Abstract
Background
Cue-elicited craving may vary due to duration of smoking history, increasing as more years of smoking strengthen associations between nicotine intake and cues. However, research on this relationship is virtually absent. This project assessed the relationship between cue reactivity and years of smoking.
Methods
Data from 53 studies (68 effect sizes) were analyzed. Eligible studies were those measuring self-reported craving following cue exposure in nontreatment seeking smokers and reporting mean years smoking. Preliminary subgroup analyses identified methodological factors influencing cue-reactivity effect sizes; primary meta-regression analysis assessed differences across years smoking; exploratory analyses assessed potential for ceiling effects.
Results
Effect sizes varied due to abstinence requirement and cue presentation modality, but not dependence severity. Unexpectedly, meta-regression analysis revealed a decline in effect sizes across years smoking. Exploratory analyses suggested declines may have been due to a ceiling effect in craving measurement for those with longer smoking histories—more experienced smokers reported higher levels of craving at baseline or following neutral cue exposure, but all reported similar levels of craving after smoking cue exposure.
Conclusions
Methodological factors and duration of smoking history influenced measurement of cue reactivity. Highlighted were important relationships between years smoking and magnitude of cue reactivity, depending on use of baseline or neutral cue comparisons. Further research is needed to assess differences in cue reactivity due to duration of smoking history using participant-level data, directly testing for ceiling effects. In addition, cue-reactivity studies are needed across young adults to assess onset of associations between nicotine intake and cues.
Implications
This meta-analysis project contributes to the cue-reactivity literature by reporting on the previously ignored relationship between duration of smoking history and magnitude of cue-elicited craving. Results suggest that declines in cue-reactivity effect sizes across years of smoking may have been due to study-level methodological factors, but not due to differences in sample-level dependence severity. Cue-reactivity effect sizes were stable across years of smoking in studies using a neutral cue comparison but declined sharply in studies when baseline assessment (typically coupled with an abstinence requirement) was used.
Introduction
For decades, researchers have known that drug craving can be triggered by environmental stimuli frequently paired with drug intake, or “cues,” in those with drug use histories.1 This effect, known as cue reactivity, has been demonstrated across a variety of drugs2 and is supported by a large body of research in which participants are systematically exposed to actual or representations of cues (eg, lighter, ashtray, lit cigarette) then report their craving.2,3 The clinical utility of cue-reactivity research in smokers lies in its relationship to indices of nicotine dependence, such as its potential to predict subsequent smoking behavior.4,5 Ecological research has found that many lapses and relapses during a quit attempt follow exposure to cues in the real world,6,7 suggesting that cue-elicited craving experienced in vivo may precipitate failed cessation attempts. Yet, cue-elicited craving assessed in lab settings is not affected by proven pharmacotherapies for smoking cessation,8,9 and does not consistently correlate with measures of dependence severity.10,11 Cue reactivity has been found to be predictive of more immediate smoking behavior; greater cue-elicited craving (relative to baseline assessment or exposure to neutral control cues) has been associated with shorter latency and greater amount of immediate subsequent smoking.12–15 Taken together, support for the clinical utility of cue reactivity is mixed—its predictive ability has been reliably demonstrated for immediate smoking behavior but not yet for more temporally distal outcomes (ie, relapse during a quit attempt4,5). Elucidating factors affecting cue reactivity in smokers may lead to a better understanding of cue reactivity’s clinical utility.
The magnitude of cue-reactivity craving response may vary due to an index of smoking persistence, duration of smoking history. A framework for this potential relationship comes from two learning-based theories. According to classical conditioning theory, repeated association of cues with nicotine intake would strengthen conditioned responses to those cues.16,17 The incentive-sensitization theory18–20 adds a psychobiological basis—repeated drug use progressively sensitizes the primary reward center in the brain, rendering this system hyperreactive to drug-associated stimuli, resulting in intense feelings of craving or wanting for drug when presented with such stimuli.18–20 Both theories predict that the magnitude of cue reactivity will increase with repeated pairings of nicotine and drug-associated stimuli, eventually reaching an asymptote.16–20 However, it is unclear how quickly cue reactivity develops and whether it reaches an asymptotic phase once established. If the magnitude of cue-elicited craving response varies due to duration of smoking history, the clinical utility of cue-reactivity research (ie, reliable prediction of lapse and relapse risk due to environmental factors) may vary due to an individual’s smoking history.
Despite many studies of cue reactivity in smokers, the potential relationship between cue-elicited craving and duration of smoking history has been largely ignored. One study—possibly the only—which did report on the relationship between cue reactivity and years of smoking in abstinent tobacco smokers found a small, but still statistically significant, inverse relationship between years of smoking and change in craving from pre- to post-cue exposure.21 In other words, after being asked to light (but not smoke) a cigarette, those with more years of smoking reported less of an increase in craving than those with fewer years of smoking. However, this effect may have been due to a ceiling effect in craving measurement. The authors reported a significant positive relationship between pre-cue craving levels and years of smoking and no relationship between post-cue craving and years of smoking. Thus, when compared to less experienced smokers, more experienced smokers reported significantly higher levels of craving before cue exposure but similar craving levels after exposure to the cue, resulting in diminished increases due to exposure to the smoking cue per se.
In light of the lack of research in this area, the current article used meta-analytical techniques to integrate data from cue-reactivity studies which reported sample mean years of smoking. The primary aim of this project was to assess the relationship between craving response to smoking cues (relative to neutral cues and/or baseline assessment) and years of smoking in the extant cue-reactivity literature. Other aims included: identifying methodological (eg, abstinence requirement, mode of cue presentation) and theoretical variables (eg, age, cigarettes per day [CPD], level of dependence) which modulate the magnitude of cue reactivity; and exploring the potential for ceiling effects in craving measurement in cue-reactivity research.
Methods
Search Procedures and Selection Criteria
Three electronic databases (PsycINFO, PubMed, and Google Scholar) were searched in January 2018 to identify relevant full-text articles. Searches used the following combination of keywords: cue-reactivity AND (self-reported craving OR urge) AND (smok* OR cigarette). The wildcard asterisk (*) was used to allow for alternate forms of the respective keyword (eg, smoker, smokers, smoking). In addition, reference lists of relevant or related review and meta-analysis papers were also examined to identify additional studies for inclusion.
Studies were eligible if they: (1) included only nontreatment seeking smokers (cue reactivity has been shown to be blunted in treatment seeking smokers22,23); (2) recruited either adolescent smokers or adult smokers (with outcomes reported separately for each group if both groups were recruited in the same study); (3) measured self-reported craving; (4) reported mean years of smoking for the sample (if mean years smoking not reported, mean age and mean age started smoking were reported); (5) exposed smokers to both smoking-related cues and a control procedure of neutral cues (or, if neutral cues were not used, a baseline assessment of craving); (6) no administration or use of another drug, medication, or nicotine replacement therapy that may have influenced cue-elicited craving; (7) presented original data published in a peer-reviewed journal; and (9) were written in English.
Data Extraction
All data were entered into a database using Comprehensive Meta-Analysis 3.0 software. Data extracted from each study included sample size, age, percent male, hours abstinent, cue presentation mode, use of neutral cue or baseline assessment, order of cue presentation, years of smoking, age started smoking, craving measure used, and craving response (ie, the dependent measure).
Data Synthesis and Analysis Plan
Comprehensive Meta-Analysis software computed Hedges’ g, a standardized within-subjects measure of effect size, calculated as the difference in craving response between smoking and neutral cues (or baseline assessment) for each study. In the sections later, “cue reactivity” will refer to the difference between smoking and neutral cues/baseline assessment; craving values obtained at a specific timepoint (eg, baseline assessment only, post smoking cue exposure only) will be referred to as “absolute craving.” When available, means and standard deviations were used to calculate the effect size for each study. Otherwise, t scores or one-way F values (converted to t; ) were used to estimate the effect size. All meta-analyses used random-effects models.24,25
Heterogeneity in effect sizes was assessed using Cochran’s Q and I2 statistics. When significant, Cochran’s Q test indicates that differences exist between studies and further testing to identify factors contributing to heterogeneity is warranted.24,26 The I2 statistic quantifies the amount of heterogeneity between studies, with values of 0%, less than 30%, and greater than 50% indicating no, moderate, and high levels of heterogeneity, respectively.26
Preliminary Analyses
Preliminary omnibus meta-analysis was performed using effect sizes from all studies to determine whether cue reactivity (ie, difference in craving measured after smoking cue exposure vs. baseline assessment/neutral cue exposure) was different from zero. Publication bias was then assessed across all studies using Kendall’s tau,27 Duval and Tweedie’s trim and fill method,28,29 and funnel plots. Subsequent preliminary subgroup meta-analyses examined the influence of methodologically relevant variables: use of baseline versus neutral cue comparisons, craving measure, abstinence requirement (any abstinence vs. no abstinence), cue presentation mode (in vivo, images, scripted imagery, video, or virtual reality), and order of cue presentation (fixed order vs. other). Variables found to significantly influence cue-reactivity effect sizes were included as covariates in the primary analyses.
Primary and Secondary Analyses
The primary analyses used random effects meta-regression to assess whether mean years of smoking (entered as a continuous covariate) was related to cue-reactivity effect sizes, controlling for variables identified in the preliminary subgroup analyses. Secondary analyses examined the effect of theoretically relevant variables: age, CPD, and level of dependence (Fagerström Test for Nicotine Dependence [FTND] scores).
Exploratory Analyses
In their review on craving measurement, Sayette et al.30 identified methodological factors which may facilitate ceiling effects for self-reported craving in cue-reactivity studies. According to the authors, use of baseline assessment only (ie, not including neutral control cues) and requiring abstinence prior to cue exposure procedures may lead to ceiling effects. A series of exploratory analyses examined whether these methodological variables contributed to ceiling effects in the current sample of studies. First, two separate meta-regression models were run; the first model tested use of study abstinence requirement and the second model examined use of baseline versus neutral cue comparison. Both models included cue presentation mode, years of smoking, and the interaction of years of smoking and abstinence (model 1) or use of baseline assessment or neutral cue exposure (model 2). Simple slopes analysis was used to follow up significant interaction effects.
Additional exploratory regression analyses were conducted in SPSS 25.0. These analyses examined the relationship between absolute craving scores (expressed as percent of scale max to standardize scores across different measures23) and years of smoking, controlling for abstinence requirement, use of baseline or neutral cue comparison, and the interaction between mean years smoking and use of baseline/neutral cue. Following Donny et al.,21 separate regression models were used to test effects on absolute craving assessed at baseline or following neutral cue exposure, absolute craving following smoking cue exposure, and change in absolute craving from baseline or neutral cue to smoking cue.
Results
Characteristics of Included Studies
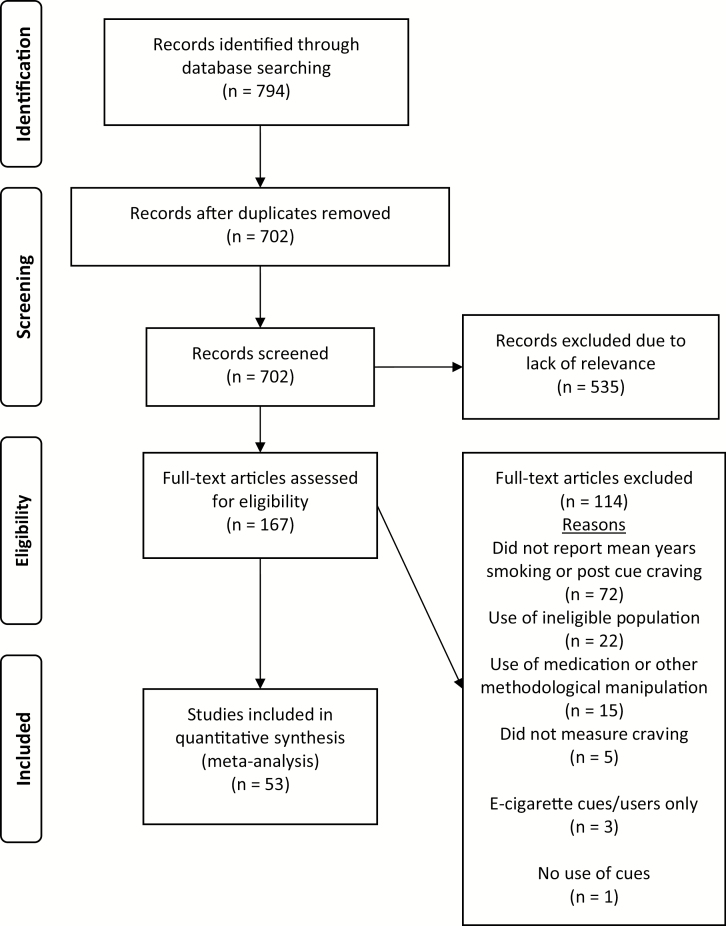
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.31 A total of 807 articles were identified using the procedures described earlier. After removing 105 duplicates, titles and abstracts of the remaining 702 articles were screened and an additional 535 were excluded due to lack of relevance to the topic of interest. Of the 167 remaining full-text articles reviewed for eligibility, 53 studies were included in the analysis.12,14,15,32–81
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection.
Sample-Level Characteristics
All study characteristics are presented in Supplementary Table 1. Of the 53 studies included in the analyses, seven presented data from the same participants under differing conditions (eg, abstinent and nonabstinent, standardized and personalized cues, in vivo and imaginal cues) and eight presented data from separate samples (ie, pilot study and main study, heavy smokers and lighter smokers, abstinent and nonabstinent groups), for a total of 68 sets of data (k = 68). The following sample characteristics do not repeatedly count participants measured more than once (k = 60, unless otherwise noted). Sample sizes ranged from 8 to 225 (combined n = 3372), with a mean (SD) sample size of 56.2 (47.75). The mean sample age ranged from 16.2 to 55 years (grand mean 31.11 [8.63] years). Overall, samples tended to have slightly more males (mean 52.06% [17.58] male). Mean (SD) smoking characteristics across samples were 18.42 (5.46) CPD, smoking for 14.10 (7.95) years, and FTND score of 4.93 (1.26) (k = 35).
Study Methodologies
Across all studies (k = 68), 28 did not require abstinence before cue exposure procedures. Abstinence requirements in the other 40 studies included durations of 0.5–5 hours (k = 14), 6–12 hours (k = 23) and more than 12 hours (k = 3). Most studies included a neutral cue condition (k = 55), whereas a few others only reported baseline craving before exposure to smoking cues (k = 13). Most studies (k = 51) reported whether the cues were presented in a fixed or other order (ie, randomized or counterbalanced). Of those reporting, fewer studies presented cues in a fixed order (k = 14) than other cue presentations (k = 37). Craving was measured using the Shiffman–Jarvik Craving Scale82 (k = 2; separate samples in the same study), the Cigarette Craving Questionnaire83 (k = 7), a single item (k = 24), Factor 1 of the Questionnaire on Smoking Urges84 (k = 1), Questionnaire on Smoking Urges-1085 (k = 23), or the Questionnaire on Smoking Urges-486 (k = 11). Cue modes included photographic images (k = 24), handling or seeing smoking paraphernalia (ie, in vivo; k = 26), scripted imagery (k = 13), virtual reality (k = 3), and video (k = 2).
Preliminary Analyses
Omnibus Effect Size
Overall, participants reported significantly more craving following exposure to smoking cues versus baseline or neutral cues, Hedge’s g = 0.77, 95% CI = 0.65 to 0.89, Z = 12.96, p < .001. There was high heterogeneity of effect sizes, Q(67) = 667.35, p < .001 with between-study variance, τ2, estimated at 0.20. Thus, the true effect size most likely varied from study to study and the observed variance was not due to sampling error. Almost all observed variance (I2 = 90.0%) reflected differences in study effects.
Publication Bias
Kendall’s tau was marginally significant, τ = 0.15, Zτ = 1.82, p = .07, indicating potential publication bias. To explore this further, Duval and Tweedie’s trim and fill method was used to estimate the impact of potential publication bias, resulting in an adjusted Hedge’s g = 0.47, 95% CI = 0.34 to 0.60). This adjusted omnibus effect size retained statistical significance (ie, 95% CI does not overlap with 0), despite the reduction in magnitude. A funnel plot illustrating possible publication bias is presented in Supplementary Figure 1.
Subgroup Analyses
Results of the subgroup analyses organized by methodologically relevant variables are displayed in Supplementary Table 2. There was low heterogeneity among (i.e., similar) effect sizes between studies across most variables, including cue presentation order (fixed vs. other) and craving measure used. However, effect sizes varied due to abstinence requirement, Q(1) = 5.29, p = .02, and cue presentation mode, Q(4) = 15.25, p = .004. As shown in Supplementary Table 2, studies requiring any abstinence prior to cue exposure had lower effect sizes than those without an abstinence requirement. For cue presentation mode, each cue modality resulted in significantly greater reactivity following smoking cue presentation versus neutral control cue/baseline, except for video. After excluding studies that used video cue presentation, there was still significant heterogeneity due to cue mode, Q(3) = 10.31, p = .02. Both the abstinence requirement (any vs. none) and cue presentation mode variables were included as covariates in subsequent analyses.
Primary Meta-regression Analysis
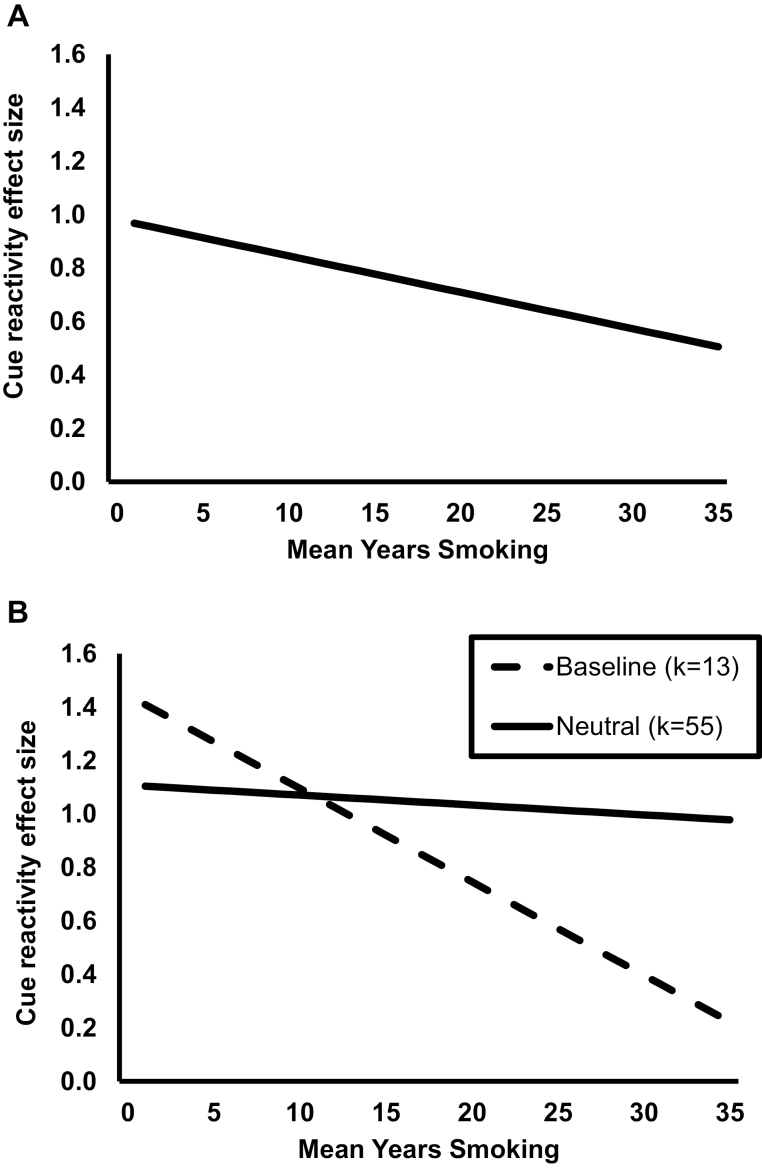
Results of the meta-regression analysis are presented in Table 1. An omnibus test of the model indicated it as a whole was related to effect size, Q(6) = 25.01, p = .0003. There was a high level of between-study variance in effect sizes observed in the model, I2 = 86.2%, p < .0001. Unexpectedly, mean years of smoking had a significant negative linear relationship with cue-reactivity effect size, β = −0.01, p = .04. As shown in Figure 2A, the magnitude of cue-reactivity effect sizes declined 0.01 units for each one-year increase in mean years of smoking.
Table 1.
Meta-regression Results
| 95% CI | |||||
|---|---|---|---|---|---|
| Q | β | SE | LL | UL | |
| Overall model | 25.01*** | ||||
| Intercept | 0.98*** | 0.15 | 0.69 | 1.27 | |
| Mean years smoking | |||||
| Linear effect | −0.01* | 0.007 | −0.0268 | −0.0005 | |
| Cue mode | 16.34** | ||||
| Images | 0.27* | 0.13 | 0.02 | 0.52 | |
| Scripted imagery | 0.44** | 0.15 | 0.15 | 0.73 | |
| Video | −0.54† | 0.31 | −1.14 | 0.07 | |
| Virtual reality | 0.16 | 0.27 | −0.37 | 0.69 | |
| In vivo (reference) | |||||
| Abstinence requirement | |||||
| None | 0.21† | 0.11 | −0.01 | 0.43 | |
| Any (reference) | |||||
CI = confidence interval; LL = lower limit; Q = Cochran’s Q; SE = standard error; UL = upper limit.
†p < .10, *p < .05, **p < .01, ***p < .001.
Figure 2.
(A) Plot of the significant linear relationship between cue-elicited craving effect size and mean years smoking, estimated using meta-regression analysis controlling for effect of cue presentation mode and abstinence requirement. (B) Plot of simple slopes for cue-reactivity effect size and mean years smoking, comparing between studies using a baseline comparison (dashed line) versus studies using a neutral cue comparison condition (solid line).
Secondary Analyses
Separate meta-regression analyses tested whether CPD (k = 68), age (k = 67), and FTND (k = 40) were related to effect size heterogeneity, controlling for cue mode and abstinence requirement. None of these theoretically relevant variables had significant influence on effect size heterogeneity: CPD β = 0.01, 95% CI = −0.01 to 0.04, p = .24; age β = −0.01, 95% CI = −0.021 to 0.004, p = .18; FTND β = −0.07, 95% CI =−0.20 to 0.07, p = .33.
Exploratory Analyses
Exploratory Meta-regressions
Results of the exploratory meta-regression analyses are displayed in Table 2. The first model examined the relationship between years of smoking and use of an abstinence requirement (controlling for cue presentation mode), with specific interest in the interaction between these variables. The interaction between years smoking and abstinent requirement was not significant, β = −0.02, 95% CI = −0.04 to 0.01, p = .17.
Table 2.
Exploratory Meta-regression Results
| 95% CI | |||||
|---|---|---|---|---|---|
| Q | β | SE | LL | UL | |
| Exploratory model 1 | 27.32*** | ||||
| Intercept | 1.07*** | 0.18 | 0.70 | 1.44 | |
| Cue mode | 17.61** | ||||
| In vivo | −0.29* | 0.13 | −0.54 | −0.04 | |
| Scripted imagery | 0.14 | 0.16 | −0.17 | 0.46 | |
| Video | −0.88** | 0.31 | −1.49 | −0.26 | |
| Virtual reality | −0.09 | 0.27 | −0.63 | 0.44 | |
| Images (reference) | |||||
| Mean years smoking | |||||
| Linear effect | −0.003 | 0.010 | −0.023 | 0.017 | |
| Abstinence requirement | |||||
| Abstinent | 0.05 | 0.22 | −0.38 | 0.48 | |
| Not abstinent (reference) | |||||
| Years smoking × abstinence | −0.02 | 0.01 | −0.04 | 0.01 | |
| Exploratory model 2 | 29.26*** | ||||
| Intercept | 1.07*** | 0.18 | 0.73 | 1.42 | |
| Cue mode | 14.37** | ||||
| In vivo | −0.25† | 0.13 | −0.50 | 0.01 | |
| Scripted imagery | 0.17 | 0.16 | −0.15 | 0.49 | |
| Video | −0.79* | 0.32 | −1.41 | −0.17 | |
| Virtual reality | −0.14 | 0.28 | −0.69 | 0.41 | |
| Images (reference) | |||||
| Mean years smoking | |||||
| Linear effect | −0.04** | 0.01 | −0.06 | −0.01 | |
| Abstinence requirement | |||||
| Abstinent | −0.28* | 0.12 | −0.51 | −0.04 | |
| Not abstinent (reference) | |||||
| Baseline/neutral comparison | |||||
| Baseline | 0.47† | 0.27 | −0.07 | 1.01 | |
| Neutral (reference) | |||||
| Years Smoking × Baseline/neutral | 0.03* | 0.015 | 0.003 | 0.062 | |
CI = confidence interval; LL = lower limit; Q = Cochran’s Q; SE= standard error; UL = upper limit.
†p <.10, *p < .05, **p < .01, ***p < .001.
The second exploratory model focused on the interaction between years of smoking and use of baseline versus neutral cue comparison, which was significant, β = 0.032, 95% CI = 0.003 to 0.062, p = .03. Simple slopes follow-ups are displayed in Figure 2B. In studies using a baseline comparison (k = 13), there was a significant negative relationship between years of smoking and cue-reactivity effect sizes, β = −0.03, 95% CI =−0.06 to −0.01, p = .01. Looking at studies using a neutral cue comparison (k = 55), the relationship between years of smoking and cue-reactivity effect sizes was not significant, β = −0.004, 95% CI = −0.021 to 0.013, p = .67. In sum, cue-reactivity effect sizes were consistent over years of smoking in studies using a neutral cue comparison and declined over years of smoking in studies using baseline assessment.
Exploratory Regression Analyses of Absolute Craving Scores
The final set of exploratory analyses examined the relationship between mean years of smoking and absolute craving ratings obtained at two timepoints: baseline/neutral cue exposure and smoking cue exposure. Abstinence requirement, use of baseline/neutral cue comparison, and the interaction between use of baseline/neutral cue and years of smoking were also entered into the regression model. Only studies which reported absolute mean craving at these timepoints were included in this series of exploratory analyses (k = 51). To standardize craving scores across the various measures used, mean craving ratings were converted into percent of scale maximum value.22
Somewhat consistent with Donny et al.,21 there was a marginal positive relationship between years of smoking and baseline/neutral cue craving ratings, B = 0.69, 95% CI = −0.09 to 1.46, p = .08, no relationship between mean years of smoking and smoking cue craving, B = −0.04, 95% CI = −0.76 to 0.68, p = .92, and a significant negative relationship between years of smoking and change in craving from baseline/neutral cue to smoking cue, B = −0.72, 95% CI = −1.39 to −0.05, p = .04. In other words, more experienced smokers reported marginally higher craving at baseline or following neutral cue exposure compared to less experienced smokers, and all smokers (regardless of amount of smoking history) had similar craving ratings following smoking cue exposure.
Discussion
It is well established that smoking-associated stimuli can elicit drug craving in those with smoking histories.1–3 Less well known is whether the magnitude of cue-elicited craving varies due to amount of smoking history. Both classical conditioning and Incentive-Sensitization theories support a hypothesized increase in cue-elicited craving as years of smoking accumulate, eventually reaching an asymptote.16–20 As noted, this specific relationship has been ignored by the large body of cue-reactivity research in smokers. The current project aimed to address this gap in the literature using meta-analytical procedures.
An omnibus meta-analysis indicated that smokers had moderate to large increases in self-reported craving following exposure to smoking cues, relative to neutral cue exposure or baseline assessment. Subgroup and secondary meta-analyses indicated that cue-reactivity effect sizes were consistent within most methodologically and theoretically relevant variables, although they did vary between cue presentation modes and abstinence requirements. Among the various cue presentation modes, scripted imagery was found to elicit the largest magnitude of cue reactivity, followed by pictorial images, virtual reality, in vivo, and video. Further, studies requiring any abstinence prior to cue exposure procedures had a lower magnitude of cue reactivity than studies not requiring abstinence.
Contrary to expectations, meta-regression analyses revealed a significant negative linear relationship between cue-elicited craving effect sizes and mean years of smoking. One possible explanation—supported by a series of exploratory analyses—is that the observed declines in cue reactivity over years of smoking were due to a ceiling effect for more experienced smokers.
The relationship between mean years of smoking and cue reactivity varied between studies depending on whether they used a baseline or neutral cue comparison. As shown in Figure 2B, cue-reactivity effect sizes declined sharply across years of smoking in studies using baseline craving measurement (k = 13) but remained steady in those using neutral cue exposure (k = 55). This suggests that use of baseline measurement in cue-reactivity research may be less stable across durations of smoking histories compared to use of a neutral cue comparison.
Consistent with the current findings, an earlier study reported declines in cue reactivity across durations of smoking histories.21 Although not addressed by the authors, examination of the reported relationship between years of smoking and absolute craving scores separately by timepoint (ie, baseline and post-cue exposure) supports a hypothesized ceiling effect. The researchers reported a significant positive relationship between years of smoking and pre-cue craving, but no relationship between years of smoking and post-cue rating. In other words, more experienced smokers rated their baseline craving significantly higher than less experienced smokers, but all smokers rated their post-cue craving similarly. When cue reactivity responses were calculated as the change in craving from baseline to post-cue exposure, more experienced smokers appeared to have a smaller magnitude response to smoking cues than less experienced smokers. This effect appears to have been due to higher craving at baseline for the more experienced smokers—leaving less room toward the top of the scale for subsequent increases due to smoking cue exposure leading to an artificial decline in cue reactivity over years of smoking when the difference between timepoints was computed. A similar pattern was observed in exploratory analyses among a subset of studies (51 of 68) in the current project. Absolute craving scores obtained at baseline/following neutral cue exposure had a marginally positive relationship with years of smoking, but no relationship was found between absolute craving following smoking cue exposure and years of smoking. This pattern suggests that ceiling effects likely contributed to declines in cue reactivity across years of smoking. However, without participant-level data this cannot be directly determined. It is possible that using aggregated, sample-level data may have reduced the ability to detect differences in cue reactivity across varying durations of smoking histories.
The lack of relationship between absolute craving elicited by smoking cues and years of smoking may have been due smokers developing an asymptotic craving response, as predicted by classical conditioning and Incentive-Sensitization theories.16–20 Cue-reactivity effect sizes were already robust at the lowest end of years smoking for studies included in the project, suggesting that associations between nicotine intake and environmental cues were firmly established within 2 years of smoking. Although the timeline of when these associations were formed is unclear, the observed consistency in craving response following exposure to smoking cues across smokers—regardless of how long they have been smoking—suggests that cue-elicited craving reached asymptote within this time as well.
Implications
The findings of the current meta-analysis can inform design of future cue-reactivity studies. The results suggest a possible ceiling effect may have reduced the magnitude of cue reactivity in more experienced smokers and for all smokers in studies requiring abstinence before cue exposure procedures. Owing to the possible risk of ceiling effects, abstinence may raise the pre-cue level of craving so high that the magnitude of craving due to cue reactivity per se cannot be fully evaluated. Somewhat relevant to this is peak provoked Craving, which focuses on extreme craving states triggered by the combination of abstinence and smoking cues to model real-world antecedents to lapse and relapse.87 Thus, including an abstinence requirement—which itself clearly raises craving in the absence of any control cue exposure—may increase the risk of a ceiling effect. Further, baseline craving may not be adequate when used as the only point of comparison. Studies may need to use a neutral cue condition to compare against smoking cues to better control for the effects of time per se while also isolating reactions to cues specific to smoking per se. In the current study, 10 of the 13 studies using a baseline comparison also had an abstinence requirement, limiting evaluation of the magnitude of cue reactivity.
The magnitude of cue reactivity was found to be stable across years of smoking when using a neutral cue for comparison. These findings add to the cue-reactivity literature to show that this phenomenon is relatively consistent across the duration of smoking histories contained in the studies assessed here and are important for researchers to take into consideration when designing and reporting future cue-reactivity studies.
Limitations and Future Directions
Limitations of the current study need to be taken into consideration when interpreting the results. The search terms used to identify relevant studies may have been too narrow, potentially excluding studies that would have otherwise qualified for inclusion. Reference sections from related review studies were also examined to identify studies absent from the literature search, but there is a potential that not all possible studies were included in the present analysis. Also, there was no formal method used to assess the quality of each study included in the analysis. Analyses suggested a potential for publication bias, but after adjusting the omnibus effect size for publication bias, the resulting overall effect size was still statistically significant. Thus, although the potential for publication bias was not trivial, the omnibus results were still valid.88
Participant characteristics included as covariates in the analyses (eg, mean years of smoking, mean age) were aggregates of sample means. As noted earlier, use of this study-level data rather than participant-level data may have led to ecological bias (ie, discrepancies between associations made using aggregate-level vs. individual-level data89). Without participant-level data, the extent of ecological bias cannot be determined.89,90
Another limitation was that studies included in the meta-analysis were mostly those on smokers with extensive smoking histories—there were a limited number of studies with smokers at the earlier spectrum of years of smoking. Of the 60 studies, 12 had samples with less than 6 mean years of smoking, with only two studies coming close to initial smoking at approximately 2 mean years of smoking. There is a lack of cue-reactivity research in early smokers—possibly due to the ethical issues that arise when studying youth substance use.91
Despite the limitations, results of the current meta-analysis updated our knowledge of methodological factors affecting the measurement of cue reactivity, highlighting important relationships between years of smoking and magnitude of cue reactivity, depending on use of baseline or neutral cue comparisons and abstinence requirements. Additional research is needed to replicate and extend these findings and fill gaps in the literature. The current results suggest a ceiling effect, but this was not able to be directly tested without participant-level data. Additional research directly testing for a ceiling effect in cue-reactivity research is needed to confirm the implications from the current meta-analysis. One such study could measure cue reactivity in a large sample of smokers with heterogenous durations of smoking histories, to directly assess how cue reactivity varies due to years of smoking. As also noted earlier, there is a lack of cue-reactivity research using early or adolescent smokers. This is a glaring hole in the literature that needs to be addressed with additional studies to more fully understand how cues come to promote smoking persistence in earlier smokers.
Funding
Research reported in this publication was supported by National Institutes of Health Grant R01 DA035774 from National Institute on Drug Abuse
Supplementary Material
Acknowledgments
This research is based on a project completed to fulfill a Comprehensive Specialty Exam PhD program milestone by JLK. The author thanks Kenneth Perkins, Michael Sayette, Cynthia Conklin, and Saul Shiffman for serving on the Comprehensive Specialty Exam committee and Kenneth Perkins for providing mentorship and helpful feedback on the milestone project and earlier drafts of this article. Declaration of Interests None.
References
- 1. Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91(2):251–268. [PubMed] [Google Scholar]
- 2. Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- 3. Reynolds EK, Monti PM. The cue reactivity paradigm in addiction research. In: MacKillop J, de Wit H, eds. The Wiley‐Blackwell Handbook of Addiction Psychopharmacology. West Sussex, UK: John Wiley & Sons; 2013:381–410. [Google Scholar]
- 4. Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104(10):1610–1616. [DOI] [PubMed] [Google Scholar]
- 5. Perkins KA. Subjective reactivity to smoking cues as a predictor of quitting success. Nicotine Tob Res. 2012;14(4):383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. [DOI] [PubMed] [Google Scholar]
- 7. Ferguson SG, Shiffman S, Blizzard L. Triggers of smoking lapses over the course of a quit attempt. J Smok Cessat. 2016;12(4):205–212. [Google Scholar]
- 8. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235–243. [DOI] [PubMed] [Google Scholar]
- 9. Hitsman B, Hogarth L, Tseng LJ, et al. Dissociable effect of acute varenicline on tonic versus cue-provoked craving in non-treatment-motivated heavy smokers. Drug Alcohol Depend. 2013;130(1-3):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hogarth L, Chase HW. Evaluating psychological markers for human nicotine dependence: tobacco choice, extinction, and Pavlovian-to-instrumental transfer. Exp Clin Psychopharmacol. 2012;20(3):213–224. [DOI] [PubMed] [Google Scholar]
- 11. Hardy L, Mitchell C, Seabrooke T, Hogarth L. Drug cue reactivity involves hierarchical instrumental learning: evidence from a biconditional Pavlovian to instrumental transfer task. Psychopharmacology (Berl). 2017;234(13):1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conklin CA, Vella EJ, Joyce CJ, Salkeld RP, Perkins KA, Parzynski CS. Examining the relationship between cue-induced craving and actual smoking. Exp Clin Psychopharmacol. 2015;23(2):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conklin CA, McClernon FJ, Vella EJ, et al. Combined smoking cues enhance reactivity and predict immediate subsequent smoking. Nicotine Tob Res. 2019;21(2):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiffman S, Dunbar M, Kirchner T, et al. Smoker reactivity to cues: effects on craving and on smoking behavior. J Abnorm Psychol. 2013;122(1):264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevenson JG, Oliver JA, Hallyburton MB, Sweitzer MM, Conklin CA, McClernon FJ. Smoking environment cues reduce ability to resist smoking as measured by a delay to smoking task. Addict Behav. 2017;67:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rees VW, Heather N. Individual differences and cue reactivity. In: Drummond DC, Tiffany ST, Glautier S, Remington B, eds. The Wiley series in clinical psychology addictive behaviour: Cue exposure theory and practice. Oxford, England: John Wiley & Sons; 1995:99–118. [Google Scholar]
- 17. Rupprecht L, Smith T, Schassburger R, Buffalari D, Sved A, Donny EC. Behavioral mechanisms underlying nicotine reinforcement. In: Balfour D, Munafo M, eds. The Neuropharmacology of Nicotine Dependence. Cham, Switzerland: Springer, 2015:19–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. [DOI] [PubMed] [Google Scholar]
- 19. Berridge KC, Robinson TE. The mind of an addicted brain: neural sensitization of wanting versus liking. Curr Dir Psychol Sci. 1995;4(3):71–75. [Google Scholar]
- 20. Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(8s2):S91–S 117. [DOI] [PubMed] [Google Scholar]
- 21. Donny EC, Griffin KM, Shiffman S, Sayette MA. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine Tob Res. 2008;10(3):447–455. [DOI] [PubMed] [Google Scholar]
- 22. Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7(3):211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Exp Clin Psychopharmacol. 2001;9(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borenstein M, Rothstein M, Cohen J.. Comprehensive Meta-analysis Manual. Biostat Inc.; 2001. https://www.meta-analysis.com/downloads/Meta-Analysis Manual V3.pdf. Accessed August 31, 2018. [Google Scholar]
- 25. Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health. 2014;17(2):53–57. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 28. Duval S. The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta-analysis: Prevention, Assessment and Adjustments. Oxford, England: John Wiley; 2005:127–144. [Google Scholar]
- 29. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 30. Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95 (8s2):S189–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafò MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103(11):1875–1882. [DOI] [PubMed] [Google Scholar]
- 33. Bidwell LC, Leventhal AM, Tidey JW, Brazil L, Niaura RS, Colby SM. Effects of abstinence in adolescent tobacco smokers: withdrawal symptoms, urge, affect, and cue reactivity. Nicotine Tob Res. 2013;15(2):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brandon TH, Drobes DJ, Unrod M, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl). 2011;218(2):391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92(1):15–26. [PubMed] [Google Scholar]
- 36. Carpenter MJ, Saladin ME, DeSantis S, Gray KM, LaRowe SD, Upadhyaya HP. Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addict Behav. 2009;34(6-7):536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cepeda-Benito A, Tiffany ST. The use of a dual-task procedure for the assessment of cognitive effort associated with cigarette craving. Psychopharmacology (Berl). 1996;127(2):155–163. [DOI] [PubMed] [Google Scholar]
- 38. Chiuccariello L, Boileau I, Guranda M, et al. Presentation of smoking-associated cues does not elicit dopamine release after one-hour smoking abstinence: a [11C]-(+)-PHNO PET study. PLoS One. 2013;8(3):e60382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conklin CA, Salkeld RP, Perkins KA, Robin N. Do people serve as cues to smoke? Nicotine Tob Res. 2013;15(12):2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Exp Clin Psychopharmacol. 2001;9(4):399–408. [DOI] [PubMed] [Google Scholar]
- 41. Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111(1-2):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16(3):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. David SP, Munafò MR, Johansen-Berg H, et al. Effects of acute nicotine abstinence on cue-elicited ventral striatum/nucleus accumbens activation in female cigarette smokers: a functional magnetic resonance imaging study. Brain Imaging Behav. 2007;1(3-4):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I—effects on incentive motivation. Psychopharmacology (Berl). 2006;189(3):355–367. [DOI] [PubMed] [Google Scholar]
- 45. Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology (Berl). 2007;194(2):279–288. [DOI] [PubMed] [Google Scholar]
- 46. Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: self-report, psychophysiological, and startle probe responses. Exp Clin Psychopharmacol. 1995;3(2):156. [Google Scholar]
- 47. Erblich J, Bovbjerg DH. In vivo versus imaginal smoking cue exposures: is seeing believing? Exp Clin Psychopharmacol. 2004;12(3):208–215. [DOI] [PubMed] [Google Scholar]
- 48. Erblich J, Montgomery GH. Cue-induced cigarette cravings and smoking cessation: the role of expectancies. Nicotine Tob Res. 2012;14(7):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78(3):647–652. [DOI] [PubMed] [Google Scholar]
- 50. Gass JC, Tiffany ST. Craving and tobacco use: development of the choice behavior under cued conditions (CBUCC) procedure. Psychol Addict Behav. 2017;31(3):276–283. [DOI] [PubMed] [Google Scholar]
- 51. Germeroth LJ, Wray JM, Tiffany ST. Response time to craving-item ratings as an implicit measure of craving-related processes. Clin Psychol Sci. 2015;3(4):530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heishman SJ, Lee DC, Taylor RC, Singleton EG. Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: effects of tobacco deprivation and sex. Exp Clin Psychopharmacol. 2010;18(3):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hutchison KE, Monti PM, Rohsenow DJ, et al. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl). 1999;142(2):139–143. [DOI] [PubMed] [Google Scholar]
- 54. Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Exp Clin Psychopharmacol. 1998;6(1):45–53. [DOI] [PubMed] [Google Scholar]
- 55. Kang OS, Kim SY, Jahng GH, et al. Neural substrates of acupuncture in the modulation of cravings induced by smoking-related visual cues: an fMRI study. Psychopharmacology (Berl). 2013;228(1):119–127. [DOI] [PubMed] [Google Scholar]
- 56. LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32(12):2888–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li X, Sahlem GL, Badran BW, et al. Transcranial magnetic stimulation of the dorsal lateral prefrontal cortex inhibits medial orbitofrontal activity in smokers. Am J Addict. 2017;26(8):788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Littel M, Franken IH. Implicit and explicit selective attention to smoking cues in smokers indexed by brain potentials. J Psychopharmacol. 2011;25(4):503–513. [DOI] [PubMed] [Google Scholar]
- 59. Littel M, Franken IH. Intentional modulation of the late positive potential in response to smoking cues by cognitive strategies in smokers. PLoS One. 2011;6(11):e27519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Litvin EB, Brandon TH. Testing the influence of external and internal cues on smoking motivation using a community sample. Exp Clin Psychopharmacol. 2010;18(1):61–70. [DOI] [PubMed] [Google Scholar]
- 61. Lochbuehler K, Engels RC, Scholte RH. Influence of smoking cues in movies on craving among smokers. Addiction. 2009;104(12):2102–2109. [DOI] [PubMed] [Google Scholar]
- 62. Lopez EN, Drobes DJ, Thompson JK, Brandon TH. Effects of a body image challenge on smoking motivation among college females. Health Psychol. 2008;27(3S):S243–S251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maude-Griffin P, Tiffany ST. Production of smoking urges through imagery: the impact of affect and smoking abstinence. Exp Clin Psychopharmacol. 1996;4(2):198. [Google Scholar]
- 64. McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl). 2009;204(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miranda R Jr, Rohsenow DJ, Monti PM, Tidey J, Ray L. Effects of repeated days of smoking cue exposure on urge to smoke and physiological reactivity. Addict Behav. 2008;33(2):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morissette SB, Gulliver SB, Kamholz BW, Spiegel DA, Tiffany ST, Barlow DH. Transdermal nicotine during cue reactivity in adult smokers with and without anxiety disorders. Psychol Addict Behav. 2012;26(3):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Paris MM, Carter BL, Traylor AC, et al. Cue reactivity in virtual reality: the role of context. Addict Behav. 2011;36(7):696–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rohsenow DJ, Monti PM, Hutchison KE, et al. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol. 2007;15(1):81–92. [DOI] [PubMed] [Google Scholar]
- 69. Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. J Abnorm Psychol. 1994;103(4):812–818. [DOI] [PubMed] [Google Scholar]
- 70. Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96(10):1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sayette MA, Parrott DJ. Effects of olfactory stimuli on urge reduction in smokers. Exp Clin Psychopharmacol. 1999;7(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Singleton EG, Anderson LM, Heishman SJ. Reliability and validity of the Tobacco Craving Questionnaire and validation of a craving-induction procedure using multiple measures of craving and mood. Addiction. 2003;98(11):1537–1546. [DOI] [PubMed] [Google Scholar]
- 73. Smolka MN, Bühler M, Klein S, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl). 2006;184(3-4):577–588. [DOI] [PubMed] [Google Scholar]
- 74. Thompson-Lake DG, Cooper KN, Mahoney JJ III, et al. Withdrawal symptoms and nicotine dependence severity predict virtual reality craving in cigarette-deprived smokers. Nicotine Tob Res. 2015;17(7):796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addict Behav. 1990;15(6):531–539. [DOI] [PubMed] [Google Scholar]
- 76. Tong C, Bovbjerg DH, Erblich J. Smoking-related videos for use in cue-induced craving paradigms. Addict Behav. 2007;32(12):3034–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Traylor AC, Bordnick PS, Carter BL. Assessing craving in young adult smokers using virtual reality. Am J Addict. 2008;17(5):436–440. [DOI] [PubMed] [Google Scholar]
- 78. Watson NL, Carpenter MJ, Saladin ME, Gray KM, Upadhyaya HP. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addict Behav. 2010;35(7):673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weinberger AH, McKee SA, George TP. Smoking cue reactivity in adult smokers with and without depression: a pilot study. Am J Addict. 2012;21(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci. 2013;8(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yalachkov Y, Kaiser J, Görres A, Seehaus A, Naumer MJ. Sensory modality of smoking cues modulates neural cue reactivity. Psychopharmacology (Berl). 2013;225(2):461–471. [DOI] [PubMed] [Google Scholar]
- 82. Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl). 1976;50(1):35–39. [DOI] [PubMed] [Google Scholar]
- 83. Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003;98(5):657–664. [DOI] [PubMed] [Google Scholar]
- 84. Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–1476. [DOI] [PubMed] [Google Scholar]
- 85. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 86. Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9(2):183–190. [DOI] [PubMed] [Google Scholar]
- 87. Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108(6):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Borenstein M, Hedges LV, Higgins JP, Rothstein HR.. Introduction to Meta-analysis. West Sussex, UK: John Wiley & Sons; 2011. [Google Scholar]
- 89. Greenland S, Morgenstern H. Ecological bias, confounding, and effect modification. Int J Epidemiol. 1989;18(1):269–274. [DOI] [PubMed] [Google Scholar]
- 90. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. [DOI] [PubMed] [Google Scholar]
- 91. Moolchan ET, Mermelstein R. Research on tobacco use among teenagers: ethical challenges. J Adolesc Health. 2002;30(6):409–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.