Abstract
Introduction
The co-use of cannabis and alcohol among tobacco-using youth is common. Alcohol co-use is associated with worse tobacco cessation outcomes, but results are mixed regarding the impact of cannabis on tobacco outcomes and if co-use leads to increased use of non-treated substances. This secondary analysis from a youth smoking cessation trial aimed to (1) evaluate the impact of cannabis or alcohol co-use on smoking cessation, (2) examine changes in co-use during the trial, and (3) explore secondary effects of varenicline on co-use.
Methods
The parent study was a 12-week, randomized clinical trial of varenicline for smoking cessation among youth (ages 14–21, N = 157; Mage = 19, 40% female; 76% White). Daily cigarette, cannabis, and alcohol use data were collected via daily diaries during treatment and Timeline Follow-back for 14 weeks post-treatment.
Results
Baseline cannabis co-users (68%) had double the odds of continued cigarette smoking throughout the trial compared with noncannabis users, which was pronounced in males and frequent cannabis users. Continued smoking during treatment was associated with higher probability of concurrent cannabis use. Baseline alcohol co-users (80%) did not have worse smoking outcomes compared with nonalcohol users, but continued smoking was associated with higher probability of concurrent drinking. Varenicline did not affect co-use.
Conclusions
Inconsistent with prior literature, results showed that alcohol co-users did not differ in smoking cessation, whereas cannabis co-users had poorer cessation outcomes. Youth tobacco treatment would benefit from added focus on substance co-use, particularly cannabis, but may need to be tailored appropriately to promote cessation.
Implications
Among youth cigarette smokers enrolled in a pharmacotherapy evaluation clinical trial, alcohol and/or cannabis co-use was prevalent. The co-use of cannabis affected smoking cessation outcomes, but more so for males and frequent cannabis users, whereas alcohol co-use did not affect smoking cessation. Reductions in smoking were accompanied by concurrent reductions in alcohol or cannabis use. Substance co-use does not appear to affect all youth smokers in the same manner and treatment strategies may need to be tailored appropriately for those with lower odds of smoking cessation.
Introduction
Nearly 90% of all cigarette smokers initiate tobacco use in adolescence.1 Among adolescents in the United States (ages 12–17), estimates of past 30-day cigarette smoking are 4.6%,2 whereas rates among emerging adults (ages 18–25) are estimated at 22.3%.3 The majority of adolescent and emerging adult smokers have an interest in quitting, but cessation attempts rarely result in sustained abstinence, even when evidence-based tobacco treatment is used.4–6 As with adult smokers,7 one barrier to long-term abstinence among youth may be the role of co-occurring substance use.
Cannabis and/or alcohol use is prevalent among adolescents and emerging adults. Recent estimates of past month cannabis and alcohol use in the United States among 12–17 year olds were 6.5% and 9.9%, respectively, and among 18–25 year olds were 22.1% and 56.3%, respectively.3 Cannabis and/or alcohol use are particularly prevalent and problematic among youth smokers. Adolescents who use tobacco are more likely to use cannabis and alcohol on a daily basis compared with nonsmoking adolescents,8 and are at increased risk of developing cannabis and alcohol problems in young adulthood.9 The co-use of tobacco, cannabis, and alcohol prior to the age of 16, compared with use of a single substance, is predictive of problematic substance use outcomes in young adulthood (e.g., nonmedical prescription drug use, transition to illicit drug use and dependence).10 Although the co-use of these substances in adolescence is problematic in the long-term, there are also immediate treatment-related concerns associated with co-use and a potentially detrimental impact on cessation.
The co-use of cannabis and tobacco occurs frequently among youth,11–13 with initial use of a single substance (cannabis or tobacco) predicting subsequent use of the other.14,15 Results from cross-sectional studies or secondary analyses evaluating the impact of cannabis co-use on tobacco outcomes have been mixed. Some studies have found that cannabis co-use is associated with worse tobacco cessation outcomes compared with tobacco-only users.14,16,17 Consistent with those findings, a recent secondary analysis from a tobacco cessation study with emerging adults found that cannabis co-use was associated with a decreased likelihood of tobacco reduction or cessation, though co-use was not associated with motivation to quit tobacco or quit attempts.18 Other studies have shown no adverse impact of cannabis use on tobacco outcomes.19–21 Additionally, tobacco treatment may be associated with continued or even increased use of cannabis among co-users, and prospective data to address this are lacking.
Similar to cannabis, alcohol co-use is common among smokers, and may be particularly deleterious for youth. Approximately three quarters of youth smokers report hazardous drinking in the past year, compared with less than half of nonsmokers.22,23 Evidence indicates that early tobacco initiation is related to subsequent alcohol use,24 including excessive alcohol consumption and more severe alcohol use disorder symptoms.25 Smoking cessation outcomes tend to be poorer for both adolescent26–28 and adult alcohol co-users.19,29 Although alcohol use is often associated with increased cigarette craving and vice versa,30,31 a recent review of human laboratory studies concluded nicotine deprivation results in mixed findings with respect to increased alcohol self-administration.31
Although the relationship between alcohol co-use and worse smoking cessation outcomes is supported in the literature, results are mixed regarding the impact of cannabis co-use on smoking cessation outcomes. Furthermore, continued or increased use of a nontreated substance is an important, yet understudied issue among co-users, and daily alcohol and cannabis use is typically not collected within smoking cessation studies. Within the context of an adolescent and emerging adult smoking cessation pharmacotherapy clinical trial,32 we conducted a secondary analysis of the impact of cannabis and/or alcohol co-use on smoking cessation outcomes. Primarily, the aims of this secondary analysis were to (1) evaluate the impact of any cannabis or alcohol co-use/severity of co-use at baseline on smoking cessation outcomes during treatment and at follow-up and (2) examine changes in cannabis and/or alcohol use during the trial and at follow-up. Secondarily, since the parent study evaluated varenicline (Chantix) for smoking cessation, and there is some evidence that varenicline may have efficacy in the treatment of alcohol use disorder,33 and potentially for cannabis use disorder,34 the third aim of this study was to assess the effects of varenicline on alcohol and cannabis co-use during the trial.
Methods
Participants and Procedures
Youth smokers (N = 157) were recruited from the community in Charleston, SC from September 2012 through November 2017. To meet inclusion criteria, participants had to be between the ages of 14 and 21, were required to smoke daily or near-daily (25 out of the past 30 days) for ≥6 months, report a desire to quit, and have at least one past failed quit attempt. Female participants agreed to use birth control methods throughout the study. Exclusion criteria included lifetime history of any mood or psychotic disorder based on criteria from the Diagnostic and Statistical Manual-IV; 35 lifetime history of suicidality or homicidality; significant hostility/aggression per medical clinician judgment; current substance dependence (other than nicotine); unstable medical disorder; pregnant/breastfeeding; current use of medications with smoking cessation efficacy; or known hypersensitivity to varenicline. Written and informed consent/assent was obtained prior to study participation. For participants under the age of 18, parent(s) or guardian(s) participated in informed consent and initial assessment. All study procedures were approved by the university’s Institutional Review Board. This trial was registered with clinicaltrials.gov (NCT01509547). Primary outcomes from the parent study have been described elsewhere.32 Briefly, the parent study found that rates of abstinence from smoking at the 12-week end of treatment (EOT) visit did not differ between varenicline or placebo groups, though participants randomized to the varenicline group achieved smoking abstinence earlier in the trial and exhibited higher overall rates of abstinence throughout the trial. Varenicline was well-tolerated among the study cohort.
Eligible participants were randomized to receive a 12-week course of varenicline or matched placebo (1.0 mg twice per day), which included 1 week of dose titration. Randomization was stratified by age (14–17 years old versus 18–21 years old) and baseline smoking level (<12 versus 12 or more cigarettes per day). All participants received brief cessation counseling during weekly visits. Participants returned for weekly study visits (±3 day visit window) for 12 weeks and then returned for three post-treatment follow-up (F/U) visits at weeks 13, 16, and 26.
Measures
Screening Assessments
Assessments were conducted to collect demographics, psychiatric and medical histories, and substance use history.
Cigarette and Substance Use Measures
Calendar-based Timeline Follow-Back (TLFB)36 was used to capture self-reported substance use in the 30 days preceding screening, and at all post-treatment follow-ups for: (1) number of cigarettes per day (CPD), (2) other tobacco use, (3) cannabis use (yes/no), and (4) standard alcoholic drinks consumed [per National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines]. The use of electronic cigarettes was discouraged throughout the study and was not systematically collected. Daily cannabis use estimates were not standardized in their collection (e.g., no gram estimation), and amount of cannabis used per day was not considered in analyses. During the 12-week treatment phase, participants recorded their substance use via paper daily diaries. If daily diaries were incomplete or were not returned at the weekly study visit, research staff collected substance use data for the previous week (or since the last study visit) using retrospective TLFB. These methods yielded continuous, daily self-reports of smoking, and substance use throughout the treatment period and follow-up.
The modified Fagerström Tolerance Questionnaire (mFTQ)37 is a 7-item measure, validated in adolescents, to assess nicotine dependence. Scores range from 0 to 9, with higher scores reflecting greater nicotine dependence. The 5-item Penn Alcohol Craving Scale (PACS)38 was administered at each study visit (scores range from 0 to 30) for all participants, regardless of their endorsement of alcohol use during screening. The PACS was added to the assessment battery after we became aware of preliminary findings in the literature regarding varenicline’s effects on alcohol use.39 The PACS was added after study enrollment had commenced and was only administered to 155 study participants.
Biological Measures
Biological confirmation of smoking was assessed using: (1) breath carbon monoxide (CO) at all visits (≤8 parts per million were considered abstinent), and (2) urinary cotinine collected at screening, week 12, and week 26 (≤50 ng/mL considered abstinent). Biochemical verification of abstinence was not used in this analysis since: (1) breath CO is elevated by combustible cannabis use,40 (2) the use of blunts (loose leaf cannabis rolled in a cigar wrapper) was prevalent among cannabis users, which may have elevated their urinary cotinine, and (3) self-reported tobacco use among adolescents has been shown to correlate with biological verification.41 Taken together, self-reported tobacco use served as our outcome measure here.
An instant-read, 6-panel urine drug screen (UDS) was administered at screening, randomization, weeks 4, 8, 12, and 26. Cannabis tests measured the presence of Δ 9-tetrahydrocannabinol (THC) metabolites in the urine sample (>50 ng/mL considered positive). No biochemical verification of alcohol use was conducted in this study.
Statistical Analyses
Cannabis co-users were defined as any participant with a positive urine cannabinoid UDS at screening or any self-reported cannabis use in the 30 days preceding screening. Alcohol co-users were defined as participants who self-reported consuming at least one standard drink in the past 30 days before screening. Standard descriptive statistics were used to summarize baseline demographics, tobacco, cannabis, and alcohol use characteristics for all participants (N = 157), as well as cannabis co-users (n = 107) and alcohol co-users (n = 125). Co-use groups were not mutually exclusive (Table 1; Supplementary Figure 1), and many participants used both cannabis and alcohol (n = 91; 58%).
Table 1.
Demographics and Clinical Characteristics for the Overall Sample (N = 157) and Separated by Cannabis Co-Users and Alcohol Co-Users at Screening (Not Mutually Exclusive Groups)
| Demographics and Clinical Characteristics at Screening | Entire Cohort N=157 | Cannabis Users n=107 | Alcohol Users n=125 |
|---|---|---|---|
| Age | 19.1 (1.5) | 19.0 (1.5) | 19.2 (1.4) |
| Male %(n) | 59.9 (94) | 64.5 (69) | 57.6 (72) |
| Race %(n) | |||
| White | 76.4 (120) | 74.8 (80) | 80.0 (100) |
| African American | 14.0 (22) | 16.8 (18) | 11.2 (14) |
| Other / More than 1 Race | 9.6 (15) | 8.4 (9) | 8.8 (11) |
| Tobacco Use Characteristics | |||
| Cigarettes per Day (past 30 days) | 11.5 (6.8) | 11.9 (7.2) | 11.5 (6.9) |
| Breath CO (ppm) | 15.2 (10.4) | 16.0 (11.2) | 15.5 (10.6) |
| Urinary Cotinine (ng/mL) | 1011 (652) | 1040 (622) | 993 (645) |
| Days Smoking (past 30 days) | 29.2 (2.7) | 29.1 (2.8) | 29.3 (2.5) |
| mFTQ Total Score | 4.3 (1.7) | 4.4 (1.8) | 4.3 (1.7) |
| Age at First Smoking | 14.7 (2.4) | 14.7 (2.7) | 15.0 (2.3) |
| Age at Regular Smoking | 16.3 (1.9) | 16.3 (2.1) | 16.5 (1.7) |
| Past Quit Attempts | 2.5 (2.3) | 2.2 (1.5) | 2.5 (2.4) |
| Cannabis/Alcohol Use Characteristics | |||
| Positive UDS for THC % (n) | 58.6 (92) | 86.0 (92) | 62.4 (78) |
| Any Self-Reported Cannabis Use (past 30 days) % (n) | 66.2 (104) | 97.2 (104) | 73.4 (91) |
| Days of Cannabis Use (past 30 days; n = 107) | 7.3 (10.1) | 11.0 (10.6) | 7.9 (10.2) |
| Any Self-Reported Drinking (past 30 days) % (n) | 79.6 (125) | 85.1 (91) | 100.0 (125) |
| Drinking Days (past 30 days) | 4.9 (5.4) | 5.8 (5.8) | 6.2 (5.3) |
| Self-Reported Standard Drinks (past 30 days) | 31.7 (42.2) | 38.9 (47.1) | 39.8 (43.7) |
| Drinks per Drinking Day* (past 30 days) | 6.2 (4.3) | 6.5 (3.9) | 6.2 (4.3) |
| Heavy Drinker (5+ binge episodes in the past 30 days) % (n) | 24.2 (38) | 29.9 (32) | 30.4 (38) |
| PACS Total Score (n = 155) | 5.5 (4.9) | 6.2 (5.2) | 6.3 (4.9) |
It should be noted that co-users of both alcohol and cannabis made up 58% of the study cohort and are represented here in both cannabis and alcohol groups.
CO = Carbon monoxide; mFTQ = Modified Fagerström Tolerance Questionnaire; ppm = parts per million; UDS = Urine drug screen; THC = Δ 9-tetrahydrocannabinol; PACS = Penn Alcohol Craving Scale.
*Reported only for participants who self-report any drinking (n = 125).
Beyond simple assessment of any alcohol use in the past 30 days, we also assessed: (1) binge drinking, defined as four or more drinks per day for females and five or more drinks per day for males (based on NIAAA definitions),42 and (2) heavy drinking, based on definitions from the National Survey on Drug Use and Health as being five or more binge drinking days in the past 30.43
The analyses conducted in this report were secondary and not powered based on the outcomes presented. The primary hypothesis was that baseline co-use of cannabis or alcohol would adversely affect weekly point prevalence abstinence (PPA) from cigarette smoking during treatment; specifically that co-users would have decreased probability of weekly abstinence from smoking. PPA at each study visit during treatment (weeks 1–12) was defined as no self-reported smoking since the last visit, whereas PPA at the follow-up visits (weeks 18 and 26) was defined as no self-reported smoking in the last 7 days. An intent-to-treat approach that included all randomized participants was used in the modeling process and all missing cigarette data were considered not abstinent. A logistic regression model with a sandwich variance estimate44 was used to assess the primary end-of-treatment efficacy outcome (week 12). Additionally, repeated measures logistic regression models were constructed to assess abstinence at weekly treatment visits (weeks 1–12) and at post-treatment follow-up visits (weeks 18 and 26), using the methods of generalized estimating equations.45 Risk ratios (RR) and asymptotic 95% confidence intervals (CI) were computed for all efficacy estimates. Models were adjusted for treatment assignment (varenicline or placebo), baseline CPD, gender, and study visit (when appropriate). All models simultaneously tested the effect of baseline cannabis co-use status (yes/no) and alcohol co-use status (yes/no) on smoking cessation outcomes. Additional models tested the interaction of cannabis and alcohol co-use with treatment efficacy, as well as the modifying effect of gender on tobacco outcomes.
Next, severity of cannabis or alcohol use was investigated to determine if dose-dependent relationships between co-use frequency at baseline and smoking abstinence were present. The relationship between self-reported smoking abstinence and baseline cannabis or alcohol use severity as continuous variables (days of use) was analyzed in the primary models as predictors in place of the binary co-use variables. When evidence of a significant dose-dependent relationship was found, severity categories were created based on cannabinoid-positive tests and self-reported cannabis use to elucidate changes in the probability of smoking abstinence with variations in co-use severity. Severity categorizations were based on the clinical judgment of the investigative team.
Finally, changes in cannabis or alcohol co-use during treatment were examined, specifically assessing any secondary effects of varenicline on co-use. Cannabis use data were reported as any use since the last study visit (yes/no) and number of days used. Daily alcohol consumption throughout the trial was collected as standard alcoholic drinks consumed per day. For alcohol co-use analyses, self-reported standard drinks were used to calculate any drinking since the last visit (yes/no), number of drinking days, standard drinks per drinking day, and binge drinking days (defined as 4 or more drinks per day for females and 5 or more drinks per day for males). Finally, alcohol craving was measured via the PACS, which was administered weekly.
Between-visit smoking abstinence (yes/no) and self-reported CPD were independently tested for associations with co-occurring cannabis and alcohol use with generalized linear mixed effects models (GLMM) using methods of maximum likelihood (Poisson distribution). Adjusted models controlled for randomized treatment assignment, study visit, age, gender, concurrent cigarette smoking status (yes/no, CPD), and baseline cannabis/alcohol use severity. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc. Cary, NC).
Results
Demographics and Clinical Characteristics
The randomized study sample had a mean age of 19.1 years (SD = 1.5), was predominantly male (60%), White (76%), and reported smoking 11.5 (SD = 6.8) cigarettes per day at screening (Table 1). The majority of the sample (n = 107; 68%) either self-reported cannabis use in the past 30 days (n = 104) or submitted a cannabinoid-positive UDS (n = 92) at screening. Most participants (n = 125; 80%) reported drinking alcohol in the past 30 days. Cannabis and alcohol co-users were compared with the overall cohort (Table 1). Co-use groups in Table 1 are not mutually exclusive and co-users of both alcohol and cannabis are represented in both groups. A majority of the study cohort (58%) reported co-use of both cannabis and alcohol (Supplementary Figure 1). Cannabis co-users were similar in age, gender, and racial distribution to the entire cohort and reported using cannabis an average of 10.7 (SD = 10.6) days in the past 30. Alcohol co-users reported an average of 6.2 (SD = 4.3) drinks per drinking day and 6.2 drinking days (SD = 5.3) at baseline, and were similar to nonalcohol using participants with respect to demographic and smoking characteristics at baseline. Cannabis and alcohol co-users as exclusive categories are shown in Supplementary Figure 1.
Study Retention
Among randomized participants, 57% completed the week 12 EOT study visit (90/157) and 53% completed the week 26 F/U study visit (83/157). The majority of participants (93%) completed at least one study visit following randomization. Rates of study retention at EOT and F/U visits were similar among cannabis co-users (EOT = 57%, 61/107; F/U = 55%, 59/107) and alcohol co-users (55%, 69/125; F/U = 51%, 64/125) compared with the entire study cohort.
Cannabis or Alcohol Co-Use and Smoking Cessation
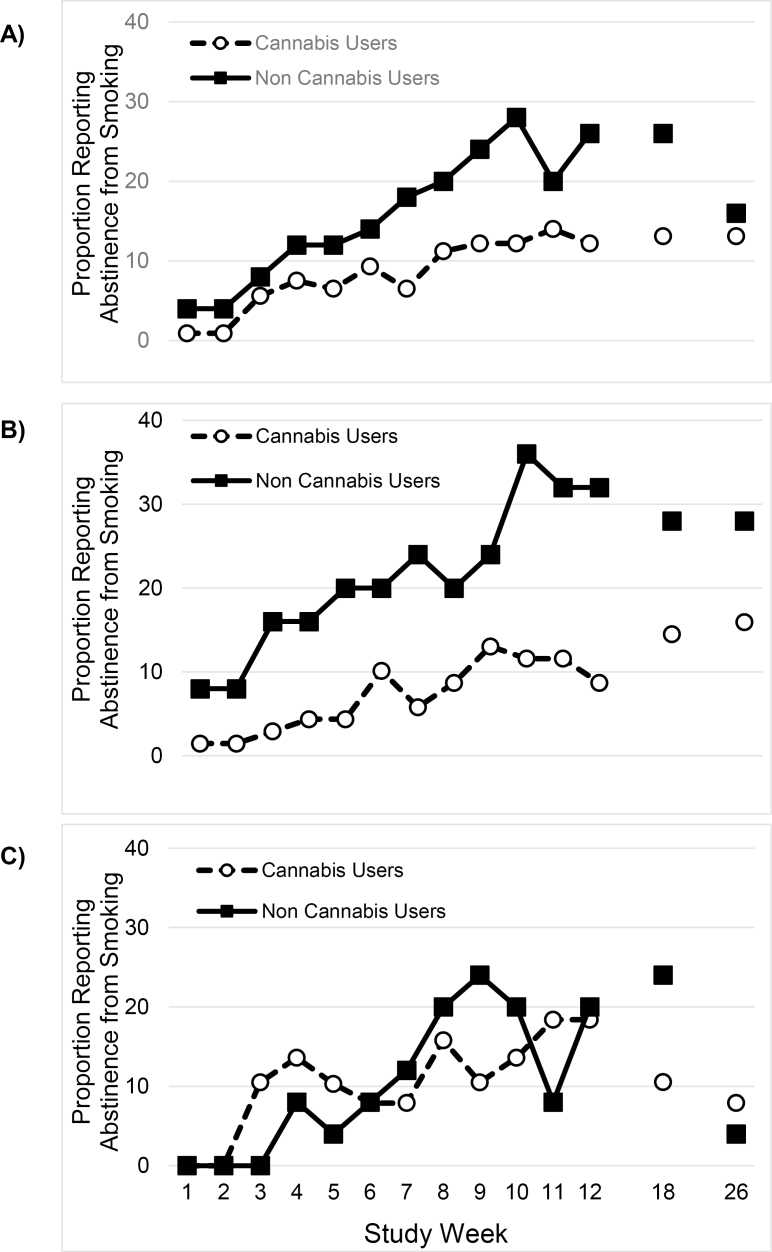
The percentage of weekly self-reported PPA from smoking is shown in Figure 1A. Compared with cannabis co-using participants, noncannabis using participants had double the probability of any smoking abstinence (PPA) during treatment (RR 2.0 95%; CI, 1.1–3.6; p = .021). At the week 12 EOT visit, the difference between cannabis co-users and nonusers was still present (26.0% versus 12.2%; RR = 2.2 [1.1–4.5]; p = .025). Differences at the week 18 follow-up persisted (26.0% versus 13.1%; RR = 2.1 [1.1–3.9]; p = .021) but not at the week 26 follow-up (16.0% versus 13.1%; RR = 1.3 [0.6–2.7]; p = .49). However, participant gender modified the relationship between cannabis use status and smoking abstinence during treatment (cannabis use x gender, p = .022): cannabis co-use adversely affected smoking cessation among male participants during the study (Figure 1B; RR = 3.6 [1.9–6.6]; p < .001), but did not similarly affect female participants (Figure 1C; RR = 0.9 [0.4–2.1]; p = .77). At follow-up visits, gender did not modify the relationship between cannabis use status and smoking abstinence (p = .61). Baseline cannabis use status did not modify treatment efficacy of varenicline on weekly smoking abstinence during study treatment (p = .32) or at follow-up (p = .72).
Figure 1.
Between-visit point prevalence abstinence (PPA) during treatment and 7-day PPA at post-treatment follow-up visits for the intent to treat sample by cannabis co-use status (A) and by (B) male participants and (C) female participants. (A) All participants; Cannabis co-users (n = 107) compared with noncannabis co-users (n = 50). (B) Male Participants; cannabis co-users (n = 69) vs. noncannabis users (n = 25). (C) Female Participants; cannabis users (n = 38) vs. noncannabis users (n = 25).
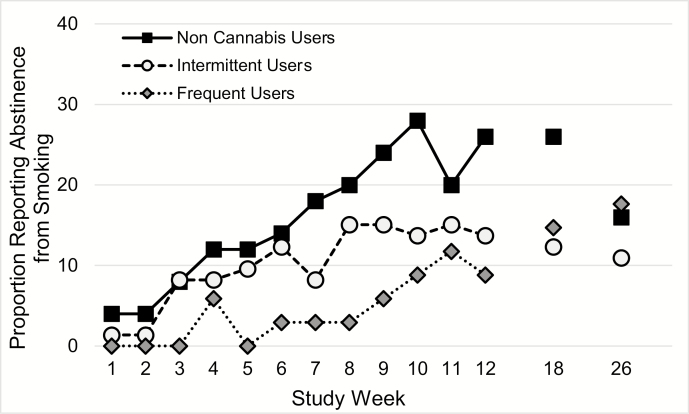
A greater number of cannabis use days in the 30 days prior to screening was significantly associated with decreased probability of smoking abstinence during the study (for a 10 day increase in cannabis use days; RR = 0.7 [0.4–1.0]; p = .04). Given this significant relationship and the preponderance of zero cannabis use days (among noncannabis co-users), participants were further categorized into three cannabis use severity groups: (1) >50% days of use at baseline and positive urine cannabinoid test = frequent users, n = 34; (2) positive urine cannabinoid test and <50% days of use at baseline = intermittent users, n = 73; and (3) negative urine cannabinoid test and no self-reported use = nonusers, n = 50. Frequent cannabis users had significantly lower rates of weekly PPA from smoking when compared with nonusers (Figure 2; RR = 3.5 [1.6–8.0]; p = .003) but not when compared with intermittent users (RR = 2.1 [0.9–4.8]; p = .075). Intermittent cannabis users were numerically similar, but not statistically less likely to achieve abstinence when compared with cannabis nonusers (RR = 1.7 [0.9–3.1]; p = .11).
Figure 2.
Between-visit point prevalence abstinence during treatment and 7-day point prevalence abstinence at post-treatment follow-up visits for the intent to treat sample of cannabis users by baseline cannabis use frequency. Noncannabis users (n = 50), infrequent cannabis users (n = 73), and frequent cannabis users (n = 34).
The percentage of weekly self-reported smoking PPA among alcohol co-users and nonusers is shown in Supplementary Figure 2. Baseline alcohol co-use was not significantly associated with abstinence from smoking during treatment (RR = 0.6 [0.3–1.3]; p = .21). No effects of alcohol co-use were found at the week 18 follow-up visit (RR = 1.0 [0.5–2.0]; p = .96) or at the week 26 follow-up visit (RR = 1.0 [0.5–2.4]; p = .93). Participant gender did not modify the effect of alcohol co-use on smoking abstinence during treatment (Alcohol co-use × gender, p = .56). Baseline drinking days (χ 21 = 0.5, p = .47), standard drinks per drinking day (χ 21 = 2.1, p = .15), binge drinking days (χ 21 = 1.8, p = .18), and being a heavy drinker at baseline (χ 21 = 1.9, p = .17) were not significantly associated with changes in the probability of abstinence from smoking during the study. Similar to cannabis outcomes above, alcohol co-use did not modify the efficacy of varenicline on abstinence during treatment (p = .34).
Additionally, the interaction between cannabis and alcohol co-use was added to the model to assess the relationship of cannabis and alcohol co-use on abstinence from smoking. When included in the model, the interaction between co-use variables on smoking abstinence during the study was insignificant (any co-use χ 21 = 1.4, p = .24). Although not powered to do so, we assessed the influence of cannabis co-users, who also met criteria for heavy drinking at baseline on smoking abstinence (n = 32/107 = 30% of cannabis co-users). Cannabis co-users who were also heavy drinkers had a further increased risk of continued smoking during treatment when compared with noncannabis co-users (RR = 2.6 [1.2–5.6]; p = .02), whereas cannabis co-users not meeting the criteria for heavy drinking at baseline were not at an increased risk of continued smoking (RR = 1.6 [0.8–3.0]; p = .14). Although cannabis co-users with heavy baseline alcohol use had a numerically increased risk of nonabstinence when compared with cannabis co-users without heavy alcohol use, the difference was not statistically significant (χ 21 = 1.5, p = .22).
Changes in Cannabis or Alcohol Use During Treatment
Decreased rates of smoking during study treatment were associated with a moderate decrease in cannabis use days (5 CPD reduction; RR = 0.9 [0.8–1.0]; p = .046) during the concurrent between-visit time period. Treatment with varenicline did not modify any self-reported cannabis use (RR = 1.0 [0.8–1.4]; p = .82) or number of cannabis use days (RR = 0.9 [0.6–1.4]; p = .65) between study visits. Participants who did not abstain from smoking between visits were more likely to also report drinking between visits (RR = 1.3 [1.0–1.5]; p = .019). Treatment with varenicline did not modify self-reported drinking during the study (RR = 1.0 [0.8–1.2]; p = .64), number of drinking days (RR = 0.9 [0.7–1.2]; p = .57), number of binge drinking days (RR = 1.1 [0.7–1.5]; p = .76), or alcohol craving as assessed via the PACS (F11,874 = 0.57, p = .85). These results indicate that reductions in smoking were not associated with increases in cannabis or alcohol use; rather, continued smoking was associated with increased probability of co-occurring cannabis and alcohol use during treatment.
Discussion
This secondary analysis evaluated cannabis and/or alcohol co-use among a sample of adolescent and emerging adult cigarette smokers enrolled in a smoking cessation pharmacotherapy clinical trial. Results showed that cannabis co-users were half as likely to achieve any weekly abstinence from smoking compared with tobacco-only users. The adverse impact of cannabis co-use on smoking abstinence was particularly pronounced in male co-using participants and among more frequent cannabis users. Alcohol co-users, however, did not differ in smoking abstinence during treatment or at follow-up. Similarly, smoking abstinence across male and female alcohol co-users versus nonalcohol using participants did not vary. Findings revealed no evidence of increased alcohol or cannabis use associated with a reduction or cessation from smoking. Rather, results showed associations between continued smoking and concurrent cannabis use and alcohol consumption. Finally, varenicline did not exert moderating effects on cannabis or alcohol use during the treatment trial.
Results for cannabis co-users in this study are consistent with some previous work showing that co-users have worse tobacco outcomes.14,17,18 Our results demonstrate that severity of cannabis use (days of use in the past 30 at baseline), beyond mere status of cannabis use (yes/no), had a pronounced effect on failure to abstain from smoking compared with intermittent users of cannabis. This nuanced distinction in defining cannabis co-use may help us to explain the inconsistent literature on cannabis co-use affecting smoking cessation outcomes. In addition, gender emerged as an important variable in this association. Rates of baseline cannabis use were similar across male and female participants (7.5 ± 10.2 days of cannabis use in the past 30 for males compared with 6.9 ± 10.0 days of use for females), which is unlikely to explain this finding. More detailed measures of cannabis use frequency and amount were not collected in this study, and thus, we are unable to determine if there were gender differences in the quantity of cannabis consumption in this sample, which may have contributed to lower rates of smoking cessation for male co-users. Some studies suggest that males and females have different patterns of cannabis use, with females being more likely to report use of cannabis to cope with stress or negative mood.46,47 One study found that individuals who report coping motives are more likely to use substances interchangeably rather than concurrently.48 Although speculative, perhaps males’ cannabis-tobacco co-use occurs concurrently (therefore tracking more closely together), whereas females may be more likely to use cannabis or tobacco interchangeably. Clarifying patterns of co-use, and whether such patterns differ by individual characteristics (gender, severity of use, or other) may better explain the mixed literature on the impact of cannabis on tobacco outcomes and is a critical future direction. More work is necessary to contextualize the characteristics of co-use in order to identify which co-users will have the most difficulty with tobacco cessation and develop methods to mitigate those challenges.
Relatedly, among co-users engaged in tobacco treatment, there may also be individual differences predicting concurrent increases or decreases in the use of other substances. Results from the current study indicate that increased use of alcohol or cannabis did not occur during smoking reduction or cessation. Rather, we found concurrent reductions in these substances with rate of smoking, which occurred in both the varenicline and placebo groups. It is likely that individual differences exist in co-use patterns during treatment such that some may reduce all substance use concurrently, whereas others may increase use of nontreated substances. This is an area of study that requires further exploration. Obtaining detailed, prospective measures of other drug use during tobacco treatment will allow for the exploration of compensatory use and individual differences and characteristics that may lead to these associated changes.
Finally, this study found no impact of alcohol co-use on smoking outcomes, even when taking heavier drinking patterns into consideration, which is inconsistent with previous literature.26,29 This may be due, in part, to relatively low rates of alcohol use in this sample, coupled with only 20% of individuals reporting no alcohol use. Although we did see relatively high rates of binge episodes in study participants, those meeting diagnostic criteria for alcohol dependence were excluded and it is possible that recruiting a heavy drinking sample of tobacco users would have likely yielded different outcomes.
Limitations
This study had several limitations. First, as an opportunistic analysis based on a smoking cessation trial, we did not specifically recruit co-users of cannabis or alcohol. Participants were excluded from study procedures if they met criteria for substance dependence, as well as certain mood and depressive disorders. Although severe co-users of cannabis and/or alcohol were excluded from study participation, the majority of enrolled participants were using cannabis and alcohol at least once in the past 30 days. Second, our study is limited by the lack of frequent biochemical verification to confirm self-reported cannabis and/or alcohol use and the limitations to the biochemical verification of smoking that precluded their use in analyses. UDS were conducted at key time points in the study to detect the presence of THC metabolites, but tests did not provide a quantitative measure of cannabis use, only positive or negative for the presence of THC metabolites. Alcohol co-use was not biochemically verified in this trial. Although standard drinks were collected daily, a similar standard metric does not currently exist for cannabis. The quantification of cannabis use is challenging, and as such, we relied on cannabis use days without added granularity. Cannabis co-use also affected biochemical measures of smoking (breath CO and cotinine), which prevented their use in this analysis. Finally, the retention of study participants for 12 weeks of treatment and 14 weeks of follow-up was challenging in this trial.32 Cannabis and alcohol use data presented in this report were only available for those participants retained in the study (57% at week 12). This limits our findings regarding co-use changes during the study and the effects of varenicline on co-use.
Conclusions
Rates of cannabis and alcohol co-use in this sample of youth smokers enrolled in a smoking cessation pharmacotherapy clinical trial were high. The co-use of cannabis negatively affected smoking outcomes, more so for males and frequent cannabis users, whereas alcohol use did not affect smoking cessation. No evidence of increased rates of alcohol or cannabis co-use was found, and varenicline did not appear to affect co-use. It is critically important to continue work on polysubstance use among youth smokers and address barriers to cessation that may exist as a result of their co-use. Research using fine-grained data assessment methods (e.g., ecological momentary assessment) that examine affective state and motivations for use is warranted, particularly at the event level (i.e., per each episode of co-use). Advancing this literature is necessary to develop more informed treatment guidelines and recommendations for youth co-users, which is currently limited. For cannabis and tobacco co-use, treatment interventions to date have been pilot/feasibility trials and all have focused on adults.49–52 For alcohol and tobacco co-use, treatment strategies have a stronger evidence base, but work is still limited in terms of approved or widely disseminated treatments that target both substances.27,53 Tobacco treatment guidelines for youth co-users may also need to take into consideration the motivation to quit or reduce other substance use and how to manage a potential lack of motivation to quit, while still promoting tobacco cessation. Future work should integrate substance co-use information into youth tobacco treatment, which may need to be tailored specifically by certain variables in order to improve rates of cessation.
Funding
This study was supported by a grant from the National Institutes of Health (NIDA U01 DA031779, PI Gray). Additional funding and support came from the National Center for Advancing Translational Sciences (NCATS UL1TR001450, PI Brady), and NIH grants K01 DA036739 (McClure), K12 HD055885 (Tomko), K23 AA025399 (Squeglia), and K23 AA023845 (Flanagan), as well as NIH grant T32DA035200 (Hood). Study medication and matched placebo was provided at no cost by Pfizer, Inc. No other funding or support from Pfizer, Inc. was provided.
Declaration of Interests
Drs. Gray and Carpenter have provided consultation to Pfizer, Inc. No other authors have conflicts of interest to declare.
Supplementary Material
Acknowledgments
The authors would like to thank the medical and research staff at the Medical University of South Carolina for the successful execution of this study protocol. Specifically, we would like to thank Lori Ann Ueberroth, Jessica Hinton, Jaclyn Condo, Patrick Cato, Kathryn Meltzer, Casy Johnson, Christine Horne, Danielle Paquette, Priscilla Muldrow, and Elizabeth Kryway. We would also like to thank the study participants for their involvement.
References
- 1. U.S. Department of Health Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health;2014. [Google Scholar]
- 2. Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME.. Monitoring the Future national survey results on drug use, 1975–2018: Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan;2019. [Google Scholar]
- 3.Center for Behavioral Health Statistics and Quality. 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.pdf. Published 2018. Accessed January 31, 2019.
- 4. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol. 2006;25(5):549–557. [DOI] [PubMed] [Google Scholar]
- 5. Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone-Banks J, Hartmann-Boyce J. Tobacco cessation interventions for young people. Cochrane Database Syst Rev. 2017;11:Cd003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myung SK, Park JY. Efficacy of pharmacotherapy for smoking cessation in adolescent smokers: a meta-analysis of randomized controlled trials. Nicotine Tob Res. 2018. [DOI] [PubMed] [Google Scholar]
- 7. Weinberger AH, Funk AP, Goodwin RD. A review of epidemiologic research on smoking behavior among persons with alcohol and illicit substance use disorders. Prev Med. 2016;92:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duhig AM, Cavallo DA, McKee SA, George TP, Krishnan-Sarin S. Daily patterns of alcohol, cigarette, and marijuana use in adolescent smokers and nonsmokers. Addict Behav. 2005;30(2):271–283. [DOI] [PubMed] [Google Scholar]
- 9. Palmer RH, Young SE, Hopfer CJ, et al. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102(1–3):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. [DOI] [PubMed] [Google Scholar]
- 11. Ramo DE, Liu H, Prochaska JJ. Tobacco and marijuana use among adolescents and young adults: a systematic review of their co-use. Clin Psychol Rev. 2012;32(2):105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schauer GL, Peters EN. Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug Alcohol Depend. 2018;185:238–244. [DOI] [PubMed] [Google Scholar]
- 13. Goodwin RD, Pacek LR, Copeland J, et al. Trends in Daily Cannabis Use Among Cigarette Smokers: United States, 2002–2014. Am J Public Health. 2018;108(1):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinberger AH, Platt J, Copeland J, Goodwin RD. Is Cannabis Use Associated With Increased Risk of Cigarette Smoking Initiation, Persistence, and Relapse? Longitudinal Data From a Representative Sample of US Adults. J Clin Psychiatry. 2018;79(2):e1-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen TQ, Ebnesajjad C, Stuart EA, Kennedy RD, Johnson RM. Does Marijuana use at ages 16–18 predict initiation of daily cigarette smoking in late adolescence and early adulthood? A propensity score analysis of add health data. Prev Sci. 2019;20(2):246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abrantes AM, Lee CS, MacPherson L, Strong DR, Borrelli B, Brown RA. Health risk behaviors in relation to making a smoking quit attempt among adolescents. J Behav Med. 2009;32(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schauer GL, King BA, McAfee TA. Prevalence, correlates, and trends in tobacco use and cessation among current, former, and never adult marijuana users with a history of tobacco use, 2005–2014. Addict Behav. 2017;73:165–171. [DOI] [PubMed] [Google Scholar]
- 18. Vogel EA, Rubinstein ML, Prochaska JJ, Ramo DE. Associations between marijuana use and tobacco cessation outcomes in young adults. J Subst Abuse Treat. 2018;94:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Humfleet G, Munoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addict Behav. 1999;24(1):149–154. [DOI] [PubMed] [Google Scholar]
- 20. Rabin RA, Ashare RL, Schnoll RA, et al. Does cannabis use moderate smoking cessation outcomes in treatment-seeking tobacco smokers? Analysis from a large multi-center trial. Am J Addict. 2016;25(4): 291–296. [DOI] [PubMed] [Google Scholar]
- 21. Hendricks PS, Delucchi KL, Humfleet GL, Hall SM. Alcohol and marijuana use in the context of tobacco dependence treatment: impact on outcome and mediation of effect. Nicotine Tob Res. 2012;14(8):942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haug S, Schaub MP, Salis Gross C, John U, Meyer C. Predictors of hazardous drinking, tobacco smoking and physical inactivity in vocational school students. BMC Public Health. 2013;13:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison EL, Desai RA, McKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol Clin Exp Res. 2008;32(12):2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002;97(5):517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10(1):59–73. [DOI] [PubMed] [Google Scholar]
- 26. Van Zundert RM, Kuntsche E, Engels RC. In the heat of the moment: Alcohol consumption and smoking lapse and relapse among adolescents who have quit smoking. Drug Alcohol Depend. 2012;126(1–2): 200–205. [DOI] [PubMed] [Google Scholar]
- 27. Haug S, Paz Castro R, Kowatsch T, Filler A, Schaub MP. Efficacy of a technology-based, integrated smoking cessation and alcohol intervention for smoking cessation in adolescents: Results of a cluster-randomised controlled trial. J Subst Abuse Treat. 2017;82:55–66. [DOI] [PubMed] [Google Scholar]
- 28. Haug S, Schaub MP, Schmid H. Predictors of adolescent smoking cessation and smoking reduction. Patient Educ Couns. 2014;95(3):378–383. [DOI] [PubMed] [Google Scholar]
- 29. Weinberger AH, Gbedemah M, Goodwin RD. Cigarette smoking quit rates among adults with and without alcohol use disorders and heavy alcohol use, 2002–2015: A representative sample of the United States population. Drug Alcohol Depend. 2017;180:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA. Alcohol and tobacco cessation in alcohol-dependent smokers: analysis of real-time reports. Psychol Addict Behav. 2007;21(3):277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verplaetse TL, McKee SA. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am J Drug Alcohol Abuse. 2017;43(2):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gray KM, Baker NL, McClure EA, et al. Varenicline for adolescent smoking cessation: A randomized clinical trial. JAMA Pediatrics. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Malley SS, Zweben A, Fucito LM, et al. Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solinas M, Scherma M, Fattore L, et al. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007;27(21):5615–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., Text Revision). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 36. Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, Monti PM. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug Alcohol Depend. 2005;79(1):33–43. [DOI] [PubMed] [Google Scholar]
- 37. Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified Fagerstrom tolerance questionnaire with salivary cotinine among adolescents. Addict Behav. 2000;25(3):429–433. [DOI] [PubMed] [Google Scholar]
- 38. Flannery BA, Volpicelli JR, Pettinati HM. Psychometric Properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- 39. Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology(Berl). 2011;215(4):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moolchan ET, Zimmerman D, Sehnert SS, Huestis MA, Epstein DH. Recent marijuana blunt smoking impacts carbon monoxide as a measure of adolescent tobacco abstinence. Subst Use Misuse. 2005;40(2):231–240. [DOI] [PubMed] [Google Scholar]
- 41. Boykan R, Messina CR, Chateau G, Eliscu A, Tolentino J, Goniewicz ML. Self-Reported Use of Tobacco, E-cigarettes, and Marijuana Versus Urinary Biomarkers. Pediatrics. 2019;143(5). [DOI] [PubMed] [Google Scholar]
- 42. National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed March 25, 2019.
- 43. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration;2018. [Google Scholar]
- 44. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 45. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 46. Bujarski SJ, Norberg MM, Copeland J. The association between distress tolerance and cannabis use-related problems: The mediating and moderating roles of coping motives and gender. Addict Behav. 2012;37(10):1181–1184. [DOI] [PubMed] [Google Scholar]
- 47. Simons J, Correia CJ, Carey KB, Borsari BE. Validating a five-factor marijuana motives measure: Relations with use, problems, and alcohol motives. J Couns Psychol. 1998;45(3):265–273. [Google Scholar]
- 48. O’Hara RE, Armeli S, Tennen H. Alcohol and cannabis use among college students: Substitutes or complements? Addict Behav. 2016;58:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter JF, Stanger C. Outcomes from a computer-assisted intervention simultaneously targeting cannabis and tobacco use. Drug Alcohol Depend. 2015;155:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hill KP, Toto LH, Lukas SE, et al. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. Am J Addict. 2013;22(3):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Becker J, Haug S, Sullivan R, Schaub MP. Effectiveness of different web-based interventions to prepare co-smokers of cigarettes and cannabis for double cessation: a three-arm randomized controlled trial. J Med Internet Res. 2014;16(12):e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beckham JC, Adkisson KA, Hertzberg J, et al. Mobile contingency management as an adjunctive treatment for co-morbid cannabis use disorder and cigarette smoking. Addict Behav. 2018;79:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roche DJ, Ray LA, Yardley MM, King AC. Current insights into the mechanisms and development of treatments for heavy drinking cigarette smokers. Curr Addict Rep. 2016;3(1):125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.