Cousins et al. assess the 2018 ATN framework and find that non-amnestic patients with Alzheimer’s disease have lower CSF phosphorylated tau and total tau than patients with amnestic Alzheimer’s disease, while CSF amyloid-β accurately stratifies both non-amnestic and amnestic variants from frontotemporal lobar degeneration.
Keywords: non-amnestic Alzheimer’s disease, frontotemporal degeneration, cerebrospinal fluid, ATN
Abstract
Under the ATN framework, CSF analytes provide evidence of the presence or absence of Alzheimer’s disease pathological hallmarks: amyloid plaques (A), phosphorylated tau (T), and accompanying neurodegeneration (N). Still, differences in CSF levels across amnestic and non-amnestic variants or due to co-occurring pathologies might lead to misdiagnoses. We assess the diagnostic accuracy of CSF markers for amyloid, tau, and neurodegeneration in an autopsy cohort of 118 Alzheimer’s disease patients (98 amnestic; 20 non-amnestic) and 64 frontotemporal lobar degeneration patients (five amnestic; 59 non-amnestic). We calculated between-group differences in CSF concentrations of amyloid-β1–42 peptide, tau protein phosphorylated at threonine 181, total tau, and the ratio of phosphorylated tau to amyloid-β1–42. Results show that non-amnestic Alzheimer’s disease patients were less likely to be correctly classified under the ATN framework using independent, published biomarker cut-offs for positivity. Amyloid-β1–42 did not differ between amnestic and non-amnestic Alzheimer’s disease, and receiver operating characteristic curve analyses indicated that amyloid-β1–42 was equally effective in discriminating both groups from frontotemporal lobar degeneration. However, CSF concentrations of phosphorylated tau, total tau, and the ratio of phosphorylated tau to amyloid-β1–42 were significantly lower in non-amnestic compared to amnestic Alzheimer’s disease patients. Receiver operating characteristic curve analyses for these markers showed reduced area under the curve when discriminating non-amnestic Alzheimer’s disease from frontotemporal lobar degeneration, compared to discrimination of amnestic Alzheimer’s disease from frontotemporal lobar degeneration. In addition, the ATN framework was relatively insensitive to frontotemporal lobar degeneration, and these patients were likely to be classified as having normal biomarkers or biomarkers suggestive of primary Alzheimer’s disease pathology. We conclude that amyloid-β1–42 maintains high sensitivity to A status, although with lower specificity, and this single biomarker provides better sensitivity to non-amnestic Alzheimer’s disease than either the ATN framework or the phosphorylated-tau/amyloid-β1–42 ratio. In contrast, T and N status biomarkers differed between amnestic and non-amnestic Alzheimer’s disease; standard cut-offs for phosphorylated tau and total tau may thus result in misclassifications for non-amnestic Alzheimer’s disease patients. Consideration of clinical syndrome may help improve the accuracy of ATN designations for identifying true non-amnestic Alzheimer’s disease.
Introduction
The 2018 ATN research framework is a systematic method to determine Alzheimer’s disease continuum designation, and can be applied using CSF analytes as markers of Alzheimer’s disease pathology (Jack et al., 2018). Low CSF levels of amyloid-β1–42 peptide (Aβ1–42) are associated with amyloid deposition in the brain, while high CSF levels of tau protein phosphorylated at threonine 181 (p-tau) are associated with intracellular hyperphosphorylated tau aggregation (Andreasen et al., 2003; Tapiola et al., 2009; Hampel et al., 2018). Measures of CSF total tau (t-tau) have been interpreted as a marker of neurodegeneration that is not specific to Alzheimer’s disease; within the ATN framework, CSF t-tau can serve as a marker of disease severity (Jack et al., 2016). Cut-off points for CSF Aβ1–42, p-tau, and t-tau determine if patients should be judged as positive or negative for pathological amyloid (A status), phosphorylated tau (T status), and neurodegeneration (N status), respectively (Jack et al., 2018); these cut-off points have been established in large autopsy cohorts (Shaw et al., 2009). Under the ATN framework, A status determines whether an individual is positive or negative for Alzheimer’s continuum disease, while more fine-grained designations are determined by T and N status. Designations within Alzheimer’s continuum disease are based on the biological definition of Alzheimer’s disease that includes both amyloid plaques and tau tangles, and observations that changes in CSF Aβ1–42 typically precede CSF p-tau, followed by neurodegeneration and cognitive decline (Jack et al., 2013). Thus, abnormal A and T markers (A+T+N− or A+T+N+) are interpreted as Alzheimer’s disease, while abnormal A alone (A+T−N−) is interpreted as early stage Alzheimer’s continuum or ‘Alzheimer’s pathological change’ and abnormal A and N (A+T−N+) is suspected copathology or ‘Alzheimer’s and suspected non-Alzheimer’s pathological change’. Individuals who are A− are interpreted as not having Alzheimer’s continuum disease, and can have either normal biomarkers (A−T−N−) or biomarkers indicative of non-Alzheimer’s disease pathological change (A−T+N−, A−T−N+, or A−T+N+). In this way, ATN provides a framework to interpret CSF biomarkers and to obtain a diagnosis in life beyond binary Alzheimer’s disease or non-Alzheimer’s disease.
Diagnostic sensitivity and specificity to typical, amnestic Alzheimer’s disease is high for CSF Aβ1–42, p-tau, and t-tau (Shaw et al., 2009; Ewers et al., 2015; Palmqvist et al., 2015; Hampel et al., 2018), and studies have shown that the ratio of p-tau to Aβ1–42 (p-tau/Aβ1–42) is especially accurate at stratifying patients with Alzheimer’s disease pathology from another form of neurodegenerative disease, frontotemporal lobar degeneration (FTLD) (Struyfs et al., 2015; Oeckl et al., 2016; Lleó et al., 2018). Still, these studies demonstrate partial overlap in CSF levels between pathological Alzheimer’s disease and FTLD. Thus, cut-off points that determine ATN status still lead to a small but consequential subset of false-negative Alzheimer’s disease and false-positive FTLD cases. Such misdiagnoses can interfere with enacting appropriate clinical management strategies for patients and their families, and hinder the development of new therapeutic treatments and strategies by adding unexplained variance in a research population.
One possible source of diagnostic error is that broadly-applied cut-offs for Alzheimer’s disease pathology may better capture amnestic than non-amnestic variants of Alzheimer’s disease due to differences in CSF levels (Teng et al., 2014; Paterson et al., 2015; Wellington et al., 2018; Pillai et al., 2019). Most patients with Alzheimer’s disease pathology clinically present with amnestic Alzheimer’s disease, the most common form of dementia, characterized by profound loss of episodic memory (Dubois et al., 2007). However, there are several non-amnestic variants of Alzheimer’s disease that instead present with visuospatial, language, or behavioural/executive impairments (Galton et al., 2000; Murray et al., 2011; Dickerson et al., 2017). Patients with non-amnestic Alzheimer’s disease can phenotypically mimic and are often misdiagnosed as a clinical variant of frontotemporal dementia (FTD) (Koedam et al., 2010), the second most common dementia. FTD is associated with pathological FTLD (Hodges et al., 2004; Perry and Miller, 2013) and, like non-amnestic Alzheimer’s disease, is characterized by language, behavioural, executive, and/or visuospatial dysfunction with relatively spared episodic memory. Both FTD and non-amnestic Alzheimer’s disease are characterized by a younger age of symptom onset than typical, amnestic Alzheimer’s disease (Koedam et al., 2010; Crutch et al., 2012; Lam et al., 2013; Onyike and Diehl-Schmid, 2013; Mendez, 2017). Despite common Alzheimer’s disease pathology, amnestic and non-amnestic variants have relatively distinct patterns of anatomical disease distribution and protein accumulation (Galton et al., 2000; Wolk, 2013; Phillips et al., 2018a, b, 2019). Differences in disease dynamics between clinical syndromes may explain part of the observed variance in CSF levels amongst patients with Alzheimer’s disease pathology (Teng et al., 2014; Paterson et al., 2015).
Another potential source of diagnostic error is that CSF-derived ATN status may be relatively insensitive to primary pathologies other than Alzheimer’s disease. While the ATN framework interprets positive T and/or N status in the absence of A as non-Alzheimer’s disease pathology, these designations may not represent true A− status (Pouclet-Courtemanche et al., 2019) or successfully detect other neurodegenerative pathologies, like FTLD. Moreover, co-occurring pathologies are common in patients with dementia, as well as in our brain bank (Robinson et al., 2018). While an A+T−N+ profile is associated with Alzheimer’s and concomitant suspected non-Alzheimer’s pathological change, it is unknown if this profile captures the majority of co-pathological cases with primary non-Alzheimer’s disease pathology. Thus, secondary Alzheimer’s disease pathology—resulting in positive biomarkers for Alzheimer’s disease—may obscure a primary pathology of FTLD and reduce diagnostic specificity (Toledo et al., 2012).
Here we used a sample of amnestic and non-amnestic patients with post-mortem pathological diagnoses of primary Alzheimer’s disease or FTLD to test whether levels of CSF Aβ1–42, p-tau, and t-tau differ across amnestic and non-amnestic phenotypes, and whether these differences lead to errors in patients’ classification under the ATN framework. Similarly, we assessed pathological and phenotypic differences for p-tau/Aβ1–42, a single biomarker that represents a commonly used alternative to the ATN framework for Alzheimer’s disease pathological change and to stratify Alzheimer’s disease from FTLD (Struyfs et al., 2015; Oeckl et al., 2016; Lleó et al., 2018). For each CSF analyte, we compared the frequency and clinical characteristics of true- and false-negative cases in non-amnestic and amnestic Alzheimer’s disease, as well as true and likely false positives in FTLD. Receiver operating characteristic (ROC) curve assessments tested the diagnostic accuracy of CSF markers when stratifying patients with autopsy-confirmed primary Alzheimer’s disease from primary FTLD pathology. Between-group comparisons adjusted for age, sex, global cognitive impairment, APOE status, and presence of copathology. We compared A and T status during life to amyloid and tangle pathological severity at autopsy, and compared N status during life to post-mortem atrophic severity. Our goals were to test the diagnostic accuracy of the ATN framework and the p-tau/Aβ1–42 ratio by comparing CSF markers across phenotypes and identifying likely sources of diagnostic error.
Materials and methods
Participants
Participants were 182 patients with a lumbar puncture and autopsy-confirmed primary Alzheimer’s disease or FTLD pathology identified through the Integrated Neurodegenerative Disease Biobank and Database (Xie et al., 2011; Toledo et al., 2014). All patients were autopsied at the University of Pennsylvania Center for Neurodegenerative Disease Research. Autopsy analyses classified the level of Alzheimer’s disease pathological change from ‘not’ to ‘high’ using established ‘ABC’ scoring methods (Montine et al., 2012) for amyloid-β (modified Thal scoring on a 4-point 0–3 scale), tau neurofibrillary tangles (modified Braak scoring on a 4-point 0–3 scale), and neuritic plaques (4-point CERAD scale) when available (Mirra et al., 1991). For FTLD-tau cases, Braak staging was determined using monoclonal antibodies GT-7 and GT-38 immunohistochemistry that is specific to both 3 and 4 microtubule-binding repeat (3R; 4R) isoforms characteristic of Alzheimer’s disease neurofibrillary tangles (Gibbons et al., 2018, 2019). Thirty cases lacked scoring for amyloid-β but had neurofibrillary tangle and CERAD neuritic plaque scores; cases with both CERAD and Braak scores of 2 or 3 were judged to have a non-zero likelihood of Alzheimer’s disease pathology, while cases with both CERAD and Braak scores of 0 or 1 were judged unlikely for Alzheimer’s disease pathological change. For six cases, the level of Alzheimer’s disease pathological change was unable to be estimated, and the presence of Alzheimer’s disease pathology was based on pathologists’ judgment. FTLD pathology was assessed using established histopathological methods by accumulations of misfolded 3R or 4R tau (FTLD-Tau) associated with corticobasal degeneration, progressive supranuclear palsy, Pick’s disease, argyrophilic grain disease, and frontotemporal dementia with parkinsonism linked to chromosome 17; or transactive response DNA-binding protein of 43 kDa (TDP-43) associated with frontotemporal dementia and amyotrophic lateral sclerosis with cognitive impairment (FTLD-TDP) (Igaz et al., 2008; Mackenzie et al., 2010). Gross atrophic severity was visually evaluated by pathologists at autopsy on a 4-point scale from none to severe. For a subset of patients, neuronal loss ratings (0–3) were available, and for each patient we calculated the median neuronal loss across five regions (left or right hemispheres): CA1/subiculum, entorhinal cortex, middle frontal gyrus, angular gyrus, and superior/middle temporal gyrus. Pathological data are summarized in Table 1.
Table 1.
Pathological characteristics of phenotypes in Alzheimer's disease and FTLD
| Alzheimer's disease |
FTLD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Amnestic (n = 98) | Non-amnestic (n = 20) | Chi-square |
Amnestic (n = 5) | Non-amnestic (n = 59) | Chi-square |
|||
| χ 2 | P | χ 2 | P | |||||
| APOE ε4 alleles | ||||||||
| 0 | 32 (33%) | 11 (55%) | 2 (40%) | 50 (86%) | ||||
| 1 | 48 (49%) | 8 (40%) | 4.4 | 0.12 | 2 (40%) | 8 (14%) | 14.8 | 0.011 |
| 2 | 18 (18%) | 1 (5%) | 1 (20%) | 0 (0%) | ||||
| Thal stage | ||||||||
| 0 | 0 (0%) | 0 (0%) | 1.9 | 0.55 | 0 (0%) | 26 (51%) | 1.9 | 0.50 |
| 1 | 2 (3%) | 0 (0%) | 1(100%) | 17 (33%) | ||||
| 2 | 1 (1%) | 1 (6%) | 0 (0%) | 5 (10%) | ||||
| 3 | 76 (96%) | 16 (94%) | 0 (0%) | 3 (6%) | ||||
| CERAD stage | ||||||||
| 0 | 1 (1%) | 0 (0%) | 2.9 | 0.41 | 2 (50%) | 44 (75%) | 9.0 | 0.08 |
| 1 | 1 (1%) | 0 (0%) | 0 (0%) | 10 (17%) | ||||
| 2 | 5 (5%) | 3 (15%) | 1 (25%) | 4 (7%) | ||||
| 3 | 90 (93%) | 17 (85%) | 1 (25%) | 1 (2%) | ||||
| Braak stage | ||||||||
| 0 | 0 (0%) | 0 (0%) | 0.17 | 1.0 | 2 (50%) | 20 (42%) | 1.2 | 0.70 |
| 1 | 0 (0%) | 0 (0%) | 2 (50%) | 17 (35%) | ||||
| 2 | 3 (3%) | 1 (5%) | 0 (0%) | 10 (21%) | ||||
| 3 | 93 (97%) | 19 (95%) | 0 (0%) | 1 (2%) | ||||
| Atrophy rating | ||||||||
| 0 | 5 (5%) | 2 (10%) | 1.9 | 0.62 | 0 (0%) | 2 (3%) | 1.5 | 0.71 |
| 1 | 31 (32%) | 7 (35%) | 0 (0%) | 14 (24%) | ||||
| 2 | 29 (30%) | 7 (35%) | 2 (50%) | 20 (34%) | ||||
| 3 | 33 (34%) | 4 (20%) | 2 (50%) | 22 (38%) | ||||
| Wilcox | Wilcox | |||||||
| W | P | W | P | |||||
| Median neuronal loss | ||||||||
| Median | 2.0 | 2.5 | 1.0 | 2.0 | ||||
| IQR | 1.0 | 1.3 | 262.5 | 0.10 | 1.0 | 1.4 | 50 | 0.43 |
| n | 61 | 12 | 3 | 46 | ||||
Contingency table by primary pathology (Alzheimer’s disease, FTLD) and phenotype (amnestic, non-amnestic) for number of APOE ε4 alleles, Thal Stage, CERAD stage, Braak stage, and atrophy. Median, interquartile range (IQR), and sample size (n) are reported for median neuronal loss. Chi-square and Mann-Whitney-Wilcox tests compare phenotypes (amnestic and non-amnestic) within each pathological group (Alzheimer’s disease and FTLD); χ2, W, and P-values are reported. Information was not available for all subjects.
Patients were followed clinically at the Penn Frontotemporal Degeneration Center or the Penn Memory Center and were clinically diagnosed by board-certified neurologists according to published diagnostic criteria (Gorno-Tempini et al., 2011; McKhann et al., 2011; Rascovsky et al., 2011; Armstrong et al., 2013; Höglinger et al., 2017; Strong et al., 2017). Participants’ consent was obtained according to the Declaration of Helsinki and approved by the University of Pennsylvania’s Institutional Review Board. Exclusion criteria included primary pathology other than Alzheimer’s disease or FTLD, patients without cognitive impairment, co-occurring neurological conditions (e.g. stroke, hydrocephalus, chronic traumatic encephalopathy, vascular disease discovered by a clinical MRI or at autopsy), and primary psychiatric disorders (e.g. depression, anxiety).
We next determined if Alzheimer’s disease and FTLD patients had mixed pathology, with primary pathological diagnosis based on pathologist’s judgement. Alzheimer’s disease patients were classified as having ‘mixed’ pathology if they had α-synuclein-positive Lewy bodies/neurites or TDP-43 inclusions (1–3, mild–severe) in one or more neocortical regions (McKeith et al., 2005; Montine et al., 2012). FTLD patients were classified as having ‘mixed’ pathology if they were positive for any level of Alzheimer’s disease pathology (ABC scores of low/intermediate/high) or neocortical α-synuclein (McKeith et al., 2005; Montine et al., 2012). Copathological conditions included hippocampal sclerosis, limbic-predominant age-related TDP-43 encephalopathy (LATE), argyrophilic grain disease, Lewy body disease, primary age-related tauopathy (PART), and globular glial tauopathy. If no form of clinically meaningful copathology was detected, patients were classified as having ‘negligible’ copathology. Alzheimer’s disease patients with amygdala-predominant Lewy bodies or cerebral amyloid angiopathy were considered to have ‘negligible’ copathology. Of the 118 patients with confirmed primary Alzheimer’s disease pathology, 63 were classified as having mixed pathology. Three of these mixed cases had high levels of both FTLD and Alzheimer’s disease (high ABC rating), and primary pathology could not be determined; because of their high levels of Alzheimer’s disease pathological change, these cases were classified as Alzheimer’s disease with mixed pathology in analyses. Of 64 patients with confirmed primary FTLD pathology, 34 were classified with mixed pathology. Of these FTLD with mixed pathology cases, three had ABC scores suggesting no Alzheimer’s disease, 22 had low Alzheimer’s disease pathological change, and four had intermediate pathological change. Of the remaining five FTLD cases missing ABC scores, copathology was determined by pathologists’ assessment; two were determined to have Alzheimer’s disease, one had argyrophilic grain disease, and two had PART.
Of 118 patients with confirmed Alzheimer’s disease pathology, 98 had an amnestic phenotype at presentation (clinical diagnoses: Alzheimer’s disease, n = 95; amnestic mild cognitive impairment, n = 3), and 20 had a non-amnestic phenotype at presentation [clinical diagnoses: behavioural variant Alzheimer’s disease, n = 1; behavioural variant FTD, n = 2; unspecified FTD, n = 5; unspecified primary progressive aphasia (PPA), n = 2; logopenic variant PPA, n = 3; corticobasal syndrome, n = 7]. Of 64 patients with confirmed FTLD pathology, five had an amnestic phenotype at presentation (clinical diagnoses: Alzheimer’s disease, n = 5), and 59 had a non-amnestic phenotype at presentation (clinical diagnoses: non-amnestic MCI, n = 1; behavioural variant FTD, n = 16; unspecified FTD, n = 8; unspecified PPA, n = 3; logopenic variant PPA, n = 3; non-fluent/agrammatic PPA, n = 3; semantic variant PPA, n = 5; corticobasal syndrome, n = 9; progressive supranuclear palsy, n = 7; and ALS with FTD, n = 4).
Demographic comparisons
Across pathology and phenotype, we compared patients for number of copies of the APOE ε4 allele, sex, age at CSF, age at onset (earliest reported symptom), age at death, CSF to death interval (years from CSF to death), disease duration at CSF (years from onset to CSF), disease severity [Mini-Mental State Examination (MMSE)] (Folstein et al., 1975), survival (years from earliest symptom onset to death), and years of education (Table 2). A chi-squared test indicated significant differences between Alzheimer’s disease and FTLD pathology in the frequency of individuals with zero, one, or two copies of the APOE ε4 allele [χ2(2) = 35.70, P = 1.77 × 10−8]. Further testing indicated that the distribution of allele carriers did not differ between amnestic and non-amnestic Alzheimer’s disease groups [χ2(2) = 4.4, P = 0.11], but that ε4 alleles were more frequent among the small group of amnestic FTLD patients than non-amnestic FTLD patients [χ2(2) = 14.78, P = 0.0006]. There was no significant difference in sex distribution across amnestic and non-amnestic Alzheimer’s disease and FTLD groups [χ2(3) = 1.17, P = 0.76]. Because quantitative demographic measures were not normally distributed, non-parametric Mann-Whitney-Wilcox tests compared Alzheimer’s disease and FTLD groups. Alzheimer’s disease and FTLD patients were not significantly different for years of education or disease duration (both P > 0.1). Alzheimer’s disease patients were older at CSF compared to FTLD (W = 5067.5, P = 0.0001), had a longer interval from CSF to death than FTLD (W = 5365.5, P = 2.4 × 10−6), and had marginally lower MMSE than FTLD (W = 2939.5, P = 0.065). Kruskal-Wallis tests were used to compare across amnestic Alzheimer’s disease, non-amnestic Alzheimer’s disease, amnestic FTLD, and non-amnestic FTLD (Table 2). Patients were matched for MMSE, disease duration, education (all P > 0.1), but were significantly different for age [χ2(3) = 19.9, P = 0.00018], and CSF to death [χ2(3) = 21.6, P = 7.9 × 10−5]. In pairwise comparisons using Mann-Whitney-Wilcox tests, amnestic Alzheimer’s disease patients had a longer interval to death than non-amnestic FTLD (U = 4230.5, P = 1.0 × 10−6), were significantly older than non-amnestic FTLD (U = 4108.5, P = 1.0 × 10−5), and were marginally older than non-amnestic Alzheimer’s disease (U = 1235, P = 0.068). Non-amnestic Alzheimer’s disease patients also had a longer interval from CSF to death than non-amnestic FTLD (U = 765, P = 0.47); no other comparisons for age and interval to death were significant. We observed similar differences for all other age-related variables (Table 2), including age at disease onset, age at death and survival, consistent with findings that non-amnestic variants tend to have an earlier disease onset than amnestic variants (Koedam et al., 2010).
Table 2.
Sample characteristics
| Alzheimer's disease |
FTLD |
Chi-square/Kruskal-Wallis |
|||||
|---|---|---|---|---|---|---|---|
| Amnestic (n = 98) | Non-amnestic (n = 20) | Amnestic (n = 5) | Non-amnestic (n = 59) | ||||
| χ 2 | P | ||||||
| Sex, % male | 48 | 50 | 33 | 54 | 1.2 | 0.76 | |
| Co-pathology, % mixed | 54% | 50% | 60% | 53% | 0.2 | 0.98 | |
| Age at CSF, years | Median | 73.5 | 63.5 | 71.0 | 65.0 | 19.9 | <0.001 |
| IQR | 14.5 | 13.5 | 4.0 | 10.0 | |||
| Duration at CSF, years | Median | 3.0 | 2.5 | 6.0 | 4.0 | 2.7 | 0.44 |
| IQR | 3 | 4 | 3 | 3 | |||
| Age at onset, years | Median | 69 | 61 | 66 | 61 | 19.8 | <0.001 |
| IQR | 15.75 | 13.0 | 6.0 | 11.5 | |||
| Age at death, years | Median | 79.0 | 72.5 | 76.0 | 69.0 | 31.9 | <0.001 |
| IQR | 15.0 | 11.5 | 8.0 | 10.0 | |||
| Survival, years | Median | 9.0 | 8.5 | 9.0 | 7.0 | 9.5 | 0.02 |
| IQR | 5 | 6 | 1 | 5 | |||
| CSF to death, years | Median | 6.0 | 5.5 | 4.0 | 4.0 | 23.9 | <0.001 |
| IQR | 3.75 | 4.25 | 1.00 | 3.00 | |||
| Education, years | Median | 15.5 | 15.0 | 16.0 | 16.0 | ||
| IQR | 5.0 | 4.0 | 0.0 | 5.75 | 1.8 | 0.62 | |
| n | 98 | 19 | 5 | 58 | |||
| MMSE (max = 30) | Median | 23 | 19 | 24 | 25 | ||
| IQR | 9 | 9 | 8 | 7 | 5.6 | 0.13 | |
| n | 97 | 17 | 5 | 57 | |||
Median and interquartile range (IQR) by primary pathology (Alzheimer’s disease, FTLD) and phenotype (amnestic, non-amnestic). Sample size (n) is provided for education and MMSE because of missing values. For each variable, χ2 and P-values are reported for chi-square or Kruskal-Wallis tests comparing the four patient groups (Alzheimer’s disease and FTLD by amnestic and non-amnestic).
CSF analysis
CSF samples were collected following a standard lumbar puncture typically following an overnight fast. Samples were measured for Aβ1–42, p-tau, t-tau, and p-tau/Aβ1–42 using the xMAP Luminex platform (INNO-BIA AlzBio3 for research-only reagents; Innogenetics), according to standardized procedures (Shaw et al., 2011). Analyses were performed for research purposes by laboratory technicians who were blinded to clinical data; assay results were not considered in the diagnostic process. Published cut-off points for all measures are based on best thresholds reported by Shaw et al. (2009); amyloid (A) positivity was based on an Aβ1–42 cut-off of 192 pg/ml; tau (T) positivity was based on a p-tau cut-off of 23 pg/ml; neurodegeneration (N) positivity was based on a t-tau cut-off of 93 pg/ml; and a cut-off of 0.10 was applied to the p-tau/Aβ1–42 ratio to determine positivity for Alzheimer’s disease. Outlier checks were performed on CSF values, and one case in the pathological Alzheimer’s disease group was excluded for a CSF t-tau value >5 standard deviations above the sample mean.
Statistical analysis
ATN classification based on CSF biomarkers
For phenotypic and pathological groups, we evaluated ATN designation of patients on the Alzheimer’s disease continuum based on positive or negative A, T, or N status and examined the profiles of cases that were likely misclassified. Patients with CSF Aβ1–42 ≤ 192 pg/ml were considered A+; those with CSF p-tau ≥ 23 pg/ml were considered T); and those with CSF t-tau ≥ 93 pg/ml were considered N+. In addition, we tested classification using the p-tau/Aβ1–42 ratio; patients with p-tau/Aβ1–42 ≥ 0.10 were considered positive for Alzheimer’s disease.
Between-group comparisons of CSF
To elucidate the sources of misclassification within the ATN framework or the p-tau/Aβ1–42 ratio, analyses of covariance (ANCOVAs) compared each CSF analyte across pathology (Alzheimer’s disease, FTLD) and phenotype (amnestic, non-amnestic), covarying for number of APOE ε4 alleles, copathology status (negligible, mixed), age at CSF, MMSE, and sex (α = 0.05). To ensure that the presence of mixed pathology was not driving differences, models were also run excluding patients with high levels of copathology. MMSE scores were unavailable for five participants (two non-amnestic Alzheimer’s disease, one amnestic Alzheimer’s disease, and two non-amnestic FTLD), and education data were unavailable for two participants (one non-amnestic Alzheimer’s disease and one non-amnestic FTLD). These missing data were imputed based on the mean of each patient’s respective pathology and phenotype group. In initial statistical analysis of CSF measures, model residuals were not normally distributed, violating an assumption of multivariate normality; thus, a log transformation was applied to each measure. Because of the unbalanced design, type II sum of squares were calculated for ANCOVAs. CSF levels were compared through post hoc linear contrasts.
For each marker, true- and false-negative and true- and likely false-positive cases were identified based on established cut-off points (Shaw et al., 2009), and Fisher’s exact tests were used to test whether the likelihood of being classified as positive for each marker differed according to memory phenotype in Alzheimer’s disease. We did not compare CSF marker positivity between FTLD phenotypes due to the small number of amnestic FTLD cases (n = 6).
Discrimination of pathological Alzheimer’s disease from FTLD
Finally, ROC curve and area under the curve (AUC) analyses assessed diagnostic accuracy of each CSF marker when discriminating non-amnestic patients with Alzheimer’s disease from FTLD pathology, compared to discriminating amnestic patients with Alzheimer’s disease from FTLD pathology. Optimal cut-offs were calculated for each CSF marker based on Youden’s index, which maximized the sum of sensitivity and specificity in the current sample.
All statistical analyses were conducted in the R statistical environment, using the Companion to Applied Regression (car), multcomp, and partial ROC (pROC) packages (Robin et al., 2011; Fox et al., 2012; R Core Team, 2017).
Data availability
All qualified investigators are welcome to view data through an established algorithm at the CNDR.
Results
ATN classification based on CSF biomarkers
Table 3 outlines patient classifications according to A, T, and N positivity based on published cut-offs for CSF biomarkers (Shaw et al., 2009). According to the ATN framework, of the 98 amnestic patients with autopsy-confirmed Alzheimer’s disease pathology, 91 (93%) had CSF biomarkers positive for Alzheimer’s continuum disease. A similarly high proportion of non-amnestic patients with Alzheimer’s disease pathology, 17 of 20 (85%), were positive for Alzheimer’s continuum disease. However, the likelihood of more specific ATN designations was different across amnestic phenotype in patients with Alzheimer’s disease pathology. Fisher’s tests indicated that non-amnestic Alzheimer’s disease patients were significantly less likely to be positive for all three markers than amnestic Alzheimer’s disease patients [20% versus 52%; odds ratio (OR) = 0.23; confidence interval (CI) = 0.05–0.79; P = 0.013], and were significantly more likely to be negative for all three markers and classified as having normal biomarkers than amnestic Alzheimer’s disease patients (15% versus 2%; OR = 8.23; CI = 0.88–105.4; P = 0.034).
Table 3.
Results of ATN classification using CSF biomarkers
| Interpretation | Biomarker profile | Amnestic Alzheimer’s disease (n = 98) | Non-amnestic Alzheimer’s disease (n = 20) | Amnestic FTLD (n = 5) | Non-amnestic FTLD (n = 59) |
|---|---|---|---|---|---|
| Alzheimer's continuum | |||||
| Alzheimer’s disease | A+T+N+ | 51 (52%) | 4 (20%) | 0 (0%) | 0 (0%) |
| A+T+N− | 16 (16%) | 5 (25%) | 0 (0%) | 0 (0%) | |
| Alzheimer’s pathological change | A+T−N− | 13 (13%) | 6 (30%) | 2 (40%) | 7 (12%) |
| Alzheimer’s disease and concomitant suspected non-Alzheimer’s disease pathological change | A+T−N+ | 11 (11%) | 2 (10%) | 1 (20%) | 2 (3%) |
| Non-Alzheimer’s pathological change | A−T+N− | 2 (2%) | 0 (0%) | 0 (0%) | 2 (3%) |
| A−T−N+ | 0 (0%) | 0 (0%) | 0 (0%) | 7 (12%) | |
| A−T+N+ | 3 (3%) | 0 (0%) | 0 (0%) | 3 (5%) | |
| Normal biomarkers | A−T−N− | 2 (2%) | 3 (15%) | 2 (40%) | 38 (64%) |
| Alzheimer’s disease | p-tau/Aβ1–42 ≥ 0.1 | 88 (90%) | 13 (65%) | 1 (20%) | 9 (15%) |
Interpretation of biomarkers using ATN framework and p-tau/Aβ1–42 ratio (bottom row).
Only 10 of 118 Alzheimer’s disease cases (8%) were negative for Alzheimer’s continuum disease. Of these, five had profiles indicative of non-Alzheimer’s disease pathological change (A−T+N− or A−T+N+); all five were amnestic; two had no co-occurring pathology detected at autopsy, two had co-occurring Lewy body pathology, and one had co-occurring TDP-43 pathology. ABC scores indicated high (n = 2) or intermediate (n = 3) Alzheimer’s disease pathological change. Another five (three non-amnestic; two amnestic) had normal profiles (A−T−N−); ABC scores show high (n = 3), intermediate (n = 1), and low (n = 1) levels of Alzheimer’s disease pathological change; two patients had negligible copathology and three had co-occurring TDP-43 pathology.
We also examined accuracy of the A+T−N+ classification to indicate concomitant Alzheimer’s and non-Alzheimer’s pathological change. Thirteen of 118 patients (11 amnestic; two non-amnestic) were classified as A+T−N+. ABC scores indicated high (n = 12) or intermediate (n = 1) Alzheimer’s disease pathological change. Four of these 13 (31%) patients were found to have negligible levels of co-occurring pathology at autopsy and represent probable misclassifications.
Of the 64 FTLD patients, only 12 (19%; 12 non-amnestic; zero amnestic) had an ATN profile suggestive of non-Alzheimer’s disease pathological change (A−T+N+, A−T−N+, or A−T+N−). The majority of FTLD patients (63%) were classified as having normal ATN biomarkers. Nine (14%) FTLD patients had an A+T−N− profile, suggesting Alzheimer’s disease pathological change only. Of these nine, two were determined to not have Alzheimer’s disease (ABC scores, n = 1; pathologist, n = 1); these cases represent probable misclassifications. The other six were determined to have low Alzheimer’s disease pathological change (ABC scores, n = 5; pathologist, n = 1); ATN biomarkers in these cases were insensitive to patients’ primary FTLD pathology. Three FTLD patients had an A+T−N+ profile, suggesting concomitant Alzheimer’s disease and non-Alzheimer’s disease pathological change; two were found to have negligible copathology at autopsy, and the final patient had likely Alzheimer’s disease pathology.
Finally, we compared the ATN framework to the p-tau/Aβ1–42 ratio in amnestic and non-amnestic Alzheimer’s disease patients. Performance was equivalent in patients with autopsy-confirmed Alzheimer’s disease pathology. Of 98 amnestic Alzheimer’s disease patients, 91 (93%) were classified as positive for Alzheimer’s continuum using the ATN framework (as reported above), and 88 (90%) were classified as positive for Alzheimer’s disease using the p-tau/Aβ1–42 ratio. Of the 20 non-amnestic patients with Alzheimer’s disease pathology, 17 (85%) were classified as positive for Alzheimer’s continuum using the ATN framework (as reported above), while 13 (65%) were classified as positive for Alzheimer’s disease using the p-tau/Aβ1–42 ratio. Comparing the false positives in FTLD patients with negligible copathology (Supplementary Table 1), 2 of 30 FTLD patients (7%) were positive for Alzheimer’s disease pathology using the p-tau/Aβ1–42 ratio, and 4 of 30 FTLD patients (13%) were positive for Alzheimer’s continuum disease according to the ATN framework. While ATN identified a numerically larger proportion of non-amnestic Alzheimer’s disease cases, and the p-tau/Aβ1–42 ratio had fewer false positives, Fisher’s tests determined these proportions were not significantly different (all P > 0.2).
Chi-squared tests examined if there were differences in amyloid and tau tangle burden between amnestic and non-amnestic Alzheimer’s disease patients that might explain differences in ATN status observed; there were no significant differences between amnestic and non-amnestic Alzheimer’s disease patients in Thal, Braak, or CERAD staging (Table 1; all P < 0.4). Because Thal, Braak, or CERAD staging formed the basis of patients’ pathological diagnosis (Alzheimer’s disease or FTLD), we did not consider these measures as potential explanatory variables. As expected however, chi-squared tests confirmed that Alzheimer’s disease patients had a higher number of APOE ε4 alleles [χ2(3) = 35.7, P = 1.8 × 10−8] than FTLD patients (Table 1). Importantly, there were also no differences in gross atrophy ratings (P = 0.41) between Alzheimer’s disease and FTLD, or between non-amnestic Alzheimer’s disease and amnestic Alzheimer’s disease (P = 0.62) that might explain the high rates of N− patients we see in non-amnestic Alzheimer’s disease or in FTLD compared to amnestic Alzheimer’s disease; Alzheimer’s disease patients did have a marginally higher neuronal loss score than FTLD patients (W = 2109, P = 0.080), but there was no difference in neuronal loss between non-amnestic Alzheimer’s disease and amnestic Alzheimer’s disease (P = 0.10) (Table 2). To elucidate the sources of ATN classification differences between non-amnestic and amnestic Alzheimer’s disease, we subsequently compared levels of each CSF marker parametrically across pathology and phenotype. If CSF Aβ1–42, p-tau, and the p-tau/Aβ1–42 ratio are accurate markers of likely amyloid, tangle, and Alzheimer’s disease pathology, respectively, they are expected to be significantly different across pathology (Alzheimer’s disease, FTLD) but invariant to phenotype (amnestic, non-amnestic). If CSF t-tau is an accurate non-specific staging marker of N, all patient groups would be hypothesized to have a high proportion of N+ cases; while we do not know severity of neurodegeneration in life, all patients were symptomatic at time of CSF sample (Jack et al., 2013).
Between-group comparisons of CSF amyloid-β1–42
We compared levels of CSF Aβ1–42 parametrically across pathology and phenotype (Fig. 1). An ANCOVA (type II) showed that Aβ1–42 levels differed by pathology [F(1,172) = 53.2, P = 1.0 × 10−11], with lower values in the pathological Alzheimer’s disease group than the pathological FTLD group. Additionally, Aβ1–42 levels were significantly associated with APOE status [F(1,172) = 6.0, P = 0.015], with lower concentrations associated with greater numbers of the ε4 allele. Phenotypic differences in Aβ1–42 levels were non-significant [F(1,172) = 1.1, P = 0.29]. Copathology status (P = 0.22), age (P = 0.98), interval to death (0.97), sex (P = 0.78), and MMSE (P = 0.85) were likewise not significantly associated with Aβ1–42 levels. Omnibus results when restricting the cohort to patients with negligible copathology also showed the effect of pathology type was significant [F(1,76) = 31.7, P = 2.9 × 10−7] while the effect of phenotype was not (P = 0.82); all other effects were non-significant including the association with APOE (all P < 0.32).
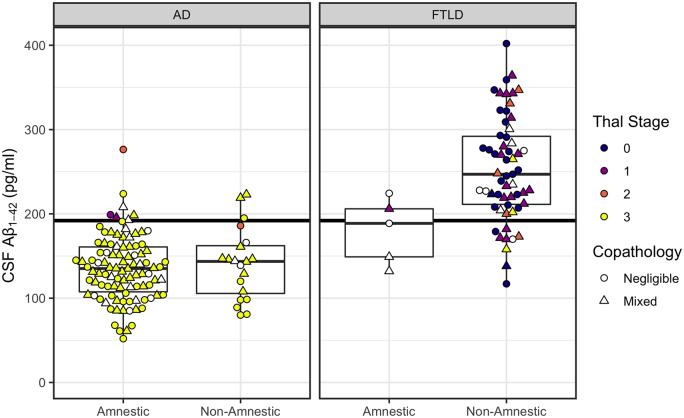
Figure 1.
CSF Aβ1–42 concentrations according to phenotype (amnestic, non-amnestic) and post-mortem ratings of amyloid-β pathology. Left: Plots for patients with primary Alzheimer’s disease (AD) pathology. Right: Plots for patients with primary FTLD pathology. Horizontal line indicates CSF Aβ1–42 = 192 pg/ml, with patients below the line designated as A+. CSF levels above the line are considered normal. Colour indicates modified Thal staging of amyloid-β pathology on a 0–3 scale (white indicates cases with no available Thal staging). Shape indicates presence or absence of copathology.
A status
False negative rates for A status were low for both amnestic (7 of 98) and non-amnestic (3 of 20) patients with primary Alzheimer’s disease pathology, with Fisher’s test showing no difference across phenotype (P = 0.37). In the patients with primary FTLD pathology, 12 of 64 were CSF A+ (Supplementary material).
Between-group comparisons of CSF p-tau
CSF p-tau levels were compared parametrically across pathology and phenotype (Fig. 2). A type II ANCOVA showed that p-tau levels differed by pathology [F(1,172) = 24.3, P = 1. 9 × 10−6] and memory phenotype [F(1,172) = 7.2, P = 0.01], with APOE status (P = 0.41), copathology status (P = 0.80), age [F(1,172) = 3.4, P = 0.068], interval to death (0.20), sex (P = 0.18), and MMSE (P = 0.18) included as covariates. Post hoc linear contrasts confirmed that the pathological FTLD group had lower CSF p-tau than the pathological Alzheimer’s disease group [t(172) = −4.9, P = 1.9 × 10−6], and that non-amnestic Alzheimer’s disease patients had lower CSF p-tau than amnestic Alzheimer’s disease patients [t(172) = −3.2, P = 0.0076]. Non-amnestic FTLD patients had lower CSF p-tau than both amnestic Alzheimer’s disease [t(172) = −9.1, P = 1.0 × 10−16] and non-amnestic Alzheimer’s disease [t(172) = −3.5, P = 0.0028] patients. Amnestic FTLD patients had lower CSF p-tau than amnestic Alzheimer’s disease [t(172) = −4.5, P = 7.7 × 10−5]; no other comparisons were significant (P < 0.11). Similar results were obtained when restricting the cohort to patients with negligible secondary pathology: we observed significant effects of pathology type [F(1,76) = 24.0, P = 5.3 × 10−6], phenotype [F(1,76) = 5.2, P = 0.025], and age [F(1,78) = 10.0, P = 0.0022]. All other effects were non-significant (all P < 0.09).
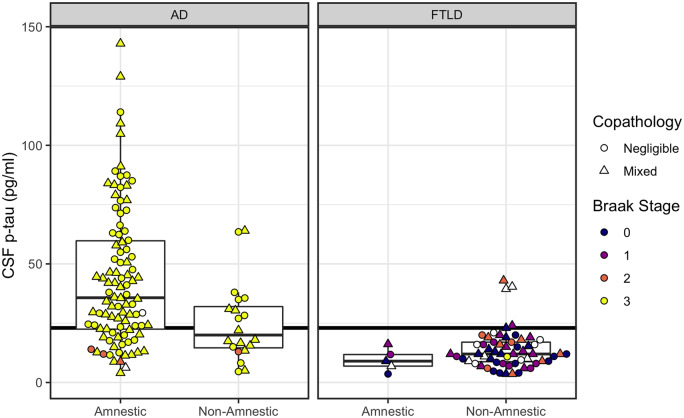
Figure 2.
CSF p-tau concentrations according to phenotype (amnestic, non-amnestic) and post-mortem ratings of tau neurofibrillary tangle pathology. Left: Plots for patients with primary Alzheimer’s disease (AD) pathology. Right: Plots for patients with primary FTLD pathology. Horizontal line indicates CSF p-tau = 23 pg/ml, with patients at or above the line designated as positive for pathological tau (T+). CSF levels below the line are considered normal. Colour indicates post-mortem Braak staging of tau tangles (white indicates cases with no available Braak staging). Shape indicates presence or absence of copathology.
T status
Fisher’s test showed significantly lower rates for T-positivity in non-amnestic patients (9 of 20; 45%) compared to amnestic patients (72 of 98; 73%) with confirmed Alzheimer’s disease pathology (OR = 0.30; CI: 0.10–0.89; P = 0.018). Five of 64 FTLD patients were T+ (Supplementary material).
CSF p-tau/t-tau ratio
Given the differences observed between amnestic and non-amnestic Alzheimer’s disease in CSF p-tau, we examined if the p-tau/t-tau ratio would provide a more informative metric of elevated tau pathology relative to overall degeneration (Supplementary Fig. 1). A type II ANCOVA showed that p-tau/t-tau ratios differed by pathology [F(1,172) = 6.6, P = 0.01] but not by memory phenotype [F(1,172) = 0.0063, P = 0.94]. Despite this, ROC analyses indicated that the difference across pathology was very small compared to other CSF measures, and that discrimination was poor (AUC = 0.65). See the Supplementary material for full results.
Between-group comparisons of CSF t-tau
CSF t-tau levels were compared parametrically across pathology and phenotype (Fig. 3). A type II ANCOVA showed that CSF t-tau differed by pathology [F(1,172) = 8.1, P = 0.0049], phenotype [F(1,172) = 8.9, P = 0.0033], with APOE status (P = 0.94), copathology status (P = 0.15), age (P = 0.46), interval to death (P = 0.84), sex [F(1,172) = 11.0, P = 0.0011], and MMSE (P = 0.49) included as covariates. In post hoc contrasts, the pathological FTLD group had lower CSF t-tau than the Alzheimer’s disease group [t(172) = −2.8, P = 0.0049]. Compared to amnestic Alzheimer’s disease, we observed lower t-tau in non-amnestic Alzheimer’s disease [t(172) = −2.9, P = 0.018] and in non-amnestic FTLD patients [t(172) = −6.1, P = 2.3 × 10−8]; all other comparisons were non-significant (P < 0.22). CSF t-tau results were similar when restricting the cohort to patients with negligible copathology: the effect of pathology type was significant [F(1,76) = 5.7, P = 0.019], although the effect of memory phenotype was marginal [F(1,76) = 3.6, P = 0.062]; all other effects were non-significant (all P < 0.20).
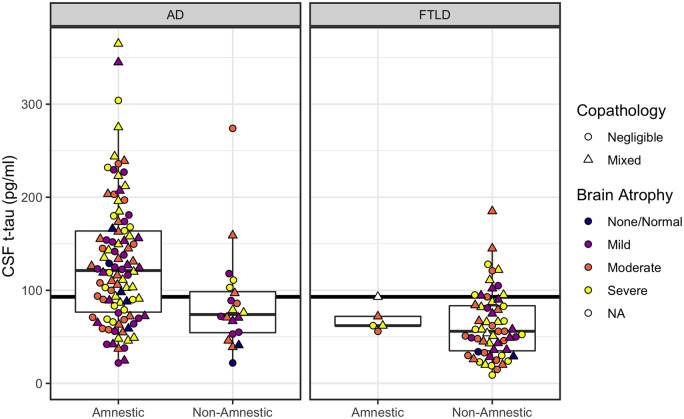
Figure 3.
CSF t-tau concentrations according to phenotype (amnestic, non-amnestic) and post-mortem ratings of brain atrophy. Left: Plots for patients with primary Alzheimer’s disease (AD) pathology. Right: Plots for patients with primary FTLD pathology. Horizontal line indicates CSF t-tau = 93 pg/ml, with patients at or above the line designated as positive for neurodegeneration (N+). CSF levels below the line are considered normal. Colour indicates severity of gross brain atrophy (white indicates cases with no available atrophy). Shape indicates presence/absence of copathology.
N status
Fisher’s test showed significantly lower rates of N-positivity for non-amnestic patients (6 of 20, or 30%) compared to amnestic patients (65 of 98, or 66%) with confirmed Alzheimer’s disease pathology (OR = 0.22; CI: 0.06–0.68; P = 0.005). Only 13 of 64 (20%) FTLD patients were positive for N. Two (3%) FTLD cases were true negatives and had no atrophy at autopsy. The remaining 49 cases (77%) were likely false negatives for N, showing mild to severe atrophy at autopsy (Supplementary material).
Between-group comparison of CSF p-tau/Aβ1–42 levels
The p-tau/Aβ1–42 ratio was compared parametrically across pathology and phenotype (Fig. 4). A type II ANCOVA showed that p-tau/Aβ1–42 levels differed by pathology [F(1,172) = 57.9, P = 1.7 × 10−12] and memory phenotype [F(1,172) = 7.9, P = 0.0040], with APOE status (P = 0.82), copathology status (P = 0.90), age (P = 0.094), interval to death (P = 0.22), sex (P = 0.27), and MMSE (P = 0.24) included as covariates. Post hoc testing confirmed that the pathological FTLD group had lower p-tau/Aβ1–42 ratios than the Alzheimer’s disease group [t(172) = −7.6, P = 1.7 × 10−12]. Further, non-amnestic Alzheimer’s disease patients had significantly lower p-tau/Aβ1–42 ratios than amnestic Alzheimer’s disease patients [t(172) = −2.9, P = 0.020]. Non-amnestic FTLD patients had lower p-tau/Aβ1–42 ratios than both amnestic Alzheimer’s disease [t(172) = −11.1, P < 0.0001] and non-amnestic Alzheimer’s disease [t(172) = −5.7, P = 2.1 × 10−7]; amnestic FTLD patients also had lower p-tau/Aβ1–42 ratios than both amnestic Alzheimer’s disease [t(172) = −4.7, P = 2.6 × 10−5] and non-amnestic Alzheimer’s disease [t(172) = −2.6, P = 0.043]. No difference was observed between non-amnestic and amnestic FTLD patients (P = 0.88). Omnibus results were similar when restricting the cohort to patients with no copathology: there was a significant effect of pathology [F(1,76) = 50.7, P = 4.91 × 10−10], and a marginal effect of phenotype [F(1,76) = 3.7, P = 0.058]; APOE status (P = 0.98), age [F(1,76) = 8.3, P = 0.0051], interval to death (P = 0.31), sex (P = 0.71), and MMSE [F(1,76) = 3.9, P = 0.053] were included as covariates.
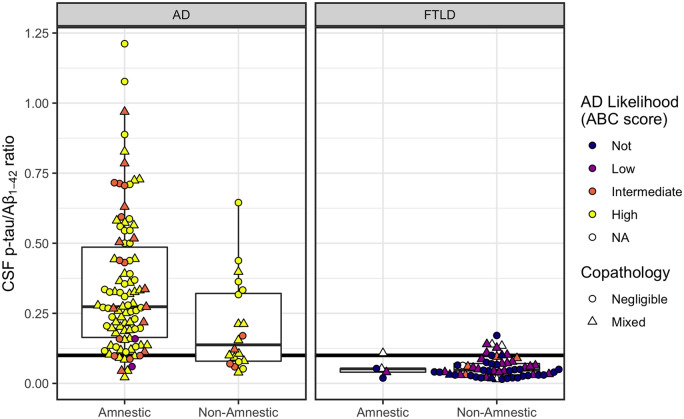
Figure 4.
CSF p-tau/Aβ1–42 according to phenotype (amnestic, non-amnestic) and post-mortem ratings of Alzheimer’s disease likelihood. Left: Plots for patients with primary Alzheimer’s disease (AD) pathology. Right: Plots for patients with primary FTLD pathology. Horizontal line indicates CSF p-tau/Aβ1–42 = 0.10, with patients at or above the line designated as positive for Alzheimer’s disease pathology. Ratios below the line are considered negative for Alzheimer’s disease. Colour indicates level of Alzheimer’s disease pathological change given by ABC score (white indicates cases with no available ABC score). Shape indicates presence/absence of co-pathology.
Alzheimer’s disease status
A Fisher’s test showed significantly higher false-negative rates for non-amnestic patients (7 of 20, or 35%) compared to amnestic patients (10 of 98, or 10%) with confirmed Alzheimer’s disease pathology (OR = 4.7; CI: 1.27–16.58; P = 0.009). In the pathological FTLD group, 10 of 64 (16%) were Alzheimer’s disease positive (Supplementary material).
Discrimination of pathological Alzheimer’s disease from frontotemporal lobar degeneration
We computed ROCs for each CSF measure stratifying amnestic or non-amnestic Alzheimer’s disease from all patients with FTLD pathology (Table 4). In an overall analysis distinguishing all Alzheimer’s disease from FTLD patients, CSF Aβ1–42 and the p-tau/Aβ1–42 ratio produced similar AUCs showing excellent discrimination of both markers (0.938 versus 0.942). Of the two measures, CSF Aβ1–42 had the greatest sensitivity, while the p-tau/Aβ1–42 ratio yielded higher specificity (i.e. fewer primary FTLD patients classified as Alzheimer’s disease) than all other markers.
Table 4.
ROC results for stratification of patients with Alzheimer’s disease from FTLD pathology
| CSF measure | AUC | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|
| All AD from FTLD | ||||
| Aβ1–42 | 0.938 | 199.5 pg/ml | 0.958 | 0.812 |
| p-tau | 0.863 | 20.1 pg/ml | 0.737 | 0.906 |
| t-tau | 0.792 | 96.0 pg/ml | 0.585 | 0.875 |
| p-tau/amyloid-β1–42 | 0.942 | 0.110 | 0.831 | 0.922 |
| Amnestic AD from FTLD | ||||
| Aβ1–42 | 0.941 | 199.5 pg/ml | 0.969 | 0.812 |
| p-tau | 0.888 | 20.1 pg/ml | 0.786 | 0.906 |
| t-tau | 0.822 | 96.5 pg/ml | 0.643 | 0.875 |
| p-tau/amyloid-β1–42 | 0.958 | 0.110 | 0.888 | 0.922 |
| Non-amnestic with AD from FTLD | ||||
| Aβ1–42 | 0.925 | 168.0 pg/ml | 0.80 | 0.922 |
| p-tau | 0.743 | 12.5 pg/ml | 0.85 | 0.578 |
| t-tau | 0.647 | 66.5 pg/ml | 0.70 | 0.625 |
| p-tau/Aβ1–42 | 0.866 | 0.076 | 0.80 | 0.781 |
Includes AUC, best threshold or cut-off point, and sensitivity and specificity at best threshold for each CSF measure. Table shows stratification of all patients with Alzheimer’s disease (AD) from all patients with FTLD pathology (top); stratification of amnestic patients with Alzheimer's disease pathology from FTLD pathology (middle); and stratification of non-amnestic patients with Alzheimer’s disease pathology from all FTLD pathology (bottom).
DeLong’s test for two ROC curves compared diagnostic accuracy by Alzheimer’s disease phenotype for each marker. No difference in accuracy was observed between non-amnestic and amnestic Alzheimer’s disease when Aβ1–42 was used to discriminate groups from pathological FTLD (P = 0.64), which maintained high accuracy for both amnestic and non-amnestic Alzheimer’s disease (AUC = 0.94 versus AUC = 0.92). However, CSF p-tau [D(105.1) = −1.9, P = 0.057] and the p-tau/Aβ1–42 ratio [D(99.6) = −1.9, P = 0.059] were both marginally less accurate for discriminating non-amnestic Alzheimer’s disease than amnestic Alzheimer’s disease from all FTLD patients. Although CSF t-tau is not a diagnostic marker, DeLong’s test shows a significantly lower AUC for non-amnestic Alzheimer’s disease than amnestic Alzheimer’s disease patients when discriminating from all FTLD patients [D(119.9) = −2.3, P = 0.025].
For discrimination of amnestic Alzheimer’s disease from all FTLD patients, optimal cut-offs in the current study were close to the thresholds published by Shaw et al. (2009): for Aβ1–42, 199.5 pg/ml versus 192 pg/ml; for p-tau, 20.1 pg/ml versus 23 pg/ml; for t-tau, 96.5 pg/ml versus 93 pg/ml; and for the p-tau/Aβ1–42 ratio, 0.11 versus 0.10. However, for discrimination of non-amnestic Alzheimer’s disease from all FTLD patients, optimal cut-offs were substantially lower than published thresholds for Aβ1–42 (168.0 pg/ml), p-tau (12.5 pg/ml), t-tau (66.5 pg/ml), and the p-tau/Aβ1–42 ratio (0.076). ROC analyses were repeated excluding cases with copathology and produced similar results, although the p-tau/Aβ1–42 ratio had a high AUC (0.93) when discriminating non-amnestic Alzheimer’s disease from FTLD patients, albeit with a much lower cut-off of 0.051 (versus 0.10) (Supplementary Table 2).
Secondary analysis of p-tau and t-tau associations with age, sex, and disease severity
We performed secondary analyses to investigate three possible factors—age, sex and disease severity—that might explain the lower CSF p-tau and t-tau levels observed in non-amnestic Alzheimer’s disease compared to amnestic Alzheimer’s disease. First, in the current study, non-amnestic Alzheimer’s disease patients were younger at age of symptom onset and at death than amnestic Alzheimer’s disease patients, consistent with previous reports that atypical Alzheimer’s disease phenotypes are associated with a younger age of onset (Lam et al., 2013; Mendez, 2017). While there was no effect for age in analysing Aβ1–42 or t-tau, this demographic difference raised the question of whether lower p-tau values in the non-amnestic Alzheimer’s disease sample were simply a consequence of their younger age, which would be reflected by a positive association between age and p-tau levels among non-amnestic Alzheimer’s disease patients. Second, models also revealed significantly lower t-tau in males than females, which warranted further examination. Finally, it is theorized that low CSF Aβ1–42 in the absence of elevated p-tau and t-tau (A+T−N−) represents an early stage of Alzheimer’s disease pathological change, and that increases in CSF p-tau and t-tau occur in more advanced stages of disease (A+T+N−, A+T+N+). If so, higher p-tau and/or t-tau concentrations would be expected to relate to features of advanced disease, such as lower MMSE, higher age, or shorter interval to death. Conversely, longitudinal decreases in CSF p-tau (Seppälä et al., 2011) and t-tau (Lleó et al., 2019) have been observed in patients with Alzheimer’s disease (although concentrations remain elevated compared to MCI and controls), and thus somewhat lower concentrations may be indicative of advanced disease in Alzheimer’s disease. Type II ANOVAs were performed to test these factors in Alzheimer’s disease and revealed that lower concentrations of CSF p-tau and t-tau in non-amnestic Alzheimer’s disease patients could not be explained by sex, younger age, longer interval to death or higher MMSE, compared to amnestic Alzheimer’s disease (Supplementary material). Instead, secondary analyses found that higher concentrations of p-tau were associated with higher MMSE and younger age, and that T− status in non-amnestic Alzheimer’s disease patients was associated with older age, compared to T+ non-amnestic Alzheimer’s disease (Supplementary material).
Discussion
Recent diagnostic strategies, such as the ATN framework, have emphasized biomarkers over traditional clinical evaluations to improve antemortem predictions of Alzheimer’s disease pathology, as well as stratification from other pathologies such as FTLD. Even with the improved accuracy of antemortem predictions of Alzheimer’s disease pathology, differences in CSF levels across Alzheimer’s disease variants might lead to a small number of diagnostic errors (Teng et al., 2014; Paterson et al., 2015; Wellington et al., 2018; Pillai et al., 2019). To characterize the full spectrum of CSF profiles in Alzheimer’s disease continuum disease and evaluate diagnostic accuracy of CSF biomarkers, we compared the accuracy of CSF-based ATN classifications in Alzheimer’s disease patients with amnestic and non-amnestic clinical phenotypes, and in FTLD. In autopsy-confirmed primary Alzheimer’s disease pathology, non-amnestic Alzheimer’s disease patients were less likely than amnestic Alzheimer’s disease patients to be classified as A+T+N+, but more likely to be classified as normal (A−T−N−) according to CSF biomarkers. Parametric comparisons revealed that differences in designation are likely because non-amnestic Alzheimer’s disease patients have lower concentrations of both p-tau and t-tau in their CSF than amnestic Alzheimer’s disease. Likewise, ROC curve analyses showed that CSF p-tau and the p-tau/Aβ1–42 ratio have somewhat reduced AUC and lower sensitivity in non-amnestic Alzheimer’s disease than amnestic Alzheimer’s disease when discriminating from FTLD patients. While t-tau is not a diagnostic marker, it also had a lower AUC when discriminating non-amnestic Alzheimer’s disease from FTLD, emphasizing that concentrations of t-tau differ by amnestic phenotype. Unlike CSF p-tau and t-tau, CSF Aβ1–42 levels were equivalent between amnestic and non-amnestic Alzheimer’s disease patients, and were significantly lower than in all FTLD patients. ROC curve analyses confirmed that CSF Aβ1–42 is a highly sensitive marker to the presence of Alzheimer’s disease in both amnestic and non-amnestic Alzheimer’s disease, and is excellent at stratifying both Alzheimer’s disease groups from FTLD patients. Thus, our findings indicate that A status is consistent across memory phenotypes in Alzheimer’s disease, and accurately detects individuals who are likely positive for pathological amyloid deposition and thus Alzheimer’s disease continuum disease. However, more specific designations of T and N status based on CSF p-tau and t-tau may inaccurately classify non-amnestic Alzheimer’s disease patients as negative for tau aggregation or neurodegeneration. These findings highlight the need for careful implementation of the ATN framework for in vivo diagnosis, as markers optimized for the most common amnestic presentations of Alzheimer’s disease may not capture the full phenotypic spectrum of the pathological disease.
Our findings are corroborated by other studies that have also found lower CSF p-tau and/or t-tau in non-amnestic Alzheimer’s disease compared to amnestic Alzheimer’s disease (Teng et al., 2014; Wellington et al., 2018). However, this pattern has not been seen by all, and higher concentrations of t-tau in non-amnestic variants (Pillai et al., 2019) or no difference between Alzheimer’s disease variants (Ossenkoppele et al., 2015) have also been observed. One critical difference between our study and prior studies is inclusion criteria: previous examinations of living patients used clinical diagnosis and/or Alzheimer’s disease positive biomarkers as inclusion criteria for non-amnestic Alzheimer’s disease (Teng et al., 2014; Ossenkoppele et al., 2015; Wellington et al., 2018; Pillai et al., 2019). Because our inclusion criteria were based on post-mortem pathological diagnosis, we were able to observe non-amnestic Alzheimer’s disease cases who were not positive for Alzheimer’s disease biomarkers despite having Alzheimer’s disease pathology at autopsy (as well as cases with positive Alzheimer’s disease biomarkers), and Alzheimer’s disease cases who were diagnosed with a clinical disease associated with FTLD; cases such as these might have been excluded by previous studies. Another potential source of variability is that many pre- and post-analytical factors may influence biomarker values (Vos et al., 2014; Fourier et al., 2015). For example, while the enzyme-linked immunosorbent assay (ELISA) is commonly used to analyse CSF samples (Teng et al., 2014; Ossenkoppele et al., 2015; Wellington et al., 2018; Pillai et al., 2019); in this study we used the Luminex platform. While we cannot be sure how the use of different analytical platforms affects ATN classification, one strength of our study is that we used standardized CSF collection and analysis methods to improve reliability and replicability (Shaw et al., 2011). Finally, fine-grained differences in p-tau and t-tau may exist across non-amnestic subtypes, with some subtypes showing decreased levels and others showing elevated levels of p-tau and t-tau compared to amnestic Alzheimer’s disease (Paterson et al., 2015); no differences across subtype has also been observed (Ossenkoppele et al., 2015). Thus, the proportion of clinical subtypes present may also influence aggregated findings.
While a primary goal of the ATN framework is to identify patients with Alzheimer’s disease pathology, a secondary goal of the ATN framework may be the characterization of individuals with non-Alzheimer’s disease pathology (Jack et al., 2018). Under the ATN framework, detection of non-Alzheimer’s disease pathology relies on N+ status in the absence of either A or T, or T+ status in the absence of A. Our results show that patients with FTLD pathology were not reliably classified by ATN; only 12 of 64 were identified as having non-Alzheimer’s disease pathology (A−T+N−, A−T−N+, A−T+N+). Three FTLD patients were identified as having suspected co-occurring Alzheimer’s disease and non-Alzheimer’s disease pathologies; however, two of these had ABC scores of 0 at autopsy. Instead, a majority of FTLD patients were N− due to lower CSF t-tau levels, despite showing atrophy at autopsy (only two cases of FTLD were judged not to be atrophic); 9 of 64 were classified as having Alzheimer’s pathological change, and the majority were classified as having normal biomarkers (40 of 64). It is noteworthy that a majority of non-amnestic Alzheimer’s disease patients were also N− (14 of 20). Discrepancies between t-tau results and autopsy assessments of atrophy in non-amnestic Alzheimer’s disease and in FTLD may indicate that brain structure was relatively preserved at time of lumbar puncture; alternatively, negative t-tau results may indicate a failure to detect true degeneration. While CSF t-tau is interpreted as a non-specific marker of neurodegeneration (Jack et al., 2016), our results indicate that t-tau may be relatively insensitive to neurodegeneration in non-amnestic pathological cases, both Alzheimer’s disease and FTLD.
We additionally tested discrimination based on p-tau/Aβ1–42 ratio as an alternative to the ATN framework. This composite marker had the highest AUC (0.96) and highest specificity (0.92) when stratifying amnestic Alzheimer’s disease from pathological FTLD, a finding in agreement with previous studies (Vergallo et al., 2017; Lleó et al., 2018). Indeed, this ratio score correctly identified 90% of amnestic Alzheimer’s disease patients (Table 3). Results using the ATN framework were comparable: 93% of amnestic Alzheimer’s disease patients were classified as being on the Alzheimer’s disease continuum because of A+ status. However, the p-tau/Aβ1–42 ratio had a lower AUC than Aβ1–42 alone (0.87 versus 0.93) when discriminating non-amnestic Alzheimer’s disease patients from all FTLD patients, correctly identifying only 65% cases. By comparison, ATN designated 85% of non-amnestic Alzheimer’s disease patients as being on the Alzheimer’s disease continuum, although this increase was not significant.
The optimal thresholds for CSF Aβ1–42, p-tau, and p-tau/Aβ1–42 specific to our entire cohort were broadly consistent with cut-off points that were established in an independent cohort to distinguish Alzheimer’s disease from healthy controls (Shaw et al., 2009). However, all optimal cut-offs were lower when stratifying non-amnestic Alzheimer’s disease patients from FTLD, including CSF Aβ1–42. A caveat to this statement is that CSF t-tau is not intended to discriminate pathological Alzheimer’s disease from pathological FTLD; to calculate a true cut-off for t-tau in non-amnestic patients, we would need a reference control sample without degeneration, which is beyond the scope of this study. Still, lower optimal cut-offs of p-tau, t-tau, and the p-tau/Aβ1–42 reflect the lower concentrations of p-tau and t-tau in non-amnestic Alzheimer’s disease compared to amnestic Alzheimer’s disease. Even so, the sensitivity and specificity of these adjusted cut-offs is poor, and does little to improve diagnostic specificity of these markers because of overlap with pathological FTLD. The exception is CSF Aβ1–42 which maintains high sensitivity and specificity in non-amnestic Alzheimer’s disease. Unlike p-tau and t-tau, the lower optimal cut-off of CSF Aβ1–42 in non-amnestic Alzheimer’s disease is a more conservative cut-off than in the entire sample, and may in part be an artefact of small cohort size; changing the cut-off from 192 to 166 would change the designation of only one subject (Fig. 1).
Instead of adjusting CSF cut-offs, our findings suggest that selection of the appropriate marker based on phenotype (amnestic or non-amnestic) and diagnostic goals (increase sensitivity or specificity) would do more to improve diagnostic accuracy. The p-tau/Aβ1–42 ratio appears to perform best in a cohort of amnestic Alzheimer’s disease dementia patients, and has slightly higher specificity for excluding non-Alzheimer’s disease cases. However, it performs less well in non-amnestic Alzheimer’s disease because of their lower levels of CSF p-tau. By comparison, the ATN framework has excellent sensitivity to the presence of Alzheimer’s continuum disease due in part to the reliable performance of A status as measured by Aβ1–42 in both amnestic and non-amnestic Alzheimer’s disease. However, it may be that more specific designations within the ATN framework incorrectly classify non-amnestic Alzheimer’s disease patients as normal or Alzheimer’s pathological change. Potential limitations of classification using the ATN framework in non-amnestic Alzheimer’s disease should be considered for candidate treatment trials. Furthermore, our results indicate that the ATN framework is relatively insensitive to non-Alzheimer’s disease diseases such as FTLD, and these patients were likely to be erroneously classified as having normal biomarkers or Alzheimer’s disease pathological change alone. Biomarkers suggestive of co-occurring pathologies (A+T−N+) were unreliable: A+T−N+ failed to capture the majority of FTLD with secondary Alzheimer’s disease pathology and two of three A+T−N+ FTLD cases had no detected level of Alzheimer’s disease (zero ABC scores). In sum, both the ATN framework and the p-tau/Aβ1–42 were excellent at identifying amnestic Alzheimer’s disease cases, while the single marker of CSF Aβ1–42 had the most consistent performance across memory phenotypes in Alzheimer’s disease, and was sensitive to both amnestic and non-amnestic Alzheimer’s disease.
In secondary analyses, we investigated how factors relating to sex, age, or disease severity might affect CSF levels. Significantly lower levels of t-tau were observed in males compared to females in amnestic Alzheimer’s disease, but secondary analyses showed no interaction between sex and CSF t-tau that explain group differences. Still, the current study adds to a growing body of results on sex differences in CSF; previous research has found that females have elevated CSF t-tau (Hohman et al., 2018) and higher Alzheimer’s disease pathological burden and tau tangle density (Oveisgharan et al., 2018) compared to males. Analysis of age effects did not support the interpretation that non-amnestic Alzheimer’s disease patients had less tau accumulation due to their younger age. On the contrary, younger non-amnestic Alzheimer’s disease patients were had higher levels of p-tau and were more likely to be T+ than older patients (these trends were marginally significant in amnestic Alzheimer’s disease). Models also showed that higher p-tau levels were associated with higher MMSE in Alzheimer’s disease patients. Likewise, a longitudinal study of CSF trajectories in Alzheimer’s disease suggests that, CSF p-tau levels first peak and then decline over time, and that this decline relates to declining MMSE (Seppälä et al., 2011). It is therefore possible that the high rate of T-negativity in non-amnestic Alzheimer’s disease patients is a consequence of rapid disease progression, and that CSF was measured after p-tau levels have begun to decline.
Caveats and limitations
There are a number of caveats to the current study. First, although autopsy studies suggest that mixed pathology is highly common in dementia, no current CSF analytes can determine the presence of co-occurring or ‘mixed’ pathology. To increase the relevance and application of our findings, we examined patients who had Alzheimer’s disease and FTLD with negligible levels of secondary pathology (thought to not contribute significantly to phenotype), as well as those with mixed pathology, including Alzheimer’s disease and FTLD patients with co-occurring α-synuclein pathology. To ensure that mixed pathology cases were not driving diagnostic errors, analyses included copathology status as a covariate and were repeated in patients with negligible copathology. Results were largely consistent across models with and without copathology cases, indicating that differences observed across phenotypes in Alzheimer’s disease are not explained by copathological status. We were insufficiently powered to examine subgroups of secondary pathology or subgroups of FTLD pathology. Second, while we interpret A− or T− status in pathological Alzheimer’s disease as likely false-negative cases, we cannot entirely rule out that CSF markers accurately reflect a true absence of amyloid or tau pathology that developed only later in disease course and after obtaining the CSF sample. However, we believe this is an unlikely possibility, given that patients were symptomatic at the time of CSF, and that the accumulation of these histopathological features is thought to begin much earlier in the presymptomatic course of disease. For the same reason, it is likely that both Alzheimer’s disease and FTLD patients had significant degeneration at the time of CSF, and that the high proportion of N-negativity seen in non-amnestic Alzheimer’s disease and FTLD patients is not representative of their disease. Indeed, gross atrophy and neuronal loss ratings indicate that degeneration at autopsy was equivalent across groups. However, we note that these are coarse measurements of degeneration, and that fine-grained differences in the regional distribution and burden of disease may influence CSF levels. Third, amnestic and non-amnestic patients differed in age-related factors (age at CSF, at onset, and survival). These differences are expected given the dementia population: amnestic patients tend to be older at onset than non-amnestic variants of both Alzheimer’s disease and FTLD (Koedam et al., 2010; Mendez, 2017). To account for age and the course of disease at the time of lumbar puncture, age at CSF, interval from CSF to death, and MMSE were included as covariates in models. Still, one possibility is that the lower p-tau and t-tau concentrations in non-amnestic Alzheimer’s disease compared with amnestic Alzheimer’s disease are due to younger age or differences in disease severity. Disease trajectories differ significantly across non-amnestic Alzheimer’s disease, amnestic Alzheimer’s disease, and FTLD, with different syndromes showing different average ages of onset and years of survival, different rates and modalities of cognitive decline, and different distributions of atrophy and pathology. We report several measures of disease severity in this paper, including MMSE, interval from CSF to death, survival, amyloid burden (Thal/CERAD), tangle burden (Braak), atrophy, or neuronal loss ratings. While none of these measures differ across amnestic and non-amnestic Alzheimer’s disease, there are currently no agreed upon metrics that can equate disease severity across syndromes, and MMSE and other non-specific measures of disease severity may not capture differences across groups (Santana et al., 2016; Pillai et al., 2019). We find no evidence that non-amnestic Alzheimer’s disease patients were less impaired than amnestic Alzheimer’s disease at time of lumbar puncture. Instead, we saw evidence that lower p-tau concentrations were associated with older age and lower MMSE in Alzheimer’s disease, signs of advanced disease. Fourth, our non-amnestic Alzheimer’s disease group was composed of six clinical subtypes, which all have distinct clinical and anatomical features. It is possible that aggregation obscures fine-grained differences in p-tau and t-tau concentrations across non-amnestic subtypes. However, we were underpowered to investigate if CSF p-tau and t-tau levels differ across non-amnestic Alzheimer’s disease variants. Fifth, t-tau has been criticized as a marker of neurodegeneration, in part because t-tau is highly correlated with p-tau, and thus characterizations of T-status and N-status may not be truly independent (Hampel et al., 2018). Moreover, recent longitudinal work shows little prognostic value to t-tau in controls (Soldan et al., 2019; Vos and Duara, 2019). While imaging-based markers of neurodegeneration may provide an attractive alternative to CSF t-tau, patients in the current study were not selected based on availability of MRI or PET data; sparse MRI data, variability in the temporal intervals between patients’ scans and lumbar punctures, coupled with differences in scan acquisition parameters, prevented meaningful comparative analysis. However, we note that the shortcomings of t-tau as a marker of N are orthogonal to the phenotypic differences in ATN classification reported in the current study. Finally, this is a single-centre study, and thus may be limited in generalizability.
In conclusion, results show that only CSF Aβ1–42 is consistent across amnestic and non-amnestic patients with Alzheimer’s disease pathology, and is highly accurate at discriminating Alzheimer’s disease pathology from FTLD in both memory groups. Even so, ROC analyses show that CSF Aβ1–42 has slightly decreased specificity compared to other CSF biomarkers when stratifying amnestic Alzheimer’s disease from FTLD, such as the p-tau/Aβ1–42 ratio. By comparison, CSF p-tau and t-tau levels differ across amnestic and non-amnestic patients with Alzheimer’s disease pathology, and show a much lower accuracy for non-amnestic patients. Because of these differences, specific ATN designations within the Alzheimer’s continuum indicating early Alzheimer’s disease or concomitant pathologies may be less accurate for non-amnestic variants of Alzheimer’s disease. Findings also show that the ATN framework had poor sensitivity to detecting primary FTLD, erroneously classifying them as Alzheimer’s pathological change alone or as having normal biomarkers. Together, our results indicate that CSF Aβ1–42 alone more accurately classifies non-amnestic Alzheimer’s disease than the ATN framework or the p-tau/AB ratio, and that consideration of clinical phenotype can improve diagnostic accuracy of CSF biomarkers.
Funding
This work was supported in part by the National Institute of Aging AG054519, AG017586, K01-AG061277, National Institute of Neurological Disorders and Stroke NS088341, Alzheimer's Association Research Fellowships AARFD-619473, AARF-16–443681, Brightfocus Foundation A2016244S, and Penn Institute on Aging. K.A.Q.C. is a Fellow for Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) and a recipient of the Alzheimer’s Association Research Fellowship, AARFD-619473. J.S.P. is the recipient of an Alzheimer’s Association Research fellowship, AARF-16–443681, and an NIA-sponsored K01-AG061277 Mentored Research Scientist Career Development Award.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- ALS =
amyotrophic lateral sclerosis
- AUC =
area under the curve
- Aβ1–42 =
amyloid-β1–42 peptide
- FTD =
frontotemporal dementia
- FTLD =
frontotemporal lobar degeneration
- LATE =
limbic-predominant age-related TDP-43 encephalopathy
- MMSE =
Mini-Mental State Examination
- PART =
primary age-related tauopathy
- PPA =
primary progressive aphasia; p-tau = tau protein phosphorylated at threonine 181
- ROC =
receiver operating characteristic
- TDP-43 =
transactive response DNA-binding protein of 43 kDa
References
- Andreasen N, Sjögren M, Blennow K.. CSF markers for Alzheimer’s disease: total tau, phospho-tau and Aβ42. World J Biol Psychiatry 2003; 4: 147–55. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC.. Posterior cortical atrophy. Lancet Neurol 2012; 11: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, McGinnis SM, Xia C, Price BH, Atri A, Murray ME, et al. Approach to atypical Alzheimer’s disease and case studies of the major subtypes. CNS Spectr 2017; 22: 439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B Feldman HH Jacova C Dekosky ST Barberger-Gateau P Cummings J, et al. . Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. The Lancet Neurology 2007; 6: 734–46. [DOI] [PubMed] [Google Scholar]
- Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, et al. CSF biomarkers for the differential diagnosis of Alzheimer’s disease: a large-scale international multicenter study. Alzheimers Dement 2015; 11: 1306–15. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘ Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Fourier A, Portelius E, Zetterberg H, Blennow K, Quadrio I, Perret-Liaudet A.. Pre-analytical and analytical factors influencing Alzheimer’s disease cerebrospinal fluid biomarker variability. Clin Chim Acta 2015; 449: 9–15. [DOI] [PubMed] [Google Scholar]
- Fox J Weisberg S Adler D Bates D Baud-Bovy G Ellison S et al. Package ‘car’ Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR.. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 2000; 123: 484–98. [DOI] [PubMed] [Google Scholar]
- Gibbons GS, Banks RA, Kim B, Changolkar L, Riddle DM, Leight SN, et al. Detection of Alzheimer disease (AD)-specific tau pathology in AD and nonAD tauopathies by immunohistochemistry with novel conformation-selective tau antibodies. J Neuropathol Exp Neurol 2018; 77: 216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons GS, Kim SJ, Robinson JL, Changolkar L, Irwin DJ, Shaw LM, et al. Detection of Alzheimer’s disease (AD) specific tau pathology with conformation-selective anti-tau monoclonal antibody in co-morbid frontotemporal lobar degeneration-tau (FTLD-tau). Acta Neuropathol Commun 2019; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez MF, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Toschi N, Baldacci F, Zetterberg H, Blennow K, Kilimann I, et al. Alzheimer’s disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: aβ1–42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimers Dement 2018; 14: 492–501. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004; 56: 399–406. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017; 32: 853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, et al. Sex-specific association of apolipoprotein e with cerebrospinal fluid levels of tau. JAMA Neurol 2018; 75: 989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K, Neumann M, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol 2008; 173: 182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016; 87: 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam E, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg Y.. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimer’s Dis 2010; 19: 1401–8. [DOI] [PubMed] [Google Scholar]
- Lam B, Masellis M, Freedman M, Stuss DT, Black SE.. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther 2013; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimer’s Dement 2019; 15: 742–53. [DOI] [PubMed] [Google Scholar]
- Lleó A, Irwin DJ, Illán-Gala I, Mcmillan CT, Wolk DA, Lee EB, et al. A 2-step cerebrospinal algorithm for the selection of frontotemporal lobar degeneration subtypes. JAMA Neurol 2018; 75: 738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010; 119: [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005; 65: 1863–72. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF. Early-onset Alzheimer disease. Neurol Clin 2017; 35: 263–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41: 479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW.. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 2011; 10: 785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckl P, Steinacker P, Feneberg E, Otto M.. Neurochemical biomarkers in the diagnosis of frontotemporal lobar degeneration: an update. J Neurochem 2016; 138: 184–92. [DOI] [PubMed] [Google Scholar]
- Onyike CU, Diehl-Schmid J.. The epidemiology of frontotemporal dementia. Int Rev Psychiatry 2013; 25: 130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Mattsson N, Teunissen CE, Barkhof F, Pijnenburg Y, Scheltens P, et al. Cerebrospinal fluid biomarkers and cerebral atrophy in distinct clinical variants of probable Alzheimer’s disease. Neurobiol Aging 2015; 36: 2340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA.. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol 2018; 136: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Minthon L, Blennow K, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 2015; 85: 1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson RW, Toombs J, Slattery CF, Nicholas JM, Andreasson U, Magdalinou NK, et al. Dissecting IWG-2 typical and atypical Alzheimer’s disease: insights from cerebrospinal fluid analysis. J Neurol 2015; 262: 2722–30. [DOI] [PubMed] [Google Scholar]
- Perry DC, Miller BL.. Frontotemporal dementia In: seminars in neurology. New York: Thieme Medical Publishers; 2013. p. 336–41. [DOI] [PubMed] [Google Scholar]
- Phillips JS, Da Re F, Dratch L, Xie SX, Irwin DJ, McMillan CT, et al. Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer’s disease. Neurobiol Aging 2018; 63: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JS, Da Re F, Irwin DJ, McMillan CT, Vaishnavi SN, Xie SX, et al. Longitudinal progression of grey matter atrophy in non-amnestic Alzheimer’s disease. Brain 2019; 142: 1701–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JS, Das SR, McMillan CT, Irwin DJ, Roll EE, Da Re F, et al. Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease. Hum Brain Mapp 2018; 39: 691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JA, Bonner-Jackson A, Bekris LM, Safar J, Bena J, Leverenz JB.. Highly elevated cerebrospinal fluid total tau level reflects higher likelihood of non-amnestic subtype of Alzheimer’s disease. J Alzheimer’s Dis 2019; 70: 1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouclet-Courtemanche H, Nguyen T-B, Skrobala E, Boutoleau-Bretonnière C, Pasquier F, Bouaziz-Amar E, et al. Frontotemporal dementia is the leading cause of “true” A−/T+ profiles defined with Aβ42/40 ratio. Alzheimer’s Dement 2019; 11: 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. [Internet]. 2017; Available from: https://www.r-project.org/. [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018; 141: 2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana I, Duro D, Lemos R, Costa V, Pereira M, Simões MR, et al. Mini-mental state examination: screening and diagnosis of cognitive decline, using new normative data. Acta Med Port 2016; 29: 240–8. [DOI] [PubMed] [Google Scholar]
- Seppälä TT, Koivisto AM, Hartikainen P, Helisalmi S, Soininen H, Herukka S-K.. Longitudinal changes of CSF biomarkers in Alzheimer’s disease. J Alzheimer’s Dis 2011; 25: 583–94. [DOI] [PubMed] [Google Scholar]
- Shaw LM Vanderstichele H Knapik-Czajka M Figurski M Coart E Blennow K, et al. . Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011; 121: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009; 65: 403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Fagan AM, Schindler SE, Moghekar A, Fowler C, et al. ATN profiles among cognitively normal individuals and longitudinal cognitive outcomes. Neurology 2019; 92: e1567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18: 153–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyfs H, Niemantsverdriet E, Goossens J, Fransen E, Martin J-J, De Deyn PP, et al. Cerebrospinal fluid P-Tau181P: biomarker for improved differential dementia diagnosis. Front Neurol 2015; 6: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka S-K, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009; 66: 382–9. [DOI] [PubMed] [Google Scholar]
- Teng E, Yamasaki TR, Tran M, Hsiao JJ, Sultzer DL, Mendez MF.. Cerebrospinal fluid biomarkers in clinical subtypes of early-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 37: 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, et al. CSF biomarkers cut-offs: the importance of coincident neuropathological diseases. Acta Neuropathol 2012; 124: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, et al. A platform for discovery: the University of Pennsylvania integrated neurodegenerative disease biobank. Alzheimer’s Dement 2014; 10: 477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergallo A, Carlesi C, Pagni C, Giorgi FS, Baldacci F, Petrozzi L, et al. A single center study: aβ42/p-Tau 181 CSF ratio to discriminate AD from FTD in clinical setting. Neurol Sci 2017; 38: 1791–7. [DOI] [PubMed] [Google Scholar]
- Vos SJB, Duara R. The prognostic value of ATN Alzheimer biomarker profiles in cognitively normal individuals. Neurology 2019; 92: 643–4. [DOI] [PubMed] [Google Scholar]
- Vos SJB, Visser PJ, Verhey F, Aalten P, Knol D, Ramakers I, et al. Variability of CSF Alzheimer’s disease biomarkers: implications for clinical practice. PLoS One 2014; 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington H, Paterson RW, Suárez-González A, Poole T, Frost C, Sjöbom U, et al. CSF neurogranin or tau distinguish typical and atypical Alzheimer disease. Ann Clin Transl Neurol 2018; 5: 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA. Amyloid imaging in atypical presentations of Alzheimer’s disease. Curr Neurol Neurosci Rep 2013; 13: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie SX, Baek Y, Grossman M, Arnold SE, Karlawish J, Siderowf A, et al. Building an integrated neurodegenerative disease database at an academic health center. Alzheimer’s Dement 2011; 7: e84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All qualified investigators are welcome to view data through an established algorithm at the CNDR.