Abstract
Background and Purpose
Periventricular (PVWMH) and deep white matter hyperintensities (DWMH) are regional classifications of white matter hyperintensities (WMH) and reflect proposed differences in etiology. In the first study to date, we undertook genome-wide association analyses (GWAS) of DWMH and PVWMH to show that these phenotypes have different genetic underpinnings.
Methods
Participants were aged 45 years and older; free of stroke and dementia. We conducted GWAS of PVWMH and DWMH in 26,654 participants from CHARGE, ENIGMA, and the UK Biobank (UKB). Regional correlations were investigated using the GWAS-pairwise method. Cross-trait genetic correlations between PVWMH, DWMH, stroke, and dementia were estimated using LDSC.
Results
In the discovery and replication analysis, for PVWMH only, we found associations on chromosomes (Chr) 2 (NBEAL), 10q23.1 (TSPAN14/FAM231A), and 10q24.33 (SH3PXD2A). In the much larger combined meta-analysis of all cohorts, we identified ten significant regions for PVWMH: Chr 2 (3 regions), 6, 7, 10 (2 regions), 13, 16 and 17q23.1. New loci of interest include 7q36.1 (NOS3) and 16q24.2. In both the discovery/replication and combined analysis, we found genome-wide significant associations for the 17q25.1 locus for both DWMH and PVWMH. Using gene-based association analysis, 19 genes across all regions where identified for PVWMH only, including the new genes: CALCRL (2q32.1), KLHL24 (3q27.1), VCAN (5q27.1) and POLR2F (22q13.1). Thirteen genes in the 17q25.1 locus were significant for both phenotypes. More extensive genetic correlations were observed for PVWMH with small vessel ischemic stroke. There were no associations with dementia for either phenotype.
Conclusions
Our study confirms these phenotypes have distinct and also shared genetic architectures. Genetic analyses indicated PVWMH was more associated with ischemic stroke whilst DWMH loci were implicated in vascular, astrocyte and neuronal function. Our study confirms these phenotypes are distinct neuroimaging classifications and identifies new candidate genes associated with PVWMH only.
Keywords: Genome-wide association study, white matter, neuroimaging, brain, risk factors, genomics
Introduction
Radiological white matter hyperintensities of presumed ischemic origin (WMH) are the most prevalent sign of cerebral small vessel disease (SVD) and represent 40% of all SVD disease burden1. They are detected as incidental lesions on T2-weighted MRI1. WMH are associated with increased risk for ischemic and hemorrhagic stroke, cognitive decline, and motor gait disorders 2–6. Two regional classifications, based on their anatomical relationship to the lateral ventricles in the brain, are periventricular (PVWMH) and deep WMH (DWMH)5, 7–9. PVWMH have been associated with declines in cognitive performance and increased systolic and arterial pressure, while DWMH are linked to BMI, mood disorders, gait impairment and arterial hypertension10–12. This categorization reflects proposed differences in underlying pathophysiology5, 7, 8. DWMH lesions occur in the subcortex, areas primarily supplied by long microvessels, with lower estimated blood pressures, possibly subject to damage secondary to hypertension and possibly with consequent hypoperfusion.1, 8, 13, 14. PVWMH are related to alterations in short penetrating microvessels ending in close approximation to larger arterial blood vessels with different vascular architecture such as two leptomeningeal layers and enlarged perivascular spaces1, 15. They are hypothesized to be affected more directly by hypertension and risk factors associated with stroke1, 8, 13, 14.
These sub-classifications may also reflect differences in associated underlying genetic factors16. Twin and family studies report that both PVWMH and DWMH have high heritability and genetic correlations16, 17. Recently, GWAS for total WMH volume identified a major genetic risk locus on chromosome 17q25.118–21 and several other loci (e.g., 10q24, 2p21, 2q33, 6q25.1)19, 21, 22. However, the genetic determinants of regional WMH burden, specifically DWMH and PVWMH, remain elusive.
We combined all available participants aged 45 and above with both DWMH and PVWMH measurements from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) and the Enhancing Neuro-Imaging Genetics through Meta-Analysis (ENIGMA) consortia, and the UK Biobank (UKB). This is the only GWAS to date examining WMH subclassifications. We hypothesized that separating the two WMH subclassifications would mitigate phenotype heterogeneity, allowing us to identify additional risk loci and show that DWMH and PVWMH have different genetic underpinnings and pathophysiology.
Materials and Methods
Summary data for this meta-analysis will be available through the database of Genotypes and Phenotypes Cohorts for Heart and Aging Research in Genomic Epidemiology Summary Results site, which can be downloaded via authorized access.
Study Cohorts
Study participants (total N~26,654) were drawn from cohorts in the CHARGE and ENIGMA consortia and the UKB. Detailed methods are in the Data Supplement. All cohorts followed standardized procedures for participant inclusion, genotype calling, phenotype harmonization, covariate selection and study-level analysis. Participants were included if they had phenotype, genotype and covariate data available and were aged 45 years and over without stroke, dementia or any neurological abnormality at the time of MRI scanning. All participants provided written informed consent and each study received ethical approval to undertake this work.
Phenotype and covariates
The MRI and WMH extraction methods for each study are detailed in the Data Supplement. In brief, PVWMH and DWMH volumetric data were extracted using automated methods for all studies except HUNT, LBC and AGES, which used visual rating scales (Supplementary Table I). Hypertension was defined as systolic blood pressure ≥140mm Hg and diastolic blood pressure ≥90mm Hg or on current antihypertensive treatment.
Statistical analysis
Each study fitted linear regression models to test the association of DWMH and PVWMH (continuous measures) with individual SNPs. Additive genetic effects were assumed and the models were adjusted for age (years), sex and ICV (where applicable). In addition, principal components for population stratification and other covariates, such as familial structure, were included if necessary. Models were also fitted with hypertension as an additional covariate.
Fixed-effects, inverse-variance-weighted meta-analysis was carried out in METAL23, with correction for genomic control. Two meta-analyses were carried out: all cohorts excluding UKB (discovery, phase I) and all cohorts (phase II). Post meta-analysis QC was also performed (see Data Supplement).
Genetic correlations with stroke and dementia
Cross-trait genetic correlation between the two sub-classifications of WMH, stroke and dementia were estimated using LDSC24 on the GWAS summary statistics from phase II, MEGASTROKE (European ancestry-only)25. LD scores were based on the HapMap3 European reference panel. Regional level correlation was investigated using the GWAS-PW and HESS methods26, 27.
Results
Detailed study descriptions are provided in Supplementary Tables I-III. The discovery cohort was comprised of ~18,234 older adults (≥45 years, 16 studies) and was primarily Caucasian, with 736 African Americans and 658 Hispanics. The predominantly Caucasian UKB was used as the replication cohort (n=8,428).
In the discovery analysis (Phase I), genome-wide significant associations (p<5e-8) were observed in the 17q25.1 region for both phenotypes (Supplementary Tables IV-V). Only the PVWMH analysis found additional genome-wide significant associations on chromosomes 2 and 10 (2 regions). Two of these regions had previously been described for total WMH burden (chr 2, NBEAL19, 21, 10q24.33, SH3PXD2A21) whilst 10q23.1 had not been described. Adjusting for hypertension made little difference to our findings (Supplementary Tables VI-VII). Replication of the majority of genome-wide significant results for both phenotypes was observed after adjustment for multiple testing (DWMH p <3.6e-4, PVWMH p < 2.76e-4, Supplementary Tables VIII-IX).
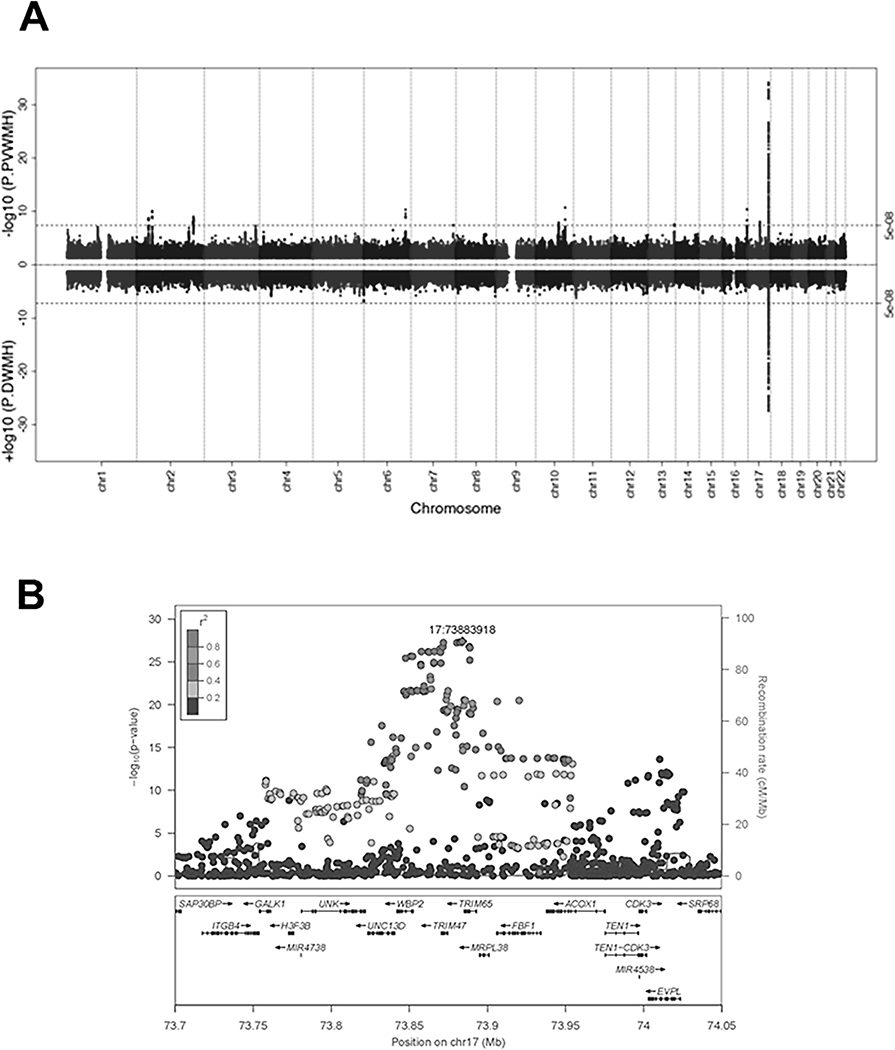
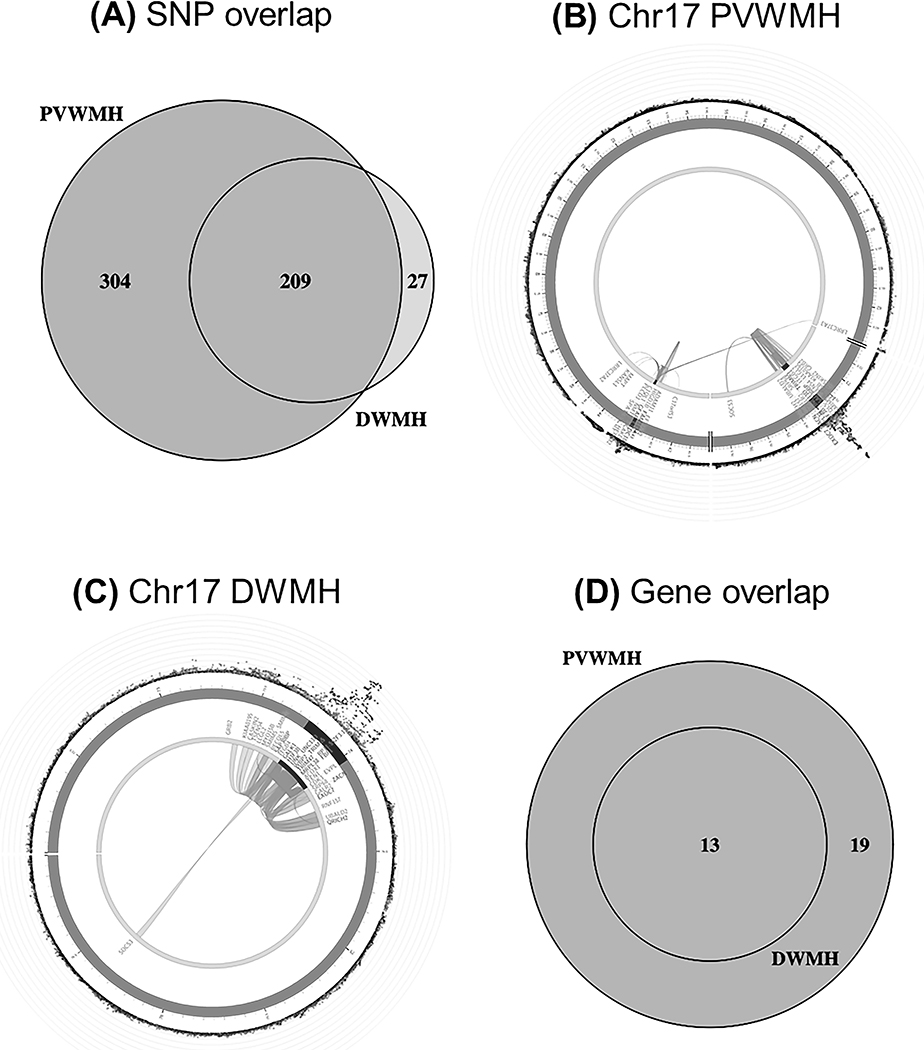
Given the relatively large size of the replication cohort, a combined meta-analysis (Phase II) was undertaken using all samples (n~26,654). Removing either the small subsample of non-Caucasians, or the cohorts with visual ratings, did not substantially change the findings (beta value r2 > 0.93). The Phase II GWAS meta-analyses identified 236 for DWMH and 513 genome-wide significant SNPs for PVWMH (Figure 1a, Table 1, Supplementary Tables X-XI respectively). Figure 1b shows the zoom plot of the single locus identified for DWMH on chr17q25.1. The associations of the identified genome-wide and suggestive associations for each phenotype for the alternate trait are also provided in Supplementary Tables X-XI. The only SNPs genome-wide significant for both phenotypes (n=209) were located on 17q25.1 (Figure 2a).
Figure 1.
(A) Phase II GWAS meta-analysis. Miami plot for PVWMH (upper panel) and DWMH (lower panel). Dashed line shows genome-wide significance threshold (p<5e-8). (B) Chr17 regional plot of genome-wide significant SNPs for DWMH. Colors of the SNPs indicate the level of LD with the top SNP (purple), rs35392904.
Table 1.
Top genome-wide significant SNP results from each genomic locus identified from the Phase II GWAS meta-analysis for deep and periventricular (PV) WMH.
| WMH | rsID | CHR | POS | Nearest gene | Function/Position | A1 | A2 | Freq (A1) | Beta (SE) | N | Direction | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PV | rs3744020 | 17q25.1 | 73871773 | TRIM47 | Intronic | A | G | 0.1897 | 0.0899 (0.0073) | 26438 | +++-+++?+++++++++++++ | 7.06E-35 |
| Deep | rs35392904 | 17q25.1 | 73883918 | TRIM65 | Intronic | T | C | 0.7981 | −0.0765 (0.0070) | 26642 | -------+--------+---- | 3.99E-28 |
| PV | rs3758575 | 10q24.33 | 105454881 | SH3PXD2A | Intronic | A | G | 0.4904 | 0.0388 (0.0058) | 26654 | +++-++-++++++++++++++ | 2.00E-11 |
| PV | rs12928520 | 16q24.2 | 87237568 | C16orf95 | Inter-genic | T | C | 0.4252 | 0.0431 (0.0065) | 26327 | +++++-?-+--+++++++-++ | 4.22E-11 |
| PV | rs275350 | 6q25.1 | 151016058 | PLEKHG1 | Intronic | C | G | 0.4202 | 0.0374 (0.0057) | 26654 | +-+-+++++-++++++---++ | 4.86E-11 |
| PV | rs7596872 | 2p16.1 | 56128091 | EFEMP1 | Intronic | A | C | 0.0975 | 0.0642 (0.0099) | 25730 | -++++++-+--+++???++++ | 8.66E-11 |
| PV | rs72934583 | 2q33.2 | 204009057 | NBEAL1 | Intronic | T | G | 0.8740 | 0.0529 (0.0087) | 25730 | -+++++++++-+++???+-++ | 1.03E-09 |
| PV | rs57242328 | 2p21 | 43073247 | AC098824.6 | Intergenic | A | G | 0.3317 | −0.0368 (0.0061) | 25730 | ----+----+----???-++- | 1.85E-09 |
| PV | rs7213273 | 17q21.31 | 43155914 | NMT1 | Intronic | A | G | 0.6668 | 0.0341 (0.0059) | 26111 | +++++-??++-++-+++++++ | 8.89E-09 |
| PV | rs1993484 | 10q23.1 | 82222698 | TSPAN14 | Intronic | T | C | 0.2388 | 0.0378 (0.0067) | 26654 | ++++-+--++-++-+++++++ | 1.36E-08 |
| PV | rs11838776 | 13q34 | 111040681 | COL4A2 | Intronic | A | G | 0.2793 | 0.0350 | 26654 | -+++++-++-+++++++-+-+ | 2.82E-08 |
| PV | rs1799983 | 7q36.1 | 150696111 | NOS3 | Exonic | T | G | 0.3201 | 0.0373 | 26654 | ++++++--++-++-++----+ | 3.68E-08 |
Notes: Effect allele is allele 1 (A1). A2 = allele 2. SE = standard error. Those loci bolded have not been previously associated with total WMH.
Figure 2.
(A) Overlap between genome-wide significant SNPs (p<5e-8) for DWMH and PVWMH. (B - C) Circos plots for chr17 for both phenotypes, showing two identified regions for PVWMH (B) but only one for DWMH (C). Outer ring shows SNPs <0.05 with the most significant SNPs located towards the outermost ring. SNPs in high LD with the independent significant SNPs in each locus are colored in red (r2>0.8)-blue (r2>0.2); no LD (grey). Genomic risk loci are colored in dark blue (2nd layer). Genes are mapped by chromatin interaction (orange), eQTL (green) or both (red). (D) Overlap between significant genes identified by MAGMA for both phenotypes.
Ten chromosomal regions containing 290 genome-wide significant SNPs for PVWMH only were identified on chromosomes 2 (3 regions), 6, 7, 10 (2 regions), 13, 16 and 17q23.1 (Supplementary Results; Supplementary Table XI; Supplementary Figure I-II). Four loci had not been previously reported for associations with total WMH at the genome-wide significant level: (i) 7q36.1 (7.2kb) containing 2 exonic SNPs in the NOS3 gene; (ii) 10q23.1 (50.5kb) containing 4 intronic SNPs in TSPAN14 & FAM231A; (iii) 16q24.2 (1.2kb) containing 2 intergenic SNPs; (iv) 17q21.31 (27.2kb) containing 8 SNPs, most of which are intronic and in the NMT1 gene. Many of these are eQTLs or participate in long-range chromatin interactions (Figure 2b). Further descriptions of the PVWMH findings are found in the Supplementary Results.
As expected, the association of the 17q25.1 locus with both phenotypes was confirmed. The size of this region, including genome-wide significant SNPs only, was similar for both DWMH (236 SNPs, BP 73757836–74025656, Figure 1b) and PVWMH (223 SNPs, BP 73757836–74024711, Supplementary Figure Ia). The top results in this locus were rs3744020 for DWMH, (p=7.06e-35, TRIM47 intronic SNP) and rs35392904 for PVWMH (p=3.989e-28, TRIM65 intronic SNP), which are in high linkage disequilibrium (LD, R2=0.902) (Table 1). Many of these SNPs are eQTLs or have long-range chromatin interactions (Figure 2b-c). For further details see the Supplementary Results.
Using gene-based tests, 13 genes in the 17q25.1 locus reached genome-wide significance (p<2.66e-6) with both phenotypes (Table 2, Figure 2d, Supplementary Tables XII-XIII). For PVWMH, an additional 19 genes were identified, covering the majority of regions/loci found in the SNP-based analysis (Figure 2d, Table 2, Supplementary Table XIII). Four genes were located in previously unidentified regions: CALCRL (2q32.1), KLHL24 (3q27.1), VCAN (5q27.1) and POLR2F (22q13.1).
Table 2.
Thirty-two significant genes were identified for PVWMH using gene-based tests (p<2.66e-6). Thirteen of these genes (chr17) were also significant for DWMH (*).
| GENE | CHR | START | STOP | N SNPS | N | p PVWMH | p DWMH |
|---|---|---|---|---|---|---|---|
| WBP2 | 17 | 73841780 | 73852588 | 28 | 24682 | 3.19E-26 | 1.16E-21* |
| TRIM65 | 17 | 73876416 | 73893084 | 52 | 24555 | 7.73E-24 | 9.12E-19* |
| TRIM47 | 17 | 73870242 | 73874656 | 13 | 24185 | 1.70E-23 | 9.04E-19* |
| RP11–552F3.12 | 17 | 73894726 | 73926210 | 53 | 24351 | 2.15E-20 | 1.76E-15* |
| FBF1 | 17 | 73905655 | 73937221 | 55 | 24338 | 3.98E-17 | 1.23E-13* |
| GALK1 | 17 | 73747675 | 73761792 | 36 | 24307 | 6.34E-16 | 3.23E-14* |
| MRPL38 | 17 | 73894724 | 73905899 | 21 | 24481 | 7.62E-15 | 1.18E-13* |
| UNC13D | 17 | 73823306 | 73840798 | 73 | 23788 | 3.10E-14 | 1.22E-13* |
| UNK | 17 | 73780681 | 73821886 | 120 | 22768 | 3.28E-13 | 4.85E-10* |
| H3F3B | 17 | 73772515 | 73781974 | 23 | 24009 | 4.43E-12 | 1.41E-10* |
| SH3PXD2A | 10 | 105348285 | 105615301 | 788 | 24847 | 8.43E-12 | 0.21731 |
| ACOX1 | 17 | 73937588 | 73975515 | 151 | 24198 | 7.72E-11 | 1.1E-09* |
| EVPL | 17 | 74000583 | 74023533 | 67 | 24582 | 1.26E-10 | 2.82E-14* |
| PLEKHG1 | 6 | 150920999 | 151164799 | 1022 | 24922 | 1.59E-10 | 0.011765 |
| WDR12 | 2 | 203739505 | 203879521 | 322 | 23753 | 2.53E-10 | 0.00104 |
| ICA1L | 2 | 203640690 | 203736708 | 224 | 23843 | 8.44E-10 | 0.001301 |
| CARF | 2 | 203776937 | 203851786 | 157 | 24076 | 2.41E-09 | 0.001763 |
| NMT1 | 17 | 43128978 | 43186384 | 221 | 24766 | 7.18E-08 | 0.00034 |
| CDK3 | 17 | 73996987 | 74002080 | 12 | 24433 | 8.54E-08 | 1.82E-08* |
| OBFC1 | 10 | 105642300 | 105677963 | 99 | 25461 | 1.41E-07 | 0.054127 |
| NOS3 | 7 | 150688083 | 150711676 | 58 | 24608 | 1.73E-07 | 0.000371 |
| DCAKD | 17 | 43100708 | 43138473 | 111 | 25229 | 2.60E-07 | 0.000363 |
| DYDC2 | 10 | 82104501 | 82127829 | 91 | 25050 | 2.88E-07 | 0.003460 |
| NBEAL1 | 2 | 203879602 | 204091101 | 367 | 23413 | 3.83E-07 | 0.040539 |
| NEURL1 | 10 | 105253736 | 105352309 | 296 | 25038 | 4.84E-07 | 0.098303 |
| MAT1A | 10 | 82031576 | 82049440 | 66 | 25295 | 4.90E-07 | 0.002421 |
| TSPAN14 | 10 | 82213922 | 82292879 | 213 | 24731 | 6.73E-07 | 0.006605 |
| CALCRL | 2 | 188207856 | 188313187 | 278 | 24309 | 7.87E-07 | 0.000574 |
| KLHL24 | 3 | 183353356 | 183402265 | 207 | 24356 | 1.29E-06 | 0.002571 |
| POLR2F | 22 | 38348614 | 38437922 | 105 | 23525 | 1.94E-06 | 0.252540 |
| VCAN | 5 | 82767284 | 82878122 | 316 | 24248 | 2.52E-06 | 0.065044 |
| COL4A2 | 13 | 110958159 | 111165374 | 1140 | 24876 | 2.61E-06 | 0.365300 |
Notes: Those loci bolded have not been previously associated with total WMH.
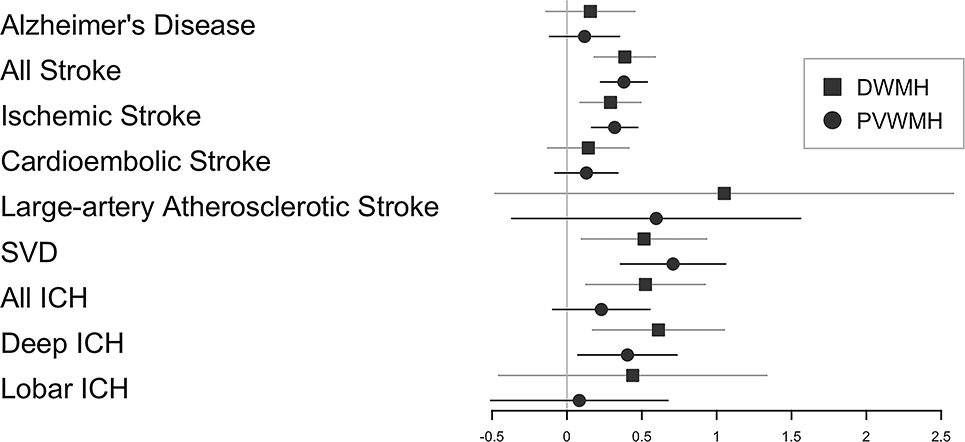
Heritability analyses revealed low to moderate heritability for both traits (see Supplementary Results). A high genetic correlation between DWMH and PVWMH was observed (rg= 0.927, p=1.1e-65), indicating a shared genetic architecture. Figure 3 shows the genetic correlations with DWMH, PVWMH, stroke and Alzheimer’s disease (AD). Positive genetic correlations with both phenotypes were found for ‘all stroke’, ischemic stroke and SVD. Intracerebral haemorrhage (ICH, all types) was correlated with DWMH only. No significant correlations were found with AD (Supplementary Table XIV).
Figure 3.
Genetic correlations (rg) between DWMH, PVWMH, Alzheimer’s disease (AD) and stroke phenotypes. Horizontal bars represent standard errors and the size of the square corresponds precision. SVD = small vessel disease stroke, All ICH = All intracranial hemorrhage, Deep ICH = deep intracranial hemorrhage, Lobar ICH = lobar intracranial hemorrhage
Using GWAS-PW26, we observed several regions with high probability (>90%) for harboring a shared genetic variant between PVWMH and DWMH (Supplementary Table XV). These regions encompass several genome-wide significant loci that were identified for PVWMH (2p16.1 (EFEMP1), 2q33.2 (CARF & NBEAL), 6q25.1 (PLEKHGI22), 16q24.2 (C16orf95), and 17q25.1 (TRIM47, TRIM65). Additionally, by using HESS27 regional level correlation estimates were derived for those regions identified by the Bayesian approach (GWAS-PW).
Finally, we investigated local regions of a shared genetic variant between the WMH subtypes and stroke (Supplementary Table XV). A region on chromosome 7 (encompassing the PVWMH NOS3 exonic SNP) exhibited shared genetic influence of ‘all stroke’ with both phenotypes. Other regions of shared influence with all stroke were observed for PVWMH only. For the sub-types of stroke, significant regions were identified for DWMH and PVWMH, but none were found for both phenotypes except the chromosome 7 region for ischemic stroke (also identified for all stroke). Similar to the GW level correlation, a positive regional level genetic correlation was observed between the WMH subtypes and stroke (all stroke, all-ischemic, cardio-embolic and small-vessel), by using HESS27.
Discussion
In our meta-analyses using all available individuals (n=26,654, Phase II), PVWMH had significant independent associations with loci containing genes implicated in large and small vessel disease, as well as ischemic and deep hemorrhagic stroke suggesting a unique genetic and pathophysiological underpinning. While our Phase II GWAS were only slightly larger than the previous biggest GWAS on total WMH burden with 21,079 participants21, our detection rate of significant SNPs was substantially higher18, 19, 21. This improved detection may be the result of reduced heterogeneity by separately analyzing the DWMH and PVWMH phenotypes.
We identified 11 independent loci for PVWMH and one locus for DWMH. Significant genes associated with WMH for the first time in PVWMH include CALCRL, VCAN, TSPAN, and NOS3. Most genes and loci previously reported as significant in total WMH.28–32 were now found to be associated with PVWMH alone, including PLEKHG122, SH3PXD2A25, 28, 33 and COL4A233. Similarly, genes viewed as potential candidates18, 19, 21 in prior studies we now find to be significantly associated only with PVWMH including DYDC2 and NEURL1 as well as NMT1, GALK1, H3F3B, UNK, UNC13D, EVPL, ICAL1, WDR12/CARF, NBEAL1, and EFEMP1.
Many of these genes associated with PVWMH affect vascular function or vascular disease such as ischemic stroke, or coronary artery disease. The NOS3 gene is associated with coronary artery disease, migraine, vascular dysfunction, SVD, and ischemic stroke22, 29, 30, 34. PLEKHG1 is associated with dementia and ischemic stroke35 and SH3PXD2A has been previously associated with total WMH and ischemic stroke19, 25.
The most notable associated vascular gene is COL4A2 that encodes for a subunit of type IV collagen, which has been associated with SVD, ischemic stroke, intracranial hemorrhage, and coronary artery disease31, 35–38. It is a proposed therapeutic target for the prevention of intracranial hemorrhage32, 39. The association of this vascular gene with PVWMH and deep ICH is suggestive of underlying regional gene effects of the COL4A2 gene on the microvasculature affecting the risk of vascular injury in the periventricular region. These include potential weakening of the structural integrity of the regional microvasculature by altered collagen type 4 structural integrity, dysregulated gene expression of COL4A1 and COL4A2, and toxic cytosolic accumulations of COL4A2 within microvascular structural cells40. When comparing PVWMH and DWMH anatomy these mechanisms may enhance the direct mechanical effects of hypertension, or the other stroke risk factors, on the unique microvascular structure of the PVWMH region that also has predicted higher ambient blood pressure1, 6, 13.
We also discovered a new set of putative PVWMH genes. These include: TSPAN14, which encodes one of the tetraspanins which organize a network of interactions referred to as the tetraspanin web, ADAM10 - a metalloprotease that cleaves the precursor of cell surface proteins41, KLHL24 encodes a ubiquitin ligase substrate receptor42, VCAN encodes a large chondroitin sulfate proteoglycan that is found in the extracellular matrix. In a recent meta-analysis, VCAN was associated with white matter microstructural integrity43. These candidate genes for PVWMH may influence the immediate tissues surrounding microvessels and may contribute to SVD-associated biological changes.
The only significant locus observed for DWMH was the previously reported total WMH 17q25.1 locus18, 19, 21, 22, which was also found for PVWMH. This locus contained the SNPs with the largest effect sizes for both phenotypes. The top genome-wide significant hits for DWMH and PVWMH (17q25.1) were either identical with the SNP recently reported by Traylor et al22 for total WMH (PVWMH rs3744020) or in high LD (R2>0.9) with the previously identified top ranked SNPs in the same locus (rs3744028, Fornage et al.18, rs7214628, Verhaaren et al.21). Our identified SNPs were only in moderate LD (R2≤0.396) with the top SNP (rs3760128) identified in a recent exome association analysis19. All of these SNPs fall within or between the previously reported TRIM47 and TRIM65 genes18, 21, 22, 35. This gene-rich locus contains genes that influence glial cell proliferation and have been hypothesized to influence gliosis, which is a histological and MRI marker of microvascular injury1. It includes previously identified total WMH genes, such as TRIM47/TRIM65 (glial proliferation, astrocytoma’s)18, 21, ACOX1 (cell replication, hepatic cancer)18, 19, 21 and MRPL38 (protein synthesis)19. Genes associated with neuronal injury and/or neurodegenerative disorders are also found in the 17q25.1 locus, including CDK3 (neuronal cell death in stroke)44, H3F3B (schizophrenia pathogenesis) and GALK1 (galactosemia)45. Interestingly, two genome-wide significant intronic UNC13D SNPs identified in this study and reported previously for total WMH burden21, rs9894244 and rs7216615, have been reported as eQTLs for GALK1 and H3F3B respectively46. The PVWMH specific loci also contained genes that potentially influence astrocytic function and gliosis, several previously reported for total WMH. These include NBEAL119, 21, WDR1219, NEURL118, 19, 21, CARF47, and EFEMP137. Newly identified PVWMH genes potentially affecting astrocytic functioning include NMT148, ICA1L49, POLR2F, OBFC1 and DYDC2.
Shortcomings of this study include the potential variability due to the different WMH extraction algorithms used, with a minority of samples using visual ratings. However, this is a common problem encountered in this type of study18, 19, 21. Even though our results suggest improved power and reduction in potential bias through the discrimination of PVWMH from DWMH, the Euclidean methodology used by the majority of studies undoubtedly missed PVWMH lesions outside this boundary. The majority of the participants in this study were Caucasian and hence these results may not apply to other ethnicities. Sex differences have been previously reported but were not examined in the current study50. For the Phase II meta-analysis, we did not have an independent replication cohort. Older adults were included in this study and the majority of participants had both DWMH and PVWMH and not one or the other. However, selection of individuals with only one of subtype of these lesions present may be more appropriate to identify differences but would only be possible in younger cohorts. Future studies should aim to address these shortcomings, including continuing to improve and harmonize WMH measurement methods but also using consistent DWMH and PVWMH measurement methods across studies.
Summary/Conclusion
Our study confirms PVWMH and DWMH have distinct and shared genetic architecture. Genetic analyses indicated PVWMH was more associated with ischemic stroke and vascular function (PLEKHG1, SH3PXD2, COL4A2, CALCRL, VCAN, NOS3), while DWMH loci were implicated in vascular, astrocyte and neuronal function (TRIM47/TRIM 65, ACOX1, MRPL38, H3F3B, GALK, UNC13D, GALK1). New genes for PVWMH, potentially affecting the extravascular connective tissue, where also identified (TSPAN14, ADAM10, KLHL24, VCAN). Our study confirms that PVWMH and DWMH are distinct neuroimaging classifications and identifies new candidate genes associated with PVWMH only.
Supplementary Material
Acknowledgements
Sources of Funding
This work is supported by the NINDS, NIH, R01AG059874, P41EB015922, R56AG058854, U54 EB020403, R01AG022381. Medical Research Council, Age UK, Scottish Funding Council, Row Fogo Trust, The Welcome Trust, Age UK, Cross Council Lifelong Health and Wellbeing Initiative, Leverhulme Trust, National Institute for Health Research, Biotechnology and Biological Sciences Research Council, UK Medical Research Council, Icelandic Heart Association, and the Althingi, Austrian Science Fund (FWF), Australian National Health and Medical Research Council, Austrian National Bank, Anniversary Fund, European Commission FP6 STRP, European Community’s 5th and 7th Framework Program, Netherlands Organization for Scientific Research, Netherlands Consortium for Healthy Aging, Russian Foundation for Basic Research, Russian Federal Agency of Scientific Organizations, Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, Norwegian National Advisory Unit for functional MRI, Leipzig Research Center for Civilization Diseases (LIFE), Bristol-Myers Squibb, Netherlands Heart Foundation, French National Research Agency (ANR), Foundation Leducq, Joint Programme of Neurodegenerative Disease research, Bordeaux University, Institut Pasteur de Lille, the labex DISTALZ and the Centre National de Génotypage. Deutsche Forschungsgesellschaft (DFG) no. WI 3342/3–1 and grants from European Union, European Regional Development Fund, Free State of Saxony within the framework of the excellence initiative, LIFE-Leipzig Research Center for Civilization Diseases no. 100329290, 713–241202, 14505/2470, 14575/2470. Max Planck Society, State of Saxony, Brain Foundation, Bristol Myers Squibb, NHMRC of Australia, NHMRC of Australia, Parkinson’s UK; Medical Research Council - Dementias Platform UK. Full details of funding support for each cohort are detailed in the online Data Supplement
Conflict-of-Interest/Disclosure
Jonathan Marchini is an employee of, and owns stock and stock options for, Regeneron Pharmaceuticals; Perminder Sachdev received personal fees from Biogen; Henry Brodaty, Advisory Board member, Nutricia Australia; Philippe Amouyel, advisor for Foundation Alzheimer, Occupational medicine, Oil company, and Genoscreen Biotech company; Alzheimer’s Foundation, Occupational Medicine, Oil company, Genoscreen personal fees, Biotech company; Christine Fennema-Notestine, received finding from National Institutes of Health Grants NIA R01AG022381; Rebecca F. Gottesman, American Academy of Neurology, Associate Editor, Neurology; Paul Thompson, funded by NIH grant U54 EB020403 and received partial research support from Biogen, Inc., Charles DeCarli, consultant to Novartis; Markus Scholz disclosed Pfizer Inc. grants; Ralph Sacco, grant support from Boehringer Ingelheim, NINDS Grants; Neda Jahanshad received funding from the NIH grants, NIH R01AG059874,NIH R01AG059874; Joanna Wardlaw, grant support from the Medical Research Council, Age UK, Scottish Funding Council, and Row Fogo Trust during the conduct of the study and grant support from Fondation Leducq, Wellcome Trust, EPSRC, Chest Heart Stroke Scotland, British Heart Foundation, Stroke Association, Alzheimer’s Society and Alzheimer’s Research UK outside the submitted work.
Footnotes
See online Data Supplement.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The rotterdam scan study. Stroke. 2003;34:1126–1129 [DOI] [PubMed] [Google Scholar]
- 3.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: A systematic review of mri and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135 [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental mri white matter signal hyperintensities. Neurology. 1993;43:1683–1689 [DOI] [PubMed] [Google Scholar]
- 6.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 7.Griffanti L, Jenkinson M, Suri S, Zsoldos E, Mahmood A, Filippini N, et al. Classification and characterization of periventricular and deep white matter hyperintensities on mri: A study in older adults. Neuroimage. 2018;170:174–181 [DOI] [PubMed] [Google Scholar]
- 8.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398 [DOI] [PubMed] [Google Scholar]
- 9.Albrecht M, Zitta K, Bein B, Wennemuth G, Broch O, Renner J, et al. Remote ischemic preconditioning regulates hif-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: A pilot experimental study. Basic Res Cardiol. 2013;108:314. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis risk in communities (aric) study. Stroke. 2010;41:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan MS, O’Brien JT, Firbank MJ, Pantoni L, Carlucci G, Erkinjuntti T, et al. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The ladis study. Int J Geriatr Psychiatry. 2006;21:983–989 [DOI] [PubMed] [Google Scholar]
- 12.Kreisel SH, Blahak C, Bazner H, Inzitari D, Pantoni L, Poggesi A, et al. Deterioration of gait and balance over time: The effects of age-related white matter change--the ladis study. Cerebrovasc Dis. 2013;35:544–553 [DOI] [PubMed] [Google Scholar]
- 13.Blanco PJ, Muller LO, Spence JD. Blood pressure gradients in cerebral arteries: A clue to pathogenesis of cerebral small vessel disease. Stroke Vasc Neurol. 2017;2:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Reuck J The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur Neurol. 1971;5:321–334 [DOI] [PubMed] [Google Scholar]
- 15.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachdev PS, Thalamuthu A, Mather KA, Ames D, Wright MJ, Wen W, et al. White matter hyperintensities are under strong genetic influence. Stroke. 2016;47:1422–1428 [DOI] [PubMed] [Google Scholar]
- 17.Fennema-Notestine C, McEvoy LK, Notestine R, Panizzon MS, Yau WW, Franz CE, et al. White matter disease in midlife is heritable, related to hypertension, and shares some genetic influence with systolic blood pressure. Neuroimage Clin. 2016;12:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornage M, Debette S, Bis JC, Schmidt H, Ikram MA, Dufouil C, et al. Genome-wide association studies of cerebral white matter lesion burden: The charge consortium. Ann Neurol. 2011;69:928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jian X, Satizabal CL, Smith AV, Wittfeld K, Bis JC, Smith JA, et al. Exome chip analysis identifies low-frequency and rare variants in mrpl38 for white matter hyperintensities on brain magnetic resonance imaging. Stroke. 2018;49:1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaaren BF, de Boer R, Vernooij MW, Rivadeneira F, Uitterlinden AG, Hofman A, et al. Replication study of chr17q25 with cerebral white matter lesion volume. Stroke. 2011;42:3297–3299 [DOI] [PubMed] [Google Scholar]
- 21.Verhaaren BF, Debette S, Bis JC, Smith JA, Ikram MK, Adams HH, et al. Multiethnic genome-wide association study of cerebral white matter hyperintensities on mri. Circ Cardiovasc Genet. 2015;8:398–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traylor M, Tozer DJ, Croall ID, Lisiecka Ford DM, Olorunda AO, Boncoraglio G, et al. Genetic variation in plekhg1 is associated with white matter hyperintensities (n = 11,226). Neurology. 2019;92:e749–e757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willer CJ, Li Y, Abecasis GR. Metal: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics C, et al. Ld score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi H, Mancuso N, Spendlove S, Pasaniuc B. Local genetic correlation gives insights into the shared genetic architecture of complex traits. Am J Hum Genet. 2017;101:737–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traylor M, Zhang CR, Adib-Samii P, Devan WJ, Parsons OE, Lanfranconi S, et al. Genome-wide meta-analysis of cerebral white matter hyperintensities in patients with stroke. Neurology. 2016;86:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen XJ, Qiu CG, Kong XD, Ren SM, Dong JZ, Gu HP, et al. The association between an endothelial nitric oxide synthase gene polymorphism and coronary heart disease in young people and the underlying mechanism. Mol Med Rep. 2018;17:3928–3934 [DOI] [PubMed] [Google Scholar]
- 30.Bastian C, Zaleski J, Stahon K, Parr B, McCray A, Day J, et al. Nos3 inhibition confers post-ischemic protection to young and aging white matter integrity by conserving mitochondrial dynamics and miro-2 levels. J Neurosci. 2018;38:6247–6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rannikmae K, Sivakumaran V, Millar H, Malik R, Anderson CD, Chong M, et al. Col4a2 is associated with lacunar ischemic stroke and deep ich: Meta-analyses among 21,500 cases and 40,600 controls. Neurology. 2017;89:1829–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray LS, Lu Y, Taggart A, Van Regemorter N, Vilain C, Abramowicz M, et al. Chemical chaperone treatment reduces intracellular accumulation of mutant collagen iv and ameliorates the cellular phenotype of a col4a2 mutation that causes haemorrhagic stroke. Hum Mol Genet. 2014;23:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traylor M, Malik R, Nalls MA, Cotlarciuc I, Radmanesh F, Thorleifsson G, et al. Genetic variation at 16q24.2 is associated with small vessel stroke. Ann Neurol. 2017;81:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik R, Rannikmae K, Traylor M, Georgakis MK, Sargurupremraj M, Markus HS, et al. Genome-wide meta-analysis identifies 3 novel loci associated with stroke. Ann Neurol. 2018;84:934–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traylor M, Lewis CM. Genetic discovery in multi-ethnic populations. Eur J Hum Genet. 2016;24:1097–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rannikmae K, Davies G, Thomson PA, Bevan S, Devan WJ, Falcone GJ, et al. Common variation in col4a1/col4a2 is associated with sporadic cerebral small vessel disease. Neurology. 2015;84:918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Qu B, Xia X, Kuang Y, Li J, Fan K, et al. Impact of interaction between the g870a and efemp1 gene polymorphism on glioma risk in chinese han population. Oncotarget. 2017;8:37561–37567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbeek E, Meuwissen ME, Verheijen FW, Govaert PP, Licht DJ, Kuo DS, et al. Col4a2 mutation associated with familial porencephaly and small-vessel disease. Eur J Hum Genet. 2012;20:844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo DS, Labelle-Dumais C, Gould DB. Col4a1 and col4a2 mutations and disease: Insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:R97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, et al. Col4a2 mutations impair col4a1 and col4a2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saint-Pol J, Eschenbrenner E, Dornier E, Boucheix C, Charrin S, Rubinstein E. Regulation of the trafficking and the function of the metalloprotease adam10 by tetraspanins. Biochem Soc Trans. 2017;45:937–944 [DOI] [PubMed] [Google Scholar]
- 42.Has C The “kelch” surprise: Klhl24, a new player in the pathogenesis of skin fragility. J Invest Dermatol. 2017;137:1211–1212 [DOI] [PubMed] [Google Scholar]
- 43.Rutten-Jacobs LCA, Tozer DJ, Duering M, Malik R, Dichgans M, Markus HS, et al. Genetic study of white matter integrity in uk biobank (n=8448) and the overlap with stroke, depression, and dementia. Stroke. 2018;49:1340–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osuga H, Osuga S, Wang F, Fetni R, Hogan MJ, Slack RS, et al. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci U S A. 2000;97:10254–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmers I, Zhang H, Bastiani M, Jansma BM, Roebroeck A, Rubio-Gozalbo ME. White matter microstructure pathology in classic galactosemia revealed by neurite orientation dispersion and density imaging. J Inherit Metab Dis. 2015;38:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild PS, Felix JF, Schillert A, Teumer A, Chen MH, Leening MJG, et al. Large-scale genome-wide analysis identifies genetic variants associated with cardiac structure and function. J Clin Invest. 2017;127:1798–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, carf, that regulates neuronal activity-dependent expression of bdnf. Neuron. 2002;33:383–395 [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Selvakumar P, Ali K, Shrivastav A, Bajaj G, Resch L, et al. Expression of n-myristoyltransferase in human brain tumors. Neurochem Res. 2005;30:9–13 [DOI] [PubMed] [Google Scholar]
- 49.Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, et al. A gene encoding a putative gtpase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001;29:166–173 [DOI] [PubMed] [Google Scholar]
- 50.Sachdev P, Chen X, Wen W. White matter hyperintensities in mid-adult life. Curr Opin Psychiatry. 2008;21:268–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.