Abstract
Coronavirus disease 2019 (COVID-19) is a global pandemic, and it is increasingly important that physicians recognize and understand its atypical presentations. Neurological symptoms such as anosmia, altered mental status, headache, and myalgias may arise due to direct injury to the nervous system or by indirectly precipitating coagulopathies. We present the first COVID-19 related cases of carotid artery thrombosis and acute PRES-like leukoencephalopathy with multifocal hemorrhage.
Keywords: COVID-19, Infection, Leukoencephalopathy, Carotid thrombosis, Stroke
Highlights
-
•
COVID-19 may manifest with neurological symptoms
-
•
SARS-CoV-2 may have direct neurologic effects due to endothelial invasion, such as our case of PRES-like leukoencephalopathy.
-
•
Indirect effects may include coagulation cascade disruption, resulting in unusual thrombosis and large vessel occlusions.
1. Introduction
The SARS-CoV-2 virus (severe acute respiratory syndrome-coronavirus 2) and the resultant Coronavirus disease 2019 (COVID-19) was first identified in December 2019, and has subsequently spread into a world-wide pandemic with at least 1.5 million cases in 212 countries [1]. The point of entry for the SARS-CoV-2 virus is the ACE2 cell surface receptor, which is variably found in many organs including the lungs and gastrointestinal tissues, which may explain COVID-19 transmission and symptom manifestation, typically reported to be fever, cough, shortness of breath, and diarrhea [[2], [3], [4], [5]].
Recent reports have additionally identified COVID-19-related neurologic symptoms such as anosmia, altered mental status, headache, and myalgias [[6], [7], [8], [9]]. These neurologic findings may be attributed to both direct and indirect effects of the SARS-CoV-2 virus (Fig. 1 ) [10,11]. Direct nervous system injury may occur through hematogenous spread or retrograde CNS invasion as the ACE2 receptor is also expressed in skeletal muscles, vascular endothelial cells, and nerve cells such as in the olfactory bulb [[12], [13], [14]]. Indirectly, SARS-CoV-2 may affect coagulation cascade functionality, leading to thrombus formation or hemorrhage, including strokes and acute hemorrhagic necrotizing encephalopathy [[15], [16], [17], [18], [19], [20]].
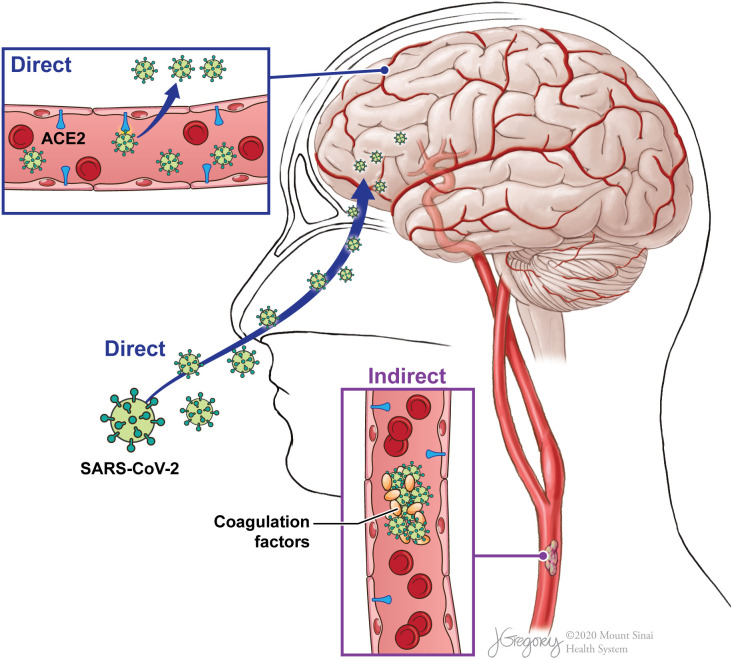
Fig. 1.
Potential pathways for SARS-CoV-2 nervous system involvement. SARS-CoV-2 virus may directly invade through hematogenous spread or retrograde effect through ACE2 receptors on vascular endothelial cells or the nervous system. SARS-CoV-2 may also affect coagulation cascade functionality, indirectly precipitating thrombus formation or hemorrhage.
(Illustration credit to Jill Gregory, printed with permission from ©Mount Sinai Health System).
We report two unusual neurological presentations which may be related to these potential direct and indirect effects of COVID-19 infection, with clinicoradiological correlations. First, carotid thrombosis with large ischemic stroke; large ischemic strokes have previously been identified in the prior SARS outbreak, and may be an initial presenting finding in COVID-19 [15]. Secondly, while posterior reversible encephalopathy syndrome (PRES) has been reported in other viral illnesses, this is the first PRES-like case in COVID-19 diagnosed with imaging findings of cortical/subcortical edema in a symmetric and more dominant distribution in the parieto-occipital lobes [[21], [22], [23]].
2. Case presentations
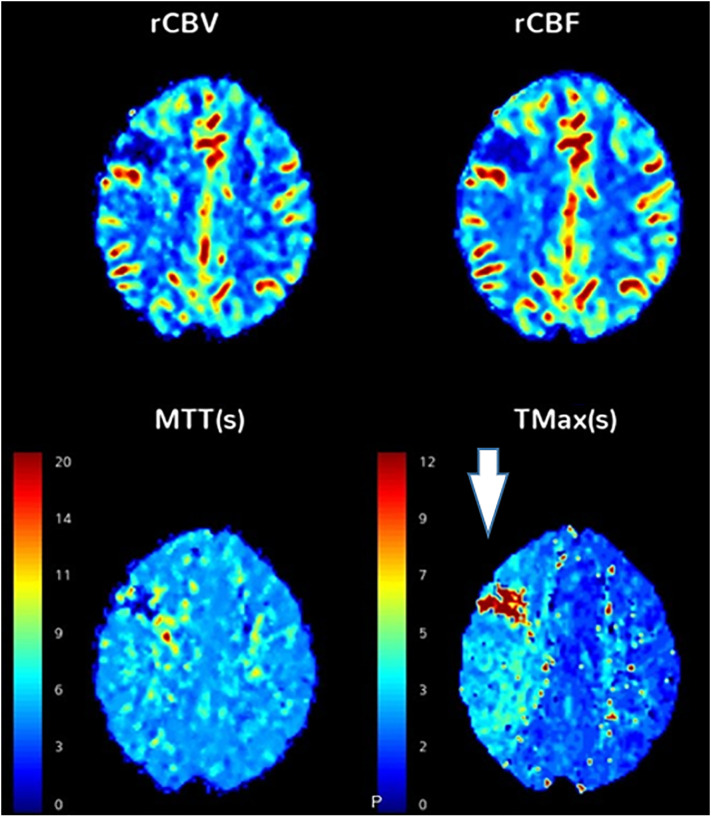
The first case is that of a 55 year-old male with past medical history of insulin-dependent diabetes mellitus, who presented to the emergency department with left wrist droop. Initial CT head was negative. While in the emergency department, the patient developed new-onset left gaze preference, left facial droop and left arm weakness. A CT angiogram of the head and neck was obtained, which showed large thrombus in the right common carotid artery, and CT head perfusion study showed acute right frontal ischemic infarct and surrounding penumbra (Fig. 2 ), with lung apical findings raising suspicion for COVID-19. Catheter angiography confirmed a large thrombus in the right carotid artery for which chemical thrombolysis was performed with eptifibatide. Patient was found to be COVID-19 positive on reverse transcriptase polymerase chain reaction test of a nasopharyngeal swab. He developed fevers, and was started on aspirin, atorvastatin, plaquenil, and azithromycin. The patient's dysarthria and left gaze preference began to improve. A follow-up MR angiogram (MRA) of the head and neck was performed which demonstrated significantly decreased thrombus burden.
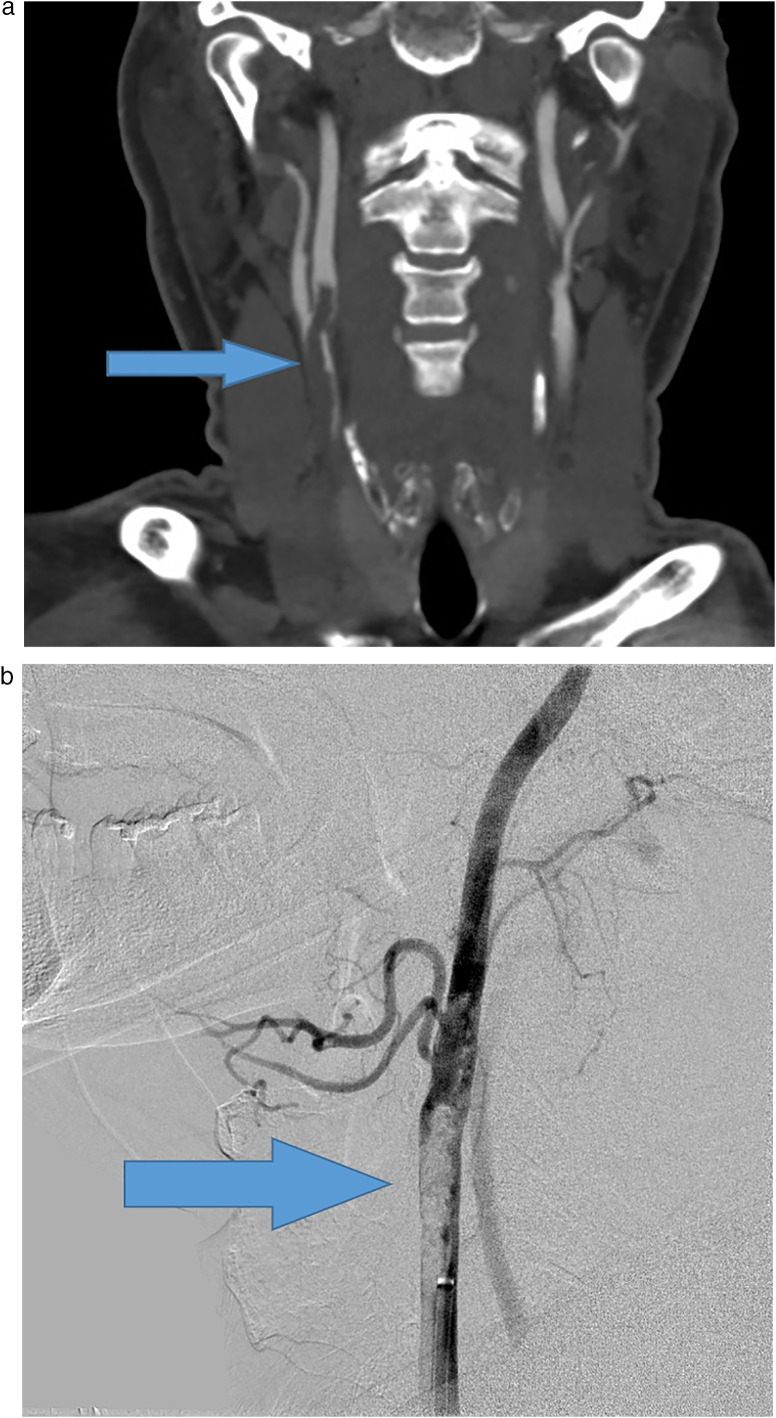
Fig. 2.
Large thrombosis of the carotid artery. A 55 year-old male, who presented with left wrist drop, found to be COVID-19 positive by serology. (A) CT angiogram of the head and neck showed a large subocclusive thrombosis of the right common carotid artery extending into the internal and external carotid arteries (arrows). (B) CT perfusion study showed acute infarct in the right superior frontal lobe, with a large area of increased Tmax in the right cerebral hemisphere, involving the right frontal and parietal lobes, suggesting an area at risk for further infarction (box). (C) Subsequently, a right carotid endovascular chemical thrombolysis was performed.
The second case is that of a 64 year-old male, former smoker with no other past medical history or prescribed medications, who presented to the hospital with one week history of ongoing productive cough, worsening shortness of breath, generalized fatigue, decreased appetite, myalgias, fevers, chills, and malaise. In the emergency department, the patient was found to be mildly tachycardic, tachypneic, and saturating at 88% on room air which improved to 96% on 4 liters nasal cannula. The patient tested positive for SARS-CoV2, and was admitted. Subsequently while on the floor, the patient continued to desaturate with higher oxygen requirements, and was transferred to the intensive care unit (ICU) for close monitoring. Given worsening respiratory failure, the patient was intubated, and placed on maximal ventilatory support. Due to ongoing desynchrony and desaturation, the patient was paralyzed with a vecuronium drip, started on inhaled nitric oxide and subsequently proned for 12 h. Early ICU course was further complicated by persistent fevers up to 103, acute kidney injury with electrolyte abnormalities, and transaminitis.
The patient was administered hydroxychloroquine, azithromycin, vancomycin and ceftriaxone. Given the severity of his condition, the patient was given two doses of tocilizumab. As the patient began to improve, ventilator support was decreased and paralysis was weaned off. Thereafter the patient developed rhythmic jerking movements that were refractory to midazolam pushes, but stopped with vecuronium push. Video electroencephalogram was consistent with status epilepticus. Multiple antiepileptic drugs including intravenous levetiracetam, lacosamide and valproic acid, along with a midazolam drip were required to stop the seizure activity.
Initial CT head showed striking hypoattenuation of the white matter consistent with bilateral posterior cerebral vasogenic edema, and repeat CT head a few days later revealed interval devolvement of multiple small foci of brain parenchymal hemorrhage, right greater than left (Fig. 3 ). Initial brain MRI re-demonstrated extensive symmetric cerebral edema with a parieto-occipital dominance and scattered small foci of recent hemorrhage. MRA and MR venography were both negative for any acute abnormalities. Repeat brain MRI showed slightly decreased but persistent extensive cerebral edema with stable foci of hemorrhage. Lumbar puncture was performed and cerebrospinal fluid analysis was within normal limits except for slight nonspecific elevation of protein.
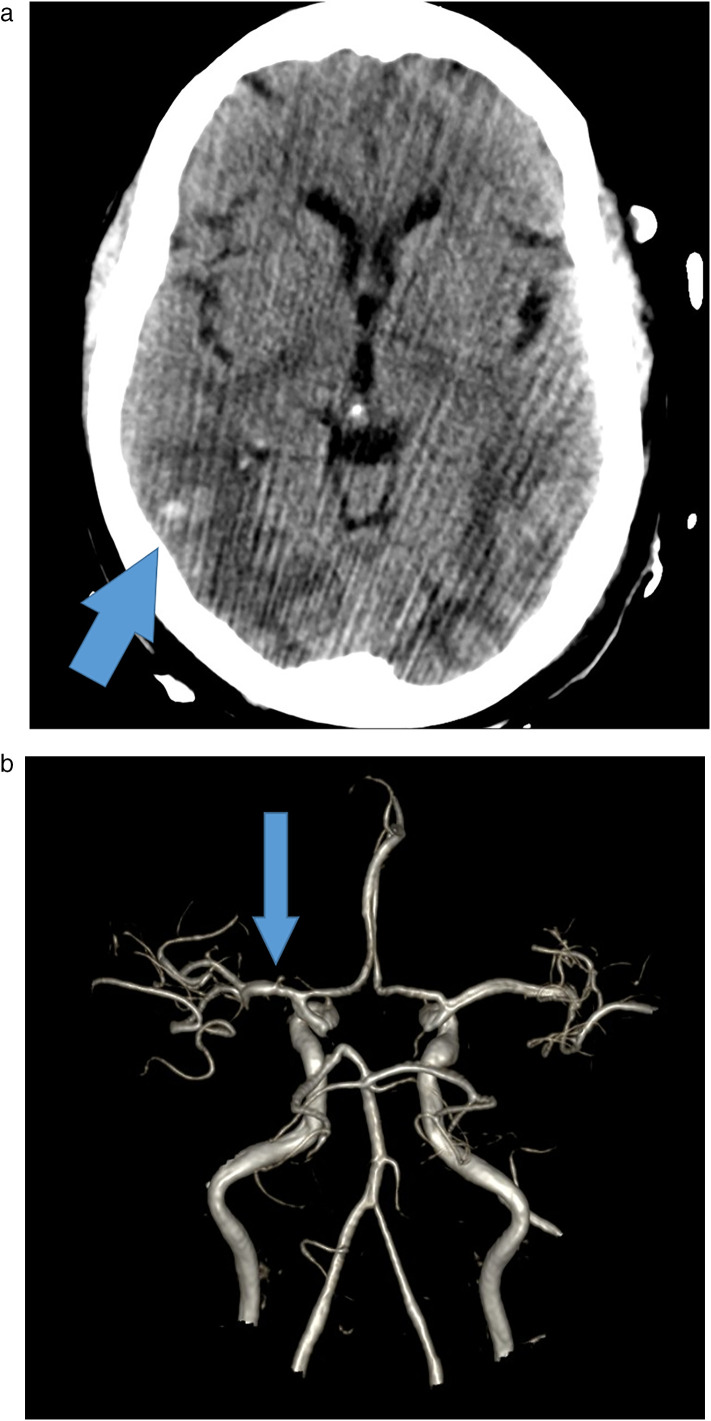
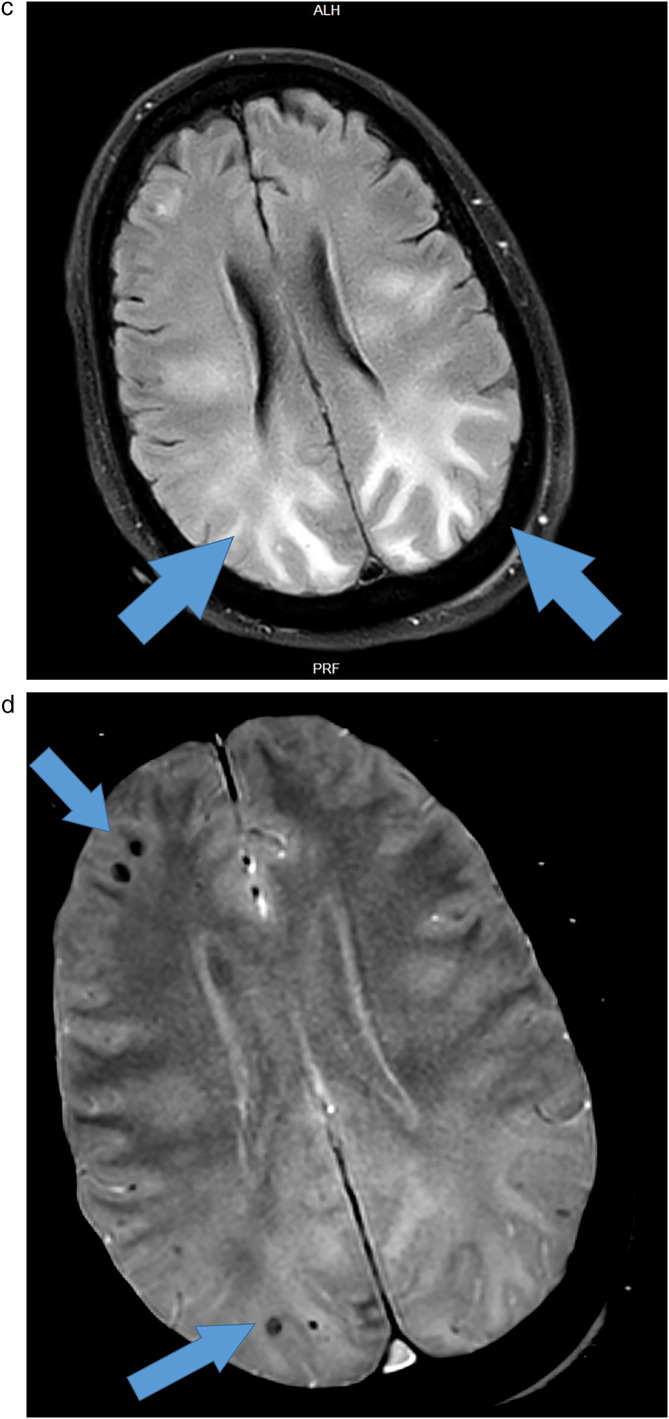
Fig. 3.
Acute PRES-like leukoencephalopathy with multifocal hemorrhage. A 64 year-old male, COVID-19 positive by serology, who initially presented to ED with cough, fever, and chills, with hypoxic respiratory failure requiring admission to MICU, subsequently had a change in mental status. (A) CT head demonstrated foci of hemorrhage with bilateral posterior cerebral vasogenic edema. (B) MRA of the brain, 3D reconstruction showing mild narrowing of the left MCA but no acute abnormalities. (C) MRI of the brain, FLAIR sequence showing symmetric cerebral edema greatest in the parieto-occipital white matter, and (D) same MR of the brain, GRE sequence showing multifocal foci of hemorrhage, together likely reflecting posterior reversible encephalopathy syndrome (PRES)-like leukoencephalopathy with hemorrhagic component.
3. Discussion
We demonstrate two patients with unusual neurologic clinical presentations and imaging findings in the setting of COVID-19. While neurologic symptoms such as altered mental status may be an atypical presentation of COVID-19, it may be more commonly seen as the incidence of COVID-19 increases.
As seen in our first case, COVID-19 may indirectly precipitate unusual thrombotic or hemorrhagic findings in patients without other predisposing factors. Disruption of the coagulation cascade may result in thrombus formation in proximal large vessel branch points such as the distal common and cervical internal carotid arteries, causing intracranial large vessel occlusions or unusual cerebrovascular accidents. Potential SARS-CoV-2 related coagulation cascade dysfunction is beginning to be recognized for thrombus formation in COVID-19 patients, and as recent studies have shown, may adversely affect prognosis [24,25].
Our second case may be attributable to the direct entry and invasion of the SARS-CoV-2 virus. The pathophysiology of PRES itself is not entirely understood, however is thought to be related to hyperperfusion or vasospasm, with fluid extravasation through the blood-brain barrier resulting in cortical or subcortical edema [26]. In our second patient, this PRES-like appearance may be attributable to direct uptake of SARS-CoV-2 via the olfactory bulb and surface ACE2 targets on vascular endothelial cells, injuring the blood-brain barrier [14]. Additionally, the ACE2 enzyme degrades angiotensin-II in the renin-angiotensin system, which affects blood pressure regulation, and may be another potential pathophysiological explanation. The rare hemorrhagic component may have been due to superimposed COVID-19 coagulopathic abnormalities.
Further research is needed to confirm the mechanisms underlying SARS-CoV-2 invasion and its resultant neurological effects. Due to the potential for SARS-CoV-2 to directly and indirectly affect the nervous system as described above, physicians should be aware of these atypical neurologic presentations in COVID-19 patients.
Declaration of competing interest
None, for all listed authors and contributors.
References
- 1.World Health Organization Coronavirus [internet] https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available from.
- 2.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. https://pubmed.ncbi.nlm.nih.gov/32133578/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. https://pubmed.ncbi.nlm.nih.gov/32142651/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H.-Y., Li X.-L., Yan Z.-R., Sun X.-P., Han J., Zhang B.-W. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord. 2020;13 doi: 10.1177/1756286420917830. [1756286420917830] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7149362/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin H., Hong C., Chen S. Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke Vasc Neurol. 2020;94(19):809–810. doi: 10.1212/WNL.0000000000009455. https://pubmed.ncbi.nlm.nih.gov/32229625/ [svn-2020-000382] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020 doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 10.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conde G., Pájaro L.D.Q., Marzola I.D.Q., Villegas Y.R., Salazar L.R.M. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J Neurol Sci. 2020;0(0) doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58(3):299–301. doi: 10.4193/Rhin20.114. https://pubmed.ncbi.nlm.nih.gov/32240279/ [DOI] [PubMed] [Google Scholar]
- 13.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Nerosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 14.Politi L.S., Salsano E., Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2125. (Published online May 29) [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;0(0) doi: 10.1056/NEJMc2007575. [null] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han M., Yan W., Huang Y. The nucleocapsid protein of SARS-CoV induces transcription of hfgl2 prothrombinase gene dependent on C/EBP alpha. J Biochem (Tokyo) 2008;144(1):51–62. doi: 10.1093/jb/mvn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoniak S., Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123(17):2605–2613. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. https://pubmed.ncbi.nlm.nih.gov/32228363/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. https://www.sciencedirect.com/science/article/pii/S1386653220301049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai L.-K., Hsieh S.-T., Chang Y.-C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14(3):113–119. [PubMed] [Google Scholar]
- 21.Bartynski W.S. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. Am J Neuroradiol. 2008;29(6):1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney A.M., Short J., Truwit C.L. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. Am J Roentgenol. 2007;189(4):904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 23.Bartynski W.S., Upadhyaya A.R., Boardman J.F. Posterior reversible encephalopathy syndrome and cerebral vasculopathy associated with influenza a infection: report of a case and review of the literature. J Comput Assist Tomogr. 2009;33(6):917–922. doi: 10.1097/RCT.0b013e3181993a43. [DOI] [PubMed] [Google Scholar]
- 24.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. https://pubmed.ncbi.nlm.nih.gov/32291094/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartynski W.S. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol. 2008;29(6):1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]