Abstract
Objectives
The effect of age on the response of peripheral blood mononuclear cells (PBMCs) to immunosuppression induced by human periodontal ligament stem cells (hPDLSCs) is unclear. The identity of the cytokines most effective in inducing the PBMC immune response remains unknown. This study investigated the effects of age on immunophenotype, proliferation, activation, and cytokine secretion capacities of PBMCs following co-culture with hPDLSCs.
Methods
PBMCs were collected from younger (16–19 years) and older (45–55 years) donors, then co-cultured with confirmed hPDLSCs for various lengths of time. T lymphocyte proliferation and cell surface marker expression were analyzed by flow cytometry. Cytokine expression levels were measured by quantitative polymerase chain reaction assays and enzyme-linked immunosorbent assays.
Results
CD28 expression by T lymphocytes decreased with age, indicating reduced proliferation; CD95 expression increased with age, indicating enhanced apoptosis. Moreover, hPDLSCs inhibited T lymphocyte proliferation in both age groups; this inhibition was stronger in cells from older donors than in cells from younger donors. Age reduced the secretion of interleukin-2 and interferon-γ, whereas it increased the secretion of tumor necrosis factor-β by PBMCs cultured with hPDLSCs.
Conclusions
Aging may have a robust effect on the response of PBMCs towards hPDLSC-induced immunosuppression.
Keywords: Periodontal ligament stem cells, peripheral blood mononuclear cells, T lymphocytes, immunosuppression, immunosenescence, immunophenotyping, cytokines, coculture, age
Introduction
Recently, it has been established that mesenchymal stem cells (MSCs) have great potential for use in allotransplantation processes and treatment of severe autoimmune diseases because of their low immunogenicity and strong immunomodulatory effects.1 Human periodontal ligament stem cells (hPDLSCs), one kind of MSC, are easily harvested; they also exhibit strong self-renewal and multilineage differentiation capacities and thus constitute a reliable cell origin for the clinical application of periodontal regeneration therapy.2 According to Wei et al.,3 hPDLSCs can reconstruct periodontal tissues and bone defects; they also exhibit low immunogenicity and strong immunoregulatory ability, as observed in other MSCs.4 Ding et al.5 found that hPDLSCs can inhibit the activation and proliferation of T lymphocytes, thus avoiding rejection of allogeneic transplanted cells. Considering the increase in life expectancy in many nations worldwide, the number of older people with degenerative diseases (e.g., periodontitis) is expected to grow substantially in the future.6 Currently, studies of hPDLSC immunoregulation involve samples mainly derived from young animal models or young human donors.7 However, both younger and older patients may undergo allotransplantation.
Previous studies have revealed many aging-related changes in most components of the immune system.8 The decline in immune response associated with aging is often referred to as “immunosenescence.”9 The earliest study to show a relationship between immune function and age reported that T cell proliferation was reduced in older individuals, compared with younger individuals.10 Subsequently, T cells were found to undergo age-related changes in phenotype and function; these included reduced production of naïve T cells, enhanced generation of memory and effector T cells, weak activation of T cells, and impairment of cytokine secretion.11,12 Wu et al.13 demonstrated that peripheral blood mononuclear cells (PBMCs) differ between older and younger individuals. Additionally, immune cells in older people generally lack a coordinated response; their gene expression patterns are variable and unstable.12,14
Despite extensive research concerning immunosenescence, the difference in immune responses between older and younger PBMCs is not yet fully understood. This study was performed to compare the responses of younger and older PBMCs to hPDLSC-mediated immunosuppression and assess the underlying mechanism, with the aim of providing an important theoretical basis for the application of hPDLSC allotransplantation.
Materials and methods
Culture and identification of hPDLSCs
hPDLSCs were harvested from intact third molars that had been extracted from 14 systemically healthy donors aged 16 to 20 years; all donors and their parents provided written informed consent for use of their tissues in this study. The samples were cultured in accordance with previously described methods.15 All procedures performed herein, regarding third molars, were approved by the Research Ethics Committee of Shandong University (approval no: G201401601). For osteogenic or adipogenic differentiation assays, hPDLSCs were cultured with osteogenic or adipogenic inductive medium, respectively. After 7 days of culture, hPDLSCs were identified by staining with alkaline phosphatase; after 21 days of culture, mineralized nodules were identified by staining with alizarin red. Additionally, after 14 days of culture, lipid droplets were identified by staining with oil red O. To confirm their identity, hPDLSCs were incubated with phycoerythrin-conjugated anti-STRO-1 (cat. no. FAB1038G, R&D Systems, Minneapolis, MN, USA), anti-CD146 (cat. no. 12-1469-41, eBioscience, San Diego, CA, USA), anti-CD31 (cat. no. 11-0319-41, eBioscience), anti-CD45 (cat. no. 12-0459-41, eBioscience), anti-HLA-I (cat. no. phhad-25, BioLegend, San Diego, CA, USA), anti-HLA-II DR (cat. no. phhd-25, BioLegend), anti-CD80 (cat. no. phc80-25, BioLegend), and anti-CD86 (cat. no. phc86-25, BioLegend) antibodies; all antibodies were used without a dilution step. Cells without antibody were used as blank controls. Subsequently, the cells were analyzed by flow cytometry (BD Accuri C6, BD Biosciences, Franklin Lakes, NJ, USA).
Culture and identification of PBMCs
Peripheral blood was collected from 24 systemically healthy donors who were either younger (age range: 16–19 years) or older (age range: 45–55 years); all donors provided written informed consent. All procedures performed herein, regarding peripheral blood, were approved by the Research Ethics Committee of Shandong University (approval no: G20180506). The large gap between the two age groups was expected to ensure a clear difference in the characteristics of their cells.16 Two milliliters of fresh heparinized peripheral blood were diluted with phosphate-buffered saline (PBS; HyClone, Logan, UT, USA), carefully layered onto 5 mL Ficoll (1.077 g/mL), then centrifuged at 900 × g for 30 minutes. The lymphocyte layer was separated and washed with phosphate-buffered saline, and the precipitated cells were suspended in RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Gibco BRL), 100 U/mL penicillin (Hyclone), and 100 µg/mL streptomycin (Hyclone). PBMCs were incubated with phycoerythrin-conjugated anti-CD3 (cat. no. 12-0038-41, eBioscience), anti-CD28 (cat. no. 12-9039-82, eBioscience), and anti-CD95 (cat. no. 12-0959-41, eBioscience) antibodies; all antibodies were used without a dilution step. Cells without antibody were used as blank controls. Subsequently, the cells were analyzed by flow cytometry.
Immune assays
Effect of hPDLSCs on T lymphocyte proliferation, compared between age groups
hPDLSCs were seeded in six-well plates (5.0 × 104 cells per well) in RPMI-1640 medium (Gibco BRL) containing 10% fetal bovine serum (Gibco BRL), 100 U/mL penicillin (Hyclone), and 100 µg/mL streptomycin (Hyclone). After 2 hours of culture, allogeneic PBMCs from donors in the different age groups were added (5.0 × 105 cells per well) and stimulated with 5 µg/mL phytohemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO, USA). The mixtures were cocultured for 5 days. Supernatants of PMBCs from the different age groups were collected and stored at −80°C for use in enzyme-linked immunosorbent assays (ELISAs) to examine cytokine production. Following the collection of supernatant (8 hours before cells were harvested), fresh medium containing 5-ethynyl-2′-deoxyuridine (EdU; 10 µM; RiboBio, Guangzhou, China) was added to the cultures; T lymphocyte proliferation was then analyzed by flow cytometry using an anti-CD3 antibody (as described in the “Culture and identification of PBMCs” section.
Effect of delayed hPDLSC addition on T lymphocyte proliferation, compared between age groups
PBMCs from different age groups were seeded in six-well plates (5.0 × 105 cells per well), stimulated with 5 µg/mL PHA, and cultured in RPMI-1640 medium. After 2 days of culture, hPDLSCs (5.0 × 104 cells per well) were added to each well; the mixtures were then cocultured for 3 days. Eight hours before cells were harvested, EdU (10 µM) was added to the cultures; T lymphocyte proliferation was then analyzed by flow cytometry.
Repeated stimulation of T lymphocytes, compared between age groups
hPDLSCs were seeded in six-well plates (5.0 × 104 cells per well) in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. After 2 hours of culture, allogeneic PBMCs from donors in the different age groups were added (5.0 × 105 cells per well) and stimulated with 5 µg/mL PHA. The mixtures were cocultured for 5 days; then, suspended PBMCs were separated and 5 µg/mL PHA was added to reactivate PBMCs. Two days later, EdU (10 µM) was added to the cultures and incubated for 8 hours; cells were then harvested for analysis of T lymphocyte proliferation by flow cytometry.
Transwell culture of T lymphocytes, compared between age groups
hPDLSCs (5.0 × 104 cells per well) were seeded in 12-well plates and cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Allogeneic PBMCs from donors in the different age groups (5.0 × 105 cells per well) were seeded with PHA in the top Transwell chambers. Cells were cocultured for 5 days. Eight hours before cells were harvested, EdU (10 µM) was added to the cultures; T lymphocyte proliferation was then analyzed by flow cytometry.
Effect of hPDLSCs on two-way mixed lymphocyte response, compared between age groups
hPDLSCs were seeded in six-well plates (5.0 × 104 cells per well) were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. After 2 hours of culture, PBMCs from donors in the different age groups (PBMC donors in this specific step were confirmed to be distinct from hPDLSC donors) were added (5.0 ×105 cells per well) and stimulated with 5 µg/mL PHA. The mixtures were cocultured for 5 days. Eight hours before cells were harvested, EdU (10 µM) was added to the cultures; T lymphocyte proliferation was then analyzed by flow cytometry.
Quantitative polymerase chain reaction analysis
Total RNA was isolated from PBMCs from donors in the different age groups using RNAios Plus reagent (Takara, Shiga, Japan), then reverse transcribed to synthesize complementary DNA using the Prime Script RT reagent Kit (Takara). Primer sequences for quantitative polymerase chain reaction analysis were as follows: interleukin (IL)-2, sense: 5′-TGCTGATGAGACAGCAACCAT-3′, antisense: 5′-TCAAGTCAGTGTTGAGATGATGC-3′; interferon (IFN)-γ, sense: 5′-AAGTGATGGCTGAACTGTCG-3′, antisense: 5′-TACTGGGATGCTCTTCGACC-3′; tumor necrosis factor (TNF)-β, sense: 5′-TCTGGAGAGCAAACACGGAC-3′, antisense: 5′-ACCACCTGGGAGTAGACGAA-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), sense: 5′-TCATGGGTGTGAACCATGAGAA-3′, antisense: 5′-GGCATGGACTGTGGTCATGAG-3′. GAPDH was used to quantify and normalize the results of all samples. Relative transcript levels were measured with quantitative polymerase chain reaction analysis using a Roche LightCycler®480 sequence detection system (Roche, Basel, Switzerland), in accordance with the manufacturer’s protocol. Each 20-μL reaction volume contained 10 µL of SYBR® Premix Ex Taq™ (Takara), 0.4 µL of 10 µM forward primer (0.4 µM final), 0.4 µL of 10 µM reverse primer (0.4 µM final), 200 ng of template cDNA, and diethylpyrocarbonate-treated water. The amplification protocol was as follows: 95°C for 30 s, followed by 45 cycles of 95°C for 5 s and 60°C for 30 s. All amplification analyses were performed in triplicate.
ELISA
Concentrations of IL-2, IFN-γ, and TNF-β secreted by PBMCs from donors in the different age groups were determined using an ELISA kit (Elabscience, Wuhan, China). Supernatants were screened and analyzed in accordance with the manufacturer’s instructions. Standard curves were generated based on absorbance values of standard samples; regression equations were established to calculate the concentrations of IL-2, IFN-γ, and TNF-β in tested samples.
Statistical analysis
All experiments were performed independently at least three times, and the data are expressed as mean ± standard deviation. Student’s t-test was used to determine statistical differences. GraphPad Prism software, version 5.0 (GraphPad Inc., La Jolla, CA, USA) was used to conduct statistical analyses. Differences with p < 0.05 were considered statistically significant.
Results
Culture and identification of hPDLSCs
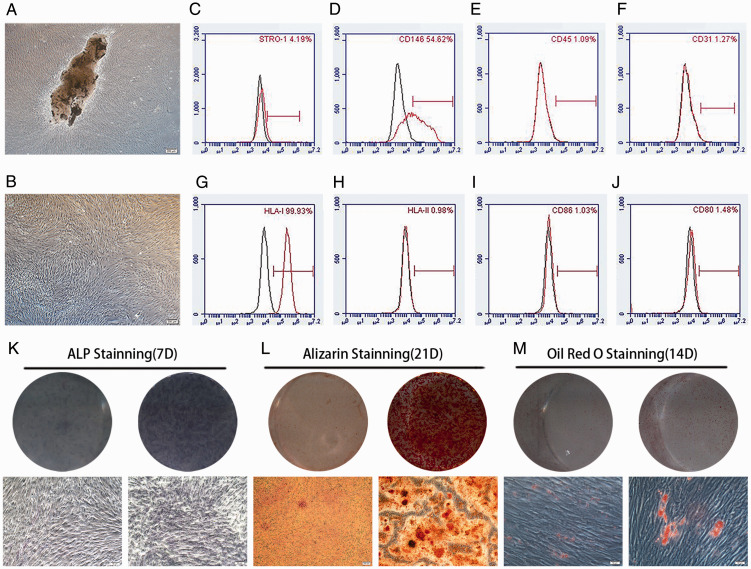
Figure 1A and B shows evenly distributed and proliferated hPDLSCs with a long spindle-like shape. Flow cytometry analyses revealed that 4.19% and 54.62% of these cells possessed STRO-1 and CD146 MSC-specific surface markers, respectively. However, hPDLSCs did not exhibit the leukocyte-specific molecule CD45 or platelet endothelial cell-specific molecule CD31 (Figure 1C–F). These results suggest that hPDLSCs are the source of MSCs. Similarly, flow cytometry analyses of hPDLSCs showed that 99.93% of these cells expressed HLA-I, whereas they did not express HLA-II DR or costimulatory molecules CD80 and CD86 (Figure 1G–J). These characteristics may contribute to the low immunogenicity of hPDLSCs. Observations of alkaline phosphatase activity (Figure 1K), alizarin red-positive calcium deposits (Figure 1L), and oil red O-positive lipid droplets (Figure 1M) after osteogenic and adipogenic induction confirm the multilineage differentiation ability of hPDLSCs.
Figure 1.
Isolation, culture, and identification of hPDLSCs. (A): hPDLSCs were cultured for 14 days (scale bar: 200 μm). (B): hPDLSCs of third generation (P3) (scale bar: 200 μm). (C–F): hPDLSCs were positive for CD146 and STRO-1, but negative for CD31 and CD45. (G–J): hPDLSCs were positive for HLA-I, but negative for HLA-II DR, CD80, and CD86. (K–M): hPDLSCs could undergo osteogenic and adipogenic differentiation when cultured in inductive medium (scale bars: 50 μm [panels K and M] and 200 μm [panel L]).
Abbreviations: ALP, alkaline phosphatase; CD, cluster of differentiation; HLA, human leukocyte antigen; hPDLSCs, human periodontal ligament stem cells.
Phenotypic changes of T lymphocytes according to donor age
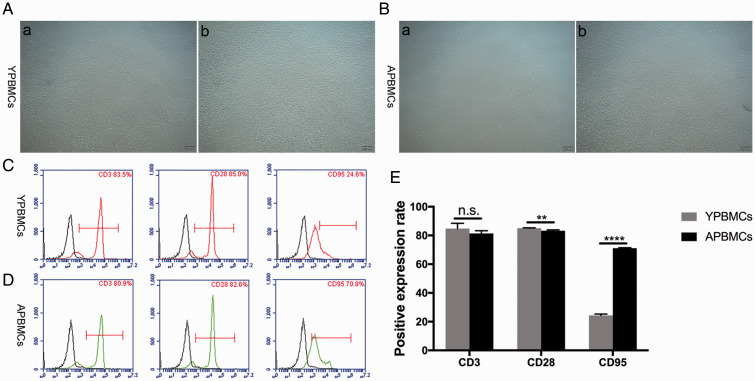
PBMCs were isolated from blood samples from younger and older donors, then stimulated with PHA to achieve T lymphocyte activation (Figure 2A, B). Flow cytometry analysis revealed similar rates of CD3 expression in T lymphocytes from younger and older donors. However, the rates of CD28 and CD95 expression in these groups were significantly different; the rate of CD28 expression decreased with age (p < 0.01), whereas the rate of CD95 expression increased with age (p < 0.001; Figure 2C–E). This finding implied that aging reduces the proliferation of T lymphocytes, while promoting apoptosis in these cells.
Figure 2.
Extraction, culture, and identification of PBMCs from donors of different ages. (A): (a) PBMCs isolated from younger donors, (b) PHA-activated PBMCs from younger donors (scale bar: 200 μm). (B): (a) PBMCs isolated from older donors, (b) PHA-activated PBMCs from older donors (scale bar: 200 μm). (C): Analysis of CD3, CD28, and CD95 surface markers in PBMCs from younger donors. (D): Analysis of CD3, CD28, and CD95 surface markers in PBMCs from older donors. (E): Rate of CD28 expression decreased with age and rate of CD95 expression increased with age. Data represent three independent experiments. Values are expressed as mean ± standard deviation (**p < 0.01, ****p < 0.0001, n.s., no significance).
Abbreviations: CD, cluster of differentiation; hPDLSCs, human periodontal ligament stem cells; OPBMCs, older peripheral blood mononuclear cells; PBMCs, peripheral blood mononuclear cells; PHA, phytohemagglutinin; YPBMCs, younger peripheral blood mononuclear cells.
T lymphocyte proliferation according to donor age
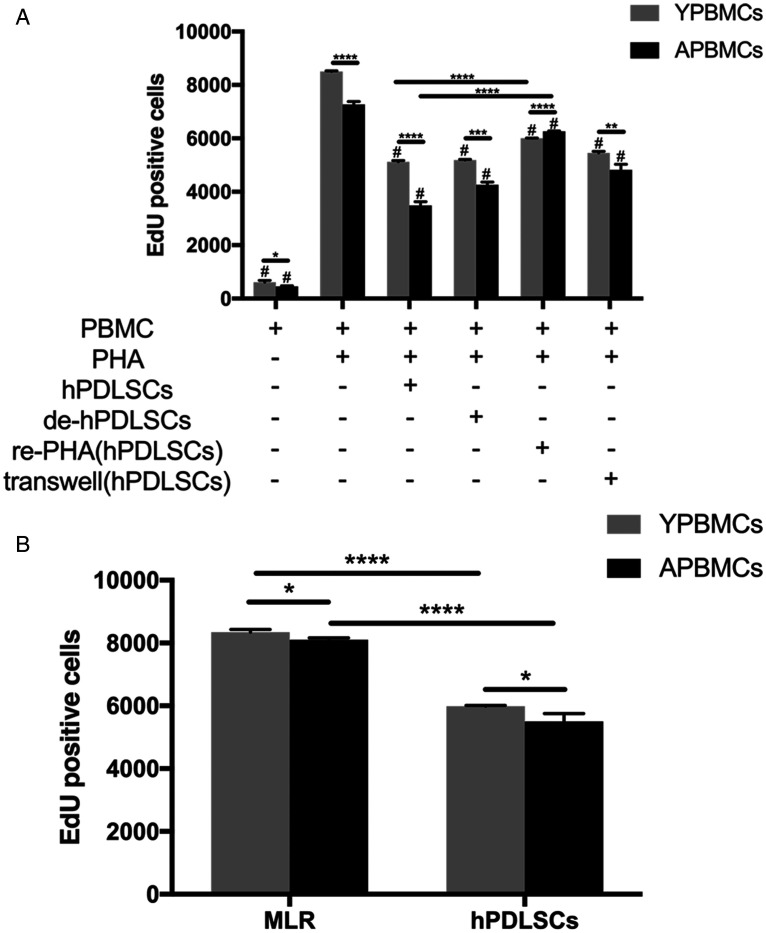
To determine the effect of allogeneic hPDLSCs on mitogen-activated T lymphocyte proliferation according to donor age, hPDLSCs were cultured with PHA-stimulated PBMCs collected from older and younger donors. The results are shown in Figure 3A. hPDLSCs were able to significantly suppress the proliferation of PHA-stimulated T lymphocytes from both younger and older donors (both p < 0.05); however, the proliferation of T lymphocytes from older donors was weaker than the proliferation of T lymphocytes from younger donors (p < 0.0001). Notably, delayed addition of hPDLSCs also inhibited T lymphocyte proliferation (i.e., hPDLSCs could inhibit the proliferation of activated T lymphocytes; both p < 0.05); however, the proliferation of T lymphocytes from older donors was weaker than the proliferation of T lymphocytes from younger donors (p < 0.001). Furthermore, T lymphocytes from both younger and older donors that had been inhibited by hPDLSCs could be reactivated when stimulated with PHA; T lymphocytes from younger donors were more easily reactivated and proliferated more rapidly than T lymphocytes from older donors (p < 0.0001). Additionally, hPDLSCs could inhibit the proliferation of T lymphocytes in Transwell culture (both p < 0.05), but the proliferation of T lymphocytes from older donors was weaker than the proliferation of T lymphocytes from younger donors (p < 0.01), suggesting that variation in T lymphocyte proliferation rates between younger and older donors was dependent on cell–cell contact and on soluble factors (e.g., cytokines). Two-way mixed lymphocyte response analysis revealed that hPDLSCs collected from a third party were also capable of inhibiting the proliferation of T lymphocytes in allogeneic PBMCs collected from younger and older donors (both p < 0.0001), and that this effect was stronger in T lymphocytes from older donors (p < 0.05; Figure 3B). These results suggest that T lymphocytes from older people have a weak immune response and are poorly reactivated.
Figure 3.
T lymphocyte proliferation from donors of different ages. (A): Effects of hPDLSC addition on PHA-stimulated PBMC proliferation under different experimental conditions. (B): Effect of hPDLSCs on two-way mixed lymphocyte reaction. Number of EdU+ cells per 10,000 viable lymphocytes after incubating cells. Data represent three independent experiments. Values are expressed as mean ± standard deviation (#, p < 0.05, compared with PHA-stimulated PBMCs in same age group; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Abbreviations: EdU, 5-ethynyl-2′-deoxyuridine; de-hPDLSCs, delayed addition of human periodontal ligament stem cells; hPDLSCs, human periodontal ligament stem cells; MLR, mixed lymphocyte reaction; OPBMCs, older peripheral blood mononuclear cells; PBMCs, peripheral blood mononuclear cells; PHA, phytohemagglutinin; re-PHA(hPDLSCs), peripheral blood mononuclear cells reactivated by phytohemagglutinin after inhibition by human periodontal ligament stem cells); transwell(hPDLSCs), transwell culture of peripheral blood mononuclear cells and human periodontal ligament stem cells; YPBMCs, younger peripheral blood mononuclear cells.
Levels of IL-2, IFN-γ and TNF-β according to donor age
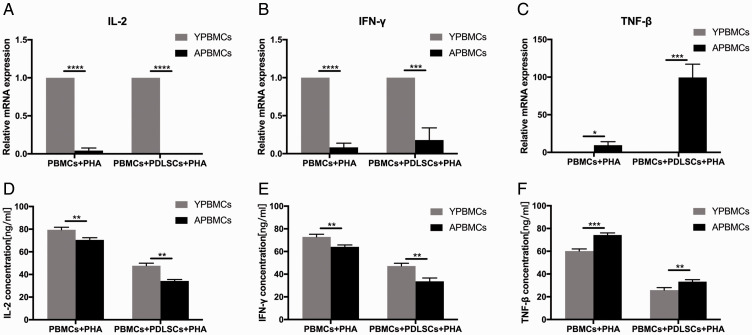
The mechanism by which hPDLSCs may inhibit T lymphocyte proliferation was investigated, using PBMCs from donors in both age groups. The expression levels of three T lymphocyte immunoregulatory cytokines (IL-2, IFN-γ, and TNF-β) were examined by quantitative polymerase chain reaction. The results showed significant differences between T lymphocytes from younger donors and those from older donors. Moreover, mRNA levels of these cytokines within activated T lymphocytes in both younger and older groups were significantly different, based on the presence or absence of hPDLSCs (Figure 4). Compared with the younger group, the mRNA expression levels of IL-2 (p < 0.0001 for the presence and absence of hPDLSCs) and IFN-γ were down-regulated (p < 0.0001 for the absence of hPDLSCs and p < 0.001 for the presence of hPDLSCs; Figure 4A, B) in the older group, whereas expression levels of TNF-β were up-regulated (p < 0.05 for the absence of hPDLSCs and p < 0.001 for the presence of hPDLSCs; Figure 4C). ELISA revealed that cultures of allogeneic hPDLSCs in mixed lymphocyte reactions of different age groups secreted significantly lower concentrations of IL-2 and IFN-γ proteins (for both, p < 0.01 for the presence and absence of hPDLSCs; Figure 4D, E) and higher concentrations of TNF-β protein (p < 0.001 for the absence of hPDLSCs and p < 0.01 for the presence of hPDLSCs; Figure 4F) in the older group, compared with the younger age group. The age-induced changes in the expression levels of these three immunoregulatory cytokines may lead to reduced T lymphocyte proliferation and activation, as well as other alterations of immunologic functions.
Figure 4.
Quantitative polymerase chain reaction analysis of (A) IL-2, (B) IFN-γ, and (C) TNF-β gene expression levels in PBMCs from younger and older donors. Enzyme-linked immunosorbent assays of (D) IL-2, (E) IFN-γ, and (F) TNF-β protein levels in supernatant of PBMCs from younger and older donors. Data represent three independent experiments. Values are expressed as mean ± standard deviation (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Abbreviations: hPDLSCs, human periodontal ligament stem cells; IFN, interferon; IL, interleukin; OPBMCs, older peripheral blood mononuclear cells; PBMCs, peripheral blood mononuclear cells; PHA, phytohemagglutinin; TNF, tumor necrosis factor; YPBMCs, younger peripheral blood mononuclear cells.
Discussion
According to Clarkson et al.,17,18 adverse bodily reactions to allogeneic or xenogeneic transplants are highly complex immunological processes that involve many mechanisms of immune damage mediated by cells and antibodies. An important antigen-specific immune response is controlled by recipient T lymphocytes.17,18 hPDLSCs exhibit low immunogenicity and potent immunoregulatory capacity;4 thus, they can inhibit the proliferation of T lymphocytes either by direct contact or through secretion of soluble factors.5 Accordingly, hPDLSCs have great potential for use in allogeneic applications. However, previous studies have shown that immune aging (also known as immunosenescence), an inevitable stage of a body’s metabolic lifespan,19 leads to changes in the numbers of T lymphocytes and cell subsets, as well as changes in the surface molecules and functions of the cellular membrane.20 Ultimately, immunosenescence results in diminished and abnormal T lymphocyte immune functions.
Thus far, it has been established that aging triggers a complex remodeling of the immune system, whereby the functions of lymphocytes, especially T lymphocytes, exhibit the greatest change.21 These aging-related alterations, including imbalanced T lymphocyte subsets and modified proliferation responses to antigen and mitogen stimulation,21 are partly due to variations in expression patterns of marker molecules on the cell surface.22 CD28 and CD95 are currently recognized as potential biomarkers for the evaluation of immune aging. Previous studies have shown that the age-induced decline in CD28 expression is associated with reduced T lymphocyte responsiveness to mitogens, which affects the abilities of these cells to proliferate and secrete various cytokines.23 Conversely, CD95 expression increases with age, ultimately leading to excessive T lymphocyte apoptosis.24 Consistent with previous findings, our results showed that the CD3-positive rates of T lymphocytes from younger and older donors in this study were relatively similar; however, the rate of CD28 expression was higher in T lymphocytes from younger donors, whereas the rate of CD95 expression was higher in T lymphocytes from older donors. CD28– T cells are characterized by oligoclonal expansion, which results in reduced diversity of corresponding antigen receptors, narrowing of the T cell antigen recognition spectrum, weakening of the pathogen-clearing ability, and the presence of mutant cells in vivo.25 An elevated proportion of CD95+ T cells and substantial splitting of regular DNA fragments are indicative of higher rates of cellular apoptosis.26 Therefore, suppression of the immune response in older people is partly caused by reduced CD28 expression and elevated CD95 expression.
The lymphocyte proliferation experiment and mixed lymphocyte reaction can be used as an in vitro model to simulate in vivo cellular immune regulation and allogeneic transplantation; this constitutes a reliable method to study the immunosuppressive ability of MSCs. Therefore, we used lymphocyte proliferation and mixed lymphocyte reaction experiments to compare immune responses produced by PBMCs from younger and older donors exposed to allogeneic hPDLSCs. The results of this study showed that hPDLSCs have a strong immunosuppressive effect, both through cell–cell contact and Transwell (diffusion) mechanisms; moreover, the immune response is weaker in older PBMC donors than in younger donors. Thus, the activity and proliferation of T lymphocytes from older recipients are less robust, compared with T lymphocytes from younger recipients. Indeed, previous studies have shown that younger recipients exhibit less PBMC suppression by bone marrow MSCs, compared with older recipients, or that there are no significant differences between the two groups.16,27 Considering that the same cell ratio (1:10) was used in the previous and current studies,5,28 the observed inconsistency is attributable to variation in the immunosuppressive capacities of hPDLSCs and bone marrow MSCs.28,29 The weaker immune rejection of hPDLSC allotransplantation in older recipients, compared with younger recipients, may be due to poor immune functions (e.g., activation, proliferation, and differentiation) in older recipients.
Our results also demonstrate that aging may interfere with the immune response of allogeneic hPDLSCs by affecting the ability of T lymphocytes to secrete cytokines. Importantly, age gradually changes cytokines, thereby altering the normal activation, proliferation, and differentiation of T lymphocytes; these changes lead to reduced or abnormal immune functions.30 Previous reports have shown that the main cytokines related to T lymphocyte functions are IL-2, IL-3, IL-4, IL-5, IL-10, IFN-γ, and TNF-β;31 however, the current study did not investigate which factors play a major role in this process. Previous studies have shown that IL-2—a cytokine signaling molecule that plays an important role in the body’s immune response and can promote the production of other cytokines—is mainly produced by activated T lymphocytes.32 Similarly, IFN-γ, an important immunoregulatory factor in vivo, can only be secreted when T lymphocytes are activated by antigens or mitogens during an immune response.33 T lymphocytes can also produce high levels of TNF-β following stimulation by antigens and mitogens.34 Here, our findings indicated that the expression levels of IL-2 and IFN-γ in T lymphocytes were negatively correlated with age after co-culture with hPDLSCs, while the expression level of TNF-β was positively correlated. These results are consistent with those of previously published reports, indicating that reduced IL-2 and IFN-γ secretion in older individuals impairs the production and further activation of T lymphocytes that are activated by specific antigens or mitogens;35 conversely, elevated TNF-β secretion by T lymphocytes in older individuals can enhance T lymphocyte proliferation and promote the production of other cytokines.34 Therefore, the reduced levels of IL-2 and IFN-γ in older recipients may be the main reason for the weakened immune rejection of T lymphocytes.
In summary, PBMCs from younger and older donors exhibited different responses to hPDLSC-induced immunosuppression. Allogeneic hPDLSCs inhibited T lymphocyte proliferation more strongly in PBMCs from older donors than in PBMCs from younger donors, due to changes in the expression levels of T lymphocyte surface molecules and secretion of cytokines. Further understanding regarding the mechanism of age-related differences in T lymphocyte immunity is critical for the development of hPDLSC allotransplantation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos: 81771030 and 81570945) and the Construction Engineering Special Fund of Taishan Scholars (Grant No: ts201611068).
ORCID iDs
Xiaoyu Li https://orcid.org/0000-0002-8177-1813
Fulan Wei https://orcid.org/0000-0001-6827-8293
References
- 1.Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016; 7: e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004; 364: 149–155. [DOI] [PubMed] [Google Scholar]
- 3.Wei F, Song T, Ding G, et al. Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev 2013; 22: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada N, Menicanin D, Shi S, et al. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 2009; 219: 667–676. [DOI] [PubMed] [Google Scholar]
- 5.Ding G, Liu Y, Wang W, et al. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 2010; 28: 1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez R, Smith PC, Gostemeyer G, et al. Ageing, dental caries and periodontal diseases. J Clin Periodontol 2017; 44: S145–S152. [DOI] [PubMed] [Google Scholar]
- 7.Liu O, Xu J, Ding G, et al. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells 2013; 31: 1371–1382. [DOI] [PubMed] [Google Scholar]
- 8.Pawelec G. Age and immunity: what is “immunosenescence”? Exp Gerontol 2018; 105: 4–9. [DOI] [PubMed] [Google Scholar]
- 9.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol 2007; 211: 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson JS, Wekstein DR, Rhoades JL, et al. The immune status of healthy centenarians. J Am Geriatr Soc 1984; 32: 274–281. [DOI] [PubMed] [Google Scholar]
- 11.Salam N, Rane S, Das R, et al. T cell ageing: effects of age on development, survival & function. Indian J Med Res 2013; 138: 595–608. [PMC free article] [PubMed] [Google Scholar]
- 12.Bektas A, Schurman SH, Sen R, et al. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol 2017; 102: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Liu Z, Zhang Y, et al. Age-dependent alterations of HLA-DR expression and effect of lipopolysaccharide on cytokine secretion of peripheral blood mononuclear cells in the elderly population. Scand J Immunol 2011; 74: 603–608. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre-Gamboa R, Joosten I, Urbano PCM, et al. Differential effects of environmental and genetic factors on T and B cell immune traits. Cell Rep 2016; 17: 2474–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G. Aging and its impact on innate immunity and inflammation: implications for periodontitis. J Oral Biosci 2014; 56: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan YQ, Zhao CS, Zou HQ, et al. Aging, rather than Parkinson’s disease, affects the responsiveness of PBMCs to the immunosuppression of bone marrow mesenchymal stem cells. Mol Med Rep 2019; 19: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarkson MR, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Transplantation 2005; 80: 555–563. [DOI] [PubMed] [Google Scholar]
- 18.Moreau A, Varey E, Anegon I, et al. Effector mechanisms of rejection. Cold Spring Harb Perspect Med 2013; 3: a015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solana R, Tarazona R, Gayoso I, et al. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012; 24: 331–341. [DOI] [PubMed] [Google Scholar]
- 20.Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol Rev 2005; 205: 5–6. [DOI] [PubMed] [Google Scholar]
- 21.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des 2013; 19: 1680–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingender G, Kronenberg M. OMIP-030: characterization of human T cell subsets via surface markers. Cytometry A 2015; 87: 1067–1069. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev 2005; 205: 158–169. [DOI] [PubMed] [Google Scholar]
- 24.Potestio M, Pawelec G, Di Lorenzo G, et al. Age-related changes in the expression of CD95 (APO1/FAS) on blood lymphocytes. Exp Gerontol 1999; 34: 659–673. [DOI] [PubMed] [Google Scholar]
- 25.Messaoudi I, Lemaoult J, Guevara-Patino JA, et al. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med 2004; 200: 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahlavani MA, Vargas DA. Aging but not dietary restriction alters the activation-induced apoptosis in rat T cells. FEBS Lett 2001; 491: 114–118. [DOI] [PubMed] [Google Scholar]
- 27.Landgraf K, Brunauer R, Lepperdinger G, et al. The suppressive effect of mesenchymal stromal cells on T cell proliferation is conserved in old age. Transpl Immunol 2011; 25: 167–172. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Jo CH, Kim HR, et al. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells Int 2018; 2018: 8429042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valencia J, Blanco B, Yanez R, et al. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy 2016; 18: 1297–1311. [DOI] [PubMed] [Google Scholar]
- 30.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today 2000; 21: 515–521. [DOI] [PubMed] [Google Scholar]
- 31.Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol 2016; 7: 2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemmers S, Schizas M, Azizi E, et al. IL-2 production by self-reactive CD4 thymocytes scales regulatory T cell generation in the thymus. J Exp Med 2019; 216: 2466–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano S, Ghosh P, Kusaba H, et al. Effect of promoter methylation on the regulation of IFN-gamma gene during in vitro differentiation of human peripheral blood T cells into a Th2 population. J Immunol 2003; 171: 2510–2516. [DOI] [PubMed] [Google Scholar]
- 34.Uhrlaub JL, Pulko V, DeFilippis VR, et al. Dysregulated TGF-beta production underlies the age-related vulnerability to chikungunya virus. PLoS Pathog 2016; 12: e1005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pourgheysari B, Karimi L, Bagheri R, et al. Low IL-2 expressing T cells in thalassemia major patients: is it immune aging. Indian J Hematol Blood Transfus 2018; 34: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]