Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040.1
Keywords: pathology competencies, diagnostic medicine, transfusion medicine, concepts of blood transfusion, transfusion reactions, febrile transfusion reactions
Primary Objective
Objective TM1.2: Transfusion Reactions. Compare and contrast the pathophysiology, presentations, prophylaxis, and acute management of the different types of transfusion reactions.
Competency 3: Diagnostic Medicine and Therapeutic Pathology; Topic TM: Transfusion Medicine; Learning Goal 1: Concepts of Blood Transfusion.
Secondary Objective
Objective IM1.3: Cytokines. Discuss, with examples, the production of different cytokines by different immune cells, the roles that cytokines play in effecting the immune response, and how knowledge of cytokine action can be exploited in the treatment of disease.
Competency 1: Disease Mechanisms and Processes; Topic IM: Immunological Mechanisms; Learning Goal 1: Immune Dysfunction
Patient Presentation
A 35-year-old woman presents to the emergency department complaining of lightheadedness and dizziness. She reports a history of menometrorrhagia and that she had been diagnosed with uterine fibroids 6 months ago, which has been managed medically with oral contraceptives. She does not take any other medications, including supplements. Review of systems is positive for fatigue and restless legs. She says her appetite has been suboptimal but does not complain of dysphagia. On physical examination, she appears pale and has dry skin. There is conjunctival pallor. The tongue appears moist, mildly atrophic, and smooth with no ulceration. There is mild angular cheilitis and koilonychia. The patient’s blood pressure and heart rate lying down were 108/75 mmHg and 67 beats per minute, and after 5 minutes, her standing blood pressure and heart rate were 105/76 mmHg and 73 beats per minute.
A metabolic panel including electrolytes, glucose, calcium, serum urea nitrogen, and creatinine was within normal limits. Beta-human chorionic gonadotropin was undetectable. A complete blood count and iron studies are summarized in Tables 1 and 2.
Table 1.
Complete Blood Count.
| Laboratory test | Result | Reference interval |
|---|---|---|
| White blood cell count (cells × 103/μL) | 5.2 | 3.7-11.0 |
| Red blood cell count (cells × 106/μL) | 3.52 | 4.50-6.10 |
| Hemoglobin (g/dL) | 6.5 | 13.4-17.5 |
| Hematocrit (%) | 18.5 | 38.9-52.0 |
| Mean corpuscular volume (fL) | 74.1 | 78.0-100.0 |
| Mean corpuscular hemoglobin (pg) | 22.6 | 26.0-32.0 |
| Mean corpuscular hemoglobin concentration (g/dL) | 29.6 | 31.0-35.5 |
| Red cell distribution width (%) | 18.9 | 11.5-15.5 |
| Platelet count (cells × 103/μL) | 450 | 150-400 |
Table 2.
Iron Studies.
| Laboratory test | Result | Reference interval |
|---|---|---|
| Ferritin (ng/mL) | 10 | 20-300 |
| Iron (μg/dL) | 20 | 55-175 |
| Iron-binding capacity (μg/dL) | 410 | 210-330 |
| Iron saturation (%) | 6 | 20-50 |
| Transferrin (mg/dL) | 360 | 160-340 |
Given the patient’s symptomatic anemia, the attending physician ordered a unit of red blood cells (RBCs) to be transfused. Thirty minutes into the transfusion, the patient developed chills.
Diagnostic Findings
The patient’s vital signs before the transfusion and when the patient began to experience chills are summarized in Table 3. The patient reports no other new or worsening clinical symptoms.
Table 3.
Vital Signs Before Transfusion and After Onset of Symptoms.
| Vital signs | Pretransfusion | After symptoms |
|---|---|---|
| Temperature (°C) | 36.6 | 38.2 |
| Blood pressure | 90/75 | 105/85 |
| Heart rate (beats per minute) | 75 | 77 |
| Respiratory rate (breaths per minute) | 8 | 10 |
| Oxygen saturation (%) | 100, room air | 99, room air |
Question/Discussion Points
What Is the Clinical Concern at This Point?
Given the onset of fever and chills within a short period of time after starting a blood transfusion, the possibility of a transfusion reaction must be considered. Her other physical examination findings and review of systems noted upon presentation are likely chronic in nature and noncontributory to her sudden change in clinical status.
What Is the First Step in Managing a Patient with a Suspected Transfusion Reaction?
Stop the transfusion!
What Blood Product Attributes Can Mechanistically Invoke a Transfusion Reaction?
Anything that can be found circulating in the bloodstream may be collected as part of a blood donation. Blood donations are either autologous or allogeneic depending on whether the product is intended to be transfused back to the donor (autologous) or to another person (allogeneic). All blood products are stored in a bag that contains anticoagulant (citrate) and preservative nutrients such as citric acid, dextrose, and phosphate.2 In the setting of hemorrhagic shock resuscitation with blood component therapy, a significant transfusion of citrate can lead to hypocalcemia and metabolic alkalosis.3 Over time, cellular blood components (RBCs and platelets) develop biochemically altering storage lesions that can adversely affect transfusion recipients through a variety of complex pathophysiologic mechanisms and clinical sequelae.4,5 They may express antigens that are foreign to the recipient, which can result in their destruction where the recipient has corresponding alloantibodies. They may also contain plasma proteins and white blood cells that contribute to inflammation despite leukoreduction, where the maximum total number of leukocytes must be less than 5 × 106 cells.2 Regarding specifically RBCs, some alloantibodies to ABO blood group and non-ABO antigens have the potential to elicit acute or delayed hemolytic transfusion reactions and/or hemolytic disease of the fetus and newborn.6 Transfusion reactions may be classified as acute (less than 24 hours) or delayed (after 24 hours) depending on their onset relative to the inciting transfusion (Figure 1). Furthermore, most transfusion reactions are associated with the host’s immune system responding innately (acute) or adaptively (delayed).
Figure 1.
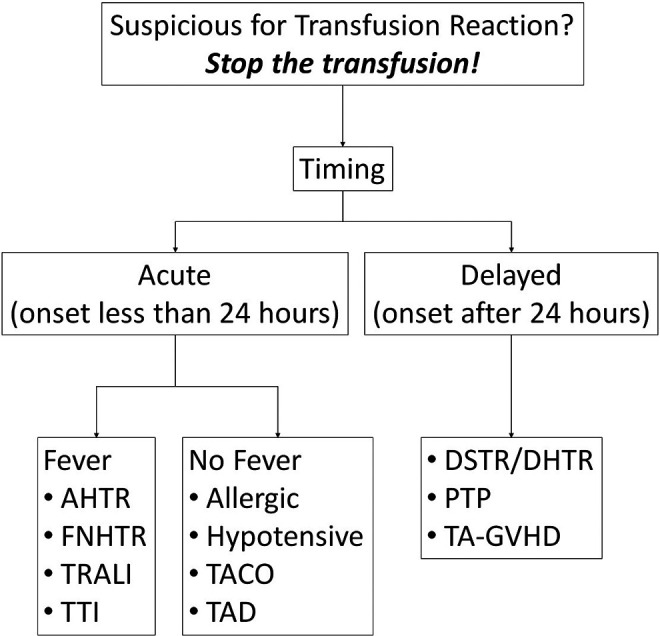
Clinical differential diagnosis of transfusion reactions. AHTR indicates acute hemolytic transfusion reaction; DSTR/DHTR, delayed serologic/hemolytic transfusion reaction; FNHTR, febrile non-hemolytic transfusion reaction; PTP, post-transfusion purpura; TA-GVHD, transfusion-associated graft-versus-host disease TACO, transfusion-associated circulatory overload; TAD, transfusion-associated dyspnea; TRALI, transfusion-related acute lung injury; TTI, transfusion-transmitted infection.
What Is the Workup for a Suspected Transfusion Reaction?
The workup for a suspected transfusion reaction includes 2 parts: clinical and laboratory. First, assess the patient clinically for concerning signs and symptoms of unstableness such as chest pain, dyspnea, back pain, and so on, depending on the specific reaction. The timing of a reaction relative to the transfusion is critical as it can be immediate or delayed (Figure 1), depending on the pathophysiologic response. Delayed reactions are often mediated by donor and/or recipient alloantibodies, which require days to produce. Acute reactions are often mediated by preformed antibodies.
A consultation to a transfusion service and/or blood bank is warranted in order to perform the necessary laboratory workup, which includes 3 steps. The first step is a clerical check to ensure there is no discrepancy between patient information and blood product labeling. The second step is visual inspection of the patient’s plasma to assess for icterus and hemolysis. Normal plasma appears straw colored. Icteric samples with increased bilirubin exhibit dark or bright yellow plasma. Hemolyzed samples suggest RBC destruction as evidenced by free hemoglobin, which gives a pale to cherry red color to the plasma. Icteric and hemolyzed samples can potentially interfere with laboratory testing in a number of ways. For example, many laboratory assays, such as for bilirubin and for hemoglobin, utilize spectrophotometry. Analyzers can flag samples as icteric or hemolyzed if the sample absorption exceeds a permitted threshold for a given analyte.7 Hemolyzed specimens further complicate test results as a result of a leakage in RBC contents that are also found (and measured) in plasma. For hemolyzed specimens, analytes that have a higher concentration in the red cell matrix compared to plasma (eg, potassium, aspartate aminotransferase, and folate) would artificially elevate the true analyte concentration, whereas analytes that have a lower concentration in the red cell matrix compared to plasma (eg, calcium and sodium) would dilute and artificially decrease the true analyte concentration.7 The third step is the direct antiglobulin test (DAT). The DAT incorporates antihuman globulin (“Coombs”) reagent in detecting sensitized RBCs by binding complement C3 and/or the Fc portion of immunoglobulin (IgG). A positive DAT result is visually characterized by agglutination in vitro when the test is performed in a test tube.
What Is the Differential Diagnosis of a Suspected Transfusion Reaction Causing Fever and Chills?
The differential diagnosis of fever attributable to a transfused blood product includes febrile nonhemolytic transfusion reaction (FNHTR), transfusion-transmitted infection (TTI), transfusion-associated acute lung injury (TRALI), and acute hemolytic transfusion reaction (AHTR).
The Differential Diagnosis of Fever and Chills Is Broad. What Else Can Cause These Symptoms and Why Is a Transfusion Reaction Favored?
The differential diagnosis of fever (and chills) in the adult population includes infection, malignancy, inflammatory disorders such as rheumatoid arthritis, and other miscellaneous conditions.8 There is no other evidence in the clinical history to suggest any of these conditions, though a thorough workup could include blood cultures and focused laboratory and imaging studies.
It is important to recognize the underlying problem as iron deficiency anemia secondary to menometrorrhagia (Tables 1 and 2) because iron deficiency is frequently associated with chronic inflammatory disease. In chronic inflammation, elevated levels of cytokines such as interleukin 6 enhance hepcidin and degrade its associated transmembrane receptor, ferroportin, resulting in decreased circulating levels of iron.9,10 This is more of a functional iron deficiency whereby iron remains sequestered within the reticuloendothelial system as opposed to more rarely observed absolute iron deficiency whereby overall iron storage and availability is reduced.
How Do Cytokines Result in Fever and Chills?
Fever and chills are a clinical manifestation of cytokine production in the setting of (acute) inflammation. Endogenous pyrogens including tumor necrosis factor and interleukins 1 and 6 indirectly reset the core thermoregulatory center in the hypothalamus to a higher temperature; the body then responds with chills because the original set point is now perceived as cold compared to its new temperature setting.11,12 Many circulating blood leukocytes such as monocytes, lymphocytes, and natural killer cells as well as blood vessel-lining endothelial cells contribute to the production of endogenous pyrogens.
What Are The Pathophysiologies of Febrile Nonhemolytic Transfusion Reaction, Transfusion-Transmitted Infection, Transfusion-Associated Acute Lung Injury, and Acute Hemolytic Transfusion Reaction? How Do These Reactions Lead to the Production of Endogenous Pyrogens?
Although the pathophysiological mechanisms underlying transfusion reactions are highly variable,13 transfused foreign and/or toxic substances in blood products can stimulate an innate immune/inflammatory response mediated by endothelial cells and circulating leukocytes. A byproduct of this response often includes the generation of endogenous pyrogens.
Febrile nonhemolytic transfusion reaction
The transfusion of any blood product can elicit a febrile reaction, which is one of the most prevalent transfusion reactions.13 Febrile reactions may be caused by increased leukocyte production of cytokines as a manifestation of a storage lesion.14 They may also be caused by incompatible donor antibodies recognizing recipient antigens as foreign.14 Given the major role leukocytes play in the pathophysiology of FNHTR removing them from blood products, leukoreduction has been shown to reduce the risk of febrile reactions.13
Transfusion-transmitted infection
Pathogenic organisms are prone to surviving in different temperatures and nutritional environments catered by various blood products. They produce pathogen-associated molecular patterns through a variety of complex mechanisms culminating in septic shock and potentially death (see summary Figure 4-20 in Kumar et al).11 Given that RBC products are stored at 1 °C to 6 °C, they can harbor organisms such as Yersinia enterocolitica, which thrive in cold temperatures.15 On the other hand, platelets are stored at room temperature and are particularly susceptible to bacterial contamination from organisms such as Staphylococcus epidermidis, which is part of normal skin flora.15 In order to donate blood, the skin must be punctured in order to access a vein. A diversion pouch, which siphons the first 30 mL of blood away from the collection bag, has been shown to reduce the risk of bacterial contamination.16
Transfusion-associated acute lung injury
This is classically characterized as a sequela of “2 hits.”17 The first hit underscores the patient’s underlying hyperinflammatory clinical condition, which primes leukocytes and endothelial cells. The second hit occurs as a result of a transfusion of direct antibody-mediated (eg, antibodies to human neutrophil antigens and class I major histocompatibility [MHC] antigens), indirect antibody-mediated (eg, monocytes and antibodies to class II MHC antigens), and antibody-independent (eg, CD40 ligand) mechanisms culminating in neutrophil activation and extravasation into the lungs.17 This is characterized histologically by diffuse alveolar damage and clinically as acute respiratory distress syndrome. Antibodies to MHC antigens are of particular frequency and concern in women who may have been knowingly or unknowingly exposed to corresponding foreign MHC antigens expressed by a fetus. Manufacturing plasma-containing blood products from males, females who have never been pregnant, or females who have tested negative for MHC antibodies has been a successful TRALI mitigation strategy and remains standard practice.18,19 Recently, TRALI has been redefined to distinguish cases attributable to transfusion (type I) from cases attributable to underlying or concurrent risk factors for acute respiratory distress syndrome (type II) that often are concomitant with hyperinflammatory states such as pneumonia and pancreatitis.20
Acute hemolytic transfusion reaction
These reactions occur as a result of preformed recipient alloantibodies eliciting a severe immunologic response against corresponding transfused donor RBC antigens such as ABO blood group antigens. Take for example donor RBCs demonstrating A-blood group antigen transfused to a recipient whose blood type is group O. The recipient has circulating preformed alloantibodies to A-antigen (allo-anti-A) that recognize the A-antigen on transfused RBCs as foreign. Allo-anti-A is predominantly IgM in nature and briskly fixes complement to the RBC membrane.6 This initiates a chain reaction mediated by the complement system that terminates in the production of complement C5-C9 membrane attack complex, culminating in the lysis of RBCs. Note that during this process, activated complement proteins C3a and C5a (anaphylatoxins) contribute to a shock-like response by recruiting and activating endogenous pyrogen-producing leukocytes as well as histamine-producing basophils and platelets, which promote vasodilation and vascular permeability vis-à-vis histamine H1 receptor on microvascular endothelial cells.11
What Are the Diagnostic Criteria and Management Opportunities for Patients Who Present With Transfusion Reactions That Cause Fever and Chills?
The Centers for Disease Control and Prevention National Healthcare Safety Network Hemovigilance Module defines transfusion reactions in the Biovigilance Component Protocol.21 The most common initial presentation of AHTR includes fever and/or chills,13 though the reaction typically occurs within minutes after receiving an ABO or non-ABO incompatible blood product. Additional signs and symptoms due to intravascular RBC hemolysis include back or flank pain, disseminated intravascular coagulation, epistaxis, hematuria, hypotension, oliguria/anuria, pain and/or oozing at intravenous site(s), and renal failure. At least 2 of the following laboratory studies must be present: decreased fibrinogen, decreased haptoglobin, elevated bilirubin, elevated lactate dehydrogenase, hemoglobinemia, hemoglobinuria, plasma discoloration consistent with hemolysis, or spherocytes on blood film. For immune-mediated hemolysis, a positive DAT for anti-IgG or anti-C3 is also required in addition to a positive elution test with alloantibody present on the transfused RBCs. Acute hemolytic transfusion reaction occurs during or within 24 hours of cessation of transfusion. The management of AHTR is largely supportive.
Sepsis can occur relatively rapidly after transfusion of a blood product contaminated with a pathogenic organism (TTI). In order to definitively prove TTI, the inflicting organism must be identified in the blood donor (at the time of donation) or donated blood product and newly identified in the transfusion recipient with evidence that the strains are related by molecular or extended phenotypic comparison testing with statistical confidence (P < .05). Management of TTI includes appropriate antimicrobial therapy in addition to supportive care where clinically indicated.
Patients with TRALI become severely hypoxic and exhibit radiographic evidence of pulmonary edema. Importantly, there is no evidence of left atrial hypertension (ie, circulatory overload) so as to distinguish from the transfusion reaction transfusion-associated circulatory overload. The onset of TRALI is during or within 6 hours of cessation of transfusion. Management is largely supportive. Diuretics are not helpful and in fact may potentiate hypotension.18 The data on corticosteroids which are used to suppress the immune system are equivocal with respect to efficacy and survival outcomes.18
By definition, FNHTR occurs during or within 4 hours of cessation of transfusion and either fever (greater than or equal to 38 °C/100.4 °F oral and a change of at least 1 °C /1.8 °F) or chills/rigors are present. This is a diagnosis of exclusion.13 For fever, acetaminophen (an antipyretic) is typically used. For rigors, meperidine has been used.22
It is important to remember that some patients may already be febrile or chilled before receiving blood products. Exhibiting these symptoms is not a contraindication for blood transfusion.
What Transfusion Reaction Did This Patient Have?
The patient meets the diagnostic criteria for FNHTR. There are no other clinical or laboratory signs and symptoms to suggest an alternative transfusion reaction.
Is This Patient at Risk for a Subsequent Transfusion Reaction?
The risk of FNHTR is 1 to 3 per 100 transfusions.13 Although patients can experience more than one transfusion reaction in their lifetime, they are not necessarily at increased risk. This, in part, is due to the fact that usually a single product may be responsible for the reaction and no 2 products are the same. Premedicating patients with acetaminophen can be considered to prevent recurrent FNHTRs, though there is no strong evidence supporting this practice.23
Patient Follow-Up
Once the patient developed a fever and a transfusion reaction was suspected, the transfusion was stopped immediately. The blood product and tubing were submitted to the laboratory for a transfusion reaction workup. The clerical check was without error. A posttransfusion sample of patient blood revealed no hemolysis and no icterus. The DAT was negative. The patient was given acetaminophen 325 mg orally, shortly after which point her temperature subsided to 36.9 °C. The patient continued to exhibit symptomatic anemia. The clinical team requested another unit of RBCs, which was reviewed and approved by the transfusion medicine service. This unit was transfused to the patient in its entirety. The patient tolerated the transfusion without any complications.
Teaching Points
The first step in managing a suspected transfusion reaction is to stop the transfusion!
The blood bank performs a transfusion reaction workup to include clerical check, visual inspection, and DAT.
Transfusion reactions can be classified as either acute or delayed, depending on whether the inciting transfusion occurred before or after 24 hours.
Febrile and allergic transfusion reactions are the most common transfusion reactions.
Different transfusion reactions can present with similar pathophysiologic mechanisms involving cytokines, resulting in fever and chills. These include FNHTR, TTI, TRALI, and AHTR. Additional clinical signs and symptoms and laboratory studies can help to further distinguish one transfusion reaction from another.
Tumor necrosis factor, interleukin 1, and interleukin 6 are endogenous pyrogens that affect body temperature.
Correlate signs and symptoms suspicious for a transfusion reaction in the context of the patient’s underlying clinical history.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aaron D. Shmookler  https://orcid.org/0000-0001-7789-7184
https://orcid.org/0000-0001-7789-7184
References
- 1. Knollmann Ritschel BEC, Regula DP, Borowitz MJ, Conran R, Prystowsky MB. Pathology competencies for medical education and educational cases. Acad Pathol. 2017;4 doi:10.1177/2374289517715040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fung MK, Eder A, Spitalnik SL, Westhoff CM, eds. Technical Manual. 19th ed AABB; 2017. [Google Scholar]
- 3. Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137:209–220. doi:10.1378/CHEST.09-0252 [DOI] [PubMed] [Google Scholar]
- 4. Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. doi:10.2450/2019.0217-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng MSY, Tung JP, Fraser JF. Platelet storage lesions: what more do we know now? Transfus Med Rev. 2018;32:144–154. doi:10.1016/j.tmrv.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 6. Reid M, Francis CL, Olsson M. The Blood Group Antigen FactsBook. 3rd ed Academic Press; 2012. [Google Scholar]
- 7. Bailey DN, Fitzgerald RL. Clinical Chemistry: Practical Laboratory Diagnosis of Disease. American Society for Clinical Pathology Press; 2017. [Google Scholar]
- 8. Cunha BA, Lortholary O, Cunha CB. Fever of unknown origin: a clinical approach. Am J Med. 2015;128:1138.e1-1138.e15 doi:10.1016/j.amjmed.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 9. Cappellini MD, Colet JC, de Francisco A, et al. Iron deficiency across chronic inflammatory conditions: international expert opinion on definition, diagnosis, and management. Am J Hematol. 2017;92:1068–1078. doi:10.1002/ajh.24820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31:225–233. doi:10.1016/j.blre.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 11. Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed Saunders Elsevier; 2015. [Google Scholar]
- 12. Hall JE. Guyton and Hall Textbook of Medical Physiology. 13th ed Saunders Elsevier; 2016. [Google Scholar]
- 13. Delaney M, Wendel S, Bercovitz RS, et al. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388:2825–2836. doi:10.1016/S0140-6736(15)01313-6 [DOI] [PubMed] [Google Scholar]
- 14. Heddle NM. Pathophysiology of febrile nonhemolytic transfusion reactions. Curr Opin Hematol. 1999;6:420–426. [DOI] [PubMed] [Google Scholar]
- 15. Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18:195–204. doi:10.1128/CMR.18.1.195-204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee CK, Wong HK, Ho PL, et al. Significant bacterial contamination risk reduction with the use of diversion pouch. Transfus Med. 2012;22:404–408. doi:10.1111/j.1365-3148.2012.01194.x [DOI] [PubMed] [Google Scholar]
- 17. Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: from bedside to bench and back. Blood. 2011;117:1463–1471. doi:10.1182/blood-2010-04-278135 [DOI] [PubMed] [Google Scholar]
- 18. Kuldanek SA, Kelher M, Silliman CC. Risk factors, management and prevention of transfusion-related acute lung injury: a comprehensive update. Expert Rev Hematol. 2019;12:773–785. doi:10.1080/17474086.2019.1640599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Standards for Blood Banks and Transfusion Services. 32nd ed AABB; 2020. [PubMed] [Google Scholar]
- 20. Vlaar APJ, Toy P, Fung M, et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion. 2019;59:2465–2476. doi:10.1111/trf.15311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Centers for disease control and prevention The National Healthcare Safety Network (NHSN) Manual: Biovigilance Component Protocol v2.5. 2018. Accessed October 3, 2019 https://www.cdc.gov/nhsn/acute-care-hospital/bio-hemo/index.html.
- 22. Burks LC, Aisner J, Fortner CL, Wiernik PH. Meperidine for the treatment of shaking chills and fever. Arch Intern Med. 1980;140:483–484. doi:10.1001/archinte.1980.00330160043024 [PubMed] [Google Scholar]
- 23. Martí Carvajal AJ, Solà I, González LE, Leon de Gonzalez G, Malagon NR. Pharmacological interventions for the prevention of allergic and febrile non-haemolytic transfusion reactions. Cochrane Database Syst Rev. 2010;CD007539 doi:10.1002/14651858.CD007539.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]


