Abstract
Introduction
This study examined cortical thickness (CTX) in World Trade Center (WTC) responders with cognitive impairment (CI).
Methods
WTC responders (N = 99) with/without CI, recruited from an epidemiologic study, completed a T1‐MPRAGE protocol. CTX was automatically computed in 34 regions of interest. Region‐based and surface‐based morphometry examined CTX in CI versus unimpaired responders. CTX was automatically computed in 34 regions of interest. Region‐based measures were also compared to published norms.
Results
Participants were 55.8 (SD = 0.52) years old; 48 had CI. Compared to unimpaired responders, global mean CTX was reduced in CI and across 21/34 cortical subregions. Surface‐based analyses revealed reduced CTX across frontal, temporal, and parietal lobes when adjusting for multiple comparisons. Both CI and unimpaired WTC groups had reduced CTX in the entorhinal and temporal cortices compared to published normative data.
Discussion
Results from the first structural magnetic resonance imaging study in WTC responders identified reduced CTX consistent with a neurodegenerative disease of unknown etiology.
Keywords: cognitive impairment, cortical thickness, World Trade Center responders
1. INTRODUCTION
Studies have documented the extreme exposures and concomitant high burden of psychiatric illness among the men and women (currently aged 45 to 65) who responded to the World Trade Center (WTC) attacks on September 11, 2001. 1 Despite their relative youth, currently available data suggest that this population is also at an increased risk for age‐related cognitive impairment (CI). 2 Age‐related CI with dysfunction in episodic memory is most commonly attributed to neurodegenerative diseases including Alzheimer's disease and related dementias (ADRD). Earlier reports indicate that post‐traumatic stress disorder (PTSD) among veterans of the Vietnam, Iraq, and Afghanistan wars was associated with cortical atrophy. 3 , 4 Studies have also increasingly suggested that severe and/or chronic exposure to inhaled fine particulate matter (PM < 2.5μm) as was experienced on‐site at the WTC disaster, 5 may also cause neurodegeneration in the temporal lobe. 6 To date, no studies have used neuroimaging in CI in WTC responders. Yet, risk of CI in WTC responders is more common than in the general population and is associated with risk factors for ADRD including aging, apolipoprotein‐ε4 allele possession, and is also associated with WTC exposures and PTSD. 2 , 7 , 8 , 9 , 10 , 11 , 12
Cortical thickness (CTX) measurement from magnetic resonance imaging (MRI) provides a powerful non‐invasive method for quantifying neurodegenerative disease risk including for AD. 13 For example, prior studies have shown that individuals with AD experience reductions in CTX in the medial temporal and posterior cingulate/retro‐splenial cortices, 14 with reductions in mean CTX estimated at –0.04 mm in mild CI (regional range: [0.00 to ‐0.16]), 15 and –0.13 mm in AD (regional range: [0.00 to –0.48]), 16 with effects concentrated in the temporal lobe. The objective of the current study was to determine whether CI in WTC responders was potentially attributable to reduced CTX, and whether the pattern of CTX reductions matched known disease profiles. A secondary objective was to examine whether CI among responders with PTSD differed in extent and/or distribution compared to CI alone. Finally, because many cognitively unimpaired WTC responders also spent significant amounts of time near the WTC site, a final objective was to examine the potential for population‐wide differences in comparison to population norms.
2. METHODS
Participants were recruited from a single clinic‐based monitoring program in the WTC Health Program 17 who participated in an epidemiologic study of cognitive aging involving serial administration of the Montreal Cognitive Assessment (MoCA). 18 Responders were contacted if they had previously consented to being contacted to participate in research studies and met inclusion criteria.
RESEARCH IN CONTEXT
To date, little is known about the reasons for cognitive impairment in World Trade Center (WTC) responders.
In this study of WTC responders aged 55.8 years, whole‐brain and regional cortical thickness was reduced in cognitively impaired responders with and without posttraumatic stress disorder.
Compared to published norms, cognitively unimpaired and impaired responders had reduced cortical thickness throughout the temporal lobe.
The study used a two‐by‐two design including CI (present/absent) and PTSD (present/absent). Inclusion criteria were ages 44 to 65, fluent in English, current cognitive assessment indicative of unimpaired (MoCA ≥ 26) or CI (MoCA ≤ 20), and a diagnostic assessment of WTC‐PTSD. While not an inclusion/exclusion criterion, all but one responder with CI had functional limitations consistent with mild dementia. Cognitive status was re‐assessed at the screening visit to ensure case status, and only responders who maintained cognitive status were invited to complete imaging. Responders whose case status was not confirmed were excluded from the neuroimaging study. Eligible responders also had to have diagnoses of either non‐PTSD or PTSD at screening. To account for differences in design across these four general categories, responders’ status was matched across groups based on age, sex, and race/ethnicity as well as occupation and education.
Exclusion criteria: history of psychosis, history of diagnosed neurological conditions including diagnosed ADRD, other dementias, major stroke, multiple sclerosis, and Parkinson's disease; any head injury during the WTC or a history of military head trauma including combustive blasts; current liver disease; and current use of cognitively active medications. Subjects also satisfied eligibility criteria for MRI scanning including body mass index ≥ 40, no known claustrophobia, and no known metal implants or shrapnel that was not deemed MRI‐safe. Upon enrollment, eligible responders were screened to provide more detailed information about functioning and also to ensure case status.
In total, 598 responders were contacted because they fit preliminary inclusion criteria. After screening to determine interest in study participation, 176 were scheduled for screening visits. Of those who were scheduled, 88.6% (n = 156) completed screening visits of whom 27.6% (n = 43) were deemed ineligible, and 7.7% declined participation. The most frequent reason for screened participants being deemed ineligible was failure to screen into appropriate study groups; for example, 34.9% (n = 15) of responders who had previously been diagnosed with PTSD failed to endorse PTSD at screening interviews. Finally, 100 WTC responders commenced neuroimaging; however, only 99 responders completed the imaging used herein. On average, 26.2 (standard deviation [SD] = 17.2) days (inter‐quartile range = 14 to 35 days) elapsed between screening and neuroimaging.
2.1. Image acquisition
Three‐dimensional T1‐weighted magnetization‐prepared rapid gradient echo (MPRAGE) images were acquired using a 3T Siemens Biograph mMR (TR = 1900s, TE = 2.49 ms, TI = 900 ms; Flip Angle = 9°; acquisition matrix: 256 × 256; voxel resolution: 0.89 × 0.89 × 0.89 mm). For incidental pathology screening, T2‐weighted anatomical scans used a turbo spin‐echo pulse sequence (34 axial slices, TR = 6170s, TE = 96 ms; Flip angle = 150°; acquisition matrix = 320 × 320; voxel size = 0.36 × 0.36 × 3 mm) were acquired and read by a board‐certified radiologist to determine incidental findings.
2.2. Image processing
Image processing was completed in two steps. Region‐based analysis was completed using FreeSurfer (version 5.3) to process T1‐weighted images, because it provides the best regional estimate of CTX both compared to other quantification programs, 19 and compared to histological data. 20 Regional CTX was computed automatically using the standard, automated cortical reconstruction pipeline of FreeSurfer, as described in previous publications. 21 , 22 Mean CTX was measured as the average distance between gray and white matter bounds and the outer surface of the cortex of the brain for a given region. CTX values were extracted for 34 regions of interest (ROIs) in each hemisphere based on the "Desikan‐Killiany" atlas. 23 ROI measures of CTX across the two hemispheres were averaged to compute bilateral mean CTX values for each of the 34 ROIs included in the analyses. Visual inspection was performed during quality control efforts. All scans passed global visual inspection in both FreeSurfer and Computational Anatomy Toolbox (CAT12) for severe motion artifacts and low gray‐white matter contrast, which can undermine segmentation accuracy.
Surface‐based morphometry (SBM) was conducted using the CAT12. 24 CAT12 provides better signal to noise calculation in SBM compared to FreeSurfer, 16 and also facilitates threshold‐free cluster analysis. A fully automated projection‐based thickness estimation method incorporating partial volume correction was used to measure CTX. 25 Topological defects were repaired using spherical harmonics as part of CAT12. 26 Analyses followed standard guidelines for pre‐processing including cortical segmentation and Dartel registration prior to CTX being bilaterally merged and smoothed (at 15 mm). For descriptive purposes, total intracranial volume, gray and white matter volume, and white matter hypointensity volume were also extracted using CAT12 automated volumetrics.
2.3. Norms
For external comparisons of region‐based analyses generated by FreeSurfer, we extracted CTX norms derived from 2799 MRI images of cognitively unimpaired (CU) controls aged 18 to 94. 27 Published norms were matched to our protocol using norms adjusted for age, sex, scanner type, and scanner resolution demographic characteristics.
2.4. Measures
2.4.1. Cortical thickness
CTX is a consistent measure of brain atrophy that is commonly used in studies of AD and other related dementias. 28 CTX compares favorably with gray matter volume, whole‐brain volume, and hippocampal volumes because, while all of these measures can be indicators of neurodegeneration, CTX can be quantified across multiple brain regions, and is unrelated to intracranial volume, or sex.
2.4.2. Cognitive impairment
The MoCA is a widely used measure of cognitive functioning developed to objectively and reliably identify age‐related CI. A conservative cutoff (MoCA ≤ 20) was used to identify CI, at a level consistent with possible mild dementia. 29 CU controls had scores in the normal range (MoCA > 26).
2.4.3. Cognitive functioning
A validated computer assisted battery consisted of repeated game‐like tasks. 30 , 31 A total of five tasks were used to measure cognitive dysfunction across domains of episodic memory (accuracy), visuospatial functioning and recall (total number of errors), working memory (accuracy), throughput (accurate responses per second), intra‐individual item‐response variability (measured in standard deviations), processing speed (responses per millisecond), response speed (answers per millisecond), and attention (accuracy). Each task includes 30 to 88 independent trials with an overall score being averaged across valid trials within each task.
2.4.4. Posttraumatic stress disorder
Diagnosis was assessed using the Structured Clinical Interview for the DSM‐IV (SCID‐IV) 32 , a semi‐structured interview schedule administered by trained clinical interviewers. Symptom subdomains were measured using subscales calculated using reported symptom severity in the SCID for the following symptom domains: re‐experiencing symptoms, avoidance, hyperarousal, and negative life experiences.
2.4.5. WTC exposure severity
An interviewer‐administered exposure assessment questionnaire (EAQ) was administered to all responders upon enrollment into the epidemiologic study, that is, at baseline, to record exposures to harmful physical and psychological conditions during work on the WTC recovery effort. 17 In this study, we examined time spent on site among individuals who worked at the WTC pile. 10 , 33 High exposure was defined as the top quintile of exposure duration (≥ 15 weeks).
2.4.6. Demographics
Age in years, sex (male vs female), race (white, black, and Hispanic), educational attainment (high school or less, some college, and university degree), and occupation on September 11 (New York Police vs Other) were included for matching purposes.
2.5. Statistical analyses
Descriptive characteristics were provided using mean and standard deviations, or frequencies and percentages where noted. In this study, confounding from central variables including age was completed by the use of matching in the design phase. One‐way analysis of variance and χ2 tabulations were used to examine differences in matching and diagnostic variables across diagnostic groups.
The analytic plan focused on studying first global mean CTX, then examining CTX in subregions, before finally examining whole‐brain SBM. In each section, the study compared WTC responders to CI versus unimpaired responders, and then compared PTSD to non‐PTSD responders. Analyses then sought to compare subgroups, PTSD‐CI to CI alone, as well as PTSD‐CI and CI alone compared to unimpaired responders without PTSD. Regional differences in CTX in CI were examined using generalized linear modeling. Because main covariates were included in matching efforts, analyses did not include additional adjustments for matched variables. Additionally, because both CI and PTSD were independent effects, generalized linear modeling was additionally used to examine associations between CTX and both PTSD and CI status; however, because CI emerged as the predominant predictor while PTSD was consistently non‐significant, associations with PTSD were described as sensitivity analyses. A similar approach was used in other sensitivity analyses examining stability in the predictive value of CI while adjusting for potential confounders. Analyses initially included total intracranial volume as a confounder; however, since it was not associated with the outcome in any analyses and made no substantial changes to interpretation presented, it was not included in final reported analyses.
Whole‐brain SBM relied on threshold‐free cluster enhancement (TFCE; E = 0.5, H = 2.0) using Draper‐Stoneman (10,000 permutations) to detect vertex clusters; TFCE‐calculated t‐values were reported (tTFCE). 34 Cluster‐based analyses were reported mapped onto central surface maps; cluster locations were reported in Montreal Neurological Institute (MNI) coordinates and mapped onto Desikan‐Killiany ROIs.
One‐sample t‐tests were used to determine differences between WTC responders and published norms. In ROI‐based analyses, standardized mean differences between groups were calculated and both nominal differences (α = 0.05), and differences that were statistically significant after accounting for the false discovery rate (FDR = 0.05) were reported. 35 Generalized linear modeling was used to estimate effect sizes while adjusting for PTSD status in dimensional analyses. Sensitivity analyses further examined results when controlling for matching criteria including age; however, because the design ensured that similar results would be evident the results of these analyses were not reported. Statistical analyses of whole‐brain mean CTX and ROI‐based differences in CTX were implemented using Stata 15/SE (StataCorp).
2.6. Power analysis
CTX is reduced by 0.50 to 1.50 SDs in mild CI and ADRD. 36 This study was created to detect differences in mean CTX with a signal intensity of 0.57 SDs between two groups, and 0.82 SD in 34 subregions.
2.7. Ethics
The Institutional Review Boards at both Stony Brook University and the Icahn School of Medicine at Mount Sinai approved study procedures; participants provided informed written consent.
3. RESULTS
Responders who completed T1‐weighted imaging in this study (Table 1) were in their mid‐50s at the time of the scan (max age was 65 years), and most were male. By design, sample subgroups were matched in terms of age at scan, sex, race/ethnicity, occupation, educational attainment (Table S1 in supporting information for subgroup table).
TABLE 1.
Sample characteristics
| Characteristic | Overall (N = 99) | Cognitively unimpaired (N = 51) | Cognitively impaired (N = 48) | P |
|---|---|---|---|---|
| Age | 56.38 (0.52) | 56.42 (0.63) | 56.32 (0.86) | .926 |
| Intracranial volume | 1516.23 (15.51) | 1532.05 (18.58) | 1498.72 (25.36) | .292 |
| Cognitive domains | ||||
| Response speed | 0.08 (0.01) | 0.08 (0.01) | 0.07 (0.01) | 8.61E‐05 |
| Processing speed | 0.06 (0.01) | 0.07 (0.00) | 0.06 (0.01) | 2.32E‐04 |
| Episodic memory | 0.64 (0.17) | 0.72 (0.19) | 0.56 (0.10) | 2.68E‐06 |
| Intra‐individual response variability | 0.10 (0.04) | 0.09 (0.03) | 0.12 (0.04) | 2.34E‐05 |
| Visuospatial function | 75.08 (47.36) | 57.08 (15.42) | 95.04 (61.21) | 4.40E‐05 |
| Visuospatial memory | 13.28 (9.09) | 9.47 (4.33) | 17.5 (10.99) | 5.42E‐06 |
| Throughput | 0.32 (0.04) | 0.33 (0.04) | 0.30 (0.03) | 3.74E‐05 |
| Working memory | 0.95 (0.12) | 0.99 (0.12) | 0.91 (0.10) | 3.39E‐04 |
| Posttraumatic stress disorder | ||||
| No | 52.5% | 53.9% | 51.1% | .782 |
| Yes | 47.5% | 46.2% | 48.9% | |
| Posttraumatic stress symptomatology | ||||
| Re‐experiencing | 17.48 (6.73) | 17.16 (7.02) | 17.83 (6.45) | .620 |
| Avoidance | 24.07 (9.54) | 24.18 (10.56) | 23.96 (8.44) | .910 |
| Hyperarousal | 17.76 (6.73) | 17.45 (7.25) | 18.08 (6.21) | .643 |
| Negative experiences | 12.23 (4.59) | 12.41 (4.83) | 12.04 (4.36) | .691 |
| Severe WTC exposure | ||||
| No | 87.4% | 90.2% | 84.1% | .172 |
| Yes | 12.6% | 9.8% | 15.9% | |
| Sex | ||||
| Male | 85.3% | 80.8% | 76.6% | .612 |
| Female | 14.7% | 19.2% | 23.4% | |
| Minority status | ||||
| White | 77.8% | 86.5% | 68.1% | .087 |
| Black | 10.1% | 5.8% | 14.9% | |
| Hispanic | 12.1% | 7.7% | 17.0% | |
| Occupation | ||||
| NYPD | 56.6% | 60.8% | 52.1% | .383 |
| Other | 43.4% | 39.2% | 47.9% | |
| Education | ||||
| High school or less | 23.2% | 17.7% | 29.2% | .359 |
| Some college | 47.5% | 49.0% | 45.8% | |
| University degree | 29.3% | 33.3% | 25.0% |
Note: Means (standard deviations) or percentages (%) reported. P‐values examine the extent to which noted characteristics differ across cognitive status and were derived using χ2 tests for categorical variables, and Student's t‐tests for continuous variables. All significant effects passed the false discovery rate.
Abbreviations: NYPD, New York Police Department; WTC, World Trade Center.
Not shown in Table 1, when comparing WTC‐CI to unimpaired responders, total intracranial volume (1528.05 [SD = 132.50] cm3 vs 1498.72 [173.88]] cm3; P = .286), white matter volume (551.37 [62.00] cm3 vs 547.51 [10.67] cm3; P = .778), and white matter hypointensity volume (1.92 [1.09] cm3 vs 2.12 [2.14]cm3; P = .554) were similar across groups, while gray matter volume (629.22 cm3 vs 603.20 cm3; P = .030) and mean whole‐brain mean CTX (2.48 [0.08] mm vs 2.41 [0.08] mm; P = .0003) were significantly reduced in CI.
While mean CTX was reduced in CI, PTSD status did not predict reduced CTX across groups (Figure 1). For example, no differences were evident in mean CTX when comparing PTSD to non‐PTSD groups (P = .261), or in analyses comparing PTSD‐CI to CI alone (P = .521), justifying combining PTSD with non‐PTSD groups in further analyses. In addition, multivariable analyses controlling for cognitive status showed PTSD was not associated with mean CTX (P = .216) further justifying a focus on CI in this study; however, because PTSD‐related differences were potentially important in this population, additional analyses of PTSD‐related reductions in CTX were completed (Figure S1 in supporting information).
FIGURE 1.
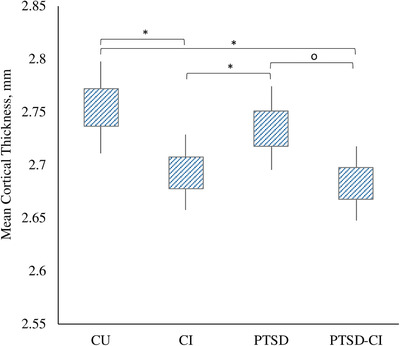
Absolute differences in mean whole‐brain mean cortical thickness expressed in mm by patient diagnostic group. Note: Group differences were evident across groups when using one‐way analysis of variance across all group profiles (P = .002) that was concentrated in cognitively impaired responders. 95% confidence intervals shown using capped error bars. *Statistically significant at P < .05; o P = .055
3.1. CTX in WTC‐CI
In region‐based analyses of the 34 ROIs (Table 2), CTX was significantly reduced in WTC‐CI in 23/34 regions throughout the frontal, temporal, and occipital lobes of which 21/23 (91.3%) of these ROIs remained significantly different after accounting for multiple comparisons. ROIs with the largest CTX reductions included the precentral, supramarginal, lateral occipital, superior temporal, and transverse temporal regions.
TABLE 2.
Regional estimates of mean cortical thickness and estimated standardized mean differences among cognitively impaired and cognitively unimpaired responders
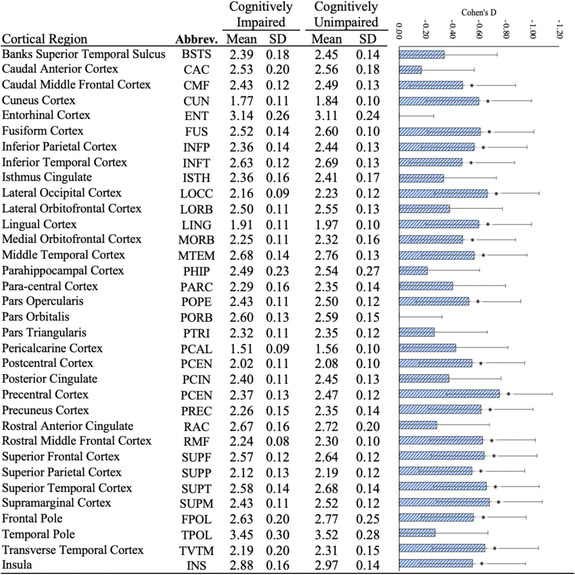
|
Note: Because there were no significant positive results, only negative associations are reported. Standardized mean differences (Cohen's D) shown using blue bars with 95% confidence intervals. *Statistically significant in bivariate analyses after adjusting for the false discovery rate.
Heat maps examining associations between dimensional measures of cognitive performance and PTSD symptomatology (Figure 2) showed significant differences showing that worse performance in episodic memory, throughput, visual memory, and the continuance MoCA score were significantly associated with reduced CTX across similar regions to those noted above; however, more CTX subregions were significantly associated cognitive subdomains including episodic memory (17 ROIs), throughput (25 ROIs), intra‐individual response variability (one ROI), working memory (16 ROIs), and MoCA score (25 ROIs). PTSD symptom subdomains were not associated with CTX.
FIGURE 2.
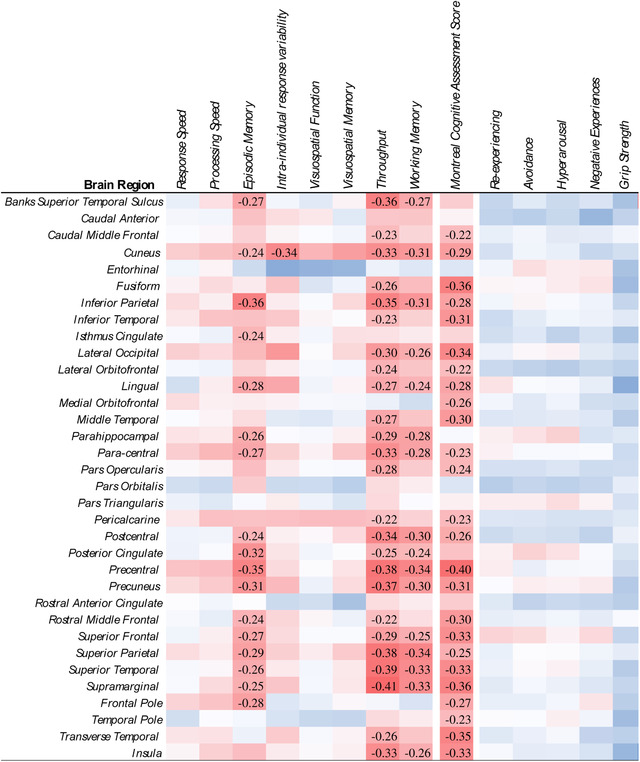
Heat map showing levels of association between dimensional measures of cognition and posttraumatic stress disorder and regional measures of cortical thickness (CTX). Note: Coefficients were transformed so that increases in dimensional scores are consistent with worse outcomes. Stanardized Mean Differences (Cohen's D) were estimated from generalized linear modeling; all coefficients deemed statistically significant upon adjusting for the false discovery rate (FDR = 0.05) were reported. Red indicates reduced CTX; blue indicates increased CTX.
Results from surface‐based analysis identified broad reductions in CTX throughout the brain (Figure 3). This report reflects three dominant statistically significant clusters with 11 cluster‐peaks that remained after adjustment for multiple comparisons (FDR = 0.05). Clusters‐peaks were evident across the frontal, temporal, and occipital lobes with the most severe reductions, ordered by location, in the right medial orbitofrontal region (MNI coordinate: 8/67/‐6 mm, tTFCE = 31,662.6, P = .003). Other cluster‐peaks were identified in the left insula, left posterior cingulate, left superior frontal, left and right precentral, left precuneus, left inferior parietal, left superior parietal, right pericalcarine, left medial orbitofrontal, and left rostral anterior cingulate (Table S2 in supporting information).
FIGURE 3.
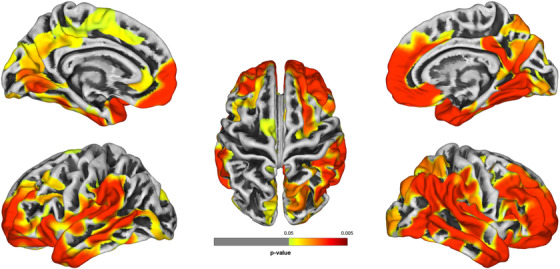
Group‐wise analyses using surface‐based morphometry comparing cognitively impaired to cognitively unimpaired responders
Note: Group‐wise analyses using surface‐based morphometry identified areas of reduced cortical thickness (CTX) are shown since no regions with increased cortical thickness were identified. Regions lacking significant differences in CTX between groups are shown in gray; cluster locations provided in Table S2 in supporting information. Figure generated using Computational Anatomy Toolbox (CAT12)
3.2. Comparison to published norms
When compared to published norms, CTX was significantly reduced in 7/34 ROIs in CU responders, and in 14/34 ROIs in WTC responders with CI (Table 3). Differences in CU responders included regions within the temporal lobe including, in order of effect size, reduced CTX in the entorhinal cortex, fusiform gyrus, middle temporal, parahippocampal gyrus, temporal pole, inferior temporal, and superior temporal regions. Regions that were reduced in CU responders were further reduced in WTC‐CI, while other regions such as the medial orbitofrontal, precentral, pars opercularis, supramarginal, transverse temporal, and insula, which were not reduced in CU WTC responders, were decreased in WTC responders with CI.
TABLE 3.
Region‐based standardized mean differences comparing cognitively unimpaired and cognitively impaired responders to norms from 2799 cognitively normal individuals in the general population
| CU versus norms | CI versus norms | |||
|---|---|---|---|---|
| Region | D | P | D | P |
| Banks superior temporal sulcus | −0.26 | .362 | −0.80 | .007 |
| Caudal anterior | 0.71 | .015 | 0.32 | .262 |
| Caudal middle frontal | 0.77 | .009 | −0.17 | .543 |
| Cuneus | 1.29 | <.001 | −0.05 | .874 |
| Entorhinal | −3.33 | <.001 | −2.64 | <.001 |
| Fusiform | −1.75 | <.001 | −2.25 | <.001 |
| Inferior parietal | 0.92 | .002 | −0.28 | .332 |
| Inferior temporal | −0.98 | .001 | −1.92 | <.001 |
| Isthmus cingulate | 0.77 | .009 | 0.07 | .796 |
| Lateral occipital | 1.26 | <.001 | 0.00 | .998 |
| Lateral orbitofrontal | 0.39 | .173 | −0.45 | .122 |
| Lingual | 0.71 | .015 | −0.53 | .066 |
| Medial orbitofrontal | 0.20 | .489 | −0.92 | .002 |
| Middle temporal | −1.22 | <.001 | −2.12 | <.001 |
| Parahippocampal | −1.16 | <.001 | −1.71 | <.001 |
| Para‐central | 0.72 | .014 | −0.11 | .706 |
| Pars opercularis | 0.23 | .418 | −0.85 | .004 |
| Pars orbitalis | −0.01 | .981 | 0.11 | .687 |
| Pars triangularis | 0.44 | .130 | −0.09 | .747 |
| Pericalcarine | 0.41 | .148 | −0.44 | .125 |
| Postcentral | 1.28 | <.001 | 0.08 | .772 |
| Posterior cingulate | 0.82 | .006 | 0.15 | .596 |
| Precentral | 0.40 | .160 | −1.11 | <.001 |
| Precuneus | 0.77 | .009 | −0.49 | .088 |
| Rostral anterior cingulate | 0.03 | .905 | −0.58 | .047 |
| Rostral middle frontal | 1.05 | .001 | −0.23 | .412 |
| Superior frontal | 0.99 | .001 | −0.31 | .276 |
| Superior parietal | 1.04 | .001 | −0.15 | .603 |
| Superior temporal | −0.95 | .002 | −2.23 | <.001 |
| Supramarginal | 0.72 | .014 | −0.74 | .012 |
| Frontal pole | 1.06 | <.001 | −0.14 | .625 |
| Temporal pole | −1.15 | <.001 | −1.50 | <.001 |
| Transverse temporal | 0.60 | .039 | −0.73 | .013 |
| Insula | 0.32 | .260 | −0.81 | .006 |
Note: P‐values provided in the rightmost column were derived from one‐sample t‐tests. Regions where cortical thickness was significantly reduced upon accounting for the false discovery rate were highlighted in Bold. Codes to abbreviations are provided in Table 2.
Abbreviations: CU, cognitively unimpaired; CI, Cognitively impaired; D, Standardized Mean Differences (Cohen's D).
3.3. Sensitivity analyses
Matching variables were not incorporated as covariables in these analyses due to concerns of over‐matching. Sensitivity analyses using generalized linear modeling comparing the association between CI and CTX when adjusting for matching variables including age, sex, occupation, education, and intracranial volume did not explain the association between CI and CTX shown above. Additional efforts to consider cardiovascular and metabolic risk factors identified associations with white matter hypointensities but did not explain the results shown in this study. While the study was not sufficiently large to power analyses examining interactions, preliminary analyses examining interactions between domains of cognitive dysfunction and PTSD symptoms identified a small number of interaction effects (Figure S2 in supporting information). For example, slower processing speed among responders with more severe re‐experiencing symptoms was associated with lower CTX in the lateral orbitofrontal and pars opercularis. Additional associations were found between increased avoidance and hyperarousal symptoms with poorer visuospatial learning and higher CTX in some parts of the temporal lobe.
4. DISCUSSION
WTC responders were exposed to a range of inhaled neurotoxicants and also to extreme psychosocial stressors, 37 exposures that have been linked to increased risk for CI. 33 The goal of the current study was to determine whether CI was associated with reduced CTX in WTC responders at midlife. Results suggested that CI was characterized by reduced CTX, a parameter that is often used in the adult brain as a surrogate marker for neurodegeneration. Affected regions of the brain for both WTC responders with CI and those with PTSD‐CI involved a wide array of regions that have been previously implicated in conditions such as early onset ADRD or a related dementia, sporadic ADRD, inhaled particulate matter exposures, aging, and PTSD (Table S3 in supporting information).
The pattern of cortical thinning arising from the SBM reported results appear to be consistent, in part, with a relatively uncommon parietal‐dominant subtype of ADRD. 38 Interestingly, parietal‐dominant ADRD is more common in early onset ADRD patients and is characterized by cognitive deficits in episodic memory alongside changes in a wide range of cognitive domains such as executive function, visuospatial functioning, visuospatial and self‐processing, as is also common in amnestic hippocampal‐sparing early‐onset ADRD. 39 The spread of neuropathology in parietal‐dominant ADRD does not match the topography of amyloid beta (Aβ) deposition or neurofibrillary tauopathy of late‐onset ADRD. 38 Prognosis for both parietal‐dominant and hippocampal‐sparing subtypes of ADRD is also often poor, irrespective of age of onset, potentially because Aβ deposits more broadly throughout the brain 40 thereby causing cognitive declines across a broader range of cognitive domains than seen in other AD subtypes. 41
Relatively little is known about causes of CI at midlife, partially because dementing diseases at midlife are fairly rare with estimates at < 1/1000 individuals. 42 CI at midlife carries an increased risk of mortality when compared to non‐demented individuals or patients with late‐onset dementia. 43 Affected ROIs, identified as those with reduced CTX, were not limited to cortical regions commonly associated with ADRD including, for example, the inferior temporal gyrus and precuneus. 13 Analyses also identified additional ROIs such as the postcentral gyri that are associated with neuroinflammatory disease, 44 and the peri‐calcarine cortex, which has been associated with sensory dysfunction in aging. 13
Studies have recently suggested that severe and/or chronic exposure to inhaled fine particulate matter (PM < 2.5 μm) may also lead to neurodegeneration. 6 Exposure to fine particulate matter has been linked to similar processes as those discussed in PTSD including systemic inflammation, 45 changes to glutamatergic functioning, 46 increased amyloid accumulation 47 and deposition, 48 with the end result being cognitive decline 49 and increased risk of dementia. 50 In our analyses, we found that compared to normative data even unimpaired responders had reduced CTX in the entorhinal and temporal cortices, previously shown to be vulnerable to exposure to inhaled neurotoxins, 6 when adjusting for age, sex, scanner type, and scanner resolution. Further work that integrates information about vulnerability to ADRD and related dementias derived from genotypes shown to be sensitive to air pollution may, therefore, be critical in future analyses.
Prior studies have reported associations between chronic PTSD and increased risk of CI in this population. 18 , 33 CI in responders with PTSD was indistinguishable from CI without PTSD in this study, though analyses were suggestive of greater severity in PTSD‐CI. While PTSD is not an accepted risk factor for neurodegenerative diseases, it has been linked to many of the features of neurodegenerative diseases, including widespread cortical thinning, 3 neural network dysfunction, 51 and accumulation of Aβ and the microtubule‐associated protein tau. 52 , 53 , 54 Evidence in combat veterans has linked chronic PTSD with widespread cortical thinning across regions including the frontal, temporal, occipital, and insular regions 3 as well as the prefrontal cortex, and anterior and posterior cingulate. 51 It is unclear why PTSD might increase risk of neurodegenerative disease, but current theories suggest that it may engage a chronic stress response 55 by activating the hypothalamic‐pituitary‐adrenal axis 56 and engaging an immune response 57 , 58 when processing emotionally stressful memories. 59 An additional possibility is that PTSD may cause the proliferation of norepinephrine engaging concomitant microglial 60 and tau 61 response.
In this study, trends toward reduced CTX were evident in 21 regions after adjusting for multiple comparisons. The entorhinal cortex and pars orbitalis were the only regions where trends were not indicative of reduced CTX in WTC responders. The entorhinal cortex is concerning because it is commonly influenced by most dementing conditions, and thus the lack of results in the entorhinal cortex in particular is unusual. One interpretation of this finding may be that these regions are commonly reduced across the WTC responder population, but may not be sufficient to cause cognitive symptoms on their own. 62 Results comparing CTX in cognitively unimpaired WTC responders with published normative data from 2,799 cognitively normal adults 27 identified substantial reductions in the entorhinal cortex and across the temporal lobe despite revealing thicker cortices on average across many other regions; results may suggest that cognitively unimpaired WTC responders also have some abnormalities in this region of the brain. Reduced CTX and volume of the entorhinal cortex is commonly interpreted as an early marker of the spread of tauopathy in ADRD, 63 which is often associated with greater neurodegeneration. 64 Because the temporal lobe has been implicated recently in exposure‐based neurodegenerative diseases, 6 the potential for proliferation of tauopathy in CU WTC responders is concerning. Future research should examine the extent to which these results are supported by neuropathology using molecular neuroimaging techniques.
4.1. Limitations
Limitations include small sample size, the unique nature of the exposure, and a lack of non‐WTC external control group. This study also examined severe CI and focused on PTSD status irrespective of exposure to inhaled neurotoxicants during response efforts. Genotype has been shown to be relevant to pathogenesis of exposure‐related CI, thus the lack of genotype measures in this manuscript is a limitation. Though we did make efforts to increase recruitment of minorities and women to the point of doubling the numbers of both in this sample compared to the responder population, the sample would benefit from improved integration of persons from different backgrounds. This cross‐sectional study could not provide information about rates of CTX reduction, and therefore does not allow us to estimate the age of onset of cortical thinning. Consequently, we could not determine whether atrophy occurred pursuant to WTC exposures. Further research is warranted to examine more mildly impaired responders to determine the prevalence of neurodegenerative disease in responders. The identification of reduced CTX does not clarify the extent to which neurodegeneration is ongoing or resulted instead from a one‐time massive systemic shock or, instead, if chronic stressors involved in PTSD caused changes to the brain's structure. While relying on a well‐established published normative database (N = 2799), results from comparisons with normative data may be subject to a range of selection biases. Though providing good specificity for multiple diagnostic categories, CTX cannot be used to definitively determine etiology of neurodegeneration.
5. CONCLUSIONS
The WTC disaster exposed tens of thousands of individuals including employees at, and near, the WTC site; residents of the area; and responders to a host of traumatic experiences and, at the same time, exposed many to the toxic detritus of the towers after they collapsed. Current estimates suggest that at midlife, a growing number of WTC responders are experiencing early CI and dementia. 33 The current study showed for the first time in a sample of WTC responders that WTC‐CI was accompanied by reduced CTX encompassing regions commonly influenced by neurodegenerative diseases. However, results also suggested that WTC‐CI had an architecture that, while reminiscent of ADRD, was also inconsistent with signatures developed for known neurodegenerative diseases. This work supports the view that WTC‐CI may be a WTC‐specific encephalopathy with an unknown etiology characterized by widespread cortical atrophy.
CONFLICTS OF INTEREST
The authors have no disclosures to report.
Supporting information
Supplementary materials
ACKNOWLEDGMENTS
The authors would like to acknowledge support from the Centers for Disease Control and Prevention for supporting the neuroimaging study (CDC/NIOSH U01 OH011314), the National Institute on Aging that supports research on characterization and treatment of Alzheimer's disease (NIH/NIA P50 AG005138), and aging‐related work in this population (NIH/NIA R01 AG049953). We would also like to acknowledge ongoing funding to monitor World Trade Center responders as part of the WTC Health and Wellness Program (CDC 200‐2011‐39361).
Clouston SAP, Deri Y, Horton M. Reduced cortical thickness in World Trade Center responders with cognitive impairment. Alzheimer's Dement. 2020;12:e12059 10.1002/dad2.12059
REFERENCES
- 1. Bromet E, Hobbs M, Clouston S, Gonzalez A, Kotov R, Luft B. DSM‐IV post‐traumatic stress disorder among World Trade Center responders 11‐13 years after the disaster of 11 September 2001 (9/11). Psychol Med. 2016;46:771‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clouston S, Kotov R, Pietrzak RH, et al. Cognitive impairment among World Trade Center responders: long‐term implications of re‐experiencing the 9/11 terrorist attacks. Alzheimer's dementia. 2016;4:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wrocklage KM, Averill LA, Scott JC, et al. Cortical thickness reduction in combat exposed US veterans with and without PTSD. Eur Neuropsychopharmacol. 2017;27:515‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lioy PJ, Georgopoulos P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann N Y Acad Sci. 2006;1076:54‐79. [DOI] [PubMed] [Google Scholar]
- 6. Cho J, Noh Y, Kim SY, Sohn J, Noh J, Kim W, Cho S‐K, Seo H, Seo G, Lee S‐K, Seo S, Koh S‐B, Oh SS, Kim HJ, Seo SW, Shin D‐S, Kim N, Kim HH, Lee JI, Kim C. Effects of Long‐Term Exposure to Ambient Air Pollution on Brain Cortical Thinning Among Elderly Individuals Without Neurological Diseases. SSRN Electronic Journal. 10.2139/ssrn.3402016. [DOI] [Google Scholar]
- 7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenberg MS, Tanev K, Marin MF, Pitman RK. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10:S155‐65. [DOI] [PubMed] [Google Scholar]
- 9. Flatt JD, Gilsanz P, Quesenberry CP, Albers KB, Whitmer RA. Post‐traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimer's Dementia. 2018;14:28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clouston SAP, Pietrzak RH, Kotov R, et al. Traumatic exposures, posttraumatic stress disorder, and cognitive functioning in World Trade Center responders. Alzheimers Dement (N Y). 2017;3:593‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clouston SAP, Deri Y, Diminich E, et al. Posttraumatic stress disorder and total amyloid burden and amyloid‐beta 42/40 ratios in plasma: results from a pilot study of World Trade Center responders. Alzheimers Dement (Amst). 2019;11:216‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukherjee S, Clouston S, Kotov R, Bromet E, Luft B. Handgrip Strength of World Trade Center (WTC) responders: the role of re‐experiencing Posttraumatic Stress Disorder (PTSD) symptoms. Int J Environ Res Public Health. 2019;16:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickerson BC, Bakkour A, Salat DH, et al. The Cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild ad dementia and is detectable in asymptomatic amyloid‐positive individuals. Cereb Cortex. 2009;19:497‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho H, Jeon S, Kang SJ, et al. Longitudinal changes of cortical thickness in early‐versus late‐onset Alzheimer's disease. Neurobiol Aging. 2013;34:1921. e9‐. e15. [DOI] [PubMed] [Google Scholar]
- 15. Cheng CP‐W, Cheng S‐T, Tam CW‐C, Chan W‐C, Chu WC‐W, Lam LC‐W. Relationship between cortical thickness and neuropsychological performance in normal older adults and those with mild cognitive impairment. Aging Dis. 2018;9:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seiger R, Ganger S, Kranz GS, Hahn A, Lanzenberger R. Cortical thickness estimations of freesurfer and the CAT12 toolbox in patients with Alzheimer's disease and healthy controls. J Neuroimaging. 2018;28:515‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dasaro CR, Holden WL, Berman KD, et al. Cohort profile: world trade center health program general responder cohort. Int J Epidemiol. 2017;46:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clouston SA, Kotov R, Pietrzak RH, et al. Cognitive impairment among World Trade Center responders: long‐term implications of re‐experiencing the 9/11 terrorist attacks. Alzheimers Dement (Amst). 2016;4:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tustison NJ, Cook PA, Klein A, et al. Large‐scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166‐179. [DOI] [PubMed] [Google Scholar]
- 20. Cardinale F, Chinnici G, Bramerio M, et al. Validation of FreeSurfer‐estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics. 2014;12:535‐542. [DOI] [PubMed] [Google Scholar]
- 21. Dale AM, Fischl B, Sereno MI. Cortical surface‐based analysis. I. segmentation and surface reconstruction. Neuroimage. 1999;9:179‐194. [DOI] [PubMed] [Google Scholar]
- 22. Fischl B, Sereno MI, Dale AM. Cortical surface‐based analysis. II: inflation, flattening, and a surface‐based coordinate system. Neuroimage. 1999;9:195‐207. [DOI] [PubMed] [Google Scholar]
- 23. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968‐980. [DOI] [PubMed] [Google Scholar]
- 24. Gaser C, Dahnke R. CAT—a computational anatomy toolbox for the analysis of structural MRI data. HBM. 2016;2016:336‐348. [Google Scholar]
- 25. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336‐348. [DOI] [PubMed] [Google Scholar]
- 26. Yotter RA, Nenadic I, Ziegler G, Thompson PM, Gaser C. Local cortical surface complexity maps from spherical harmonic reconstructions. Neuroimage. 2011;56:961‐973. [DOI] [PubMed] [Google Scholar]
- 27. Potvin O, Dieumegarde L, Duchesne S. Normative morphometric data for cerebral cortical areas over the lifetime of the adult human brain. Neuroimage. 2017;156:315‐339. [DOI] [PubMed] [Google Scholar]
- 28. Lerch JP, Pruessner J, Zijdenbos AP, et al. Automated cortical thickness measurements from MRI can accurately separate Alzheimer's patients from normal elderly controls. Neurobiol Aging. 2008;29:23‐30. [DOI] [PubMed] [Google Scholar]
- 29. Freitas S, Simoes MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:37‐43. [DOI] [PubMed] [Google Scholar]
- 30. Lim YY, Pietrzak RH, Bourgeat P, et al. Relationships between performance on the cogstate brief battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol. 2015;30:49‐58. [DOI] [PubMed] [Google Scholar]
- 31. Hammers D, Spurgeon E, Ryan K, et al. Reliability of repeated cognitive assessment of dementia using a brief computerized battery. Am J Alzheimer's Disease Other Demen. 2011;26:326‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. First MB. Structured clinical interview for the DSM (SCID). Encyclopedia Clin Psychol. 2014:1‐6. [Google Scholar]
- 33. Clouston SAP, Diminich ED, Kotov R, et al. Incidence of mild cognitive impairment in World Trade Center responders: long‐term consequences of re‐experiencing the events on 9/11/2001. Alzheimers Dement (Amst). 2019;11:628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83‐98. [DOI] [PubMed] [Google Scholar]
- 35. Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289‐300. [Google Scholar]
- 36. Julkunen V, Niskanen E, Muehlboeck S, et al. Cortical thickness analysis to detect progressive mild cognitive impairment: a reference to Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28:389‐397. [DOI] [PubMed] [Google Scholar]
- 37. Neria Y, DiGrande L, Adams BG. Posttraumatic stress disorder following the September 11, 2001, terrorist attacks a review of the literature among highly exposed populations. Am Psychol. 2011;66:429‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, et al. Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology. 2014;83:1936‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mendez MF. Early‐onset Alzheimer's disease: nonamnestic subtypes and type 2 AD. Arch Med Res. 2012;43:677‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hwang J, Kim CM, Jeon S, et al. Prediction of Alzheimer's disease pathophysiology based on cortical thickness patterns. Alzheimer's Dementia. 2016;2:58‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Na HK, Kang DR, Kim S, et al. Malignant progression in parietal‐dominant atrophy subtype of Alzheimer's disease occurs independent of onset age. Neurobiol Aging. 2016;47:149‐156. [DOI] [PubMed] [Google Scholar]
- 42. Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD. The diagnosis of young‐onset dementia. Lancet Neurol. 2010;9:793‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koedam EL, Pijnenburg YA, Deeg DJ, et al. Early‐onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord. 2008;26:147‐152. [DOI] [PubMed] [Google Scholar]
- 44. Arfanakis K, Fleischman DA, Grisot G, et al. Systemic inflammation in non‐demented elderly human subjects: brain microstructure and cognition. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng H, Saffari A, Sioutas C, Forman HJ, Morgan TE, Finch CE. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Persp. 2016;124:1537‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morgan TE, Davis DA, Iwata N, et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Persp. 2011;119:1003‐1009. [Google Scholar]
- 47. Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7:e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim SH, Knight EM, Saunders EL, et al. Rapid doubling of Alzheimer's amyloid‐β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Res. 2012;1:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172:219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kilian J, Kitazawa M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer's disease–evidence from epidemiological and animal studies. Biomed J. 2018;41:141‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clouston SAP, Deri Y, Diminich E, et al. Posttraumatic stress disorder associated with total amyloid burden and amyloid‐ß 42/40 ratios in plasma: results from a pilot study of World Trade Center responders. Alzheimer's Dementia. 2019;11:216‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mohamed AZ, Cumming P, Gotz J, Nasrallah F. Department of defense Alzheimer's disease neuroimaging I. tauopathy in veterans with long‐term posttraumatic stress disorder and traumatic brain injury. Eur J Nucl Med Mol Imaging. 2019;46:1139‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mohamed AZ, Cumming P, Srour H, et al. Amyloid pathology fingerprint differentiates post‐traumatic stress disorder and traumatic brain injury. Neuroimage Clin. 2018;19:716‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lawrence‐Wood E, Van Hooff M, Baur J, McFarlane AC. Re‐experiencing phenomena following a disaster: the long‐term predictive role of intrusion symptoms in the development of post‐trauma depression and anxiety. J Affect Disord. 2016;190:278‐281. [DOI] [PubMed] [Google Scholar]
- 56. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55‐89. [DOI] [PubMed] [Google Scholar]
- 57. Passos IC, Vasconcelos‐Moreno MP, Costa LG, et al. Inflammatory markers in post‐traumatic stress disorder: a systematic review, meta‐analysis, and meta‐regression. Lancet Psychiatry. 2015;2:1002‐1012. [DOI] [PubMed] [Google Scholar]
- 58. Kuan P‐F, Yang X, Clouston S, et al. Cell type‐specific gene expression patterns associated with posttraumatic stress disorder in World Trade Center responders. Transl Psychiatry. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Dell TJ, Connor SA, Guglietta R, Nguyen PV. Beta‐Adrenergic receptor signaling and modulation of long‐term potentiation in the mammalian hippocampus. Learn Mem. 2015;22:461‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heneka MT, Nadrigny F, Regen T, et al. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci. 2010;107:6058‐6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang F, Gannon M, Chen Y, et al. β‐amyloid redirects norepinephrine signaling to activate the pathogenic GSK3β/tau cascade. Sci Transl Med. 2020;12:eaay6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eich T, Parker D, Gazes Y, Razlighi Q, Habeck C, Stern Y. Towards an ontology of cognitive processes and their neural substrates: a structural equation modeling approach. PLoS One. 2020;15:e0228167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Okamura N, Harada R, Furumoto S, Arai H, Yanai K, Kudo Y. Tau PET imaging in Alzheimer's disease. Curr Neurol Neurosci Rep. 2014;14:500. [DOI] [PubMed] [Google Scholar]
- 64. La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau‐PET. Sci Transl Med. 2020;12:eaau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials


