Abstract
Background:
Patellar chondral defects represent up to 34.6% of defects found during routine arthroscopy. Surgical management has evolved during the past 20 years in an effort to develop techniques to replace hyaline cartilage. Currently, the only technique that achieves this is osteochondral autologous transfer (OAT). Although good and excellent results have often been reported at midterm and long-term follow-up for femoral lesions, little is known about isolated patellar defects.
Purpose:
To assess clinical and imaging results of patients treated with OAT for high-grade patellar defects.
Study Design:
Case series; Level of evidence, 4.
Methods:
This was a retrospective study on all patients who received OAT for high-grade symptomatic patellar chondral defects between 2010 and 2018 at our institution. The study included patients younger than 40 years of age with anterior knee pain and a grade 4 International Cartilage Repair Society patellar chondral defect between 1 and 2.5 cm2. Patients with surgery in other knee compartments, concomitant anterior cruciate ligament ruptures, infection, rheumatoid arthritis, and degenerative lesions were excluded. Six months postoperatively, all patients underwent magnetic resonance imaging (MRI) to allow assessment of graft integrity via the MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) score to evaluate morphologic features and integration. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Kujala scores were used to assess functional outcomes at final follow-up.
Results:
A total of 26 patients who received a patellar OAT were included. Most patients were male (88.4%), and the mean ± SD age was 28.5 ± 9.7 years. Patellar chondral defects had a median size of 180 mm2 (range, 64-250 mm2), and patients received a median of 1 autograft (range, 1-3). Functional outcomes assessed at a minimum of 1 year after surgery showed a mean Kujala score of 90.42 ± 6.7 and a mean WOMAC score of 95 ± 3.6. MRI revealed a median MOCART score of 75 points (range, 20-90 points).
Conclusion:
To our knowledge, this is the largest series to date regarding isolated patellar OAT. At midterm follow-up, most patients reported good and excellent results regarding symptoms and activity levels. Most autografts showed good osseous integration and excellent filling of the chondral surface, as evidenced on MRI. OAT is a good alternative to treat high-grade patellar chondral defects, especially among young patients.
Keywords: patella, autologous, transplantation, osteochondral, chondral, cartilage
Knee chondral defects are frequent findings in routine arthroscopy, representing a challenge for the orthopaedic surgeon. This is especially true in young athletes due to high demands on returning to previous levels of sports activity.12 These defects usually cause pain and articular effusion and eventually lead to the development of early osteoarthritis.6
The patella is a sesamoid bone that presents the thickest articular cartilage in the body, with a mean thickness of 6 to 7 mm. This allows the patella to bear the load to which the patellofemoral joint is exposed, which can be 6 to 7 times the total body weight.17 Thus, any defect on the patella reduces the normal distribution of forces on its surface, which can lead to defect enlargement, increased pain, and further functional deterioration.
It is well known that articular cartilage has a low regeneration capacity given its avascular and hypocellular characteristics.19,22 Special concern exists regarding chondral defects on the patella given the treatment difficulty that these pose.22 Furthermore, patellar chondral defects can be evidenced in approximately 34.6% of defects found during routine arthroscopy.10
Management of these defects depends on a variety of patient features, such as age; size, thickness, and location of the defect; injury mechanism (trauma or degenerative); and muscle balance, among others. However, independent of the treatment offered, results achieved are controversial in the literature.5,13
In recent years, different treatment options have been developed for these defects, although success rates have varied. Nonoperative management may not be able to achieve sufficient symptom relief, whereas surgical management has the relative advantage of restoring, to some extent, the articular cartilage.22 Osteochondral autologous transfer (OAT), popularized by Hangody et al16 in the 1990s, is a 1-stage procedure that transplants mature hyaline cartilage. This procedure entails a relatively easy surgical technique and has demonstrated cost-effectiveness.9 Since then, multiple studies have shown good and excellent results at long-term follow-up.3,8,12,14 Only a few case series have focused exclusively on patellar OAT, leading to the current controversial results.
The purpose of this study was to assess clinical, functional, and imaging outcomes of patients with high-grade patellar chondral defects treated with OAT.
Methods
We conducted a retrospective study of all patients who underwent OAT for the treatment of high-grade patellar chondral defects in our center. These patients were treated between 2010 and 2018.
Inclusion criteria were patients younger than 40 years of age, presence of anterior knee pain, a physical examination compatible with patellar chondral defect, International Cartilage Repair Society (ICRS) grade 4 patellar chondral defect shown on magnetic resonance imaging (MRI), traumatic origin, and defect size between 1 and 2.5 cm2. We excluded patients who underwent any other procedure in another knee compartment (anterior cruciate ligament reconstruction or multiligament reconstructions) or any other cartilage resurfacing procedure different from OAT in order to isolate the effect of this procedure (mosaicplasty was included because this has not been shown to significantly affect results, as >80% of the diseased area is covered); we further excluded patients with a medical history of rheumatoid arthritis or degenerative lesions. Preoperatively, patients underwent bilateral knee computed tomography so we could measure tibial tuberosity–trochlear groove (TT-TG) distance and decide whether there would be a benefit of tibial osteotomy. We included patients who underwent concomitant procedures to address patellar instability. Patients with a history of patellar instability and a normal TT-TG distance underwent a medial patellofemoral ligament reconstruction via autologous semitendinosus graft. Patients with a TT-TG distance of 20 mm or greater underwent a distal realignment through use of the Elmslie-Trillat technique.23
All patients received a trial of nonoperative management with physical therapy, exercise, and activity modification for at least 3 months before surgical intervention.
Surgical Procedure
All patients underwent standard knee arthroscopy with a standard thigh tourniquet, where an ICRS grade 4 defect was confirmed. Once the defect was debrided, a longitudinal medial or lateral parapatellar incision 3 to 5 cm long was made from the superior to the inferior patellar pole. An arthrotomy was performed through this incision, and the patella was mobilized and everted to provide direct visualization of the articular surface (Figure 1). Defect diameter was measured to determine the size of the osteochondral plug needed. All patients were treated with the Osteochondral Autograft Transfer System (Arthrex).
Figure 1.

Patellar eversion revealing a large high-grade patellar chondral defect.
The receiving area on the patella was drilled 10 mm deep in all cases (Figure 2). The harvesting device is 0.5 mm wider than the device that drills the receiving area, allowing for a press-fit of the transferred osteochondral plug. Mean plug height was 10 mm, and the plug was selected from the superior nonweightbearing trochlear surface, from an area that most resembled the shape of receiving patellar defect (Figure 3A). The plug was inserted in the patella until it was level with the native adjacent cartilage (Figure 3B). Bone extracted from the drilled patellar defect was used as a graft to fill the trochlear donor sites.
Figure 2.
(A) The patellar defect was drilled 10 mm deep. (B) Two holes were drilled into the patellar articular cartilage.
Figure 3.
(A) Osteochondral plugs were harvested from the femoral trochlea. (B) Osteochondral plugs were inserted press-fit into the patella.
All patients were asked to undergo a knee MRI (1.5-T) 6 months postsurgically so we could to assess the degree of repair and filling of the defect; integration of the plug; area, structure, and signal of the repair tissue; and presence of synovitis, using the MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) classification system.20 This score uses 9 categories to describe morphologic features and signal intensity within the repair tissue in comparison with adjacent native articular cartilage. A complete repair was considered when repair tissue looked as thick as the native adjacent articular cartilage, with complete margin integration, a smooth articular surface that reproduced the original articular contour without adhesions, and an intact subchondral bone plate. Signal intensity was determined on fast spin-echo sequences and gradient-echo with fat suppression. A complete repair regarding signal intensity was considered when repair tissue presented isointensity with native adjacent cartilage (Table 1).
Table 1.
MOCART Scoring Categoriesa
| Category | Item | Points |
|---|---|---|
| Defect fill | Subchondral bone exposed | 0 |
| Incomplete <50% | 5 | |
| Incomplete >50% | 10 | |
| Complete | 20 | |
| Cartilage interface | Complete | 15 |
| Demarcating border visible | 10 | |
| Defect visible <50% | 5 | |
| Defect visible >50% | 0 | |
| Surface | Surface intact | 10 |
| Surface damaged <50% of depth | 5 | |
| Surface damaged >50% of depth | 0 | |
| Adhesions | Absent | 5 |
| Yes | 0 | |
| Structure | Homogeneous | 5 |
| Inhomogeneous or cleft formation | 0 | |
| Signal intensity | Normal | 30 |
| Nearly normal | 10 | |
| Abnormal | 0 | |
| Subchondral lamina | Intact | 5 |
| Not intact | 0 | |
| Subchondral bone | Intact | 5 |
| Granulation tissue, cyst, sclerosis | 0 | |
| Effusion | Absent | 5 |
| Yes | 0 | |
| Total | 100 |
aThis table is adapted from the publication by Marlovits et al,20 which validates the numeric score to assess repair cartilage characteristics on magnetic resonance imaging. MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue.
A total of 26 patients who received OAT for a high-grade isolated patellar chondral defect were included during the study period.
At final follow-up, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Kujala scores were obtained to assess knee functionality.
Demographic and statistical analysis was performed with IBM SPSS Statistics 22. This study was approved by the ethics committee of our institution, and each patient gave written informed consent prior to participation.
Results
Of the patients included, 88.4% were male, and the mean ± SD age was 28.5 ± 9.7 years. Laterality was 16 right knees and 10 left knees. Defects were on the medial facet in 12 patients (46.2%), on the lateral facet in 9 patients, and on the central ridge in 5 patients. Mean follow-up was 2.5 years (range, 1-4 years). Patients who completed 2 years of follow-up were considered to have achieved maximum recovery potential; 4 patients were lost to follow-up before the 2-year mark (15%).
The median defect area was 180 mm2 (range, 64-250 mm2); 14 patients received 1 OAT plug, 11 patients received 2 plugs, and 1 patient received 3 plugs. The mean diameter of the harvested plugs was 9.5 mm (range, 8-10 mm), and the mean depth was 10.65 mm (range, 10-15 mm). A total of 15 patients who had a previous history of recurrent dislocation underwent associated procedures to the patellofemoral compartment. A total of 10 patients underwent an isolated medial patellofemoral ligament reconstruction, 4 had an isolated tibial tubercle transfer, and 1 had both procedures performed (Table 2).
Table 2.
Patient Demographic Data
| Variable | Result |
|---|---|
| Sex, % male | 88.4 |
| Age, y, mean ± SD | 28.5 ± 9.7 |
| Defect size, mm2, median (range) | 180 (64-250) |
| No. of osteochondral autologous transfers received, median (range) | 1 (1-3) |
| Laterality, % right knees | 61.5 |
| Location of defect | 46.2% medial facet, 34.6%lateral facet, 19.2% vertex |
Regarding functional outcomes, at the time of final follow-up, the patients had a mean Kujala score of 90.42 ± 6.7 points and a mean WOMAC score of 95 ± 3.6 points (Table 3).
Table 3.
Functional Outcome Score Resultsa
| Score | Mean ± SD, points |
|---|---|
| WOMAC | 95 ± 3.6 |
| Kujala | 90.42 ± 6.7 |
aWOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
We independently assessed each WOMAC dimension, specifically pain, stiffness, and functional capacity. Most patients (80.7%) reported mild pain while walking on flat terrain, 15.3% reported moderate pain while using stairs, and 3.9% reported moderate pain while sitting. Less than one-third of patients (30.7%) reported stiffness during daily activities. Functional capacity regarding the use of stairs was moderately difficult for 11.5% of patients and severely difficult for 7.6% of patients. Lateral knee pain associated with the graft harvest was not a common complaint among our patients; only 3 patients mentioned this complication, noting it did not affect daily life or sports activities. Regarding return to sport, 70% of our patients reported returning to sport at a level equal to their preinjury level.
Imaging outcomes assessed via the MOCART score at 6-month MRI showed a median of 75 points (range, 20-90 points). There were 2 cases with unsatisfactory imaging outcomes, with 20 and 25 points on the MOCART score. Figures 4 and 5 show examples of sagittal and axial cuts from MRI scans.
Figure 4.
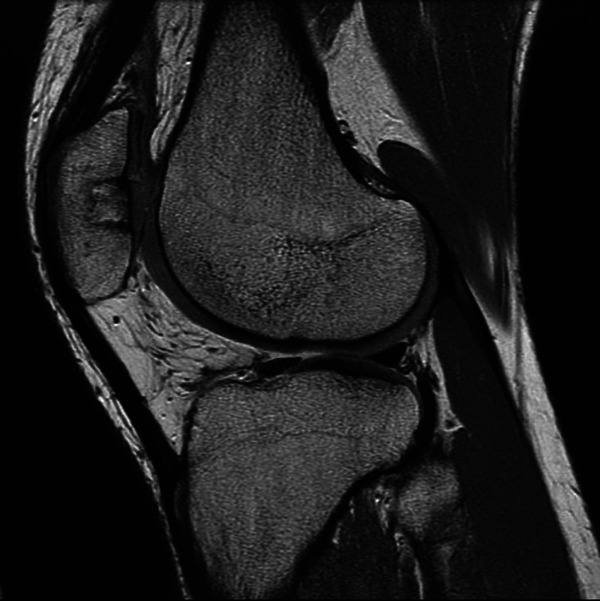
Sagittal high-resolution magnetic resonance imaging scan (T2-weighted, fast spin-echo, dual sagittal). This cut shows the complete filling of the patellar defect with complete integration of the bone plug. The repair surface and structure remain intact with intact subchondral bone.
Figure 5.

Axial high-resolution magnetic resonance imaging scan (T2-weighted, fast spin-echo, dual sagittal). This cut reveals complete filling and integration of the patellar osteochondral autologous transfer plug to the native adjacent cartilage. The surface and structure of the repair tissue remain intact, as does the subchondral bone.
Analysis of each category included in the MOCART score showed that nearly 70% of patients had a complete hypertrophic repair of the defect. Most patients revealed a visible plug border (23/26 patients), which is considered normal at 6-month follow-up. Furthermore, 77% of patients had a defect filling greater than 50%, with plug characteristics similar to adjacent native cartilage (isointensity) (Table 4).
Table 4.
MOCART Results for 26 Patientsa
| MOCART Categories | No. of Patients |
|---|---|
| Defect fill | |
| Complete | 13 |
| Hypertrophy | 5 |
| Incomplete | |
| >50% of adjacent cartilage | 2 |
| <50% of adjacent cartilage | 6 |
| Exposed subchondral bone | 0 |
| Cartilage interface | |
| Complete | 0 |
| Visible plug border | 23 |
| Visible defect | |
| <50% of depth | 3 |
| >50% of depth | 0 |
| Surface | |
| Surface intact | 20 |
| Surface damaged <50% of depth | 5 |
| Surface damaged >50% of depth | 1 |
| Adhesions | |
| Absent | 23 |
| Yes | 3 |
| Structure | |
| Homogeneous | 15 |
| Inhomogeneous or cleft formation | 11 |
| Signal intensity | |
| Normal | 20 |
| Nearly normal | 4 |
| Abnormal | 2 |
| Subchondral lamina | |
| Intact | 24 |
| Not intact | 2 |
| Subchondral bone | |
| Intact | 6 |
| Granulation tissue, cyst, sclerosis | 20 |
| Effusion | |
| Absent | 19 |
| Yes | 7 |
aMOCART, Magnetic Resonance Observation of Cartilage Repair Tissue.
Discussion
Patellar osteochondral defects are a relatively frequent knee pathology and pose a challenge to the treating orthopaedic surgeon. These are common injuries in young athletes, compromising their performance in sports activities. The problem with this tissue is that spontaneous healing is not part of the defect’s natural history, which can be explained by the low regeneration capacity of cartilage.
OAT is a technique used for treating high-grade chondral defects, allowing for the restoration of damaged hyaline cartilage. This technique possesses the advantage of providing structural subchondral bone support. Hangody and Füles15 presented a series of 831 patients who underwent OAT for focal osteochondral defects between 1 and 4 cm2. Their series showed good and excellent results in 92% of femoral defects, 87% of tibial defects, and 79% of patellofemoral defects. When the investigators performed second-look arthroscopy in 83 patients, biopsy demonstrated hyaline cartilage survival in 83% (69 patients).
Other surgical options available for treating these defects include autologous chondrocyte implantation (ACI), microfracture, and osteochondral allograft. When these techniques were compared with OAT, recent evidence showed no significant difference with microfracture and ACI regarding pain or functional scores and showed better Lysholm scores with OAT versus ACI, revealing a trend toward better outcomes with OAT.22 Osteochondral allograft is an alternative but is generally used for larger defects, making a comparison with OAT difficult.
Regarding OAT for the management of patellar chondral defects, Jakob et al18 reported on 52 chondral defects treated with OAT; however, only 1 patient in that series had an isolated patellar defect. There were 23 patients classified as ICRS grade 3 and 29 patients as ICRS grade 4. After 2-year follow-up, 86% of patients reported a significant recovery regarding functional aspects of the ICRS score. After second-look arthroscopy in 10 patients of the same case series, biopsies revealed that transplanted cartilage kept its hyaline characteristics.
Miniaci et al21 evaluated 8 cases with patellar high-grade defects treated with OAT. These authors observed that adequate orientation of the harvested graft and angulations with the femoral trochlea were technically demanding through arthroscopically performed surgery. Given these findings, this group recommended performing a mini arthrotomy for correct visualization of the defect, reporting 88% good and excellent functional outcomes in their series.
Bentley et al4 presented results on a randomized prospective series of 100 patients with chondral defects; 5 of these patients had patellar chondral defects. The 5 patients received OAT but were considered to have experienced treatment failure because all of them required revision surgery during the first and second year after initial intervention.
Our study presents a series of young, mostly male patients who reported good and excellent postoperative functional outcome scores at the end of follow-up. These excellent results could be explained by correct patient selection (young athletic patients, without the presence of degenerative disease, with no patellar tilt or reciprocal trochlear defects). All patients presented good and excellent results regarding each dimension of the WOMAC score, analyzed independently.
Imaging outcomes at 6 months revealed good results in almost all patients (92%). These showed adequate plug integration to adjacent bone and good cartilage characteristics compared with adjacent native cartilage. The most important imaging outcome is to achieve a repaired cartilage surface similar to adjacent native cartilage (>50% of filling and isointense). This was achieved in 96.1% of our cases. In this regard, other recent studies have analyzed the same outcomes and revealed that most plugs (67%-100%, depending on the analyzed series) remained flush with surrounding native articular cartilage, with full osteochondral integration at 12 months of follow-up.2,3,11,22
These findings follow the same line as recent published literature. Cohen et al7 evaluated a series of 17 patients treated with OAT for a patellar chondral defect, reporting significant improvements in functional assessments including Lysholm, Kujala, Fulkerson, and 36-Item Short Form Health Survey scores. Furthermore, in a recently published study by the same group, 20 patients treated with patellar OAT were followed for 2 years.3 The study revealed that patients presented a significant improvement in pain, gait pattern, and knee swelling at 6 and 24 months postoperatively.
Regarding pain outcomes, a common concern is donor site morbidity. Usual donor sites include the femoral trochlea, intercondylar notch, patellofemoral joint, and upper tibiofibular joint. Recent systematic reviews seeking to clarify this concern revealed that, on average, donor site morbidity for transfers from the knee to other knee compartments was 5.9%,1 with the most common complaints being patellofemoral disturbances, knee stiffness, and persistent pain. Some interventions to diminish these complications have been proposed, such as filling the donor site with a bone graft and harvesting from potential morbidity-free areas such as the lower weightbearing area of the patellofemoral joint or the upper tibiofibular joint.
Another outcome of interest in this population is return to sports. Recent studies that analyzed this outcome in patients who received OAT for high-grade patellar chondral defects showed that at 2 years of follow-up, patients did not report changes in sports activities due to knee pain. The Tegner sports scale revealed a preoperative score range of 0 to 5, which increased to 5 to 9 at 2 years of follow-up.3,11
A recent systematic review that aimed to assess patellar OAT outcomes arrived at a similar conclusion.8 Patients showed significant improvement in patient-reported outcomes at final follow-up, with most of them being able to return to previous levels of sports activities (Tegner score range, 5-9 at 2 years of follow-up). Regarding imaging outcomes, MRI scans taken during the first year postoperatively (12 months on average) revealed that most plugs were completely integrated and correctly positioned, thus highlighting the good results this technique offers for patients in this setting.8
Nonetheless, our study presents limitations. Results could be attributable to the retrospective nature of our analysis. We did not undertake preoperative assessment of functional scores to compare with preoperative scores, so we are unable to state the magnitude of the improvement from this procedure. Additionally, more than half of our patients had concomitant surgery to correct patellar instability, which may have influenced the results. Also, our results could be influenced by the relatively small sample size.
Conclusion
Our series showed that OAT was a good alternative for treating high-grade patellar chondral defects, especially among young patients. This study also revealed that at midterm follow-up, most patients reported good results regarding symptoms and activity levels. On the 6-month follow-up MRI scan, most plugs showed good integration and excellent filling of the patellar chondral surface. To our knowledge, this is the largest series of patellar defects treated exclusively with OAT. Further prospective midterm and long-term follow-up studies should be performed to corroborate our findings, but this study provides promising results for treating surgeons.
Footnotes
Final revision submitted February 8, 2020; accepted February 26, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: D.F. is a paid consultant of Smith & Nephew and Stryker. A.V. has received educational support from Arthrex, AO, and DePuy Synthes. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Clínica Alemana de Santiago.
References
- 1. Andrade R, Vasta S, Pereira R, et al. Knee donor-site morbidity after mosaicplasty—a systematic review. J Exp Orthop. 2016;3(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries. J Bone Joint Surg. 2014;96(10):816–823. [DOI] [PubMed] [Google Scholar]
- 3. Astur DC, Bernardes A, Castro S, et al. Functional outcomes after patellar autologous osteochondral transplantation. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3084–3091. [DOI] [PubMed] [Google Scholar]
- 4. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85-B(2):223–230. [DOI] [PubMed] [Google Scholar]
- 5. Brix MO, Stelzeneder D, Chiari C, et al. Treatment of full-thickness chondral defects with hyalograft C in the knee: long-term results. Am J Sports Med. 2014;42(6):1426–1432. [DOI] [PubMed] [Google Scholar]
- 6. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 7. Cohen M, Amaro JT, Fernandes R de SC, et al. Osteochondral autologous transplantation for treating chondral lesions in the patella. Rev Bras Ortop. 2012;47(3):348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donoso R, Figueroa D, Espinoza J, Yañez C, Saavedra J. Osteochondral autologous transplantation for treating patellar high-grade chondral defects: a systematic review. Orthop J Sports Med. 2019;7(10):232596711987661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emre TY, Atbasi Z, Demircioglu DT, Uzun M, Kose O. Autologous osteochondral transplantation (mosaicplasty) in articular cartilage defects of the patellofemoral joint: retrospective analysis of 33 cases. Musculoskelet Surg. 2017;101(2):133–138. [DOI] [PubMed] [Google Scholar]
- 10. Figueroa D, Calvo R, Vaisman A, Carrasco MA, Moraga C, Delgado I. Knee chondral lesions: incidence and correlation between arthroscopic and magnetic resonance findings. Arthroscopy. 2007;23(3):312–315. [DOI] [PubMed] [Google Scholar]
- 11. Figueroa D, Meleán P, Calvo R, Gili F, Zilleruelo N, Vaisman A. Osteochondral autografts in full thickness patella cartilage lesions. Knee. 2011;18(4):220–223. [DOI] [PubMed] [Google Scholar]
- 12. Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M. Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop. 2014;38(9):1905–1912. [DOI] [PubMed] [Google Scholar]
- 13. Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21(9):1066–1075. [DOI] [PubMed] [Google Scholar]
- 14. Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125–1133. [DOI] [PubMed] [Google Scholar]
- 15. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 1):25–32. [DOI] [PubMed] [Google Scholar]
- 16. Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects: a preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5(4):262–267. [DOI] [PubMed] [Google Scholar]
- 17. Huberti HH, Hayes WC. Patellofemoral contact pressures: the influence of Q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66(5):715–724. [PubMed] [Google Scholar]
- 18. Jakob RP, Franz T, Gautier E, Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop Relat Res. 2002;401:170–184. [DOI] [PubMed] [Google Scholar]
- 19. Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagnini S. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee. Am J Sports Med. 2007;35(12):2014–2021. [DOI] [PubMed] [Google Scholar]
- 20. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. [DOI] [PubMed] [Google Scholar]
- 21. Miniaci A, Jambor C, Petrigliano FA. Autologous osteochondral transplantation In: Williams RJ, ed. Cartilage Repair Strategies. Humana Press; 2007:105–114. [Google Scholar]
- 22. Mundi R, Bedi A, Chow L, et al. Cartilage restoration of the knee. Am J Sports Med. 2016;44(7):1888–1895. [DOI] [PubMed] [Google Scholar]
- 23. Trillat A, Dejour H, Couette A. Diagnostic et traitement des subluxations r'ecidivantes de la rotule [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:813–824. [PubMed] [Google Scholar]




