2011). Our EEG results also point in the same direction by showing higher theta and alpha spectral power, as well as higher values of cortico-cortical functional connectivity, and in particular higher prefronto-temporal connectivity, in ASR-EX as compared to ASR-IN patients. These results are coherent with the previous reports of reduced ASR habituation in patients suffering from temporal and frontal lesions (Liegeois-Chauvel et al., 1989), and of a significant correlation between reduced hASR and lower midfrontal theta activity in parkinsonian patients (Chen et al., 2016). Interestingly, several studies linked this midfrontal theta activity to attentional mechanisms (Clayton et al., 2015; Fries, 2015) and to inhibition of behavioural responses to external stimuli (Wessel and Aron, 2013). The prefrontal origin of ASR inhibition is also reinforced by the finding of a reduced prepulse inhibition of the startle response (a behaviour closely related to hASR) in schizophrenic patients (Dawson et al., 2000; Hazlett et al., 2007). Note also that this prepulse inhibition behaviour correlated with prefrontal cortex activity in control subjects, as measured by functional MRI, and that the alteration of this behaviour in schizophrenic patients treated with neuroleptics paralleled a decrease of this prefrontal activity (Kumari et al., 2007). In addition to strengthening the crucial role of the frontal cortex in this behaviour, these works also suggest a relationship between ASR habituation and integrity of dopaminergic pathways, which are altered in Parkinson’s disease and schizophrenia, and are targeted by antipsychotic drugs, as postulated by the meso-circuit hypothesis proposed as a integrative neurophysiological framework of disorders of consciousness (Schiff, 2010; Fridman and Schiff, 2014). A detailed and dedicated fine anatomical study of the patients explored in the present study could further test the cortical underpinnings of hASR. Note, however, that eyelid apraxia is unlikely to affect hASR (Supplementary material).
Links between ASR habituation and consciousness
Once we linked hASR to the activity of cortical networks, we were able to discuss more precisely the relationship prevailing between this new CMS clinical sign and consciousness. Our main ERP result consisted in the presence of a response to violations of global regularity in the auditory local-global paradigm in ASR-EX patients. Such a ‘global effect’ was previously proposed as a neural signature of conscious access to this violation. In healthy volunteers performing a passive version of this task, only those who could consciously report the global structure of the stimuli and of their violations showed a global effect in ERPs (Bekinschtein et al., 2009) or in pupillometry (Quirins et al., 2018). In a group of 31 patients in a behavioural VS/UWS, only the two individuals who showed a global effect improved to a behavioural MCS within 3–4 days following ERP recordings (Faugeras et al., 2011; Raimondo et al., 2017). Neural sources of the global effect correspond to a large-scale cortical network including frontal regions and coherent with the global neuronal workspace (Dehaene and Naccache, 2001) and fronto-parietal (Laureys and Schiff, 2012) theories of consciousness. Therefore, the discovery of a global effect in both MCS-EX and in VS/UWS-EX groups strongly suggests that most patients of these groups may have consciously accessed these violations, in contrast with both MCS-IN and VS/UWS-IN patients. Note however, that the scalp topography of these global effects differed between MCS and VS/UWS ASR-EX patients. While MCS-EX patients showed the typical P3b topography observed in conscious controls when they are aware of global regularity violations or of any other visual or auditory stimulus (Naccache et al., 2016), VS/UWS-EX patients showed a central negativity topography that occurred earlier than the P3b. This pattern is reminiscent of the visual awareness negativity that has been reported primarily in the visual modality (Koivisto et al., 2008), and that was recently extended to the auditory modality (Eklund and Wiens, 2019). The ultimate interpretation of this difference of topography between the two groups of ASR-EX patients is beyond the scope of the present study, but the mere and specific presence of such a global effect when ASR habituation is preserved suggests a tight link between this sign and conscious state. Note also that the presence of a sustained and stronger CNV response in ASR-EX patients than in ASR-IN patients further suggests that the former were more prone to actively and consciously expect the auditory stimuli (Faugeras et al., 2012; Sergent et al., 2017; Rozier et al., 2020). Future studies should better assess this relationship at the individual level.
In a complementary and coherent way, our PET results revealed that precuneus and posterior cingulate cortices, which belong to the major node of the DMN (Fransson and Marrelec, 2008), correlated with hASR. Reduced activity within these two regions has been repeatedly associated with loss of consciousness in various physiological and pathological conditions such as slow wave sleep (Horovitz et al., 2009; Boly et al., 2012), general anaesthesia (Boveroux et al., 2010; Schrouff et al., 2011), and disorders of consciousness (Cauda et al., 2009; Vanhaudenhuyse et al., 2010; Soddu et al., 2012; Demertzi et al., 2014, 2015).
Taken together, our results strongly suggest that preserved hASR indexes the residual function of large-scale cortical networks subserving conscious states, conscious access to stimuli, and voluntary ‘top-down’ inhibition of automatic behavioural responses such as the ASR, which will later foster consciousness recovery, as shown by the better 6-month outcome in patients with ASR-EX.
Habituation of auditory startle reflex is a powerful sign of CMS
In accordance with the recent proposal to reinterpret the MCS as a CMS (Naccache, 2018a), hASR appears as a new additional behavioural sign that can be used to differentiate MCS from VS/UWS patients. We showed that habituation to ASR is a powerful new MCS item compared to the current items included in the CRS-R. Not only the presence of hASR discriminated MCS from VS/UWS patients, but this sign also showed the best prevalence and sensitivity values as compared to the other current MCS items (Wannez et al., 2018). Moreover, we showed that hASR correlated with MCS-like patterns of brain activity on two validated neuroimaging tools (EEG-based classification and metabolic index of the FDG-PET), and that its presence was associated with an increase of white matter structural integrity (FA on DTI) that is predictive of motor and cognitive recovery (Galanaud et al., 2012; Luyt et al., 2012; Velly et al., 2018). The convergence between these two independent brain imaging techniques is crucial as it satisfies the requirement of consilience between tests in this situation of imperfect gold standard (Peterson, 2016), where ∼15–20% of clinically VS/UWS patients show patterns of brain activity suggestive of MCS or conscious state (Owen et al., 2006; Sitt et al., 2014; Kondziella et al., 2016; Edlow et al., 2017; Claassen et al., 2019). Interestingly, hASR not only mirrored the VS/UWS versus MCS prognostic value (Multi-Society Task Force on PVS, 1994; Luauté et al., 2010; Faugeras et al., 2018), but we also found that it predicted consciousness recovery even in unresponsive and VS/UWS patients, in a similar fashion as cognitive-motor dissociation was recently shown to predict outcome (Claassen et al., 2019). Such a situation calls for a new classification of disorders of consciousness that would include some of these neural measures (Bayne et al., 2017; Naccache, 2018b), but it also underlines the importance to expand the range of MCS/CMS behaviours that can be tested at the bedside. Indeed, contrary to the simple hASR, functional brain imaging techniques (PET, functional MRI, quantitative EEG and cognitive ERPs) show a very limited availability in most routine care structures.
Conclusion
In this study, we defined the administration guidelines of a new clinical sign, the ASR habituation, and explored its physiological underpinnings as well as its links with consciousness. We showed that its presence was suggestive of a MCS or conscious state, and that it correlated with the functional preservation of large-scale cortical networks related to conscious processing and to voluntary inhibitory control of behaviour, as measured by quantitative high-density EEG, with cognitive ERPs and with resting state PET imaging. Moreover, hASR was able to predict 6-month consciousness improvement, in particular the recovery of command following in initially unresponsive patients. In the light of these findings, ASR habituation satisfies the criteria required to label it as a new MCS/CMS item. Its simplicity would make it easily implemented in various clinical settings, either as a screening tool or as a complement to the CRS-R. In fact, ASR habituation could be integrated into the auditory subscale hierarchy of the CRS-R, between localization to sound and command following.
Supplementary Material
Acknowledgements
We thank the patients and their relatives. We are grateful to the Editor and to the Reviewers for their very constructive comments and suggestions.
Funding
B.H. was funded by a “Bourse poste d’Accueil” from “INSERM”. This work was also supported by the ‘Human Brain Project' (F.R.), the FRM 2015 (L.N.), the Académie des Sciences-Lamonica Prize 2016 (L.N.) and by the ‘Recovery of consciousness after severe brain injury Phase II’ grant of the James S. McDonnell Foundation. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10- IAIHU-06.
Competing interests
The authors report no competing interests.
Glossary
- CMS =
cortically mediated state
- CNV =
contingent negative variation
- CRS-R =
Coma Recovery Scale-Revised
- ERP =
event-related potential
- EX =
extinguishable
- hASR =
habituation of the auditory startle reflex
- IN =
inextinguishable
- MCS =
minimally conscious state
- UWS =
unresponsive wakefulness syndrome
- VS =
-
vegetative state
;
wSMI = weighted Symbolic Mutual Information
References
- Bareham CA, Allanson J, Roberts N, Hutchinson PJA, Pickard JD, Menon DK, et al. Longitudinal assessments highlight long-term behavioural recovery in disorders of consciousness. Brain Commun 2019; 1. doi: 10.1093/braincomms/fcz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne T, Hohwy J, Owen AM.. Reforming the taxonomy in disorders of consciousness. Ann Neurol 2017; 82: 866–72. [DOI] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L.. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci USA 2009; 106: 1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat JL. Questions remaining about the minimally conscious state. Neurology 2002; 58: 337–8. [DOI] [PubMed] [Google Scholar]
- Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, et al. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci USA 2012; 109: 5856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci USA 2012; 109: 4690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, Bruno M-A, Noirhomme Q, Lauwick S, Luxen A, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010; 113: 1038–53. [DOI] [PubMed] [Google Scholar]
- Bruno M-A, Majerus S, Boly M, Vanhaudenhuyse A, Schnakers C, Gosseries O, et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol 2012; 259: 1087–98. [DOI] [PubMed] [Google Scholar]
- Bruno M-A, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S.. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011; 258: 1373–84. [DOI] [PubMed] [Google Scholar]
- Cauda F, Micon BM, Sacco K, Duca S, D’Agata F, Geminiani G, et al. Disrupted intrinsic functional connectivity in the vegetative state. J Neurol Neurosurg Psychiatry 2009; 80: 429–31. [DOI] [PubMed] [Google Scholar]
- Chen K-H, Okerstrom KL, Kingyon JR, Anderson SW, Cavanagh JF, Narayanan NS.. Startle Habituation and midfrontal theta activity in Parkinson disease. J Cogn Neurosci 2016; 28: 1923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennu S, Noreika V, Gueorguiev D, Blenkmann A, Kochen S, Ibáñez A, et al. Expectation and attention in hierarchical auditory prediction. J Neurosci 2013; 33: 11194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J, Doyle K, Matory A, Couch C, Burger KM, Velazquez A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019; 380: 2497–505. [DOI] [PubMed] [Google Scholar]
- Clayton MS, Yeung N, Cohen Kadosh R.. The roles of cortical oscillations in sustained attention. Trends Cogn Sci 2015; 19: 188–95. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernández-Espejo D, Pickard JD, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2012; 378: 2088–94. [DOI] [PubMed] [Google Scholar]
- Curley WH, Forgacs PB, Voss HU, Conte MM, Schiff ND.. Characterization of EEG signals revealing covert cognition in the injured brain. Brain 2018; 141: 1404–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Hazlett EA, Nuechterlein KH, Filion DL.. On the clinical and cognitive meaning of impaired sensorimotor gating in schizophrenia. Psychiatry Res 2000; 96: 187–97. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L.. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001; 79: 1–37. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Antonopoulos G, Heine L, Voss HU, Crone JS, de Los Angeles C, et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 2015; 138: 2619–31. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Gómez F, Crone JS, Vanhaudenhuyse A, Tshibanda L, Noirhomme Q, et al. Multiple fMRI system-level baseline connectivity is disrupted in patients with consciousness alterations. Cortex 2014; 52: 35–46. [DOI] [PubMed] [Google Scholar]
- Du Y, Wu X, Li L.. Differentially organized top-down modulation of prepulse inhibition of startle. J Neurosci 2011; 31: 13644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Chatelle C, Spencer CA, Chu CJ, Bodien YG, O’Connor KL, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017; 140: 2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund R, Wiens S.. Auditory awareness negativity is an electrophysiological correlate of awareness in an auditory threshold task. Conscious Cogn 2019; 71: 70–8. [DOI] [PubMed] [Google Scholar]
- Engemann DA, Raimondo F, King J-R, Rohaut B, Louppe G, Faugeras F, et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain J Neurol 2018; 141: 3179–92. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Valente M, Sitt J, Demeret S, Bolgert F, et al. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Inj 2018; 32: 72–7. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein T, Galanaud D, Puybasset L, et al. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia 2012; 50: 403–18. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein TA, Galanaud D, Puybasset L, et al. Probing consciousness with event-related potentials in the vegetative state. Neurology 2011; 77: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage 2011; 54: 103–12. [DOI] [PubMed] [Google Scholar]
- Fischer DB, Truog RD.. What is a reflex? A guide for understanding disorders of consciousness. Neurology 2015; 85: 543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JE. Habituation and prestimulus inhibition of the auditory startle reflex in decerebrate rats. Physiol Behav 1979; 23: 291–7. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G.. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 2008; 42: 1178–84. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Schiff ND.. Neuromodulation of the conscious state following severe brain injuries. Curr Opin Neurobiol 2014; 29: 172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: communication through Coherence. Neuron 2015; 88: 220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanaud D, Perlbarg V, Gupta R, Stevens RD, Sanchez P, Tollard E, et al. Assessment of white matter injury and outcome in severe brain trauma: a prospective multicenter cohort. Anesthesiology 2012; 117: 1300–10. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state definition and diagnostic criteria. Neurology 2002; 58: 349–53. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K.. Diagnostic and prognostic guidelines for the vegetative and minimally conscious states. Neuropsychol Rehabil 2005; 15: 166–74. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J.. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004; 85: 2020–9. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018; 91: 450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, et al. MNE software for processing MEG and EEG data. Neuroimage 2014; 86: 446–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci 2008; 9: 934–46. [DOI] [PubMed] [Google Scholar]
- Hammond F, Giacino J, Nakase-Richardson R, Sherer M, Zafonte RD, Whyte J, et al. Disorders of consciousness due to traumatic brain injury: functional status ten years post-injury. J Neurotrauma 2019; 36: 1136–46. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Romero MJ, Haznedar MM, New AS, Goldstein KE, Newmark RE, et al. Deficient attentional modulation of startle eyeblink is associated with symptom severity in the schizophrenia spectrum. Schizophr Res 2007; 93: 288–95. [DOI] [PubMed] [Google Scholar]
- Hermann B, Raimondo F, Hirsch L, Huang Y, Denis-Valente M, Pérez P, et al. Combined behavioural and electrophysiological evidence for a direct cortical effect of prefrontal tDCS on disorders of consciousness. Sci Rep 2020; 10: 4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, et al. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci USA 2009; 106: 11376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T-C, Lin W-Y, Ding H-J, Sun S-S, Wu Y-C, Yen K-Y, et al. Sex- and age-related differences in brain FDG metabolism of healthy adults: an SPM analysis. J Neuroimaging 2012; 22: 21–7. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: separating automatic from intentional uses of memory. J Mem Lang 1991; 30: 513–41. [Google Scholar]
- King J-R, Sitt JD, Faugeras F, Rohaut B, El Karoui I, Cohen L, et al. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr Biol 2013; 23: 1914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto M, Lähteenmäki M, Sørensen TA, Vangkilde S, Overgaard M, Revonsuo A.. The earliest electrophysiological correlate of visual awareness? Brain Cogn 2008; 66: 91–103. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K.. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016; 87: 485–92. [DOI] [PubMed] [Google Scholar]
- Kuhn M. Building predictive models in R using the caret package. J Stat Softw 2008; 28: 1–26.27774042 [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SCR, Sharma T.. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharm 2007; 10: 463–77. [DOI] [PubMed] [Google Scholar]
- Lant ND, Gonzalez-Lara LE, Owen AM, Fernández-Espejo D.. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clin 2016; 10: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Schiff ND.. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 2012; 61: 478–91. [DOI] [PubMed] [Google Scholar]
- Leaton RN, Cassella JV, Borszcz GS.. Short-term and long-term habituation of the acoustic startle response in chronic decerebrate rats. Behav Neurosci 1985; 99: 901–12. [DOI] [PubMed] [Google Scholar]
- Libet B. Unconscious cerebral initiative and the role of conscious will in voluntary action. Behav Brain Sci 1985; 8: 529–39. [Google Scholar]
- Liegeois-Chauvel C, Morin C, Musolino A, Bancaud J, Chauvel P.. Evidence for a contribution of the auditory cortex to audiospinal facilitation in man. Brain J Neurol 1989; 112: 375–91. [DOI] [PubMed] [Google Scholar]
- Luauté J, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010; 75: 246–52. [DOI] [PubMed] [Google Scholar]
- Luyt C-E, Galanaud D, Perlbarg V, Vanhaudenhuyse A, Stevens RD, Gupta R, et al. Diffusion tensor imaging to predict long-term outcome after cardiac arrest: a bicentric pilot study. Anesthesiology 2012; 117: 1311–21. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R.. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007; 164: 177–90. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab 1996; 16: 385–98. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010; 362: 579–89. [DOI] [PubMed] [Google Scholar]
- Multi-Society Task Force on PVERSUS. Medical aspects of the persistent vegetative state (2). N Engl J Med 1994; 330: 1572–9. [DOI] [PubMed] [Google Scholar]
- Naccache L. Minimally conscious state or cortically mediated state? Brain J Neurol 2018. a; 141: 949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L. Reply: response to ‘Minimally conscious state or cortically mediated state? ’ Brain 2018. b; 141: e27–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Marti S, Sitt JD, Trübutschek D, Berkovitch L.. Why the P3b is still a plausible correlate of conscious access? A commentary on Silverstein et al., 2015. Cortex 2016; 85: 126–8. [DOI] [PubMed] [Google Scholar]
- Nakase-Richardson R, Whyte J, Giacino JT, Pavawalla S, Barnett SD, Yablon SA, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma 2012; 29: 59–65. [DOI] [PubMed] [Google Scholar]
- Newcombe VFJ, Williams GB, Scoffings D, Cross J, Carpenter TA, Pickard JD, et al. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J Neurol Neurosurg Psychiatry 2010; 81: 552–61. [DOI] [PubMed] [Google Scholar]
- Noé E, Olaya J, Navarro MD, Noguera P, Colomer C, García-Panach J, et al. Behavioural recovery in disorders of consciousness: a prospective study with the Spanish version of the Coma Recovery Scale-Revised. Arch Phys Med Rehabil 2012; 93: 428–33.e12. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD.. Detecting awareness in the vegetative state. Science 2006; 313: 1402. [DOI] [PubMed] [Google Scholar]
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine Learning in Python. J Mach Learn Res 2011; 12: 2825–30. [Google Scholar]
- Persaud N, Cowey A.. Blindsight is unlike normal conscious vision: evidence from an exclusion task. Conscious Cogn 2008; 17: 1050–5. [DOI] [PubMed] [Google Scholar]
- Peterson A. Consilience clinical validation, and global disorders of consciousness. Neurosci Conscious 2016; 1. 10.1093/nc/niw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Taboué MC, Landeau B, Desson JF, Desgranges B, Baron JC.. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage 1998; 7: 176–84. [DOI] [PubMed] [Google Scholar]
- Quirins M, Marois C, Valente M, Seassau M, Weiss N, El Karoui I, et al. Conscious processing of auditory regularities induces a pupil dilation. Sci Rep 2018; 8: 14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo F, Rohaut B, Demertzi A, Valente M, Engemann DA, Salti M, et al. Brain–heart interactions reveal consciousness in noncommunicating patients. Ann Neurol 2017; 82: 578–91. [DOI] [PubMed] [Google Scholar]
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozier C, Seidel Malkinson T, Hasboun D, Baulac M, Adam C, Lehongre K, et al. Conscious and unconscious expectancy effects: a behavioural, scalp and intracranial electroencephalography study. Clin Neurophysiol 2020; 131: 385–400. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 2010; 33: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015; 72: 1413. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioural assessment. BMC Neurol 2009; 9: 2017–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, et al. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage 2011; 57: 198–205. [DOI] [PubMed] [Google Scholar]
- Schultze-Kraft M, Birman D, Rusconi M, Allefeld C, Görgen K, Dähne S, et al. The point of no return in vetoing self-initiated movements. Proc Natl Acad Sci USA 2016; 113: 1080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Faugeras F, Rohaut B, Perrin F, Valente M, Tallon-Baudry C, et al. Multidimensional cognitive evaluation of patients with disorders of consciousness using EEG: A proof of concept study. Neuroimage Clin 2017; 13: 455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain 2011; 134: 2233–47. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 2010; 107: 6106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu H, Hu Z, Hu H, Shi P.. The relationship between cerebral glucose metabolism and age: report of a large brain PET data set. PLoS One 2012; 7: e51517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitt JD, King J-R, El Karoui I, Rohaut B, Faugeras F, Gramfort A, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014; 137: 2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soddu A, Vanhaudenhuyse A, Bahri MA, Bruno M-A, Boly M, Demertzi A, et al. Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Hum Brain Mapp 2012; 33: 778–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender J, Mortensen KN, Thibaut A, Darkner S, Laureys S, Gjedde A, et al. The minimal energetic requirement of sustained awareness after brain injury. Curr Biol 2016; 26: 1494–9. [DOI] [PubMed] [Google Scholar]
- Van Der Eerden AW, Khalilzadeh O, Perlbarg V, Dinkel J, Sanchez P, Vos PE, et al. White matter changes in comatose survivors of anoxic ischemic encephalopathy and traumatic brain injury: comparative diffusion-tensor imaging study. Radiology 2014; 270: 506–16. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda L-F, Bruno M-A, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010; 133: 161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velly L, Perlbarg V, Boulier T, Adam N, Delphine S, Luyt C-E, et al. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol 2018; 17: 317–26. [DOI] [PubMed] [Google Scholar]
- Wannez S, Gosseries O, Azzolini D, Martial C, Cassol H, Aubinet C, et al. Prevalence of coma-recovery scale-revised signs of consciousness in patients in minimally conscious state. Neuropsychol Rehabil 2018; 28: 1350–9. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR.. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J Neurosci 2013; 33: 18481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW.. The acoustic startle reflex: neurons and connections. Brain Res Rev 1995; 21: 301–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patients’ main demographic and clinical data are available in Supplementary Table 2. Other data, including brain imaging data, are available upon reasonable request, but cannot be made open because of ethics protocol requirement and the sensitive nature of patient data.