Abstract
Introduction
A nicotine-reduction policy could have major benefits for smokers with serious mental illness (SMI). However, potential unintended consequences, such as compensatory smoking, should be considered to ensure that such a policy does not negatively affect this population. The purpose of this secondary analysis was to examine the impact of smoking very low nicotine content (VLNC) cigarettes for 6 weeks on smoking topography characteristics, indicators of compensatory smoking, among smokers with SMI.
Aims and Methods
After a baseline usual brand smoking phase, smokers with SMI (N = 58) were randomly assigned under double-blind conditions to receive either VLNC (0.4 mg nicotine per g tobacco) or normal nicotine content (NNC; 15.8 mg nicotine per g tobacco) research cigarettes for 6 weeks. During two study visits scheduled 6 weeks apart, participants smoked either their usual brand (baseline) or assigned study cigarettes (postrandomization) through a handheld smoking topography device. Univariate analysis of variance compared smoking topography indices with cigarette condition (VLNC vs. NNC) as the between-subjects factor with corresponding baseline topography results included as covariates.
Results
At week 6, participants in the VLNC condition smoked fewer puffs per cigarette and had shorter interpuff intervals compared to participants in the NNC condition (ps < .05). There were no differences between research cigarette conditions at week 6 for cigarette volume, puff volume, puff duration, peak flow rate, or carbon monoxide boost.
Conclusions
Findings are consistent with acute VLNC cigarette topography studies and indicate that a nicotine-reduction policy is unlikely to lead to compensation among smokers with SMI.
Implications
Given the high smoking rates among people with SMI, understanding how a nicotine-reduction policy may affect this population is critically important. When considering the smoking topography results as a whole, smokers with SMI did not engage in compensatory smoking behavior when using VLNC cigarettes during a 6-week trial. Study findings suggest that compensatory smoking is not likely to occur among smokers with SMI if nicotine content is lowered to minimally addictive levels.
Introduction
Cigarette smoking remains the leading cause of premature death in the United States, contributing to approximately 480 000 deaths annually.1 One novel approach to reduce the public health burden of smoking is for the Food and Drug Administration to set the maximum allowable nicotine content in cigarettes to a minimally addictive level.2 By mandating that all cigarettes sold in the US have very low nicotine levels, fewer adolescents may start smoking and current smokers may be more successful at quitting. Smokers provided with very low nicotine content (VLNC; 0.4 mg nicotine per g tobacco) cigarettes during clinical trials have experienced significant reductions in smoking behavior, toxicant exposure, and cigarette dependence,3,4 indicating that a nicotine-reduction policy may be a feasible public health approach.
One potential concern regarding a nicotine-reduction policy is that smokers may engage in compensatory smoking in order to maintain their desired nicotine level. The tobacco in VLNC cigarettes used in these trials contains drastically reduced nicotine levels thereby making it difficult for people to increase their nicotine exposure via cigarette smoking.5 Despite this, smokers may still attempt to compensate for the lower nicotine levels either by increasing the number of cigarettes smoked each day or by changing the manner in which they smoke each cigarette (ie, smoking topography) in order to maximize nicotine delivery. A previous smoking topography study found evidence of compensation after acute laboratory exposure to VLNC cigarettes,6 but these effects were not sustained with repeated exposure to VLNC cigarettes.7 Additionally, when smokers used VLNC cigarettes during clinical trials, they did not compensate by increasing the total number of cigarettes smoked per day.3,4 Although these findings are encouraging, they may not be representative of all smoking populations.
Compensatory smoking is especially concerning for smokers with serious mental illness (SMI; ie, those with schizophrenia or bipolar disorder) because this population already experiences a greater burden of disease from tobacco use. Life expectancies for people with SMI are approximately 15 years shorter compared to people without psychiatric conditions and cigarette smoking is a major contributing factor in this disparity.8–10 Smoking rates for people with schizophrenia and bipolar disorder are three to five times higher than the general smoking population, and the overall cessation rates for people with SMI are quite low despite similar interest in quitting.9,11 In laboratory studies, smokers with schizophrenia had more intense smoking topography characteristics, leading to greater smoke exposure, when smoking their usual brand cigarettes compared to smokers without psychiatric conditions.12,13 A recent laboratory study in smokers with SMI reported that participants had longer puff durations and shorter interpuff intervals when smoking VLNC cigarettes compared to their usual brand, but they took fewer puffs, resulting in a decrease in total cigarette volume.14 Likewise, a laboratory study in smokers with affective and other disorders found that participants smoked fewer puffs and had lower mean peak flow rates when using VLNC cigarettes compared to normal nicotine content (NNC) research cigarettes.15 Furthermore, in both studies, breath carbon monoxide (CO) levels were not affected by cigarette condition, indicating that VLNC use did not increase smoke exposure.14,15
There are a limited number of smoking topography studies among smokers using VLNC cigarettes for extended periods. In one study, when participants smoked VLNC cigarettes in the real world for 7 days, laboratory assessments of total puff count and total cigarette volume significantly decreased relative to their usual brand smoking topography.16 During a 6-week clinical trial of cigarettes varying in nicotine content, when smoking topography was assessed during laboratory visits, total cigarette volume was reduced among smokers assigned to VLNC cigarettes, which is encouraging.3 However, these studies excluded people with significant psychiatric illnesses including schizophrenia3,16 or those prescribed psychotropic medications,16 so little is known about whether extended use of VLNC cigarettes leads to compensatory smoking behavior among smokers with SMI. This secondary analysis examined the effects of 6-week use of VLNC cigarettes on smoking topography characteristics in smokers with SMI.17
Materials and Methods
Participants
Daily smokers with SMI were recruited from the community via advertisements to participate in a 6-week clinical trial assessing the impact of cigarette nicotine content on smoking behavior, toxicant exposure, subjective cigarette effects, and psychiatric symptoms. Study enrollment required a clinical diagnosis of either schizophrenia, schizoaffective disorder, and/or bipolar disorder as determined by the Structured Clinical Interview for DSM-IV. Eligibility criteria included: 18–70 years old, smoking at least 10 cigarettes or little cigars or cigarillos (LCC) per day for the past year, preferring cigarettes when available, expired breath CO levels greater than 8 ppm or NicAlert urinary cotinine tests equal to 6. Exclusion criteria included: seeking treatment for smoking cessation or planning to quit in the next month, medically or psychiatrically unstable, medication changes in the past 3 months, pregnancy or breastfeeding, positive urine toxicology tests (excluding cannabis) or breath alcohol level greater than 0.02%, having a legal guardian and/or power of attorney, and prior participation in VLNC cigarette studies.
Study Design and Procedures
After a 1-week baseline usual brand cigarette smoking phase, participants (N = 58) were randomized under double-blind conditions to receive either NNC research cigarettes with 15.8 mg nicotine per g tobacco or VLNC research cigarettes with 0.4 mg nicotine per g tobacco for 6 weeks. At each weekly visit, participants received free 2-week supplies of their assigned research cigarettes. (Two weeks’ worth of supplies were provided to accommodate for potential changes in smoking behavior and/or potential missed visits.) Research assistants instructed participants to smoke only their assigned research cigarettes for the duration of the trial and emphasized the importance of research cigarette adherence during the weekly visits. The Brown University Institutional Review Board approved all study procedures, and an external Data and Safety Monitoring Board reviewed the trial on a semi-annual basis. All participants provided written informed consent prior to enrollment. Additional information on the study protocol as well as the primary trial outcomes were reported previously.17
Smoking Topography
During the baseline visit, participants smoked one of their usual brand cigarettes through a CReSS handheld topography device (Borgwaldt, KC, Richmond, VA). At the weeks 2 and 6 visits, participants smoked one of their assigned research cigarettes using the topography device. The topography variables assessed in this study include: (1) total cigarette volume (mL of smoke inhaled), (2) total puff count (number of cigarette puffs), (3) mean puff volume (cigarette volume divided by puff count), (4) mean interpuff interval (time between puffs), (5) mean puff duration (length of time inhaling the smoke), and (6) mean peak flow rate (smoke inhalation velocity). Additionally, expired breath CO readings were obtained from participants immediately before and 15 min after smoking the cigarettes. We calculated the CO boost variable by subtracting the pretopography CO reading from the posttopography CO reading.
Statistical Methods
We analyzed baseline demographic and smoking characteristics using t tests for continuous outcome variables and Fisher’s exact tests for categorical outcome variables. For this secondary analysis, we compared smoking topography variables at week 6 using univariate analysis of variance tests with cigarette condition (NNC vs. VLNC) as the between-subject factor. Models adjusted for baseline smoking by including the corresponding usual brand topography indices as covariates. Week 2 topography indices were not included in the present analyses. Effect sizes (partial eta squared, ) are provided when ps < .05, with indicating small, indicating medium, and indicating large effect sizes.18 Analyses were conducted using SPSS statistical software version 25 (IBM).
Results
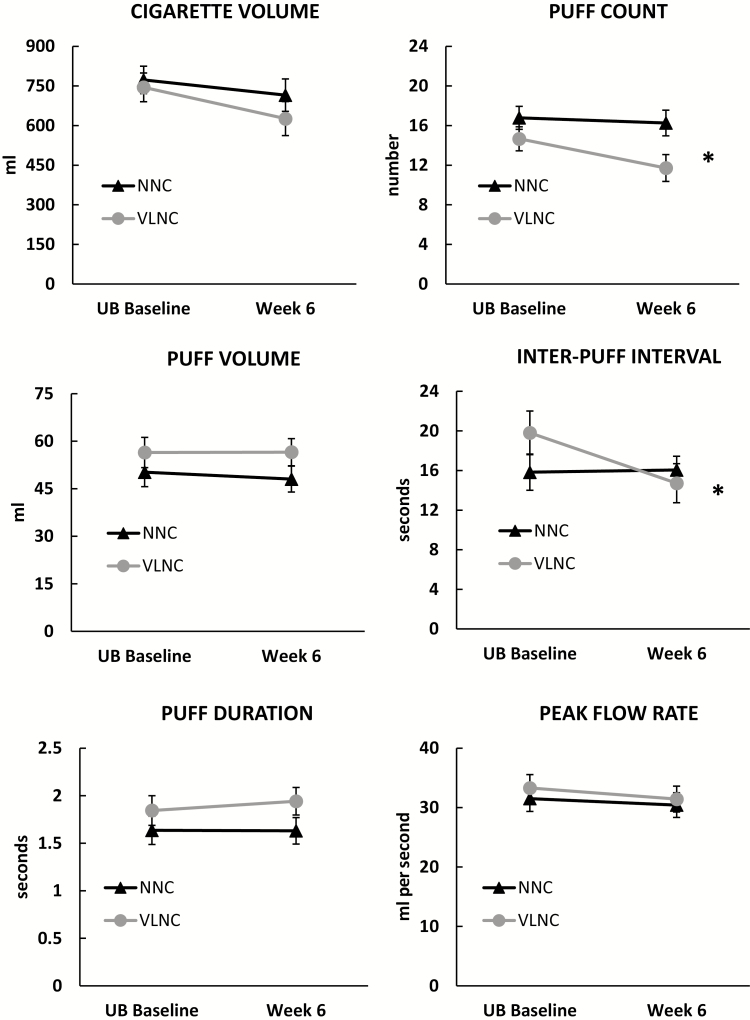
Baseline demographic and smoking characteristic by cigarette condition are reported in Table 1. No significant differences were observed between the two cigarette conditions (NNC vs. VLNC) for any demographic or smoking history variable. The effects of cigarette condition on topography measures are shown in Figure 1. We found significant differences between research cigarette conditions for puff count [F(1,43)= 5.23, p = .027, ] and interpuff interval [F(1,42) = 6.42, p = .015, ], such that participants in the VLNC condition smoked fewer puffs per cigarette and had shorter interpuff intervals relative to the NNC condition at the week 6 visit. One participant in the VLNC condition was excluded from the interpuff interval analysis because his baseline response was more than three standard deviations above the baseline mean. The results remain significant with or without the outlier included in the analysis. No significant differences between research cigarette conditions were observed for cigarette volume, puff duration, puff volume, peak flow rate, or CO boost.
Table 1.
Baseline Demographic and Smoking Characteristics by Cigarette Nicotine Conditiona
| VLNC (n = 30) | NNC (n = 28) | p | |
|---|---|---|---|
| Age (years) | 43.4 (9.6) | 43.1 (11.0) | .924 |
| Gender (female), no. (%) | 12 (40.0) | 12 (42.9) | 1.00 |
| Race, no. (%) | |||
| White | 15 (50.0)c | 19 (67.9) | .412b |
| Black | 6 (20.0) | 5 (17.9) | |
| Multiracial | 6 (20.0) | 4 (14.3) | |
| Hispanic ethnicity, no. (%) | 3 (10.0) | 0 (0) | .237 |
| Diagnosis, no. (%) | |||
| Schizophrenia and/or schizoaffective | 22 (73.3) | 23 (82.1) | .534 |
| Bipolar | 8 (26.7) | 5 (17.9) | |
| Menthol use, no. (%) | 23 (76.7) | 18 (64.3) | .390 |
| Cigarettes per day | 20.1 (8.8) | 18.2 (7.7) | .374 |
| Carbon monoxide level (ppm) | 20.4 (13.0) | 21.1 (11.9) | .835 |
| FTCD | 6.8 (1.5) | 6.5 (1.5) | .438 |
Baseline demographics for all participants randomized to the very low nicotine content condition (VLNC) or normal nicotine control condition (NNC).
FTCD = Fagerström Test of Cigarette Dependence.
aUnless otherwise indicated, values represent mean (standard deviation).
b p value is based on the comparison between whites and nonwhites.
cThree participants did not report race.
Figure 1.
Smoking topography characteristics by cigarette nicotine condition. Mean (±SEM) total cigarette volume, total puff count, mean puff volume, mean interpuff interval, mean puff duration, and mean peak flow rate observed during baseline and week 6 by cigarette nicotine condition (VLNC or NNC). * indicates a significant difference between the cigarette conditions at week 6 (p < .05). NNC = normal nicotine content; UB = usual brand; VLNC = very low nicotine content. Baseline results are included for reference.
Discussion
In this study, the nicotine content of the research cigarettes affected two smoking topography characteristics. Participants assigned to the VLNC condition smoked fewer puffs per cigarette and had significantly shorter interpuff intervals at week 6 compared to these variables at baseline. Shorter interpuff intervals could be consider an indicator of potential compensation (ie, more rapid smoking behavior). However, the VLNC group had higher (slower) interpuff intervals at baseline (ie, when smoking their usual brand cigarettes) and the conditions had similar interpuff intervals at week 6, indicating no increased risk in absolute level of harm for the VLNC condition relative to the NNC condition. Importantly, a reduction in the number of puffs smoked per cigarette is in the direction of less smoke exposure after smoking VLNC cigarettes. Furthermore, total cigarette volume and CO boost were similar across cigarette conditions at week 6, indicating that smoking VLNC cigarettes did not increase overall smoke exposure compared to the NNC condition. However, one important consideration is that total cigarette volume decreased in both conditions at week 6, suggesting that switching to research cigarettes, regardless of nicotine content, affects smoking topography behavior.
Overall, this study indicates that when smokers with SMI use VLNC cigarettes for 6 weeks, they did not engage in compensatory smoking behavior as measured by smoking topography characteristics. These outcomes bolster the trial’s primary findings of significant reductions in cigarette smoking and CO exposure in the VLNC condition, which indicates reduced smoke exposure for smokers with SMI.17 Additionally, the study results are consistent with previous VLNC smoking topography laboratory studies among smokers with mental health conditions, which reported that VLNC cigarette use reduced puffs compared to usual brand or NNC research cigarettes.14,15 The current study extends this literature by analyzing smoking topography characteristics after smoking VLNC cigarettes in the natural environment for several weeks. This study design is more informative for a nicotine-reduction policy because participants were instructed to only use their assigned study cigarettes for the duration of the trial in an attempt to simulate a regulated tobacco environment in which only VLNC cigarettes are available. Under such conditions, smokers using VLNC cigarettes could attempt to alter their smoking behavior in an effort to increase their per cigarette nicotine exposure. However, the results indicate that smokers with SMI did not modify their smoking behavior over time to compensate for the lower nicotine levels.
There are a few limitations to consider in this study. First, with a modest sample size, the trial may have been underpowered to test the effects of cigarette nicotine condition on smoking topography variables. However, the average total cigarette volume observed in the VLNC condition was approximately 80 mL lower than the NNC condition at the week 6 visit, which is in the direction of less exposure. Second, adherence to smoking only VLNC cigarettes during the 6-week trial was low,17 which could lead to an underestimation of compensation. However, an in-patient study of participants who only had access to VLNC cigarettes also found reductions in cigarettes per day and number of puffs smoked per cigarette, suggesting that even when compliance is assured, smoke exposure does not increase with VLNC cigarettes.7
Despite these limitations, this study contributes meaningful information to the VLNC cigarette literature. Given the high rates of smoking in people with SMI, a nicotine-reduction policy could have major benefits. Results from this study are encouraging in that they suggest that a nicotine-reduction policy may not lead to compensatory smoking behavior among smokers with SMI.
Funding
This research was supported by grant U54DA031659 from the National Institute on Drug Abuse (NIDA) and the Food and Drug Administration Center for Tobacco Products (FDA). Additional author support during the preparation of this paper was provided by NIDA grants R36DA045183 and U54DA036114, and the National Cancer Institute (NCI) grants K01CA189300 and T32CA122061. Research reported in this publication was also supported by National Institute of Health (NIH) grant P30CA077598 utilizing the Biostatistics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1-TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, NCI or the FDA.
Declaration of Interest
None declared.
Acknowledgments
We thank Mollie Miller, Netesha Reid, Emily Xavier, Ashley Marzullo, Kimberly Duguay, Tonya Lane, the Center for Evaluation of Nicotine in Tobacco (CENIC) Administrative, Biomarkers and Biostatistics Cores, and the study participants for their essential contributions to this research. We also want to thank Jasjit Ahluwalia for his feedback during manuscript development.
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. [DOI] [PubMed] [Google Scholar]
- 3. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richter P, Steven PR, Bravo R, et al. Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86(2–3):294–300. [DOI] [PubMed] [Google Scholar]
- 7. Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. [DOI] [PubMed] [Google Scholar]
- 8. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. [DOI] [PubMed] [Google Scholar]
- 9. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38(1):165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tam J, Warner KE, Meza R. Smoking and the reduced life expectancy of individuals with serious mental illness. Am J Prev Med. 2016;51(6):958–966. [DOI] [PubMed] [Google Scholar]
- 11. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. [DOI] [PubMed] [Google Scholar]
- 12. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend. 2005;80(2):259–265. [DOI] [PubMed] [Google Scholar]
- 13. Williams JM, Gandhi KK, Lu SE, et al. Shorter interpuff interval is associated with higher nicotine intake in smokers with schizophrenia. Drug Alcohol Depend. 2011;118(2–3):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tidey JW, Cassidy RN, Miller ME. Smoking topography characteristics of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 2016;18(9):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104(1–2):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tidey JW, Colby SM, Denlinger-Apte RL, et al. Effects of 6-week use of very low nicotine content cigarettes in smokers with serious mental illness. Nicotine Tob Res. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New Jersey: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]