To the Editor: COVID-19, which is due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a worldwide pandemic on March 11, 2020.1 , 2
Recently, numerous cases of acute chilblains-like lesions have been reported on social networks from Belgium, France, and Italy. Despite weak evidence, particularly the absence of serologic studies, the hypothesis that these lesions were potentially related to the COVID-19 infection, as a paucisymptomatic form, rapidly grew.3, 4, 5 An alert letter was even sent to the French Ministry of Health and to French dermatologists by the National Union of Dermatologists.
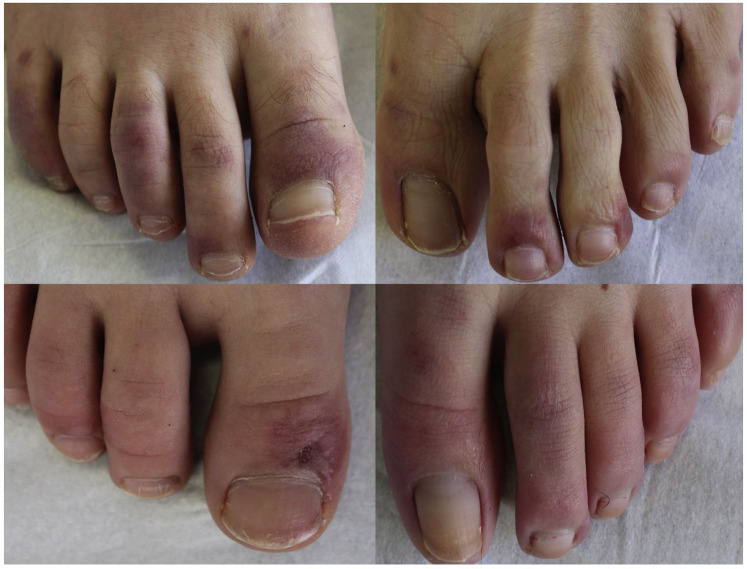
Because 33 patients (14 women, 19 men) with chilblains were referred to our dermatology department within 1 week, we studied the relationship between these lesions and the COVID-19 infection. All patients (mean ± standard deviation age, 23.4 ± 8.7 y) presented erythematous and purpuric papules localized on the toes alone or toes and fingers (12 patients, 36.4%) (Fig 1 ). Edema was present in 12 (36.4%) and pruritus or burning sensation in 18 (54.5%) patients. The median delay between the onset of dermatologic features and the first consultation was 8 days (interquartile range, 6.5-18 d). Chilblains were the only clinical manifestation in 23 cases (69.7%); 10 (30.3%) patients presented other symptoms, which occurred before (n = 6), concomitantly with (n = 1), or after skin symptoms (n = 3): asthenia (n = 4), cough (n = 3), diarrhea (n = 3), fever (n = 2), myalgia (n = 2), headache (n = 1), and odynophagia (n = 1). No patient reported contact with COVID-19–infected patients. Three patients had recently been tested for COVID-19 infection and had negative results on sinus reverse-transcription polymerase chain reaction (RT-PCR).
Fig 1.
Examples of 4 patients referred for chilblains.
Blood cell count results were normal in 26 patients. A mild lymphopenia (mean, 1.15 ± 0.21 giga per liter) was observed in 7 patients. C-reactive protein and erythrocyte sedimentation rate results were negative for all patients. Two patients had positive results for antinuclear antibodies, and 3 patients had antibodies for a type III cryoglobulinemia.
Histology performed in 5 patients showed lymphocytic infiltrate in the superficial dermis around the vessels and eccrine glands in all cases, reminiscent of idiopathic chilblains. Direct immunofluorescence showed fibrinogen and C3 deposits on endothelial cells in 2 cases. Results of indirect immunofluorescence assay using the serum from a patient with anti–SARS-CoV-2 immunoglobulin (Ig) G antibodies and RT-PCR on lesional skin were negative.
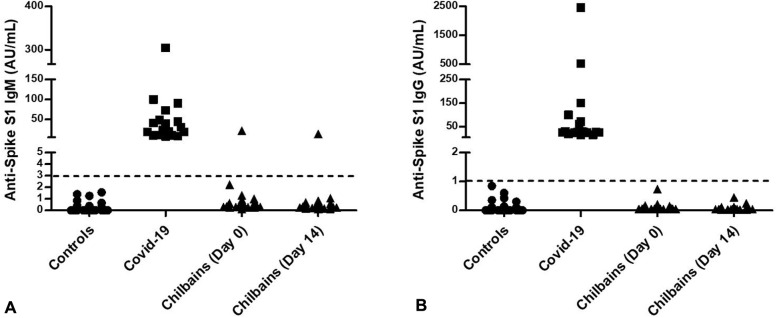
We developed an ALBIA-Spike S1 for detecting anti–SARS-CoV-2 antibodies that allowed a 96% sensitivity and 98% specificity (Drouot et al, personal communication, May 2020). Test results for anti–SARS-CoV-2 IgG antibodies were negative in all 33 sera, and in 1 of 33, the result was positive for IgM at the first consultation and at the second consultation 14 days later. No seroconversion was observed (Fig 2 ). Our findings were then confirmed by using the Abbott (Abbott Park, IL) SARS-CoV-2 IgG immunoassay performed on the day 14 sera, without detectable seropositivity.
Fig 2.
Severe acute respiratory syndrome coronavirus 2 serology. Sera from negative control patients (circles, n = 130; health donors of blood collected by the French Établissement Franҫais du Sang before the pandemic), patients with COVID-19 (squares, n = 18; patients with reverse-transcription polymerase chain reaction results from Rouen University Hospital), and patients with chilblains (triangles, n = 33 at day 0 and n = 31 at day 14) were assayed for the presence of anti-Spike S1 antibodies. A, Immunoglobulin M. B, Immunoglobulin G. The dotted line marks the threshold of positivity of the assay. AU, Arbitrary units; Ig, immunoglobulin.
The present data provide no argument for any link between these chilblains and infection with SARS-CoV-2. Clinical and histologic features were those of idiopathic chilblains. The results of RT-PCR and indirect immunofluorescence on lesional skin, when performed, were negative. None of the 33 sera tested contained anti–SARS-CoV-2 IgG antibodies, and only 1 had IgM twice (3%), consistent with the current estimation of a rate of SARS-CoV-2 infection of 5% to 10% in the general population in France.6
We think that this hypothesis of COVID-19–related chilblains could be explained by a cumulation of (1) a temporality bias in this early spring period, when the average temperature differences were the widest; (2) a confounding bias related to the young age, because paucisymptomatic forms of the infection are observed in young people; and (3) a recruitment bias related to the shortening of dermatology consultation delays due to the quarantine period.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Institute for Research and Innovation in Biomedicine.
Reprints not available from the authors.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouaziz J., Duong T., Jachiet M., et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Masson A., Bouaziz J.-D., Sulimovic L., et al. Chilblains are a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolivras A., Dehavay F., Delplace D., et al. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institut Pasteur Une modélisation indique qu'entre 3% et 7% des Français ont été infectés. 2020. https://www.pasteur.fr/fr/espace-presse/documents-presse/covid-19-modelisation-indique-que-pres-6-francais-ont-ete-infectes