Abstract
Introduction
Alzheimer's disease (AD) is a growing public health concern affecting millions of patients worldwide and costing billions of dollars annually. We review the pipeline of drugs and biologics in clinical trials for the treatment of AD. We use the Common Alzheimer's and Related Dementias Research Ontology (CADRO) to classify treatment targets and mechanisms of action. We review our annual pipeline reports for the past 5 years to provide longitudinal insight into clinical trials and drug development for AD.
Methods
We reviewed ClinicalTrials.gov as of February 27, 2020, and identified all trials of pharmacologic agents currently being developed for treatment of AD as represented on this widely used U.S. Food and Drug Administration registry.
Results
There are 121 agents in clinical trials for the treatment of AD. Twenty‐nine agents are in 36 Phase 3 trials, 65 agents are in 73 Phase 2 trials, and 27 agents are in 27 Phase 1 trials. Twelve agents in trials target cognitive enhancement and 12 are intended to treat neuropsychiatric and behavioral symptoms. There are 97 agents in disease modification trials. Compared to the 2019 pipeline, there is an increase in the number of disease‐modifying agents targeting pathways other than amyloid or tau.
Discussion
The 2020 pipeline has innovations in clinical trials and treatment targets that provide hope for greater success in AD drug development programs. Review of clinical trials over the past 5 years show that there is progressive emphasis on non‐amyloid targets, including candidate treatments for inflammation, synapse and neuronal protection, vascular factors, neurogenesis, and epigenetic interventions. There has been a marked growth in repurposed agents in the pipeline.
Keywords: Alzheimer's disease, biomarkers, clinical trials, Common Alzheimer's and Related Dementias Research Ontology (CADRO), drug development, NIH, pharmaceutical companies, repurposed drugs
1. INTRODUCTION
Alzheimer’ disease (AD) is a progressive neurodegenerative disease that currently produces dementia in 5.8 million U.S. citizens and this number will increase to 13.5 million by 2050. 1 AD dementia is projected to have a devastating impact on global populations by 2050 with 131 million affected. The costs of AD are accelerating—rising from $1 trillion globally in 2018 to a projected $2 trillion in 2030. 2 Means of preventing, delaying the onset, slowing the progression, and improving the symptoms of AD are urgently needed. This annual review describes the pipeline of drugs in development for AD; discusses innovations in drug development; and provides an update on new targets, drugs, and biomarkers represented in current clinical trials. We call attention to notable recent advances in the field.
This is the 5th year of the pipeline review, presenting an opportunity to describe changes in AD drug development from a longitudinal perspective. 3 , 4 , 5 , 6 To better present the targets of AD therapeutics in this review, we adopted the terminology of the Common Alzheimer's and Related Dementias Research Ontology (CADRO). 7 , 8 The CADRO identifies the following potential targets for AD from early‐stage to late‐stage clinical drug development: amyloid, tau, apolipoprotein E (apoE)/lipids/lipoprotein receptors, neurotransmitter receptors, neurogenesis, inflammation, oxidative stress, cell death, proteostasis/proteinopathies, metabolism/bioenergetics, vasculature, growth factors/hormones, synaptic plasticity/neuroprotection, epigenetics, and “others.” While this classification was not conceived primarily as a means of capturing drug mechanisms, the CADRO systematizes the processes of AD that are the current drug targets relevant to AD and provides a framework for classifying treatment mechanisms. We reclassified the drug mechanisms from previous reviews using the CADRO approach. Some agents have more than one mechanism of action and, in these cases, we noted both mechanisms and depended on the available literature to identify a dominant mechanism. Infection and immunity were not included in the original CADRO system and we included any agents targeting infection or immunity with inflammation for the purpose of this review. We kept the terminology of “symptomatic” treatments for agents whose purpose was cognitive enhancement or control of neuropsychiatric symptoms without claiming to impact the biological causes of cell death in AD, and we used “disease‐modifying” for treatments intended to change the biology of AD and produce neuroprotection (often through a variety of intermediate mechanisms such as effects or amyloid or tau). 9 , 10 AD is now recognized to have preclinical, prodromal, and dementia phases, 11 and we note if the studies are prevention trials including cognitively normal participants with preclinical AD; prodromal trials involving participants with mild cognitive impairment (MCI) but not meeting criteria for dementia; or treatment trials for participants with mild, moderate, or severe AD dementia.
2. METHODS
The U.S. Food and Drug Administration (FDA) website ClinicalTrials.gov is the source of information for this review. The “Common Rule” governing ClinicalTrials.gov requires registration on this site of all trials from sponsors with an investigational new drug (IND) or investigational new device (IDE) being assessed in the United States. Compliance with the required trial registration is high among trial sponsors. 12 , 13 , 14 , 15 There are other clinical trial registries with some treatments not present on the ClinicalTrials.gov website, and our review is not an exhaustive listing of every clinical trial or every drug in trials for the treatment of AD. The United States has more clinical trials than any other nation; ClinicalTrials.gov includes the majority of agents currently in clinical trials for AD globally. Phase 1 trials are often conducted outside the United States and may not be captured on clinicaltrials.gov. Comparison to the World Health Organization registry suggests that clinicaltrials.gov includes 90% of worldwide Phase 3 trials; 86% of global Phase 2 trials; 43% of Phase 1 trials.
HIGHLIGHTS
In 2020, there are 121 unique therapies in clinical trials for Alzheimer's disease (AD) as registered on clinicaltrials.gov.
The largest number of drugs in the AD pipeline are putative disease‐modifying agents targeting disease onset or progression.
There is a growing number of repurposed agents (approved from non‐AD indication) in the pipeline; repurposed agents now comprise 43% of the pipeline.
The total number of participants required for currently recruiting trials is 31,314.
RESEARCH IN CONTEXT
Systematic review: We reviewed all drugs currently in clinical trials for Alzheimer's disease (AD) listed in the federal government database, ClinicalTrials.gov.
Interpretation: There are 121 agents in clinical trials for the treatment for AD. Ninety‐seven of these drugs are disease‐modifying agents intended to change the underlying biology of AD. Twelve of the drugs are putative cognitive enhancing agents, and 12 are being developed for the treatment of neuropsychiatric symptoms. Over the past 5 years, there has been an increase in the number of disease‐modification treatment candidates, greater diversification of the targets for drugs in the pipeline, more repurposed agents, and greater integration of biomarkers into development programs.
Future directions: Progress is occurring in new drug development for AD with potential new treatments for cognitive decline, insomnia, and psychosis. Trial methodology is being advanced, improved biomarkers to report on drug effects are emerging, and novel outcomes and designs reflect innovations that are assisting in development of new treatments for AD.
This pipeline report is based on trials present on ClinicalTrials.gov as of February 27, 2020; the tables and text of the review apply to the information available at that time. On average, clinical trial results are published in peer‐reviewed literature 25 months after completion of the trial, 16 and in our discussion we include recently published trial results of agents previously noted to be in the pipeline. We include all trials of all agents in Phase 1, 2, and 3; if trials are presented as Phase 1/2 or Phase 2/3 in the ClinicalTrials.gov database we use that terminology in the review. Our trial database tracks trial title; trial number in ClinicalTrials.gov; beginning date; projected end date; primary completion date; actual end date if completed or terminated; calculated trial duration; duration of treatment exposure; number of subjects planned for enrollment; number of arms of the study (usually a placebo arm and one or more treatment arms with different doses); whether a biomarker was described; whether the agent was repurposed; subject characteristics (inclusion and exclusion criteria); trial location; assessment tools used for outcome measures; and sponsorship (a biopharmaceutical company, National Institutes of Health [NIH], academic medical center, “other” entity such as a consortium, a philanthropic organization or other federal agencies, or a combination of these sponsors). We used the ClinicalTrials.gov labeling and included trials that were recruiting, active but not recruiting (eg, trials that have completed recruiting and are continuing with the exposure portion of the trial), enrolling by invitation (eg, open label extension trials), and not yet recruiting. We did not include trials listed as completed, suspended, unknown, or withdrawn. Information on these trials and reasons for their current status are often not publicly revealed. We do not include terminated trials in the analyses; we comment on them if the information is publicly available but is not yet reflected on ClinicalTrials.gov. We do not include trials of non‐pharmacologic therapeutic approaches such as cognitive therapies, caregiver interventions, supplements, and medical foods. We do not include trials of biomarkers; we note whether biomarkers were used in the trials discussed. We include stem cell therapies among the interventions reviewed (they are not integrated into Figure 1 nor included in the analyses).
FIGURE 1.
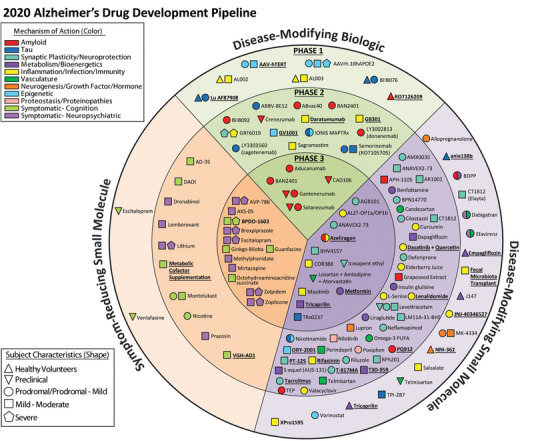
Agents in clinical trials for treatment of Alzheimer's disease in 2020 (from ClinicalTrials.gov as of February 27, 2020. The inner ring shows Phase 3 agents; the middle ring is comprised of Phase 2 agents; the outer ring presents Phase 1 compounds; agents in green areas are biologics; agents in purple are disease‐modifying small molecules; agents in orange areas are symptomatic agents addressing cognitive enhancement or behavioral and neuropsychiatric symptoms; the shape of the icon shows the population of the trial; the icon color shows the class of target for the agent. Agents underlined are new to the pipeline since 2019 (Figure by Mike de la Flor)
We used the search terms “Alzheimer's” as the condition/disease and “interventional studies” as the study type, and included trials in Phase 1, Phase 1/2, Phase 2, Phase 2/3, and Phase 3. Most Phase 1 trials include healthy participants and some trials list “healthy” as the condition/disease rather than listing both “Alzheimer's” and “healthy.” These trials may have escaped capture in our search.
Drug targets and mechanisms of action (MOA) are important aspects of this review. MOA of listed agents was determined from the information on ClinicalTrials.gov or from a comprehensive literature search. In a few cases, the mechanism is undisclosed and could not be identified in the literature; we note these agents as having an “unknown” or “undisclosed” MOA. We grouped the mechanisms into symptomatic agents or disease‐modifying therapies (DMTs). We divided the symptomatic agents into those that are putative cognitive enhancing agents or those that address neuropsychiatric and behavioral symptoms. DMTs were divided into small molecules or biologics, including immunotherapies. DMTs were further categorized using the CADRO system. The distinction between symptomatic and disease‐modifying agents can be arbitrary, and some agents may have both properties. For purposes of this review, we chose what appears to be the principal MOA.
3. RESULTS
3.1. Overview
As of February 27, 2020, there were 121 agents in 136 trials of AD therapies. Figure 1 shows all pharmacologic compounds currently in clinical trials for AD. Twelve (9.9%) agents in trials target cognitive enhancement and 12 (9.9%) are intended to treat neuropsychiatric and behavioral symptoms. There are 97 (80.2%) agents that intend to achieve disease modification; 16 (16.5%) of these have amyloid and 11 (11.3%) have tau as the primary target or as one of several effects seen in non‐clinical or previous clinical studies. Six of the anti‐amyloid agents are small molecules and ten are monoclonal antibodies or biological therapies. Anti‐tau agents include four small molecules and seven biologics.
3.2. Phase 3
In Phase 3 there are 29 agents in 36 trials (Figures 1 and 2, Table 1). There are 12 symptomatic agents (41%) in Phase 3; 4 cognitive enhancers (13.8%) and 8 targeting behavioral symptoms (27.6%). Of the 17 (59%) putative disease‐modifying agents in Phase 3, there are 5 biological therapies and 12 oral agents/small molecules. All five of the biological therapies, and one of the small molecules have amyloid as the primary or one of several targets (35.3% of DMTs). Other CADRO mechanisms represented among Phase 3 DMT molecules include tau (n = 1; 5.9%), inflammation/infection/immunity (n = 3; 17.6%), metabolism and bioenergetics (n = 2; 11.8%), vasculature (n = 2; 5.9%), and synaptic plasticity/neuroprotection (n = 4; 23.5%). Of the drugs with amyloid targets, there were five immunotherapies and one anti‐aggregation agent. Figure 2 shows the MOAs of agents in Phase 3. Six (35%) of the DMT agents are repurposed agents approved for use in another indication. 17 , 18 , 19 There are five new agents in the Phase 3 pipeline compared to 2019.
FIGURE 2.
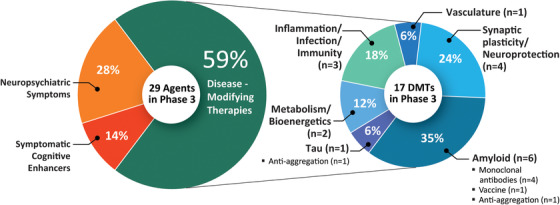
Mechanisms of action of agents in Phase 3 of the Alzheimer's disease drug development pipeline (ClinicalTrials.gov accessed February 27, 2020) (Figure by Mike de la Flor)
TABLE 1.
Agents in Phase 3 of Alzheimer's disease drug development (ClinicalTrials.gov accessed February 27, 2020)
| Agent | CADROmechanism class | Mechanism of action | Therapeutic purpose | Status(CT.gov ID) | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| Aducanumab | Amyloid | Monoclonal antibody directed at plaques and oligomers | Remove amyloid (DMT) |
Not yet recruiting |
Biogen | Mar 2020 | Sep 2023 |
|
AGB101 (low‐dose levetiracetam) |
Synaptic plasticity/neuroprotection | SV2A modulator | Improve synaptic function; reduce amyloid‐induced neuronal hyperactivity (DMT) |
Recruiting |
AgeneBio, NIA | Jan 2019 | Nov 2022 |
|
ALZT‐OP1 (cromolyn + ibuprofen) |
Inflammation | Mast cell stabilizer (cromolyn), anti‐inflammatory (ibuprofen) | Microglial modulation; promote microglial clearance of amyloid (DMT) |
Active, not recruiting |
AZTherapies | Sep 2015 | Dec 2020 |
|
ANAVEX2‐73 (blarcamesine) |
Synaptic plasticity/neuroprotection | Sigma‐1 receptor agonist, M2 autoreceptor antagonist | Enhances cell signaling to ameliorate oxidative stress, protein misfolding, mitochondrial dysfunction and inflammation (DMT) |
Recruiting a |
Anavex life sciences | Jul 2018 | Dec 2021 |
| AVP‐786 | Neurotransmitter receptors | Sigma 1 receptor agonist; NMDA receptor antagonist | Improve neuropsychiatric symptoms (agitation) |
Recruiting |
Avanir | Oct 2017 | Jun 2021 |
|
Recruiting, extension study |
Avanir | Dec 2015 | Jun 2022 | ||||
| AXS‐05 | Neurotransmitter receptors | Sigma 1 receptor agonist; NMDA receptor antagonist (dextromethorphan); dopamine‐norepinephrine reuptake inhibitor (bupropion) | Improve neuropsychiatric symptoms (agitation) |
Recruiting a |
Axsome therapeutics | Jul 2017 | Jun 2020 |
| Azeliragon | Amyloid, inflammation | RAGE antagonist | Reduce amyloid transport into the brain; reduce inflammation (DMT) |
Recruiting a |
vTv Therapeutics | Jun 2019 | Jul 2023 |
| BAN2401 | Amyloid | Monoclonal antibody directed at protofibrils | Reduce protofibrillar amyloid and amyloid plaques (DMT) |
Recruiting |
Eisai, Biogen | Mar 2019 | Mar 2024 |
|
BHV4157 (troriluzole) |
Synaptic plasticity/neuroprotection | Glutamate modulator; prodrug of riluzole | Reduce synaptic levels of glutamate; improve synaptic functioning (DMT) |
Active, not recruiting a |
Biohaven pharma, ADCS | Jul 2018 | Dec 2020 |
| BPDO‐1603 | Undisclosed | Undisclosed | Undefined mechanism (cognitive enhancer) |
Not yet recruiting |
Hyundai pharmaceutical | Feb 2020 | Mar 2023 |
| Brexpiprazole | Neurotransmitter Receptors | D2 receptor partial agonist, serotonin‐dopamine modulator | Improve neuropsychiatric symptoms (agitation) |
Recruiting a |
Otsuka | Aug 2018 | Nov 2021 |
|
Recruiting, extension study |
Otsuka | Oct 2018 | Aug 2021 | ||||
|
Recruiting |
Otsuka | May 2018 | Dec 2020 | ||||
|
Recruiting, extension study |
Otsuka | Nov 2018 | May 2021 | ||||
| CAD106 b | Amyloid | Amyloid vaccine | Remove amyloid (DMT) |
Active, not recruiting a |
Novartis, Amgen, NIA, Alzheimer's Association, Banner Alzheimer's Institute |
Nov 2015 | Mar 2025 |
| COR388 | Inflammation/infection | Bacterial protease inhibitor targeting gingipain produced by P. gingivalis | Reduce neuroinflammation and hippocampal degeneration (DMT) |
Recruiting a |
Cortexyme | Mar 2019 | Dec 2022 |
| Escitalopram | Neurotransmitter receptors | SSRI | Improve neuropsychiatric symptoms (agitation) |
Recruiting |
Johns Hopkins University, NIA | Jan 2018 | Aug 2022 |
| Gantenerumab | Amyloid | Monoclonal antibody directed at plaques and oligomers | Remove amyloid (DMT) |
Active, not recruiting |
Roche | Mar 2014 | Apr 2021 |
|
Active, not recruiting |
Roche | Nov 2010 |
Aug 2020 |
||||
|
Recruiting |
Roche | Jun 2018 | May 2023 | ||||
|
Recruiting |
Roche | Aug 2018 | May 2023 | ||||
| Gantenerumab and solanezumab | Amyloid | Monoclonal antibody directed at plaques and oligomers (gantenerumab); Monoclonal antibody directed at monomers (solanezumab) | Remove amyloid; reduce amyloid production (DMT) | Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | Dec 2012 | Mar 2021 | |
| Ginkgo biloba | Metabolism and bioenergetics | Plant extract with antioxidant properties | Improve brain blood flow and mitochondrial function (cognitive enhancer) |
Recruiting a |
Nanjing Medical University | Aug 2016 | Mar 2020 |
| Guanfacine | Neurotransmitter receptors | Alpha‐2 adrenergic agonist | Modulation of noradrenergic deficit (cognitive enhancer) |
Recruiting |
Imperial College London, UK National Institute of Health Research | Jan 2019 | Mar 2021 |
| Icosapent ethyl (IPE) | Synaptic plasticity/neuroprotection | Purified form of the omega‐3 fatty acid EPA | Improve synaptic function; reduce inflammation (DMT) |
Recruiting a |
VA Office of Research and Development, University of Wisconsin, Madison | Jun 2017 | Nov 2021 |
| Losartan and amlodipine and atorvastatin + exercise | Vasculature | Angiotensin II receptor blocker (losartan), calcium channel blocker (amlodipine), cholesterol agent (atorvastatin) | Vascular risk reduction; preservation of cognitive function (DMT) |
Active, not recruiting a |
University of Texas Southwestern | Sep 2016 | Mar 2022 |
| Masitinib | Inflammation/immunity | Tyrosine kinase inhibitor | Modulation of mast cell‐related inflammatory processes; reduce amyloid protein and tau phosphorylation (DMT) |
Active, not recruiting |
AB Science | Jan 2012 | Dec 2019 |
| Metformin | Metabolism and bioenergetics | Insulin sensitizer | Improve CNS glucose metabolism (DMT) |
Not yet recruiting a |
Columbia University, NIA, EMD Serono | Apr 2020 | Apr 2024 |
| Methylphenidate | Neurotransmitter receptors | Dopamine reuptake inhibitor | Improve neuropsychiatric symptoms (apathy) |
Active, not recruiting |
Johns Hopkins University, NIA | Jan 2016 | Jun 2020 |
| Mirtazapine | Neurotransmitter Receptors | Alpha‐1 antagonist | Improve neuropsychiatric symptoms (agitation) |
Recruiting |
University of Sussex | Jan 2017 | Jul 2020 |
| Octohydro‐aminoacridine Succinate | Neurotransmitter receptors | Acetylcholinesterase inhibitor | Improve acetylcholine signaling (cognitive enhancer) |
Recruiting |
Shanghai Mental Health Center, Changchun‐Huayang High‐tech, Jiangsu Sheneryang High‐tech | Aug 2017 | Feb 2021 |
| Solanezumab | Amyloid | Monoclonal antibody directed at monomers | Remove amyloid and prevent aggregation (DMT) |
Active, not recruiting |
Eli Lilly, ATRI | Feb 2014 | Jul 2022 |
|
Tricaprilin |
Metabolism and bioenergetics | Ketone body stimulant; caprylic triglyceride | Induce ketosis to improve mitochondrial function and neuronal metabolism (DMT) |
Not yet recruiting |
Cerecin | Jul 2020 | Dec 2022 |
|
TRx0237 (LMTX) |
Tau |
Tau protein aggregation inhibitor | Reduce tau mediated neuronal damage (DMT) |
Recruiting |
TauRx Therapeutics | Jan 2018 | Dec 2022 |
| Zolpidem and zoplicone | Neurotransmitter receptors | Positive allosteric modulator of GABA‐A receptors | Improve neuropsychiatric symptoms (sleep disorders) |
Recruiting |
Brasilia University Hospital | Oct 2016 | Dec 2020 |
Abbreviations: ADCS, Alzheimer's disease cooperative study; ATRI, Alzheimer's Therapeutic Research Institute; BACE, beta‐site amyloid precursor protein cleaving enzyme; CADRO, Common Alzheimer's Disease and Related Disorders Research Ontology; DMT, disease‐modifying therapy; EPA, eicosapentaenoic acid; GABA, gamma‐aminobutyric acid; NIA, National Institute on Aging; SSRI, selective serotonin reuptake inhibitor; SV2A, synaptic vesicle protein 2A
Note: Twenty‐nine agents in 36 Phase 3 clinical trials currently ongoing as of February 27, 2020 according to ClinicalTrials.gov.
Note: Bolded terms represent new agents into the 2020 Phase 3 pipeline since 2019.
Note: The following agents have been identified as terminated per company press releases and have been removed from the current pipeline although they are still listed as ongoing on ClinicalTrials.gov: CNP520/umibecestat (NCT03131453), E2609/elenbecestat (NCT02956486, NCT03036280).
Phase 2/3 trials.
CNP520 (umibecestat) has been removed from the GENERATION 1 trial.
DIAN‐TU trial has been completed and failed to meet its clinical outcomes for both gantenerumab and solanezumab. Secondary analyses are pending.
In Phase 3, there are 4 prevention trials enrolling cognitively normal participants; 11 trials enrolling participants with prodromal AD/MCI or prodromal‐to‐mild AD; 1 trial enrolling both cognitively normal patients and patients with MCI to mild AD; 11 trials of patients with mild‐to‐moderate AD; and 9 trials of patients with mild‐to‐severe AD.
Phase 3 trials included a mean of 554 participants and had a mean duration of 240 weeks (including the recruitment and the treatment period). The mean treatment exposure period was 64 weeks. DMT trials were longer and larger than trials of symptomatic agents with a mean duration of 279 weeks comprising 98 treatment weeks and including an average of 689 participants. The mean duration of cognitive enhancer trials was 162 weeks (19 treatment weeks), and they included an average of 428 participants. Trials of agents for behavioral symptoms had a mean duration of 193 weeks (15 treatment weeks) and included a mean of 342 subjects. For the average DMT trial, the recruitment period (average 160 weeks) substantially exceeds the exposure period (average 98 weeks) indicating that drug development timelines are more related to the success of recruitment than to the period required to assess the efficacy and safety of the agent.
When examined by trial population, DMT prevention trials are 375 weeks in duration (178 treatment weeks); trials for patients with MCI/prodromal/prodromal‐to‐mild AD are 275 weeks in duration (99 treatment weeks); and trials for patients with mild to moderate AD are 223 weeks in duration (38 treatment weeks).
3.3. Phase 2
Phase 2 has a larger number of therapies with more diverse mechanisms that are being assessed compared to the Phase 3 repertoire of agents. There are 65 agents in 73 trials (Figure 1 and 3, Table 2). Of these, there are ten symptomatic agents: six cognitive enhancers and four agents targeting behavioral symptoms. There are 55 potential DMTs in Phase 2 trials; 14 biologics and 41 small molecules. Four of the small molecules and four of the biologics have amyloid reduction as one of the mechanisms observed in non‐clinical studies (14.5% of DMTs). One small molecule and five biologics in Phase 2 target tau‐related processes as one of their mechanisms (10.9% of DMTs). There are 15 small molecules with synaptic plasticity/neuroprotection as one of the mechanisms (27.3% of DMTs). Four of the biologics and seven of the small molecules have inflammation/infection/immunity as their mechanism (20% of DMTs). Among other CADRO mechanisms represented in Phase 2, there were two agents targeting proteostasis/proteinopathies, six agents with metabolism and bioenergetic targets, four agents addressing vascular factors, one hormonal agent, and two epigenetic agents. Of the drugs with amyloid targets, there were four immunotherapies, two anti‐aggregation agents, one alpha‐secretase modulator, and one involving amyloid clearance. Figure 3 shows the MOAs of agents in Phase 2. There are five trials involving stem cell therapies in Phase 2 (see Table 4). Twenty‐three (42%) of the Phase 2 DMT candidates are repurposed agents approved for use in another indication. There are 14 new agents in the Phase 2 pipeline compared to 2019.
FIGURE 3.
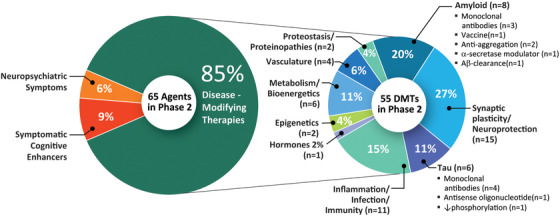
Mechanisms of action of agents in Phase 2 of the Alzheimer's disease drug development pipeline (ClinicalTrials.gov accessed February 27, 2020)(Figure by Mike de la Flor)
TABLE 2.
Agents in Phase 2 of Alzheimer's disease drug development (ClinicalTrials.gov accessed February 27, 2020)
| Agent | CADRO mechanism class | Mechanism of action | Therapeutic purpose | Status(CT.gov ID) | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| ABBV‐8E12 | Tau | Monoclonal antibody | Remove tau and prevent tau propagation (DMT) |
Active, not recruiting |
AbbVie | Oct 2016 | Jul 2021 |
|
Recruiting, extension study |
AbbVie | Mar 2019 | Aug 2026 | ||||
| ABvac40 | Amyloid | Active immunotherapy | Remove amyloid (DMT) |
Recruiting |
Araclon biotech | Feb 2018 | Feb 2022 |
| AD‐35 | Neurotransmitter Receptors | Acetylcholinesterase inhibitor | Improve acetylcholine signaling (cognitive enhancer) |
Recruiting |
Zhejiang hisun pharmaceutical | Oct 2018 | Jul 2020 |
|
Active, not recruiting |
Zhejiang hisun pharmaceutical | Dec 2018 | Jul 2021 | ||||
| AMX0035 | Neuroprotection, cell death | Combination of sodium phenylbutyrate and tauroursodeoxycholic acid | Reduce cell death associated with mitochondrial dysfunction; modulate neuroinflammation (DMT) |
Recruiting |
Amylyx pharmaceuticals, ADDF, Alzheimer's association | Aug 2018 | Sep 2020 |
|
ANAVEX 2‐73 (blarcamesine) |
Synaptic plasticity/Neuroprotection | Sigma‐1 receptor agonist; M2 autoreceptor antagonist | Enhance cell signaling to ameliorate oxidative stress, protein misfolding, mitochondrial dysfunction and inflammation (DMT) |
Active, not recruiting, extension study |
Anavex life sciences | Mar 2016 | Nov 2020 |
| APH‐1105 | Amyloid | Alpha‐secretase modulator | Reduce amyloid (DMT) |
Not yet recruiting |
Aphios | Jun 2021 | Dec 2022 |
| AR1001 | Synaptic plasticity/neuroprotection | PDE‐5 inhibitor | Improve synaptic plasticity (DMT) |
Recruiting |
AriBio Co. | Jan 2019 | Aug 2020 |
| BAN2401 | Amyloid | Monoclonal antibody directed at protofibrils | Remove amyloid protofibrils and reduce amyloid plaques (DMT) |
Active, not recruiting |
Eisai | Dec 2012 | Jul 2022 |
| Benfotiamine | Metabolism and bioenergetics | Synthetic thiamine | Improve glucose use (DMT) |
Active, not recruiting |
Burke Medical Research Institute, Columbia University, NIA, ADDF | Nov 2014 | Nov 2019 |
| BIIB092 | Tau | Monoclonal antibody targeting truncated form of tau | Remove tau and reduce tau propagation (DMT) |
Active, not recruiting |
Biogen | May 2018 | Mar 2024 |
| BPN14770 | Synaptic plasticity/neuroprotection |
PDE‐4 inhibitor |
Prolongs cAMP activity and improves neuronal plasticity (DMT) |
Active, not recruiting |
Tetra Discovery Partners | Apr 2019 | Feb 2020 |
| Candesartan | Vasculature | Angiotensin receptor blocker | Improve cerebrovascular functioning (DMT) |
Recruiting |
Emory University | Jun 2016 | Sep 2021 |
| Cilostazol | Synaptic plasticity/neuroprotection | PDE‐3 inhibitor | Improve cerebral circulation; reduce accumulation of amyloid and tau phosphorylation (DMT) |
Recruiting |
National cerebral and cardiovascular center, Japan | May 2015 | Dec 2020 |
|
Crenezumab |
Amyloid | Monoclonal antibody targeting soluble oligomers | Remove amyloid (DMT) |
Active, not recruiting |
Genentech, NIA Banner Alzheimer's Institute | Dec 2013 | Feb 2022 |
| CT1812 | Synaptic plasticity/neuroprotection | Sigma‐2 receptor antagonist; competes with oligomeric Aβ binding | Preserve synaptic plasticity and protect against Aβ‐induced synaptic toxicity (DMT) |
Recruiting |
Cognition therapeutics | Oct 2018 | Jul 2020 |
|
Active, not recruiting a |
Cognition therapeutics | Apr 2018 | Mar 2021 | ||||
| Curcumin + aerobic yoga | Inflammation | Herb with antioxidant and anti‐inflammatory properties | Decrease inflammation and oxidation‐related neurotoxicity (DMT) |
Active, not recruiting |
VA office of research and development | Jan 2014 | Mar 2020 |
| DAOI | Neurotransmitter receptors | NMDA receptor modulation | Enhance NMDA activity (cognitive enhancer) |
Recruiting |
Chang Gung Memorial Hospital, Taiwan | May 2015 | Dec 2019 |
| Dapagliflozin | Metabolism and bioenergetics | SGLT2 inhibitor |
Improve insulin sensitivity and CNS glucose metabolism (DMT) |
Recruiting a |
University of Kansas | Jan 2019 | Oct 2020 |
| Daratumumab | Inflammation/Immunity | Monoclonal antibody targeting CD38 | Immunomodulatory effects; regulates microglial activity (DMT) |
Recruiting |
Janssen, Northwell health | Nov 2019 | Jun 2022 |
| Dasatinib + Quercetin | Inflammation/Immunity | Tyrosine kinase inhibitor (dasatinib); flavonoid (quercetin) | Senolytic therapy approach to reduce senescent cells and tau aggregation (DMT) |
Not yet recruiting a |
The University of Texas Health Science Center at San Antonio, Mayo Clinic | Mar 2020 | Dec 2022 |
| Deferiprone | Synaptic plasticity/neuroprotection | Iron chelating agent | Reduce reactive oxygen species that damage neurons (DMT) |
Recruiting |
Neuroscience trials Australia | Jan 2018 | Dec 2021 |
| Dronabinol | Neurotransmitter Receptors | CB1 and CB2 endocannabinoid receptor partial agonist | Improve neuropsychiatric symptoms (agitation) |
Recruiting |
Mclean Hospital, Johns Hopkins University | Mar 2017 | Dec 2020 |
| Elderberry Juice | Inflammation | Antioxidant rich in anthocyanins | Improve mitochondrial function (DMT) |
Recruiting |
University of Missouri | Sep 2016 | Apr 2020 |
| GB301 | Inflammation/Immunity | Regulatory T cells | Promote immune cell homeostasis and reduce neuroinflammation (DMT) |
Not yet recruiting a |
GMP BIO, BHT Lifescience Australia | Dec 2019 | Dec 2021 |
| Grapeseed Extract | Amyloid | Polyphenolic compound; antioxidant | Anti‐oligomerization agent; prevents aggregation of amyloid and tau (DMT) |
Recruiting |
Mount Sinai School of Medicine, NCCIH | Nov 2014 | Sep 2020 |
| GRF6019 | Synaptic plasticity/neuroprotection, Inflammation | Blood plasma protein fractions from young adult donors | Young blood parabiosis can counteract inflammatory and age‐related degeneration in the brain (DMT) |
Active, not recruiting |
Alkahest | Jan 2019 | Mar 2020 |
| GV1001 | Epigenetic | hTERT peptide vaccine | Mimics the extra‐telomeric functions of hTERT to inhibit neurotoxicity, apoptosis, and the production of reactive oxygen species induced by Aβ (DMT) |
Not yet recruiting |
GemVax & Kael | Sep 2019 | Feb 2022 |
| Insulin glulisine intranasal | Metabolism and bioenergetics | Increase insulin signaling in the brain | Enhance cell signaling and growth; promote neuronal metabolism (DMT) |
Active, not recruiting |
HealthPartners Institute | Aug 2015 | Feb 2020 |
| IONIS MAPTRx (BIIB080) | Epigenetic, Tau | Antisense oligonucleotide targeting tau expression; MAPT RNA inhibitor | Reduce tau production (DMT) |
Active, not recruiting a |
Ionis pharmaceuticals |
Jun 2017 | May 2022 |
|
Lemborexant (E2006) |
Neurotransmitter receptors | Dual antagonist of orexin OX1 and OX2 receptors | Improve neuropsychiatric symptoms (sleep‐wake disorders) | Active, not recruiting (NCT03001557) | Eisai, purdue pharma | Dec 2016 | Apr 2020 |
| Lenalidomide | Inflammation/Immunity | Anti‐neoplastic; immunomodulator | Reduce inflammatory cytokines (TNF‐a, IL‐6, IL‐8); modulate both innate and adaptive immune responses (DMT) |
Not yet recruiting |
Cleveland Clinic, NIA | Feb 2020 | Sep 2024 |
| Levetiracetam | Synaptic plasticity/neuroprotection | SV2A modulator | Improve synaptic function; reduce amyloid‐induced neuronal hyperactivity (DMT) |
Recruiting |
University of California, San Francisco | Jun 2014 | Aug 2020 |
|
Recruiting |
UCB Pharma, University of Oxford, NHS Foundation Trust | Nov 2018 | Jan 2020 | ||||
|
Recruiting |
Medical College of Wisconsin, NIA | Apr 2019 | Mar 2020 | ||||
|
Recruiting |
Beth Israel Deaconess Medical Center | Aug 2019 | Nov 2023 | ||||
| Liraglutide | Metabolism and bioenergetics | Glucagon‐like peptide 1 receptor agonist | Improve CNS glucose metabolism (DMT) |
Active, not recruiting |
Imperial College London | Jan 2014 | Dec 2019 |
| Lithium | Neurotransmitter receptors | Ion channel modulator | Improve neuropsychiatric symptoms (agitation, aggression, psychosis) |
Recruiting |
New York State Psychiatric Institute, NIA | Jun 2014 | Jan 2020 |
| LM11A‐31‐BHS | Synaptic plasticity/neuroprotection, cell Death | Non‐peptide ligand of the p75 neurotrophin receptor (p75NTR) | Inhibits apoptosis signaling and reduces cell death; reduces Aβ‐induced synaptic impairment (DMT) |
Recruiting a |
Pharmatrophix, NIA |
Feb 2017 | Oct 2019 |
| L‐Serine | Inflammation | Naturally occurring dietary amino acid | Reduces brain inflammation and preserves nerve cells (DMT) |
Recruiting |
Dartmouth‐Hitchcock Medical Center | Mar 2017 | Dec 2020 |
| Lupron (leuprolide acetate depot) | Growth factors and hormones | GnRH receptor agonist | Reduces negative effects of elevated GnRH and gonadotropins on the brain (DMT) |
Not yet recruiting |
New York University | Feb 2020 | Feb 2026 |
|
LY3002813 (donanemab) |
Amyloid | Monoclonal antibody specific for pyroglutamic peptide fragment | Remove amyloid (DMT) |
Recruiting |
Eli Lilly | Dec 2017 | Nov 2021 |
|
LY3303560 (zagotenemab) |
Tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) |
Active, not recruiting |
Eli Lilly | Apr 2018 | Oct 2021 |
| Metabolic cofactor supplementation | Metabolism and bioenergetics | Mixture of N‐acetylcysteine, L‐carnitine tartrate, nicotinamide roboside, and serine | Enhance hepatic‐B oxidation and increase mitochondrial activity (cognitive enhancer) |
Recruiting |
Istanbul Medipol University Hospital, ScandiBio Therapeutics | Dec 2019 | Sep 2020 |
| Montelukast | Inflammation | Leukotriene receptor antagonist | Reduce inflammatory pathways and neuronal injury (cognitive enhancer) |
Recruiting (NCT03402503)–buccal film |
IntelGenx Corp. | Nov 2018 | Jul 2021 |
|
Recruiting (NCT03991988)–tablet |
Emory University | Sep 2019 | Aug 2021 | ||||
|
Neflamapimod (VX‐745) |
Synaptic plasticity/neuroprotection | p38 MAPK‐α inhibitor | Enhances endolysosomal function to reduce synaptic dysfunction (DMT) |
Recruiting |
EIP Pharma | Oct 2018 | Jan 2021 |
| Nicotinamide |
Epigenetic, Tau |
Histone deacetylase (HDAC) inhibitor; microtubule protein modulator | Reduce tau‐induced microtubule depolymerization and tau phosphorylation (DMT) |
Recruiting |
University of California, Irvine | Jul 2017 | Jun 2020 |
| Nicotine transdermal patch | Neurotransmitter receptors | Nicotinic acetylcholine receptor agonist | Enhance acetylcholine signaling (cognitive enhancer) |
Recruiting |
University of Southern California, NIA, ATRI, Vanderbilt University | Jan 2017 | Dec 2020 |
| Nilotinib | Proteostasis/proteinopathies | Tyrosine kinase inhibitor; Abl inhibition | Autophagy enhancer; promotes clearance of amyloid and tau proteins (DMT) |
Active, not recruiting |
Georgetown University | Jan 2017 | Feb 2020 |
| Omega‐3 PUFA | Vasculature | Fish oil concentrate standardized to long chain in n‐3 PUFA content | Reduces inflammation and glial activation; enhances amyloid removal (DMT) |
Active, not recruiting |
Oregon Health and Science University, NIA | May 2014 | Aug 2019 |
| ORY‐2001 (vafidemstat) | Epigenetic | HDAC demethylase (LSD1) inhibitor and MAO‐B inhibitor | Targets two enzymes: LSD1, which downregulates HDAC demethylase, and MAO‐B, which has neuroprotective properties (DMT) |
Recruiting |
Oryzon genomics, ADDF | May 2019 | Nov 2020 |
| Posiphen | Proteostasis/Proteinopathies | Selective inhibitor of APP to reduce amyloid; reduces synthesis of tau and α‐synuclein proteins | Reduce amyloid, tau and α‐synuclein production (DMT) |
Recruiting a |
QR Pharma, ADCS | Mar 2017 | Dec 2020 |
| Prazosin | Neurotransmitter receptors | Alpha‐1 adrenoreceptor antagonist | Improve neuropsychiatric symptoms (agitation) |
Recruiting |
ADCS, NIA | Jul 2019 | Dec 2022 |
| PTI‐125 | Amyloid | Filamin A protein inhibitor | Stabilize the interaction of soluble amyloid and the α7 nicotinic acetylcholine receptor, reducing tau hyperphosphorylation and synaptic dysfunction (DMT) |
Recruiting |
Cassava Sciences, NIA | Aug 2019 | Apr 2020 |
| PQ912 | Amyloid | Glutaminyl cyclase (QC) enzyme inhibitor | Reduce pyroglutamate Aβ (pGlu‐Aβ) production and amyloid plaques (DMT) |
Not yet recruiting |
Probiodrug, ADCS, NIA | Jan 2020 | Apr 2023 |
| Riluzole | Synaptic Plasticity/Neuroprotection | Glutamate receptor antagonist | Reduce glutamate‐mediated excitotoxicity (DMT) |
Active, not recruiting |
Rockefeller University | Nov 2013 | Sep 2020 |
| Rifaximin | Inflammation/Infection/Immunity | Antibiotic | Reduce pro‐inflammatory cytokines secreted by harmful gut bacteria (DMT) |
Recruiting |
Duke University, Bausch Health | Apr 2019 | Feb 2021 |
| RPh201 | Synaptic plasticity/neuroprotection | Undisclosed; extract from a botanical source | Neuroprotective from amyloid and vascular‐related neuropathology (DMT) |
Recruiting |
Regenera pharma | Mar 2018 | Jun 2020 |
|
Sargramostim (GM‐CSF) |
Inflammation/Immunity |
Granulocyte macrophage colony stimulating factor | Immune system stimulator that removes amyloid and improves synaptic function (DMT) |
Active, not recruiting |
University of Colorado, Denver, The Dana Foundation |
Mar 2011 | May 2020 |
|
Semorinemab (RO7105705) |
Tau | Monoclonal antibody | Remove extracellular tau (DMT) |
Active, not recruiting |
Genentech | Oct 2017 | Sep 2022 |
|
Recruiting |
Genentech | Jan 2019 | Jun 2023 | ||||
|
S‐equol (AUS‐131) |
Metabolism and bioenergetics | Agonist of non‐hormonal estrogen receptor B located on mitochondria | Mitochondrial function potentiation; improve synaptic functioning and neuronal survival (DMT) |
Recruiting |
Ausio pharmaceuticals | May 2017 | Jun 2020 |
| T3D‐959 | Metabolism and bioenergetics | Dual agonist of PPAR‐δ and PPAR‐γ | Regulate glucose and lipid metabolism; reduce insulin resistance (DMT) |
Recruiting |
T3D therapeutics, NIA | Feb 2020 | Aug 2021 |
|
T‐817MA (edonerpic) |
Synaptic Plasticity/Neuroprotection | Activates sigma receptors | Promotes neurite outgrowth, preserves synaptic plasticity; protects against amyloid toxicity (DMT) |
Recruiting |
Toyama Chemical | Dec 2019 | Oct 2022 |
| Tacrolimus | Synaptic plasticity/neuroprotection | Calcineurin inhibitor | Prevents amyloid‐induced dendritic spine loss and synaptic dysfunction (DMT) |
Not yet recruiting |
Massachusetts General Hospital | Mar 2020 | Dec 2021 |
| Telmisartan & Perindopril | Vasculature | Angiotensin II receptor blocker (telmisartan); angiotensin converting enzyme inhibitor (perindopril) | Improve vascular functioning (DMT) |
Recruiting |
Sunnybrook Health Sciences Centre, ADDF |
Mar 2014 | Mar 2021 |
| Thiethylperazine (TEP) | Amyloid | Activates transport protein ABCC1 | Remove amyloid (DMT) |
Active, not recruiting |
Immungenetics AG | Nov 2017 | Jul 2021 |
| Valacyclovir | Infection/Immunity | Antiviral against HSV‐1 and ‐2 infection | Prevents amyloid aggregation and plaque deposition (DMT) |
Recruiting |
Umea University | Dec 2016 | Apr 2020 |
|
Recruiting |
New York State Psychiatric Institute, NIH, NIA | Feb 2018 | Aug 2022 | ||||
| VGH‐AD1 | Undisclosed | Traditional Chinese herbal medicine | Undisclosed (cognitive enhancer) |
Not yet recruiting (NCT04249869) a |
Taipei Veterans General Hospital, Taiwan | Feb 2020 | Dec 2020 |
Abbreviations: Aβ, amyloid beta; ABCC1, ATP binding cassette subfamily C member 1; ADCS, Alzheimer's Disease Cooperative Study; ADDF, Alzheimer's Drug Discovery Foundation; APP, amyloid precursor protein; ATRI, Alzheimer's Therapeutic Research Institute; CADRO, Common Alzheimer's Disease and Related Disorders Research Ontology; cAMP, cycling adenosine monophosphate; CB, cannabinoid; DMT, disease‐modifying therapy; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; GnRH, gonadotropin‐releasing hormone; HSV, herpes simplex virus; hTERT, human telomerase reverse transcriptase; HT, hydroxytryptamine; MAPK, mitogen‐activated protein kinase; NCCIH, National Center for Complementary and Integrative Health; NIA, National Institute on Aging; NMDA, N‐methyl‐D‐aspartate; PDE, phosphodiesterase; PPAR, peroxisome proliferator‐activated receptor; PUFA, polyunsaturated fatty acids; SGLT2, sodium glucose transporter 2; SV2A, synaptic vesicle protein 2A.
Note: Sixty‐five agents in 73 Phase 2 clinical trials currently ongoing as of February 27, 2020 according to ClinicalTrials.gov.
Note: Bolded terms represent new agents into the 2020 Phase 2 pipeline since 2019.
Note: The following agents have been identified as completed/terminated per company press releases and have been removed from the current pipeline although they are still listed as ongoing on ClinicalTrials.gov: elenbecestat (NCT02322021), NA‐831 (NCT03538522).
Phase 1/2 trials.
TABLE 4.
Stem cell therapy in clinical trials for Alzheimer's disease (ClinicalTrials.gov accessed February 27, 2020)
| Agent | Phase | Status(CT.gov ID) | Sponsor | Subjectcharacteristics | Amyloid evidence at entry |
|---|---|---|---|---|---|
| Allogeneic human MSCs | 1 |
Recruiting |
University of Miami | Mild to moderate AD with MMSE of 20‐26 | Amyloid PET |
| Allogeneic human MSCs | 1 |
Active, not recruiting |
Longeveron | Mild to moderate AD with MMSE of 18‐24 | Amyloid PET |
| Autologous adipose‐derived MSCs | 1/2 |
Active, not recruiting |
Hope biosciences | Preclinical/MCI | Amyloid PET |
| Human umbilical cord blood‐derived MSCs (NEUROSTEM) | 1/2 |
Recruiting |
Medipost | Probable AD with KMMSE of 18‐26 | Amyloid PET |
| 1/2 |
Recruiting, extension study |
Medipost | Probable AD with KMMSE of 18‐26 | Amyloid PET | |
| Human umbilical cord blood‐derived MSCs | 1/2 |
Ongoing |
South China research center, Sun Yat‐Sen University | Probable AD with MMSE of 10‐26 | Not required |
| Allogeneic human MSCs | 2 |
Recruiting |
Stemedica | Mild to moderate AD with MMSE of 12‐24 | Amyloid PET |
Abbreviations: AD, Alzheimer's disease; KMMSE, Korea Mini‐Mental State Examination; MMSE, Mini‐Mental State Examination; MSC, mesenchymal stem cell; PET, positron emission tomography.
Two of the Phase 2 trials were prevention trials; 37 trials involved patients with MCI/prodromal or prodromal‐to‐mild AD; 32 were trials for mild‐to‐moderate AD; one trial was for patients with severe AD; and one trial included patients with mild‐to‐severe AD.
Phase 2 trials are shorter in duration and smaller in terms of participant number than Phase 3 trials. Phase 2 trials had a mean duration of 192 weeks, average treatment period of 43 weeks and included an average of 131 subjects in each trial. Phase 2 trials of DMTs had a mean duration of 201 weeks, average treatment period of 45 weeks, and included an average of 137 subjects in each trial.
3.4. Phase 1
Phase 1 has 27 agents in 27 trials (Figure 1, Table 3). There are two cognitive enhancers being assessed in Phase 1 and no agents addressing neuropsychiatric symptoms. There are 18 DMT small molecules and 7 DMT biologics being assessed in Phase 1. One of the small molecules and one of the biologics have amyloid as a primary target or one among several targets. Tau is targeted by two small molecules and two biologics in Phase 1 studies. Other CADRO mechanisms represented in Phase 1 include targeting inflammation/infection/immunity (n = 6), metabolism/bioenergetics (n = 3), growth factors/hormones (n = 2), epigenetics (n = 3), neurogenesis (n = 1), vasculature (n = 1), synaptic plasticity/neuroprotection (n = 1), and combination of metabolism/bioenergetics and vasculature (n = 2) as the primary or one of a combination of effects. There are two stem cell therapy trials in Phase 1 (Table 4).
TABLE 3.
Agents in Phase 1 of Alzheimer's disease drug development (ClinicalTrials.gov accessed February 27, 2020)
| Agent | CADRO mechanism class | Mechanism of action | Therapeutic purpose | Status(CT.gov ID) | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| AAV‐hTERT | Epigenetic | hTERT delivered via transduction using AAV | Extending telomeres may benefit AD; reduce amyloid‐induced neurotoxicity; effects on multiple cellular pathways (DMT) |
Recruiting |
Libella gene therapeutics | Oct 2019 | Jan 2021 |
| AAVrh.10hAPOE2 | Epigenetic | Serotype rh. 10 AAV gene transfer vector expressing the cDNA coding for ApoE2 | Conversion of the ApoE protein isoforms in the CSF of ApoE4 homozygotes from ApoE4 to ApoE2‐ApoE4 (DMT) |
Recruiting |
Cornell University | Oct 2019 | Dec 2021 |
| AL002 | Inflammation | Monoclonal antibody targeting TREM2 receptors | Promote microglial clearance of amyloid and other toxic proteins (DMT) |
Recruiting |
Alector | Nov 2018 | Mar 2020 |
| AL003 | Inflammation | Monoclonal antibody targeting SIGLEC‐3 (CD33) | Reactivates microglia and immune cells in the brain; improve microglial clearance of toxic proteins (DMT) |
Recruiting |
Alector | Mar 2019 | Jul 2020 |
| Allopregnanolone (Allo) | Growth factors/hormones | GABA‐A receptor modulator; neurosteroid | Promote neurogenesis; reduce inflammation (DMT) |
Recruiting |
University of Southern California, University of Arizona, Alzheimer's Association | Oct 2019 | Oct 2020 |
| anle138b | Tau | Aggregation inhibitor |
Prevents/reduces aggregation of tau, α‐synuclein and prion proteins (DMT) |
Recruiting |
MODAG, quotient sciences | Dec 2019 |
Oct 2020 |
| BDPP (bioactive dietary polyphenol preparation) | Metabolism and bioenergetics, amyloid | Combination of grape seed polyphenolic extract and resveratrol | Prevents amyloid and tau aggregation (DMT) |
Recruiting |
Johns Hopkins University, Mount Sinai School of Medicine | Jun 2015 | Jun 2020 |
| BIIB076 | Tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) |
Active, not recruiting |
Biogen | Feb 2017 | Mar 2020 |
| CT1812 | Synaptic plasticity/neuroprotection | Sigma‐2 receptor antagonist; competes with oligomeric Aβ binding | Preserve synaptic plasticity and protect against Aβ‐induced synaptic toxicity (DMT) |
Recruiting |
Cognition therapeutics | May 2018 | Mar 2021 |
| Dabigatran | Metabolism and bioenergetics, vasculature | Direct thrombin inhibitor | Reduce neurovascular damage (DMT) |
Not yet recruiting |
University of Rhode Island, ADDF, boehringer ingelheim | Nov 2018 | Dec 2021 |
| Efavirenz | Metabolism and bioenergetics, vasculature | Antiretroviral; non‐nucleoside reverse transcriptase inhibitor | Promote cholesterol removal from the brain and enhance amyloid reduction (DMT) |
Recruiting |
Case Western Reserve University, Cleveland Medical Center, Massachusetts General Hospital | May 2018 | Dec 2020 |
| Empagliflozin | Metabolism and bioenergetics | SGLT2 inhibitor | Improve glycemic control and enhance neuronal function (DMT) |
Recruiting |
NIA | Mar 2019 | Dec 2022 |
| Escitalopram and Venlafaxine | Neurotransmitter receptors | SSRI (escitalopram), SNRI (venlafaxine) | Improve neurotransmission (cognitive enhancer) |
Recruiting |
New York University | Jul 2017 | Jan 2020 |
| Fecal microbiota transplant (FMT) | Inflammation | Oral FMT intervention | Improve gut microbiota; reduce AD pathology (DMT) |
Recruiting |
University of Wisconsin, Madison | Nov 2019 | May 2022 |
| J147 | Metabolism and bioenergetics | Mitochondrial ATP synthase inhibitor | Increases use of free fatty acid to increase ketones for energy use; vascular protective effects (DMT) |
Recruiting |
Abrexa |
Jan 2019 | Jan 2020 |
| JNJ‐40346527 | Inflammation | CSF‐1R antagonist | Attenuates microglial proliferation and neurodegeneration (DMT) |
Not yet recruiting |
Janssen, University of Oxford | Nov 2019 | Nov 2021 |
| Lu AF87908 | Tau | Monoclonal antibody | Remove tau (DMT) |
Recruiting |
Lundbeck | Sep 2019 | Mar 2021 |
| MK‐4334 | Growth Factors and Hormones | Corticosteroid | Reduce inflammation (DMT) |
Not yet recruiting |
Merck | Sep 2019 | Feb 2020 |
| NNI‐362 | Neurogenesis | Nerve cell proliferation | Enhance neurogenesis; activates progenitor cells (DMT) |
Recruiting |
Neuronascent, NIA | Aug 2019 | Apr 2020 |
| RO7126209 | Amyloid | Monoclonal antibody; “brain‐shuttle” gantenerumab | Remove amyloid (DMT) |
Recruiting |
Roche | Aug 2019 | Jul 2020 |
| Salsalate | Inflammation | Non‐steroidal anti‐inflammatory | Reduce inflammation and neuronal injury (DMT) |
Recruiting |
University of California, San Francisco | Jul 2017 | Oct 2019 |
| Telmisartan | Vasculature | Angiotensin II receptor blocker | Improve vascular function with effects on amyloid pathology (DMT) |
Recruiting |
Emory University | Apr 2015 | Jun 2020 |
| TPI‐287 | Tau |
Tubulin‐binding and microtubule‐stabilization |
Reduce tau‐mediated cellular damage (DMT) |
Active, not recruiting |
University of California, San Francisco |
Nov 2013 |
Nov 2019 |
|
Tricaprilin (AC‐DS‐03) |
Metabolism and bioenergetics | Caprylic triglyceride; ketone body stimulant | Induce ketosis to improve mitochondrial metabolism (DMT) |
Recruiting |
Cerecin | Aug 2019 | Aug 2020 |
|
Not yet recruiting |
Cerecin | Feb 2020 | Jul 2020 | ||||
| Vorinostat | Epigenetic | Histone deacetylase (HDAC) inhibitor | Neuroprotection and enhanced synaptic plasticity (DMT) |
Recruiting |
German Center for Neurodegenerative Diseases, University Hospital, Bonn, University of Gottingen | Sep 2017 | Mar 2022 |
| XPro1595 | Inflammation | TNF inhibitor | Reduce neuroinflammation |
Recruiting |
Immune bio, Alzheimer's association | Nov 2019 | Dec 2020 |
Abbreviations: AAV, adeno‐associated virus; Aβ, amyloid beta; ADDF, Alzheimer's Drug Discovery Foundation; ApoE, apolipoprotein E; CADRO, Common Alzheimer's Disease and Related Disorders Research Ontology; CSF, cerebrospinal fluid; CSF‐1R, colony‐stimulating factor 1 receptor; DMT, disease‐modifying therapy; GABA, gamma‐aminobutyric acid; hTERT, human telomerase reverse transcriptase; NIA, National Institute on Aging; RIPK1, receptor‐interacting serine/threonine‐protein kinase 1; SGLT2, sodium glucose co‐transporter 2; SIGLEC‐3, sialic acid‐binding Ig‐like lectin 3; SNRI, serotonin‐norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TREM2, triggering receptor expressed on myeloid cells 2.
Note: Twenty‐seven agents in 27 Phase 1 clinical trials currently ongoing as of February 27, 2020 according to ClinicalTrials.gov.
Note: Bolded terms represent new agents into the 2020 Phase 1 pipeline since 2019.
Phase 1 trials have an average duration of 116 weeks (recruitment and treatment period) and include a mean number of 43 participants in each trial.
3.5. Trial sponsors
Across all trials, 46% are sponsored by the biopharma industry, 39% by academic medical centers (with funding from NIH, industry, and/or other entities), and 15% by others. Table 5 shows the sponsor of agents in each phase of development.
TABLE 5.
Trial sponsor for each phase of Alzheimer's disease drug development (ClinicalTrials.gov accessed February 27, 2020)
| N of trials (%) | ||||
|---|---|---|---|---|
| Sponsor | Phase 1 | Phase 2 | Phase 3 | Total |
| Biopharma industry | 12 (44%) | 28 (38%) | 22 (61%) | 62 (46%) |
| Academic medical centers | 12 (44%) | 33 (45%) | 8 (22%) | 53 (39%) |
| NIH | 1 (4%) | 0 | 0 | 1 (1%) |
| Other federal agencies (eg, VA) | 0 | 3 (4%) | 1 (3%) | 4 (3%) |
| Industry and NIH | 1 (4%) | 3 (4%) | 1 (3%) | 5 (4%) |
| Industry and consortium/foundation | 1 (4%) | 3 (4%) | 2 (6%) | 6 (4%) |
| NIH and consortium/foundation | 0 | 1 (1%) | 0 | 1 (1%) |
| NIH and consortium/foundation and industry | 0 | 2 (3%) | 1 (3%) | 3 (2%) |
| NIH and consortium/foundation and industry and academic | 0 | 0 | 1 (3%) | 1 (3%) |
Abbreviations: NIH, National Institutes of Health; VA, veterans affairs.
Repurposed agents have promise to accelerate drug development because the results of non‐clinical studies, dosing, safety, tolerability, formulation, manufacturing, and distribution are known. 17 , 18 , 19 Of the 57 trials for 52 repurposed agents across all phases, 9 trials (16%) are by the biopharma industry, 42 trials (74%) are hosted by academic medical centers (with funding from NIH, industry, and/or other entities), and 6 trials (11%) are by other entities. Figure 4 shows the sponsor of repurposed agents compared to non‐repurposed agents in Phase 2 and Phase 3 trials of the AD pipeline.
FIGURE 4.
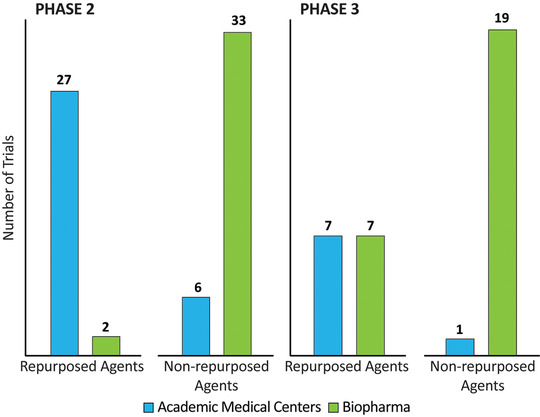
Trial sponsor for repurposed versus non‐repurposed agents in the Alzheimer's disease pipeline (ClinicalTrials.gov accessed February 27, 2020) (Figure by Mike de la Flor)
3.6. Trial locations
Clinical trials require many sites to participate in trials to recruit a sufficient number of participants in a short enough period of time to make the trial feasible. 20 Figure 5 shows that 30% of Phase 3 and 61% of Phase 2 trials include sites only in North America; 28% of Phase 3 and 25% of Phase 2 trials involve only non‐North American clinical trial sites; and 42% of Phase 3 and 14% of Phase 2 trials include sites in both North America and non‐North American countries.
FIGURE 5.
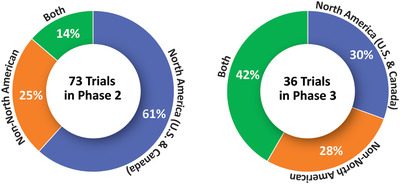
Location of sites for Phase 2 and Phase 3 trials in the Alzheimer's disease drug development pipeline (ClinicalTrials.gov accessed February 27, 2020) (Figure by Mike de la Flor)
3.7. Clinical trial recruitment
When considering the total number of sites involved in Phase 3 DMT trials, the total number of participants to be recruited, and the average number of months allowed for recruitment, the calculated average productivity of sites is 0.19 participants/site/month. In trials of symptomatic agents in Phase 3, the calculated average productivity of sites is 0.25 participants/site/month. Some types of trials are more difficult to enroll: Phase 3 prevention trials involving asymptomatic at‐risk individuals treated with disease‐modifying agents recruit at a rate of 0.26 participants/site/month; prodromal/mild AD trials recruit at a rate of 0.16 participants/site/month; and mild‐to‐moderate AD dementia trials have a rate of 0.29 participants/site/month. The total number of participants required for all currently recruiting trials is 31,314 participants.
3.8. Trial completion date
Thirty‐six trials are listed as “completed” on ClinicalTrials.gov since our last report in 2019. The actual completion date of a trial is typically much later than the anticipated completion date at trial initiation. The mean difference between the actual completion date and the anticipated completion date was 30 weeks for completed trials in Phase 1, 32 weeks for Phase 2, and 72 weeks for Phase 3, respectively.
3.9. Biomarkers
Table 6 shows the biomarkers used as outcome measures in current Phase 2 and Phase 3 AD clinical trials of DMTs as described in the federal website; not all trial descriptions in ClinicalTrials.gov note if biomarkers are included in the trial.
TABLE 6.
Biomarkers as outcome measures in Phase 2 and Phase 3 disease‐modifying therapies trials (ClinicalTrials.gov accessed February 27, 2020)
| N of trials (%) | ||
|---|---|---|
| Biomarker | Phase 2 | Phase 3 |
| CSF amyloid | 15 (25%) | 10 (48%) |
| CSF tau | 17 (28%) | 9 (43%) |
| FDG‐PET | 7 (11%) | 1 (5%) |
| Vmri | 8 (13%) | 8 (38%) |
| Plasma amyloid | 7 (11%) | 2 (10%) |
| Plasma tau | 2 (3%) | 1 (5%) |
| Amyloid PET | 5 (8%) | 7 (33%) |
| Tau PET | 4 (7%) | 3 (14%) |
Abbreviations: CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose; PET, positron emission tomography; vMRI, volumetric magnetic resonance imaging.
AD biomarkers served as secondary outcome measures in 14 Phase 3 trials of DMTs and 27 Phase 2 trials of DMTs. The most common biomarkers used were cerebrospinal fluid (CSF) amyloid, CSF tau, volumetric magnetic resonance imaging (MRI), and amyloid positron emission tomography (PET). Tau imaging is increasingly involved in AD drug development programs and was included as a secondary outcome in three (14%) Phase 3 and four (7%) Phase 2 trials of DMTs. Of the 21 Phase 3 DMT trials, 5 (24%) used amyloid PET as an entry criterion, 2 (10%) used CSF‐amyloid, and 4 (19%) used either amyloid PET or CSF‐amyloid. Nine (15%) of 61 Phase 2 DMT trials used amyloid PET as an entry criterion, nine (15%) used CSF‐amyloid, and nine (15%) used either amyloid PET or CSF‐amyloid. Ten (47%) DMT trials in Phase 3 and 34 (55%) in Phase 2 did not require biomarker confirmation of AD for trial entry.
3.10. Trial entry criteria and primary outcomes
The initiation of prevention trials in preclinical patients and treatment trials of patients with very early symptoms of AD has led to new trial population definitions and novel outcome measures (Table 7). Entry criteria and outcomes must be appropriate to identify the population of interest. As shown in Table 7, trials with similar descriptions of the population (eg, prodromal AD/mild AD dementia) have slightly different Mini‐Mental State Examination (MMSE) criteria for entry into the study, creating slightly different cohorts and possibly different disease trajectories. The Clinical Dementia Rating–sum of boxes (CDR‐sb) is the most widely used outcome for trials of prodromal or prodromal/mild disease, but some trials have dual outcomes traditionally used in AD dementia trials. There is substantial heterogeneity among the instruments used as primary outcomes in prevention trials although the elements of the tools overlap.
TABLE 7.
Trial entry criteria and primary outcome measures for Phase 2/3 and 3 disease‐modifying therapies trials (ClinicalTrials.gov accessed February 27, 2020)
| Agent | Sponsor | CT.gov ID | Trial name | Subject population | MMSE | Primary outcome assessment tool |
|---|---|---|---|---|---|---|
| Aducanumab | Biogen | EMBARK | MCI due to AD or mild AD | — | Safety | |
| AGB101 | AgeneBio, NIA | NCT03486938 | HOPE4MCI | MCI due to AD | 24–30 | CDR‐SB |
| ALZT‐OP1 | AZTherapies | NCT02547818 | COGNITE | Early AD | — | CDR‐SB |
|
ANAVEX2‐73 (blarcamesine) |
Anavex Life Sciences | NCT03790709 |
ANAVEX2‐73‐AD‐004 |
MCI due to AD or mild AD | 20–28 | ADAS‐Cog, ADCS‐ADL |
| Azeliragon | vTv therapeutics | Elevage | Mild AD with elevated HbA1c | 21–26 | ADAS‐Cog14, CDR‐SB | |
| BAN2401 | Eisai, biogen | NCT03887455 | Clarity AD | MCI due to AD or mild AD | 22–30 | CDR‐SB |
|
BHV4157 (troriluzole) |
Biohaven pharma, ADCS | NCT03605667 | T2 Protect | Mild to moderate AD | — | ADAS‐Cog11, CDR‐SB |
| CAD106 | Novartis, banner Alzheimer's institute, NIA, Alzheimer's association, amgen | NCT02565511 | Generation S1 | Preclinical; homozygous ApoE4 genotype | ≥24 | Time to diagnosis of MCI or dementia due to AD, APCC |
| COR388 | Cortexyme | GAIN | Mild to moderate AD | 12–24 | ADAS‐Cog11, CDR‐SB | |
| Gantenerumab | Roche | NCT02051608 | Marguerite road | Mild AD | — | ADAS‐Cog13, ADCD‐ADL |
| NCT01224106 | SCarlet road | Prodromal AD | ≥24 | CDR‐SB | ||
| NCT03444870 | GRADUATE I | Prodromal or mild AD | ≥22 | CDR‐SB | ||
| NCT03443973 | GRADUATE II | Prodromal or mild AD | ≥22 | CDR‐SB | ||
| Gantenerumab and solanezumab | Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | NCT01760005 | DIAN‐TU‐001 | Carriers of dominantly inherited AD mutations who are cognitively normal or with MCI or mild dementia | — | DIAN‐TU cognitive composite score |
| Icosapent ethyl | VA office of research and development, University of Wisconsin, Madison | NCT02719327 | BRAVE‐EPA | Cognitively normal with parental history of AD and increased prevalence of ApoE4 | — | Brain blood flow using arterial spin‐labeling MRI |
| Losartan and amlodipine and atorvastatin + exercise | University of Texas Southwestern | NCT02913664 | rrAD | Preclinical; family history of dementia or subjective cognitive decline with high blood pressure | ≥26 | ADCS‐PACC, NIH‐TB Cognition Battery |
| Mastinib | AB Science | NCT01872598 | AB09004 | Mild to moderate AD | 12–25 | ADCS‐ADL, ADAS‐Cog |
| Metformin | Columbia University, NIA, EMD serono | NCT04098666 | MAP | aMCI, overweight or obese | ≥24 | FCSRT |
| Solanezumab | Eli Lilly, ATRI | NCT02008357 | A4 | Preclinical with amyloid evidence | 25–30 | ADCS‐PACC |
| Tricaprilin | Cerecin | NCT04187547 | AC‐19‐020 | Mild to moderate AD who are ApoE4 non‐carriers | 14–26 | ADAS‐Cog11 |
| TRx0237 | TauRx therapeutics | NCT03446001 | LUCIDITY | Probable AD or MCI due to AD | 16–27 | ADAS‐Cog11, ADCS‐ADL |
Abbreviations: ADAS‐Cog, Alzheimer's Disease Assessment Scale‐Cognitive Subscale; ADCS‐ADL, Alzheimer's Disease Cooperative Study‐Activities of Daily Living; ADCS‐PACC, Alzheimer's Disease Cooperative Study‐Preclinical Alzheimer Cognitive Composite; APCC, Alzheimer's Prevention Initiative Composite Cognitive; ApoE, apolipoprotein E; ATRI, Alzheimer's Therapeutic Research Institute; CDR‐SB, Clinical Dementia Eating‐Sum of Boxes; FCSRT, Free and Cued Selective Reminding Test; HbA1c, hemoglobin A1c; MCI, mild cognitive impairment; NIA, National Institute on Aging; NIH‐TB, National Institutes of Health toolbox.
3.11. Longitudinal observations
Figure 6 shows the pipeline activity over the past 5 years by CADRO category. Amyloid and tau mechanisms are further divided into small molecule therapies and monoclonal antibodies. There is a trend for increasing diversification of the pipeline with a greater number of tau‐targeted, anti‐inflammatory, synaptic and neuroprotective, metabolic, neurogenesis, and epigenetic agents over the 5 years of observation.
FIGURE 6.
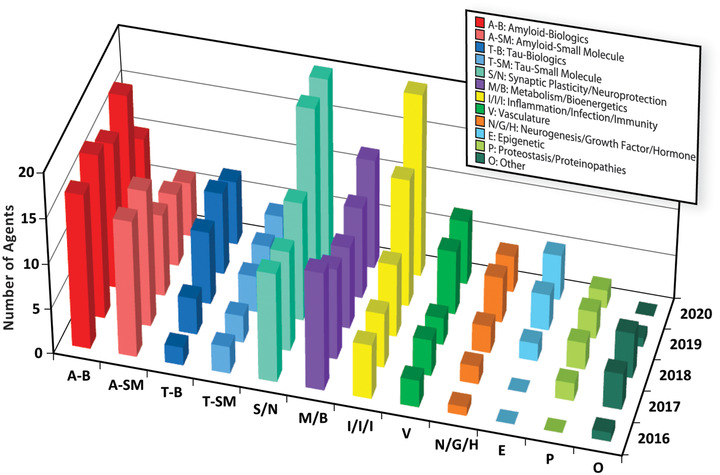
Targets of Alzheimer's disease therapeutics by Common Alzheimer's Disease and Related Disorders Research Ontology (CADRO) category: 2016–2020 (ClinicalTrials.gov accessed February 27, 2020) (Figure by Mike de la Flor)
4. DISCUSSION
The U.S. FDA approved 53 new novel therapies in 2019, including 48 new molecular entities and 3 therapies and 2 vaccines representing biological products. 21 Twelve agents for neurological disorders were among the 48 approved therapies. There were three sleep disorder treatments; three drugs for psychiatric conditions; two anti‐migraine therapies; two drugs for childhood neuromuscular disorders; and one treatment each for partial onset seizures, Parkinson's disease with excessive “off” episodes, and relapsing multiple sclerosis. The two neuromuscular disorder therapies and the agent for relapsing multiple sclerosis can be regarded as DMTs. The approved diagnostic tests included 18‐F fluorodopa PET for the diagnosis of parkinsonian disorders. There were no treatments approved in the United States for AD and no DMTs for any primary neurodegenerative disorder.
GV‐971 (Oligomannate) became the first drug approved for treatment of AD since 2003. 22 , 23 , 24 The agent was approved in China for improvement of cognition in patients with mild‐to‐moderate AD dementia not treated with cholinesterase inhibitors or memantine based on a Phase 3 clinical trial that demonstrated a significant drug‐placebo difference on the Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS‐cog) and a trend toward a difference on the Clinician Interview‐Based Impression of Change with caregiver input (CIBIC‐plus). These outcomes satisfied the requirements of the National Medical Products Administration (NMPA; Chinese equivalent of the FDA) for approval as an AD therapy. Non‐clinical studies suggest that GV‐971 has an effect on the dysbiosis of the gut microbiome to decrease secreted amino acids (phenylalanine and isoleucine) that stimulate proliferation of peripheral pro‐inflammatory T‐helper cells and cross the blood‐brain barrier and contribute to neuroinflammation. 25
Aducanumab is a monoclonal antibody developed to remove fibrillar amyloid beta (Aß) as a means of ameliorating progression of cognitive impairment in AD. The agent had a successful Phase 1B trial demonstrating a dose‐ and time‐dependency for Aß reduction with a beneficial impact on some clinical measures after 12 months of treatment. 26 Two large Phase 3 clinical trials were initiated to confirm the clinical and biological effects. A planned futility analysis concluded that continuing the trials was futile and both were stopped. Further analyses that included participants exposed for longer periods of time at higher antibody doses indicated that aducanumab reduced brain amyloid and decreased the rate of decline on the CDR‐sb, the pre‐specified primary outcome. On the basis of these analyses, the sponsor has initiated discussions with the FDA regarding marketing approval for aducanumab. 27
BAN2401, a monoclonal antibody targeting prefibrillar amyloid, 28 completed a Phase 2 trial in 2018 with evidence of amyloid reduction and slowing of cognitive decline. 29 This agent has now entered Phase 3. Crenezumab, a monoclonal antibody targeting oligomers, had a Phase 2 trial suggesting efficacy in participants with mild AD; 30 , 31 a Phase 3 program was recently halted due to futility. Crenezumab is being assessed in a prevention trial involving a Colombian kindred with autosomal dominant AD. 32 Gantenerumab is being assessed in Phase 3 trials after a trial in prodromal disease stopped for futility suggested that higher doses might be efficacious. 33 Gantenerumab and solanezumab failed to show drug‐placebo differences in clinical outcomes of the Dominantly Inherited Alzheimer Disease–Treatment Unit (DIAN‐TU) study of individuals with autosomal dominant AD. Biomarker studies showed that gantenerumb decreased brain amyloid and ameliorated the increase of CSF markers of neurodegeneration.
Several tau‐targeting monoclonal antibodies are in trials for AD and some are in trials for other tauopathies (ABBV‐8E12, BIIB076, BIIB092, Lu AF87908, LY3303560 [zagotenemab], RO7105705 [semorinemab]). A trial of ABBV‐8E12 in progressive supranuclear palsy (PSP) was recently halted for futility; the antibody remains in trials for AD. A trial of the tau antibody, gosuranemb, in a PSP population, failed to meet its primary endpoints and development of this antibody for tauopathies has been halted.
Several trials of beta‐site amyloid precursor protein cleaving enzyme (BACE) inhibitors were stopped for futility or toxicity. Verubecestat trials of mild‐to‐moderate AD and prodromal AD were discontinued for futility. 34 , 35 Atabecestat was stopped for hepatotoxicity. Umibecestat (CNP520) was stopped when it was found to cause accelerated cognitive decline. Elenbecestat trials were suspended for an unfavorable harm/benefit ratio. Analyses of data from the verubecestat trial in prodromal AD showed increased cognitive decline and greater atrophy on volumetric MRI in the active treatment group. 35 Retrospective analyses of atabecestat also demonstrated increased cognitive impairment compared to the placebo group. While it is possible that less complete BACE inhibition or use of BACE inhibitors earlier in the course of the AD continuum might define a niche for these agents, the cumulative evidence of cognitive toxicity makes it difficult to design development programs that ensure participant safety.
A 1‐year, double‐blind, placebo‐controlled Phase 2 trial of edonerpic maleate (T‐817MA)—an agent that in animal models protected against amyloid‐induced neurotoxicity, promoted neurite outgrowth, and preserved hippocampal synapses in tau transgenic mice—had no clinical effect in participants with mild to moderate AD. 36 A Phase 2 trial of T‐817MA has been initiated to evaluate the drug's effect on CSF‐tau in patients with MCI due to AD or mild AD.
Intepirdine and idalopirdine are 5‐HT6 inhibitors that failed to establish efficacy in recent trials and development of these agents was stopped. 37 In both cases, dosing issues remained unresolved by the trials. Masuperdine (SUVN‐502), another 5‐HT6 inhibitor, completed a Phase 2 clinical trial in 2019 and was shown not to be efficacious for cognition in patients receiving donepezil and memantine.
Xanamem an 11‐ß‐hyrodroxysteroid receptor inhibitor whose development program was based on the adverse effects of steroids on hippocampal function and the evidence of steroid dysregulation in AD 38 failed in a Phase 2 trial to establish a drug‐placebo difference. The negative outcome was similar to that observed with an earlier drug in this class, ABT‐854. 39
Infections and inflammation are targeted by several drugs in the current pipeline. COR388 antagonized gingipain produced by P. gingivalis and blocked Aβ1‐42 production, reduced neuroinflammation, and rescued neurons in the hippocampus of mice. 40 Substantial evidence links herpes virus infection to AD and valacyclovir targets this relationship. 41 GV‐971 and rifaxamin may reduce brain inflammation through effects on the microbiome. 25 These trials are based on theories that infections or inflammation induced in other ways are central to causing or exacerbating AD. The outcomes of the trials will help inform these underlying concepts.
Treatments for neuropsychiatric symptoms of AD had successes in 2019/2020. The Harmony trial of pimavanserin for dementia‐related psychosis (DRP) was discontinued early on the basis of a robust drug‐placebo difference in patient relapse after withdrawal from drug or placebo in a relapse prevention trial. This trial was unique in including five types of dementia with psychosis–AD, Parkinson's disease dementia, dementia with Lewy bodies, frontotemporal dementia, vascular dementia–and using a randomized withdrawal design to demonstrate drug efficacy. 42 There are several ongoing trials of agitation in AD. A recently reported trial of nabilone (a partial agonist of cannabinoid receptors 1 and 2) showed reduced agitation and improvement on the MMSE but poorer cognition on the Severe Impairment Battery and sedation in association with active treatment compared to placebo. 43 A fixed dose and a flexible dose study of brexpiprazole for agitation in AD demonstrated that in both studies the 2 mg dose produced a significant reduction in agitation while the 1 mg dose did not. 44 A confirmatory trial is in progress. Two trials of dextromethorphan/quinidine that had a positive Phase 2 trial 45 failed to reduce agitation in a Phase 3 program. The selective serotonin reuptake inhibitor (SSRI) citalopram has previously shown to reduce agitation in AD but also prolonged the QT interval. An ongoing study will assess the effects of the S(+)‐enantiomer escitalopram using an identical study design. 46 , 47
Insomnia in AD, a major challenge for patients and caregivers, was shown to respond to treatment with suvorexant, a dual orexin antagonist, in a randomized clinical trial. 48 The trial demonstrated that participants receiving active therapy had increased time asleep and decreased wakefulness after sleep onset (WASO). The package insert has been modified to include the efficacy findings and the side effects observed in the AD trial.
Proof‐of‐concept (POC) trials are essential as a means of generating data to inform go/no go decisions for larger trials. Rasagiline, an agent approved for the treatment of motor disturbances in Parkinson's disease, was assessed in a POC trial using fluorodeoxyglucose (FDG) PET as the primary outcome. 49 The pre‐specified primary outcome was met, with less decline of metabolism in the group receiving active treatment. Another monoamine oxidase inhibitor—ladostigil—that has neuroprotective effects in cell preparations and animal models was found not to delay the progression from MCI to AD dementia when given in low doses for 3 years. 50
Repurposed agents are increasingly included in the AD drug development pipeline. 16 , 17 , 18 There are 14 repurposed agents in Phase 3 trials, 28 in Phase 2 trials, and 10 in Phase 1 trials. The difficulty of generating intellectual property protection for repurposed agents makes them less attractive as development candidates for biopharmaceutical companies and, because of their lower costs, more attractive to academic drug developers. Biopharmaceutical companies are sponsors of 44% of Phase 3 repurposing trials and 6% of Phase 2 repurposing trials; this compares to their sponsorship of 95% of non‐repurposed Phase 3 and 80% of non‐repurposed Phase 2 trials (Figure 4). Repurposed agents represent a larger fraction of the AD drug development compared to 5 years ago: there were 32 repurposed agents in 2016 (33% of the pipeline) compared to 52 repurposed agents in 2020 (43% of the pipeline).
Biomarkers play increasingly important roles in AD drug development. 51 Figure 7 shows the percentage of trials of disease‐modifying agents (biologics and small molecules) that required confirmation of the presence of amyloid at baseline over the past 5 years. This reflects the recognition that the amnestic dementia phenotype is a phenocopy without corresponding AD‐continuum pathology in 20% to 30% of patients. 52 Demonstration of the presence of AD pathology creates the appropriate population for assessment for agents that require AD‐related biological targets for their mechanism of action. Similarly, substantiation of disease‐modifying effects is expected to rely on a combination of clinical trial design, clinical outcome measures, and biomarkers of AD, especially markers of neurodegeneration. 9 Figure 8 shows the 5‐year trend in use of biomarkers as outcomes in trials of disease modifying agents. The number of trials of DMTs not using biomarkers for diagnostic confirmation, demonstration of target engagement, and support of disease modification is surprisingly high.
FIGURE 7.
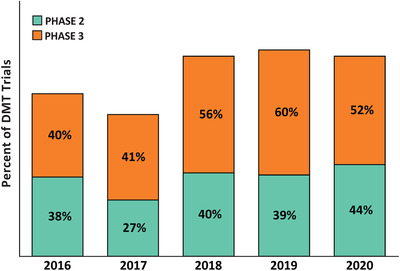
Percent of Phase 2 and 3 disease‐modifying therapy trials requiring amyloid evidence (positron emission tomography, cerebrospinal fluid or either) at entry: 2016–2020 (ClinicalTrials.gov accessed February 27, 2020) (Figure by Mike de la Flor)
FIGURE 8.
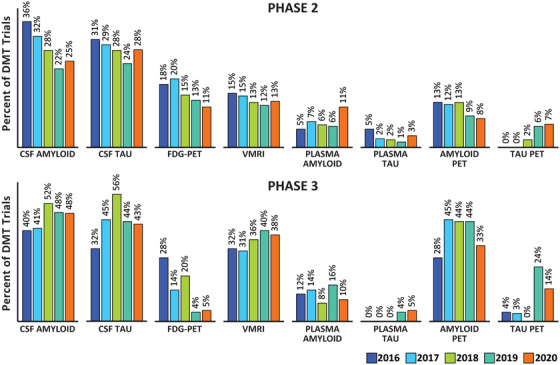
Phase 2 and Phase 3 disease‐modifying therapy trials using Alzheimer's disease biomarkers as outcome measures: 2016–2020 (ClinicalTrials.gov accessed February 27, 2020) (Figure by Mike de la Flor)
Basket trials can improve efficiency by including more than one disorder that has a characteristic biomarker or endophenotype. 53 TPI‐287 was assessed in a basket trial comprised of patients with tau pathology including AD, PSP, and corticobasal degeneration. 54 The trial of pimavanserin used a basket trial strategy with five types of dementia. Basket trials can facilitate recruitment by having less narrow inclusion criteria, provide insight into the responsiveness of different conditions to the intervention, and facilitate understanding of the biology of the diseases involved in the studies. 53
The World Health Organization registry indicates that there are 170 drugs in development for AD worldwide, contrasting with 6833 for malignant neoplasms and 433 for diabetes. These disparities reflect the less well defined target biology, limited availability of biomarkers, longer trial durations, greater expense, and higher risk of failure of AD drug development programs.
In summary, there are fewer agents in the AD pipeline in 2020 than in 2019 (121 vs 134). There are 29 agents in Phase 3 (compared to 29 in 2019), 65 agents in Phase 2 (compared to 75 in 2019), and 27 in Phase 1 (compared to 30 in 2019). All BACE inhibitors—prominent in the 2019 pipeline—have been discontinued for futility or toxicity. Several agents have shown robust reductions of amyloid using amyloid PET and new trials will provide insight into the relationship of anti‐amyloid and clinical effects. Biomarkers provide increasing data linking the MOA of the candidate agent to the biology of AD and promise to inform drug development decisions. The 5‐year perspective captured in this pipeline review shows that over this period there have been trends for increased pipeline target diversity, greater reliance on repurposed agents, engagement of participants in more mild stages of the continuum of AD with a corresponding change of trial entry criteria and outcome measures, and increasing use of biomarkers to define trial populations.
CONFLICTS OF INTEREST
Jeffrey Cummings has provided consultation to Acadia, Accera, Actinogen, Agenebio, Alkahest, Allergan, Alzheon, Annovis, Avanir, Axsome, Cassava, Cerecin, Cerevel, Cortexyme, EIP Pharma, Eisai, Foresight, GemVax, Green Valley, Grifols, Hisun, Karuna, MapLight, Nutricia, Otsuka, ReMYND, Resverlogix, Roche, Samus, Samumed, Sunovion, Suven, Third Rock, and United Neuroscience pharmaceutical and assessment companies. Marwan Sabbagh receives Royalties from Harper Collins, stock/equity from uMethodHealth, BrainHealthInc, Athira, Optimal Cognitive Health Company, and Versanum; Speakers Bureau from Peerview and Rockpointe; and Consult/Advisor fees from Biogen, Signant, Eisai, Neurotrope, Cortexyme, NeuroReserve, Grifols, Acadia, Roche, Regeneron, VTV Therapeutics, and Alzheon. Kate Zhong is the CEO of CNS Innovations and has provided consultation to Green Valley Pharmaceuticals, Otsuka, and Home Instead. Garam Lee has no disclosures. Aaron Ritter has no disclosures.
ACKNOWLEDGMENTS
JC acknowledges funding from the National Institute of General Medical Sciences (Grant: P20GM109025) and support from Keep Memory Alive.
Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2020. Alzheimer's Dement. 2020;6:e12050 10.1002/trc2.12050
REFERENCES
- 1. Alzheimer's Association . 2019 Alzheimer's Facts and Figures. Chicago, IL: Alzheimer's Association, 2019. [Google Scholar]
- 2. Alzheimer's Disease International . World Alzheimer's report 2015: the global impact of dementia. London, UK: Alzheimer's Disease International; 2015. [Google Scholar]
- 3. Cummings J, Morstorf T, Lee G. Alzheimer's drug‐development pipeline: 2016. Alzheimers Dement (N Y). 2016;2:222‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimers Dement (N Y). 2017;3:367‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cummings J, Lee G, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement (N Y). 2018;4:195‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (N Y). 2019;5:272‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Refolo LM, Snyder H, Liggins C, et al. Common Alzheimer's disease research ontology: national Institute on Aging and Alzheimer's Association collaborative project. Alzheimers Dement. 2012;8:372‐375. [DOI] [PubMed] [Google Scholar]
- 8. National Institute on Aging, Alzheimer's Association . International Alzheimer's and Related Dementias Research Portfolio: Common Alzheimer's and Related Dementias Research Ontology (CADRO). Bethesda, MD: National Institutes of Health; 2020. [Google Scholar]
- 9. Cummings J. Disease modification and neuroprotection in neurodegenerative disorders. Transl Neurodegener. 2017;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummings J, Fox N. Defining disease modifying therapy for Alzheimer's disease. J Prev Alzheimers Dis. 2017;4:109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 12. Lassman SM, Shopshear OM, Jazic I, Ulrich J, Francer J. Clinical trial transparency: a reassessment of industry compliance with clinical trial registration and reporting requirements in the United States. BMJ Open. 2017;7:e015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller JE, Wilenzick M, Ritcey N, Ross JS, Mello MM. Measuring clinical trial transparency: an empirical analysis of newly approved drugs and large pharmaceutical companies. BMJ Open. 2017;7:e017917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372:1031‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou CX, Becker JE, Phillips AT, et al. Registration, results reporting, and publication bias of clinical trials supporting FDA approval of neuropsychiatric drugs before and after FDAAA: a retrospective cohort study. Trials. 2018;19:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sreekrishnan A. Reporting and dissemination of clinical trials in neurology‐reply. JAMA Neurol. 2018;75:1573‐1574. [DOI] [PubMed] [Google Scholar]
- 17. Appleby BS, Nacopoulos D, Milano N, Zhong K, Cummings JL. A review: treatment of Alzheimer's disease discovered in repurposed agents. Dement Geriatr Cogn Disord. 2013;35:1‐22. [DOI] [PubMed] [Google Scholar]
- 18. Appleby BS, Cummings JL. Discovering new treatments for Alzheimer's disease by repurposing approved medications. Curr Top Med Chem. 2013;13:2306‐2327. [DOI] [PubMed] [Google Scholar]
- 19. Corbett A, Pickett J, Burns A, et al. Drug repositioning for Alzheimer's disease. Nat Rev Drug Discov. 2012;11:833‐846. [DOI] [PubMed] [Google Scholar]
- 20. Cummings J, Reynders R, Zhong K. Globalization of Alzheimer's disease clinical trials. Alzheimers Res Ther. 2011;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morrison C. Fresh from the biotech pipeline‐2018. Nat Biotechnol. 2019;37:118‐123. [DOI] [PubMed] [Google Scholar]
- 22. Xiao S, Zhang Z, Geng M. Phase 3 clinical trial of a novel and multi‐targeted oligosaccharide in patients with mild‐moderate AD in China. Barcelona, Spain: Clinical Trials on Alzheimer's Disease; 2018. [Google Scholar]
- 23. Syed YY. Sodium oligomannate: first approval. Drugs. 2020;80:441‐444. [DOI] [PubMed] [Google Scholar]
- 24. Syed YY. Correction to: sodium Oligomannate: first Approval. Drugs. 2020;80:445‐446. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Sun G, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids‐shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. 2019;29:787‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces abeta plaques in Alzheimer's disease. Nature. 2016;537:50‐56. [DOI] [PubMed] [Google Scholar]
- 27. AlzForum . Aducanumab. Alzforum.org; February 3, 2020.
- 28. Logovinsky V, Satlin A, Lai R, et al. Safety and tolerability of BAN2401–a clinical study in Alzheimer's disease with a protofibril selective abeta antibody. Alzheimers Res Ther. 2016;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abbasi J. Promising results in 18‐month analysis of Alzheimer drug candidate. JAMA. 2018;320:965. [DOI] [PubMed] [Google Scholar]
- 30. Cummings JL, Cohen S, van Dyck CH, et al. ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90:e1889‐e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang T, Dang Y, Ostaszewski B, et al. Target engagement in an Alzheimer trial: crenezumab lowers amyloid beta oligomers in cerebrospinal fluid. Ann Neurol. 2019;86:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tariot PN, Lopera F, Langbaum JB, et al. The Alzheimer's Prevention Initiative Autosomal‐Dominant Alzheimer's Disease Trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal‐dominant Alzheimer's disease, including a placebo‐treated noncarrier cohort. Alzheimers Dement (N Y). 2018;4:150‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ostrowitzki S, Lasser RA, Dorflinger E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild‐to‐moderate Alzheimer's disease. N Engl J Med. 2018;378:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Egan MF, Kost J, Voss T, et al. Randomized trial of verubecestat for prodromal Alzheimer's disease. N Engl J Med. 2019;380:1408‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider LS, Thomas RG, Hendrix S, et al. Safety and efficacy of edonerpic maleate for patients with mild to moderate Alzheimer disease: a phase 2 randomized clinical trial. JAMA Neurol. 2019;76(11):1330‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atri A, Frolich L, Ballard C, et al. Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients with Alzheimer disease: three randomized clinical trials. JAMA. 2018;319:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Libro R, Bramanti P, Mazzon E. Endogenous glucocorticoids: role in the etiopathogenesis of Alzheimer's disease. Neuro Endocrinol Lett. 2017;38:1‐12. [PubMed] [Google Scholar]
- 39. Marek GJ, Katz DA, Meier A, et al. Efficacy and safety evaluation of HSD‐1 inhibitor ABT‐384 in Alzheimer's disease. Alzheimers Dement. 2014;10:S364‐73. [DOI] [PubMed] [Google Scholar]
- 40. Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small‐molecule inhibitors. Sci Adv. 2019;5:eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Readhead B, Haure‐Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64‐82.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cummings J, Ballard C, Tariot P, et al. Pimavanserin: potential treatment for dementia‐related psychosis. J Prev Alzheimers Dis. 2018;5:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herrmann N, Ruthirakuhan M, Gallagher D, et al. Randomized placebo‐controlled trial of nabilone for agitation in Alzheimer's disease. Am J Geriatr Psychiatry. 2019;27:1161‐1173. [DOI] [PubMed] [Google Scholar]
- 44. Grossberg GT, Kohegyi E, Mergel V, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer's dementia: two 12‐week, randomized, double‐blind, placebo‐controlled trials. Am J Geriatr Psychiatry. 2019;28(4):383‐400. [DOI] [PubMed] [Google Scholar]
- 45. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan‐quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314:1242‐1254. [DOI] [PubMed] [Google Scholar]
- 46. Ehrhardt S, Porsteinsson AP, Munro CA, et al. Escitalopram for agitation in Alzheimer's disease (S‐CitAD): methods and design of an investigator‐initiated, randomized, controlled, multicenter clinical trial. Alzheimers Dement. 2019;15:1427‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311:682‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer's disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16:541‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matthews DC, Ritter A, Thomas RG, et al. The effects of rasagiline on glucose metabolism and cognition and their relationship to tau burden in a double‐blind, placebo‐controlled phase II clinical trial of participants with Alzheimer's dementia. Submitted, in review.
- 50. Schneider LS, Geffen Y, Rabinowitz J, et al. Low‐dose ladostigil for mild cognitive impairment: a phase 2 placebo‐controlled clinical trial. Neurology. 2019;93:e1474‐e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cummings J. The role of biomarkers in Alzheimer's disease drug development. Adv Exp Med Biol. 2019;1118:29‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sevigny J, Suhy J, Chiao P, et al. Amyloid PET screening for enrichment of early‐stage Alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30:1‐7. [DOI] [PubMed] [Google Scholar]
- 53. Renfro LA, Sargent DJ. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann Oncol. 2017;28:34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsai RM, Miller Z, Koestler M, et al. Reactions to multiple ascending doses of the microtubule stabilizer TPI‐287 in patients With Alzheimer disease, progressive supranuclear palsy, and corticobasal syndrome: a randomized clinical trial. JAMA Neurol. 2019;77(2):215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]


