Abstract
Background:
The evidence base for optimum third-line therapy for metastatic colorectal cancer (mCRC) is not conclusive. Recent studies have demonstrated the efficacy of regorafenib as third-line therapy in mCRC. This indirect meta-analysis compared the efficacy and safety of regorafenib with other available third-line therapies for mCRC.
Methods:
A literature search for randomized controlled trials (RCTs) was conducted in PubMed, Embase, and Cochrane Library for studies evaluating the efficacy and safety of fruquintinib, regorafenib, TAS-102, and nintedanib as third-line therapies in patients with mCRC. Overall survival (OS) and progression-free survival (PFS) were the primary outcomes, while objective response rate (ORR) and safety were the secondary outcomes. Hazard ratio (HR) and relative risk (RR) with their respective 95% confidence interval (CI) were used for analysis of survival, clinical response, and safety data. An adjusted indirect meta-analysis with placebo as the common comparator was performed.
Results:
We identified eight RCTs comparing regorafenib (two studies), fruquintinib (two studies), TAS-102 (three studies), and nintedanib (one study) against placebo. The OS with regorafenib was significantly better when compared with nintedanib (HR = 0.66; 95% CI: 0.45, 0.95, p = 0.02) but was similar to that of fruquintinib (HR = 1.01; 95% CI: 0.67, 1.52, p = 0.94) and TAS-102 (HR = 0.97; 95% CI: 0.68, 1.38, p = 0.88). The PFS and ORR for regorafenib were slightly better than those of TAS-102 (PFS: HR = 0.86, 95% CI: 0.54, 1.37, p = 0.5; ORR: RR = 1.13, 95% CI: 0.11, 11.05, p = 0.92) and nintedanib (PFS: HR = 0.68, 95% CI: 0.42, 1.10, p = 0.12; ORR: not reported) but were lower than those for fruquintinib (PFS: HR = 1.53, 95% CI: 0.93, 2.52, p = 0.08; ORR: RR = 0.68269, 95% CI: 0.045, 10.32, p = 0.79). Safety analysis showed that the RR of adverse events (AEs) was lesser in patients treated with regorafenib in comparison with that in patients treated with fruquintinib, but was similar to that in patients treated with nintedanib and TAS-102.
Conclusion:
Regorafenib has efficacy similar to that of TAS-102 and better safety when compared with fruquintinib. Considering the mechanism of action of regorafenib, which targets multiple factors in the angiogenic pathway, it could be an ideal option for treatment in the beyond second-line setting.
Keywords: fruquintinib, meta-analysis, metastatic colorectal cancer, nintedanib, regorafenib
Introduction
Colorectal cancer (CRC) is the third and second most commonly occurring cancer in men and women, respectively.1 On the basis of the localization and metastases, CRC is divided into different stages that correspond to localized, regional, and distant metastases.2 The 5-year relative survival ranges from 90% for localized CRC to 14% for metastatic colorectal cancer (mCRC). The most common cause of death in patients with CRC is metastases to distant organs.3,4 Owing to better treatment options in early treatment setting, the median survival in patients with mCRC is reported to be approximately 30 months in recent trials.5
The accumulated toxicity of chemotherapeutic drugs used for first- and second-line treatments of mCRC limits the options available for later lines of therapy.6 Regorafenib is an oral multikinase inhibitor that is indicated for heavily pretreated patients with mCRC by the United States Food and Drug Administration (FDA) in 2012.7 TAS-102 is the other drug that has been approved for treatment in the beyond second-line setting in mCRC.8 Other drugs that have been evaluated include nintedanib and fruquintinib. Fruquintinib has been evaluated, and was approved for the treatment in Chinese patients in 2018.9
Among all the treatment options, regorafenib was the first to be approved and used in real-world settings, with modest efficacy and manageable adverse events (AEs).10 Different AE management strategies for regorafenib, including altering the dose regimen and dosage schedule, have been evaluated and found to be effective in distinct subgroup of patients mCRC.11 By virtue of getting approval from the FDA in 2012, there is an accumulated real-world evidence base that establishes statistically significant improvement in the survival for regorafenib therapy.12 However, recent trials with multiple drugs had broadened the repertoire of therapeutic options in third-line treatment of patients with mCRC. The increase in options has also led to a therapeutic conundrum among clinicians for the most optimum choice of drug in third-line setting.
In this context, it is essential to appraise and compare the evidence for regorafenib against newer drugs in the treatment of mCRC in third-line setting. Unfortunately, there are no head-on trials comparing regorafenib with other drugs. Given the lack of direct evidence, evidence from properly conducted indirect meta-analyses could be used for clinical decision-making.13 In recent years, statistical methods for indirect analysis have also matured to include the effect estimates from direct evidence, thereby preserving randomization.14 Hence, to aid clinical decision-making, we have performed an adjusted indirect meta-analysis to compare regorafenib with the available treatment options for patients with mCRC in a third-line setting.
Methods
Ethical review
This meta-analysis (CRD42018099548) was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.15 It is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Search strategy
We conducted a systematic literature search in PubMed, Embase, and Cochrane Library up to January, 2019 using the following search string: [(“metastatic colorectal cancer” OR “mCRC”) OR (“colorectal neoplasm” OR “colorectal cancer” AND “metastatic”)] AND (“regorafenib” OR “BAY 73-4506” OR “BAY73-4506” OR “BAY-73-4506” OR “fruquintinib” OR “TAS-102” OR “TAS102” OR “raltitrexed” OR “ZD1694” OR “cetuximab” OR “Erbitux” OR “C225” OR “Apatinib” OR “YN968D1” OR “Anlotinib” OR “AL3818” OR “nivolumab” OR “ONO5438” OR “MDX1106” OR “pembrolizumab” OR “MK-3475” OR “atezolizumab” OR “RG-7446” OR “RG7446” OR “avelumab” OR “MSB0010718C” OR “durvalumab” OR “MEDI4736” OR “MEDI-4736”) AND “Placebo” (supplemental Table 1). Two independent reviewers screened the articles published in English language for randomized control trials (RCTs) comparing the efficacy and safety of different third-line therapies in patients with mCRC against the best supportive care (BSC). Studies other than RCTs, that is, case reports, retrospective studies, reviews, and meta-analysis were excluded. The reference lists of the included articles were screened manually for relevancy.
Data extraction
A data extraction protocol was defined, and a customized data extraction sheet designed. Separate analysis sheets were created for direct and indirect pairwise comparisons. Data extraction and assessment were performed by two independent reviewers from the full-text articles selected for inclusion. Disagreements in inclusion and data extraction were resolved after discussion with a third reviewer. The data extracted include demographic, survival, tumor response, and safety data. Hazard ratios (HRs) and relative risk (RR), along with their 95% confidence intervals (CIs), were extracted for survival [overall survival (OS) and progression-free survival (PFS)] and response outcomes [objective response rate (ORR)], respectively. The number and type of AEs in the intervention and comparator arms were also extracted for assessing safety. OS and PFS were the primary outcomes, whereas ORR and safety were the secondary outcomes.
Methodological assessment and publication bias
A quality assessment of the included studies was performed using the Cochrane risk-of-bias tool for randomized trials (RoB 2) (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials). We intended to assess the publication bias using funnel plot technique, Begg’s rank test, and Egger’s regression test, as appropriate, because the methods have known limitations.
Data synthesis and statistical analysis
The objective of the study was to compare outcomes in patients with mCRC treated with regorafenib against those who received other treatment regimens. As there are no studies with head-on comparison among the different third-line regimens in mCRC, we performed an adjusted indirect comparison analysis. An analysis was conducted for individual third-line therapies (fruquintinib, regorafenib, TAS-102, and nintedanib) versus placebo (pairwise direct comparison) separately. Placebo was the common comparator for all the third-line therapies. We calculated the overall HRs for OS and PFS and overall OR for ORR in the pairwise direct comparisons by using either a fixed or random effects model depending on the heterogeneity. The HRs and ORs used were adjusted for baseline differences in the individual studies. The RRs and 95% CIs for AEs were calculated for the individual studies and used for arriving at a pooled RR for the pairwise direct comparisons.
The adjusted indirect comparisons were performed according to the method of Bucher et al.16 Accordingly, BSC was used as the common comparator. As per the method, the direct effect estimates of intervention A relative to intervention B, and intervention C relative to intervention B can be used to find the indirect effect estimate of intervention A relative to intervention C. The indirect effect estimates, which could be either logeHR or logeOR or logeRR, depending on the outcome, could be calculated using the following formula: effect estimate AC = effect estimate AB – effect estimate CB. The variance of effect estimate AC is the sum of the variances of the direct estimates: variance AB + variance CB.
The assumption of transitivity that is significant in indirect comparison meta-analysis was evaluated based on the individual study characteristics. The homogeneity of the studies in direct comparison was measured using the I2 statistic. All the analyses were performed using the “R” software version 3.4.1 (https://www.r-project.org/).
Results
Study selection
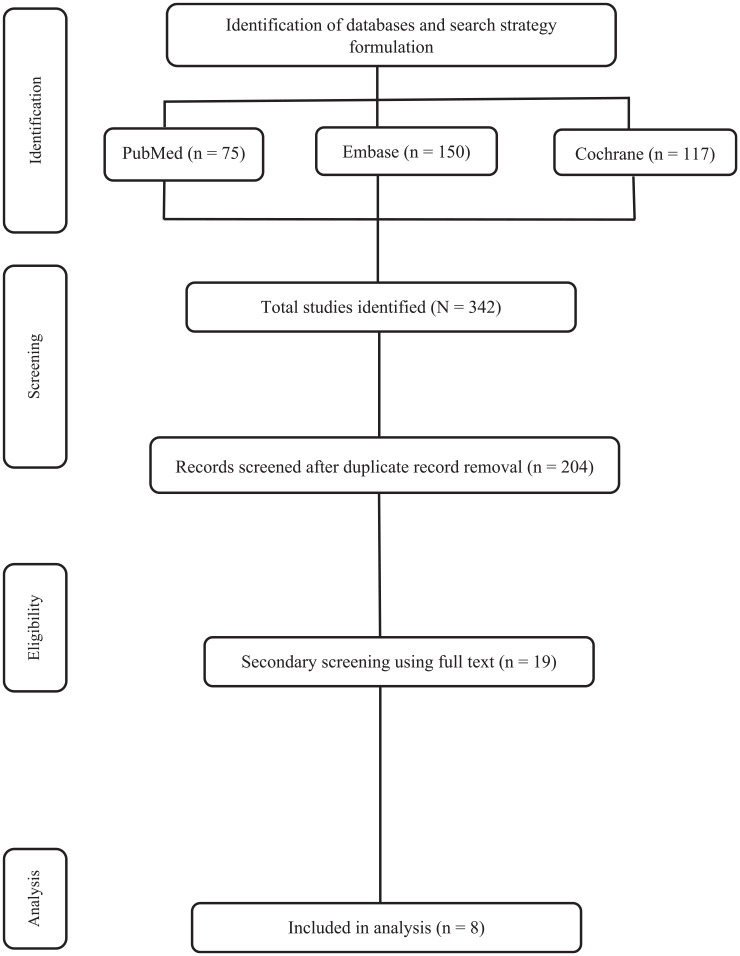
A total of 324 studies were retrieved after a thorough manual and electronic searches of all the databases. After an extensive assessment based on our inclusion and exclusion criteria, only eight studies were included for the analyses. The flow diagram of the study selection is shown in Figure 1. The eight RCTs evaluating a total of 3598 patients with mCRC for efficacy and safety of different third-line therapies were included in the analysis. The following indirect comparisons were made: regorafenib versus fruquintinib, regorafenib versus TAS-102, and regorafenib versus nintedanib. The included studies presented minimal risk of bias. Publication bias was not assessed as the number of included trials was inadequate to properly assess a funnel plot or any other more advanced regression-based assessments.
Figure 1.
PRISMA flowchart.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Transitivity
The inclusion and exclusion criteria used in the included studies were similar without substantial variation. All the studies recruited patients with histologically confirmed mCRC who had received a minimum of one second-line standard therapy. Patients previously treated with vascular endothelial growth factor receptor (VEGFR) inhibitors were excluded in the RCTs evaluating fruquintinib. The different baseline characteristics were varied across the included studies but were similar in individual trials. Overall, the distribution of effect modifiers was comparable across the direct comparisons. The different baseline characteristics of the included studies were provided in Table 1.
Table 1.
Baseline demographic characteristics of the included studies.
| Study no. | Author | No. of patients (n) | Third-line therapy |
Age |
Gender |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | No. of patients | Comparator | No. of patients | Intervention | Comparator | Intervention (M/F) | Comparator (M/F) | |||
| 1 | Jin Li et al.17 | 416 | Fruquintinib 5 mg/day | 278 | Placebo | 138 | 55 (23–75) | 57 (24–74) | 158/120 | 97/41 |
| 2 | Rui-Hua Xu et al.18 | 71 | Fruquintinib 5 mg/day | 47 | Placebo | 24 | 50 (25.0–69.0) | 54 (38.0–70.0) | 35/12 | 17/7 |
| 3 | Jin Li et al.19 | 208 | Regorafenib 160 mg/day | 136 | Placebo | 68 | 57.5 (50.0–66.0) | 55.5 (48.5–62.0) | 85/51 | 33/35 |
| 4 | Axel Grothey et al.20 | 760 | Regorafenib 160 mg/day | 505 | Placebo | 255 | 61 (54.0–67.0) | 61 (54.0–68.0) | 311/194 | 153/102 |
| 5 | Yoshino et al.21 | 169 | TAS-102 35 mg/m2/dose | 112 | Placebo | 57 | 63 (28–80) | 62 (39–79) | 64/48 | 28/29 |
| 6 | Mayer et al.22 | 800 | TAS-102 35 mg/m2/dose | 534 | Placebo | 266 | 63 (27–82) | 63 (27–82) | 326/208 | 165/101 |
| 7 | Jianming Xu et al.23 | 406 | TAS-102 35 mg/m2/dose | 271 | Placebo | 136 | 58 (26–81) | 56 (24–80) | 175/101 | 84/51 |
| 8 | Van Custem et al.24 | 768 | Nintedanib | 384 | Placebo | 381 | 62 (22–85) | 62 (23–83) | 236/150 | 218/164 |
F, female; M, male.
Survival outcomes
Overall survival
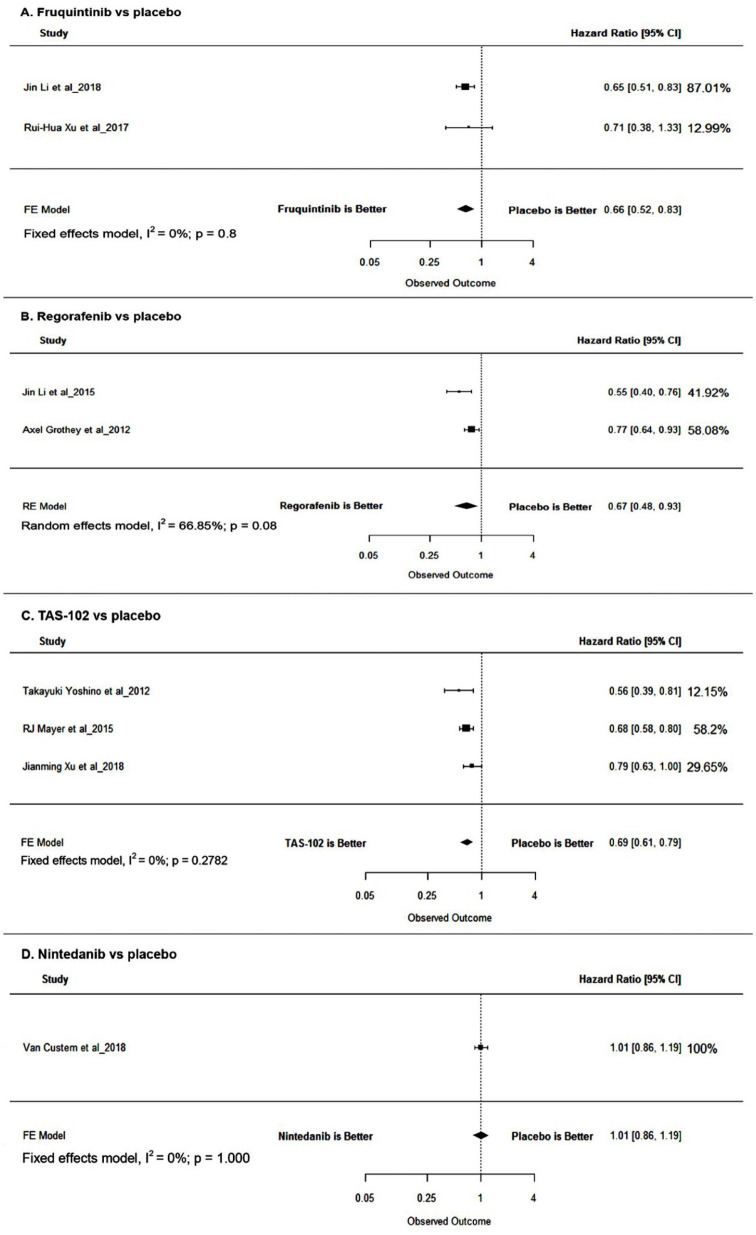
Pooled HRs for the pairwise direct comparisons were 0.67 (95% CI: 0.48–0.93, p = 0.0154), 0.66 (95% CI: 0.52–0.8, p = 0.0003), and 0.69 (95% CI: 0.61–0.79, p < 0.0001) for regorafenib, fruquintinib, and TAS-102, respectively, favoring the individual interventions. In case of nintedanib, no significant difference in OS (HR = 1.01; 95% CI: 0.86–1.19, p = 0.9044) was observed. Adjusted HR estimates were used for the analysis wherever available to rule out the difference in baseline parameters (see Figure 2 and Table 2).
Figure 2.
Forest plot for OS. From the pooled direct OS estimates, adjusted indirect comparison was performed, and it demonstrated statistically nonsignificant improvement in the OS for regorafenib in comparison with patients treated with TAS-102 (HR = 0.97; 95% CI: 0.68, 1.38, p = 0.88) and statistically significant improvement in those treated with nintedanib (HR = 0.66; 95% CI: 0.45, 0.95, p = 0.02). Regorafenib and fruquintinib had similar effect on the OS (HR = 1.01; 95% CI: 0.67, 1.52, p = 0.94).
CI, confidence interval; FE, fixed effects; HR, hazard ratio; OS, overall survival; RE, random effects.
Table 2.
Survival outcomes.
| Author | OS |
PFS |
Factors adjusted for | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median OS |
Unadjusted HR | Adjusted HR (95% CI) | Pairwise pooled HR (95% CI) | Median PFS |
Unadjusted HR | Adjusted HR (95% CI) | Pairwise pooled HR (95% CI) | ||||
| Placebo | Fruquintinib | Placebo | Fruquintinib | ||||||||
| Jin Li et al.17 | 6.57 | 9.3 | 0.71 | 0.65 (0.51–0.83) | 0.66 (0.52–0.8) | 1.84 | 3.71 | 0.50 | 0.26 (0.21–0.34) | 0.26 (0.21–0.33) | Prior use of VEGF inhibitor (yes versus no), K-Ras gene state (wild type versus mutant) |
| Rui-Hua Xu et al.18 | 5.52 | 7.72 | 0.71 (0.38–1.34) | 0.99 | 4.73 | 0.30 (95% CI 0.15–0.59) | |||||
| Placebo | Regorafenib | Placebo | Regorafenib | ||||||||
| Jin Li et al.19 | 6.3 | 8.8 | 0.72 | 0.57 (0.41–0.78) | 0.67 (0.48–0.93) | 1.7 | 3.2 | 0.53 | 0.31 (0.22–0.44) | 0.40 (0.26–0.63) | Baseline stratification factors including age, prior CT, previous therapy, and metastatic disease |
| Axel Grothey et al.20 | 5 | 6.4 | 0.78 | 0.78 (0.64–0.94) | 1.7 | 1.9 | 0.89 | 0.89 (0.42–0.58) | Baseline stratification factors including age, gender, previous anticancer treatment, previous treatment lines, and baseline ECOG score, among others | ||
| Placebo | TAS-102 | Placebo | TAS-102 | ||||||||
| Yoshino et al.21 | 6.6 | 9 | 0.73 | 0.56 (0.39–0.81) | 0.69 (0.61–0.79) | 1 | 2 | 0.5 | 0.41 (0.28–0.59) | 0.46 (0.40–0.52) | Baseline stratification factors |
| Mayer et al.22 | 5.3 | 7.1 | 0.75 | 0.68 (0.58–0.81) | 1.7 | 2 | 0.85 | 0.48 (0.41–0.57) | Baseline stratification factors | ||
| Jianming Xu et al.23 | 7.1 | 7.8 | 0.91 | 0.79 (0.62–0.99) | 1.8 | 2 | 0.9 | 0.43 (0.34–0.54) | Baseline stratification factors | ||
| Placebo | Nintedanib | Placebo | Nintedanib | ||||||||
| Van Custem et al.24 | 6 | 6.4 | 0.93 | 1.01 (0.86–1.19) | 1.01 (0.86–1.19) | 1.4 | 1.5 | 0.93 | 0.58 (0.49–0.69) | 0.58 (0.49–0.69) | Baseline stratification factors |
CI, confidence interval; CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; VEGF, vascular endothelial growth factor.
Progression-free survival
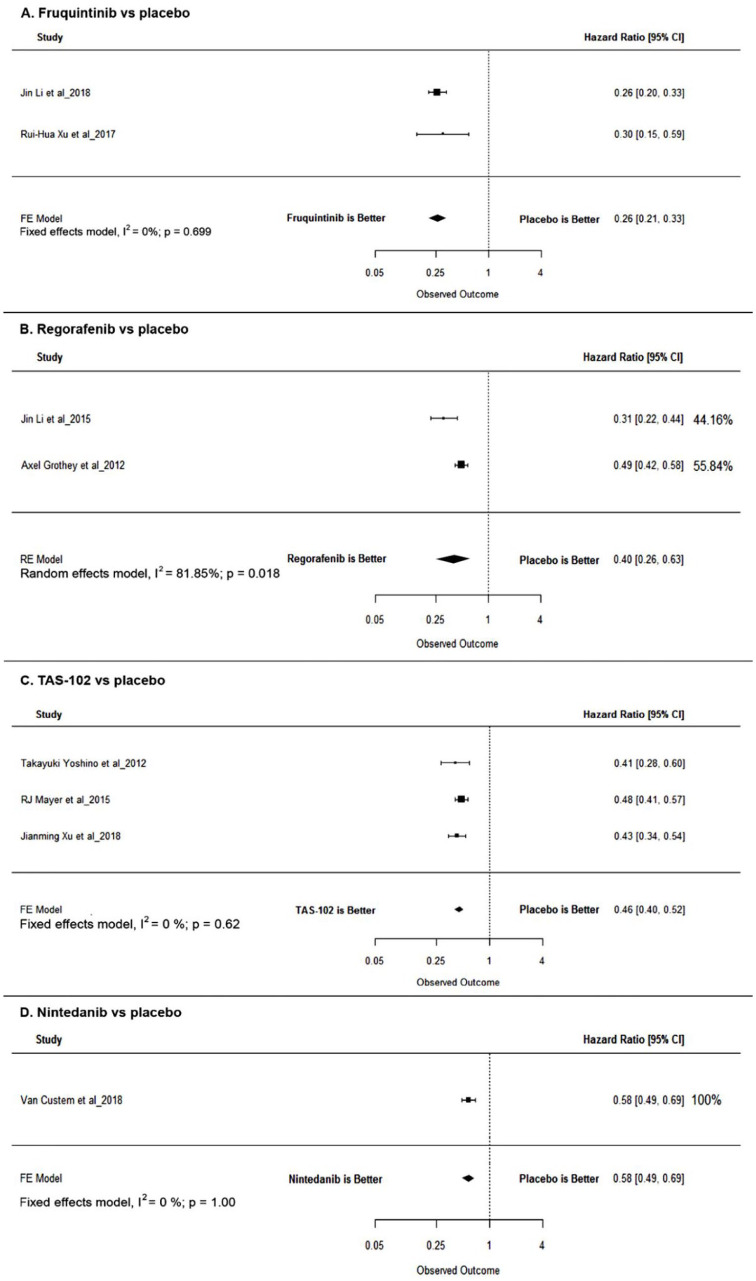
Direct pairwise analysis revealed the HRs for PFS to be 0.40 (95% CI: 0.26–0.63, p < 0.0001), 0.26 (95% CI: 0.21–0.33, p < 0.0001), 0.46 (95% CI: 0.40–0.52, p < 0.0001), and 0.58 (95% CI: 0.49–0.69, p < 0.0001) for regorafenib, fruquintinib, TAS-102, and nintedanib, respectively. Wherever available, HRs adjusted for baseline differences were used for the analysis (see Figure 3 and Table 2).
Figure 3.
Forest plots for PFS.
CI, confidence interval; FE, fixed effects; HR, hazard ratio; PFS, progression-free survival; RE, random effects.
The adjusted indirect comparison revealed statistically nonsignificant improvement in the PFS for regorafenib in comparison with that for TAS-102 (HR = 0.86; 95% CI: 0.54, 1.37, p = 0.5) and nintedanib (HR = 0.68; 95% CI: 0.42, 1.10, p = 0.12). Statistically nonsignificant improvement in the PFS was observed for fruquintinib in comparison with that for regorafenib (HR = 1.53; 95% CI: 0.93, 2.52, p = 0.08).
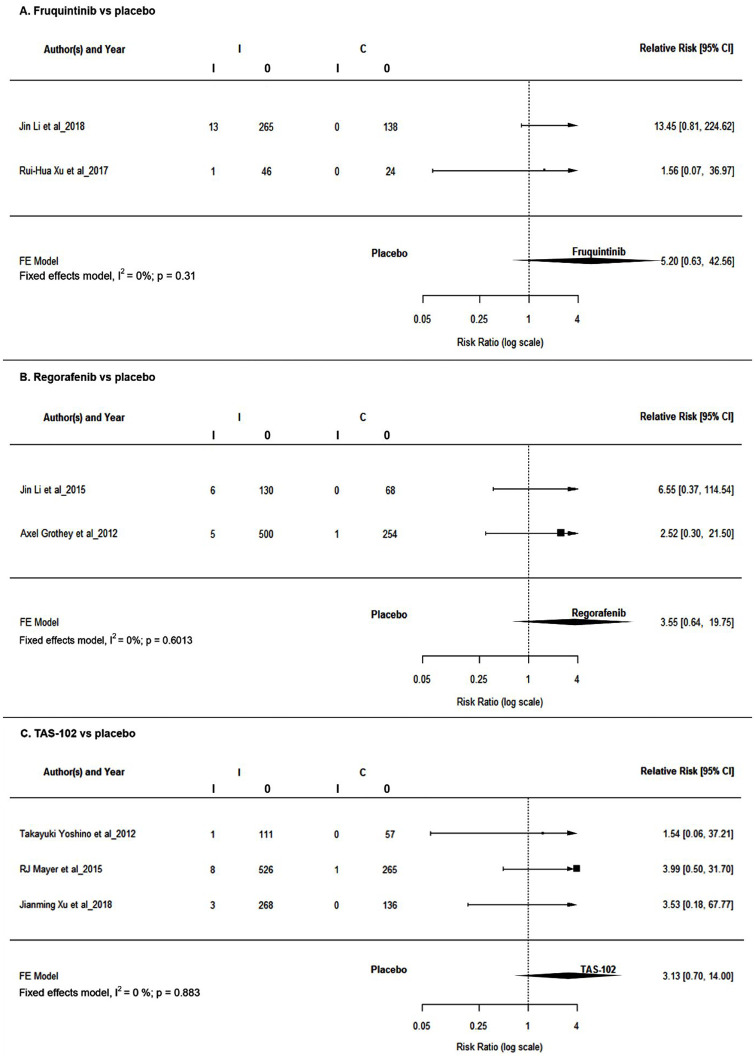
Response outcome—ORR
Tumor response was reported in trials evaluating regorafenib, fruquintinib, and TAS-102. In all the three third-line therapies, ORR was nonsignificantly better in the respective treatment arms (see Figure 4 and Table 3). Even though not significant, patients treated with regorafenib achieved better ORR in comparison with those treated with TAS-102 (RR = 1.13; 95% CI: 0.11, 11.05, p = 0.92) but was lower than that in those treated with fruquintinib (RR = 0.68269; 95% CI: 0.045, 10.32, p = 0.79) in the adjusted indirect comparison.
Figure 4.
Forest plots for tumor response rates.
CI, confidence interval; FE, fixed effects; HR, hazard ratio.
Table 3.
Spectrum of AEs in the different third-line therapies.
| Regorafenib, n (%) | Fruquintinib, n (%) | TAS-102, n (%) | Ninetedanib, n (%) | |
|---|---|---|---|---|
| Common AEs | 1. HFS: 416 (65.4) 2. Hypertension: 206 (32.38) 3. Hoarseness: 176 (28) 4. Hyperbilirubinaemia: 105 (16.5) 5. Liver enzyme increased: 64 (10) |
1. Hypertension: 168 (51.7) 2. HFS: 144 (44.30) 3. Proteinuria: 117 (36) 4. Dysphonia: 100 (30.7) 5. AST elevated: 65 (20) |
1. Anemia: 695 (76.2) 2. Leukopenia: 683 (75) 3. Neutropenia: 616 (67.54) 4. Nausea: 429 (47) 5. Liver enzyme increased: 392 (43) |
1. Liver enzyme increased: 192 (50) 2. Fatigue: 183 (47.7) 3. Diarrhea: 175 (45.6) 4. Nausea: 165 (43.0) 5. Vomiting: 151 (39.3) |
AE, adverse event; AST, aspartate aminotransferase; HFS, hand foot syndrome.
Safety analysis
The common AEs observed in all the third-line therapies included fatigue, diarrhea, and increased liver enzymes [alanine aminotransferase (ALT) and aspartate aminotransferase AST)]. Hypertension and hand foot syndrome (HFS) were commonly reported in patients treated with regorafenib and fruquintinib. Spectrum of AEs and the rate of their prevalence for the four third-line therapies are presented in Table 3. The prevalence of ⩾3 grade AEs was higer in patients treated with fruquintinib and TAS-102 (61% and 61.44%, respectively), whereas it was low in those treated with regorafenib and nintedanib (54% and 42%, respectively).
The adjusted indirect analysis of the RR for serious AEs revealed regorafenib to be better than fruquintinib (RR = 0.57; 95% CI: 0.3–1.02) and to be similar to TAS-102 (RR = 1.17; 95% CI: 0.91–1.55) and nintedanib (RR = 1.00; 95% CI: 0.78–1.3). The analysis also revealed regorafenib to be better than fruquintinib with respect to diarrhea (RR = 0.61; 95% CI: 0.194–1.91; p = 0.4). Regorafenib also had a better safety profile with respect to fatigue in comparison with TAS-102 (RR = 0.65; 95% CI: 0.31–1.35; p = 0.2580) and increased ALT in comparison with nintedanib (RR = 0.9; 95% CI: 0.33–2.41; p = 0.8551) (see Table 4).
Table 4.
Adjusted indirect comparison of AEs.
| Serious adverse events | ||
|---|---|---|
| Relative risk (95% CI) | p-value | |
| Regorafenib versus fruquintinib | 0.56 (0.31–1.02) | 0.059 |
| Regorafenib versus TAS-102 | 1.17 (0.91–1.51) | 0.19 |
| Regorafenib versus nintedanib | 1.00 (0.78–1.3) | 0.94 |
| Fatigue | ||
| Regorafenib versus fruquintinib | 1.02 (0.51–2.03) | 0.93 |
| Regorafenib versus TAS-102 | 0.65 (0.32–1.35) | 0.25 |
| Regorafenib versus nintedanib | 1.36 (1.05–1.75) | 0.01 |
| Diarrhea | ||
| Regorafenib versus fruquintinib | 0.61 (0.19–1.91) | 0.4 |
| Regorafenib versus TAS-102 | 1.84 (1.14–2.98) | 0.01 |
| Regorafenib versus nintedanib | 1.56 (0.98–2.49) | 0.06 |
| Increased aspartate aminotransferase | ||
| Regorafenib versus fruquintinib | 1.2 (0.45–3.21) | 0.72 |
| Regorafenib versus TAS-102 | 3.10 (.133–7.24) | 0.008 |
| Regorafenib versus nintedanib | 1.35 (0.56–3.27) | 0.5 |
| Increased alanine aminotransferase | ||
| Regorafenib versus fruquintinib | 1.54 (0.52–4.52) | 0.43 |
| Regorafenib versus TAS-102 | 3.63 (1.44–9.12) | 0.006 |
| Regorafenib versus nintedanib | 0.90 (0.33–2.41) | 0.85 |
AE, adverse event; CI, confidence interval.
Discussion
The selection of an optimum treatment regimen in mCRC beyond the second-line setting is a trade-off between modest efficacy and manageable AE.6 Driven by better outcome and increased therapeutic options across different lines of treatment in mCRC, there have been substantial improvements in survival and tumor response outcomes.5 Regorafenib and TAS-102 are the treatment options approved by the FDA, whereas fruquintinib has been approved in China.8,9,25 Nintedanib has also been evaluated in a recent phase III trial involving patients with mCRC,24 increasing the repertoire of treatment options available to clinicians. Unfortunately, the comparative efficacy of these drugs has not been evaluated in head-on clinical trials, which makes an adjusted indirect comparison the ideal option for evaluating the treatment options.
The homogeneity and transitivity assumptions that have no standard methods or thresholds to evaluate form the basis of indirect treatment comparisons.13 Among the four direct pairwise comparisons, there were no heterogeneities (I2 = 0) in all, except regorafenib versus placebo. Hence, a random effects model was used to address the issue. Although we cannot determine the precise source of heterogeneity in regorafenib trials, we presume that it could be due to the ethnicity of patients in the two trials (Asian versus mixed ethnicity). Furthermore, with respect to transitivity, all the included studies had the same study design and inclusion criteria. Although the patient characteristics were not identical in all the included studies, they were comparable across the treatment regimens as denoted by the baseline characteristics presented in Table 1. Therefore, the impact of potential effect modifiers may be negligible.
Among the different targets for metastatic solid tumors, tumor angiogenesis is a promising target because neovascularization is a key aspect of tumor metastasis.26–28 Continuous antiangiogenic therapy in which the targets of a drug molecule are spread across the angiogenesis pathway is considered to be a better option for the treatment of mCRC.29,30
The results of this adjusted indirect comparison revealed regorafenib to be better, in terms of efficacy in comparison to the FDA-approved TAS-102 and nintedanib. In terms of safety, regorafenib was non-inferior to TAS-102 and nintedanib. TAS-102 is a combination of α, α,α-trifluorothymidine (FTD) and 5-chloro-6-(2-iminopyrrolidin-1-yl) methyl-2,4 (1H,3H)-pyrimidinedione hydrochloride (TPI). FTD is incorporated during the DNA synthesis, which confers antitumor activity, and TPI is an inhibitor of thymidine phosphorylase, which could degrade FTD, thereby exerting synergy.31 TAS-102 has been evaluated in two trials involving Asian population and 1 trial involving patients of multiple ethnicity.21–23 Both the TERRA study for TAS-102 and the CONCUR study for regorafenib were performed involving Asian patients, with regorafenib providing a better OS in comparison with placebo (0.79 versus 0.55).19,23 Although 54% of the patients in the regorafenib arm in the CONCUR study experienced ⩾3 grade AE, the corresponding prevalence in the TERRA study was 46%. The incidence of treatment-emergent serious adverse event was 23% and 9% in the TERRA and the CONCUR studies, respectively, which suggests regorafenib to be better option in Asian patients. Owing to the difference in the mode of action, the spectrum of AEs is also markedly different for the 2 drugs, with fatigue, diarrhea, and changes in liver enzymes being the common AEs.
Fruquintinib is a selective small-molecule inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3, which was approved for third-line setting in the treatment of patients with mCRC in China.9,32 The approval was based on a randomized phase II trial and a large multicenter phase III trial conducted in China. In both trials, fruquintinib showed survival benefits in comparison with placebo.17,18 Both regorafenib and fruquintinib are potent inhibitors of angiogenesis. Although fruquintinib only inhibits VEGFR-1 to VEGFR-3, regorafenib additionally inhibits the fibroblast growth factor receptor, platelet-derived growth factor receptor-β, and angiopoietin receptors.33,34 From the “mechanism of action” perspective, the broader activity of regorafenib should lead to better efficacy when compared with fruquintinib. However, in our analysis, fruquintinib had better survival benefits in comparison with regorafenib (OS: HR = 1.01, 95% CI: 0.67, 1.52, p = 0.94; PFS: HR = 1.53, 95% CI: 0.93, 2.52, p = 0.08), which is, to a certain extent, confounding. Although the baseline characteristics were comparable across the included studies, there were a couple of anomalies with respect to the age of the patients recruited for the phase II and phase III fruquintinib trials. Although the median age was 57 and 61 years for the two trials evaluating regorafenib, it was 50 and 55 in the fruquintinib trials. Similarly, in the CONCUR trial, 30% of the patients in the treatment arm were aged ⩾65 years, whereas it was 18% in the FRESCO study.17 In general, patients aged ⩾65 years are not well represented in clinical trials,35 and hence, it is practically impossible to consider this as an effect modifier. But, on the contrary, patients aged ⩾65 years are more prone to mCRC,36 and, hence, we infer that the better representation of patients aged ⩾65 years in the CONCUR study and the lack thereof in FRESCO study might have skewed the analysis in favor of fruquintinib, which may not be the case in real-world settings. This is substantiated by the subgroup analysis of patients aged ⩾65 and <65 years in the CORRECT trial (OS: 0.86 versus 0.72; PFS: 0.65 versus 0.42) and the FRESCO trial (OS: 0.95 versus 0.56; PFS: 0.33 versus 0.26).
Fruquintinib, owing to the selective inhibition of VEGFRs, was expected to have a limited off-target toxicity in comparison with regorafenib.32 However, the RR of SAEs indicated regorafenib to be better than fruquintinib (RR = 0.56, 95% CI: 0.31–1.02), despite a higher proportion of patients in the regorafenib trials being aged ⩾65 years. Owing to the similar mechanism of action, the AE profiles were also similar, but there was no statistically significant difference in the rates of occurrence of AEs between regorafenib and fruquintinib. The efficacy and safety of regorafenib versus fruquintinib, when considered together, marginally favors regorafenib. However, we strongly feel that, in real-world settings, regorafenib might provide substantial clinical benefits when compared with fruquintinib, which requires prospective head-on comparative studies.
The main objective of third-line treatment in mCRC is to prolong the survival with manageable AEs.6 A previous meta-analysis by Xie et al. on the management of AEs in patients treated with regorafenib concluded that the AEs are more common with an initial dose of 160 mg.37 The study also concluded that an initial dose of 120 mg might be a better option for treating patients with mCRC.37 Furthermore, in a recent RCT, a dose escalation strategy for regorafenib compared with standard dosing regimen concluded that dose escalation strategy could be a viable alternative to standard dosing to control AEs.38 Further, identification of early markers of therapeutic response may also minimize toxicity in patients who are unlikely to respond. Currently, objective response to anti-cancer drugs is evaluated by the RECIST criteria, which takes into account only tumor size. Assessing efficacy by tumor size may also take substantial time before identifying non-responders. In a recent phase II study by Khan et al., dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was assessed as a tool for predicting clinical response. The results of the study suggested a combination of DCE-MRI and circulating tumor DNA to predict clinical response to regorafenib. Hence usage of imaging biomarkers, which also assess tumor density and vasculature, in future clinical trials with regorafenib might further enhance efficacy.39,40
The adjusted indirect comparison uses the summation of the individual variances from direct comparison, and, hence, arriving at a statistically significant treatment effect requires high number of studies with unambiguous results. Nevertheless, the results of our analysis, with the available RCT evidence, indicate regorafenib to be a better option for beyond second-line treatment of mCRC. But the results need to be interpreted with caution owing to the potential limitations of the study. The main limitation stems from the fact that an adjusted indirect comparison is not a substitute for head-on trials. Also, despite comparable effect modifiers, the heterogeneity of regorafenib trials was >50%, which required a mixed effects model for analysis. This highlights the existence of effect modifiers that were not accounted for in the individual trials. But this limitation pertains to the nature of adjusted indirect comparisons that are performed only in the absence of direct evidence. Nonetheless, our study also has some strengths. This is the first study to synthesize the available evidence for beyond second-line treatment of mCRC with an adjusted indirect comparison. Moreover, the studies included for analysis were also selected for controlling for potential biases arising because of the variation in effect modifiers.
To conclude, among the beyond second-line treatment options for mCRC, regorafenib has efficacy similar to that of TAS-102 and better safety when compared with fruquintinib. Considering the mechanism of action of regorafenib, which targets multiple factors in the angiogenic pathway, it could be an ideal option for the treatment in the beyond second-line setting. Nevertheless, well-designed head-on studies are required to substantiate our results.
Supplemental Material
Supplemental material, Supplemental_table_search_strategy_in_Pubmed_Regorafenib for Efficacy and safety of regorafenib as beyond second-line therapy in patients with metastatic colorectal cancer: an adjusted indirect meta-analysis and systematic review by Yinying Wu, Yangwei Fan, Danfeng Dong, Xuyuan Dong, Yuan Hu, Yu Shi, Jiayu Jing and Enxiao Li in Therapeutic Advances in Medical Oncology
Acknowledgments
Medical writing and analysis support was provided by Dr. G. Kaushik Subramanian and Dr. Amit Bhat, Indegene Pvt Ltd, Bangalore, India, under the guidance from the authors.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Enxiao Li  https://orcid.org/0000-0003-3909-211X
https://orcid.org/0000-0003-3909-211X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yinying Wu, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Yangwei Fan, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Danfeng Dong, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Xuyuan Dong, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Yuan Hu, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Yu Shi, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Jiayu Jing, Department of Medical Oncology, Xi’an Jiaotong University, Xi’an, Shaanxi, China.
Enxiao Li, Department of Medical Oncology, Xi’an Jiaotong University, 277 Yanta West Road, Xi’an, Shaanxi 710061, China.
Reference
- 1. Colorectal cancer statistics [Internet]. World cancer research fund, https://www.wcrf.org/dietandcancer/cancer-trends/colorectal-cancer-statistics (2018, accessed 13 June 2019).
- 2. Cancer.Net. Colorectal cancer - stages [Internet], https://www.cancer.net/cancer-types/colorectal-cancer/stages (2012, accessed 13 June 2019).
- 3. Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. World J Gastroenterol 2015; 21: 11767–11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crooke H, Kobayashi M, Mitchell B, et al. Estimating 1- and 5-year relative survival trends in colorectal cancer (CRC) in the United States: 2004 to 2014. J Clin Oncol 2018; 36(Suppl. 4): 587–587. [Google Scholar]
- 5. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 6. Bekaii-Saab T, Kim R, Kim TW, et al. Third- or later-line therapy for metastatic colorectal cancer: reviewing best practice. Clin Colorectal Cancer 2019; 18: e117–e129. [DOI] [PubMed] [Google Scholar]
- 7. Drugs.com. Stivarga (regorafenib) FDA approval history [Internet], https://www.drugs.com/history/stivarga.html (accessed 13 June 2019).
- 8. Marcus L, Lemery SJ, Khasar S, et al. FDA approval summary: TAS-102. Clin Cancer Res 2017; 23: 2924–2927. [DOI] [PubMed] [Google Scholar]
- 9. Shirley M. Fruquintinib: first global approval. Drugs 2018; 78: 1757–1761. [DOI] [PubMed] [Google Scholar]
- 10. O’Connor JM, Ducreux M, Petersen LN, et al. Real-world dosing of regorafenib (REG) in metastatic colorectal cancer (mCRC): final results from the prospective, observational CORRELATE study. Ann Oncol 2018; 29(Suppl. 8): Abstract 463P. [Google Scholar]
- 11. Petrioli R, Chirra M, Messuti L, et al. Efficacy and safety of regorafenib with 2/1 schedule for patients ⩾75 years with metastatic colorectal cancer (mCRC) after failure of 2 lines of chemotherapy. Clin Colorectal Cancer 2018: 17: 307–312. [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi K, Komatsu Y, Satoh T, et al. Large-scale, prospective observational study of regorafenib in Japanese patients with metastatic colorectal cancer in a real-world clinical setting. Oncologist 2019; 24: e450–e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiefer C, Sturtz S, Bender R. Indirect comparisons and network meta-analyses. Dtsch Ärztebl Int 2015; 112: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Indirect comparisons: methods and validity [Internet]. haute autorité de santé, https://www.has-sante.fr/ (2009, accessed 13 June 2019)
- 15. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–691. [DOI] [PubMed] [Google Scholar]
- 17. Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA 2018; 319: 2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu RH, Li J, Bai Y, et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase Ib study and a randomized double-blind phase II study. J Hematol Oncol 2017; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16: 619–629. [DOI] [PubMed] [Google Scholar]
- 20. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Lond Engl 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 21. Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012; 13: 993–1001. [DOI] [PubMed] [Google Scholar]
- 22. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015; 372: 1909–1919. [DOI] [PubMed] [Google Scholar]
- 23. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol 2018; 36: 350–358. [DOI] [PubMed] [Google Scholar]
- 24. Van Cutsem E, Yoshino T, Lenz HJ, et al. Nintedanib for the treatment of patients with refractory metastatic colorectal cancer (LUME-Colon 1): a phase III, international, randomized, placebo-controlled study. Ann Oncol 2018; 29: 1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. FDA approves regorafenib (Stivarga) for metastatic colorectal cancer. Oncology (Williston Park) 2012; 26: 896. [PubMed] [Google Scholar]
- 26. Massagué J, Obenauf AC. Metastatic colonization. Nature 2016; 529: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marmé D. Tumor angiogenesis: a key target for cancer therapy. Oncol Res Treat 2018; 41: 164–164. [DOI] [PubMed] [Google Scholar]
- 28. Weis SM, Cheresh DA. A wake-up call for hibernating tumour cells. Nat Cell Biol 2013; 15: 721–723. [DOI] [PubMed] [Google Scholar]
- 29. Hayashi H, Arao T, Matsumoto K, et al. Biomarkers of reactive resistance and early disease progression during chemotherapy plus bevacizumab treatment for colorectal carcinoma. Oncotarget 2014; 5: 2588–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 2016; 15: 385–403. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka N, Sakamoto K, Okabe H, et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 2014; 32: 2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Q, Zhou J, Zhang Z, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther 2014; 15: 1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer 2012; 106: 1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011; 129: 245–255. [DOI] [PubMed] [Google Scholar]
- 35. Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003; 21: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 36. Tailoring treatment for older adults with colorectal cancer - the ASCO post [Internet], https://www.ascopost.com/issues/february-25-2017/tailoring-treatment-for-older-adults-with-colorectal-cancer/ (accessed 12 June 2019)
- 37. Xie G, Gong Y, Wu S, et al. Meta-analysis of regorafenib-associated adverse events and their management in colorectal and gastrointestinal stromal cancers. Adv Ther 2019; 36: 1986–1998. [DOI] [PubMed] [Google Scholar]
- 38. Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 2019; 20: 1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan K, Rata M, Cunningham D, et al. Functional imaging and circulating biomarkers of response to regorafenib in treatment-refractory metastatic colorectal cancer patients in a prospective phase II study. Gut 2018; 67: 1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khan K, Cascinu S, Cunningham D, et al. Imaging and clinical correlates with regorafenib in metastatic colorectal cancer. Cancer Treat Rev 2020; 86: 102020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_table_search_strategy_in_Pubmed_Regorafenib for Efficacy and safety of regorafenib as beyond second-line therapy in patients with metastatic colorectal cancer: an adjusted indirect meta-analysis and systematic review by Yinying Wu, Yangwei Fan, Danfeng Dong, Xuyuan Dong, Yuan Hu, Yu Shi, Jiayu Jing and Enxiao Li in Therapeutic Advances in Medical Oncology