Abstract
Background
Smoking cannabis may potentially increase exposure to numerous toxic chemicals that are commonly associated with tobacco use. There is a paucity of data related to toxicant exposures among concurrent users of tobacco and cannabis (co-users).
Methods
Data are from the Population Assessment of Tobacco and Health Study Wave 1 Biomarker Restricted-Use Files. Analyses focused on adults who provided urine samples (N = 5859). Urine samples were analyzed for biomarkers of exposure to nicotine, tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons, and volatile organic compounds. Using weighted linear regression, we compared adjusted geometric mean concentrations of 15 biomarkers between user groups of various tobacco product types according to their self-reported past 30-day cannabis use.
Results
Past 30-day cannabis use was similar across various types of tobacco product use subgroups (range: 13%–23%) and significantly more common compared to non-tobacco users (1.0%; p < .001). Across all groups of tobacco users, those who co-used cannabis exhibited significantly higher concentrations of the biomarker of exposure to acrylonitrile (CYMA) compared to non-cannabis users (by 39%–464%). Tobacco–cannabis co-users also showed significantly elevated levels of the biomarker of exposure to acrylamide (AAMA) compared to exclusive tobacco users, and significantly higher exposure to many polycyclic aromatic hydrocarbons (including fluorene and pyrene).
Conclusions
Co-users exhibited higher concentrations for biomarkers of exposure to many combustion byproducts, compared to exclusive tobacco users. More robust measurements of cannabis use can address potential confounding in assessments of exposures to tobacco-related constituents, and potential health effects resulting from co-use.
Implications
With disproportionately greater rates of cannabis use occurring among tobacco users, it is critical to consider how concurrent cannabis use may influence health-related outcomes among smokers. Our findings suggest potential additive toxicant exposures among co-users of tobacco and cannabis. Lack of consideration and measurement of cannabis use in assessing tobacco-related exposures may confound estimates thought to be attributable to tobacco, particularly for non-specific biomarkers. Assessing tobacco and cannabis use in tandem will allow for more precise measurement of outcomes related to one or both substances, and can provide additional information on potential health effects related to co-use.
Introduction
In the United States, concurrent use of tobacco and cannabis (co-use) is more common than cannabis use alone, with 5.2% of US adults reporting past month co-use, compared to 2.3% who reported exclusive use of cannabis.1 Mechanisms of co-use are related to shared genetic and environmental influences and are thought to be reinforced by a shared route of administration (inhalation).2 This includes smoking, which remains the predominant mode of delivery for tobacco and cannabis, respectively.3,4 Co-users constitute a unique subset of the tobacco user population at risk for important health issues, including heavier frequency and quantity of cannabis and tobacco use, greater dependence on cannabis and tobacco, potentially increased negative respiratory symptoms, and diminished likelihood of cannabis cessation.2,5
To date, the literature on co-use largely focuses on descriptive epidemiologic studies that characterize these users,1,6–9 aspects of co-use related to tobacco and/or cannabis treatment and cessation,5,10 and the study of these issues as they relate to cigarette use and cigar use.1,2,11 Few studies have leveraged available data to describe co-use in the context of a diverse array of tobacco products,6,12,13 ranging from higher risk combustible products (including cigarettes, cigars, and hookah), to presumably lower risk non-combustible tobacco products (most notably, electronic cigarettes [e-cigarettes]).14 Each of these tobacco products exhibits differences in user demography and associated patterns of use, lending to differential nicotine and toxicant exposure profiles that can provide clues into potential health harms and thus, potential exposure reduction strategies.15–18
Considerably less attention has been given to toxicant exposures among co-users. A recent review on this topic found that most studies in this area focused on measures related to exhaled carbon monoxide, with no available data to describe exposure to other smoke constituents in co-users.19 The importance of this concept is suggested via laboratory data showing that tobacco and cannabis smoke contain many of the same toxic constituents.20 Although tobacco produces higher yields of many toxicants (including tobacco-specific nitrosamines and several carbonyl compounds), cannabis smoke can produce yields of other toxicants at levels equivalent to, or even higher than, those found in tobacco smoke. These include many aromatic amines, volatile organic compounds (VOCs), and equivalent levels of many polycyclic aromatic hydrocarbons (PAHs).20 Many of these toxicants are linked to illnesses such as cardiovascular and respiratory diseases, and cancer.21
Several human exposure studies have been performed to understand exposures unique to exclusive users of each substance (tobacco and cannabis, respectively).15,17,22–24 As combustion remains predominant in the delivery of both drugs,3,4,25 and the inhalation of combustion byproducts is a key contributor to the development of tobacco-related diseases,26 there is a need to discern whether use of both substances is linked to elevated toxicant exposures relative to those who are only using one substance. This is not only important from the perspective of an individual’s health risks, but also provides key insights into whether residual sources of exposure from using smoked forms of one drug may confound estimates of exposure that are intended to describe potential risks associated with use of either drug alone.
Using data from the Population Assessment of Tobacco and Health (PATH) Study, we examined levels of exposure biomarkers in a sample of US adult users of various types of tobacco products and non-tobacco users to address the following: (1) Describe concentrations for biomarkers of exposure among co-users of tobacco and cannabis across a broad spectrum of tobacco products, and (2) Compare differences in biomarker concentrations between exclusive tobacco product users and tobacco–cannabis co-users of the same tobacco product.
Methods
Data Source
Data for this analysis used the merged PATH Study Wave 1 Adult Questionnaire Restricted-Use Files, and Biomarker Restricted-Use Files, collected from 2013 to 2014.27 The Biomarker Restricted-Use File is a stratified probability sample of the larger PATH adult cohort, consisting of 11 522 adults that provided urine samples that were selected for laboratory analysis and inclusion in this subsample. Details on methods for the PATH Study, including design, sampling, interviewing procedures, sample weighting, and biospecimen subsample details have been outlined elsewhere.28 The weighted response rate for screened households at Wave 1 was 54.0%. Among screened households, the weighted Wave 1 adult interview response rate for those who provided a urine sample was 63.6%. Approval for the study was granted by the Institutional Review Board at Westat.
Analytic Sample
Among the PATH biospecimen subsample, a sample of 5859 adults aged 18 or older were selected for study inclusion and stratified into six groups. For comparison purposes, we defined a group of (1) “non-tobacco users” (N = 1736) who included those who reported never using any tobacco product, or those who reported use of cigarettes more than 6 months prior to their interview, and reported no other current tobacco product use. Non-tobacco users included those who reported past 30-day cannabis use and those who did not report cannabis use. For users of a single tobacco product, four groups were defined, including: (2) “e-cigarette-only users” (n = 181), defined as those who reported current everyday or some days use of e-cigarettes and reported no other current tobacco product use; (3) “cigarette-only users” (n = 2412), defined as those reported smoking at least 100 cigarettes in their lifetime and reported current everyday or some days use of tobacco cigarettes and reported no other tobacco product use; (4) “cigar-only users” (n = 336), defined as reported current use of traditional cigars, cigarillos, and/or filtered cigars everyday or some days, and reported no other current tobacco product use; and (5) “hookah-only users” (n = 402), defined as those who reported current everyday or some days use of hookah, and no other current tobacco product use. Single product use groups were selected due to documented associations with concurrent cannabis use (cigarettes) and parallel modes of delivery relevant to both tobacco and cannabis (e-cigarettes, cigars, hookah).4,6,11 Given that 331 poly-tobacco use combinations occur among US adults,3 we additionally selected the most prevalent poly-tobacco use group as the final analytic group: (6) “dual cigarette + e-cigarette users” (“dual users”, n = 792): defined as those who smoked 100 cigarettes in their lifetime, reported current everyday or some days use of both tobacco cigarettes and e-cigarettes, and reported no other current tobacco product use. Other poly-tobacco combinations (n = 330) were not explicitly assessed, potential differences can be viewed elsewhere.29 Smokeless users were excluded due to the small number of cannabis users within this group.13 We did not exclude all non-tobacco users with detectable levels of total nicotine equivalents-2 (TNE-2) and urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) due to the presence of these constituents in secondhand smoke (SHS) and in cigars (used for the administration of blunts.).17,18,30 However, among those reporting no combusted tobacco use (ie, non-tobacco users and e-cigarette-only users) we excluded individuals with urinary NNAL levels (tobacco-specific biomarker) exceeding 14.5 pg/mg creatinine to rule out potential misreporting, estimates that may result from excessive SHS exposure, or excessive blunt use.30 In addition, all users had to report no past 3-day use of nicotine replacement therapy to rule out ancillary exposure to nicotine from non-tobacco sources.
Biospecimen Collection, Storage, Processing, and Analysis
Participants who consented to provide a urine biospecimen self-collected full-void spot urine samples in 500 mL polypropylene containers provided by interviewers. Urine samples were immediately placed in a Crēdo Cube shipper, designed to transport samples at temperatures maintained between 2°C and 8°C. Samples were shipped overnight to the study biorepository for processing, and biomarkers were subsequently measured using highly selective mass spectrometric methods carried out by the CDC Division of Laboratory Sciences.31–34
Biomarkers of Exposure
We focused on 15 biomarkers of exposure to harmful and potentially harmful chemicals with documented associations with tobacco and/or cannabis use.20 These biomarkers come from two main chemical classes that are not specific to use of either substance, including PAHs and VOCs. To assist in disentangling effects attributable to tobacco-related exposures (including tobacco use, secondhand tobacco smoke exposure, and tobacco–cannabis coadministration practices such as blunt and spliff use), we examined biomarker data for tobacco-specific exposures, including nicotine and tobacco-specific nitrosamines in all user groups. The full set of results can be found in Supplementary Table 1. For simplicity, the article will focuses on results for six biomarkers selected for their empirical association with tobacco and/or cannabis, and their documented associations with health harms across organ systems and incident illness.20,21,26,35 These include two biomarkers of exposure to PAHs: 2-hydroxyfluorene (parent compound: fluorene), and 1-hydroxypyrene (parent compound: pyrene), and two biomarkers of exposure to VOCs CYMA (parent compound: acrylonitrile), and AAMA (parent compound: acrylamide). We also compared concentrations of two tobacco-specific biomarkers: TNE-2 (parent compound: nicotine) and NNAL (parent compound: tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone [NNK]). TNE-2 reflects the molar sum of cotinine and trans-3′hydroxycotinine in urine. The clinical significance of these parent compounds includes addiction (nicotine), effects on neurological development (nicotine), respiratory irritation (fluorene), cardiovascular toxicity (pyrene), and known or suspected carcinogen status (NNK, acrylonitrile, acrylamide), among others.21 References to analytical limits of detection (LOD) for biomarkers in the PATH urine data panel have been published elsewhere.17
Cannabis Use
Cannabis use was assessed by asking participants the following question among those reporting ever use of “marijuana”: “How long has it been since you last used marijuana, hash, THC, grass, pot, or weed?” (response options: within the past 30 days, more than 30 days ago but within the past year, more than a year ago). A binary variable was created, with those reporting use within the past 30 days categorized as “past 30-day cannabis users,” whereas those reporting cannabis use more than 30 days ago or never use of cannabis were classified as “No cannabis use within the past 30 days.” The past 30-day timeframe was chosen over longer spans of time due to the short half-life of the biomarkers examined during this study.36,37 The PATH Study does not provide biochemical measurements reflective of cannabis use (ie, delta-9 tetrahydrocannabinol [THC], cannabidiol [CBD], and cannabinol [CBN]).
Statistical Analysis
Descriptive statistics (Pearson χ 2 for categorical variables, one-way analysis of variance for continuous variables) were used to compare characteristics across the entire sample, and within tobacco use groups according to self-reported past 30-day cannabis use. Owing to the log-normal distribution of biomarker data, natural log transformations were applied to minimize the effects of skewness. Biomarker values below the LOD were imputed using a standard formula (LOD/√2). Adjusted geometric mean values (adjusted GMs) were calculated for each biomarker using multivariable linear regression models. Post-estimation procedures were run to obtain adjusted marginal mean values of the natural log of the biomarkers and their 95% confidence intervals (CIs) according to past 30-day cannabis use, and were subsequently exponentiated to produce the estimate. All analyses controlled for urinary creatinine, age, gender, race/ethnicity, and education level. To adjust for additional sources of smoke exposure, all analyses also controlled for self-reported exposure to SHS within the past 7 days. Among those using any tobacco product, additional adjustment variables were included for frequency of product use and time since last use of their tobacco product. In addition, analyses of toxicant levels adjusted for urinary TNE-2 (transformed using the natural log), which was selected as a proxy control variable for intensity of product use due to its ubiquitous existence across a diverse spectrum of tobacco products, and its association with nicotine intake and smoking topography.38–41 Covariates were evaluated for potential multicollinearity and all were found to have variance inflation factors within acceptable limits.
Analyses were completed using the svy commands in Stata v. 15.0. Balanced repeated replication with Fay’s adjustment set to 0.3 was used for variance estimation. All analyses are weighted, and p values <.05 were considered statistically significant.
Results
Demographics and Cannabis Co-Use Prevalence
Table 1 outlines sample demographic characteristics. Among the sample, estimated prevalence of past 30-day cannabis use was 6.5% (95% CI = 5.7% to 7.4%). Past 30-day cannabis use was lowest among non-tobacco users (1%, 95% CI = 0.6% to 1.6%), followed by dual users of tobacco cigarettes and e-cigarettes (13.2%, 95% CI = 10.9% to 15.9%), e-cigarette-only users (15.3%, 95% CI = 9.9% to 22.9%), cigarette-only users (16.2%, 95% CI = 13.8% to 18.7%), cigar-only users (18.0%, 95% CI = 13.7% to 23.1%), and hookah users (23.1%, 95% CI = 18.2% to 28.7%). In general, past 30-day cannabis users tended to be younger in age and male compared to non-cannabis users. Cigarette-only users and cigar-only users who reported past 30-day use of cannabis had significantly higher exposure to SHS than non-cannabis users of the same product; otherwise SHS exposure was statistically similar between groups. Daily or some days use of tobacco generally did not significantly differ according self-reported past 30-day use of cannabis. The exception to this was among cigar-only users, of which a significantly greater proportion of past 30-day cannabis users reported daily use of cigars relative to cigar-only users that did not report past 30-day use of cannabis (everyday use: 18% for past 30-day cannabis users, 6% for non-cannabis users, uncorrected χ 2 = 313.28, design-based F(1,99) = 8.47, p = .0045.) Likewise, the time since last use of tobacco was not statistically different between past 30-day cannabis users and non-users within a given group (all ps > .05). The nicotine metabolite ratio (a proxy for the rate of nicotine metabolism using the ratio of 3-hydroxycotinine to cotinine) was examined for potential differences in the rate of nicotine metabolism between past 30-day cannabis users and non-users, no statistically significant differences were detected.
Table 1.
Participant Demographics (N = 5859)
| Non-tobacco users (n = 1736) | E-cigarette-only users (n = 181) | Cigarette-only smokers (n = 2412) | All exclusive cigar users (n = 336) | Hookah-only smokers (n = 402) | Dual users (n = 792) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | 95% Lower | 95% Upper | Mean (SE) | 95% Lower | 95% Upper | Mean (SE) | 95% Lower | 95% Upper | Mean (SE) | 95% Lower | 95% Upper | Mean (SE) | 95% Lower | 95% Upper | Mean (SE) | 95% Lower | 95% Upper | ||
| Age* | 44.6 (0.46) | 43.6 | 45.5 | 39.5 (1.4) | 36.6 | 42.3 | 44.2 (0.50) | 43.2 | 45.2 | 43.6 (1.0) | 41.4 | 45.6 | 24.5 (0.46) | 23.6 | 25.5 | 43.1 (0.65) | 41.8 | 44.4 | |
| SHS exposure (hours/week)* | 1.89 | 1.43 | 2.35 | 7.34 | 4.56 | 10.11 | 18.96 | 16.70 | 21.22 | 4.78 | 2.97 | 6.59 | 3.17 | 2.12 | 4.21 | 20.00 | 17.46 | 22.54 | |
| % | 95% Lower | 95% Upper | % | 95% Lower | 95% Upper | % | 95% Lower | 95% Upper | % | 95% Lower | 95% Upper | % | 95% Lower | 95% Upper | % | 95% Lower | 95% Upper | ||
| Gender* | Male | 37.8 | 35.1 | 40.6 | 41.8 | 32.7 | 51.4 | 45.8 | 42.6 | 49.0 | 80.8 | 74.9 | 85.5 | 41.1 | 34.6 | 48 | 37.1 | 32.6 | 41.8 |
| Female | 62.2 | 59.4 | 64.8 | 58.2 | 48.5 | 67.3 | 54.2 | 51.0 | 57.3 | 19.2 | 14.5 | 25.0 | 58.9 | 57.4 | 65.4 | 62.9 | 58.2 | 67.4 | |
| Race* | White, non-Hispanic | 56.8 | 53.1 | 60.3 | 73.3 | 64.5 | 80.6 | 69.4 | 65.6 | 72.9 | 69.5 | 63.4 | 75.1 | 44.7 | 38.4 | 51.1 | 81.7 | 78.8 | 84.3 |
| Other | 43.2 | 39.7 | 46.8 | 26.7 | 19.4 | 35.5 | 30.7 | 27.1 | 34.4 | 30.4 | 24.9 | 36.6 | 55.4 | 48.9 | 61.6 | 18.3 | 15.6 | 21.2 | |
| Education* | High school or less | 40.6 | 37.0 | 44.4 | 43.8 | 35.4 | 52.6 | 58.0 | 54.6 | 61.4 | 23.2 | 18.2 | 29.1 | 25.9 | 21.7 | 30.5 | 43.8 | 39.8 | 47.8 |
| Some college/2-year degree | 28.2 | 25.2 | 31.4 | 40.6 | 33.3 | 48.2 | 32.3 | 29.2 | 35.5 | 34.3 | 28.8 | 40.3 | 49.3 | 44.1 | 54.5 | 41.9 | 37.0 | 46.8 | |
| Bachelor’s degree or higher | 31.2 | 27.3 | 35.2 | 15.6 | 10.6 | 22.3 | 9.7 | 7.9 | 11.7 | 42.5 | 35.3 | 49.9 | 24.8 | 20.0 | 30.3 | 14.4 | 11.2 | 18.2 | |
| Past 30-day cannabis use* | Yes | 1.0 | 0.6 | 1.6 | 15.3 | 9.9 | 22.8 | 16.2 | 13.9 | 18.7 | 18.0 | 13.7 | 23.1 | 23.1 | 18.3 | 28.7 | 13.2 | 11.0 | 15.9 |
All estimates are weighted using urine weights for the PATH biospecimen subsample. “Non-tobacco users” = never users of tobacco or former cigarette smokers who reported quitting 6 months prior to their interview. All tobacco user groups were defined as current, everyday, or some day users of their tobacco product, with no current or some days use of any other tobacco product (tobacco product list: cigarettes, e-cigarettes, traditional cigars, cigarillos, filtered cigars, pipe tobacco, hookah, smokeless tobacco, snus, dissolvable tobacco). Cigarette-only users and dual users had to report smoking at least 100 cigarettes in their lifetime. Dual users included those individuals who reported everyday or some days use of combusted tobacco cigarettes and e-cigarettes, and no current or some days use of any other tobacco product. All groups included exclusions for past 3-day use of nicotine replacement therapies. Non-users and e-cigarette-only users excluded those with urinary NNAL levels exceeding 14.5 pg/mg creatinine.30
*Statistically significant differences in demographic characteristic according to tobacco use status (χ 2 for categorical data, one-way analysis of variance for continuous data, p < .05). SHS = Secondhand smoke, 95% L = 95% confidence interval for lower bound, 95% U = 95% confidence interval for upper bound.
Toxicant Exposure Among Non-Tobacco Users Who Use Cannabis
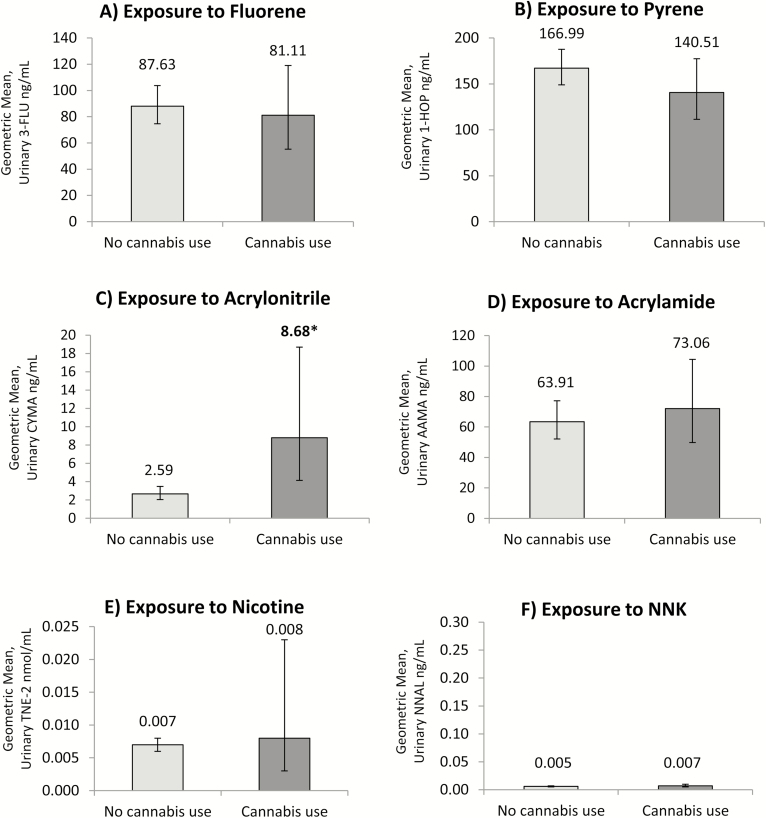
Figure 1 shows adjusted GMs representing exposure to nicotine, tobacco-specific nitrosamine NNK, fluorene, pyrene, acrylonitrile, and acrylamide among non-tobacco users. Largely, no statistically significant differences in adjusted GMs were detected according to past 30-day cannabis use or non-use. As the sole exception, adjusted GMs of acrylonitrile were statistically different, with concentrations approximately 235% higher among non-tobacco users who reported past 30-day use of cannabis relative to non-tobacco users who did not report cannabis use (t = 3.20, p = .002). Tobacco-specific biomarkers, including TNE-2 and NNAL, were statistically similar between non-users who reported past 30-day use of cannabis versus non-tobacco users who did not report cannabis use.
Figure 1.
Adjusted geometric mean concentrations representing exposure to (A) fluorene, (B) pyrene, (C) acrylonitrile, (D) acrylamide, (E) nicotine, and (F) tobacco-specific nitrosamine NNK among non-users of tobacco, stratified by self-reported past 30-day use of cannabis (n = 1736). All estimates are weighted and adjusted for urinary creatinine, age, sex, race, education, secondhand smoke exposure, and cannabis use. Toxicant biomarker analyses adjusted for logged nicotine intake (TNE-2). Bold and starred values indicate statistically significant differences between non-cannabis users and past 30-day cannabis users (p < .05).
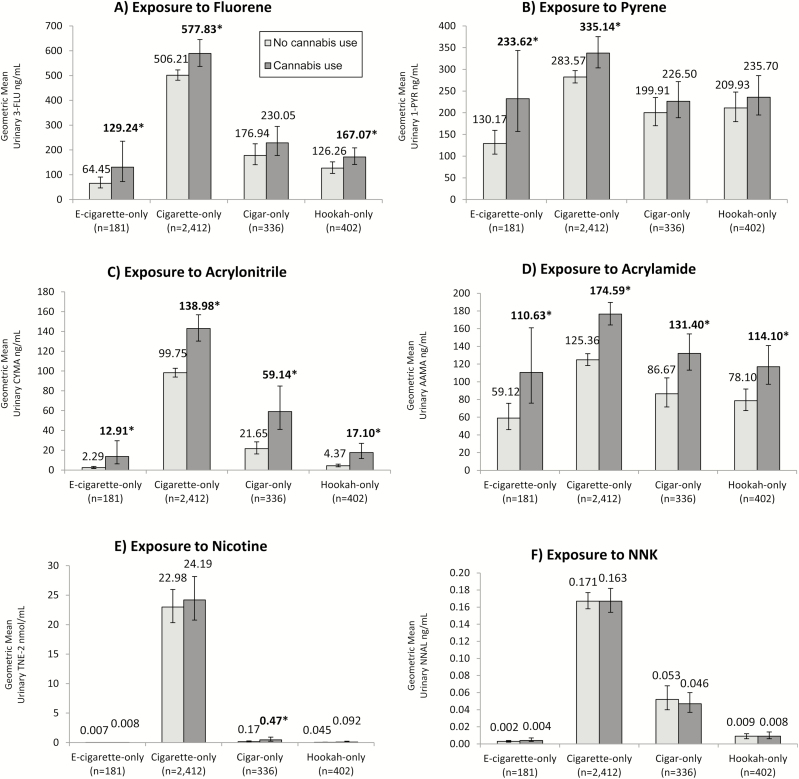
Toxicant Exposure Among Co-Users of a Single Type of Tobacco Product and Cannabis
Differences in adjusted GMs between past 30-day cannabis users and non-cannabis users were evident when examining single tobacco product users. (Figure 2) PAH exposure was significantly higher among e-cigarette-only users, cigarette-only users, (fluorene and pyrene) and hookah-only users (pyrene only) reporting past 30-day cannabis use compared to non-cannabis users. Adjusted GMs were approximately 101% (fluorene) and 79% (pyrene) greater among e-cigarette-only users who reported past 30-day cannabis use compared to non-cannabis users, whereas cigarette-only users reporting past 30-day cannabis use had 14% greater levels of the biomarker for fluorene, and 18% greater levels of the biomarker for pyrene than cigarette-only users who did not use cannabis. Hookah-only users who reported past 30-day cannabis use had levels for the biomarker of fluorene that were 32% higher than hookah-only users who did not report past 30-day cannabis use. For VOCs, all single tobacco product users that reported past 30-day cannabis use exhibited significantly higher levels of biomarkers of exposure to acrylonitrile and acrylamide than users who did not report past 30-day cannabis use. Differences were starkest among e-cigarette users; those who reported past 30-day cannabis use exhibited a 464% increase in concentrations for the biomarker for acrylonitrile, and 87% increase in biomarker concentrations for acrylamide compared to those who did not report cannabis use. Differences in adjusted GMs for VOCs were less stark. Past 30-day cannabis users had increased adjusted GMs for the biomarker for acrylonitrile ranging from 39% (cigarette-only users) to 291% (hookah-only users). Increases in levels of acrylamide ranged from 39% (cigarette-only users) to 52% (cigar-only users) among past 30-day cannabis users compared to tobacco users who had not used cannabis in the past 30 days. Tobacco-specific biomarkers of exposure (TNE-2 and NNAL) were generally equivalent between past 30-day cannabis users and non-users, with the exception of significantly higher levels of TNE-2 found in past 30-day cigar-only cannabis users relative to non-users (t = 2.43, p = .017).
Figure 2.
Adjusted geometric mean concentrations representing exposure to (A) fluorene, (B) pyrene, (C) acrylonitrile, (D) acrylamide, (E) nicotine, and (F) tobacco-specific nitrosamine NNK among single tobacco product users, stratified by self-reported past 30-day use of cannabis (n = 3331). All estimates are weighted and adjusted for urinary creatinine, age, sex, race, education, secondhand smoke exposure, cannabis use, frequency and recency of product use. Toxicant biomarker analyses adjusted for logged nicotine intake (TNE-2). Bold and starred values indicate statistically significant differences between non-cannabis users and past 30-day cannabis users (p < .05).
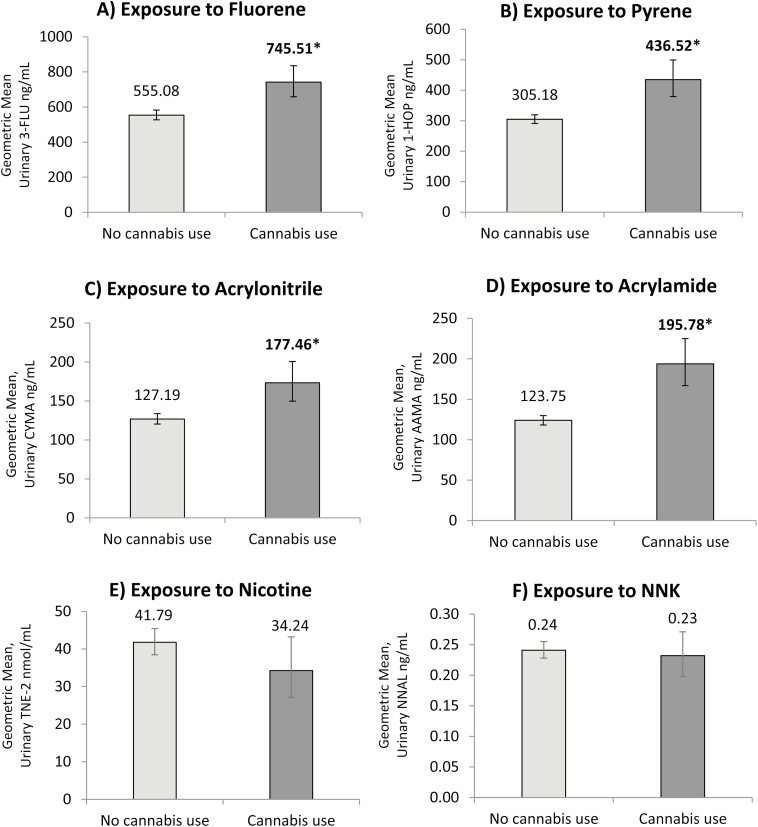
Toxicant Exposure Among Dual Users of Tobacco Cigarettes and e-Cigarettes Who Co-Use Cannabis
Differences in adjusted GMs for biomarkers among dual users of tobacco cigarettes and e-cigarettes mirror those observed for users of a single tobacco product, and can be viewed in Figure 3. Levels of exposure to tobacco-specific biomarkers were not significantly different between dual users who reported past 30-day cannabis use versus those who did not. Exposure to PAHs increased by approximately 34% for fluorene, and 43% for pyrene among past 30-day cannabis users relative to non-cannabis users. In terms of VOC exposure, levels of acrylonitrile increased by 40%, and levels of acrylamide increased by 58% among past 30-day cannabis users compared to non-cannabis users.
Figure 3.
Adjusted geometric mean concentrations representing exposure to (A) fluorene, (B) pyrene, (C) acrylonitrile, (D) acrylamide, (E) nicotine, and (F) tobacco-specific nitrosamine NNK among dual users of tobacco cigarettes and e-cigarettes, stratified by self-reported past 30-day use of cannabis (n = 792). All estimates are weighted and adjusted for urinary creatinine, age, sex, race, education, secondhand smoke exposure, cannabis use, frequency and recency of product use. Toxicant biomarker analyses adjusted for logged nicotine intake (TNE-2). Bold and starred values indicate statistically significant differences between non-cannabis users and past 30-day cannabis users (p < .05).
Discussion
To the best of our knowledge, this is the first study to examine cross-sectional differences in biomarker concentrations among tobacco product users that do and do not use cannabis using a nationally representative design covering a wide variety of tobacco products. Findings suggest that tobacco users who use cannabis are exposed to significantly higher levels of many combustion byproducts, while generally showing no differences in tobacco-specific biomarkers. Differences in biomarker concentrations between past 30-day cannabis users and non-cannabis users were most evident among e-cigarette-only users. These findings suggest that, when controlling for numerous variables related to tobacco use, tobacco–cannabis co-users have significantly greater exposure to many combustion byproducts, which is likely being driven by smoked cannabis use. Further, our findings suggest that past 30-day cannabis use can introduce confounding into studies aiming to assess tobacco-attributable exposures. These findings carry important implications related to assessing potential health effects related to co-use, as well as the need for considering cannabis use in tobacco biomonitoring studies in an era of emerging cannabis liberalization.
Studies show that co-users of tobacco and cannabis exhibit increased risks for many health-related concerns.5,11,25 Our findings demonstrate that use of cannabis in addition to tobacco could also yield additive levels of exposure to several harmful combustion byproducts linked to illnesses above and beyond levels found in those who only use tobacco. This was particularly evident among users of e-cigarettes, a group which frequently reports reduction of health harms as a reason for their e-cigarette use.42 Long-term exposure to the compounds measured in this study have linkages to health harms.43–48 Most cannabis is smoked,4,25,49 which offers a direct means of exposure, and tobacco is often consumed over sustained periods due to the addictive nature of nicotine.47 The dose of smoked cannabis has been shown to elevate levels of several of the same biomarkers measured in our study. For instance, among exclusive cannabis users, smoking two or more joints per day is associated with significantly higher levels of PAHs relative to non-cannabis users,24 and exclusive cannabis users have significantly greater concentrations of several urinary VOC metabolites (including biomarkers for acrylonitrile and acrylamide, as measured in this study).23 Although we were unable to control for frequency and mode of cannabis use in this study, our data indicate that the potential for additive exposure among co-users warrants further investigation using designs that can apply robust measures for both tobacco and cannabis, including those related to patterns of product use, time since last product use, and modes of drug delivery.
These findings stress the importance of considering the high rates of cannabis use that occur among tobacco users when planning and executing biomonitoring studies for one or both substances. Our simple stratification on self-reported past 30-day cannabis use displayed significant differences in biomarker concentrations among tobacco users as a function of cannabis use. Potential misclassification of tobacco-related exposures associated with neglecting the inclusion of cannabis-related variables may not be differential in all cases, and as such, may not result in harmful impacts on analyses, because associated error may be relatively evenly distributed throughout the sample. However, our data suggest that this may be more concerning when considering certain biomarkers where large differences have been observed, or for obtaining estimates for certain classes of tobacco products. For example, in our study, we found that urinary CYMA levels were significantly higher across all groups of co-users, with percentage differences in biomarker concentrations ranging from 39% higher (cigarettes) to 464% higher (e-cigarettes) in co-users relative to exclusive tobacco users. This difference may have less of an influential impact in assessing an absolute estimate of exposure attributable to combusted products. But in the case of e-cigarettes, which do not involve a combustion process, neglecting to consider differences in exposures attributable to other smoked products may yield inflated estimates of exposure, which in turn could yield biased interpretation of findings. As many of the biomarkers examined here have a relatively short elimination half-life, the detected elevated levels indicate that many tobacco users had used cannabis shortly before their urine samples were collected. This suggests the need to consider fairly regular or heavy use of cannabis among tobacco users. Further measurement improvements in biomonitoring studies to avoid misleading estimates may involve documenting more detailed information about use of tobacco and cannabis.
Although a cross-sectional assessment of biomarkers can only serve as an interim assessment of internal exposure, the elevated levels consistently observed for several biomarkers across a variety of tobacco user groups warrants further study on linkages to potential direct health effects using longitudinal and other designs. Our study also uses data from a large, nationally representative sample of never, former, and current tobacco users within the non-institutionalized US adult population to generate these estimates. This is further strengthened by the ability isolate focus to users of single tobacco products due to the precise and numerous measures for tobacco use used in the PATH Study. Along these lines, our analyses using this data source allowed our team the ability to control for several important confounding factors, including SHS, urinary TNE-2, frequency of product use, and time since last product use. Further, the statistical equivalence of tobacco-specific biomarkers across groups and published empirical associations of our biomarker findings with other studies examining cannabis use lend credibility to our findings.
Limitations of our research are primarily related to imprecise measures of cannabis use, including the absence of biochemical verification. Non-concordance of self-reported illicit substance use and biochemical verification is common, with one study documenting false negative rates (ie, reporting no use, but testing positive for use) from 4%–57% for cannabis.50 This suggests our findings are likely subject to underreporting of cannabis use, and so our estimates related to cannabis use are likely to be conservative. Past 30-day use of cannabis was not specific to any mode of cannabis delivery and was not assessed in the context of current or usual frequency and quantity of cannabis use. However, a past 30-day window exemplifies a standard for current use in many national surveillance studies, and despite the limitations of this measure, is likely capturing those who use cannabis with some regularity. Past year blunt use is measured as part of the PATH Study and was examined in preliminary analyses, but produced inconsistent findings. This could be due to this variable introducing smaller cell sizes into the analysis, along with the short half-lives (days, weeks) for the biomarkers assessed in this study. Therefore, a past year metric is likely too broad of a time window to obtain reasonable assessments of exposure attributable to blunt use. We cannot rule out that some of our findings may be attributable to recent blunt use, especially considering that several of the markers associated with co-use are also highly correlated with cigar use.18 However, blunt use is a specific form of tobacco–cannabis coadministration,11 which reinforces the need to capture cannabis use information in detail in assessments of tobacco exposures. Finally, we assumed exposure contributions from non-tobacco and/or cannabis occurred at comparable levels across studied categories, and we cannot rule out potential confounding from other sources of exposure for PAHs and VOCs, such as those coming from the surrounding environment or from dietary sources.
Conclusion
Co-users of tobacco and cannabis exhibited higher concentrations for biomarkers of exposure to many combustion byproducts, despite having generally equivalent levels of tobacco-specific biomarkers compared to exclusive tobacco users.
Funding
This work was supported by the National Institutes of Health, National Cancer Institute (P30 CA 016056).
Declaration of Interests
MLG serves as a paid consultant for Johnson & Johnson and has received research grant from Pfizer, manufacturers of smoking cessation medications. The other authors have no conflicts to declare.
Supplementary Material
References
- 1. Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Assessing the overlap between tobacco and marijuana: trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict Behav. 2015;49:26–32. doi:10.1016/j.addbeh.2015.05.012. Epub 2015 May 23. [DOI] [PubMed] [Google Scholar]
- 2. Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107(7):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kasza KA, Ambrose BK, Conway KP, et al. . Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. Am J Prev Med. 2016;50(1):1–8. [DOI] [PubMed] [Google Scholar]
- 5. Rabin RA, George TP. A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am J Addict. 2015;24(2):105–116. [DOI] [PubMed] [Google Scholar]
- 6. Conway KP, Green VR, Kasza KA, et al. . Co-occurrence of tobacco product use, substance use, and mental health problems among adults: findings from wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend. 2017;177:104–111. doi:10.1016/j.drugalcdep.2017.03.032. Epub 2017 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodwin RD, Pacek LR, Copeland J, et al. . Trends in daily cannabis use among cigarette smokers: United States, 2002–2014. Am J Public Health. 2018;108(1):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schauer GL, Hall CD, Berg CJ, Donovan DM, Windle M, Kegler MC. Differences in the relationship of marijuana and tobacco by frequency of use: a qualitative study with adults aged 18–34 years. Psychol Addict Behav. 2016;30(3):406–414. [DOI] [PubMed] [Google Scholar]
- 9. Schauer GL, Peters EN. Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug Alcohol Depend. 2018;185:238–244. doi:10.1016/j.drugalcdep.2017.12.007. Epub 2018 Feb 2. [DOI] [PubMed] [Google Scholar]
- 10. Peters EN, Schwartz RP, Wang S, O’Grady KE, Blanco C. Psychiatric, psychosocial, and physical health correlates of co-occurring cannabis use disorders and nicotine dependence. Drug Alcohol Depend. 2014;134:228–234. doi:10.1016/j.drugalcdep.2013.10.003. Epub 2013 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schauer GL, Rosenberry ZR, Peters EN. Marijuana and tobacco co-administration in blunts, spliffs, and mulled cigarettes: a systematic literature review. Addict Behav. 2017;64:200–211. doi:10.1016/j.addbeh.2016.09.001. Epub 2016 Sep 13. [DOI] [PubMed] [Google Scholar]
- 12. Cohn AM, Abudayyeh H, Perreras L, Peters EN. Patterns and correlates of the co-use of marijuana with any tobacco and individual tobacco products in young adults from Wave 2 of the PATH Study. Addict Behav. 2019;92:122–127. doi:10.1016/j.addbeh.2018.12.025. Epub 2018 Dec 21. [DOI] [PubMed] [Google Scholar]
- 13. Strong DR, Myers MG, Pulvers K, Noble M, Brikmanis K, Doran N. Marijuana use among US tobacco users: findings from wave 1 of the population assessment of tobacco health (PATH) study. Drug Alcohol Depend. 2018;186:16–22. doi:10.1016/j.drugalcdep.2017.12.044. Epub 2018 Mar 3. [DOI] [PubMed] [Google Scholar]
- 14. Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Public Health. 2018;39:193–213. doi:10.1146/annurev-publhealth-040617-013849. Epub 2018 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kassem NOF, Kassem NO, Liles S, et al. . Levels of urine cotinine from hookah smoking and exposure to hookah tobacco secondhand smoke in hookah lounges and homes. Int J High Risk Behav Addict. 2018;7(1). pii: e67601. doi:10.5812/ijhrba.67601. Epub 2018 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kassem NOF, Kassem NO, Liles S, et al. . Acrolein exposure in hookah smokers and non-smokers exposed to hookah tobacco secondhand smoke: implications for regulating hookah tobacco products. Nicotine Tob Res. 2018;20(4):492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goniewicz ML, Smith DM, Edwards KC, et al. . Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1(8):e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang CM, Rostron BL, Chang JT, et al. . Biomarkers of exposure among U.S. adult cigar smokers: Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Cancer Epidemiol Biomarkers Prev. 2019;28(5):943–953. doi:10.1158/1055-9965.EPI-18-0539. Epub 2019 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meier E, Hatsukami DK. A review of the additive health risk of cannabis and tobacco co-use. Drug Alcohol Depend. 2016;166:6–12. doi:10.1016/j.drugalcdep.2016.07.013. Epub 2016 Jul 25. [DOI] [PubMed] [Google Scholar]
- 20. Moir D, Rickert WS, Levasseur G, et al. . A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21(2):494–502. [DOI] [PubMed] [Google Scholar]
- 21. U.S. Department of Health and Human Services Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. Vol 77. FR 20034. Federal Register Vol. 77, No. 642012. https://www.federalregister.gov/documents/2012/04/03/2012-7727/harmful-and-potentially-harmful-constituents-in-tobacco-products-and-tobacco-smoke-established-list [Google Scholar]
- 22. Shahab L, Goniewicz ML, Blount BC, et al. . Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei B, Alwis KU, Li Z, et al. . Urinary concentrations of PAH and VOC metabolites in marijuana users. Environ Int. 2016;88:1–8. doi:10.1016/j.envint.2015.12.003. Epub 2015 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei B, Smith DM, O’Connor R, Travers MJ, Hyland A. Examining the association between body burdens of harmful chemicals and heaviness of marijuana smoking. Chem Res Toxicol. 2018;31(8):643–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Academies of Science Engineering and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 26. U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 27. NAHDAP. Population Assessment of Tobacco and Health (PATH) Study Series.2018. https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606. Accessed August 14, 2018.
- 28. Hyland A, Ambrose BK, Conway KP, et al. . Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Wong LY, Meng L, et al. . Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the U.S. Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Environ Int. 2019;123:201–208. doi:10.1016/j.envint.2018.11.068. Epub 2018 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goniewicz ML, Smith DM. Are some e-cigarette users “blowing smoke”?: assessing the accuracy of self-reported smoking abstinence in exclusive e-cigarette users. Nicotine Tob Res. 2019;21(5):699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta. 2012;750:152–160. doi:10.1016/j.aca.2012.04.009. Epub 2012 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Meng L, Pittman EN, et al. . Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(4):931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei B, Feng J, Rehmani IJ, et al. . A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436:290–297. doi:10.1016/j.cca.2014.06.012. Epub 2014 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xia B, Xia Y, Wong J, et al. . Quantitative analysis of five tobacco-specific N-nitrosamines in urine by liquid chromatography-atmospheric pressure ionization tandem mass spectrometry. Biomed Chromatogr. 2014;28(3):375–384. [DOI] [PubMed] [Google Scholar]
- 35. Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–922. [DOI] [PubMed] [Google Scholar]
- 36. Goniewicz ML, Havel CM, Peng MW, et al. . Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3421–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Z, Romanoff L, Bartell S, et al. . Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol. 2012;25(7):1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strasser AA, Souprountchouk V, Kaufmann A, et al. . Nicotine replacement, topography, and smoking phenotypes of e-cigarettes. Tob Regul Sci. 2016;2(4):352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ross KC, Dempsey DA, St Helen G, Delucchi K, Benowitz NL. The influence of puff characteristics, nicotine dependence, and rate of nicotine metabolism on daily nicotine exposure in African American Smokers. Cancer Epidemiol Biomarkers Prev. 2016;25(6):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pickworth WB, Rosenberry ZR, O’Grady KE, Koszowski B. Dual use of cigarettes, little cigars, cigarillos, and large cigars: smoking topography and toxicant exposure. Tob Regul Sci. 2017;3 (suppl 1):S72–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soule EK, Ramôa C, Eissenberg T, Cobb CO. Differences in puff topography, toxicant exposure, and subjective response between waterpipe tobacco smoking men and women. Exp Clin Psychopharmacol. 2018;26(5):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Academies of Science Engineering and Medicine. The Public Health Consequences of E-cigarettes. Washington DC National Academies Press; 2018. [PubMed] [Google Scholar]
- 43. Batterman S, Chin JY, Jia C, et al. . Sources, concentrations, and risks of naphthalene in indoor and outdoor air. Indoor Air. 2012;22(4):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. International Agency for Research on Cancer (IARC). Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 45. International Agency for Research on Cancer (IARC). Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. Lyon, France International Agency for Research on Cancer;2007. [Google Scholar]
- 46. Freitas F, Brucker N, Durgante J, et al. . Urinary 1-hydroxypyrene is associated with oxidative stress and inflammatory biomarkers in acute myocardial infarction. Int J Environ Res Public Health. 2014;11(9):9024–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 48. Vesper HW, Caudill SP, Osterloh JD, Meyers T, Scott D, Myers GL. Exposure of the U.S. population to acrylamide in the National Health and Nutrition Examination Survey 2003–2004. Environ Health Perspect. 2010;118(2):278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use—basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. 2018;52:87–96. doi:10.1016/j.drugpo.2017.11.008. Epub 2017 Dec 22. [DOI] [PubMed] [Google Scholar]
- 50. Clark CB, Zyambo CM, Li Y, Cropsey KL. The impact of non-concordant self-report of substance use in clinical trials research. Addict Behav. 2016;58:74–79. doi:10.1016/j.addbeh.2016.02.023. Epub 2016 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.