Convalescent plasma therapy is an experimental procedure, which has been used in several countries in treating patients with extreme coronavirus disease 2019 (COVID-19) [1].
The therapy's efficacy was found when the viral load was undetectable in many patients who had prior viremia with no significant adverse effects after transfusion [2], [3].
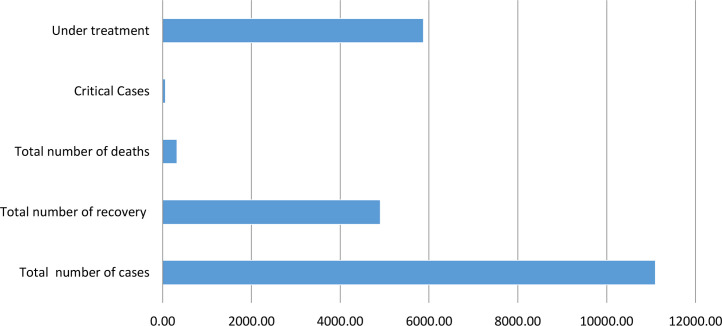
Iraq recorded 1252 confirmed cases of COVID-19 distributed on 18 states on 6 June 2020 and the dominant number was in Baghdad with 665 cases (the number of cases recorded in Iraq since the date of the first case to 6 June was 11,098 cases; their details are clarified in Fig. 1 ).
Figure 1.
COVID-19 situation in Iraq on the 6th of June, 2020.
Situation du COVID-19 en Iraq au 6 juin 2020.
The medical procedure used in Iraq relies on the symptoms being assisted and the extreme cases being treated with hydroxychloroquine.
For the critical cases, the Iraqi National Blood Bank began inviting patients who have recovered from the disease to donate their plasma to be used in treating other patients who need it.
After obtaining the consent, the donor should be without symptoms after 14–28 days, and should be checked to reassure for neutralisation of antibody titer and COVID-19 PCR [4].
400–450 mL of plasma should be used to allow for more donations and leukodepleted plasma should be used.
Iraq was faced with a major challenge when the recovered patients were contacted to donate to save the lives of others; only 50 out of 630 patients (7.9%) agreed to be donors and the others refused for many reasons as shown in Fig. 2 .
Figure 2.
Recovered patients’ stated reasons for refusing to donate their plasma.
Raisons invoquées par les patients ayant récupéré pour refuser de donner leur plasma.
The plasma is administered as 400 mL in the same day or 200 mL in two doses for each with several hours apart but should be finished within 24 hours. The plasma is only available for serious cases, and life-threatening cases depending on the symptoms [5].
The criteria for severe cases are: dyspnea, respiratory frequency 30/min, blood oxygen saturation 93%, partial arterial O2 pressure to fraction of the inspired O2 ratio < 300, and/or lung infiltration > 50% within 24 to 48 hours, respectively.
While the life-threatening disease conditions are defined as: respiratory failure, septic shock, and/or multiple organ dysfunction or failure.
Informed consent is required to undergo convalescent plasma therapy for both the patients and the donors.
In Iraq, convalescent plasma therapy provides good results in the treatment of serious cases, so the country's media, government and religious centres have begun to invite recovered people to respond to the invitation from the Iraqi National Blood Bank to donate their plasma and there is a response, but some of them are still asking for money to offer their plasma, particularly for the rare types of blood.
I should state that medical care in Iraq is free for all people and Corona patients do not pay any money for their treatment because, according to the Iraqi law, it is on the government.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jemep.2020.100564.
Disclosure of interest
The author declares that he has no competing interest.
Online supplement. Supplementary data
References
- 1.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan K., Liu B., Li Czhang H. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn J.H., Sohn Y., Lee S.H. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020 doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 5.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.