Abstract
Diabetes mellitus is an epidemic in the US and abroad. With the advent of new contact lens technology, the use of contact lenses as glucose sensors in lieu of the traditional finger stick is quickly becoming realized. This has the potential to rapidly expand the contact lens market into this growing patient population. The independent cellular and physiological effects of contact lens wear and diabetes on the corneal epithelium have been described. However, little evidence exists to date to support whether there is an increased risk associated with contact lens wear in diabetes. The focus of this review is to discuss what is known about the cellular effects of contact lenses on the corneal epithelium, the pathophysiological changes in the corneal epithelium that occur in diabetes, and whether an increased risk for corneal epithelial damage and/or infection may negatively impact safety in diabetic contact lens wearers. Available data indicates that there are inherent risks associated with contact lens wear in diabetics. Importantly, eye care practitioners fitting contact lenses in the diabetic patient need to carefully consider the duration of disease, the level of glycemic control, the presence of retinopathy, and the patient’s overall health.
Keywords: cornea, epithelium, diabetes, contact lens, infectious keratitis
Introduction
Contact lenses are widely used as an alternative to glasses to correct refractive error, for cosmetic purposes, and as bandage lenses for corneal erosions and painful epithelial defects. The development and implementation of contact lenses for new and exciting indications are rapidly exploding. These indications encompass a wide-spectrum of use, ranging from myopia prevention, drug delivery devices, and biological sensors that monitor intraocular pressure and blood glucose levels.1–10 With respect to the latter, tear glucose levels have been shown to correlate with blood glucose.11 Moreover, the implication of tear glucose monitoring using contact lenses offers the advantage of continuous monitoring and is considered to be less invasive than a traditional finger stick.4
Concurrent with the introduction of contact lenses into these new markets is the increase in the number of wearers, including children and patients with systemic diseases such as diabetes. Patients with diabetes commonly present to the clinic with damage to the tight epithelial barrier, abnormal wound healing, epithelial fragility, loss of corneal sensitivity and corneal nerves, and an overall higher risk for bacterial and fungal infections. Despite having a compromised epithelial barrier, little is known about the synergistic effects of contact lens wear on the diabetic corneal epithelium. With the reported increase in Type 1 diabetes mellitus (T1DM) and the massive increase in the numbers of patients with Type 2 diabetes mellitus (T2DM), an understanding of the interactive effects between contact lenses and diabetes on the corneal epithelium is urgently needed.12–14 Two prior reviews have addressed the ocular complications of diabetes that commonly present in clinical practice.15, 16 The scope of this review is to re-evaluate the known cellular effects of contact lens wear on the corneal epithelium and the potential impact of contact lens wear on the already abnormal diabetic corneal epithelium.
The corneal epithelium
The corneal epithelium is a non-keratinized, stratified squamous epithelium that functions to maintain the transparency of the cornea through its innate immuno-protective and tight barrier functions. The cornea is avascularized, a feature required for transparency, and as such the corneal epithelium derives oxygen (155 mmHg or 21% v/v) from air. During eyelid closure, the available oxygen drops to approximately one third open eye levels. In contrast to this, glucose and other essential nutrients are taken up from the aqueous humor. These molecules are first transported through the leaky barrier of the corneal endothelium and then diffuse anteriorly through the corneal stroma to the epithelium. Excess glucose is converted into glycogen stores, with the highest levels of glycogen residing among the basal epithelial cells to be used during times of stress. Despite the continuous bombardment of insults from shear forces due to blinking, exposure to pathogens, osmolarity changes in diabetes and dry eye, and intermittent hypoxia from overnight eyelid closure during sleep and during low oxygen transmissible contact lens wear, the corneal epithelium is able to maintain a continuous state of self-renewal.
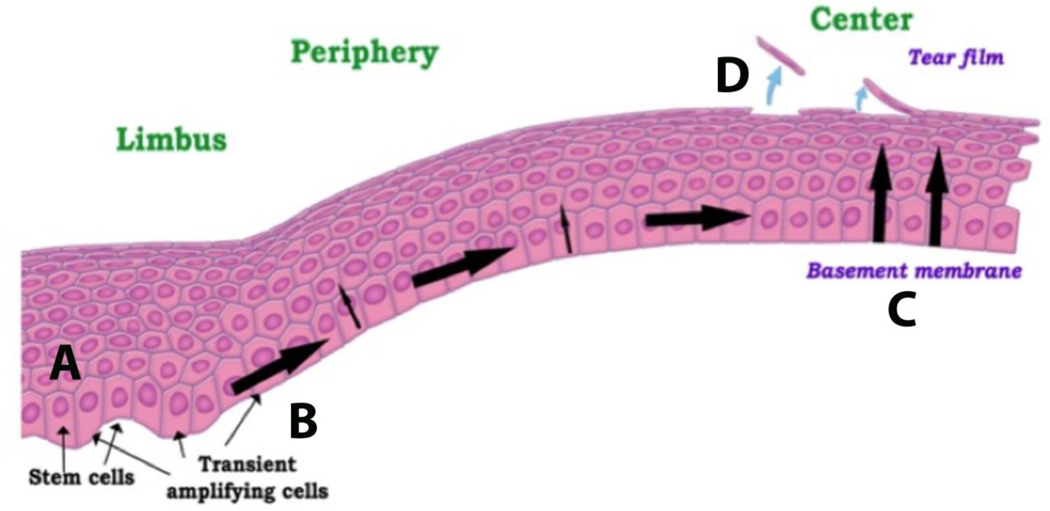
Stem cells that are necessary to replenish the corneal epithelium reside exclusively in the limbal region of cornea.17–21 Within the basal layer, these cells are protected by the pigmented Palisades of Vogt and nourished by the limbal arcades. The limbal region is unique for the source of corneal stem cells when compared to other tissues. This includes their limbal location, peripheral to the tissue they continuously replenish. Here, limbal stem cells undergo asymmetric cell division, sending one cell on a trek through the peripheral to central cornea, while the other cell is retained. This peripheral migration is associated with a continuous decrease in proliferative capacity. Upon reaching the central cornea, basal epithelial cells undergo their final round of cell division before beginning their vertical ascent towards the corneal surface as paired daughter cells.17–19 At the corneal surface, post-mitotic, fully differentiated epithelial cells slough or desquamate for clearance by the precorneal tear film (Figure 1).
Figure 1:
Schematic of corneal epithelial renewal. (A) Stem cells reside in the basal layer of the limbus. (B) Following departure from the limbus, basal epithelial cells become transient amplifying cells and exhibit a high proliferative capacity. (C) Cells continue to migrate to the central cornea, losing proliferative capacity as they go. After the final round of cell division, the paired cells move towards the corneal surface. (D) At the corneal surface, cells are shed or desquamated into the precorneal tear film. Figure taken from Ladage et al. Contact Lens Ant eye 2002.
Diabetes and the corneal epithelium
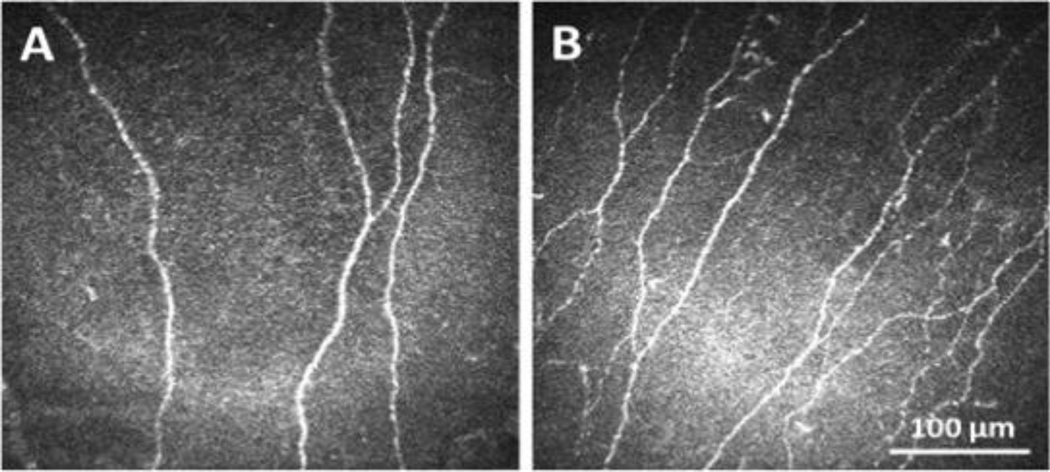
Type 1 and type 2 diabetes are increasing in frequency in young and old patients alike. Diabetes can adversely impact just about every organ system in the body, including the eye. While retinopathy is the most well-known complication of diabetes, the cornea is also adversely affected. In fact, it is estimated that the cornea is adversely affected in up to 70% of all patients.22 The most studied of the effects on the diabetic corneal epithelium include changes in the composition of the basement membrane, abnormal epithelial cell adhesion, disruption of the epithelial barrier, persistent epithelial defects, and corneal neuropathy. 23–31 These conditions can be visually devastating and unlike retinopathy, are very painful. The molecular mechanisms that underlie corneal complications in diabetes have been reviewed elsewhere (Zhu et al, manuscript in review).22 Over the past decade, the abundance of reported studies using IVCM to measure diabetes-induced damage to corneal nerves has helped to increase recognition of and the significant unmet need for novel therapies to treat corneal complications, prevent corneal nerve loss, and accelerate corneal epithelial wound healing (Figure 2).32–34
Figure 2:
In vivo confocal microscopy confirms loss of the human subbasal nerve plexus in diabetes. (A) A representative image of the subbasal nerve plexus in a patient with T2DM. (B) A representative image of the subbasal nerve plexus in a non-diabetic control. Scale bar: 100 μm. Figure taken from Stuard et al. Invest Ophthalmol Vis Sci 2018.
Corneal epithelial proliferation
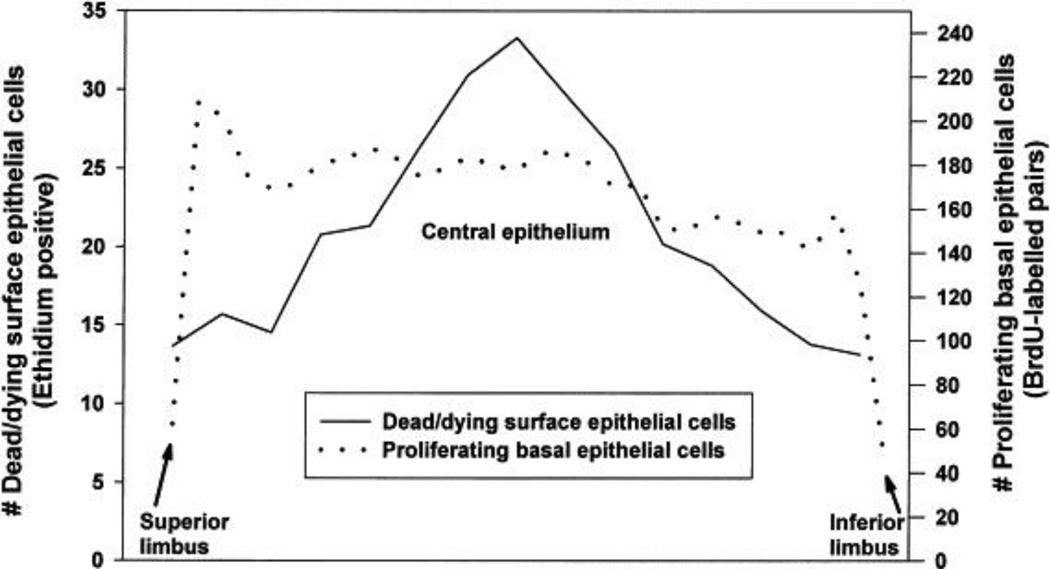
In the normal, non-lens wearing rabbit cornea, the proliferation rate is lowest in the limbus, consistent with the localization of slowly dividing basal cells known to reside in this area (Figure 3). Just adjacent to the limbus, in the peripheral cornea, the proliferation rate is highest and corresponds to the localization of transient amplifying cells.35, 36 This effect is regionally specific, as proliferation is greatest superiorly and decreased inferiorly. This vertical disparity in corneal epithelial proliferation may be explained, in part, by the regional changes in corneal epithelial thickness. Unlike proliferation, corneal epithelial thickness is greatest inferiorly and reduced superiorly (Rashdan et al, manuscript in review). This vertical shift is hypothesized to be a result of eyelid biomechanics. In the central corneal epithelium, the proliferation rate of basal epithelial cells is decreased compared to the peripheral cornea. Studies measuring the proliferation rate in the rabbit limbus have shown increased rates of proliferation in response to rigid gas permeable (RGP) contact lens wear, but not soft contact lens wear. This boost in proliferation is thought to be a direct result of mechanical stimulation from the RGP contact lens in the rabbit model.35, 37, 38 Unlike traditional RGP lens wear in humans, to facilitate lens retention in the rabbit model, the contact lens is manufactured with a much larger overall diameter that allows it to cross the limbal border.
Figure 3:
Distribution of basal epithelial cell proliferation and surface cell shedding in the rabbit cornea. 5-bromo-2’-deoxyuridine (BrdU) labeling of mitotic cells across the limbal and corneal epithelium (dotted line). Calcein-ethidium live/dead staining showing a central peak in non-viable cells in the surface epithelium (solid line). Figure taken from Ladage et al, Contact Lens Ant Eye 2002.
In the otherwise healthy cornea, basal epithelial cell proliferation is decreased with all short-term contact lens wear.37–40 This is partially mediated by hypoxia, as studies have shown significantly decreased numbers of mitotic figures (up to 90%) with very low-oxygen transmissible soft contact lenses.41 These findings were later confirmed in additional rabbit studies following two days of contact lens wear.38 Here, the authors demonstrated a greater decrease in proliferation with low oxygen transmissible RGP contact lenses (82% suppression) compared to ultra-high oxygen transmissible RGPs (21% suppression).38 This finding was also in agreement with a subsequent study that showed an 80% decrease in proliferation following 24 hour wear of low-oxygen transmissible RGP contact lenses, whereas, a 37% decrease in proliferation was shown in the ultra-high oxygen transmissible RGP lens group.37 Not only is basal epithelial cell proliferation decreased in response to hypoxic contact lens wear, but there is also a corresponding reduction in the upward movement of post-mitotic basal epithelial cells toward the surface of the central cornea.39
In recent years, there has been little to no work evaluating the effects of contemporary contact lens materials on the proliferation rate of corneal epithelial cells. The most recent study using soft silicone hydrogel contact lenses revealed that the corneal epithelial proliferation rate decreased following two days of extended wear.35 Following eight days of extended lens wear, the authors reported an increase in proliferation, which they termed “proliferative recovery”.35 These data indicate that the attenuation of proliferation of basal epithelial cells in the central cornea following the initiation of contact lens wear undergoes partial adaptation during continued wear. It is unclear whether this proliferative recovery is sustained at this intermediate level, creating a new homeostatic set point in the contact lens wearer, or if it fully returns back to baseline levels. Further studies are needed to fully understand the adaptive effects of the cornea in response to the contact lens.
It is interesting to note however, that this same group also noted changes in the proliferation rate in non-contact lens wearing control eyes when the contralateral eye was fit with either a low or high oxygen transmissible contact lens.35 A similar finding has been reported for corneal swelling in response to contact lens wear and for growth factor levels in the tear film following wounding.42, 43 Taken together, these data suggest that there is a central control mechanism regulating communication between eyes, in which perturbation to one eye triggers a similar response, albeit lower in magnitude, in the contralateral eye.
It is unknown whether the stagnation in corneal epithelial renewal that occurs in response to contact lens wear is a contributor to infection. Since the corneal epithelium functions as an innate barrier, it would be intuitive to speculate that any disruption to this barrier may lower host defenses. In the diabetic corneal epithelium, it is well established that there is functional impairment of the tight barrier and reduced adhesion of epithelial cells to the basal lamina. Abnormalities in epithelial cell proliferation have also been reported however, the data is somewhat conflicting.44, 45 Fujita and colleagues cultured corneal epithelial cells in the presence of elevated extracellular glucose and reported significant decreases in both cell number and tritiated thymidine incorporation, indicating a reduction in proliferation.44 In contrast to this, McDermott et al. demonstrated an increase in proliferation in high glucose cultures.45 Using Simian virus-40 (SV-40) transformed corneal epithelial cells, they found an increase in extracellular glucose from 5 mM to 17.5 mM increased proliferation by 44%. Further increases in glucose concentration failed to significantly alter proliferation. Our own unpublished observations using primary cultured human corneal epithelial cells from diabetic cadaveric donors have yielded mixed results on the effects of diabetes on epithelial proliferation. Growth of these cells in standard keratinocyte culture media containing 6 mM glucose ranges from a slight reduction in growth to almost completely arrested proliferation. It is important to note that these are cells that have been subject to long standing diabetes in vivo and not an acute exposure to elevated glucose that is commonly tested in cell culture models. More work is needed to fully define the effects of diabetes, in addition to glucose, that impact normal proliferation and growth of the corneal epithelium.
Apoptosis and surface epithelial cell desquamation
Previous reports have shown that in the normal, non-lens wearing eye, corneal epithelial cells are sloughed into the precorneal tear film via apoptosis, a regulated form of cell death.46 This is mediated in part, by loss of the nuclear localized anti-apoptotic protein, B-cell lymphoma-2 (Bcl2).47–49 When examining the non-lens wearing cornea, the lowest numbers of nonviable cells were found in the limbus, while there were a greater number of nonviable cells in the central cornea (Figure 3). While limited research on the mechanism(s) that regulate apoptotic shedding in the corneal epithelium is available, multiple reports using human and animal models have confirmed that there is a reduction in apoptotic shedding during contact lens wear.47, 50–54 O’Leary was the first to show that there was a decreased number of epithelial cells irrigated from the human corneal surface following contact lens wear.55 In his study, O’Leary compared cells that were presumably exfoliated from the corneal surface following soft or RGP contact lens wear compared to controls. In doing so, he found that both soft and RGP lenses disrupted normal desquamation. This work was later confirmed in multiple, prospective human clinical trials.52–54 These studies further showed that this decrease in apoptotic desquamation rate was greatest following 1 month of lens wear and similar to proliferation, showed a partial adaptive recovery after one year.53, 54, 56 Moreover, desquamation rate was not mediated by the duration of extended wear, since there were no detectable differences between 6 and 30 day wearing regimens.53, 54
It has also been proposed that during contact lens wear the contact lens may acts as a barrier to protect the corneal epithelium from the mechanical shearing forces that result during blinking and that these forces may provide the trigger that drives desquamation of terminally differentiated corneal epithelial cells from the surface of the eye. This theory is not supported by work by Ren and colleagues using nitrogen goggles that showed an inhibition of desquamation during hypoxia in the absence of a contact lens.57 It is more likely that both hypoxia and shear forces contribute to altered desquamation in response to contact lens wear. The exact mechanism(s) still remain ill defined.
There is a paucity of evidence examining the effects of diabetes on the regulation of apoptotic desquamation from the surface corneal epithelium. In dry eye, epithelial turnover increases and this is associated with a corresponding increase in non-viable surface epithelial cells.58 One could speculate that in diabetes, where there is an increase in dry eye, cellular desquamation is escalated.59–66 In a diabetic rat model, terminal deoxynucleotidyl transferase dUTP nick end (TUNEL) labeling was used to measure the number of apoptotic cells in the corneal epithelium.67 Importantly, the authors found a five-fold increase in apoptotic cells compared to the non-diabetic control. Apoptosis in this model was mediated by cleavage of caspase 3. Similar findings have been reported in cultured corneal epithelial cells.68 Specifically, reports have shown an increase in inflammatory mediators and apoptosis in response to an increase in extracellular glucose.68 Likewise, the accumulation of advanced glycation end products has also been shown to induce apoptosis in corneal epithelial cells.69 The increase in apoptotic surface shedding of surface corneal epithelial cells may be somewhat protective in the diabetic eye, at least for invasive bacterial strains that undergo lipid-raft mediated internalization.70
Corneal epithelial thickness and epithelial cell size
Clinical studies using in vivo confocal microscopy through focusing have shown that extended wear, but not daily wear, of contact lenses results in thinning of the corneal epithelium.54, 56, 71–73 This appears to be mediated partly by hypoxia as low oxygen permeable lenses result in more significant corneal epithelial thinning.54, 72, 73 Likewise, wear of RGP lenses have the greatest effect on thickness, likely due to the mechanical pressure of the lens on the cornea.53 With soft lenses, duration of wear does not appear to be a significant contributor to the corneal epithelial thinning seen in extended contact lens wear,53, 73 as there was no significant difference between 6 days versus 30 days of extended wear.53, 54 Similar to proliferation rates, there is a partial adaptive recovery after the first month of extended wear, resulting in partial restoration of epithelial thickness.53 After cessation of lens wear, thickness of the epithelium fully recovers over time. This was demonstrated by Holden and colleagues who found that after extended wear, it took 33 days for the central epithelial thickness to fully return to baseline levels.71
The reduction in epithelial proliferation and desquamation combined with epithelial thinning is thought to create a “stagnant” epithelium. Consistent with this theory, there is an increase in surface epithelial cell size in response to contact lens wear. Tsubota and Yamada were the first to demonstrate an increased epithelial cell size using specular microscopy.74 In that study they found that an increase in surface epithelial cell size was exclusively associated with extended wear of soft contact lenses.74 In a subsequent report evaluating contact lens wearers over a six month period, Tsubota et al. also demonstrated that surface epithelial cell size increased linearly with the duration of extended wear.75
Increased cell size from contact lens wear has since been confirmed using in vivo confocal microscopy.52, 53, 76 In a series of 12-month prospective clinical studies, all overnight or extended contact lens wearers showed significant increases in surface epithelial cell size.54, 77–79 This effect was greatest with rigid lenses due to the mechanical effect of the lens on the corneal surface.52, 53, 79 In contrast to prior reports however, the area of surface epithelial cells calculated from in vivo confocal microscopy (IVCM) images failed to detect a difference in cell size regardless of whether lenses were worn for 6 or 30 days. Rather than a linear increase in cell size for longer durations of wear, they also found an early rapid increase that tapered-off over time. No long-term adaptation was observed for surface cell size in this yearlong trial.53 Compared to the work by Tsubota et al, these latter two findings yielded contradictory results regarding the effect of RGP lenses and the linear relationship between duration of lens wear and surface epithelial cell size. The impact of daily lens wear on surface corneal epithelial cell size is also conflicting. While Tsubota did not detect a difference in surface area between daily lens wearers compared to controls, Ladage demonstrated a significant increase in surface epithelial cell size during 4 weeks of daily wear.52, 74
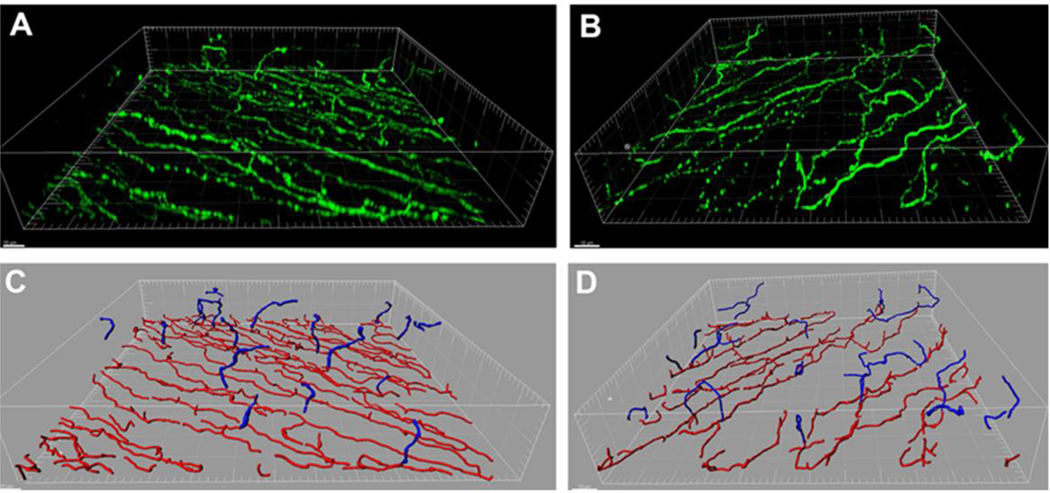
Similar to contact lens wear, at the tissue level, central corneal epithelial thinning is frequently reported in patients with diabetes. Unlike corneal epithelial thinning during contact lens wear that is thought to be driven by hypoxia and/or lens biomechanics, corneal epithelial thinning in diabetes is driven by the loss of corneal sensory nerves. Rosenberg et al were the first to use IVCM to document corneal epithelial thinning in diabetes.80 In agreement with this work, animal studies in our laboratory have also confirmed that the cornea thins in severe diabetes and is associated with damage to the subbasal nerve plexus (Figure 4).81 It should be noted that corneal epithelial thinning has not been demonstrated in all animal models of diabetes. 82, 83 This difference could be due in part to the severity of the disease, whether insulin was administered, and the method in which epithelial thickness was measured.
Figure 4:
Epithelial thinning is associated with loss of the subbasal nerve plexus in a Type 1 streptozotocin diabetic mouse model. (A) Three-dimensional surface rendering of the subbasal nerve plexus and associated terminal epithelial nerves in a control mouse. β-tubulin III staining in green. (B) Three-dimensional surface rendering of a Type 1 diabetic mouse. Scale bar: 10 μm. (C – D) Nerve modeling and segmentation using IMARIS Filament. Representative images showing the subbasal nerve plexus is shown in red, terminal epithelial nerves in blue (C, normal; D diabetic). Scale bar: 10 μm. Loss of the subbasal nerve plexus in (D) was associated with significant thinning of the corneal epithelium after 12 weeks of diabetes, 34.0 μm ± 3.0 μm (diabetes) compared to 38.6 μm ± 3.8 μm (control). Figure taken from Cai et al. Am J Pathol 2014.
Stem cells
Stem cells reside in the basal layer of the limbus, the area located at the intersection of the corneoscleral junction.20, 21 Stem cells are necessary for normal homeostasis of the epithelium and function to restore the epithelium following wounding. Clinically, the loss of stem cells is diagnosed as limbal stem cell deficiency (LSCD). There are multiple known etiologies for limbal stem cell deficiency, consisting of congenital and acquired causes and can affect one, and more rarely, both eyes. These include ectodermal dysplasia, aniridia, chemical or thermal injuries, Stevens-Johnson syndrome, and iatrogenic cases (secondary to surgery or medications). 84–86 To date, LSCD is still regarded as a complication of contact lens wear and represents a major, sight threatening complication.
Clinically, loss of limbal stem cells results in conjunctivalization of the cornea with resultant corneal opacification. 84–86 LSCD can produce signs and symptoms of decreased vision, photophobia, pain, tearing, redness, and irritation. In some wearers, particularly those in the early stages of damage, LSCD is asymptomatic.86–88 In fact, a retrospective review estimated that contact lens-induced LSCD was asymptomatic in more than 70% of patients.85 Almost all reported cases are due to many years of soft contact lens wear and are associated with significant inflammation.85, 88–90 The pathogenesis of contact lens-induced LCSD is thought to be multifactorial involving mechanical trauma,85, 91, toxicity from contact lens solutions and their preservatives,92, 93 chronic hypoxia,94 and disruption of the pre-ocular tear film.95 Contact lens induced hypoxia has been shown to be greatest in the superior cornea.96, 97 This, combined with the mechanical irritation in the superior limbus from the eyelid/contact lens interaction, may explain the increased prevalence of contact lens-induced LSCD in this region.98
Very little is known regarding the impact of diabetes on limbal stem cells. In diabetic corneal tissue, staining at the limbus for the membrane transporter protein, ATP binding cassette subfamily G member 2 (ABCG2), and the transcription factor ΔNp63α revealed a large decrease in both markers. ΔNp63α, once considered a putative stem cell marker in the cornea, is now well recognized as a known marker of proliferative capacity.99 Thus, the blunted expression of ΔNp63α seen in the diabetic cornea is consistent with the delay in corneal re-epithelialization that is frequently reported in diabetes. In support of this, expression of ΔNp63α is also significantly downregulated in the rabbit contact lens model in response to hypoxic contact lens wear, which is known to attenuate basal epithelial cell proliferation.100
Similarly, immunostaining for the cytokeratin marker K17 was almost completely abolished in diabetic corneal tissue, along with reduced expression of cytokeratins K15 and K19. Likewise, integrin β1, laminin and fibronectin were also reduced, suggesting potential mechanisms that may contribute to altered epithelial cell adhesion.101 More recently, work by this same group has shown that the miRNA profile in the diabetic limbus differs substantially from the miRNA profile in the healthy, non-diabetic limbal controls.102 While further in depth studies are needed to understand the effects of diabetes on this delicate limbal compartment, the molecular changes identified to date provide some mechanistic insight into the cellular changes that have been well described. Further, understanding the effects of continuous low-grade inflammation that arises secondary to altered aqueous tear secretion in diabetes-related dry eye, coupled with the physical pressure of a contact lens on the eye, illustrates a critical unmet need that warrants additional study.
Corneal nerves
The cornea is the most innervated tissue in the body, with approximately 7,000 nociceptors/mm2 within the central cornea alone.103 These sensory nerves are essential to drive tear production and maintain the blink reflex. In relation to the corneal epithelium, trophic factors released by corneal nerves are necessary for maintaining corneal epithelial homeostasis. In support of this view, Beuerman and colleagues in the early 1980’s demonstrated that denervation of the trigeminal nerve not only disrupted corneal epithelial integrity but also adversely affected corneal wound healing.104
The effects of contact lens wear on the subbasal nerve plexus are not well described. Early studies showed that contact lens wear triggers a reduction in corneal sensitivity; however, this decrease was primarily driven by lens-induced hypoxia.105 Murphy and colleagues also demonstrated a reduction in corneal sensitivity during contact lens wear.106 In their study, they found that loss of corneal sensitivity was independent of lens type (soft versus rigid), did not vary with duration of contact lens wear, and occurred during the first few months of wear. 106 In contrast to corneal sensitivity, more recent work using in vivo confocal microscopy to visualize the subbasal nerve plexus have failed to detect a difference in corneal nerve morphology in contact lens wearers compared to non-wearers.107 Similarly, Oliveira-Soto and Efron were unable to detect a quantitative loss of the subbasal nerve plexus following contact lens wear. Instead, they observed qualitative changes in the morphology of the subbasal nerves.108 This difference was attributed to corneal edema in response to overnight lens wear.
It is well established that damage to the subbasal nerve plexus occurs in diabetes. The literature is extensive and has been reviewed elsewhere.109–113 Loss of the subbasal nerve plexus in diabetes is thought to significantly impede corneal epithelial homeostasis. Clinically, this results in a loss of barrier function, corneal erosions, and persistent epithelial defects.27, 114, 115 Dry eye is also not uncommon in diabetics, stemming from damage to sensory nerves and the attenuation of lacrimation and blink reflexes.116 Moreover, the efficacy of treatment for dry eye is mediated in part by the density of the subbasal nerve plexus.117 This further highlights the underlying need for a healthy subbasal nerve plexus to maintain the health of the corneal epithelium. The interplay between contact lens wear, subbasal nerve loss, and corneal epithelial changes has not been well investigated. Even with the use of silicone hydrogel lens materials, which eliminate the hypoxia-driven loss in corneal sensitivity during contact lens wear, it is unknown whether diabetes–induced corneal damage may precipitate and predispose the wearer to contact lens-related corneal infections.
Infection
Hallmark studies in the late eighties established the annualized incidence of corneal infection with contact lens wear.118, 119 Since that time, lens materials have continued to evolve. The introduction of silicone hydrogel contact lenses into the mainstream population led to a major improvement in corneal physiology.120, 121 In terms of extended wear, there was a shift in the risk for corneal infection.122 Specifically, it was determined that 30 day extended wear held the same risk as 6 night extended wear. Despite this initial progress, follow up epidemiological studies found that the overall annualized incidence of contact lens-related infectious keratitis in daily and extended wear remained relatively unchanged.123 Coupled with data reported in earlier studies that showed that exposure of the surface corneal epithelium to a hypoxic environment disrupted epithelial desquamation but did not increase bacterial binding to shed cells, confirms that the actual presence of the contact lens on the eye, regardless of oxygen transmission, is the key requirement that underlies the pathophysiology of contact lens-related infection.57, 121
In diabetes, the level of complexity increases. In general, published data indicates that individuals with T2DM are more prone to infections.124 This includes infections with bacteria, fungus and yeast. Fungal and yeast infections have also been reported to be more frequent in diabetics and are a major cause of disease morbidity.125 This is thought to be due in part to altered immunity in this patient group. It has also been postulated that in poorly controlled subjects, increasing glucose levels in tears, saliva, urine, skin and blood may serve as a food source for these pathogens.126 In agreement with this, a recent large-scale retrospective study in the UK found that non-eye microbial infections were the most common in subjects with poor glycemic control.124
The relationship between adequate glycemic control and infection risk is somewhat controversial, particularly in relation to the eye. While not focused specifically on diabetes, Keay, Edwards, and Stapleton identified poor systemic health as a risk factor for microbial keratitis.127 In his landmark epidemiology study published in 1989, Schein was the first to identify diabetes as the only systemic disease that was associated with an increased risk for contact lens-related microbial keratitis.128 Eichenbaum also reported on four cases of severe corneal ulcers in aphakic contact lens wearers.129 In that study, three of the four patients reported had a positive medical history for diabetes with no evidence of retinopathy or poor glycemic control.
Somewhat conflicting with Schein, Ansari and colleagues found that in the case of eye infections, subjects with diabetes were more likely to present with conjunctivitis, but there was no increased incidence of keratitis in diabetics and the severity of infection was not related to glycemic control.130 Wang et al also examined the incidence of keratitis in T2DM compared to non-diabetics.131 Unlike Ansari’s report, Wang concluded that diabetics were more prone to bacterial keratitis but there was no difference between diabetics and controls for fungal or amoebic cases. Dan et al. also examined eyes with diabetes compared to controls and reported that diabetes was in fact an independent risk factor for severe fungal keratitis.132 Moreover, in diabetics with severe fungal infections that underwent a penetrating keratoplasty to restore vision, there was a significantly increased incidence of delayed re-epithelialization. The duration of T2DM was a major factor that impacted restoration of the corneal epithelial surface.
One potential explanation for the increased risk of microbial keratitis in diabetics stems from the shift in the conjunctival flora. Reports by multiple independent groups confirm that swabs taken from the inferior palpebral conjunctiva in diabetics have a higher rate of positive cultures compared to non-diabetics.133–136 These studies also indicate that the presence of diabetic retinopathy is associated with a higher rate of positive cultures. There is some variability however, regarding the composition of the conjunctiva flora in diabetics. Most studies have reported an increase in gram-positive cultures, principally Staphylococcus spp. in diabetics. This increase is likely due to the greater number of diabetics compared to non-diabetics that present to the clinic with blepharitis.15, 16 One report however, found an increase in gram-negative bacteria in the diabetic group compared to controls.137 It was postulated that this may be due to regional or seasonal differences. Further studies are needed to investigate these findings.
Rare cases of microbial keratitis have also been reported in diabetics (Figure 5). Prototheca wickerhamii is an alga that abounds in the environment but is rarely associated with disease. In the eye, keratitis due to P. wickerhamii has only been reported in individuals with severe immune deficiencies or following intraocular surgery. In their recent paper, Tobimatsu and colleagues reported a case of a 46-year old diabetic male who developed P. wickerhamii keratitis following an eye injury (Figure 5A).138 Similarly, a recent case report identified an 82-year old man with diabetes who presented with a severe fungal keratitis due to Roussoella solani (Figure 5B).139 This was the first known case of a fungal keratitis due to this pathogen. Of high relevance to this case, the patient had no prior history of trauma with any type of soil or vegetative matter.
Figure 5:
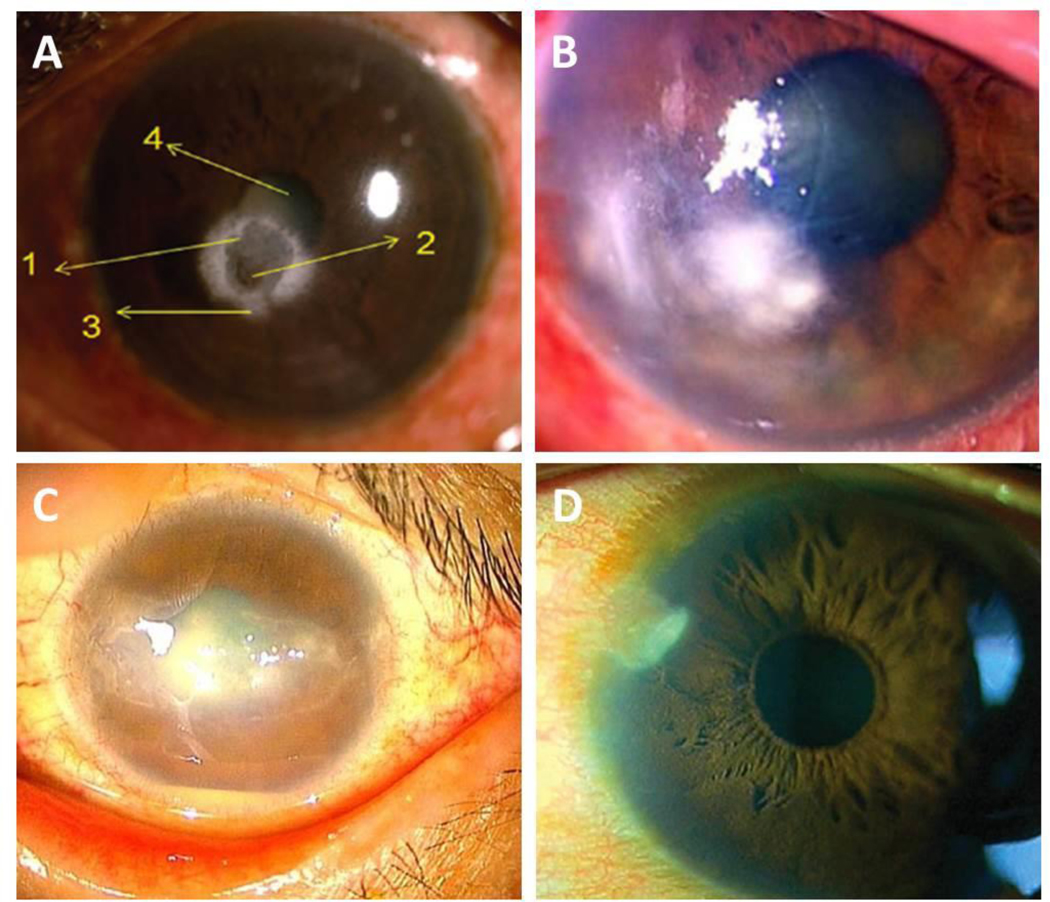
Corneal ulcers reported in diabetic patients. (A) Corneal ulcer caused by Prototheca wickerhamii. Numbers as described as detailed in the original case report. 1: central ulcer; 2: region of corneal thinning; 3: large infiltrate surrounding the ulcer; 4: lenticular changes. Image taken from Narayanan et al. Indian J Ophthalmol 2018. (B) Corneal ulcer caused by Roussoella solani. Image taken from Mochizuki et al. J Infect Chemo 2017. (C) Corneal ulcer caused by Corynebacterium propinquum. Image taken from Todokoro et al. J Clin Microbiol 2015. (D) Corneal ulcer caused by Stenotrophomonas maltophilia. Image taken from Holifield et al. Eye Contact Lens 2011.
Keratitis secondary to Corynebacterium propinquum has also been reported in a diabetic patient who was undergoing treatment with a bandage contact lens (Figure 5C).140 The subject, a 44-year old female with T1DM, nephropathy, and bilateral proliferative diabetic retinopathy, initially presented to the clinic with a persistent epithelial defect. While C. propinquum is commonly isolated from the respiratory tract, other species of Corynebacterium comprise part of the conjunctival flora. In this case, the organism was cultured from the cornea and appropriate antimicrobial therapy led to complete resolution of the infection. Not surprisingly, re-epithelialization was significantly delayed and took several months to fully heal. In another case, a 41-year old male with a history of diabetes and poor glycemic control presented to the clinic with eye pain, redness, tearing, and photophobia (Figure 5D). Cultures confirmed a diagnosis of microbial keratitis secondary to the gram-negative bacteria Stenotrophomonas maltophilia,141 Importantly, the patient denied any history of recent ocular trauma, surgery, or contact lens wear and no other comorbid disease was present. This infection was considered spontaneous and secondary to his non-controlled diabetes. While these case studies are in no way a conclusive list, they do highlight the increased risk for microbial keratitis in patients with diabetes and an increased risk for pathogens not commonly reported clinically.
Tear glucose monitoring
With an established relationship between tear and serum glucose and the reported findings that tear glucose levels are frequently elevated approximately 5 fold in diabetics compared to non-diabetic patients, the use of tear glucose-based contact lenses have received significant attention in recent years.142 Multiple designs and monitoring paradigms for these novel lenses are in development and have been described elsewhere.4 Many of these designs include embedding a glucose sensor into either conventional hydrogel or silicone hydrogel lens material, both of which are approved by the Food and Drug Administration (FDA) for daily contact lens wear. It has also been hypothesized that not only will these lenses allow for continuous tear glucose monitoring throughout the day, but they may be worn in an extended wear modality to monitor for glucose changes during sleep. Given the well-established increased infection risk with overnight or extended contact lens wear, the use of lens-based glucose sensors for continuous overnight wear greatly heightens the potential risk associated with these lenses.118, 143, 144
Infection risk aside, there are other biological considerations that may limit the use of contact lenses as glucose monitors. These include diurnal and day to day variations in tear glucose levels in addition to the effects of reflexive tear stimulation and dry eye.145 The need for calibration of these monitoring devices and their biocompatibility with contact lens care systems and cleaning regimens (such as digital rubbing to clean the lens) have not yet been reported.
Conclusions
Only a handful of studies have examined the potential for adverse ocular events in contact lens wearers that are diabetic.146–148 More large scale, prospective studies to evaluate the pathobiology of the diabetic corneal epithelium under the contact lens are needed to establish an actual level of safety. Many of the cell culture studies that function to tease out the molecular dysregulation that occurs in diabetes are performed in the presence of elevated extracellular glucose. Maintaining optimal glycemic control is necessary for preventing diabetic complications in Type 1 disease.149, 150 Unfortunately, while glycemic control is also important in Type 2 disease, it is not the only factor that predisposes this patient population to the development of complications, including those involving the eye. Other key factors include inflammation, oxidative stress, metabolic alterations, and epigenetic modifications.151–154
It is well established in the literature that diabetics do carry an inherent increased risk for infection. Likewise, microbial keratitis secondary to the presence of rare organisms has been reported. The independent effects of contact lenses and diabetes on the corneal epithelium have been reasonably well characterized. However, the scope and magnitude of these changes vary with lens material and the severity of disease. It remains unknown how epithelial homeostasis is disrupted during contact lens wear in the presence of systemic disease. Given the continued rise in the prevalence of diabetes in the US and abroad and the potential for contact lens wear to explode within this patient population, it is imperative that the clinician pay particular attention to the duration of diabetes, the level of glycemic control, the presence of retinopathy, and the patient’s overall health status when making the decision whether to fit contact lenses. While not discussed in this review, the existing level of corneal sensitivity should also be documented. This is especially important when treating diabetic patients with epithelial abnormalities or abrasions arising secondary to contact lens wear.
Highlights.
Diabetes negatively impacts the cornea in up to 70% of patients and can result in painful, sight-threatening complications.
Like diabetes, contact lens wear alters the biology of the cornea and ocular surface.
Disruption of the innate corneal epithelial barrier in diabetes and an altered immune system may predispose diabetic patients to an increased risk of infection during contact lens wear.
The implementation of glucose sensing contact lenses into the market will rapidly expand the number of diabetics wearing lenses. This creases an urgent, unmet need to elucidate the effects of contact lens wear on the diabetic cornea.
Acknowledgments
Support: EY024546 (DMR), EY024333 (DMR), Core grant for vision research EY020799, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
The funders had no role in the writing of this manuscript.
Footnotes
Conflict of interest: None of the other authors have any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badugu R, Reece EA, Lakowicz JR. Glucose-sensitive silicone hydrogel contact lens toward tear glucose monitoring. J Biomed Opt. 2018;23(5): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Hodge W, Hutnick C, Wang X. Noninvasive diagnostic devices for diabetes through measuring tear glucose. J Diabetes Sci Technol. 2011;5(1): 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.March WF, Mueller A, Herbrechtsmeier P. Clinical trial of a noninvasive contact lens glucose sensor. Diabetes Technol Ther. 2004;6(6): 782–789. [DOI] [PubMed] [Google Scholar]
- 4.Ascaso FJ, Huerva V. Noninvasive Continuous Monitoring of Tear Glucose Using Glucose-Sensing Contact Lenses. Optometry and vision science : official publication of the American Academy of Optometry. 2016;93(4): 426–434. [DOI] [PubMed] [Google Scholar]
- 5.Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012;130(12): 1534–1539. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri K, Weinreb RN. Meeting an unmet need in glaucoma: continuous 24-h monitoring of intraocular pressure. Expert Rev Med Devices. 2012;9(3): 225–231. [DOI] [PubMed] [Google Scholar]
- 7.Sankaridurg P, Holden B, Smith E, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011;52(13): 9362–9367. [DOI] [PubMed] [Google Scholar]
- 8.Ciolino JB, Dohlman CH, Kohane DS. Contact lenses for drug delivery. Semin Ophthalmol. 2009;24(3): 156–160. [DOI] [PubMed] [Google Scholar]
- 9.Guzman-Aranquez A, Colligris B, Pintor J . Contact lenses: promising devices for ocular drug delivery. J Ocul Pharmacol Ther. 2012;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Jung HJ, Chauhan A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials. 2012;33(7): 2289–2300. [DOI] [PubMed] [Google Scholar]
- 11.Lane JD, Krumholz DM, Sack RA, Morris C. Tear glucose dynamics in diabetes mellitus. Curr Eye Res. 2006;31: 895–901. [DOI] [PubMed] [Google Scholar]
- 12.Gale EAM. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51(12): 3353–3361. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Jick S, Breitenstein S, Michel A. Prevalence of diabetes and diabetic nephropathy in a large US commercially insured pediatric population, 2002–2013. Diabetes Care. 2016;39(2): 278–284. [DOI] [PubMed] [Google Scholar]
- 14.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015;314(10): 1021–1029. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell C, Efron N. Diabetes and contact lens wear. Clinical & experimental optometry. 2012;95(3): 328–337. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell C, Efron N. Contact lens wear and diabetes mellitus. Contact lens & anterior eye : the journal of the British Contact Lens Association. 1998;21(1): 19–26. [PubMed] [Google Scholar]
- 17.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. The Journal of cell biology. 1986;103(1): 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investigative ophthalmology & visual science. 1983;24(10): 1442–1443. [PubMed] [Google Scholar]
- 19.Kruse FE, Chen JJ, Tsai RJ, Tseng SC. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Investigative ophthalmology & visual science. 1990;31(9): 1903–1913. [PubMed] [Google Scholar]
- 20.Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. The journal of investigative dermatology Symposium proceedings. 2004;9(3): 202–207. [DOI] [PubMed] [Google Scholar]
- 21.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Experimental eye research. 2004;78(3): 433–446. [DOI] [PubMed] [Google Scholar]
- 22.Ljubimov AV. Diabetic complications in the cornea. Vision Res. 2017;139: 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabatabay CA, Bumbacher M, Baumgartner B, Leuenberger PM. Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 1988;226: 389–392. [DOI] [PubMed] [Google Scholar]
- 24.Taylor HR, Kimsey RA. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci. 1981;20(4): 548–553. [PubMed] [Google Scholar]
- 25.Ljubimov AV, Huang Z-S, Huang GH, et al. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998;46: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 26.Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77(2): 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobbels M, Spitznas M, Oldendoerp J. Impairment of corneal epithelial barrier function in diabetics. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1989;227(2): 142–144. [DOI] [PubMed] [Google Scholar]
- 28.Azar DT, Spurr-Michaud SJ, Tisdale A, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Archives of ophthalmology (Chicago, Ill: 1960). 1992;110(4): 537–540. [DOI] [PubMed] [Google Scholar]
- 29.Gekka M, Miyata K, Nagai Y, et al. Corneal epithelial barrier function in diabetic patients. Cornea. 2004;23(1): 35–37. [DOI] [PubMed] [Google Scholar]
- 30.Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Archives of ophthalmology (Chicago, Ill: 1960). 1979;97(6): 1076–1078. [DOI] [PubMed] [Google Scholar]
- 31.Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Decreased penetration of anchoring fibrils into the diabetic stroma. Archives of ophthalmology (Chicago, Ill: 1960). 1989;107(10): 1520–1523. [DOI] [PubMed] [Google Scholar]
- 32.Tavakoli M, Kallinikos PA, Efron N, Boulton AJM, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy is patients with diabetes. Diab Care. 2007;30(7): 1895–1897. [DOI] [PubMed] [Google Scholar]
- 33.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8): 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7): 2454–2463. [DOI] [PubMed] [Google Scholar]
- 35.Ladage PM, Ren DH, Petroll WM, Jester JV, Bergmanson JP, Cavanagh HD. Effects of eyelid closure and disposable and silicone hydrogel extended contact lens wear on rabbit corneal epithelial proliferation. Investigative ophthalmology & visual science. 2003;44(5): 1843–1849. [DOI] [PubMed] [Google Scholar]
- 36.Ladage PM, Yamamoto K, Ren DH, et al. Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear. Investigative ophthalmology & visual science. 2001;42(12): 2804–2812. [PubMed] [Google Scholar]
- 37.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 38.Ren DH, Petroll WM, Jester JV, Cavanagh HD. The effect of rigid gas permeable contact lens wear on proliferation of rabbit corneal and conjunctival epithelial cells. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 1999;25(3): 136–141. [PubMed] [Google Scholar]
- 39.Ladage PM, Jester JV, Petroll WM, Bergmanson JP, Cavanagh HD. Vertical movement of epithelial basal cells toward the corneal surface during use of extended-wear contact lenses. Investigative ophthalmology & visual science. 2003;44(3): 1056–1063. [DOI] [PubMed] [Google Scholar]
- 40.Ladage PM, Yamamoto K, Ren DH, et al. Recovery time of corneal epithelial proliferation in the rabbit following rigid gas–permeable extended contact-lens wear. Eye & contact lens. 2003;29(2): 61–64. [DOI] [PubMed] [Google Scholar]
- 41.Hamano H, Hori M. Effect of contact lens wear on the mitoses of corneal epithelial cells: preliminary report. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 1983;9(2): 133–136. [PubMed] [Google Scholar]
- 42.Woo HM, Bentley E, Campbell SF, Marfurt CF, Murphy CJ. Nerve growth factor and corneal wound healing in dogs. Experimental eye research. 2005;80(5): 633–642. [DOI] [PubMed] [Google Scholar]
- 43.Fonn D, du Toit R, Simpson TL, Vega JA, Situ P, Chalmers RL. Sympathetic swelling response of the control eye to soft lenses in the other eye. Investigative ophthalmology & visual science. 1999;40(13): 3116–3121. [PubMed] [Google Scholar]
- 44.Fujita H, Morita I, Takase H, Ohno-Matsui K, Mochizuki M. Prolonged exposure to high glucose impaired cellular behavior of normal human corneal epithelial cells. Curr Eye Res. 2003;27: 197–203. [DOI] [PubMed] [Google Scholar]
- 45.McDermott AM, Kern TS, Murphy CJ. The effect of elevated extracellular glucose on migration, adhesion and proliferation of SV40 transformed human corneal epithelial cells. Curr Eye Res. 1998;17(9): 924–932. [DOI] [PubMed] [Google Scholar]
- 46.Ren H, Wilson G. Apoptosis in the corneal epithelium. Investigative ophthalmology & visual science. 1996;37(6): 1017–1025. [PubMed] [Google Scholar]
- 47.Yamamoto K, Ladage PM, Ren DH, et al. Effects of low and hyper Dk rigid gas permeable contact lenses on Bcl-2 expression and apoptosis in the rabbit corneal epithelium. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 2001;27(3): 137–143. [PubMed] [Google Scholar]
- 48.Yamamoto K, Ladage PM, Ren DH, Li L, Jester JV, Cavanagh HD. Epitope variability of Bcl-2 immunolocalization in the human corneal epithelium. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 2001;27(4): 221–224. [PubMed] [Google Scholar]
- 49.Yamamoto K, Ladage PM, Ren DH, et al. Bcl-2 expression in the human cornea. Experimental eye research. 2001;73(2): 247–255. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Ren DH, Ladage PM, et al. Annexin V binding to rabbit corneal epithelial cells following overnight contact lens wear or eyelid closure. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 2002;28(1): 48–54. [PubMed] [Google Scholar]
- 51.Yamamoto K, Ladage PM, Ren DH, et al. Effect of eyelid closure and overnight contact lens wear on viability of surface epithelial cells in rabbit cornea. Cornea. 2002;21(1): 85–90. [DOI] [PubMed] [Google Scholar]
- 52.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108(7): 1279–1288. [DOI] [PubMed] [Google Scholar]
- 53.Ren DH, Yamamoto K, Ladage PM, et al. Adaptive effects of 30-night wear of hyper-O(2) transmissible contact lenses on bacterial binding and corneal epithelium: a 1-year clinical trial. Ophthalmology. 2002;109(1): 27–39; discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 54.Cavanagh HD, Ladage PM, Li SL, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: a 13-month clinical trial. Ophthalmology. 2002;109(11): 1957–1969. [DOI] [PubMed] [Google Scholar]
- 55.O’Leary DJ, Madgewick R, Wallace J, Ang J. Size and number of epithelial cells washed from the cornea after contact lens wear. Optometry and vision science : official publication of the American Academy of Optometry. 1998;75(9): 692–696. [DOI] [PubMed] [Google Scholar]
- 56.Robertson DM, Petroll WM, Cavanagh HD. The effect of nonpreserved care solutions on 12 months of daily and extended silicone hydrogel contact lens wear. Investigative ophthalmology & visual science. 2008;49(1): 7–15. [DOI] [PubMed] [Google Scholar]
- 57.Ren DH, Petroll WM, Jester JV, Ho-Fan J, Cavanagh HD. Short-term hypoxia downregulates epithelial cell desquamation in vivo, but does not increase Pseudomonas aeruginosa adherence to exfoliated human corneal epithelial cells. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 1999;25(2): 73–79. [PubMed] [Google Scholar]
- 58.Pflugfelder SC, de Paiva CS. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology. 2017;124(11s): S4–s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou X, Lu L, Xu Y, et al. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC ophthalmology. 2018;18(1): 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC ophthalmology. 2008;8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shujaat S, Jawed M, Memon S, Talpur KI. Determination of Risk Factors and Treatment of Dry Eye Disease in Type 1 Diabetes Before Corneal Complications at Sindh Institute of Ophthalmology And Visual Sciences. The open ophthalmology journal. 2017;11: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Najafi L, Malek M, Valojerdi AE, et al. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. Journal of diabetes and its complications. 2013;27(5): 459–462. [DOI] [PubMed] [Google Scholar]
- 63.Yu T, Shi WY, Song AP, Gao Y, Dang GF, Ding G. Changes of meibomian glands in patients with type 2 diabetes mellitus. International journal of ophthalmology. 2016;9(12): 1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nepp J, Abela C, Polzer I, Derbolav A, Wedrich A. Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea. 2000;19(4): 487–491. [DOI] [PubMed] [Google Scholar]
- 65.Lv H, Li A, Zhang X, et al. Meta-analysis and review on the changes of tear function and corneal sensitivity in diabetic patients. Acta Ophthalmol. 2014;92(2): e96–e104. [DOI] [PubMed] [Google Scholar]
- 66.Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108(3): 586–592. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Kim CS, Sohn E, Jeong IH, Kim H, Kim JS. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249(4): 529–536. [DOI] [PubMed] [Google Scholar]
- 68.Park JH, Kang SS, Kim JY, Tchah H. Nerve Growth Factor Attenuates Apoptosis and Inflammation in the Diabetic Cornea. Investigative ophthalmology & visual science. 2016;57(15): 6767–6775. [DOI] [PubMed] [Google Scholar]
- 69.Shi L, Yu X, Yang H, Wu X. Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PloS one. 2013;8(6): e66781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto N, Yamamoto N, Petroll MW, Cavanagh HD, Jester JV. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(4): 1348–1355. [DOI] [PubMed] [Google Scholar]
- 71.Holden BA, Sweeney DF, Vannas A, Nilsson KT, Efron N. Effects of long-term extended contact lens wear on the human cornea. Investigative ophthalmology & visual science. 1985;26(11): 1489–1501. [PubMed] [Google Scholar]
- 72.Jalbert I, Sweeney DF, Stapleton F. The effect of long-term wear of soft lenses of low and high oxygen transmissibility on the corneal epithelium. Eye (Lond). 2009;23(6): 1282–1287. [DOI] [PubMed] [Google Scholar]
- 73.Perez JG, Meijome JM, Jalbert I, Sweeney DF, Erickson P. Corneal epithelial thinning profile induced by long-term wear of hydrogel lenses. Cornea. 2003;22(4): 304–307. [DOI] [PubMed] [Google Scholar]
- 74.Tsubota K, Toda I, Fujishima H, Yamada M, Sugawara T, Shimazaki J. Extended wear soft contact lenses induce corneal epithelial changes. The British journal of ophthalmology. 1994;78(12): 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsubota K, Hata S, Toda I, Yagi Y, Sakata M, Shimazaki J. Increase in corneal epithelial cell size with extended wear soft contact lenses depends on continuous wearing time. The British journal of ophthalmology. 1996;80(2): 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren DH, Petroll WM, Jester JV, Ho-Fan J, Cavanagh HD. The relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended wear. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 1999;25(2): 80–100. [PubMed] [Google Scholar]
- 77.Cavanagh HD, Ladage P, Yamamoto K, Li SL, Petroll WM, Jester JV. Effects of daily and overnight wear of hyper-oxygen transmissible rigid and silicone hydrogel lenses on bacterial binding to the corneal epithelium: 13-month clinical trials. Eye & contact lens. 2003;29(1 Suppl): S14–16; discussion S26–19, S192–194. [DOI] [PubMed] [Google Scholar]
- 78.Mathers WD, Sachdev MS, Petroll M, Lemp MA. Morphologic effects of contact lens wear on the corneal surface. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc. 1992;18(1): 49–52. [PubMed] [Google Scholar]
- 79.Ladage PM, Yamamoto K, Li L, et al. Corneal epithelial homeostasis following daily and overnight contact lens wear. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2002;25(1): 11–21. [DOI] [PubMed] [Google Scholar]
- 80.Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19(9). [DOI] [PubMed] [Google Scholar]
- 81.Cai D, Zhu M, Petroll WM, Koppaka V, Robertson DM. The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am J Pathol. 2014;184(10): 2662–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of corneal epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci. 2012;53: 8067–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin JH, J., Chen C, Gao N, Wang F, Yu F-S. Corneal complications in streptozotocin-induced type 1 diabetic rats. Invest Ophthalmol Vis Sci. 2011;52: 6589–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102(10): 1476–1485. [DOI] [PubMed] [Google Scholar]
- 85.Martin R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom. 2007;90(1): 26–30. [DOI] [PubMed] [Google Scholar]
- 86.D’Aversa G, Luchs JL, Fox MJ, Rosenbaum PS, Udell IJ. Advancing wave-like epitheliopathy. Clinical features and treatment. Ophthalmology. 1997;104(6): 962–969. [DOI] [PubMed] [Google Scholar]
- 87.Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121(10): 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan CC, Holland EJ. Severe limbal stem cell deficiency from contact lens wear: patient clinical features. American journal of ophthalmology. 2013;155(3): 544–549.e542. [DOI] [PubMed] [Google Scholar]
- 89.Termote K, Schendel S, Moloney G, Holland SP, Lange AP. Focal limbal stem cell deficiency associated with soft contact lens wear. Canadian journal of ophthalmology Journal canadien d’ophtalmologie. 2017;52(6): 552–558. [DOI] [PubMed] [Google Scholar]
- 90.Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30(1): 18–23. [DOI] [PubMed] [Google Scholar]
- 91.Truong TN, Graham AD, Lin MC. Factors in contact lens symptoms: evidence from a multistudy database. Optometry and vision science : official publication of the American Academy of Optometry. 2014;91(2): 133–141. [DOI] [PubMed] [Google Scholar]
- 92.Garofalo RJ, Dassanayake N, Carey C, Stein J, Stone R, David R. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye & contact lens. 2005;31(4): 166–174. [DOI] [PubMed] [Google Scholar]
- 93.Lebow KA, Schachet JL. Evaluation of corneal staining and patient preference with use of three multi-purpose solutions and two brands of soft contact lenses. Eye & contact lens. 2003;29(4): 213–220. [DOI] [PubMed] [Google Scholar]
- 94.Holland EJ, Schwartz GS. Iatrogenic limbal stem cell deficiency. Transactions of the American Ophthalmological Society. 1997;95: 95–107; discussion 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Craig JP, Willcox MD, Argueso P, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Investigative ophthalmology & visual science. 2013;54(11): Tfos123–156. [DOI] [PubMed] [Google Scholar]
- 96.Benjamin WJ, Hill RM. Human cornea: superior and central oxygen demands. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1988;226(1): 41–44. [DOI] [PubMed] [Google Scholar]
- 97.Benjamin WJ, Rasmussen MA. Oxygen consumption of the superior cornea following eyelid closure. Acta ophthalmologica. 1988;66(3): 309–312. [DOI] [PubMed] [Google Scholar]
- 98.Rossen J, Amram A, Milani B, et al. Contact Lens-induced Limbal Stem Cell Deficiency. The ocular surface. 2016; 14(4): 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci. 2001;98(6): 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robertson DM, Zhu M, Wu YC, Cavanagh HD. Hypoxia-induced downregulation of DeltaNp63alpha in the corneal epithelium. Eye & contact lens. 2012;38(4): 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saghizadeh M, Soleymani S, Harounian A, et al. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Molecular vision. 2011;17: 2177–2190. [PMC free article] [PubMed] [Google Scholar]
- 102.Kulkarni M, Leszczynska A, Wei G, et al. Genome-wide analysis suggests a differential microRNA signature associated with normal and diabetic human corneal limbus. Scientific reports. 2017;7(1): 3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang AY, Chow J, Liu J. Corneal Innervation and Sensation: The Eye and Beyond. The Yale journal of biology and medicine. 2018;91(1): 13–21. [PMC free article] [PubMed] [Google Scholar]
- 104.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Experimental neurology. 1980;69(1): 196–201. [DOI] [PubMed] [Google Scholar]
- 105.Millodot M, O’Leary DJ. Effect of oxygen deprivation on corneal sensitivity. Acta ophthalmologica. 1980;58(3): 434–439. [DOI] [PubMed] [Google Scholar]
- 106.Murphy PJ, Patel S, Marshall J. The effect of long-term, daily contact lens wear on corneal sensitivity. Cornea. 2001;20(3): 264–269. [DOI] [PubMed] [Google Scholar]
- 107.Golebiowski B, Chao C, Stapleton F, Jalbert I. Corneal Nerve Morphology, Sensitivity, and Tear Neuropeptides in Contact Lens Wear. Optometry and vision science : official publication of the American Academy of Optometry. 2017;94(4): 534–542. [DOI] [PubMed] [Google Scholar]
- 108.Oliveira-Soto L, Efron N. Morphology of corneal nerves in soft contact lens wear. A comparative study using confocal microscopy. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2003;23(2): 163–174. [DOI] [PubMed] [Google Scholar]
- 109.Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. OculSurf. 2018;16(1): 45–57. [DOI] [PubMed] [Google Scholar]
- 110.Papanas N, Ziegler D. Corneal confocal microscopy: Recent progress in the evaluation of diabetic neuropathy. Journal of diabetes investigation. 2015;6(4): 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Current diabetes reports. 2013;13(4): 488–499. [DOI] [PubMed] [Google Scholar]
- 112.Tavakoli M, Petropoulos IN, Malik RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. Journal of diabetes science and technology. 2013;7(5): 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017;15(1): 15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Current diabetes reviews. 2012;8(4): 294–302. [DOI] [PubMed] [Google Scholar]
- 115.Bikbova G, Oshitari T, Baba T, Bikbov M, Yamamoto S. Diabetic corneal neuropathy: clinical perspectives. Clin Ophthalmol. 2018;12: 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. Journal of ophthalmology. 2016;2016: 8201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kheirkhah A, Dohlman TH, Amparo F, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122(4): 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321(12): 779–783. [DOI] [PubMed] [Google Scholar]
- 119.Schein OD, Ormerod LD, Barraquer E, et al. Microbiology of contact lens-related keratitis. Cornea. 1989;8(4): 281–285. [PubMed] [Google Scholar]
- 120.Cavanagh HD, Robertson DM, Petroll WM, Jester JV. Castroviejo Lecture 2009: 40 years in search of the perfect contact lens. Cornea. 2010;29(10): 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robertson DM. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye & contact lens. 2013;39(1): 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115(10): 1655–1662. [DOI] [PubMed] [Google Scholar]
- 123.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens related microbial keratitis in Australia. Ophthalmology. 2008,115: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 124.Hine JL, de Lusignan S, Burleigh D, et al. Association between glycaemic control and common infections in people with Type 2 diabetes: a cohort study. Diabetic medicine : a journal of the British Diabetic Association. 2017;34(4): 551–557. [DOI] [PubMed] [Google Scholar]
- 125.Poradzka A, Jasik M, Karnafel W, Fiedor P. Clinical aspects of fungal infections in diabetes. Acta poloniae pharmaceutica. 2013;70(4): 587–596. [PubMed] [Google Scholar]
- 126.Manfredi M, McCullough MJ, Vescovi P, Al-Kaarawi ZM, Porter SR. Update on diabetes mellitus and related oral diseases. Oral diseases. 2004;10(4): 187–200. [DOI] [PubMed] [Google Scholar]
- 127.Keay L, Edwards K, Stapleton F. Signs, symptoms, and comorbidities in contact lens-related microbial keratitis. Optometry and vision science : official publication of the American Academy of Optometry. 2009;86(7): 803–809. [DOI] [PubMed] [Google Scholar]
- 128.Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. The New England journal of medicine. 1989;321(12): 773–778. [DOI] [PubMed] [Google Scholar]
- 129.Eichenbaum JW, Feldstein M, Podos SM. Extended-wear aphakic soft contact lenses and corneal ulcers. The British journal of ophthalmology. 1982;66(10): 663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ansari AS, de Lusignan S, Hinton W, Munro N, McGovern A. The association between diabetes, level of glycaemic control and eye infection: Cohort database study. Primary care diabetes. 2017;11(5): 421–429. [DOI] [PubMed] [Google Scholar]
- 131.Wang B, Yang S, Zhai HL, et al. A comparative study of risk factors for corneal infection in diabetic and non-diabetic patients. International journal of ophthalmology. 2018;11(1): 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dan J, Zhou Q, Zhai H, et al. Clinical analysis of fungal keratitis in patients with and without diabetes. PloS one. 2018;13(5): e0196741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martins EN, Alvarenga LS, Hofling-Lima AL, et al. Aerobic bacterial conjunctival flora in diabetic patients. Cornea. 2004;23(2): 136–142. [DOI] [PubMed] [Google Scholar]
- 134.Karimsab D, Razak SK. Study of aerobic bacterial conjunctival flora in patients with diabetes mellitus. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2013;5(1): 28–32. [DOI] [PubMed] [Google Scholar]
- 135.Bilen H, Ates O, Astam N, Uslu H, Akcay G, Baykal O. Conjunctival flora in patients with type 1 or type 2 diabetes mellitus. Advances in therapy. 2007;24(5): 1028–1035. [DOI] [PubMed] [Google Scholar]
- 136.Kawata T, Matsuo T. Positive bacterial culture in conjunctival sac before cataract surgery with night stay is related to diabetes mellitus. BMC ophthalmology. 2017;17(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adam M, Balci M, Bayhan HA, Inkaya AC, Uyar M, Gurdal C. Conjunctival Flora in Diabetic and Nondiabetic Individuals. Turkish journal of ophthalmology. 2015;45(5): 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narayanan N, Vaidehi D, Dhanurekha L, et al. Unusual ulcerative keratitis caused by Prototheca wickerhamii in a diabetic patient. Indian journal of ophthalmology. 2018;66(2): 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mochizuki K, Nishida T, Murata K, et al. Roussoella solani causing keratomycosis, with an observed both sexual and asexual morphs. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2017;23(9): 651–654. [DOI] [PubMed] [Google Scholar]
- 140.Todokoro D, Eguchi H, Yamada N, Sodeyama H, Hosoya R, Kishi S. Contact Lens-Related Infectious Keratitis with White Plaque Formation Caused by Corynebacterium propinquum. Journal of clinical microbiology. 2015;53(9): 3092–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holifield K, Lazzaro DR. Case report: spontaneous Stenotrophomonas maltophilia keratitis in a diabetic patient. Eye Contact Lens. 2011;37(5): 326–327. [DOI] [PubMed] [Google Scholar]
- 142.Sen DK, Sarin GS. Tear glucose levels in normal people and in diabetic patients. The British journal of ophthalmology. 1980;64(9): 693–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N Engl J Med. 1989;321(12): 773–778. [DOI] [PubMed] [Google Scholar]
- 144.Choy MH, Stapleton F, Willcox MD, Zhu H. Comparison of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens- and non-contact lens-related keratitis. J Med Microbiol. 2008;57(Pt 12): 1539–1546. [DOI] [PubMed] [Google Scholar]
- 145.Baca JT, Finegold DN, Asher SA. Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul Surf. 2007;5(4): 280–293. [DOI] [PubMed] [Google Scholar]
- 146.O’Donnell C, Efron N, Boulton AJ. A prospective study of contact lens wear in diabetes mellitus. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2001;21(2): 127–138. [DOI] [PubMed] [Google Scholar]
- 147.March W, Long B, Hofmann W, Keys D, McKenney C. Safety of contact lenses in patients with diabetes. Diabetes technology & therapeutics. 2004;6(1): 49–52. [DOI] [PubMed] [Google Scholar]
- 148.Leem HS, Lee KJ, Shin KC. Central corneal thickness and corneal endothelial cell changes caused by contact lens use in diabetic patients. Yonsei medical journal. 2011;52(2): 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England journal of medicine. 1993;329(14): 977–986. [DOI] [PubMed] [Google Scholar]
- 150.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes care. 2010;33(5): 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiological reviews. 2013;93(1): 137–188. [DOI] [PubMed] [Google Scholar]
- 152.Stadler K. Oxidative stress in diabetes. Advances in experimental medicine and biology. 2012;771: 272–287. [DOI] [PubMed] [Google Scholar]
- 153.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. The Journal of clinical investigation. 2005;115(5): 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3): 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]