Abstract
Amyloid-β (Aβ) and tau proteins currently represent the two most promising targets to treat Alzheimer's disease. The most extensively developed method to treat the pathologic forms of these proteins is through the administration of exogenous antibodies, or passive immunotherapy. In this review, we discuss the molecular-level strategies that researchers are using to design an effective therapeutic antibody, given the challenges in treating this disease. These challenges include selectively targeting a protein that has misfolded or is pathological rather than the more abundant, healthy protein, designing strategic constructs for immunizing an animal to raise an antibody that has the appropriate conformational selectivity to achieve this end, and clearing the pathological protein species before prion-like cell-to-cell spread of misfolded protein has irreparably damaged neurons, without invoking damaging inflammatory responses in the brain that naturally arise when the innate immune system is clearing foreign agents. The various solutions to these problems in current clinical trials will be discussed.
Graphical abstract
1. Introduction
There are currently about 132 therapeutic agents in 156 clinical trials for Alzheimer's disease (AD) (Cummings et al. (2019)). Among these are about 29 disease-modifying monoclonal antibody therapies involved in 24 clinical trials (Cummings et al., 2018, Cummings et al., 2019), nearly all of which target two key proteins recognized as the major hallmarks in AD pathology: Aβ and tau protein. In AD pathology, Aβ forms extracellular plaques as well as oligomers that can spread the disease by propagating from cell-to-cell. Tau forms neurofibrillary tangles in neurons, and can also form oligomers that spread pathology by propagating from cell-to-cell. This review will focus on therapies targeting Aβ and tau in clinical trials, related therapies in pre-clinical development, and the underlying biochemical mechanisms that motivate researchers to hypothesize that these therapies will be effective in treating AD.
In describing the common mechanisms that underly the effectiveness of potential antibody therapeutics, we found ourselves emphasizing general themes of antibody development that various different therapeutic strategies may have in common. As well, Aβ and tau have been shown to have intimately connected pathology, and therapeutic strategies targeting Aβ exclusively have had a long history fraught with ambiguous results and minimal therapeutic benefit. For these reasons it became almost inevitable to include both Aβ and tau therapies in the same review. As some examples of biochemical similarity, both Aβ and tau have both been shown to have distinct, pathological species with conformations different from the healthy proteins, both are subject to isoform imbalance as a cause or symptom of pathology, both undergo post-translational modifications specific to pathological behavior that have been targeted by several candidate therapeutics, and both have been shown to form oligomers that propagate from cell-to-cell in prion-like fashion, which constitute therapeutic targets of specific interest.
Several excellent recent articles have reviewed current clinical developments for Aβ immunotherapies (Moreth et al. (2013); Mavoungou and Zimmerman (2013); Liu et al. (2016); van Dyck (2018); Panza et al. (2019)), tau immunotherapies (Pedersen and Sigurdsson (2015); Sigurdsson (2018); Novak et al. (2018a); Shahpasand et al. (2018); Medina (2018); Iqbal et al. (2018); Hoskin et al. (2019)), or both Aβ and tau immunotherapies (Citron (2004); Pul et al. (2011); Panza et al. (2012); Wisniewski and Goñi (2015); Hung and Fu (2017); Dolan and Zago (2018); Cummings et al. (2018); Katsinelos et al. (2019); McAlary et al. (2019b)). The widely read Alzforum domain (www.alzforum.org) is another useful source of both current and archival clinical and pre-clinical results. Our approach here has been to try to emphasize the conceptual bases underlying the strategies for the development of various immunotherapies. For example, we discuss the custom immunogens used in the active immunization phase, why they were chosen, and how they may lead to disease-selective antibodies. We also sought to describe the rationale for targeting specific epitopes, including those that appear to have disease-selective post-translational modification. The task of epitope prediction, in order maximize the efficacy of a therapeutic, is a difficult one that is understandably under-addressed. We briefly discuss a method for misfolding-specific epitope prediction (Peng et al. (2018)) here. We also discuss in detail the notions of conformational-plasticity of the target proteins Aβ and tau, and the conformational-selectivity in binding profile that an effective antibody therapeutic should possess.
1.1. Rationale for targeting Aβ and tau
There is now an enormous amount of independently gathered genetic, neuropathological, and experimental data supporting the connection between Aβ aggregation and the cognitive symptoms of AD, collectively referred to as the amyloid cascade hypothesis (Hardy and Higgins (1992); Hardy and Selkoe (2002); Hardy (2006); Karran et al. (2011); Selkoe (2012); Wisniewski and Goñi (2015)). Overexpresssion of Aβ due to trisomy 21 in individuals with Down syndrome associates with early-onset AD (Bertram and Tanzi (2005); Hartley et al. (2015)). Over 30 mutations in amyloid precursor protein, in and around the region of the Aβ peptide, are associated with inherited forms of AD (Van Cauwenberghe et al. (2016); AlzForum.org, 2020c). The mutation A673T in APP, which reduces amyloidogenic BACE1 processing of APP and to a lesser extent decreases Aβ 42 peptide aggregation, is protective against AD (Jonsson et al. (2012); Maloney et al. (2014)). γ-secretase processively cleaves APP to make Aβ peptides of appropriate length; this is dependent on the stability of its catalytic presenilin-1 (PSEN1) transmembrane domain.
Over 350 mutations in the intramembrane protease γ-secretase, more than 300 of which are in the presenilin-1 (PSEN1) domain,1 increase the production of amyloidogenic Aβ 42 and cause some of the earliest, most aggressive forms of familial AD (Scheuner et al. (1996); De Strooper et al. (1998)). The PSEN2 domain of γ-secretase, which shares 67% homology to PSEN1, also contains about 38 currently known AD-associated mutations1. PSEN2 plays a secondary role to PSEN1 in AD, and many mutations of PSEN2 are not fully penetrant (Cai et al. (2015)). Dominantly inherited AD is thus caused by mutations in either the substrate or protease enzyme in the reaction that produces Aβ. A significant correlation between the thermal stability of the PSEN1–Aβn complex, as determined by melting temperatures (T m) of various AD-linked PSEN1 mutants, and the initial age of onset of AD has been observed (Szaruga et al. (2017)). This effect is hypothesized to be due to increased dissociation rates of the complex, resulting in reduced processivity, and thus the release of longer, incompletely processed Aβ peptides.
Apolipoprotein E (APOE) is a protein involved in the metabolism of fats in the body and is the principal cholesterol carrier in the brain (Puglielli et al. (2003)). APOE exists in three main polymorphisms among the human population differing by two amino acid identities: namely the ε2 (C112, C158), ε3 (C112, R158), and ε4 (R112, R158) isoforms. The ε3 isoform is the most common (78% worldwide allele frequency). Individuals carrying APOE ε2/ε2 or ε2/ε3 (8.4% of the population) are at decreased risk of AD (Liu et al. (2013)). In vitro and in vivo evidence in APP transgenic mice has shown that APOE-ε3, but not APOE-ε4, attenuates Aβ protofibril-induced aggregation, by forming stabilizing complexes with Aβ (Hori et al. (2015)). As well, the APOE-ε4 isoform is not as effective as the others at clearing Aβ (Jiang et al. (2008); Castellano et al. (2011)), and carriers of two copies of the ε4 allele have on average 20× the risk of developing AD (Hauser and Ryan (2013)). The ε4 variant of APOE is currently the most significant known genetic risk factor for late-onset sporadic AD (Sadigh-Eteghad et al. (2012); Roda et al. (2019)).
Normally functioning TREM2, which encodes triggering receptor expressed on myeloid cells 2, facilitates microglia activation and clustering around amyloid and neurofibrillary tangles, increasing amyloid uptake, phagocytic activity, and plaque compaction in early stages of AD (D'Andrea et al. (2004); Hickman et al. (2018)). These processes are impaired in AD-associated variants of TREM2, resulting in filamentous plaques associated with increased dystrophic neurites and a possible increase of tau pathology. (Jay et al. (2017); Ulrich et al. (2017); Gratuze et al. (2018); Zheng et al. (2018)). Some variants of the TREM2 gene have been found to cause increased susceptibility to late onset AD with an odds ratio similar to that of ApoE-ε4 (Guerreiro et al. (2012); Jonsson et al. (2013)). The TREM2 mutant with the strongest AD association, R47H, has 3–4× the AD risk as wild-type, and shows significantly reduced Aβ -induced microglial responses in transgenic mouse models. Since TREM2 is exclusively expressed on immune cells, the above findings provide a direct link between dysregulation of the innate immune system as an active driver contributing to AD pathogenesis.
In summary, abundant evidence points to the progressive accumulation of Aβ in the brain, along with its impaired clearance and induced neuroinflammation, as very early features of the Alzheimer's pathogenic process.
More recent findings from genome-wide association studies (GWAS) and massive parallel resequencing (MPS) efforts emphasize the multifactorial nature of AD. There are currently over 25 genetic risk loci that contribute to the 60–80% heritability estimate for one's genetic predisposition for AD (Van Cauwenberghe et al. (2016)). Risk-associated genes roughly cluster into 3 biochemical pathways: cholesterol and lipid metabolism, immune system and inflammatory response, and endosomal vesicle cycling.
Early biomarkers of AD precede any clinically discernable changes in cognition by many years, perhaps decades (Villemagne et al. (2013)). The first known biomarker is decreased CSF Aβ 42, followed by increased brain Aβ amyloid load; This is then followed by increased concentration of CSF tau (both total and phosphorylated), then decreased glucose metabolism as measured by fluorodeoxyglucose PET (Iqbal et al. (2005); Jack et al., 2010, Jack et al., 2013). It is now accepted that accumulation of tau correlates more closely with severity of dementia than does amyloid load (Tomlinson et al. (1970); Arriagada et al. (1992); Bierer et al. (1995); Nelson et al. (2010); Serrano-Pozo et al. (2011); Nelson et al. (2012)), however there is now evidence that Aβ accumulation can exacerbate tau misfolding and pathology, and vice versa (Jucker and Walker (2011); Ashe and Aguzzi (2013); Dai et al. (2017); Rajamohamedsait et al. (2017)).
2. Immunization strategies; active immunization
Allmost all antibody therapies require some form of active immunization strategy for their generation. The immunization strategy is often a critical step in the development pipeline, as it largely determines the binding profile and selectivity of the resulting antibody.
2.1. Lessons learned from active immunization
The very first forays into antibody therapy were a form of active immunization involving inoculation with a less virulent form of the small pox virus, in the 1700's by early medical practitioners such as Benjamin Jesty and Edward Jenner (Riedel (2005)). Historically, active immunization has been used to prevent the spread of infectious diseases, before infection has occurred, while passive immunization has been used after symptoms have already manifest (Alpaugh and Cicchetti (2019)). It may be interesting and potentially fruitful to revisit this paradigm in treating AD at its various stages, either as treatment or for prophylaxis. However, in cancer, where roughly 30 passive immunotherapies are currently available and 30 more are in late stage clinical trials (Carter and Lazar (2017); Alpaugh and Cicchetti (2019); Kaplon and Reichert (2019)), only two active vaccines have been approved as therapies (Griesenauer and Kinch (2017)).
Several other active immunizations are currently in clinical trials, including CAD106 (phase III, NCT02565511), UB-311 (phase II, NCT03531710), and GV1001 (phase II, NCT03959553) for Aβ (Winblad et al. (2014); clinicaltrials.gov).
Active vaccination trials raise an interesting possibility to obtain human Aβ or tau antibodies from B-cell pools isolated from the best responders, for subsequent use as effective passive immunotherapeutics. Thus far however, there appears to be no published work seriously pursuing this possibility, albeit selection of effective clones involves significant technical challenges. Similar lines of development have been pursued for aducanumab and BIIB076, described in more detail below.
2.1.1. Active Aβ immunotherapy: AN1792
The first active immunization trials using the AN1792 vaccine, which consisted of fibrillar Aβ 42 as the immunogen as well as the QS21 adjuvant, had to be halted because approximately 6% (18/298) of the volunteers developed symptoms of aseptic meningoencephalitis (infiltration of T cells and macrophages) (Gilman et al. (2005)). Nevertheless, several important lessons were learned from these trials. First, the efficacy of the vaccine in removing Aβ load was validated: There was a dramatic clearance of plaques in the brain parenchyma of the volunteers, with broad areas of cerebral cortex devoid of plaques. Some of these patients have remained virtually plaque-free for 14 years, with the extent of plaque removal related to the degree of immune response (Nicoll et al. (2019)). Vascular amyloid and tau-related pathology were not targeted: Tau-reactive neurofibrillary tangles (NFTs) persisted, as well as amyloid in cerebral vessels. CSF tau was decreased in antibody responders however. The immune response in these cases appears to work as a double-edged sword: Anti-Aβ-specific T cells could induce significant adverse effects in AD patients vaccinated with full-length Aβ 42. Cognitive benefits were observed in a neuropsychological test battery (NTB), favoring responders versus placebo, with greater improvements from baseline associated with higher IgG antibody titers in the responders. However, other cognitive tests such as ADAS–Cog, Disability Assessment for Dementia (DAD), Clinical Dementia Rating (CDR), and MMSE showed no significant differences. After 4.6 years, the patients in this study were re-tested using the above metrics. Antibody responders demonstrated a 25.0% lower decline in daily activities as determined by the DAD, a 17.6% lower mean score in caregiver dependence, and a 20.2% less decline on the CDR scale compared with placebo-treated patients (Vellas et al. (2009)). However, no significant differences were observed in the NTB, MMSE, ADAS-Cog tests. On the other hand, in the post-mortem follow-up over a 15-year period, all patients progressed from mild/moderate dementia to moderate/severe dementia; Notably, all five patients with near complete clearance of brain plaques progressed to severe dementia prior to death. These results from the AN1792 trial—though mixed—suggest that Aβ immunotherapy, passive or active, could be helpful in current and future human trials, provided the targeting and time of application are appropriate. On the other hand, the generic targeting of Aβ that is induced from active immunization may not be sufficiently specific to result in long term cognitive benefit across multiple metrics. The observed sustained amyloid clearance over many years implies that if Aβ immunotherapy is useful as a preventative rather than a treatment, then early active immunization could in fact be an effective strategy. That is, sustained constitutive removal of all forms of Aβ (monomer, oligomer, and plaque), before it has a chance to accumulate and propagate, may be an effective strategy for treatment of AD.
2.1.2. Active tau immunotherapy: AADvac-1 and ACI-35
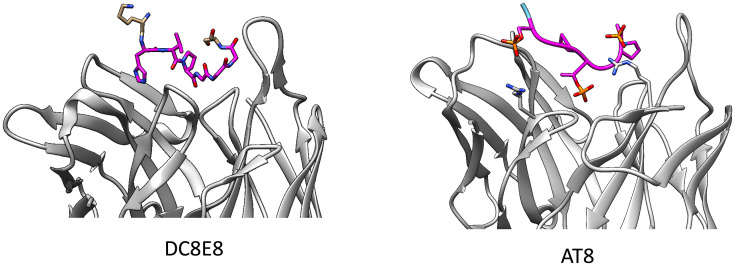
As described in more detail below in the context of tau passive immunotherapies (see Preclinical tau antibodies), mice actively immunized with tau peptides containing the HXPGGG motif generated antibodies that could block the oligomerization of tau. This approach seeks to generate antibodies that block aberrant tau–tau interactions, rather than those selective to pathological phosphorylation sites. A complementary strategy to mouse active immunization, and subsequent humanization for a passive immunotherapy, is to directly immunize humans with a similar peptide. AADvac-1 utilizes a peptide containing one of the epitopes of antibody DC8E8 (see Setion 5.10), namely 294KDNIKHVPGGGS305, conjugated to keyhole limpet hemocyanin (KLH) along with aluminum hydroxide as an adjuvant.
In phase I trials testing the immunogenicity and safety of the vaccine in patients with mild-to-moderate AD (NCT01850238), AADvac-1 was found to be well-tolerated: The vaccine elicited no aberrant immune response or microhemorrhages compared to what was observed with AN1792 (Novak et al., 2017, Novak et al., 2019). Minor injection site reactions were the most common adverse event, observed in 53% participants. In a follow-up study 72 weeks after conclusion (NCT02031198) involving 26 of the same participants, no aberrant immune responses were reported, except for microhemorrhages in one patient. Interestingly, cognitive decline as measured by baseline ADAS-cog11 value was shown to be significantly reduced in treated patients compared with placebo control (Novak et al. (2018b)). These results have prompted AADvac-1 to move into a phase II clinical trial (NCT02579252) whose preliminary results have very recently been reported (Axon Neuroscience (2019)). The vaccine was again deemed safe and tolerable. Roughly 98% of patients generated antibodies against tau. Neurofilament Light Chain (NfL) biomarkers indicated significant slowing of neurodegenerative progression. AD-specific CSF pathological tau biomarkers, including phospho-tau181 and phospho-tau217, also appeared to show moderate to large reductions. Among the younger participants in the trial, there appeared to be positive signals for cognitive endpoints according to CDR-SB, MMSE, and ADCS-MCI-ADL tests, however the strength and significance has not yet been reported.
ACI-35 utilizes a liposomal-anchored 16-amino acid tetra-palmitoylated phospho-tau peptide, 393VYKSPVVSGDTSPRHL408, with S396 and S404 phosphorylated, as they can be in pathological tau. The liposome sizes are such that they can accommodate ≈16 copies of the peptide. Presenting tau on liposomes alters the epitope conformation: Circular dichroism (CD) shows a significant amount of β-sheet structure on the liposome surface similar to that of aggregated tau (Theunis et al. (2013)).
In tau-transgenic mouse models, ACI-35 decreased both soluble and insoluble tau, increased retention of body weight, slightly extended lifespan, and improved the clinical phenotype of motor deficiency (Pihlgren et al. (2016); Theunis et al. (2013)). The vaccine also did not induce marked CNS inflammation in spite of presenting multiple (16) copies of the epitope (Theunis et al. (2013)).
3. Immunization strategies; rational engineering of antibodies and antibody design
In guiding species selectivity and thus the efficacy of an antibody, the immunization strategy is central in determining which sub-population of the conformational ensemble of an epitope that an antibody will bind to. Guiding the epitope towards a desired conformational sub-population is often referred to as “epitope scaffolding” (see e.g. Skerra (2000); Ofek et al. (2010); Correia et al. (2010); Azoitei et al. (2012)). A rational approach to immunization can save much effort by avoiding the subsequent high-throughput screens that are necessary when immunizing irrationally with generic polymorphic forms of a protein.
3.1. Advantages of using antibody-based therapies to target Aβ and tau
The protein drug targets Aβ and tau are both largely intrinsically disordered peptides when isolated as monomers in solution, and as such they are conformationally labile. Such polymorphic targets are inherently difficult to target for small-molecules, which are best-adapted to fit into well-structured binding pockets (Scott et al. (2016)). On the other hand, antibodies are well suited to bind to disordered peptide segments. The selective binding of antibodies to regions of proteins that become disordered during the course of disease has been exploited to generate misfolding-specific antibodies for several proteins wherein misfolding is correlated with neurodegeneration (Paramithiotis et al. (2003); Glabe (2004); Rakhit et al. (2007); Broering et al. (2013); Ayers et al. (2014)).
3.2. Epitope prediction
In choosing the appropriate protein sequence and conformation for active immunization, ideally one would employ a reliable method for epitope prediction, and then use an immunogen appropriately displaying that epitope as it presents in toxic species. This is an enormous challenge at present: Our understanding of what proteinic features can be ubiquitously targeted on toxic species that are involved in the spread of AD is limited at present. Soluble oligomeric species that are thought to be central to the prion-like propagation of AD are conformationally plastic—they do not have a well-defined structure that would lend themselves to structural determination and subsequently epitope identification.
As described further in the examples below, epitopes are either left unidentified when immunizations with pathogenic species such as fibrils or oligomers are used, or a disease-specific isoform is used in the immunogen (e.g. Aβ 42 rather than Aβ 40), or a known post-translational modification observed in pathogenic species is incorporated onto a peptide fragment presented on the immunogen (e.g. phosphorylation of a serine residue, or pyroglutamate cyclization of a glutamic acid).
One method of epitope prediction used for the design of antibodies targeting tau and Aβ involves computational prediction of regions likely to be selectively exposed on the surface of soluble oligomers. This molecular dynamics approach applies the concept of a misfolding-specific epitope, useful in the context of other neurodegenerative diseases (Paramithiotis et al. (2003); Rakhit et al. (2007); Peng et al. (2018)), to the problem of finding epitopes for Aβ and tau. In brief, a fibril structural model is weakened and disrupted by applying a global force along a collective coordinate. The weakest parts of the fibril complex are the first to become disordered, and constitute “stressed protofibril”-specific epitopes (Plotkin (2017)).
3.3. Epitope scaffolding for conformationally selective antibodies
We first describe an experiment providing strong evidence that Alzheimer's disease is a “conformational” disease of Aβ, which emphasizes the importance of conformation in the active immunization stage of antibody development. Peptides of Aβ 1−15 or Aβ 1−16 may be tethered to a liposome surface by conjugating two palmitoylated lysine residues at either end of the peptide, so that, for example the sequence of Aβ 1−16 is Kpal Kpal DAE…HQKKpal Kpal (Nicolau et al. (2002)). Additionally, the termini of the peptide may be PEGylated to provide an additional 77 units of spacing between the peptides and the liposome. Muhs et al., (2007) found, by CD and NMR measurements, that PEGylated, liposome-anchored Aβ preferred a random coil conformation, while non-PEGylated, liposome anchored Aβ preferred a β-sheet conformation, apparently due to enhanced proximity of the peptide to the liposome surface.
Importantly, inoculation of APP/PS-1 double-transgenic mice with liposome anchored Aβ elicited an IgG immune response that resulted in restoration of memory deficits, while inoculation with PEGylated, liposome-anchored Aβ elicited an IgM immune response with no memory benefits (Muhs et al. (2007)). This experiment indicates that antigens presenting N-terminal epitopes of Aβ in what is likely a β-sheet-like conformation will elicit antibodies that target pathogenic, memory-reducing, species of Aβ. These liposomal compounds have been developed as an active vaccine (ACI-24) and are currently in phase 1/2 clinical trials (NCT02738450) in adults with Down syndrome. The pre-humanized murine antibody (mMABT) to crenezumab was generated by liposome-anchored Aβ inoculation, using an epitope subsuming residues 13–24.
3.4. Conformational selectivity
Because of the above-mentioned disorder present in Aβ and tau, these proteins can present themselves to an antibody in multiple different conformations. It is often desirable for an antibody to be conformationally selective to a specific species (Westwood and Lawson (2015)). For example, soluble Aβ is present in normal patient brains at a concentration of about a picomolar, while in AD brains it is present at concentrations ~0.1nM (Lue et al. (1999)). Oligomer concentrations are less well-known but are thought to be about a 1000-fold lower in concentration (Yang et al. (2017)). An antibody that is not conformationally selective for oligomer would suffer from target distraction by binding to the much more abundant monomer species, and thus lack sufficient target engagement.
The brains of healthy (non-AD presenting) elderly patients may contain insoluble Aβ amyloid plaque at concentrations comparable to AD patients (Lue et al. (1999)); Diffuse senile plaques in the cerebral cortex have been considered to be age-related and unassociated with dementia (see e.g. Tagliavini et al. (1988)). Thus, antibodies that bind generically to plaque may again suffer from target distraction, as well as additional clinical risks, particularly for antibodies binding to vascular deposits. In these cases, monocytes and other lymphocytes are recruited to clear the amyloid, and binding of antibody complexes to Fc receptors on macrophage-like cells stimulates the expression of proteases, which in turn degrade the extracellular matrix at those locations. Blood-brain barriers at the vessel wall are thus weakened, insterstitial fluid can enter the brain, and microhemorrhaging can occur (Schilling et al. (2018)). This leakiness of brain vasculature manifests itself through amyloid-related imaging abnormalities (ARIA), which as mentioned above is generally accompanied by microhemorrhaging (ARIA-H) and/or edema (ARIA-E) (see Table 1 for ARIA levels for various Aβ immunotherapies).
Table 1.
Antibodies to Aβ currently in clinical development.
| Antibody | Epitope location | Immunization strategy | Binding Selectivityb | Ab species and backbone isotype | Current Status (2019-2020) | Company | Clinical outcomes |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Last completed Trial (Phase) | Patient stage | Reduced brain Ab burden | Slowing of cognitive decline | ARIA-E or ARIA-H | |||||||
| Bapineuzumab (AAB-001, 3D6) | 1–5 | Aβ (1–5) conjugated to immunoglobulin | M,O,F/P | Humanized IgG1 | NCT00998764, NCT00667810, NCT00996918 (III,D) | Janssen/Pfizer | NCT00575055, NCT00574132 (III) | Mild to moderate AD | + | − | high |
| Solanezumab (LY2062430, m266) | 16–26 | Aβ peptide 13–28 | M++,O,F/P- | Humanized IgG1 | NCT01900665 (III,halted), NCT02008357 (III) | Eli Lilly | NCT00904683, NCT00905372 (III) | Mild to moderate AD | + | +/−c | no |
| Ponezumab (PF-04360365) | 30–40 | Aβ40 | M+,O,F (Aβ40) | Humanized IgG2aa | NCT00945672 (II,D) | Janssen/Pfizer | NCT00722046 (II) | Mild to moderate AD | − | − | low ARIA-H, low ARIA-E |
| Crenezumab (MABT5102A, RG7412) | 13–24 | liposome-anchored peptides | M-,O, F/P | Humanized IgG4 | NCT02670083 (III,D), NCT01998841 (II) | Roche/Genentech | NCT01723826 (II) | Mild to moderate AD | − | − | low |
| Gantenerumab (RO4909832, RG1450) | 2–11 and 18–27 | N/A: human combinatorial antibody libraries | M-, O, F/P+ | human IgG1 | NCT03443973, NCT03444870 (III) | Hoffman-La Roche | NCT01224106, NCT02051608 (III) | Prodromal to mild AD | + | − | high: 2/6 patients (200 mg dose) |
| Donanemab (N3pG, LY-3002813, mE8) | p3–7 | Aβ pE3–42 peptide | F/P (N3pG) | Humanized IgG1 | NCT02624778 (I), NCT03367403 (II) | Eli Lilly | NCT01837641 (I) | Early/mild AD | + | n/a | moderate; immunogenic |
| Aducanumab (BIIB037) | 3–7 | N/A: B-cell libraries from healthy elderly subjects | M-,O,F/P | Human IgG1 | NCT02477800, NCT02484547 (III, D) | Neurimmune/Eisai/Biogen | NCT02477800, NCT02484547 (III) | Early AD | + | − | high |
| SAR-228810 (SAR255952, 13C3) | N-terminus (4–20) | Synthetic oligomers | O,F/P | Humanized IgG4a | NCT01485302 (I) | Sanofi | NCT01485302 (I) | Mild to moderate AD | n/a | n/a | no ARIA-E, very low ARIA-H |
| BAN-2401 (Lecanemab, mAb158) | N-terminus (1–16) | Protofibrils of E22G mutant Aβ | M-,O+, F/P | Humanized IgG1 | NCT03887455 (III) | Biogen/Eisai/BioArctic | NCT01230853 (I), (Iib) | Mild to moderate AD/Early AD | + | +/− | low |
| MEDI-1814 | Aβ42 C-terminus (29–42) | N/A: human combinatorial antibody libraries | M++,O | Human IgG1a | NCT02036645 (I) | Eli Lilly/AstraZeneca/MedImmune | NCT02036645 (I) | Mild to moderate AD | n/a | n/a | no |
| KHK6640 | Not known/Not disclosed | Not available | O,F/P | Humanized IgG4a | NCT03093519 (I) | Kyowa Hakko Kirin | NCT02127476 (I), NCT03093519 (I) | Prodromal/Mild/Moderate AD | n/a | − | no ARIA-E, moderate ARIA-H |
| Plasma Exchange/Albumen replacement, IVIgs | n/a | Serum of multiple healthy young volunteers | M,O,F/P | n/a | NCT01561053 (II/III) | Grifols Biologicals Inc. | NCT00818662 (III) | Mild to moderate AD | +/− | − | none |
| NPT088 | noncontiguous 11−12, 17–25, and 31–40 | N/A: Human IgG -viral g3p GAIM chimera | O,F/P | Human IgG1 | NCT03008161 (I) | Proclara Biosciences | NCT03008161 (I) | Mild to moderate AD | n/a | n/a | − |
With mutations to reduce effector function.
M,O,F/P = Monomers, Oligomers, fibrils/plaques respectively. Here we assume if the antibody binds fibril, it binds plaque. This is generally the case when experimental measurements exist.
For the mild AD subgroup of 2 pooled studies.
Evidence has long been accumulating that soluble Aβ and tau oligomers are key pathogenic species that propagate cellular pathology throughout the brain in Alzheimer's disease (Kane et al. (2000); Thal et al. (2002); Walsh et al. (2005); Alonso et al. (2006); Haass and Selkoe (2007); Goedert et al. (2010); Hefti et al. (2013); Bloom (2014); Goedert (2015); Cline et al. (2018); McAlary et al. (2019a)). In AD patients, the amount of soluble Aβ species correlates more closely with cognitive decline than does amyloid plaque burden (Lue et al. (1999); McLean et al. (1999); Wang et al. (1999), see also the comments on tau biomarker abnormalities below). In classic prion disease, soluble oligomers containing roughly 20 PrP molecules are by significant margin the most infectious when inoculated intracerebrally (Silveira et al. (2005)), consistent with the notion that high molecular weight species such as plaques have the potential to play a protective role (Treusch et al. (2009)). Consistently with these ideas, in brains of autopsy cases with similar amyloid load, the ratio of the amount of soluble oligomers over immunohistochemically determined plaque area fully differentiated demented vs. non-demented cases (Esparza et al. (2013)).
3.5. Energy landscape framework for conformational selectivity
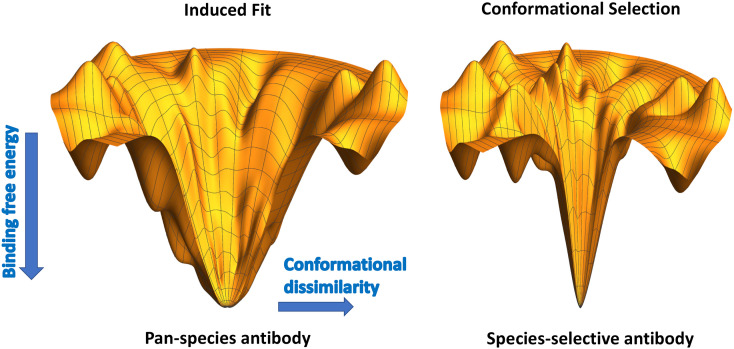
The selectivity or promiscuity of an antibody can be understood within the context of energy lanscape theory. For an antibody-peptide system, the energy landscape for binding determines the peptide conformation bound by the antibody. For high-affinity binding, the transfer free energy to the bound conformation must be significantly negative, and the overall global structure of the energy landscape will have the topography of a funnel (Tsai et al. (1999); Papoian and Wolynes (2003); Wang and Verkhivker (2003), see Fig. 1 ). There is typically a significant amount of entropy loss, which is compensated for by the (negative) enthalpy gain concomitant with binding (Lafont et al. (2007); Chodera and Mobley (2013); Mills and Plotkin (2015)). The degree to which this cancellation occurs determines how wide or how ‘bottlenecked’ the funnel is (Plotkin and Onuchic (2002)). For a wide funnel (Fig. 1 left), dissimilar conformations from the minimum free energy bound conformation also have favorable binding energy: There is more energetic guidance of dissimilar structures towards the minimum energy conformation. Conformations different from the minimal free energy one– what one may call the “active form”– are still likely to be bound, with accessible transitions into or out of the active form on a short timescale. This scenario is reminiscent of an induced-fit binding scenario (Hammes et al. (2009); Csermely et al. (2010); Zhou (2010)), where although the antibody-peptide complex has a well-defined, most-favorable bound conformation that would be observed e.g. in the crystal structure, it is not particularly energetically selective to a specific conformational species (monomer, oligomer, or fibril/plaque). The binding free energies for alternate conformations of Aβ or tau are still significant.
Fig. 1.
Schematics of energy landscapes of the binding free energy of an epitope to an antibody, as a function of conformational dissimilarity to the bound state structure, which is assumed to be at the lowest point. A conformationally-labile antibody is more prone to induced fit with different alternative conformations of a substrate ligand, and will thus lack binding selectivity (left). A conformationally-selective antibody will be unforgiving to even small conformational differences, which will be costly in terms of binding free energy.
For a steep funnel topography akin to the hole on a golf-green (Fig. 1 right), even slightly dissimilar conformations from the minimum free energy bound conformation do not have favorable binding energy, and so are not bound with significant affinity. The binding scenario is more reminiscent of conformational-selection, wherein the antibody is selective to a small ensemble of conformations consistent with a specific target species.
3.6. Fibril and oligomer polymorphism, and prion-like propagation
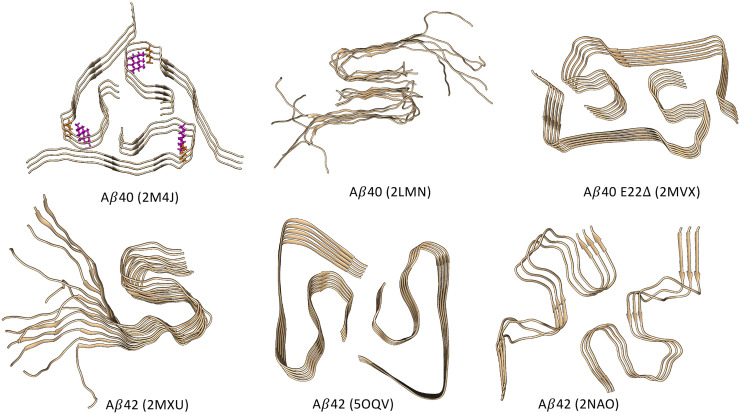
Polymorphism is an inherent aspect of Aβ fibril structures (Fig. 2 ), and ultimately it is a consequence of the absence of any evolutionary selection towards a global free energy minimum, which structured proteins generally possess (Plotkin et al. (1997); Plotkin and Onuchic (2002)). That is, the misfolding energy landscape of the fibril does not have the global topography of a funnel, with a single dominant free energy basin. In contrast to the properties inhereint in well-folded proteins, we would thus expect mutants or alternate isoforms of Aβ (or tau), or altered environmental conditions, to result in alteration of the fibril morphology, which is exactly what has been observed (Fig. 2). Structural polymorphism underlies prion-like “strains” of Aβ and tau that can propagate their own conformation (Domert et al. (2014); Watts et al. (2014); Kaufman et al. (2016); Qiang et al. (2017); Castillo-Carranza et al. (2018); Olsson et al. (2018)). An antibody targeting fibrils would have its efficacy limited by fibril polymorphism, in that the antibody may effectively bind and block propagation for one conformational species of fibril, while being ineffective in binding alternate strains. The polymorphism in oligomers is even more profound, rendering structural determination difficult or moot, and making oligomer-selective targets particularly elusive (Sengupta et al. (2016); Lee et al. (2017)).
Fig. 2.
A selection of Aβ fibril structures, illustrating their polymorphism. Species (Aβ40, Aβ42, or the mutant Aβ40(E22Δ)) are indicated for each image, along with the PDB entry: 2M4J (Lu et al. (2013)), 2LMN (Paravastu et al. (2008)), 2MVX (Schütz et al. (2015)), 2MXU (Xiao et al. (2015)), 5OQV (Gremer et al. (2017)), and 2NAO (Wälti et al. (2016)). An example of ionic salt-bridges stabilizing the fibril structure is shown for structure 2M4J (D23-K28) in licorice. Structures 2LMN and 2MXU are incompletely resolved: Residues 1–8 are disordered in 2LMN and residues 1–10 are disordered in 2MXU; These residues are thus missing from the respective solid state NMR structural models. For these structures, the missing amino acids have been added and the structures have been equilibrated using all-atom equilibrium molecular dynamics. Consistent with the solid-state NMR data, these peptide regions remain disordered when molecular dynamics is implemented for these structures. For other structures such as 2M4J and 2NAO, these N-terminal peptide regions remain structured and are largely β-sheet.
With the above caveats acknowledged, an oligomer-selective antibody that was administered at the appropriate time would have the potential to block and neutralize some or all toxic, propagating species of misfolded protein. Prion-like propagation for both Aβ and tau is supported by multiple lines of in vitro and in vivo Evidence. tau prion-like propagation is discussed further below in the context of tau therapies. Here we focus on the evidence for Aβ prion-like propagation.
It has been noted that Aβ peptide exhibits many of the hallmarks of classical prionogenesis, including the adoption of β-rich architectures that are often resistant to proteolytic or denaturing forces, amyloidogenic polymerization that may template the misfolding and aggregation of healthy protein and which results in both structurally and functionally variable “strains”, and systematic spread along neural connective networks that facilitates intercellular self-propagation (Rasmussen et al. (2017); Condello and Stöehr (2018); Watts and Prusiner (2018)). That said, there is no current evidence of host-to-host transmission and systemic uptake of toxic Aβ species in the same sense as for the canonical prion diseases (see however the comments below). We thus refer to the intercellular propagation of misfolded Aβ as “prion-like”.
Meyer-Luehmann et al. (2006) have observed that brain extract from either human AD patient or APP23 transgenic mice induced numerous Aβ deposits in APP23 murine hosts beginning ~2 months after injection. The same was not observed for WT donors or WT hosts, implying that misfolded Aβ needed to be present in the donor, and a host Aβ reservoir that is induction-competent was required for deposition of endogenous Aβ. The amyloid-inducing activity of extracts was prevented by immunodepletion of Aβ, and attenuated by pre-mixing with Aβ -specific antibodies, indicating that Aβ itself is the key species inducing deposition. Similarly, weekly interperitonial passive immunization following injection blocked amyloid deposition, reinforcing the potential efficacy of an Aβ passive immunotherapy. Interestingly, pretreatment with formic acid, which does not dissociate high molecular weight species, but which does dissociate oligomers, completely prevented amyloid deposition of endogenous data.
In the study of Meyer-Luehmann et al. (2006), no amyloid deposition was observed for aged, synthetic Aβ 40/Aβ 42 preparations, synthetic oligomers, or even synthetic Aβ mixed with brain extract from WT mice. This observation, coupled with the above-mentioned blocking activity of Aβ antibodies, suggests the presence of polymorphic conformations with significantly variable and strain-dependent seeding efficacy, reminiscent of prions. Similarly, intracerebral inoculation of hAPPwt mice—which do not develop amyloid aggregates during their lifespan—with AD patient brain extract also induced pathological Aβ deposition, after ~10 months (Morales et al. (2012)). Extending the prion analogy, peripheral inoculation intraperitoneally with Aβ -containing brain homogenates from APP23 and APP-PS1 transgenic mice into either APP23 or R1.40 transgenic mice aged 1–2 months showed induction of cerebral β-amyloidosis in a pattern consistent with the entry of Aβ -templating seeds at multiple locations in the brain (Eisele et al. (2014)).
The inability of synthetic Aβ preparations to induce cerebral amyloid deposition in the study of Meyer-Luehmann et al. (2006) is a cause for justifiable concern. However, more recently, Stöhr et al. (2012) have found that β-amyloid deposition can be induced by synthetic Aβ aggregates. In their study, either Aβ 40 or mutant Aβ 40(S26C)—which makes covalently-bonded dimers—was incubated to form aggregates, which in both cases contained both fibular and globular structures. Intracerebral inoculation of fairly high concentrations of either of these synthetic preparations induced amyloidogenic deposition of endogenous Aβ in bigenic APP23:Gfap-luc mice (these are mice with the Swedish double mutation of human APP, and a luciferase reporter under control of a glial fibrillary acidic protein (Gfap) promoter).
Induced Aβ amyloid deposition has been observed in individuals treated during childhood with cadaveric pituitary-derived growth hormone (c-hGH), which resulted in iatrogenic CJD (Swerdlow et al. (2003); Brown et al. (2012)), but with additional Aβ amyloid pathology (Jaunmuktane et al. (2015)). The samples of human c-hGH that induced Aβ pathology were shown by antibody capture and detection to contain high levels of Aβ 40, Aβ 42, and tau protein (Purro et al. (2018)). This association between peripheral administration and brain deposition of Aβ was subsequently supported in APP NL-F knock-in mice (Purro et al. (2018)). These mice were intracerebrally inoculated with the same Aβ and tau-containing samples of c-hGH that were administered to a subset of the above iatrogenic CJD patients. Intraperitoneal injections, though potentially very interesting, appear not to have been performed. The inoculated mice subsequently developed seeded formation of Aβ plaques and cerebral Aβ -amyloid angiopathy (CAA). Together, the above results provide strong support for prion-like propagation of Aβ within a single host, and in rare cases between hosts under unusual environmental exposure.
4. Passive immunotherapies for Aβ
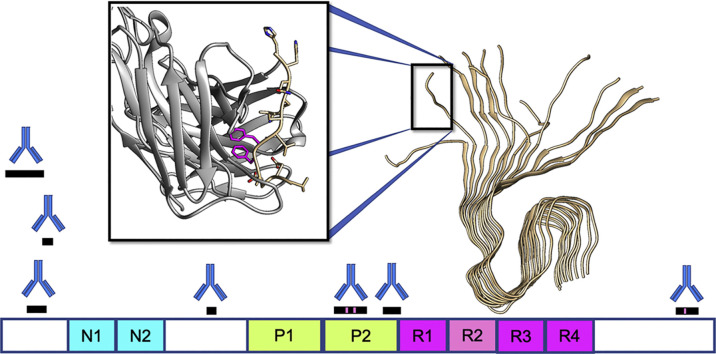
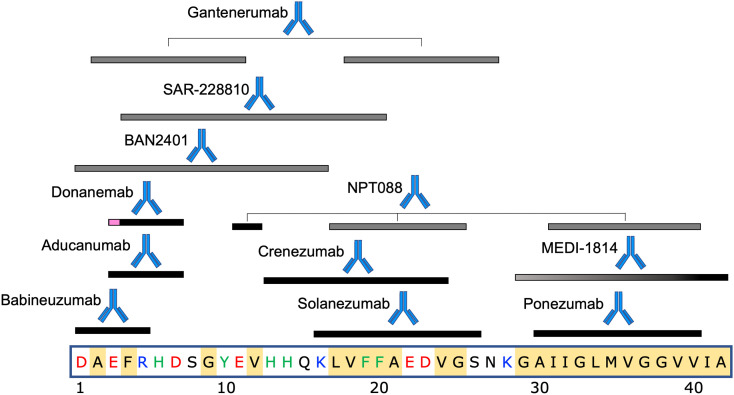
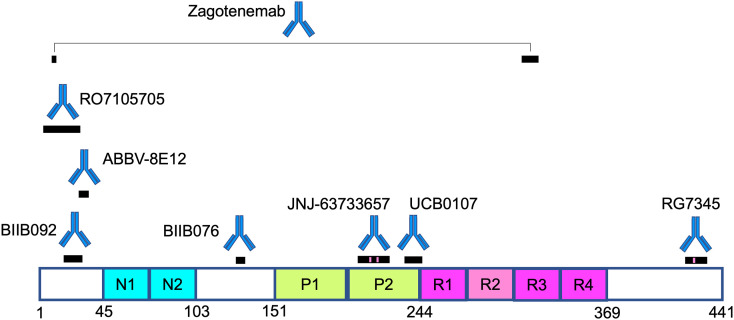
Table 1 lists the Aβ therapeutics currently in clinical trials, along with their epitopes, immunization strategies, selectivity for monomer (M), oligomer (O), or fibril/plaque (P) species. Also included are antibody backbone isotype, current clinical trials and sponsor, and results for completed trials. For antibodies currently or recently in clinical trials with known epitopes on Aβ, Fig. 3 shows the locations of those epitopes on the primary sequence. We begin by discussing antibodies whose development was relatively early historically, and/or whose clinical trials have been discontinued, moving to antibodies that have been developed more recently.
Fig. 3.
Epitope locations on the primary sequence of Aβ, for antibodies currently or recently in clinical trials. Black bars indicate epitope locations; gray bars indicate presumptive epitopes that likely subsume the actual epitope as a subset of the gray region. Gradient filling for MEDI-1814 represents the incompletely characterized epitope, but with known Aβ42 selectivity. Gantenerumab and NPT088 both have discontiguous epitopes on Aβ. Magenta region on the epitope for donanemab represents pyroglutamate at amino acid position 3. Amino acids in the primary sequence of Aβ are colored as follows. Red: negatively charged; Blue: positively charged; Green: aromatic; Yellow background: hydrophobic. The significant hydrophobicity and absence of aromatic residues in the C-terminal region is noteworthy. Specific epitope locations are listed in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.1. Bapineuzumab
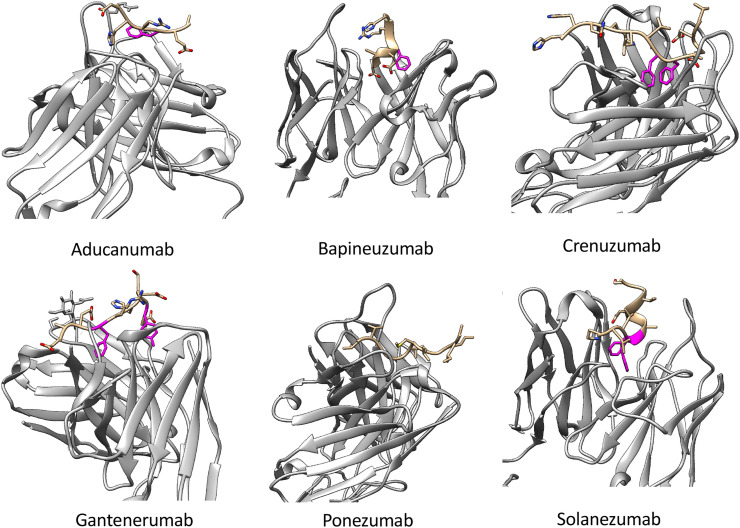
The murine version of this antibody (3D6) was generated by active immunization of mice with sequence 1DAEFR5 of Aβ conjugated to sheep anti-mouse immunoglobulin (Johnson-Wood et al. (1997)). The co-crystal structure of bapineuzumab (4HIX.pdb, see Fig. 4 ) in complex with a fragment of Aβ (residues 1–6) indicates a bound structure with a helical conformation of the epitope (Miles et al. (2013)). The side chains of the acidic residues D1 and E3 on Aβ, as well as the positive N-terminus and aromatic ring of F4, all point into the binding cleft.
Fig. 4.
Antigen-binding regions of antibodies to Aβ in clinical development, with published co-crystal structures to their epitopes. Antibodies, Protein Databank entry, and epitopes are, from top left to right: aducanumab (PDB 6CO3, structured epitope amino acids 2-7) bapineuzumab (PDB 4HIX, structured epitope aa1-6) crenezumab (PDB 5VZY, structured epitope aa13-24), gantenerumab (PDB 5CSZ, structured epitope aa1-10), ponezumab (PDB 3U0T, structured epitope aa30-40.) solanezumab (PDB 4XXD, structured epitope aa16-26). Interacting aromatic rings are rendered in magenta for visualization. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In mouse models with >10-fold expression of APP over endogenous levels (PDAPP mice), 3D6 was shown to opsonize amyloid plaques, i.e. bind, decorate, and facilitate their clearance, as well as improve synaptic function and cognitive performance in behavioral assays (Bard et al. (2000); Spires-Jones et al. (2009); Kerchner and Boxer (2010)).
Bapineuzumab was the first monoclonal antibody to enter human testing after termination of the AN1792 active vaccination trial. Patients in these trials did not demonstrate significant cognitive benefits (Salloway et al. (2009)), and MRI scans revealed significant adverse issues, including ARIA-H and ARIA-E (van Dyck (2018)). Interestingly, a retrospective review of MRI scans from the phase 2 studies revealed that about 7% (15) of participants had developed ARIA-E during the trials, but remained undetected (10% had ARIA that was detected). 13 of these 15 participants continued to receive additional immunotherapy infusions while ARIA-E was present, and these patients did not develop additional associated symptoms (Sperling et al. (2012)). The occurrence of ARIA was strongly related to the ApoE-ε4 copy number and arose predominantly during the first three infusions. All phase 3 trials with bapineuzumab were terminated in 2012 when phase III trials NCT00575055 and NCT00574132 showed no clinical benefit (Salloway et al. (2014)). This decision was not based on any new safety concerns.
Nevertheless, a variant of Babineuzumab with reduced Fc-receptor-mediated effector function, AAB-003, has been developed, and two phase I clinical trials (NCT01193608,NCT01369225) to assess safety and tolerability have been completed (Delnomdedieu et al. (2016)). To reduce binding of FcγR and complement C1q, three amino acid mutations have been introduced in the hinge region (L234A/L235A/G237A). Although these mutations are not in the complement binding region, effector activity was evidently reduced: The dose where ARIA-E was observed for AAB-003 was higher compared to bapineuzumab.
4.2. Solanezumab
The murine precursor to solanezumab, m266, was generated by immunization of mice using Aβ peptide amino acids (13–28), conjugated to anti-mouse CD3ε antibody as an immunogen (Schlossmacher and Selkoe (1993)). Solanezumab is the humanized monoclonal IgG1 antibody of m266, with epitope in the mid-region of Aβ, spanning residues 16–26 (PDB structure 4XXD.pdb, see Fig. 4, Crespi et al. (2015)). The conformation of the epitope is partly extended and partly helical (from F20-S26). Residues pointing into the binding cleft of the antibody are K16, F19, F20, E22, and D23 (F19, F20 are shown in magenta in Fig. 4). Solanezumab exhibits strong binding to monomers of Aβ 40 or Aβ 42, with affinity in the low pM range. It also exhibits cross-reactivity to other proteins from brain homogenates (Watt et al. (2014)). However, solanezumab has been thought to deplete brain Aβ stores by sequestering Aβ monomers in the blood and thus shifting the brain-blood equilibrium (the peripheral sink hypothesis, see e.g. DeMattos et al. (2001)). In humans, solanezumab treatment results in significant increases of both Aβ 40 and Aβ 42 concentrations in plasma and CSF. Subsequent observations have cast doubt on the peripheral sink mechanism, since a decrease in Aβ efflux due to m266 was observed in those experiments (Yamada et al. (2009)), suggesting that the beneficial effect of m266 is due to inhibition of Aβ forming oligomers and fibrils in the brain. Additionally, antibody binding to Aβ in plasma substantially increases the half-life of Aβ, from approximately 5 min for free peptide, to up to several days for bound Aβ (Golde and Levites (2009)). While not ruling out the peripheral sink hypothesis, such stabilizing effects must be disentangled from the potential effects of enhanced efflux from the brain.
Results from two large phase 3 trials involving over 2000 patients and completed in 2012 revealed no significant difference in cognition and memory between the solanezumab-treated and the placebo group (Doody et al. (2014), see Table 1). However, subsequent analysis of subgroups in these trials revealed a statistically significant slowing in decline for some cognitive measures (34% slowing vs. placebo for ADAS-Cog14 and MMSE) and a significant slowing for some functional measures (18% slowing vs. placebo for ADCS-iADL), for the subgroup of mild AD (Siemers et al. (2016)). This suggested positive therapeutic effects may be seen if administered at earlier stages of progression. Follow-up phase 3 clinical trials (Expedition 3, NCT01900665) in mild AD patients showed no significant benefits over placebo however, and were terminated. Currently, solanezumab is administered every 4 weeks in the Asymptomatic Alzheimer's Disease trial (A4 trial, NCT02008357), which has enrolled cognitively normal people with amyloid accumulation, to test whether earlier administration may be effective as a preventative measure. Based on modest but encouraging results from previous clinical trials, the dosage was quadrupled from 400 to 1600 mg in June 2017.
4.3. Ponezumab
Ponezumab is a humanized IgG2δA antibody with two mutations to minimize potential immune effector function (A33S and P331S). Epitope mapping by overlapping peptide scans localizes the epitope to residues 30–40 of Aβ 40 (Porte et al. (2012)). The co-crystal structure (PDB 3U0T.pdb, Fig. 4) shows an extended, linear conformation of the epitope residues 30–40, with the C-terminal more buried than the N-terminal portion (Porte et al. (2012)). The C-terminal carboxylic acid on residue 40 is critical to ponezumab binding activity: The antibody does not bind Aβ 42.
ELISA binding assays along with immunohistochemistry show that ponezumab is not species-selective, binding to monomers, oligomers, and fibrils of Aβ 40 (Porte et al. (2012)). Like solanezumab, it is hypothesized to deplete brain Aβ stores by sequestering Aβ in the blood and thus shifting the brain-blood equilibrium (the peripheral sink hypothesis, see e.g. DeMattos et al. (2001)).
Ponezumab shows low to moderate ARIA-H and low ARIA-E risk (Landen et al., 2017b, Landen et al., 2017a). Although ponezumab revealed a favorable safety profile, two subsequent phase 2 studies revealed no significant clinical benefit, and development of ponezumab for AD was discontinued.
4.4. Crenezumab
In the development of the murine precursor to crenezumab (MABT5102A or mMABT), liposomes containing anchored peptides of Aβ 1−16 were used to immunize mice (Pfeifer et al. (2008); Adolfsson et al. (2012)). Liposome presentation may present the epitope in β-sheet like conformations. Unusually, there was a shift in the binding epitope position of mMABT, from the region presented in the immunization peptide, to residues 13–24 on Aβ (Pfeifer et al. (2008)). An IgG4 backbone isotype was selected for low effector function; The mutation S228P also appears to be implemented, which stabilizes inter-heavy chain disulfide bridges preventing “half-molecule” exchange (Silva et al. (2015)).
The Aβ epitope comprising residues 13–24 is fairly linearized in the co-crystal structure 5VZY.pdb (Ultsch et al. (2016), see Fig. 4). The antibody has high affinity for higher molecular weight species such as fibrils, plaques, and oligomers, while having low affinity for monomers (Table 1).
In phase II trials, crenuzumab lowered oligomer levels in CSF for the majority of patients (89% receiving subcutaneous doses and 86% receiving intravenous doses) (Yang et al. (2019)), but PET amyloid load was not lowered, and no significant treatment-related change in cognitive outcome was observed (Table 1). Incidence of ARIA was low, which, along with the high ARIA incidence of other amyloid-clearing antibodies, suggests that activation of effector mechanisms may be a key event in the clearance of plaque amyloid. Phase 3 trials were halted in January 2019, as interim analyses indicated that the trial was unlikely to reach its primary endpoint of slowing cognitive decline according to the CDR-SB test.
Motivated by the need for preventative intervention to modify the future course of the disease, the Alzheimer Prevention Initiative (API) is currently studying the efficacy of crenezumab vs. placebo for 300 asymptomatic presenilin-1 E280A mutation carriers, who are autosomal-dominant for AD (Tariot et al. (2018)). This study will inform on the efficacy of crenezumab to either delay the onset, slow the decline, or prevent cognitive impairment in individuals with preclinical autosomal-dominant AD.
4.5. Gantenerumab
Rather than using an active immunization step, gantenerumab is a fully human IgG1 antibody selected from synthetic human combinatorial antibody libraries (HuCALs, Knappik et al. (2000)) using phage display, followed by in vitro affinity maturation using CDR cassette exchanges (Steidl et al. (2008)). In the context of antibodies targeting influenza hemagglutinin, phage display libraries from isolated B cells have been used to isolate rare lead antibodies that were not detected directly by next-generation sequencing (Rajan et al. (2018)).
Peptide screening assays (Bohrmann et al. (2012)) indicate that gantenerumab is capable of binding two discontiguous regions of Aβ, with highest affinity at residues 2-11 and 18-27. Such a binding mode to separate epitopes may allow binding to N-terminal truncated Aβ species, and facilitate avidity-enhanced binding on the fibril surface (Bohrmann et al. (2012)), potentially involving both variable domain arms of the antibody. It also implies a flexible binding pocket on the antibody that is capable of binding several sequences. This has implications for both the selectivity of the antibody for distinct Aβ species (the antibody binds all species, Table 1), and the potential for off-pathway reactions. The structure of the antibody-epitope (PDB 5CSZ.pdb) shows amino acids 1–10 of Aβ are extended in a linearized conformation (see Fig. 4). Gantenerumab exhibits sub-nanomolar binding affinity to Aβ 40 fibrils; the dissociation constants for Aβ 40 fibrils, oligomers, and monomers are 0.6 nM, 1.2 nM, and 17 nM respectively (Bohrmann et al. (2012)).
Thus, gantenerumab preferentially interacts with aggregated Aβ, and may facilitate degradation of opsonized amyloid plaques by recruiting microglia and activating phagocytosis (Bard et al. (2000)). These early studies indicated that even modest levels of peripherally administered antibody were able to cross the blood-brain barrier and enter the CNS, bind to plaques, and induce clearance of amyloid. Treatments combining BACE inhibitor R7129 with gantenerumab have shown an additive effect between the two drugs in APP transgenic mouse models, in that the combination reduced Aβ levels and plaque burden more strongly than either treatment alone (Jacobsen et al. (2014)). In phase I clinical trials, gantenerumab was found to reduce plaque burden in AD patients, prompting further trials, including two ongoing additional phase III trials for patients with prodromal (NCT01224106) and mild (NCT02051608) AD (Table 1). As well, gantenerumab and solanezumab have both been tested in patients carrying autosomal-dominant mutations for AD in the DIAN-TU clinical trial (NCT01760005), discussed further below.
4.6. Donanemab (LY3002813)
A disease-modified form of Aβ peptide (Aβ p3−42) may occur through protease-cleavage of the first two amino residues, followed by cyclization of the side chain of glutamic acid residue E3 to pyroglutamate. This cyclization occurs either spontaneously or by the enzyme glutaminyl cyclase. Aβ p3−42 plays an important role in early AD pathology by seeding toxic oligomeric species (Wirths et al. (2009)), and Aβ p3−42 deposits preferentially in amyloid plaques. There has thus been impetus to generate antibodies targeting Aβ p3−42 as plaque-specific therapeutics. The above observations have also motivated drug development for glutaminyl cyclase inhibitors (Scheltens et al. (2018)), which are currently in clinical trials.
Donanemab is a humanized IgG1 antibody developed from the murine IgG2a antibody mE8 (DeMattos et al. (2012)). mE8 is raised by actively immunizing mice with Aβ p3−42. Mutational analysis on Aβ shows that the epitope involves the truncated N-terminal residues pE3-D7. mE8 thus does not bind full-length Aβ (or full-length Aβ 42), and also shows about 300-fold greater affinity to pE3 vs. E3 in truncated Aβ 3−42. mE8 can thus be thought of as binding a disease-modified N-terminus.
Unfortunately, donanemab is itself strongly immunogenic; Nearly all (≈90%) patients who received the therapy mounted an immune response against it (NCT01837641, clinicaltrials.gov). This immunogenicity poses no serious health concerns however and patients were continued on trials. Administering the antibody resulted in significant decreases in brain amyloid burden—a six month course of 20 mg/kg dropped amyloid load by an average of 70 centiloid. Specifically, on a 100-point centiloid scale, young amyloid-negative subjects aged 31 ± 6 years have an average centiloid value of zero, and “typical” diagnosed AD patients with questionable dementia to mild cognitive impairment according to CDR-SB tests would average approximately 100 (Klunk et al. (2015)). About 25% of patients taking gantenerumab developed ARIA-E, though mostly asymptomatic.
The latest phase II trial (NCT03367403) consists of 3 arms: One with both donanemab and the BACE inhibitor LY3202626, one with donanemab and placebo, and one with two placebos. The arm of this trial involving BACE inhibitor was discontinued in October 2018, however the other two arms remain ongoing.
4.7. Aducanumab
Aducanumab is a fully human IgG1 monoclonal antibody derived from a blood lymphocyte library that was collected from elderly patients who showed either no signs of cognitive impairment or unusually slow cognitive decline. It thus relies on the assumption that these patients would generate antibodies protective against AD. B cells are isolated from peripheral blood lymphocyte preparations by anti-CD22-mediated sorting, and were cultured on gamma-irradiated human peripheral blood mononuclear cell feeder layers. Supernatants from these patients' B-cells were screened for binding to Aβ plaques in tissue sections, in vitro binding to Aβ 40 and Aβ 42, and lack of binding to full-length APP (Sevigny et al. (2016)).
Aducanumab binds to soluble Aβ aggregates and insoluble fibrils with >10,000-fold selectivity over monomers (Bussiere et al. (2013)). Weak binding to monomers has also been observed (Arndt et al. (2018); Silverman et al. (2018)). In the co-crystal structure with aducanumab (6CO3.pdb, Fig. 4), the epitope, consisting of residues 2–7 of Aβ, adopts an extended conformation (Arndt et al. (2018)). The alanine residue A2 in Aβ points away from the antibody, and so is not included in the putative epitope in Table 1. The complex is stabilized by a cation-pi interaction (Dougherty (2013)) between an arginine on the antibody and phenylalanine F4 on Aβ, which likely contributes to the high binding affinity. Otherwise, the binding pocket is relatively shallow compared to other N-terminal binding antibodies such as bapineuzumab and gantenerumab.
Biogen initially reported in March 2019 that aducanumab did not meet its primary end points for slowing cognitive decline in phase III clinical trials (NCT02477800 (ENGAGE), NCT02484547 (EMERGE)), although the antibody was effective at clearing Aβ plaque from patients, likely through FcγR-mediated phagocytosis by microglia (Sevigny et al. (2016)). The high affinity for abundant, insoluble Aβ along with significant effector function of the antibody gave rise to a 37% or 41% risk of ARIA-E,H in the two highest dosage groups (Sevigny et al. (2016)). The trial recruited patients in the early symptomatic phase of AD, however it appears that this stage is already late in deriving clinical benefit by targeting Aβ, and tau pathology and neuroinflammation may be the predominant neurodegenerative drivers at this stage.
Biogen initially halted development of aducanumab in March 2019 after the preliminary data from the EMERGE and ENGAGE trials suggested it would not meet primary endpoints. The initial conclusion that there was a failure to show cognitive benefit indicated that removal of amyloid was ineffective—at least on the time scale of 2–3 years—for patients who have progressed to mild or moderate stages of the disease (Selkoe (2019)). It should be noted however that these results do not preclude drugs such as aducanumab as potentially effective in prodromal cases of AD. There was also the speculation that, while on average there appeared to be no significant cognitive benefit, some patients could have experienced favorable effects.
On October 22, 2019, Biogen announced that the interim futility analysis was incorrect, and that subsequent analysis of a larger data set instead showed EMERGE had in fact met its primary endpoint (data was presented at the 2019 CTAD conference (Haeberlein et al. (2019))). Specifically, patients on the highest dose (titrated to 10 mg/kg over 26 weeks) had a significant reduction in decline in cognition, according to CDR-SB test–the primary endpoint. As well, the high-dose group declined less on secondary cognitive endpoints such as the MMSE, ADAS-Cog, and ADCS-ADL-MCI tests. The lower dose group (titrated to either 3 mg/kg (ApoE-ε4+) or 6 mg/kg (ApoE-ε4−)) appeared to show slowing of cognitive, but the changes did not reach statistical significance.
Oddly, the cognitive trajectories in the ENGAGE trial appear significantly different from those in the EMERGE trial, and the ENGAGE study arm did not meet its primary endpoint. This was explained through differences in the enrollment between the study arms during the dosing titration increase. Unlike the EMERGE data, the ENGAGE data also did not show dose response for phospho-tau and total tau biomarkers. That said, a subgroup analysis (post protocol version 4) of patients in both arms who had received 10 or more 10 mg/kg doses of aducanumab did show dose-dependent and statistically-significant reduction in CDR-SB-measured cognitive decline. Based on this latest data and the revised analysis, eligible patients from phase III trial arms have been asked to return for continued dosing and testing, and Biogen has announced plans to apply in early 2020 for regulatory approval for aducanumab in the U.S.
4.8. SAR-228810
SAR-228810 is a humanized IgG4 antibody based on murine antibody 13C3. 13C3 was itself raised by immunization using incubated synthetic Aβ 42, which forms multimers/oligomers of various size (Schupf et al. (2008)). 13C3 was selected by screening for antibodies specific to protofibrillar Aβ 42 (≈670kDa or ≈150 Aβ 42 molecules) over low molecular weight (LMW) species (<17kDa or 3–4 Aβ 42 molecules). SAR-228810 binds to soluble protofibrils and insoluble fibrils of Aβ.
The precise epitope location has not been determined/disclosed, but is likely in the N-terminal region between residues 4–20 (Ravetch and Fukuyama (2009)). The antibody is conformationally selective: SAR-228810 does not bind appreciably to soluble Aβ monomers or low molecular weight Aβ complexes. It does bind amyloid plaques, but not to diffuse, non-β-sheet deposits of Aβ (Pradier et al. (2018)). The antibody binds to protofibrillar and fibrillar aggregates with approximately 100-fold selectivity over Aβ monomer in ELISA assays.
SAR-228810 has two mutations on a human IgG4 backbone, one (S241P) that promotes inter-heavy chain disulfide bridges preventing “half-molecule” exchange (Angal et al. (1993)), and another (L248E) that significantly reduces effector function (Reddy et al. (2000)). The antibody has low binding affinity for activating FcγRs on human microglia, and shows no binding to complement C1q, which is a pro-inflammatory component of the innate immune system (Pradier et al. (2013)). Consistently, in phase I clinical trials (NCT01485302) of 44 single-dose and 48 multiple-dose patients (Vellas et al. (2015)), SAR-228810 was well-tolerated, an upper limiting dose as determined by adverse events was not reached, there were no reported cases of ARIA-E, and there was only one reported case of ARIA-H (a single-dose patient). No additional trials have been scheduled to date however.
4.9. BAN-2401 (Lecanemab)
Patients carrying the E22G (APP E693G) mutation of Aβ (the “Arctic” mutation) show particularly high levels of Aβ protofibrils (Nilsberth et al. (2001)), abundant parenchymal plaques but without a dense amyloid core (Basun et al. (2008)), and are autosomal-dominant for early-onset AD. (Weggen and Beher (2012)). Murine antibody mAb158 was generating by immunizing mice against E22G mutant Aβ protofibrils (Tucker et al. (2015)). Soluble protofibrils are an abundant toxic species in AD brains (Sehlin et al. (2012)). mAb158 binds to protofibrils with much higher affinity than monomers (Englund et al. (2007)), and reduces protofibrils in the brain and CSF of transgenic mice expressing both the above Arctic mutation and the “Swedish” double mutation in APP (K670N/M671L; tgArc-Swe mice) (Tucker et al. (2015)). Studies in embryonic mouse-derived co-cultures of astrocytes, neurons, and oligodendrocytes show that mAb158 can protect neurons from Aβ 42-induced death by preventing the accumulation of Aβ through astrocyte-uptake (Söllvander et al. (2018)).
BAN-2401 is the humanized version of the mAb158. In phase 2b trials, the antibody reduced plaques by 93% in patients in the highest dosage arm (Swanson et al. (2018)). This is consistent with immunohistochemical observations that the antibody binds to plaque as well as high molecular weight oligomer (ProMIS Neuroscience (2018)). As a likely consequence to plaque binding however, ARIA-E was observed in 14.6% of APOEε4 carriers in the largest, most-frequent dosage arm (10 mg/kg bi-weekly). In this dosage arm however, cognitive decline was slowed by 47% on the ADAS-Cog, and by 30% on the ADCOMS (Swanson et al. (2018)). In a controversial decision arising from safety concerns related to ARIA, european regulators limited the number of APOε4 carriers in the highest most frequent dosage arm compared to the placebo arm and other dose groups, partway through the trial. The concern arising over this imbalance was then whether it contributed significantly to the appearance of a benefit in the high dosage arm, which would then be artifactual. Subsequent independent statistical analysis of subgroups (Dickson et al. (2019)) has since indicated that the cognitive benefits were statistically significant, and that because in fact APOEε4 carriers responded better to the drug, the above regulatory limitations may have negatively (rather than positively) affected the statistical significance, leading to a potential underestimate of the drug's effects (Vellas et al. (2019); AlzForum.org, 2019c).
4.10. MEDI-1814
MEDI-1814 is a fully human antibody optimized from a clone identified from phage library selections against Aβ 42 (Billinton et al. (2017)). MEDI-1814 binds selectively to the C-terminus of Aβ 42 with very high affinity ≈50-300 pM (Billinton et al. (2017)). The epitope is broadly characterized between residues 29–42 (Groves et al. (2014)), but selective to Aβ 42 in that the antibody does not engage Aβ 40 (Bogstedt et al. (2015)). The epitope thus likely involves at least I41, A42, and possibly the charged C-terminus of A42. The antibody appears to be selective to low molecular weight species, primarily monomers. In the CSF of rats and monkeys, MEDI-1814 reduced free, antibody-unbound levels of Aβ 42, while increasing total Aβ 42 (Billinton et al. (2017)), indicating target engagement. There is no co-crystal structure reported to date.
MEDI-1814 has an IgG1 backbone, but has a triple mutation in its Fc tail to reduce effector function. Consistently, initial phase 1 results (NCT02036645, clinicaltrials.gov) report no serious adverse effects, and MRI scans showed no evidence of ARIA (Ostenfeld et al. (2017)). Participants in phase I clinical trials showed also no signs of either ARIA-H or ARIA-E.
4.11. KHK6640
KHK6640 is a humanized IgG4 antibody with mutations to limit effector function. CSF analysis indicated that the amount of KHK6640-bound Aβ oligomers increased in a dose-dependent manner, showing oligomer target engagement (Cantillon et al. (2017); Shimada et al. (2017)). About 7% of the patients were immunoreactive to the antibody. Unfortunately, there is little or no published preclinical data on this antibody or its murine precursor.
4.12. Plasma exchange therapy, albumen replacement, and IVIg
Intravenous immunoglobulin polyclonal cocktails (IVIgs) contain a small fraction of polyclonal antibodies directed against Aβ, and there is some evidence that they may enable clearance and reduce synaptic toxicity caused by Aβ (Szabo et al. (2010)). IVIgs have been examined for the treatment of many diseases including AD (Loeffler (2013)). They have an established safety record for patients with immunodeficiency or autoimmune conditions.
Previous trials involving IVIgs (Gammagard Liquid) have given overall negative results (NCT00818662, (Relkin et al. (2017))). Preliminary results from the moderate AD subgroup who were ApoE4+ taking the higher 400 mg/kg dose showed positive cognitive benefits over the placebo group. However, the trial was not powered to detect statistical significance in any of the subgroups. As well, Florbetapir PET amyloid imaging showed a modest reduction in fibrillar amyloid, the type deposited in amyloid plaques, although the study was also not powered to confirm this effect.
More recently, phase 3 clinical trials have been completed involving treatment with plasma exchange (PE) plus low or high dose therapeutic albumin replacement, with or without IVIgs (under the trade name Flebogamma) (the Alzheimer's Management by Albumin Replacement (AMBAR) trial, NCT01561053, Boada et al. (2019))). The rationale is based on the hypothesis that Aβ may be bound to albumin and the complex then circulates in plasma, so extracting this plasma could flush amyloid from the brain, similar to the peripheral sink hypothesis but without potential confounding effects of stabilized bound complexes in the blood. As well, albumin has been shown to have antioxidant, immune-modulatory, and anti-inflammatory properties (Gleeson and Dickson (2015); Bar-Or et al. (2006)), which may diminish neuroinflammation.
The AMBAR study revealed some impressive data that at the very least warrants further studies. Perhaps consistently with the performance of IVIgs in previous clinical trials for AD, there was no significant effect on whether PE was accompanied with IVIGs; There was also no significant effect on whether PE also had low or high dose albumin replacement. Cognitive endpoints such as CDR-SB showed significant difference from placebo and even potential improvement among mild AD participants (Páez et al. (2019)). Among moderate AD participants there was a statistically significant reduction in cognitive decline by CDR-SB. There were also significant differences in both groups according to psychometrics such as the Clinical Global Impression of Change (ADCS-GCIC). Among moderate but not mild AD participants, activities of daily living (ADCS-ADL) and neuropsychological Cognitive subscale (ADAS-Cog) were also significantly improved relative to placebo (61% less decline) (Páez et al. (2019)).
4.13. NPT088
Many bacteria form functional amyloid assemblies on their cell surface, which aid in biofilm formation and other community behaviors involving cell-cell interactions (Zhou et al. (2012)). These amyloids can enhance virulence, facilitate cell adherence and invasion, and aid the survival and spread of the pathogen (Gerven et al. (2018)). M13 is a filamentous bacteriophage that recognizes amyloids on the bacterial cell surface through a two-domain fragment of the phage capsid protein g3p (gene 3 protein). NMR studies have shown that g3p can also recognize Aβ fibrils, predominantly through an epitope involving the middle and C-terminal residues of Aβ (Krishnan et al. (2014)).
NPT088 is an antibody made from a fusion of g3p with the Fc region of a human IgG1. The chimeric antibody targets many different amyloids, including amyloid beta, tau, alpha-synuclein, antibody light chain, and transthyretin (Messing (2016)). The recognition portion is thus referred to as a general amyloid interaction motif (GAIM). Based on the ability of the antibody to remove Aβ plaque, reduce phospho-tau pathology, and improve cognitive performance in mouse models (Levenson et al. (2016)), NPT088 has moved into clinical trials (phase I, NCT03008161). A second candidate utilizing GAIM recognition (NPT189) is also currently in phase I clinical trials (NCT03610035).
4.14. Selected preclinical Aβ antibodies
Similar to donanemab, the antibodies 8C4 and 9D5, generated by immunizing mice with Aβ p3−38, are selective for Aβ p3−42: They bind to Aβ p3−42 but show no binding signal to Aβ 42 (Wirths et al. (2010)). Furthermore, 9D5 only binds to low molecular weight Aβ p3−42 oligomers: There was no reactivity observed to monomers or dimers, and immunohistochemistry showed intraneuronal imunoreactivity and/or vascular staining, but not broad plaque-staining as observed for other antibodies (Wirths et al. (2010)). Given the early dates of these initial findings, the prospects of the above antibodies entering clinical trials are uncertain, but would appear to be unlikely.
An Aβ oligomer-selective, humanized IgG2 antibody ACU193 (also called 19.3) was modified from a mouse monoclonal IgG1 antibody 3B3 (also known as ACU-921). 3B3 was obtained from mice immunized with synthetic Aβ -derived diffusible ligands (ADDL) of Aβ 42 (Acton et al., 2010a, Acton et al., 2010b). The antibody shows preferential binding affinity for 3-24mers of Aβ, vs. monomeric Aβ or Aβ plaque, and no visible binding to vascular amyloid. In fact, the binding epitope sequence of 3B3 was not able to be determined by linear epitope mapping in ELISA, as the antibody failed to bind any members of the overlapping peptide set, even at high concentrations. However it could bind Aβ 1–20 peptide, which was used as a positive control. Similarly, binding of 3B3 to ADDLs was not blocked by short linear peptides of ≤ 10 amino acids in Aβ 42, but interestingly binding was blocked by Aβ 1–28, indicating an epitope based on a conformational structure also found in Aβ 1–28 fragments (and possibly also Aβ 1–20). 3B3 was observed to be effective in blocking the assembly of ADDLs, as observed through fluorescence quenching of flourescein-labelled oligomers by unlabelled monomers, and fluorescence polarization increase as oligomers assembled (Acton et al., 2010a, Acton et al., 2010b). The murine precursor 3B3 was able to restore long-term potentiation in rat hippocampal slices (Cline et al. (2019)), and to reverse the dysregulation of cytosolic calcium concentration (Wang et al. (2018)).
PMN310 is a humanized IgG4 antibody that binds a conformational epitope consisting of 13HHQK16, specifically when presented on low-molecular weight oligomers and protofibrils. The antibody shows no apparent binding to Aβ monomer, amyloid plaque, or vascular deposits (Gibbs et al. (2019)). This is a potential advantage to avoid target distraction by more abundant monomers, and if clearing plaque does not correlate with cognitive benefit. The epitope was predicted based on computational modelling of Aβ oligomers, by using molecular dynamics to find the regions most-likely to be solvent-exposed in a protofibril (Peng et al. (2018); Cashman and Plotkin (2016)). Immunization proceeded by conjugating cyclic peptides containing the epitope to KLH as an immunogen, wherein the cyclic peptide constructs were chosen based on oligomer-selective epitope scaffolding (Silverman et al. (2018)). Immunohistochemical studies show that PMN310 exhibits essentially no binding to Aβ plaque in AD brain samples, supporting greater selectivity of PMN310 to Aβ oligomers and reduced risk of ARIA-related adverse effects compared to other antibodies currently in clinical development such as aducanumab and BAN2401. The murine precursor muPMN310 inhibited Aβ 42 aggregation in ThT assays, and increased viability of neurons in in vitro MTT metabolic assays (Gibbs et al. (2019)). PMN310 was also observed to block the effects of toxic oligomers on short-term memory loss in mice, as assessd by the novel object recognition test (Kaplan et al. (2019); Gibbs et al. (2019)).
5. Tau immunotherapy
Tau protein binds to and stabilizes microtubules, enabling transport of cellular cargo along neurons in the central nervous system (Drubin and Kirschner (1986)). Through microtubule regulation, tau mediates neuronal signaling and synaptic plasticity (Arendt et al. (2016)). Tau has also been observed to modulate DNA conformation and expression by both direct and indirect mechanisms, contributing to the regulation of genomic stability (Holtzman et al. (2016); Guo et al. (2017)). Microtubule binding is dynamically switched on/off by dephosphorylation/phosphorylation during the cell cycle; Tau must unbind for mitosis to occur for example.
There are more than 20 neurodegenerative diseases associated with the pathology of tau (Williams (2006)). In AD, pathologic tau manifests as neurofibrillary tangles (NFTs) in the brain. In healthy, terminally differentiated, post-mitotic cells such as neurons, cytosolic tau is phosphorylated at about 2 sites on average, while in NFTs, tau is hyperphosphorylated (Grundke-Iqbal et al. (1986)), at about 7–8 sites on average (Hanger et al. (2009); Mandelkow and Mandelkow (2012)). Phosphorylated tau can sequester normal tau and disrupt microtubule assembly (Alonso et al. (1996)), leading to neurodegeneration. Multiple (≈80) serine, threonine, and tyrosine sites on tau are engaged to varying degrees when tau is hyperphosphorylated (Arendt et al. (2016); Novak et al. (2018a)).
Tau protein is secreted from neurons; in AD it can propagate to nearby neurons along synaptic circuitry (de Calignon et al. (2012); Dujardin et al. (2014); Liu et al. (2012); Hu et al. (2016); Lewis and Dickson (2016)). Tau tangles then proliferate from the entorhinal cortex to the hippocampus and cortex as the disease progresses (Braak and Braak (1995)). Tau oligomers are highly diffusive (mobile), and are toxic to neurons (Flach et al. (2012); Tepper et al. (2014)). After prolonged incubation, human brain-derived tau oligomers can propagate the spread of abnormal tau conformation of endogenous murine tau, i.e. tau oligomers may exhibit inter-species templating of misfolding (Lasagna-Reeves et al. (2012)) reminiscent of prion-like behavior (Cashman and Caughey (2004)). There is much accumulating evidence that pathologic tau species from mouse or human tissue exhibits prion-like properties of propagation and inter-cellular infection (Clavaguera et al. (2009); Liu et al. (2012); Clavaguera et al. (2013); Sanders et al. (2014); Holmes et al. (2014); Holmes and Diamond (2014); Clavaguera et al. (2015); Guo et al. (2016); Mudher et al. (2017); Furman et al. (2017); Dujardin and Hyman (2019)).
Jackson et al. (2016) have shown that immunodepleting brain lysates from P301S transgenic mice with phospho-specific antibodies abolishes prion-like tau seeding activity in HEK cell cultures and transgenic P301S mice. Only oligomers containing more than about 10 tau molecules were seed-competant. Thus, tau hyperphosphorylation may inadvertently contribute to a conformational reorganization inducing large tau oligomers to become prion-like. The relative abundance in human brain of prion-like, phosphorylated tau, rather than the total amount of inert, insoluble tau, appears to decrease with AD patient longevity (Aoyagi et al. (2019)).
In AD, increased β-amyloid precedes the onset of symptoms and clinical disease by roughly 15 years. Years after Aβ pathology is present, tau biomarker abnormalities are detectable, and their accumulation correlates more closely with the severity of dementia than does amyloid deposits (Arriagada et al. (1992); Bierer et al. (1995); Nelson et al. (2010); Serrano-Pozo et al. (2011); Nelson et al. (2012)), as observed for example by serial PET scans of amyloid and tau, and comparing with cognition (Hanseeuw et al. (2019)). Alzheimer's disease appears to manifest as an Aβ-exacerbated tauopathy.
Roberson et al. (2007) found that in transgenic mice overexpressing human Aβ, reducing endogenous tau levels could prevent AD-like behavioral decline. High Aβ level was unaffected. Reducing tau could also protect neurons from excitotoxic dysfunction. Similar findings have been observed by administration of tau antibody 43D in triple transgenic (3 × Tg) mice, where the antibody reduced total tau and hyperphosphorylated tau, decreased APP production and thus Aβ production, and increased microglial activation and complement C1q and C9 levels (Dai et al. (2017)). These latter effects resulted in less Aβ plaque load. In another study using a phospho-specific C-terminal tau antibody, in addition to the expected decreases in soluble and insoluble tau, Aβ deposits were decreased by ≈84% (Rajamohamedsait et al. (2017)). Taken together, the above evidence suggests that reducing tau levels via passive or active immunotherapies could thus represent an effective strategy for treating AD and related tauopathies. A concern in pursing such an approach however is to avoid targeting functional tau. An antibody selective to pathological tau is most desirable as a safe and effective therapeutic.
Current immunotherapies directed against tau are listed in Table 2 , along with their epitopes, immunization strategy, binding selectivity if known, antibody backbone isotype, and current and completed trial information. For antibodies currently in clinical trials directed against tau, Fig. 5 shows the locations of their corresponding epitopes on the tau primary sequence. The order of antibodies below is loosely based on their current stage in clinical trials as well as their historical development.
Table 2.
Antibodies to tau currently in clinical development.
| Antibody | Epitope location | Immunization strategy | Binding Selectivity | Ab species and backbone isotype | Current Status (2019–2020) | Company | Clinical outcomes |
||
|---|---|---|---|---|---|---|---|---|---|
| Last completed Trial (Disease,Phase) | Patient stage | Reduced tau burden | |||||||
| Gosuranemab, BIIB092, BMS-986168, IPN007/IPN002) | 15–24 | In vitro aggregated full-length tau | M, NFT?, eTaua | humanized IgG4 | NCT03352557 (AD,II) | iPerian/Bristol-Meyers Squibb/Biogen | NCT02460094 (PSP,I), NCT03068468 (PSP,II,dd) | MCI/Mild AD | + |
| ABBV-8E12 (C2N-8E12, HJ8.5) | 25–30 (within 22–34) | recombinant full-length tau | M, NFT, eTau | humanized IgG4 | NCT03712787 (AD,II), NCT02880956(AD,II) | C2N Diagnostics/Abbvie | NCT02494024 (PSP,I) NCT03391765 (PSP,I,d) | Early AD | + |
| Zagotenemab (LY3303560, MC-1 IgG1) | 7–9/312–322 (discontiguous) | Immunopurified PHFs | M-,O,NFT | humanized IgG4 | NCT03518073 (AD,II) | Eli Lilly | NCT02754830, NCT03019536 (AD,I) | healthy, mild to moderate AD | –c |
| Semorinemab (RO7105705, MTAU9937A, RG6100) | 2–24 (C-terminal portion) | recombinant oligomers | M,NFT,eTau | humanized IgG4 | NCT03828747 (AD,II), NCT03289143 (AD,II) | AC Immune, Genentech, Hoffmann-La Roche | NCT02820896 (AD,I) | Mild to moderate AD | + |
| BIIB076 (NI-105.6C5 huIgG1λ) | probably 125−131 | N/A: B-cells from healthy subjects | M, PFF, NFT | human IgG1 | NCT03056729 (AD,I) | Biogen/Neurimmune | NCT03056729 (AD,I) | Healthy/MCI AD | +b |
| RG7345 (RO6926496) | 416–430 (pS422) | 416–430 (pS422) peptide | phospho M, PHF | humanized IgG1 or IgG4 | NCT02281786 (I,d) | Hoffmann-La Roche | NCT02281786 (I,d) | healthy males | − |
| UCB0107 (D IgG4) | 235–246 | Recombinant tau fibrils | M,O,PHF | humanized IgG4 or IgG1 | NCT03464227 (AD,I), NCT03605082 (AD,I) NCT04185415 (PSP,I,s) | UCB Biopharma | NCT03464227 (I), NCT03605082 (I) | Healthy males, healthy m/f | − |
| JNJ-63733657 (B296, PT3) | 204–225 (pT212/pT217) | PHF from AD patients | M,PHF (ptau) | humanized, likely IgG1 | NCT03375697 (prodromal, mild AD,I) | Janssen | NCT03689153 (I) | healthy m/f | + |
| NPT088 | --c | N/A: Human IgG -viral g3p GAIM chimera | O,F/P | Human IgG1 | NCT03008161 (I) | Proclara Biosciences | NCT03008161 (I) | Mild to moderate AD | − |
M = Monomers, O=Oligomers, NFT = Neurofibrillary tangles, eTau = extracellular tau, PFF = pre-formed fibrils, PHF = paired-helical filaments.
in cynomolgus monkeys.
indicates “not reported”.
d = Discontinued.
Fig. 5.
Epitope locations on the primary sequence of tau, for antibodies currently in clinical trials. Black bars indicate epitope locations; Pink bands on epitopes indicate phosphorylated sites and thus selectivity to the phosphorylated species. Domain structural features are shown for the longest isoform of tau (2N4R, 441 aa). The 2N4R isoform contains two N-terminal domains (N1 and N2 of 29 aa each), two proline-rich domains (P1 and P2 of 46 aa each), and four microtubule-binding domains (R1-R4 of 31 aa each). Zagotenemab has a discontiguous epitope. Specific epitope locations are listed in Table 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5.1. Gosuranemab (BIIB092)
As described above, extracellular tau is thought to mediate the onset, cell-to-cell propagation, and neurodegenerative progression of AD as well as the other tauopathies, including progressive supranuclear palsy (PSP), chronic traumatic encephalopathy (CTE), and some cases of frontotemporal lobar degeneration. BIIB092 was developed to target extracellular tau.
Bright et al. (2015) reprogrammed skin cells from patients with sporadic or presenilin-1-mutant AD to induced pluripotent stem cells (iPSCs), which they then differentiated into cortical neurons. Compared to age-matched control neurons, the AD-derived cells secreted N-terminal fragments of tau (eTau) into the extracellular space. Electrophysiological analysis showed that the secreted eTau induced neuronal hyperactivity, which could itself increase Aβ production in a kind of toxic pas de deux (Ittner and Götz (2010); Dai et al. (2017)).
Bright et al.'s immunization strategy was to raise a mouse monoclonal antibody by standard immunization using in vitro aggregated full-length (2N4R) tau, rather than selectively presenting a particular epitope. The purified antibodies were then screened and selected afterward for high binding affinity to both the secreted eTau and full-length tau, as well as their ability to ameliorate eTau-induced neuronal hyperactivity.
BIIB092, the humanized version of the above mouse monoclonal antibody (IPN002), is an IgG4 monoclonal that recognizes a linear, non-phosphorylated epitope in the N-terminal region of tau consisting of amino acid residues 15AGTYGLGDRK24 (Griswold-Prenner et al. (2014); Qureshi et al. (2018)).
In phase 1 trials, BIIB092 was found to be safe and well-tolerated; there were no severe adverse effects in the low and moderate dose arms, and 8% (2/24) severe adverse effects in the highest dosage arm. These were not considered to be related to the drug, and they all eventually resolved. The antibody reduced unbound N-terminal tau in CSF: A reduction of 91–97% was sustained by day 85 across all doses (Boxer et al. (2019)). However, AD biomarkers such as total tau and ptau181 (Dage et al. (2016); Tatebe et al. (2017); Yang et al. (2018)) were not reduced by BIIB092, perhaps either due to N-terminal truncation of these constructs, or the presence of antibody-bound fragments in the CSF. Such observations have motivated some of the tau mid-region binding antibodies discussed below. Two phase II clinical trials were initiated in 2017 for AD and PSP (see Table 2). The PSP trial (PASSPORT, NCT03068468) was discontinued in December 2019, for failure to meet or either primary or secondary endpoints (AlzForum.org, 2020b). The AD trial (TANGO trial, NCT03352557) is currently ongoing, and has a completion date of 2024.
5.2. ABBV-8E12
The mouse monoclonal IgG2b antibody HJ8.5 has been raised by conventional immunization using recombinant full-length human tau. The state of tau (monomeric, aggregated) as well as the adjuvant immunogen has to our knowledge not been disclosed (Holtzman et al. (2014)). The antibody is selective to human tau and does not bind to mouse tau. The epitope has been published as either 25DQGGYT30 (Yanamandra et al. (2013)) or 22DRKDQGGYTMHQD34 (Holtzman et al. (2014)). Indeed the epitopes align by BLAST primarily to one of the few gap regions in the mouse-human sequence alignment: 22DRKDQGGYTMHQD34 aligns to - - - - - - - - 19TLLQD23 in mouse, and 25DQGGYT30 aligns to - - - - - T19 in mouse. (i.e. human 30TMHQD34 aligns with mouse 19TLLQD23 above). The antibody binds both tau monomer and stains neurofibrillary tangles (Yanamandra et al. (2013)), and does not appear to be conformationally selective to oligomeric or other species.
The antibody was selected for development based on its ability to block uptake and inhibit seeded tau aggregation in in vitro assays, where neuronal cells were exposed to tau-P301S mouse brain lysates containing tau aggregates (Yanamandra et al. (2013)). Like BIIB092, it is thus screened for the ability to target extracellular tau. In human P301S tau-transgenic mice, administration of HJ8.5 reduced brain neurofibrillary pathology and phospho-tau abundance, and reduced atrophy of hippocampal brain volume (Yanamandra et al. (2015)).
ABBV-8E12 (C2N-8E12) is the humanized IgG4 antibody version of HJ8.5. It was found safe and tolerable as IV injections in phase I trials (NCT02494024, West et al. (2017)). Phase 2 trials for PSP were discontinued in July 2019 however, after futility analysis. Several press releases corroborate this (e.g. AlzForum.org, 2020a), although at present no trials are listed as halted or discontinued for 8E12 on clinicaltrials.gov. ABBV-8E12 is also in a phase 2 trial for early AD, which is continuing without changes. One rationale for continuing is that the stage of tauopathy of patients in the AD trial may be relatively early compared to those in the PSP trial. The current phase 2 trials for PSP and AD using BIIB092, which also targets an N-terminal epitope, are also continuing as originally planned.
5.3. Zagotenemab (LY3303560)
The antibody Alz-50, developed now over 34 years ago (Wolozin et al. (1986)), selectively recognizes paired helical filaments (PHFs) of tau in AD brains. Alz-50 was used to purify PHFs from AD brain homogenates by immunoaffinity, and these purified PHFs were then used as immunogens in mice to raise the monoclonal IgG1 antibody MC-1 (Vincent et al. (1996); Jicha et al., 1997). Perhaps surprisingly, these two antibodies share a similar discontiguous epitope on tau. Alz-50 also recognizes the transcriptional repressor protein FAC1 (an off-pathway target), while MC-1 does not. The discontiguous epitope requires N-terminal amino acids 7–9, and amino acids 313–322 in the third microtubule binding domain (Jicha et al. (1997)). Removing either component of the epitope ablates binding. As well, mixing chains containing only the N-terminal component with chains containing only the C-terminal component also showed no reactivity to the antibody (Jicha et al. (1997)), so it appears that both components of the epitope must be present on the same chain.
The discontiguous epitope is present in an aberrant conformation of tau that is present in a nonfilamentous, soluble form of tau indistinguishable from NFTs by ELISA and immunoblotting, and is also present in PHFs (Weaver et al. (2000)). Reactivity to the antibody appears to correlate with the severity and progression of AD (Vitale et al. (2018)). Passive immunization experiments using intra-peritoneal injections have shown that MC-1 could reduce tau pathology in the forebrain of transgenic P301L tau mice. (Chai et al. (2011); d'Abramo et al. (2013)).
LY3303560 (zagotenemab) is the humanized version of MC-1. It is likely an IgG4 isotype, with mutations in the constant region to reduce effector function and prevent subunit exchange (Alvarado et al. (2016)). The antibody recognizes a conformational epitope of tau with primary epitope located in the N-terminal region (Alam et al. (2017)), but which is also discontiguous like MC-1, and involves amino acids 7EFE9 and 312PVDLSKVTSKC322 (Alvarado et al. (2016)). One possible issue in this regard is the potential loss of reactivity to N-terminally truncated species. LY3303560 is selective for tau aggregates over monomer (Alam et al. (2017)): The affinity to soluble tau aggregates (K D < 220pM) is driven by avidity, and is much higher than the affinity for monomer (K D ≈ 235nM).
Two phase 1 trials for zagotenemab have completed, one for healthy volunteers or mild to moderate AD volunteers (NCT02754830) and another for mild to moderate AD volunteers (NCT03019536) (clinicaltrials.gov). Results have not yet been reported. A phase 2 trial is currently active for patients with early AD (NCT03518073), scheduled to complete in October 2021.
5.4. Semorinemab (RO7105705)
The development of semorinemab (RO7105705) seeks to minimize Fcγ receptor activation, and seeks to maximize binding across different extracellular tau species. The argument for a pan-tau antibody is that nearly all antibody-accessible tau is extracellular, and that any extracellular tau could drive pathology and so is a viable target for elimination.
“Effectorless” antibodies may be engineered by making D265A and N297G (DANG) mutations in the Fc region of the antibody, which when combined, abolish binding to microglial Fcγ receptors. (Couch et al. (2013)). Preclinical studies have shown that effectorless antibodies protected neurons from toxicity better than the unmodified version did, and that effectorless antibodies can remove aggregates in mouse models as well as normal antibodies (Lee et al. (2016)). Similarly, RO7105705 is a humanized IgG4, which only weakly activates microglial Fcγ receptors, minimizing inflammation. It should be noted that strict adherence to IgG4 antibodies to minimize effector response is not universally embraced. It is argued for example that the benign safety profile of the active immunotherapy AADvac-1, which induces predominantly an IgG1 antibody response, implies that at least pathological tau can be safely targeted with IgG1 antibodies (Novak et al., 2017, Novak et al., 2018a).
Antibody generation for RO7105705 likely proceeded by vaccination of mice with recombinant, phosphorylated, and oligomerized human tau (Adolfsson et al. (2016)). Antibody selection then proceeded by assaying for binding to full-length tau, then to phosphorylated tau and oligomerized tau, with the aim to find antibodies that bound equally well to both tau and post-translationally modified tau. Binding to all 6 isoforms of human tau was also used as a selection criterion. To maintain pan-tau properties, epitopes that mapped to regions with a high density of phosphorylated residues (S,T,Y) were avoided. The epitope of RO7105705 is likely within residues 2–24 (2AEPRQEFEVMEDHAGTYGLGDRK24, Adolfsson et al. (2016)). The antibody reacts with all 6 isoforms of human and primate tau, but not mouse tau (residues 19GLGDRK24 are absent in mouse tau), implying that the epitope resides in the C-terminal portion of the above sequence.
RO7105705 was found to protect neurons from tau-mediated toxicity in cell-based studies. In transgenic mice expressing human mutant P301L tau, 13 weeks of treatment with either 3, 10, or 30 mg/kg of RO7105705 reduced pathological tau in the brain in a dose-dependent fashion; The antibody also raised tau levels in blood plasma, implying target engagement and stabilization in the periphery, akin to the peripheral sink mechanism proposed for Aβ antibodies such as solanezumab and ponezumab. Chronic dosing was safe in both mice and cynomolgus monkeys (Ayalon (2017)).
Phase 1 studies have shown that the antibody is safe and tolerable in healthy volunteers, even at extraordinarily high single doses of 16,800 mg (Kerchner et al. (2017)). Two current phase II trials, one for prodromal/probable AD participants (TAURIEL trial, NCT03289143), and one for moderate AD participants (NCT03828747) are ongoing.
5.5. BIIB076
Healthy human subjects who are at risk for AD, either because of advanced age or genetic predisposition, yet who exhibit no AD symptoms or unusually slow progression, provide a valuable therapeutic resource for the isolation of antibodies to AD-related proteins. This strategy has been also exploited in the development of aducanumab, as mentioned above in Section 4.7. Tau disease-selective monoclonal antibodies isolated from memory B cells in such healthy subjects with no signs of a neurodegenerative tauopathy are expected to have excellent safety profile and lack of immunogenicity, and to be already evolutionarily optimized and affinity matured by the human immune system. This therapeutic strategy has motivated the development of 3 lead antibodies in the patent literature, NI-105.4E4, NI-105.4A3, and NI-105.24B2 (Chen et al. (2012); Nitsch et al. (2019)).
BIIB076 is cited as being derived from NI-105.6C5 (AlzForum.org, 2019a), another antibody in the patent literature (Weinreb et al. (2014)) developed similarly by Neurimmune's reverse translational approach, which mines antibody sequences isolated from healthy human B cells. The epitope of NI-105.6C5 is 125ARMVSKS131 (creativebiolabs.net, 2019; Weinreb et al. 2014), which is between N-terminal domain N2 and proline-rich domain P1.
BIIB076 is a fully human IgG1, which binds with subnanomolar affinity to both human and cynomolgus monkey recombinant tau. (Czerkowicz et al. (2017)). It is a “pan-tau” antibody, recognizing monomeric and fibrillar forms, as well as tau isolated from healthy human and Alzheimer's disease brains. No cell-based or mouse preclinical work with this antibody has been published. BIIB076 exhibited no adverse toxicology or pathology in cynomolgus monkeys, and CSF total and free tau levels were significantly reduced in the highest BIIB076 dose animals in this study (Czerkowicz et al. (2017)). These results established a positive safety profile for inclusion of BIIB076 into phase I trials. This trial (NCT03056729) recruited healthy and mild-AD volunteers to monitor adverse events and pharmacokinetics (Table 2). The trial protocol was modified in June 2019, to drop the more advanced AD cohort and adopt adverse events as the sole primary outcome. The trial has recently finished in March 2020.
5.6. RG7345 (RO6926496)
Phosphorylation at S422 is a part of the maturation process of PHFs, and generally precedes proteolytic cleavage at least at some locations such as D421 (Guillozet-Bongaarts et al. (2006)). pS422 is prominent in early stages of Alzheimer's disease and persists until late-stages, making it an attractive target. Active immunizations also support pS422 as a viable target. In a transgenic mouse model overexpressing a mutant, alternative isoform of tau (the 412 amino acid isoform missing N-terminal domain N2, with mutations G272V and P301S, under a neuron-specific promotor (Thy1.2)), active immunization with a peptide containing pS422 decreased insoluble tau in the brain, and this decrease correlated with significant memory improvement using the Y-maze spatial memory task (Troquier et al. (2011)).
Polyclonal antibodies to epitopes containing phospho-serine 422, which resides near the C-terminus of tau, have been shown to be reactive to brain extracts from patients with AD, PSP, corticobasal degeneration (CBD), and other neurodegenerative diseases, while such polyclonal antibodies are unreactive to controls (Bussière et al. (1999)). pS422 has thus been identified as a pathological epitope found in several diseases with neurofibrillary degeneration. Polyclonal pS422 antibodies recognized intra-neuronal NFTs in cells that had lost their integrity; extra-neuronal NFTs were also recognized. In contrast to some other phospho-selective antibodies with epitopes at sites pT153, pS262, and T231, staining of pre-tangles with pS422 was rare (Augustinack et al. (2002)).
RG7345 was developed from mice and/or rabbits immunized with phospho-peptide 416SIDMVD(pS)PQLATLAD430 coupled to KLH, where antibodies were subsequently screened for selective binding to the peptide with pS422 (Bohrmann et al. (2010)). The antibody was then recombinantly expressed with a murine IgG1 or a human IgG1 isotype. The isotype of the humanized antibody has not been disclosed to our knowledge, and patent protection specifies both IgG1 and IgG4 isotypes (Emrich et al. (2016)). In triple transgenic mice expressing mutant APP, PSEN2, and MAPT (TauPS2APP mice), the murine IgG1 was able to significantly reduce tau pathology compared to vehicle-treated controls, if intraperitoneal treatment persisted for 4 months (Collin et al. (2014)). In this same study, Collin et al. (2014) observed that antibodies were incorporated into neurons via endocytosis of bound antibody-antigen complex. This finding offers a possible explanation as to how anti-tau antibodies might be effective in treating intra-neuronal tau pathology, but also indicates that extracellular tau may not be exclusively targeted.
The antibody RG7345 has been advanced to phase I clinical trials (NCT02281786), which were completed in October 2015. Development of RG7345 has been discontinued by Roche however; No reasons have yet been reported.
5.7. UCB0107
There is some evidence that antibodies binding to the mid-region of tau are more effective at blocking cell-to-cell propagation than those targeting the N-terminus, though this notion is still speculative, as some N-terminal binding antibodies such as BIIB092 and ABBV-8E12 were selected for blocking cell-to-cell transmission of pathology, and findings for these antibodies as well as RO7105705 indicate at least partial efficacy of N-terminal antibodies in blocking the spread of pathogenic tau.
Courade et al., (2018) employed a high-throughput screening approach wherein they immunized rabbits with 17 different peptide constructs targeting various regions of tau, and they immunized rats with either recombinant tau fibrils or human AD brain-derived PHF tau. This resulted in 94 ELISA-reactive monoclonal antibodies, which were then screened by surface plasmon resonance (SPR) for binding to tau monomer and AD-PHFs. 51 of these antibodies were then screened for the ability to block cell-to-cell propagation of tau aggregation in HEK293 cells seeded with AD PHF. Five antibodies displayed robust and complete neutralization of pathological tau seeds.
The most potent of these 5 antibodies (antibody D) came from a rat immunization with recombinant tau fibrils. An equimolar mix of four tau isoforms (2N4R, 1N4R, 0N4R and 0N3R, see Fig. 5) containing both soluble tau and insoluble fibril tau was used for the recombinant tau fibril rat immunization (Knight et al. (2017)). Interestingly, in the cell-based assay testing propagation, recombinant antibodies using CDRs in the existing patent literature for BIIB092, ABBV-8E12, and LY3303560 were significantly less effective than antibody D in blocking seeded tau aggregation (Courade et al. (2018)). One explanation is that these antibodies target the N-terminal region of tau as that tends to be where affinity is highest, however N-terminal tails are thought to be exposed outside the core of tau fibrils where they may be cleaved by proteases (Courade et al. (2018)). Thus at least some proteopathic tau seeds may be missing N-termini.
In P301L human tau transgenic mice given hippocampal injections with AD brain homogenate or PHF from AD brains, intraperitoneal administration with high doses (30 mg/kg) of antibody D were able to block the progression of tau seeding pathology to distal brain regions (Albert et al. (2019)). This result was recapitulated for a tau fragment injectant containing only the 4 microtubule binding repeats with mutation P301L, which antibody D does not bind given its epitope location. This latter result explicitly demonstrates blockage of seeded endogenous tau.
UCB0107 is the humanized version of antibody D. Its specific isotype is likely an IgG4 (AlzForum.org, 2019b; Knight et al. (2017)). The developers are fairly agnostic as to whether at least some effector function is desirable or if it is to be avoided. The epitope of UCB0107 has been mapped to residues 234SPSSAKSRLQTA246, which is at the end of tau's second proline-rich region and just before its first microtubule-binding domain. The (undeposited) co-crystal structure contains a bound tau peptide with residues 234–244, at least part of which is in a helical structure (Knight et al. (2017)).
Two phase I clinical trials for UCB0107 (NCT03464227 and NCT03605082) were completed in December 2018 and March 2019 respectively, but results have not yet been reported. A phase I trial for PSP initiated in December 2019 (NCT04185415) has been halted as a precautionary measure due to the COVID-19 pandemic.
5.8. JNJ-63733657
Functional tau that is bound to axonal microtubules is hypo-phosphorylated, while aggregated tau in AD is hyper-phosphorylated. This post-translational process along the pathological maturation pathway can provide unique epitopes that are distinct from the physiologically active pool of tau. JNJ-63733657 is a monoclonal antibody likely of IgG1 isotype, with selective affinity for paired helical filament (PHF) tau and tau phosphorylated at select sites described below.
The murine precursor to JNJ-63733657, PT3, was derived from immunization of a Balb/c mouse with enriched PHF-tau (ePHF-tau) from AD brain. The antibody was tested for target selectivity to phospho-tau versus non-phospho-tau by ELISA, western blot, and immunohistochemistry (IHC). The humanized antibody B296 has the same CDR sequences as PT3, and was humanized by pairing variable regions of PT3 with a human IgG1/κ constant region (Mercken et al. (2018)); This antibody was affinity matured to generate JNJ-63733657. B296 has a strong affinity of 27 pM to PHF tau. B352, an IgG4 variant of B296 with the same variable regions, had an affinity of 43 pM to PHF-tau. Both of these antibodies did not bind unphosphorylated tau.
The antibody binds to a phosphorylated epitope in the proline rich domain P2 of tau protein between residues G204-K225. For high-affinity binding by the murine precursor PT3 (K D < 25nM to the phospho-peptide), either T212 or T217 must be phosphorylated. If both T212 and T217 are phosphorylated, K D < 1nM. Thus the optimal sequence is 204GTPGSRSR(pT)PSLP(pT)PPTREPKK225, although S214 may be phosphorylated as well and K D < 1nM preserved, and binding to sequences with pS214/pT217 or pS210/pT217 have affinity K D ≈ 6nM (Mercken et al. (2018)). The epitope of PT3 is distinct from other reported epitopes of phospho-dependent anti-tau antibodies, such as AT8, AT180, and anti-tau pS422.
The co-crystal structure of the antibody-epitope has been obtained (Mercken et al. (2018)) but not yet deposited on the PDB. Structurally, the bound epitope is extended and linearized when bound to the antibody in the co-crystal structure.
Similarly to the assay described above in Section 5.7, a cell-based assay may be used to measure the inhibition of tau propagation by anti-tau antibodies, when the cells are transfected with a co-incubated mixture of AD brain homogenate and anti-tau antibody. Vandermeeren et al. (2018) used HEK cells expressing two chromophore-tagged tau repeat-domain fragments, which will generate a FRET signal if they are in proximity upon aggregation. FRET-positive cells were then sorted and counted after transfection to test the efficacy of a panel of antibodies. Anti-tau antibodies tested in this system vary in their ability to immunodeplete the seeding capacity from human AD brain homogenates (Borgers et al. (2017); AlzForum.org, 2019d). Similar to the development of UCB0107, antibodies against tau's mid-region best removed pathogenic seeds, whereas antibodies to the N-terminal region of tau only weakly suppressed seeding, again likely because of N-terminal cleavage of propagative aggregates by proteases. In contrast to the findings of Courade et al. (2018), Mercken and colleagues saw some efficacy for both BIIB092 and ABBV-8E12 in their cell-based assay (AlzForum.org, 2019d).
An in vivo P301L tau transgenic mouse model was tested by Mercken et al. (2018), wherein intraperitoneal injection of PT3 (or controls) was followed by seeding induction by intracranial injection of AD-brain-derived PHF-tau. In this model, peripheral administration of PT3 was able to significantly reduce the seeded propagation of tau aggregation in mouse brains.
A recent phase I clinical trial in healthy Japanese participants aged 55–75 finished in July 2019 (NCT03689153). JNJ-63733657 was found to be generally safe and well-tolerated. CSF levels were ≈0.2% of serum levels, and a dose-dependent reduction in phospho-S217 tau was observed in the CSF following antibody administration (Galpern et al. (2019)). A 2nd phase I trial testing safety and pharmacokinetics in both healthy participants or participants with prodromal or mild Alzheimer's disease has recently completed in December 2019. Results have not yet been reported.
5.9. NPT088 and BIIB080
As mentioned above in the context of Aβ -targeting therapies (Section 4.13), NPT088 is a generic amyloid-binding antibody that is reactive to both Aβ and tau. In transgenic mouse models overexpressing mutant APP that elevates levels of Aβ (Tg2576 mice expressing K670N/M671L APP) Y-maze performance was significantly increased and novel object recognition was significantly improved after weekly intraperitoneal dosing with NPT088 for 10 weeks or 14 weeks respectively (Levenson et al. (2016)). Staining of brain sections with a PHF-tau-specific monoclonal antibody (AT8, see below) shows a reduction in PHF in these systemically treated mice compared to controls (Levenson et al. (2016)). Since NPT088 is specifically reactive to amyloid fibrils (Table 1), these results suggest that binding to monomeric tau may be unnecessary for efficacy of an immunotherapeutic, and that targeting oligomers and/or fibril species may be sufficient. This notion bears some analogy with the problem of avoiding target distraction due to abundant monomer concentration for Aβ therapeutics. Another potential advantage of a therapeutic such as NPT088 with reactivity to both Aβ and tau is that if a combination therapy is required, NPT088 alone may still be effective clinically.
BIIB080 is an antisense oligonucleotide (ASO) that silences the translation of tau mRNA as described below, so it can be thought of as a passive but not a protein immunotherapeutic. This antisense oligonucleotide targets the beginning of exon 5 (at the 5′ end). In a study investigating 31 candidate morpholinos, the morpholino targeting this specific site (extended by 3 bases to bracket the intron-exon boundary of the splice acceptor site) was one of the most effective in significantly reducing total MAPT transcript levels (Sud et al. (2014)). Exon skipping of exon 5 (as well as 1 and 7) results in changing the open reading frame of the mRNA, leading to a premature stop codon likely resulting in nonsense-mediated decay.
In preclinical studies, the ASOs of BIIB080 had a phosphorothioate backbone to improve nuclease resistance and promote cellular uptake. In human P301S tau-transgenic mice, reduction of tau expression by BIIB080 ASOs resulted in fewer tau inclusions, reversal of preexisting phosphorylated tau and Thioflavin S pathology, reduced rates of neuronal death, and extension of mouse survival time (DeVos et al. (2017)). Although the effects of tau knock-down by an anti-sense oligonucleotide may be different in humans than in a mouse model that by design is overexpressing mutant tau, these encouraging initial results have led to an active clinical trial (NCT03186989) for BIIB080.
5.10. Selected preclinical /research tau antibodies
DC8E8 will bind to any one of 4 separate epitopes on tau having similar sequence motif (Kontsekova et al. (2014)): 268HQPGGG273 (located within microtubule binding region 1 (MTBR1)), 299HVPGGG304 (in MTBR2), 330HKPGGG335 (in MTBR3) or 362HVPGGG367 (in MTBR4). Several PDB crystal structures of the DC8E8 Fab in the complex with a 14-mer tau peptide have been determined; The antigen-binding region from 5MO3.pdb is shown in Fig. 6 , which contains the epitope 298KHVPGGGS305. The epitope is mainly linear, with a β-turn in the glycine region. The backbone contacts in the glycine region are apparently important for antibody affinity: The above 6-residue epitope motifs compete with tau151−391 for binding to antibody DC8E8, but removal of the C-terminal glycine from the epitope eliminates the ability of the corresponding peptide to compete (Kontsekova et al. (2014)).
Fig. 6.
Co-crystal structures of the antigen-binding regions of two preclinical antibodies to tau, bound to their epitopes. Antibodies, Protein Databank entry, and epitopes are: (left) DC8E8, PDB 5MO3, structured epitope amino acids 298–305; (right) AT8, PDB 5E2W, structured epitope amino acids 202-209.
Binding of the antibody to this epitope on tau interfered with pathological tau-tau interactions in an in vitro assay, reducing the amount of oligomeric tau by 84% (Kontsekova et al. (2014)). In vivo, DC8E8 significantly reduced the amount of insoluble oligomerised tau and the number of early and mature neurofibrillary tangles in transgenic mouse brains. The humanized version of the murine antibody DC8E8 is AX004. AX004 has been shown to block cell-to-cell propagation of tau by preventing neuronal internalization of extracellular tau, as mediated between the microtubule binding domain containing DC8E8 epitopes, and Heparan Sulfate Proteoglycans (HSPGs) on the neuron surface (Weisová et al. (2019)). As mentioned above in Section 2.1.2, the epitope when used as an active vaccine in humans is AADvac-1.
The murine monoclonal IgG1 antibody AT8 (Mercken et al. (1992); Goedert et al. (1995)) is a widely used anti-tau antibody to probe phosphorylated PHFs and assess tau phosphorylation at the amino acids Ser 202, Thr 205 and Ser 208. AT8 recognizes an epitope doubly phosphorylated at serine 202 and threonine 205. AT8 is about 10% cross-reactive to the doubly phosphorylated epitopes S199/S202 and T205/S208. The antibody has no cross-reactivity with unphoshorylated tau. The epitope is 200PG(pS)PG(pT)PG207 (Porzig et al. (2007)). The co-crystal structure of the antigen-binding fragment of AT8 bound to a triply phosphorylated tau peptide 194Ac-RSGYSSPG(pS)PG(pT)PG(pS)RSR-OH211 (residues 202–209 are resolved in the crystal structure) is shown in Fig. 6 (Malia et al. (2016)). Currently AT8 is a useful research antibody; It is unclear if AT8 or a humanized/modified variant will be clinically-relevant.
A tau oligomer-selective monoclonal antibody (TOMA) was developed by Kayed and colleagues (Castillo-Carranza et al. (2014)), by immunizing BALB/c mice with recombinant (“synthetic”) tau oligomers. TOMA is a murine IgG1 that selectively recognizes tau oligomers over either monomeric tau or tau NFTs. Its epitope has not been determined/disclosed. In JNPL3 mice, which expresses mutant human P301L tau, a single intracerebroventricular injection of TOMA cleared tau oligomers but did not significantly affect levels of tau monomers or NFTs. The injection also reversed both rotorod locomotor and Y-maze memory deficits—improvements that persisted for 2 months post injection. The binding and clearane of extracellular tau oligomers facilitated by the antibody is thought to lead to efflux of intracellular oligomers, resulting in eventual CNS and serum clearance (Castillo-Carranza et al. (2014)).
The above are just three representative examples of many tau antibodies that are currently in preclinical development. For a more thorough list, the reader is referred to Jadhav et al. (2019).
6. Summary and discussion
It is probably too perfunctory to say that current AD therapeutics have had no effect on disease progression. More precisely, AD therapeutics in current clinical trials have generally failed to meet their desired endpoints for the slowing of cognitive decline. It must be noted however that when subgroups of patients from a full cohort have been subsequently analyzed for some therapeutics, cognitive benefits have been observed.
A recurring theme in Alzheimer's therapies is the importance of early or even preventative treatment (Dubois et al. (2016); Strobel (2010)). Treating other chronic conditions such as atherosclerosis (with statins) or hypertension (with lifestyle modification or anti-hypertensives) as early as possible has generally been advised. There are currently 9 prevention-based clinical trials ongoing since 2018 (Cummings et al. (2018)). The occurrence of molecular pathology that appears to undergo prion-like propagation long before any behavioral symptoms manifest may make presymptomatic treatment mandatory for Alzheimer's disease. It also may imply that perhaps some of the halted or discontinued therapeutics described above may be effective when used in a preemptive manner.
In line with the above notions of preemptive therapy, solanezumab and gantenerumab have both been tested in a clinical trial (the DIAN-TU trial, NCT01760005) involving subjects with known autosomal-dominant Alzheimer's disease mutations, and who are within −15 to +10 years of the predicted or actual age of cognitive symptom onset (Bateman et al. (2017); clinicaltrials.gov). Initial topline results on this trial reported in February 2020 indicated that, disappointingly, the trial had missed a cognitive endpoint consisting of a composite of four cognitive tests developed by the Dominantly Inherited Alzheimer Network. Site investigators agreed that the drugs were likely markedly underdosed, both in dosage amount and duration. Dosage was increased mid-study, but many participants received relatively short durations of the higher dose (roughly 25% of the total duration). Results reported at the April 2020 AAT-AD/PD meeting clarified that the patient cohort was also highly heterogeneous—participants who were symptomatic had descended into moderate dementia before they could be titrated up to a high dose, whereas asymptomatic participants remained stable througout the trial regardless of drug or placebo arm.
In fact, the degree of symptoms appeared to strongly determine how effective the treatment was: Participants who were presymptomatic at baseline improved significantly on logical memory tests and digit symbol substitution tests, and remained stable on MMSE, CDR-SB and functional assessment score (FAS) tests; Participants who were symptomatic did not improve on the logical memory test, and declined on all other tests. These results, though still not conclusive, appear to support the earliest possible intervention.
Additionally, gantenerumab, but not solanezumab, appeared to improve biomarkers. In addition to removing brain amyloid plaques and improving (increasing) the CSF Aβ 42/Aβ 40 ratio, gantenerumab lowered levels of CSF total tau and phospho-tau181 by ≈1/3, while maintaining the level of CSF neurofilament light. An open-label extension exploring high-dosage gantenerumab for several additional years is currently planned.
Combination therapies are now common in cancer therapeutic treatment (Miles et al. (2002); Hu et al. (2010); Woodcock et al. (2011); Saputra et al. (2018)). Combination therapies may be particularly effective in later stages of AD when multiple biochemical pathways have been altered. Even early stages of AD pathology likely involve multiple proteins and druggable pathways, including both Aβ and tau. Significant mixed pathology is present in up to 70% of dementia cases, further supporting the idea that combination therapies may be most effective and perhaps even necessary. As mentioned in the above section on fibril and oligomer polymorphism, the presence of multiple conformational strains of misfolded protein will limit the efficacy of a single antibody to block propagation and spread of all distinct conformational strains. Concerning challenges for such a combinatoric approach are the timelines required for regulatory approval for separate individual therapeutics, and the disconcerting possibility that if combination therapy is required for benefit, individual drugs will fail to meet primary end points, and support for further development will be difficult to obtain.
By achieving a more thorough understanding of the underlying biochemical mechanisms of Alzheimer's disease, we have been able to develop precision therapeutics that target key processes in the molecular pathology, including the prion-like propagative aspects that are central to the spread of the disease. With each trial, we have gained new insights and learned often painful lessons about the insidious nature of this disease. It is up to us to continue to innovate novel solutions to target and stop the spread of AD pathology, and discover the truly effective therapies that natural biology and rational design will someday provide.
Acknowledgements
We acknowledge support from the Canadian Institutes of Health Research Transitional Operating Grant 2682 and Project Grant PJT-159546 (S.S.P. and N.R.C. respectively), the Alberta Prion Research Institute, Alberta Innovates Research Team Program Grant PTM13007 (S.S.P. and N.R.C.), Compute Canada Resources for Research Groups (RRG ID 3071) (S.S.P.) and Brain Canada MIRI program PT-BCF 00001 (13-4) (N.R.C.).
Footnotes
See www.alzforum.org, 2020c; Includes missense, insertion/deletion, intronic splice-altering, and distinct nucleic but synomymous amino acid mutations.
References
- Acton P., An Z., Bett A.J., Breese R., Chang L., Dodson E.C., Kinney G., Klein W., Lambert M.P., Liang X., Shughrue P., Strohl W.R., Viola K. 2010. Anti-addl antibodies and uses thereof. Patent (US) US7780963 US Patent 7,780,963. [Google Scholar]
- Acton P., An Z., Bett A.J., Breese R., Dodson E.C., Kinney G., Klein W., Lambert M.P., Liang X., Shughrue P., Strohl W.R., Viola K., Chang L. 2010. Anti-addl antibodies and uses thereof. Patent (US) US7811563 US Patent 7,811,563. [Google Scholar]
- Adolfsson O., Pihlgren M., Toni N., Varisco Y., Buccarello A.L., Antoniello K., Lohmann S., Piorkowska K., Gafner V., Atwal J.K., Maloney J., Chen M., Gogineni A., Weimer R.M., Mortensen D.L., Friesenhahn M., Ho C., Paul R., Pfeifer A., Muhs A., Watts R.J. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfsson O., Ayalon G., Hotzel I., Di Cara D.M. 2016. Anti-tau antibodies and methods of use. Patent (US), WO2016–196726–A1. [Google Scholar]
- Alam R., Driver D., Wu S., Lozano E., Key S.L., Hole J.T., Hayashi Mansuo L., Lu J. Preclinical characterization of an antibody [LY3303560] targeting aggregated tau. Alzheimer’s Dement. 2017;13:592–593. [Google Scholar]
- Albert M., Mairet-Coello G., Danis C., Lieger S., Caillierez R., Carrier S., Skrobala E., Landrieu I., Michel A., Schmitt M., Citron M., Downey P., Courade J.P., Buèe L., Colin M. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain. 2019;142:1736–1750. doi: 10.1093/brain/awz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A.D.C., Grundke-Iqbal I., Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alonso A.D.C., Li B., Grundke-Iqbal I., Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc. Natl. Acad. Sci. 2006;103:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpaugh M., Cicchetti F. A brief history of antibody-based therapy. Neurobiol. Dis. 2019;130:104504. doi: 10.1016/j.nbd.2019.104504. [DOI] [PubMed] [Google Scholar]
- Alvarado A., Driver D., Hayashi M.L., Lu J. 2016. Antibodies to tau and uses thereof. Patent (US), WO2016–137811–A1. [Google Scholar]
- AlzForum.org THERAPEUTICS: BIIB076. 2019. https://www.alzforum.org/therapeutics/biib076 Available at. Accessed 26 Sept 2019.
- AlzForum.org Can clinical trials and longitudinal studies crack rare tauopathies? 2019. https://www.alzforum.org/news/conference-coverage/can-clinical-trials-and-longitudinal-studies-crack-rare-tauopathies Available at. Accessed 24 Sept 2019.
- AlzForum.org Second look at BAN2401 data still positive, despite snafu. 2019. https://www.alzforum.org/news/conference-coverage/second-look-ban2401-data-still-positive-despite-snafu Available at. Accessed 19 Sept 2019.
- AlzForum.org To block tau's proteopathic spread, antibody must attack its mid-region. 2019. https://www.alzforum.org/news/conference-coverage/block-taus-proteopathic-spread-antibody-must-attack-its-mid-region Available at. Accessed 27 Sept 2019.
- AlzForum.org AbbVie's Tau Antibody Flops in Progressive Supranuclear Palsy. 2020. https://www.alzforum.org/news/research-news/abbvies-tau-antibody-flops-progressive-supranuclear-palsy Available at. Accessed 30 March 2020.
- AlzForum.org Gosuranemab, Biogen's Anti-Tau Immunotherapy, Does Not Fly for PSP. 2020. https://www.alzforum.org/news/research-news/gosuranemab-biogens-anti-tau-immunotherapy-does-not-fly-psp Available at. Accessed 30 March 2020.
- AlzForum.org Mutations: This database is a repository of variants in genes linked to Alzheimer's disease (AD) 2020. https://www.alzforum.org/mutations Available at. Accessed 30 March 2020.
- Angal S., King D., Bodmer M., Turner A., Lawson A., Roberts G., Pedley B., Adair J. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol. Immunol. 1993;30:105–108. doi: 10.1016/0161-5890(93)90432-b. [DOI] [PubMed] [Google Scholar]
- Aoyagi A., Condello C., Stöhr J., Yue W., Rivera B.M., Lee J.C., Woerman A.L., Halliday G., van Duinen S., Ingelsson M., Lannfelt L., Graff C., Bird T.D., Keene C.D., Seeley W.W., DeGrado W.F., Prusiner S.B. Aβ and tau prion-like activities decline with longevity in the Alzheimer’s disease human brain. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aat8462. eaat8462; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T., Stieler J.T., Holzer M. Tau and tauopathies. Brain Res. Bull. 2016;126:238–292. doi: 10.1016/j.brainresbull.2016.08.018. Cytoskeletal proteins in health and neurodegenerative disease. [DOI] [PubMed] [Google Scholar]
- Arndt J.W., Qian F., Smith B.A., Quan C., Kilambi K.P., Bush M.W., Walz T., Pepinsky R.B., Bussière T., Hamann S., Cameron T.O., Weinreb P.H. Structural and kinetic basis for the selectivity of Aducanumab for aggregated forms of amyloid-β. Sci. Rep. 2018;8:6412. doi: 10.1038/s41598-018-24501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Ashe K.H., Aguzzi A. Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion. 2013;7:55–59. doi: 10.4161/pri.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack J.C., Schneider A., Mandelkow E.M., Hyman B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Axon Neuroscience Axon announces positive results from phase II ADAMANT trial for AADvac1 in Alzheimer's disease. 2019. http://www.axon-neuroscience.eu/docs/press_release_Axon_announces_positive_result_9-9-2019.pdf Available at:
- Ayalon G. Preclinical characterization, efficacy, pharmacokinetics and pharmacodynamics and safety of RO7105705 - an anti-tau antibody currently in clinical development for Alzheimer’s disease. Neurodegenerative Diseases. 2017;17:45. [Google Scholar]
- Ayers J.I., Xu G., Pletnikova O., Troncoso J.C., Hart P.J., Borchelt D.R. Conformational specificity of the c4f6 SOD1 antibody; low frequency of reactivity in sporadic als cases. Acta Neuropathologica Commun. 2014;2 doi: 10.1186/2051-5960-2-55. Art. 55 (13pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoitei M.L., Ban Y.E.A., Julien J.P., Bryson S., Schroeter A., Kalyuzhniy O., Porter J.R., Adachi Y., Baker D., Pai E.F., Schief W.R. Computational design of high-affinity epitope scaffolds by backbone grafting of a linear epitope. J. Mol. Biol. 2012;415:175–192. doi: 10.1016/j.jmb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F., Cannon C., Barbour R., Burke R.L., Games D., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Lieberburg I., Motter R., Nguyen M., Soriano F., Vasquez N., Weiss K., Welch B., Seubert P., Schenk D., Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bar-Or D., Thomas G.W., Bar-Or R., Rael L.T., Scarborough K., Rao N., Shimonkevitz R. Commercial human albumin preparations for clinical use are immunosuppressive in vitro. Critical Care Med. 2006;34:1707–1712. doi: 10.1097/01.CCM.0000217923.53680.4C. [DOI] [PubMed] [Google Scholar]
- Basun H., Bogdanovic N., Ingelsson M., Almkvist O., Näslund J., Axelman K., Bird T.D., Nochlin D., Schellenberg G.D., Wahlund L.O., Lannfelt L. Clinical and neuropathological features of the Arctic APP gene mutation causing early-onset Alzheimer disease. JAMA Neurol. 2008;65:499–505. doi: 10.1001/archneur.65.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman R.J., Benzinger T.L., Berry S., Clifford D.B., Duggan C., Fagan A.M., Fanning K., Farlow M.R., Hassenstab J., McDade E.M., Mills S., Paumier K., Quintana M., Salloway S.P., Santacruz A., Schneider L.S., Wang G., Xiong C. The DIAN-TU next generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement. 2017;13:8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L., Tanzi R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer L.M., Hof P.R., Purohit D.P., Carlin L., Schmeidler J., Davis K.L., Perl D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- Billinton A., Newton P., Lloyd C., Groves M., Welsh F., Bogstedt A., Eketjall S., McFarlane M., Perkinton M., Narwal R., Tan K., Dudley A., Vaughan T., Chessell I. Preclinical discovery and developement of MEDI1814, a monoclonal antibody selectively targeting beta-amyloid 42 (Aβ42) Alzheimer’s Dement. 2017;13:P266. [Google Scholar]
- Bloom G.S. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- Boada M., López O., Núñez L., Szczepiorkowski Z.M., Torres M., Grifols C., Páez A. Plasma exchange for Alzheimer’s disease management by albumin replacement (AMBAR) trial: study design and progress. Alzheimer’s Dement. 2019;5:61–69. doi: 10.1016/j.trci.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogstedt A., Groves M., Tan K., Narwal R., McFarlane M., Höglund K. Development of immunoassays for the quantitative assessment of amyloid-β in the presence of therapeutic antibody: application to pre-clinical studies. J. Alzheimers Dis. 2015;46:1091–1101. doi: 10.3233/JAD-142988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrmann B., Goepfert U., Grueninger F., Huber W., Krell H.W., Lifke V., Mundigl O., Offner S., Ozmen L., Schraeml M. 2010. Use of an anti-tau pS422 antibody for the treatment of brain diseases. Patent (US), WO2010–142423. [Google Scholar]
- Bohrmann B., Baumann K., Benz J., Gerber F., Huber W., Knoflach F., Messer J., Oroszlan K., Rauchenberger R., Richter W.F., et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloidβ. J. Alzheimers Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- Borgers M., Dockx K., Van de Weyer I., Versweyveld S., De Waepenaert K., Vandermeeren M., Van Kolen K., Vanhoof G., Ebneth A., Mercken M. Anti-tau antibody profiling for passive immunization therapy: comparing seeds derived from transgenic animals and human brain tissue. Alzheimer’s Dement. 2017;13:P1274. [Google Scholar]
- Boxer A.L., Qureshi I., Ahlijanian M., Grundman M., Golbe L.I., Litvan I., Honig L.S., Tuite P., McFarland N.R., O’Suilleabhain P., Xie T., Tirucherai G.S., Bechtold C., Bordelon Y., Geldmacher D.S., Grossman M., Isaacson S., Zesiewicz T., Olsson T., Muralidharan K.K., Graham D.L., O’Gorman J., Haeberlein S.B., Dam T. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 2019;18:549–558. doi: 10.1016/S1474-4422(19)30139-5. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. The Schmitt Symposium: The Cytoskeleton and Alzheimer’s Disease. [DOI] [PubMed] [Google Scholar]
- Bright J., Hussain S., Dang V., Wright S., Cooper B., Byun T., Ramos C., Singh A., Parry G., Stagliano N., Griswold-Prenner I. Human secreted tau increases amyloid-beta production. Neurobiol. Aging. 2015;36:693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Broering T.J., Wang H., Boatright N.K., Wang Y., Baptista K., Shayan G., Garrity K.A., Kayatekin C., Bosco D.A., Matthews C.R., et al. Identification of human monoclonal antibodies specific for human SOD1 recognizing distinct epitopes and forms of SOD1. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Brandel J.P., Sato T., Nakamura Y., MacKenzie J., Will R., Ladogana A., Pocchiari M., Leschek E., Schonberger L. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg. Infect. Dis. J. 2012;18:901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussière T., Hof P.R., Mailliot C., Brown C.D., Caillet-Boudin M.L., Perl D.P., Buée L., Delacourte A. Phosphorylated serine422 on tau proteins is a pathological epitope found in several diseases with neurofibrillary degeneration. Acta Neuropathol. 1999;97:221–230. doi: 10.1007/s004010050978. [DOI] [PubMed] [Google Scholar]
- Bussiere T., Weinreb P., Dunstan R., Qian F., Arast M., Li M., et al. Differential in vitro and in vivo binding profiles of BIIB037 and other anti-abeta clinical antibody candidates. Neurodegener. Dis. 2013;11 [Google Scholar]
- Cai Y., An S.S.A., Kim S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin. Interv. Aging. 2015;10:1163–1172. doi: 10.2147/CIA.S85808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantillon M., Wilson L., Ohta E., Prins N., Andreasen N., Tsukii K. Phase 1 study of a novel humanized anti-amyloid beta (Aβ) aggregates specific antibody KHK6640 in Alzheimer’s disease. J. Prevent. Alzheimer’s Dis. 2017;4:341. [Google Scholar]
- Carter P.J., Lazar G.A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 2017;17:197–223. doi: 10.1038/nrd.2017.227. [DOI] [PubMed] [Google Scholar]
- Cashman N.R., Caughey B. Prion diseases – close to effective therapy? Nat. Rev. Drug Discov. 2004;3:874–884. doi: 10.1038/nrd1525. [DOI] [PubMed] [Google Scholar]
- Cashman N.R., Plotkin S.S. 2016. Epitopes in amyloid beta mid-region and conformationally-selective antibodies thereto. US Patent, WO 2017/079833. [Google Scholar]
- Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W., Fagan A.M., Morris J.C., Mawuenyega K.G., Cruchaga C., Goate A.M., Bales K.R., Paul S.M., Bateman R.J., Holtzman D.M. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002156. 89ra57 (13pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carranza D.L., Sengupta U., Guerrero-Muñoz M.J., Lasagna-Reeves C.A., Gerson J.E., Singh G., Estes D.M., Barrett A.D.T., Dineley K.T., Jackson G.R., Kayed R. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J. Neurosci. 2014;34:4260–4272. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carranza, D.L., Guerrero-Muñoz, M.J., Sengupta, U., Gerson, J.E., Kayed, R., 2018. α-synuclein oligomers induce a unique toxic tau strain. Biol. Psychiatry 84, 499–508. [DOI] [PMC free article] [PubMed]
- Chai X., Wu S., Murray T.K., Kinley R., Cella C.V., Sims H., Buckner N., Hanmer J., Davies P., O’Neill M.J., Hutton M.L., Citron M. Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J. Biol. Chem. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Grimm J., Baeriswyl J.L., Nitsch R., Hock C. 2012. Human anti-tau antibodies. Patent (US), WO2012–049570. [Google Scholar]
- Chodera J.D., Mobley D.L. Entropy-enthalpy compensation: role and ramifications in biomolecular ligand recognition and design. Annu. Rev. Biophys. 2013;42:121–142. doi: 10.1146/annurev-biophys-083012-130318. 23654303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. Strategies for disease modification in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5:677–685. doi: 10.1038/nrn1495. [DOI] [PubMed] [Google Scholar]
- Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., Jucker M., Goedert M., Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Akatsu H., Fraser G., Crowther R.A., Frank S., Hench J., Probst A., Winkler D.T., Reichwald J., Staufenbiel M., Ghetti B., Goedert M., Tolnay M. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Hench J., Goedert M., Tolnay M. Invited review: prion-like transmission and spreading of tau pathology. Neuropathol. Appl. Neurobiol. 2015;41:47–58. doi: 10.1111/nan.12197. [DOI] [PubMed] [Google Scholar]
- Cline E.N., Bicca M.A., Viola K.L., Klein W.L. The amyloid-β oligomer hypothesis: beginning of the third decade. J. Alzheimers Dis. 2018;64:S567–S610. doi: 10.3233/JAD-179941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline E., Viola K., Klein W., Wang X., Bacskai B., Rammes G., Dodart J., Palop J., Siemers E., Jerecic J., Krafft G. Synaptic intervention in alzheimer’s disease: soluble aβ oligomer directed ACU193 monoclonal antibody therapeutic for treatment of early alzheimer’s disease. J. Prevent. Alzheimer’s Dis. 2019;6(Supplement 1):S151. [Google Scholar]
- Collin L., Bohrmann B., Göpfert U., Oroszlan-Szovik K., Ozmen L., Grüninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain. 2014;137:2834–2846. doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- Condello C., Stöehr J. Aβ propagation and strains: implications for the phenotypic diversity in Alzheimer’s disease. Neurobiol. Dis. 2018;109:191–200. doi: 10.1016/j.nbd.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Correia B.E., Ban Y.E.A., Holmes M.A., Xu H., Ellingson K., Kraft Z., Carrico C., Boni E., Sather D.N., Zenobia C., Burke K.Y., Bradley-Hewitt T., Bruhn-Johannsen J.F., Kalyuzhniy O., Baker D., Strong R.K., Stamatatos L., Schief W.R. Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure. 2010;18:1116–1126. doi: 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Couch J.A., Yu Y.J., Zhang Y., Tarrant J.M., Fuji R.N., Meilandt W.J., Solanoy H., Tong R.K., Hoyte K., Luk W., Lu Y., Gadkar K., Prabhu S., Ordonia B.A., Nguyen Q., Lin Y., Lin Z., Balazs M., Scearce-Levie K., Ernst J.A., Dennis M.S., Watts R.J. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005338. 183ra57 (12pp) [DOI] [PubMed] [Google Scholar]
- Courade J.P., Angers R., Mairet-Coello G., Pacico N., Tyson K., Lightwood D., Munro R., McMillan D., Griffin R., Baker T., Starkie D., Nan R., Westwood M., Mushikiwabo M.L., Jung S., Odede G., Sweeney B., Popplewell A., Burgess G., Downey P., Citron M. Epitope determines efficacy of therapeutic anti-tau antibodies in a functional assay with human Alzheimer tau. Acta Neuropathol. 2018;136:729–745. doi: 10.1007/s00401-018-1911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- creativebiolabs.net; 2019. NI-105.6C5,. Recombinant Human Anti-MAPT Antibody (NI-105.6C5). Available at: https://www.creativebiolabs.net/pdf/HPAB-0099-WJ.pdf. Accessed 26 Sept 2019.
- Crespi G.A.N., Hermans S.J., Parker M.W., Miles L.A. Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Sci. Rep. 2015;5 doi: 10.1038/srep09649. Article 9649, 5pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P., Palotai R., Nussinov R. Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem. Sci. 2010;35:539–546. doi: 10.1016/j.tibs.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J., Lee G., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkowicz J., Chen W., Wang Q., Shen C., Wager C., Stone I., Stebbins C., Lamb M., Setser J., Cantone G., Graham D. Pan-tau antibody BIIB076 exhibits promosing safety and biomarker profile in cynomolgus monkey tonxicity study. Alzheimer’s Dement. 2017;13:1271. [Google Scholar]
- d’Abramo C., Acker C.M., Jimenez H.T., Davies P. Tau passive immunotherapy in mutant p301l mice: antibody affinity versus specificity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062402. (10pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dage J.L., Wennberg A.M., Airey D.C., Hagen C.E., Knopman D.S., Machulda M.M., Roberts R.O., Jack C.R., Petersen R.C., Mielke M.M. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12:1226–1234. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C.I., Tung Y.C., Liu F., Gong C.X., Iqbal K. Tau passive immunization inhibits not only tau but also Aβ pathology. Alzheimer’s Res. Ther. 2017;9 doi: 10.1186/s13195-016-0227-5. Art. 1; 16 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea M.R., Cole G.M., Ard M.D. The microglial phagocytic role with specific plaque types in the alzheimer disease brain. Neurobiol. Aging. 2004;25:675–683. doi: 10.1016/j.neurobiolaging.2003.12.026. [DOI] [PubMed] [Google Scholar]
- de Calignon A., Polydoro M., Suárez-Calvet M., William C., Adamowicz D., Kopeikina K., Pitstick R., Sahara N., Ashe K.H., Carlson G.A., Spires-Jones T.L., Hyman B.T. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Delnomdedieu M., Duvvuri S., Li D.J., Atassi N., Lu M., Brashear H.R., Liu E., Ness S., Kupiec J.W. First-in-human safety and long-term exposure data for AAB-003 (PF-05236812) and biomarkers after intravenous infusions of escalating doses in patients with mild to moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2016;8:12. doi: 10.1186/s13195-016-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos R.B., Bales K.R., Cummins D.J., Dodart J.C., Paul S.M., Holtzman D.M. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model Alzheimer’s disease. Proc. Natl. Acad. Sci. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos R.B., Lu J., Tang Y., Racke M.M., DeLong C.A., Tzaferis J., Hole J.T., Forster B.M., McDonnell P.C., Liu F., Kinley R.D., Jordan W.H., Hutton M.L. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76:908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- DeVos S.L., Miller R.L., Schoch K.M., Holmes B.B., Kebodeaux C.S., Wegener A.J., Chen G., Shen T., Tran H., Nichols B., Zanardi T.A., Kordasiewicz H.B., Swayze E.E., Bennett C.F., Diamond M.I., Miller T.M. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson S., Ellison N., Hendrix S. Alzheimer’s association international conference (AAIC), Los Angeles. Presentation number O3-10-04. 2019. A statistical translation of the public BAN2401 study results from a Bayesian to a traditional framework. [Google Scholar]
- Dolan P.J., Zago W. In: Alzheimer’s Disease. Dorszewska J., Kozubski W., editors. IntechOpen; Rijeka: 2018. Passive immunotherapy in Alzheimer’s disease; pp. 129–151. Chapter 8. [Google Scholar]
- Domert J., Rao S.B., Agholme L., Brorsson A.C., Marcusson J., Hallbeck M., Nath S. Spreading of amyloid-β peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol. Dis. 2014;65:82–92. doi: 10.1016/j.nbd.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P.S., Siemers E., Liu-Seifert H., Mohs R. Phase 3 trials of solanezumab for mild-to-moderate alzheimer’s disease. N. Engl. J. Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Dougherty D.A. The cation-π interaction. Acc. Chem. Res. 2013;46:885–893. doi: 10.1021/ar300265y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D.G., Kirschner M.W. Tau protein function in living cells. J. Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S., Bakardjian H., Benali H., Bertram L., Blennow K., Broich K., Cavedo E., Crutch S., Dartigues J.F., Duyckaerts C., Epelbaum S., Frisoni G.B., Gauthier S., Genthon R., Gouw A.A., Habert M.O., Holtzman D.M., Kivipelto M., Lista S., Molinuevo J.L., O’Bryant S.E., Rabinovici G.D., Rowe C., Salloway S., Schneider L.S., Sperling R., Teichmann M., Carrillo M.C., Cummings J., Jack C.R. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S., Hyman B.T. In: Tau prion-like propagation: state of the art and current challenges. Takashima A., Wolozin B., Buee L., editors. Springer; Singapore: 2019. pp. 305–325. Chapter 23. [DOI] [PubMed] [Google Scholar]
- Dujardin S., Lécolle K., Caillierez R., Bégard S., Zommer N., Lachaud C., Carrier S., Dufour N., Aurégan G., Winderickx J., Hantraye P., Déglon N., Colin M., Buée L. Neuron-to-neuron wild-type tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2014;2:1–14. doi: 10.1186/2051-5960-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele Y.S., Fritschi S.K., Hamaguchi T., Obermüller U., Füger P., Skodras A., Schäfer C., Odenthal J., Heikenwalder M., Staufenbiel M., Jucker M. Multiple factors contribute to the peripheral induction of cerebral β-amyloidosis. J. Neurosci. 2014;34:10264–10273. doi: 10.1523/JNEUROSCI.1608-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich T., Grueninger F., HUGENMATTER A., Moessner E., DENGL S., Georges G., Goepfert U., Jochner A., Kettenberger H., Moelleken J., Mundigl O., Niewoehner J., Schlothauer T., MOLHOJ M., Brady K. 2016. Humanized anti-tau(pS422) antibodies and methods of use. Patent (US), WO2016–207245. [Google Scholar]
- Englund H., Sehlin D., Johansson A.S., Nilsson L.N.G., Gellerfors P., Paulie S., Lannfelt L., Pettersson F.E. Sensitive ELISA detection of amyloid-β protofibrils in biological samples. J. Neurochem. 2007;103:334–345. doi: 10.1111/j.1471-4159.2007.04759.x. [DOI] [PubMed] [Google Scholar]
- Esparza T.J., Zhao H., Cirrito J.R., Cairns N.J., Bateman R.J., Holtzman D.M., Brody D.L. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann. Neurol. 2013;73:104–119. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach K., Hilbrich I., Schiffmann A., Gärtner U., Krüger M., Leonhardt M., Waschipky H., Wick L., Arendt T., Holzer M. Tau oligomers impair artificial membrane integrity and cellular viability. J. Biol. Chem. 2012;287:43223–43233. doi: 10.1074/jbc.M112.396176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J.L., Vaquer-Alicea J., White C.L., Cairns N.J., Nelson P.T., Diamond M.I. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 2017;133:91–100. doi: 10.1007/s00401-016-1644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpern, W.R., Mercken, M., Van Kolen, K., Timmers, M., Haeverans, K., Janssens, L., Triana-Baltzer, G., Kolb, H.C., Jacobs, T., Nandy, P., Malia, T., Sun, H., Van Nueten, L., 2019. P1-052: a single ascending dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the anti-phospho-tau antibody JNJ-63733657 in healthy subjects. Alzheimers Dement. 15, P252–P253. doi: 10.1016/j.jalz.2019.06.077. [DOI]
- Gerven N.V., der Verren S.E.V., Reiter D.M., Remaut H. The role of functional amyloids in bacterial virulence. J. Mol. Biol. 2018;430:3657–3684. doi: 10.1016/j.jmb.2018.07.010. Functional Amyloids in Health and Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs E., Silverman J.M., Zhao B., Peng X., Wang J., Wellington C.L., Mackenzie I.R., Plotkin S.S., Kaplan J.M., Cashman N.R. A rationally designed humanized antibody selective for amyloid beta oligomers in Alzheimer’s disease. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-46306-5. Art. 9870 (14 pp.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G., Fox N.C., Eisner L., Kirby L., Rovira M.B., Forette F., Orgogozo J.M. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Glabe C.G. Conformation-dependent antibodies target diseases of protein misfolding. Trends Biochem. Sci. 2004;29:542–547. doi: 10.1016/j.tibs.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Gleeson M.W., Dickson R.C. Albumin gains immune boosting credibility. Clin. Transl. Gastroenterol. 2015;6 doi: 10.1038/ctg.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349 doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Vanmechelen E. Monoclonal antibody at8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 1995;189:167–170. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Goedert M., Clavaguera F., Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Golde T.E., Levites P.D., Yona Quantitative and mechanistic studies of Aβ immunotherapy. CNS Neurol. Disord. Drug Targets. 2009;8:31–49. doi: 10.2174/187152709787601830. [DOI] [PubMed] [Google Scholar]
- Gratuze M., Leyns C.E.G., Holtzman D.M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 2018;13:66. doi: 10.1186/s13024-018-0298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremer L., Schölzel D., Schenk C., Reinartz E., Labahn J., Ravelli R.B.G., Tusche M., Lopez-Iglesias C., Hoyer W., Heise H., Willbold D., Schröder G.F. Fibril structure of amyloid-β(1-42) by cryo-electron microscopy. Science. 2017;358:116–119. doi: 10.1126/science.aao2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenauer R.H., Kinch M.S. An overview of FDA-approved vaccines & their innovators. Exp. Rev. Vaccines. 2017;16:1253–1266. doi: 10.1080/14760584.2017.1383159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold-Prenner I., Stagliano N.E., Dang V. 2014. Methods of treating a tauopathy. US Patent, WO2014–028777–A2. [Google Scholar]
- Groves M., Gustavsson S., Hoglund K., Lowne D., Lloyd C., Nickson A., Niva C., Sylvia S. 2014. Antibodies to amyloid beta. US Patent, 2014–060444–A1. [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S.K., Younkin S., Hazrati L., Collinge J., Pocock J., Lashley T., Williams J., Lambert J.C., Amouyel P., Goate A., Rademakers R., Morgan K., Powell J., St. George-Hyslop P., Singleton A., Hardy J. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2012;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillozet-Bongaarts A.L., Cahill M.E., Cryns V.L., Reynolds M.R., Berry R.W., Binder L.I. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J. Neurochem. 2006;97:1005–1014. doi: 10.1111/j.1471-4159.2006.03784.x. [DOI] [PubMed] [Google Scholar]
- Guo J.L., Narasimhan S., Changolkar L., He Z., Stieber A., Zhang B., Gathagan R.J., Iba M., McBride J.D., Trojanowski J.Q., Lee V.M. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016;213:2635–2654. doi: 10.1084/jem.20160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Noble W., Hanger D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Haeberlein S.B., von Hehn C., Tian Y., Chalkias S., Muralidharan K.K., Chen T., Wu S., Li J., Skordos L., Nisenbaum L., Rajagovindan R., Dent G., Harrison K., Nestorov I., Zhu Y., Mallinckrodt C., Sandrock A. EMERGE and ENGAGE Topline Results: Two Phase 3 Studies to Evaluate Aducanumab in Patients With Early Alzheimer's Disease. 2019. http://investors.biogen.com/static-files/ddd45672-9c7e-4c99-8a06-3b557697c06f Available at:
- Hammes G.G., Chang Y.C., Oas T.G. Conformational selection or induced fit: a flux description of reaction mechanism. Proc. Natl. Acad. Sci. 2009;106:13737–13741. doi: 10.1073/pnas.0907195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger D.P., Anderton B.H., Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hanseeuw B.J., Betensky R.A., Jacobs H.I.L., Schultz A.P., Sepulcre J., Becker J.A., Cosio D.M.O., Farrell M., Quiroz Y.T., Mormino E.C., Buckley R.F., Papp K.V., Amariglio R.A., Dewachter I., Ivanoiu A., Huijbers W., Hedden T., Marshall G.A., Chhatwal J.P., Rentz D.M., Sperling R.A., Johnson K. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76:915–924. doi: 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid Cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartley D., Blumenthal T., Carrillo M., DiPaolo G., Esralew L., Gardiner K., Granholm A.C., Iqbal K., Krams M., Lemere C., Lott I., Mobley W., Ness S., Nixon R., Potter H., Reeves R., Sabbagh M., Silverman W., Tycko B., Whitten M., Wisniewski T. Down syndrome and Alzheimer’s disease: common pathways, common goals. Alzheimers Dement. 2015;11:700–709. doi: 10.1016/j.jalz.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P.S., Ryan R.O. Impact of apolipoprotein E on Alzheimer’s disease. Curr. Alzheimer Res. 2013;10:809–817. doi: 10.2174/15672050113109990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F., Goure W.F., Jerecic J., Iverson K.S., Walicke P.A., Krafft G.A. The case for soluble Aβ oligomers as a drug target in Alzheimer’s disease. Trends Pharmacol. Sci. 2013;34:261–266. doi: 10.1016/j.tips.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat. Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B.B., Diamond M.I. Prion-like properties of tau protein: the importance of extracellular tau as a therapeutic target. J. Biol. Chem. 2014;289:19855–19861. doi: 10.1074/jbc.R114.549295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B.B., Furman J.L., Mahan T.E., Yamasaki T.R., Mirbaha H., Eades W.C., Belaygorod L., Cairns N.J., Holtzman D.M., Diamond M.I. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. 2014;111:E4376–E4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D., Jiang H., Diamond M., Kfoury N., Holmes B. 2014. Antibodies to tau. US Patent, WO–2014/008404–Al. [Google Scholar]
- Holtzman D.M., Carrillo M.C., Hendrix J.A., Bain L.J., Catafau A.M., Gault L.M., Goedert M., Mandelkow E., Mandelkow E.M., Miller D.S., Ostrowitzki S., Polydoro M., Smith S., Wittmann M., Hutton M. Tau: from research to clinical development. Alzheimers Dement. 2016;12:1033–1039. doi: 10.1016/j.jalz.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Hori Y., Hashimoto T., Nomoto H., Hyman B.T., Iwatsubo T. Role of Apolipoprotein E in β-amyloidogenesis: isoform-specific effects on protofibril to fibril conversion of Aβ in vitro and brain Aβ deposition in vivo. J. Biol. Chem. 2015;290:15163–15174. doi: 10.1074/jbc.M114.622209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin J.L., Sabbagh M.N., Al-Hasan Y., Decourt B. Tau immunotherapies for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2019;28:545–554. doi: 10.1080/13543784.2019.1619694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.M.J., Aryal S., Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010;1:323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- Hu W., Zhang X., Tung Y.C., Xie S., Liu F., Iqbal K. Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimers Dement. 2016;12:1066–1077. doi: 10.1016/j.jalz.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Hung S.Y., Fu W.M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017;24 doi: 10.1186/s12929-017-0355-7. Article 47, (12pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Flory M., Khatoon S., Soininen H., Pirttila T., Lehtovirta M., Alafuzoff I., Blennow K., Andreasen N., Vanmechelen E., Grundke-Iqbal I. Subgroups of alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann. Neurol. 2005;58:748–757. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C.X. Recent developments with tau-based drug discovery. Expert Opin. Drug Discovery. 2018;13:399–410. doi: 10.1080/17460441.2018.1445084. [DOI] [PubMed] [Google Scholar]
- Ittner L.M., Götz J. Amyloid-β and tau – a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2010;12:67–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W., Petersen R.C., Trojanowski J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S., Shaw L.M., Vemuri P., Wiste H.J., Weigand S.D., Lesnick T.G., Pankratz V.S., Donohue M.C., Trojanowski J.Q. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.J., Kerridge C., Cooper J., Cavallini A., Falcon B., Cella C.V., Landi A., Szekeres P.G., Murray T.K., Ahmed Z., Goedert M., Hutton M., O’Neill M.J., Bose S. Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. J. Neurosci. 2016;36:762–772. doi: 10.1523/JNEUROSCI.3542-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen H., Ozmen L., Caruso A., Narquizian R., Hilpert H., Jacobsen B., Terwel D., Tanghe A., Bohrmann B. Combined treatment with a BACE inhibitor and anti Aβ antibody gantenerumab enhances amyloid reduction in APPlondon mice. J. Neurosci. 2014;34:11621–11630. doi: 10.1523/JNEUROSCI.1405-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S., Avila J., Schöll M., Kovacs G.G., Kövari E., Skrabana R., Evans L.D., Kontsekova E., Malawska B., de Silva R., Buee L., Zilka N. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019;7 doi: 10.1186/s40478-019-0664-z. Article no. 22 (31 pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaunmuktane Z., Mead S., Ellis M., Wadsworth J.D.F., Nicoll A.J., Kenny J., Launchbury F., Linehan J., Richard-Loendt A., Walker A.S., Rudge P., Collinge J., Brandner S. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature. 2015;525:247–250. doi: 10.1038/nature15369. [DOI] [PubMed] [Google Scholar]
- Jay T.R., von Saucken V.E., Landreth G.E. TREM2 in neurodegenerative diseases. Mol. Neurodegener. 2017;12:56. doi: 10.1186/s13024-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Lee C.D., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T.M., Collins J.L., Richardson J.C., Smith J.D., Comery T.A., Riddell D., Holtzman D.M., Tontonoz P., Landreth G.E. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha G.A., Bowser R., Kazam I.G., Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 1997;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Johnson-Wood K., Lee M., Motter R., Hu K., Gordon G., Barbour R., Khan K., Gordon M., Tan H., Games D., Lieberburg I., Schenk D., Seubert P., McConlogue L. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T., Atwal J.K., Steinberg S., Snaedal J., Jonsson P.V., Bjornsson S., Stefansson H., Sulem P., Gudbjartsson D., Maloney J., Hoyte K., Gustafson A., Liu Y., Lu Y., Bhangale T., Graham R.R., Huttenlocher J., Bjornsdottir G., Andreassen O.A., Jönsson E.G., Palotie A., Behrens T.W., Magnusson O.T., Kong A., Thorsteinsdottir U., Watts R.J., Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., Rujescu D., Hampel H., Giegling I., Andreassen O.A., Engedal K., Ulstein I., Djurovic S., Ibrahim-Verbaas C., Hofman A., Ikram M.A., van Duijn C.M., Thorsteinsdottir U., Kong A., Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M., Walker L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M.D., Lipinski W.J., Callahan M.J., Bian F., Durham R.A., Schwarz R.D., Roher A.E., Walker L.C. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Gibbs E., Silverman J.M., Zhao B., Wang J., Peng X., Plotkin S.S., Cashman N.R. Alzheimer’s association international conference (AAIC), Los Angeles. Presentation number O5-06-01. 2019. Selective targeting of amyloid-beta oligomer species by PMN310, a monoclonal antibody rationally designed for greater therapeutic potency in alzheimer’s disease. [Google Scholar]
- Kaplon H., Reichert J.M. Antibodies to watch in 2019. mAbs. 2019;11:219–238. doi: 10.1080/19420862.2018.1556465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E., Mercken M., Strooper B.D. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Katsinelos T., Tuck B.J., Mukadam A.S., McEwan W.A. The role of antibodies and their receptors in protection against ordered protein assembly in neurodegeneration. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01139. Art. 1139 (15 pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S.K., Sanders D.W., Thomas T.L., Ruchinskas A.J., Vaquer-Alicea J., Sharma A.M., Miller T.M., Diamond M.I. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron. 2016;92:796–812. doi: 10.1016/j.neuron.2016.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner G.A., Boxer A.L. Bapineuzumab. Expert. Opin. Biol. Ther. 2010;10:1121–1130. doi: 10.1517/14712598.2010.493872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner G.A., Ayalon G., Brunstein F., Chandra P., Datwani A., Fuji R.N., Manser P.T., Vaze A., Ward M., Wildsmith K.R., Foo-Atkins C. Alzheimer’s association international conference (AAIC), Los Angeles. Presentation number O2-17-03. 2017. A phase I study to evaluate the safety and tolerability of ro7105705 in and patients with mild-to-moderate AD. [Google Scholar]
- Klunk W.E., Koeppe R.A., Price J.C., Benzinger T.L., Devous M.D., Sr., Jagust W.J., Johnson K.A., Mathis C.A., Minhas D., Pontecorvo M.J., Rowe C.C., Skovronsky D.M., Mintun M.A. The centiloid project: standardizing quantitative amyloid plaque estimation by pet. Alzheimers Dement. 2015;11:1–15. doi: 10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappik A., Ge L., Honegger A., Pack P., Fischer M., Wellnhofer G., Hoess A., Wölle J., Plückthun A., Virnekäs B. Fully synthetic human combinatorial antibody libraries (hucal) based on modular consensus frameworks and cdrs randomized with trinucleotides. J. Mol. Biol. 2000;296:57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- Knight D.E.O., Baker T.S., McMillan D.J., Griffin R.A., Mairet-Coello G., Downey P., Courade J.P. 2017. Tau-binding antibodies. Patent (US), WO2017–005734. [Google Scholar]
- Kontsekova E., Zilka N., Kovacech B., Skrabana R., Novak M. Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer’s disease. Alzheimers Res. Ther. 2014;6:45. doi: 10.1186/alzrt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R., Tsubery H., Proschitsky M.Y., Asp E., Lulu M., Gilead S., Gartner M., Waltho J.P., Davis P.J., Hounslow A.M., Kirschner D.A., Inouye H., Myszka D.G., Wright J., Solomon B., Fisher R.A. A bacteriophage capsid protein provides a general amyloid interaction motif (GAIM) that binds and remodels misfolded protein assemblies. J. Mol. Biol. 2014;426:2500–2519. doi: 10.1016/j.jmb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Lafont V., Armstrong A.A., Ohtaka H., Kiso Y., Mario Amzel L., Freire E. Compensating enthalpic and entropic changes hinder binding affinity optimization. Chem. Biol. Drug Des. 2007;69:413–422. doi: 10.1111/j.1747-0285.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Landen J.W., Andreasen N., Cronenberger C.L., Schwartz P.F., Börjesson-Hanson A., Östlund H., Sattler C.A., Binneman B., Bednar M.M. a. Ponezumab in mild-to-moderate Alzheimer’s disease: randomized phase ii PET-PIB study. Alzheimer’s Dement. 2017;3:393–401. doi: 10.1016/j.trci.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen J.W., Cohen S., Billing C.B., Cronenberger C., Styren S., Burstein A.H., Sattler C., Lee J.H., Jack C.R., Kantarci K., Schwartz P.F., Duggan W.T., Zhao Q., Sprenger K., Bednar M.M., Binneman B. b. Multiple-dose ponezumab for mild-to-moderate Alzheimer’s disease: safety and efficacy. Alzheimer’s Dement. 2017;3:339–347. doi: 10.1016/j.trci.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Guerrero-Munoz M.J., Kiritoshi T., Neugebauer V., Jackson G.R., Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2012;2 doi: 10.1038/srep00700. Article no. 700 (7 pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Pichon C.E.L., Adolfsson O., Gafner V., Pihlgren M., Lin H., Solanoy H., Brendza R., Ngu H., Foreman O., Chan R., Ernst J.A., DiCara D., Hotzel I., Srinivasan K., Hansen D.V., Atwal J., Lu Y., Bumbaca D., Pfeifer A., Watts R.J., Muhs A., Scearce-Levie K., Ayalon G. Antibody-mediated targeting of tau in vivo does not require effector function and microglial engagement. Cell Rep. 2016;16:1690–1700. doi: 10.1016/j.celrep.2016.06.099. [DOI] [PubMed] [Google Scholar]
- Lee S.J.C., Nam E., Lee H.J., Savelieff M.G., Lim M.H. Towards an understanding of amyloid-β oligomers: characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017;46:310–323. doi: 10.1039/C6CS00731G. [DOI] [PubMed] [Google Scholar]
- Levenson J.M., Schroeter S., Carroll J.C., Cullen V., Asp E., Proschitsky M., Chung C.H.Y., Gilead S., Nadeem M., Dodiya H.B., Shoaga S., Mufson E.J., Tsubery H., Krishnan R., Wright J., Solomon B., Fisher R., Gannon K.S. NPT088 reduces both amyloid-β and tau pathologies in transgenic mice. Alzheimer’s Dement. 2016;2:141–155. doi: 10.1016/j.trci.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J., Dickson D.W. Propagation of tau pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:27–48. doi: 10.1007/s00401-015-1507-z. [DOI] [PubMed] [Google Scholar]
- Liu L., Drouet V., Wu J.W., Witter M.P., Small S.A., Clelland C., Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031302. 9pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yang B., Ke J., Li W., Suen W.C. Antibody-based drugs and approaches against amyloid-β species for Alzheimer’s disease immunotherapy. Drugs Aging. 2016;33:685–697. doi: 10.1007/s40266-016-0406-x. [DOI] [PubMed] [Google Scholar]
- Loeffler D.A. Intravenous immunoglobulin and Alzheimer’s disease: what now? J. Neuroinflammat. 2013;10 doi: 10.1186/1742-2094-10-70. Article 853, 7pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.X., Qiang W., Yau W.M., Schwieters C.D., Meredith S.C., Tycko R. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue L.F., Kuo Y.M., Roher A.E., Brachova L., Shen Y., Sue L., Beach T., Kurth J.H., Rydel R.E., Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malia T.J., Teplyakov A., Ernst R., Wu S.J., Lacy E.R., Liu X., Vandermeeren M., Mercken M., Luo J., Sweet R.W., Gilliland G.L. Epitope mapping and structural basis for the recognition of phosphorylated tau by the anti-tau antibody AT8. Proteins. 2016;84:427–434. doi: 10.1002/prot.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney J.A., Bainbridge T., Gustafson A., Zhang S., Kyauk R., Steiner P., van der Brug M., Liu Y., Ernst J.A., Watts R.J., Atwal J.K. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem. 2014;289:30990–31000. doi: 10.1074/jbc.M114.589069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E.M., Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006247. a006247 (26pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavoungou C., Zimmerman K.S. Immunotherapy with anti-Aβ monoclonal antibodies in Alzheimer’s disease: a critical review on the molecules in the pipelines with regulatory considerations. Front Clin Drug Res Alzheimer Disord. 2013;1:3–85. [Google Scholar]
- McAlary L., Plotkin S.S., Cashman N.R. Emerging developments in targeting proteotoxicity in neurodegenerative diseases. CNS Drugs. 2019;33:883–904. doi: 10.1007/s40263-019-00657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlary L., Plotkin S.S., Yerbury J.J., Cashman N.R. Prion-like propagation of protein misfolding and aggregation in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019;12:262. doi: 10.3389/fnmol.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C.A., Cherny R.A., Fraser F.W., Fuller S.J., Smith M.J., Vbeyreuther K., Bush A.I., Masters C.L. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Medina M. An overview on the clinical development of tau-based therapeutics. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19041160. Art. 1160 (14pp.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Van de Voorde A., Martin J.J., Gheuens J. Monoclonal antibodies with selective specificity for Alzheimer tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84:265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]
- Mercken M., Malia T., Borgers M., Van Kolen K. 2018. Anti-PHF-tau antibodies and uses thereof. US Patent, PCT/US2018/022782. [Google Scholar]
- Messing J. Phage M13 for the treatment of Alzheimer and Parkinson disease. Gene. 2016;583:85–89. doi: 10.1016/j.gene.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A.L., Vigouret J.M., Paganetti P., Walsh D.M., Mathews P.M., Ghiso J., Staufenbiel M., Walker L.C., Jucker M. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Miles D., von Minckwitz G., Seidman A.D. Combination versus sequential single-agent therapy in metastatic breast cancer. Oncologist. 2002;7:13–19. [PubMed] [Google Scholar]
- Miles L.A., Crespi G.A.N., Doughty L., Parker M.W. Bapineuzumab captures the N-terminus of the Alzheimer’s disease amyloid-beta peptide in a helical conformation. Sci. Rep. 2013;3 doi: 10.1038/srep01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.A., Plotkin S.S. Protein transfer free energy obeys entropy-enthalpy compensation. J. Phys. Chem. B. 2015;119:14130–14144. doi: 10.1021/acs.jpcb.5b09219. [DOI] [PubMed] [Google Scholar]
- Morales R., Duran-Aniotz C., Castilla J., Estrada L.D., Soto C. De novo induction of amyloid-β deposition in vivo. Mol. Psychiatry. 2012;17:1347–1353. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- Moreth J., Mavoungou C., Schindowski K. Passive anti-amyloid immunotherapy in Alzheimer’s disease: what are the most promising targets? Immunity Ageing. 2013;10 doi: 10.1186/1742-4933-10-18. Art. 18; 9pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A., Colin M., Dujardin S., Medina M., Dewachter I., Alavi Naini S.M., Mandelkow E.M., Mandelkow E., Buée L., Goedert M., Brion J.P. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 2017;5:99. doi: 10.1186/s40478-017-0488-7. 20pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhs A., Hickman D.T., Pihlgren M., Chuard N., Giriens V., Meerschman C., van der Auwera I., van Leuven F., Sugawara M., Weingertner M.C., Bechinger B., Greferath R., Kolonko N., Nagel-Steger L., Riesner D., Brady R.O., Pfeifer A., Nicolau C. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc. Natl. Acad. Sci. 2007;104:9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.T., Abner E.L., Schmitt F.A., Kryscio R.J., Jicha G.A., Smith C.D., Davis D.G., Poduska J.W., Patel E., Mendiondo M.S., Markesbery W.R. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., Tredici K.D., Duyckaerts C., Frosch M.P., Haroutunian V., Hof P.R., Hulette C.M., Hyman B.T., Iwatsubo T., Jellinger K.A., Jicha G.A., Kövari E., Kukull W.A., Leverenz J.B., Love S., Mackenzie I.R., Mann D.M., Masliah E., McKee A.C., Montine T.J., Morris J.C., Schneider J.A., Sonnen J.A., Thal D.R., Trojanowski J.Q., Troncoso J.C., Wisniewski T., Woltjer R.L., Beach T.G. Correlation of alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau C., Greferath R., Balaban T.S., Lazarte J.E., Hopkins R.J. A liposome-based therapeutic vaccine against β-amyloid plaques on the pancreas of transgenic NORBA mice. Proc. Natl. Acad. Sci. 2002;99:2332–2337. doi: 10.1073/pnas.022627199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll J.A.R., Buckland G.R., Harrison C.H., Page A., Harris S., Love S., Neal J.W., Holmes C., Boche D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain. 2019;142:2113–2126. doi: 10.1093/brain/awz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsberth C., Westlind-Danielsson A., Eckman C.B., Condron M.M., Axelman K., Forsell C., Stenh C., Luthman J., Teplow D.B., Younkin S.G., Näslund J., Lannfelt L. The‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- Nitsch R., Chen F., Grimm J., Baeriswyl J.L., Hock C. 2019. Human anti-tau antibodies. Patent (US), US2019/023506. [Google Scholar]
- Novak P., Schmidt R., Kontsekova E., Zilka N., Kovacech B., Skrabana R., Vince-Kazmerova Z., Katina S., Fialova L., Prcina M., Parrak V., Dal-Bianco P., Brunner M., Staffen W., Rainer M., Ondrus M., Ropele S., Smisek M., Sivak R., Winblad B., Novak M. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16:123–134. doi: 10.1016/S1474-4422(16)30331-3. [DOI] [PubMed] [Google Scholar]
- Novak P., Kontsekova E., Zilka N., Novak M. Ten years of tau-targeted immunotherapy: the path walked and the roads ahead. Front. Neurosci. 2018;12:1–14. doi: 10.3389/fnins.2018.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Schmidt R., Kontsekova E., Kovacech B., Smolek T., Katina S., Fialova L., Prcina M., Parrak V., Dal-Bianco P., Brunner M., Staffen W., Rainer M., Ondrus M., Ropele S., Smisek M., Sivak R., Zilka N., Winblad B., Novak M. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018;10 doi: 10.1186/s13195-018-0436-1. 16 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Zilka N., Zilkova M., Kovacech B., Skrabana R., Ondrus M., Fialova L., Kontsekova E., Otto M., Novak M. AADvac1, an active immunotherapy for alzheimer’s disease and non alzheimer tauopathies: An overview of preclinical and clinical development. J. Prevent. Alzheimer’s Dis. 2019;6:63–69. doi: 10.14283/jpad.2018.45. [DOI] [PubMed] [Google Scholar]
- Ofek G., Guenaga F.J., Schief W.R., Skinner J., Baker D., Wyatt R., Kwong P.D. Elicitation of structure-specific antibodies by epitope scaffolds. Proc. Natl. Acad. Sci. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T.T., Klementieva O., Gouras G.K. Prion-like seeding and nucleation of intracellular amyloid-β. Neurobiol. Dis. 2018;113:1–10. doi: 10.1016/j.nbd.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T., Pomfret M., Billinton A., Chessell I., Chessell T., Lindqvist E., Valencia Z.S., Groves M., Narwal R., Tatipalli M., Lee N., Turner R., Tan K., Dudley A. Evaluation of safety, tolerability, pharmacokinetics and pharmacodynamics of MEDI1814, A beta-amyloid 42 (Aβ-42)-specific antibody, in patients with mild-moderate Alzheimer’s disease. Alzheimers Dement. 2017;13:P574. [Google Scholar]
- Páez A., Boada M., López O., Szczepiorkowski Z., Costa M., Vellas B., Cummings J. AMBAR (Alzheimer’s management by albumin replacement) phase 2B/3 trial: complete clinical, biomarker and neuroimaging results. J. Prevent. Alzheimer’s Dis. 2019;6(Supplement 1):S4. [Google Scholar]
- Panza F., Frisardi V., Solfrizzi V., Imbimbo B.P., Logroscino G., Santamato A., Greco A., Seripa D., Pilotto A. Immunotherapy for alzheimer’s disease: from anti-β-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4:213–238. doi: 10.2217/imt.11.170. [DOI] [PubMed] [Google Scholar]
- Panza F., Lozupone M., Seripa D., Imbimbo B.P. Amyloid-β immunotherapy for alzheimer disease: is it now a long shot? Ann. Neurol. 2019;85:303–315. doi: 10.1002/ana.25410. [DOI] [PubMed] [Google Scholar]
- Papoian G.A., Wolynes P.G. The physics and bioinformatics of binding and folding—an energy landscape perspective. Biopolymers. 2003;68:333–349. doi: 10.1002/bip.10286. [DOI] [PubMed] [Google Scholar]
- Paramithiotis E., Pinard M., Lawton T., LaBoissiere S., Leathers V.L., Zou W.Q., Estey L.A., Lamontagne J., Lehto M.T., Kondejewski L.H., Francoeur G.P., Papadopoulos M., Haghighat A., Spatz S.J., Head M., Will R., Ironside J., O’Rourke K., Tonelli Q., Ledebur H.C., Chakrabartty A., Cashman N.R. A prion protein epitope selective for the pathologically misfolded conformation. Nat. Med. 2003;9:893–899. doi: 10.1038/nm883. [DOI] [PubMed] [Google Scholar]
- Paravastu A.K., Leapman R.D., Yau W.M., Tycko R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc. Natl. Acad. Sci. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J.T., Sigurdsson E.M. Tau immunotherapy for Alzheimer’s disease. Trends Mol. Med. 2015;21:394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Peng X., Cashman N.R., Plotkin S.S. Prediction of misfolding-specific epitopes in SOD1 using collective coordinates. J. Phys. Chem. B. 2018;122:11662–11676. doi: 10.1021/acs.jpcb.8b07680. [DOI] [PubMed] [Google Scholar]
- Pfeifer A., Pihlgren M., Muhs A., Watts R. 2008. Humanized antibody. US Patent, WO2008–011348–A2. [Google Scholar]
- Pihlgren M., Vukicevic M., Gafner V., Piorkowska K., Chuard N., Giriens V., Valdes P., Nazeeruddin S., Colin P., Pfeifer A., Schneider B.L., Muhs A. Efficacy of ACI-35, a lipsomal anti-phospho tau vaccine in two different mouse models of Alzheimer’s disease. Alzheimer’s Dement. 2016;12:260–261. [Google Scholar]
- Plotkin S. 2017. Systems and methods for predicting misfolded protein epitopes by collective coordinate biasing. Patent (US), WO2017–079836A1. [Google Scholar]
- Plotkin S.S., Onuchic J.N. Understanding protein folding with energy landscape theory i: basic concepts. Q. Rev. Biophys. 2002;35:111–167. doi: 10.1017/s0033583502003761. [DOI] [PubMed] [Google Scholar]
- Plotkin S.S., Wang J., Wolynes P.G. Statistical mechanics of a correlated energy landscape model for protein folding funnels. J. Chem. Phys. 1997;106:2932–2948. [Google Scholar]
- Porte S.L.L., Bollini S.S., Lanz T.A., Abdiche Y.N., Rusnak A.S., Ho W.H., Kobayashi D., Harrabi O., Pappas D., Mina E.W., Milici A.J., Kawabe T.T., Bales K., Lin J.C., Pons J. Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer’s disease. J. Mol. Biol. 2012;421:525–536. doi: 10.1016/j.jmb.2011.11.047. Amyloid Structure, Function, and Molecular Mechanisms (Part II) [DOI] [PubMed] [Google Scholar]
- Porzig R., Singer D., Hoffmann R. Epitope mapping of mabs at8 and tau5 directed against hyperphosphorylated regions of the human tau protein. Biochem. Biophys. Res. Commun. 2007;358:644–649. doi: 10.1016/j.bbrc.2007.04.187. [DOI] [PubMed] [Google Scholar]
- Pradier L., Cohen C., Blanchard V., Debeir T., Barneoud P., Canton T., Menager J., Bohme A., Rooney T., Guillet M.C., Cameron B., Shi Y., Naimi S., Ravetch J., Claudel S., Alam J. SAR228810: An antiprotofibrillar beta-amyloid antibody designed to reduce risk of amyloid-related imaging abnormalities (ARIA) Alzheimers Dement. 2013;9:P808–P809. [Google Scholar]
- Pradier, L., Blanchard-Brégeon, V., Bohme, A., Debeir, T., Menager, J., Benoit, P., Barneoud, P., Taupin, V., Bertrand, P., Dugay, P., Cameron, B., Shi, Y., Naimi, S., Duchesne, M., Gagnaire, M., Weeden, T., Travaline, T., Reczek, D., Khiroug, L., Slaoui, M., Brunel, P., Fukuyama, H., Ravetch, J., Canton, T., Cohen, C., 2018. SAR228810: an antibody for protofibrillar amyloid β-peptide designed to reduce the risk of amyloid-related imaging abnormalities (ARIA). Alzheimers Res. Ther. 10. [DOI] [PMC free article] [PubMed]
- ProMIS Neuroscience ProMIS Neurosciences oligomer selective antibody therapeutic for alzheimer's disease, PMN310, shows potential for improved safety profile in direct comparison to other amyloid beta-directed antibodies in clinical development (aug 21, 2018) 2018. https://promisneurosciences.com/news/promis-neurosciences-oligomer-selective-antibody-therapeutic-for-alzheimers-disease-pmn310-shows-potential-for-improved-safety-profile-in-direct-comparison-to-other-amyloid-beta-directed-an Available at.
- Puglielli L., Tanzi R.E., Kovacs D.M. Alzheimer’s disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Pul R., Dodel R., Stangel M. Antibody-based therapy in Alzheimer’s disease. Expert. Opin. Biol. Ther. 2011;11:343–357. doi: 10.1517/14712598.2011.552884. [DOI] [PubMed] [Google Scholar]
- Purro S.A., Farrow M.A., Linehan J., Nazari T., Thomas D.X., Chen Z., Mengel D., Saito T., Saido T., Rudge P., Brandner S., Walsh D.M., Collinge J. Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature. 2018;564:415–419. doi: 10.1038/s41586-018-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W., Yau W.M., Lu J.X., Collinge J., Tycko R. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature. 2017;541:217–221. doi: 10.1038/nature20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi I.A., Tirucherai G., Ahlijanian M.K., Kolaitis G., Bechtold C., Grundman M. A randomized, single ascending dose study of intravenous BIIB092 in healthy participants. Alzheimer’s Dement. 2018;4:746–755. doi: 10.1016/j.trci.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohamedsait H., Rasool S., Rajamohamedsait W., Lin Y., Sigurdsson E.M. Prophylactic active tau immunization leads to sustained reduction in both tau and amyloid-β pathologies in 3xTg mice. Sci. Rep. 2017;7:17034. doi: 10.1038/s41598-017-17313-1. (15pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Kierny M.R., Mercer A., Wu J., Tovchigrechko A., Wu H., Dall’Acqua W.F., Xiao X., Chowdhury P.S. Recombinant human B cell repertoires enable screening for rare, specific, and natively paired antibodies. Commun. Biol. 2018;1:8. doi: 10.1038/s42003-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhit R., Robertson J., Velde C.V., Horne P., Ruth D.M., Griffin J., Cleveland D.W., Cashman N.R., Chakrabartty A. An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nat. Med. 2007;13:754–759. doi: 10.1038/nm1559. [DOI] [PubMed] [Google Scholar]
- Rasmussen J., Jucker M., Walker L.C. Aβ seeds and prions: how close the fit? Prion. 2017;11:215–225. doi: 10.1080/19336896.2017.1334029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J., Fukuyama H. 2009. Antibodies specific for the protofibril form of beta-amyloid protein. US Patent, WO2009–065054–A2. [Google Scholar]
- Reddy M.P., Kinney C.A.S., Chaikin M.A., Payne A., Fishman-Lobell J., Tsui P., Dal Monte P.R., Doyle M.L., Brigham-Burke M.R., Anderson D., Reff M., Newman R., Hanna N., Sweet R.W., Truneh A. Elimination of fc receptor-dependent effector functions of a modified (IgG4) monoclonal antibody to human CD4. J. Immunol. 2000;164:1925–1933. doi: 10.4049/jimmunol.164.4.1925. [DOI] [PubMed] [Google Scholar]
- Relkin N.R., Thomas R.G., Rissman R.A., Brewer J.B., Rafii M.S., van Dyck C.H., Jack C.R., Sano M., Knopman D.S., Raman R., Szabo P., Gelmont D.M., Fritsch S., Aisen P.S. A phase 3 trial of iv immunoglobulin for alzheimer disease. Neurology. 2017;88:1768–1775. doi: 10.1212/WNL.0000000000003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel S. Edward Jenner and the history of smallpox and vaccination. Baylor Univ. Med. Center Proc. 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Roda A.R., Montoliu-Gaya L., Villegas S. The role of apolipoprotein e isoforms in alzheimer’s disease. J. Alzheimers Dis. 2019;68:459–471. doi: 10.3233/JAD-180740. [DOI] [PubMed] [Google Scholar]
- Sadigh-Eteghad S., Talebi M., Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer’s disease. A meta-analysis. Neurosciences (Riyadh) 2012;17:321–326. [PubMed] [Google Scholar]
- Salloway S., Sperling R., Gilman S., Fox N.C., Blennow K., Raskind M., Sabbagh M., Honig L.S., Doody R., van Dyck C.H., Mulnard R., Barakos J., Gregg K.M., Liu E., Lieberburg I., Schenk D., Black R., Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Ferris S., Reichert M., Ketter N., Nejadnik B., Guenzler V., Miloslavsky M., Wang D., Lu Y., Lull J., Tudor I.C., Liu E., Grundman M., Yuen E., Black R., Brashear H.R. Two phase 3 trials of bapineuzumab in mild-to-moderate alzheimer’s disease. N. Engl. J. Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Kaufman S., DeVos S., Sharma A., Mirbaha H., Li A., Barker S., Foley A., Thorpe J., Serpell L., Miller T., Grinberg L., Seeley W., Diamond M. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saputra E.C., Huang L., Chen Y., Tucker-Kellogg L. Combination therapy and the evolution of resistance: the theoretical merits of synergism and antagonism in cancer. Cancer Res. 2018;78:2419–2431. doi: 10.1158/0008-5472.CAN-17-1201. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Hallikainen M., Grimmer T., Duning T., Gouw A.A., Teunissen C.E., Wink A.M., Maruff P., Harrison J., van Baal C.M., Bruins S., Lues I., Prins N.D. Safety, tolerability and efficacy of the glutaminyl cyclase inhibitor PQ912 in Alzheimer’s disease: results of a randomized, double-blind, placebo-controlled phase 2a study. Alzheimer’s Res. Ther. 2018;10 doi: 10.1186/s13195-018-0431-6. Article 107; 14pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T.D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad L., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. Secreted amyloid ββ-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schilling S., Rahfeld J.U., Lues I., Lemere C.A. Passive Aβ immunotherapy: current achievements and future perspectives. Molecules. 2018;23 doi: 10.3390/molecules23051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmacher M.G., Selkoe D.J. 1993. Methods of screening for compounds which inhibit soluble β-amyloid peptide production. US Patent, 5,766,846. [Google Scholar]
- Schupf N., Tang M.X., Fukuyama H., Manly J., Andrews H., Mehta P., Ravetch J., Mayeux R. Peripheral Aβ subspecies as risk biomarkers of Alzheimer’s disease. Proc. Natl. Acad. Sci. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz A.K., Vagt T., Huber M., Ovchinnikova O.Y., Cadalbert R., Wall J., Güntert P., Böckmann A., Glockshuber R., Meier B.H. Atomic-resolution three-dimensional structure of amyloid β fibrils bearing the Osaka mutation. Angew. Chem. Int. Ed. 2015;54:331–335. doi: 10.1002/anie.201408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.E., Bayly A.R., Abell C., Skidmore J. Small molecules, big targets: drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016;15:533–550. doi: 10.1038/nrd.2016.29. [DOI] [PubMed] [Google Scholar]
- Sehlin D., Englund H., Simu B., Karlsson M., Ingelsson M., Nikolajeff F., Lannfelt L., Pettersson F.E. Large aggregates are the major soluble Aβ species in AD brain fractionated with density gradient ultracentrifugation. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0032014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D.J. Preventing Alzheimer’s disease. Science. 2012;337:1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J. Alzheimer disease and Aducanumab: adjusting our approach. Nat. Rev. Neurol. 2019:365–366. doi: 10.1038/s41582-019-0205-1. [DOI] [PubMed] [Google Scholar]
- Sengupta U., Nilson A.N., Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:1–24. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., O’Gorman J., Qian F., Arastu M., Li M., Chollate S., Brennan M.S., Quintero-Monzon O., Scannevin R.H., Arnold H.M., Engber T., Rhodes K., Ferrero J., Hang Y., Mikulskis A., Grimm J., Hock C., Nitsch R.M., Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- Shahpasand K., Shamloo A.S., Nabavi S.M., Lu K.P., Zhou X.Z. Tau immunotherapy: hopes and hindrances. Hum. Vaccines Immunotherapeut. 2018;14:277–284. doi: 10.1080/21645515.2017.1393594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Sugiyama K., Ouchi Y., Tsukii K. A single dose study of a novel humanized anti-amyloid beta (Aβ) aggregate specific antibody KHK6640 in Japanese patients with Alzheimer’s disease. J. Prevent. Alzheimer’s Dis. 2017;4:342. [Google Scholar]
- Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H., Dowsett S.A., Pontecorvo M.J., Dean R.A., Demattos R. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- Sigurdsson E.M. Tau immunotherapies for Alzheimer’s disease and related tauopathies: Progress and potential pitfalls. J. Alzheimers Dis. 2018;64:S555–S565. doi: 10.3233/JAD-179937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.P., Vetterlein O., Jose J., Peters S., Kirby H. The s228p mutation prevents in vivo and in vitro igg4 fab-arm exchange as demonstrated using a combination of novel quantitative immunoassays and physiological matrix preparation. J. Biol. Chem. 2015;290:5462–5469. doi: 10.1074/jbc.M114.600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira J.R., Raymond G.J., Hughson A.G., Race R.E., Sim V.L., Hayes S.F., Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J.M., Gibbs E., Peng X., Martens K.M., Balducci C., Wang J., Yousefi M., Cowan C.M., Lamour G., Louadi S., Ban Y., Robert J., Stukas S., Forloni G., Hsiung G.Y.R., Plotkin S.S., Wellington C.L., Cashman N.R. A rational structured epitope defines a distinct subclass of toxic amyloid-beta oligomers. ACS Chem. Neurosci. 2018;9:1591–1606. doi: 10.1021/acschemneuro.7b00469. [DOI] [PubMed] [Google Scholar]
- Skerra A. Engineered protein scaffolds for molecular recognition. J. Mol. Recognit. 2000;13:167–187. doi: 10.1002/1099-1352(200007/08)13:4<167::AID-JMR502>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Söllvander S., Nikitidou E., Gallasch L., Zysk M., Söderberg L., Sehlin D., Lannfelt L., Erlandsson A. The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death. J. Neuroinflammat. 2018;15 doi: 10.1186/s12974-018-1134-4. Article 98; 15pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R., Salloway S., Brooks D.J., Tampieri D., Barakos J., Fox N.C., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Lieberburg I., Arrighi H.M., Morris K.A., Lu Y., Liu E., Gregg K.M., Brashear H.R., Kinney G.G., Black R., Grundman M. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T.L., Mielke M.L., Rozkalne A., Meyer-Luehmann M., de Calignon A., Bacskai B.J., Schenk D., Hyman B.T. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol. Dis. 2009;33:213–220. doi: 10.1016/j.nbd.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S., Ratsch O., Brocks B., Dürr M., Thomassen-Wolf E. In vitro affinity maturation of human gm-csf antibodies by targeted cdr-diversification. Mol. Immunol. 2008;46:135–144. doi: 10.1016/j.molimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Stöhr J., Watts J.C., Mensinger Z.L., Oehler A., Grillo S.K., DeArmond S.J., Prusiner S.B., Giles K. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. 2012;109:11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel G. Alzheimer’s prevention initiative. J. Alzheimers Dis. 2010;21:1025–1035. doi: 10.3233/JAD-2010-101429. [DOI] [PubMed] [Google Scholar]
- Sud R., Geller E.T., Schellenberg G.D. Antisense-mediated exon skipping decreases tau protein expression: a potential therapy for tauopathies. Mol. Ther. 2014;3 doi: 10.1038/mtna.2014.30. (11 pp.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C.J., Zhang Y., Dhadda S., Wang J., Kaplow J., Lai R.Y., Lannfelt L., Kramer L.D., Luthman J. Treatment of early AD subjects with BAN2401, an anti-Aβ protofibril monoclonal antibody, signficantly clears amyloid plaque and reduces clinical decline. Alzheimer’s Dement. 2018;14:P1668. [Google Scholar]
- Swerdlow A., Higgins C., Adlard P., Jones M., Preece M. Creutzfeldt-Jakob disease in United Kingdom patients treated with human pituitary growth hormone. Neurology. 2003;61:783–791. doi: 10.1212/01.WNL.0000084000.27403.15. [DOI] [PubMed] [Google Scholar]
- Szabo P., Mujalli D.M., Rotondi M.L., Sharma R., Weber A., Schwarz H.P., Weksler M.E., Relkin N. Measurement of anti-beta amyloid antibodies in human blood. J. Neuroimmunol. 2010;227:167–174. doi: 10.1016/j.jneuroim.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Szaruga M., Munteanu B., Lismont S., Veugelen S., Horré K., Mercken M., Saido T.C., Ryan N.S., De Vos T., Savvides S.N., et al. Alzheimer’s-causing mutations shift Aβ length by destabilizing γ-secretase-Aβn interactions. Cell. 2017;170:443–456. doi: 10.1016/j.cell.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer’s disease and nondemented individuals. Neurosci. Lett. 1988;93:191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Tariot P.N., Lopera F., Langbaum J.B., Thomas R.G., Hendrix S., Schneider L.S., Rios-Romenets S., Giraldo M., Acosta N., Tobon C., Ramos C., Espinosa A., Cho W., Ward M., Clayton D., Friesenhahn M., Mackey H., Honigberg L., Bohorquez S.S., Chen K., Walsh T., Langlois C., Reiman E.M. The Alzheimer’s prevention initiative autosomal-dominant Alzheimer’s disease trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimer’s Dement. 2018;4:150–160. doi: 10.1016/j.trci.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe H., Kasai T., Ohmichi T., Kishi Y., Kakeya T., Waragai M., Kondo M., Allsop D., Tokuda T. Quantification of plasma phosphorylated tau to use as a biomarker for brain alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol. Neurodegeneration. 2017;12:63. doi: 10.1186/s13024-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper K., Biernat J., Kumar S., Wegmann S., Timm T., Hübschmann S., Redecke L., Mandelkow E.M., Müller D.J., Mandelkow E. Oligomer formation of tau protein hyperphosphorylated in cells. J. Biol. Chem. 2014;289:34389–34407. doi: 10.1074/jbc.M114.611368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D.R., Rüb U., Orantes M., Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Theunis C., Crespo-Biel N., Gafner V., Pihlgren M., López-Deber M.P., Reis P., Hickman D.T., Adolfsson O., Chuard N., Ndao D.M., Borghgraef P., Devijver H., Van Leuven F., Pfeifer A., Muhs A. Efficacy and safety of a liposome-based vaccine against protein tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson B., Blessed G., Roth M. Observations on the brains of demented old people. J. Neurol. Sci. 1970;11:205–242. doi: 10.1016/0022-510X(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Treusch S., Cyr D.M., Lindquist S. Amyloid deposits: protection against toxic protein species? Cell Cycle. 2009;8:1668–1674. doi: 10.4161/cc.8.11.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troquier L., Caillierez R., Burnouf S., Kervoaze G., Grosjean M.E., Nadege Z., Sergeant N., Lassalle P., Blum D., Buee L. Targeting phospho-ser422 by active tau immunotherapy in THY-tau22, a suitable therapeutic approach. Alzheimers Dement. 2011;7:S482–S483. [Google Scholar]
- Tsai C.j., Kumar S., Ma B., Nussinov R. Folding funnels, binding funnels, and protein function. Protein Sci. 1999;8:1181–1190. doi: 10.1110/ps.8.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S., Möller C., Tegerstedt K., Lord A., Laudon H., Sjödahl J., Söderberg L., Spens E., Sahlin C., Waara E.R., et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-arcswe mice. J. Alzheimers Dis. 2015;43:575–588. doi: 10.3233/JAD-140741. [DOI] [PubMed] [Google Scholar]
- Ulrich J.D., Ulland T.K., Colonna M., Holtzman D.M. Elucidating the role of TREM2 in Alzheimer’s disease. Neuron. 2017;94:237–248. doi: 10.1016/j.neuron.2017.02.042. [DOI] [PubMed] [Google Scholar]
- Ultsch M., Li B., Maurer T., Mathieu M., Adolfsson O., Muhs A., Pfeifer A., Pihlgren M., Bainbridge T.W., Reichelt M., Ernst J.A., Eigenbrot C., Fuh G., Atwal J.K., Watts R.J., Wang W. Structure of crenezumab complex with Aβ shows loss of β-hairpin. Sci. Rep. 2016;6 doi: 10.1038/srep39374. 39374 EP –. URL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck C.H. Anti-amyloid-β monoclonal antibodies for alzheimer’s disease: Pitfalls and promise. Biol. Psychiatr. 2018;83:311–319. doi: 10.1016/j.biopsych.2017.08.010. Mechanisms of Alzheimer’s Disease and Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermeeren M., Borgers M., Van Kolen K., Theunis C., Vasconcelos B., Bottelbergs A., Wintmolders C., Daneels G., Willems R., Dockx K., Delbroeka L., Marreiro A., Donck L.V., Sousa C., Nanjunda R., Lacy E., Casteele T.V.D., Dam D.V., Deyn P.P.D., Kemp J.A., Malia T.J., Mercken M.H. Anti-tau monoclonal antibodies derived from soluble and filamentous tau show diverse functional properties in vitro and in vivo. J. Alzheimers Dis. 2018;65:265–281. doi: 10.3233/JAD-180404. [DOI] [PubMed] [Google Scholar]
- Vellas B., Black R., Thal L.J., Fox N.C., Daniels M., McLennan G., Tompkins C., Leibman C., Pomfret M., Grundman M. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr. Alzheimer Res. 2009;6:144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellas B., Dahmen R., Nicolas O., Martin V., Cohen C., Vermet L., Barakos J., Suhy J., Börjesson-Hanson A., Östlund H., Groeneveld G.J., Prins N., Tens P.S., Blennow K., Andreasen N. Safety and pharmacokinetics of anti-protofibrillar mAb SAR228810 in first-in-man study after single and multiple ascending IV and SC dosing in patients with mild to moderate Alzheimer’s disease. J. Prev. Alz. Dis. 2015;2:348–349. [Google Scholar]
- Vellas B., Bain L.J., Touchon J., Aisen P.S. Advancing alzheimer’s disease treatment: lessons from ctad 2018. J. Prevent. Alzheimer’s Dis. 2019;6:198–203. doi: 10.14283/jpad.2019.11. [DOI] [PubMed] [Google Scholar]
- Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., Szoeke C., Macaulay S.L., Martins R., Maruff P., Ames D., Rowe C.C., Masters C.L. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Vincent I., Rosado M., Davies P. Mitotic mechanisms in alzheimer’s disease? J. Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale F., Giliberto L., Ruiz S., Steslow K., Marambaud P., d’Abramo C. Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice. Acta Neuropathol. Commun. 2018;6:82. doi: 10.1186/s40478-018-0585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Klyubin I., Shankar G., Townsend M., Fadeeva J., Betts V., Podlisny M., Cleary J., Ashe K., Rowan M., Selkoe D. The role of cell-derived oligomers of Aβ in Alzheimer’s disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- Wälti M.A., Ravotti F., Arai H., Glabe C.G., Wall J.S., Böckmann A., Güntert P., Meier B.H., Riek R. Atomic-resolution structure of a disease-relevant Aβ amyloid fibril. Proc. Natl. Acad. Sci. 2016;113:E4976–E4984. doi: 10.1073/pnas.1600749113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Verkhivker G.M. Energy landscape theory, funnels, specificity, and optimal criterion of biomolecular binding. Phys. Rev. Lett. 2003;90:188101. doi: 10.1103/PhysRevLett.90.188101. [DOI] [PubMed] [Google Scholar]
- Wang J., Dickson D.W., Trojanowski J.Q., Lee V.M.Y. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Wang X., Kastanenka K.V., Arbel-Ornath M., Commins C., Kuzuya A., Lariviere A.J., Krafft G.A., Hefti F., Jerecic J., Bacskai B.J. 2018. An acute functional screen identifies an effective antibody targeting amyloid-β oligomers based on calcium imaging. Sci. Rep. 8, 4634 (15 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt A.D., Crespi G.A.N., Down R.A., Ascher D.B., Gunn A., Perez K.A., McLean C.A., Villemagne V.L., Parker M.W., Barnham K.J., Miles L.A. Do current therapeutic anti-Aβ antibodies for alzheimer’s disease engage the target? Acta Neuropathol. 2014;127:803–810. doi: 10.1007/s00401-014-1290-2. [DOI] [PubMed] [Google Scholar]
- Watts J.C., Prusiner S.B. β-amyloid prions and the pathobiology of Alzheimer’s disease. Cold Spring Harb. Perspect. Med. 2018;8:1–14. doi: 10.1101/cshperspect.a023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J.C., Condello C., Stöhr J., Oehler A., Lee J., DeArmond S.J., Lannfelt L., Ingelsson M., Giles K., Prusiner S.B. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc. Natl. Acad. Sci. 2014;111:10323–10328. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C.L., Espinoza M., Kress Y., Davies P. Conformational change as one of the earliest alterations of tau in alzheimer’s disease. Neurobiol. Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Weggen S., Beher D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimers Res. Ther. 2012;4 doi: 10.1186/alzrt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb P.H., Chen F., Garber E.A., Grimm J., Montrasio F. 2014. Human anti-tau antibodies. Patent (US), WO2014–100600. [Google Scholar]
- Weisová P., Cehlár O., Skrabana R., Zilková M., Filipcík P., Kovácech B., Prcina M., Wojciaková L., Fialová L., Smolek T., Kontseková E., Zilka N., Novák M. Therapeutic antibody targeting microtubule-binding domain prevents neuronal internalization of extracellular tau via masking neuron surface proteoglycans. Acta Neuropathol. Commun. 2019;7:22. doi: 10.1186/s40478-019-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T., Hu Y., Verghese P., Bateman R., Braunstein J., Fogelman I., Budur K., Florian H., Mendonca N., Holtzman D. Preclinical and clinical development of ABBV-8E12, a humanized anti-tau antibody, for treatment of Alzheimer’s disease and other tauopathies. J Prev Alzheimers Dis. 2017;4:236–241. doi: 10.14283/jpad.2017.36. [DOI] [PubMed] [Google Scholar]
- Westwood M., Lawson A.D.G. Opportunities for conformation-selective antibodies in amyloid-related diseases. Antibodies. 2015;4:170–196. [Google Scholar]
- Williams D.R. Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 2006;36:652–660. doi: 10.1111/j.1445-5994.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- Winblad B., Graf A., Riviere M.E., Andreasen N., Ryan J.M. Active immunotherapy options for Alzheimer’s disease. Alzheimers Res. Ther. 2014;6 doi: 10.1186/alzrt237. 12 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O., Breyhan H., Cynis H., Schilling S., Demuth H.U., Bayer T.A. Intraneuronal pyroglutamate-Aβ 3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O., Erck C., Martens H., Harmeier A., Geumann C., Jawhar S., Kumar S., Multhaup G., Walter J., Ingelsson M., Degerman-Gunnarsson M., Kalimo H., Huitinga I., Lannfelt L., Bayer T.A. Identification of low molecular weight pyroglutamate Aβ oligomers in Alzheimer disease: a novel tool for therapy and diagnosis. J. Biol. Chem. 2010;285:41517–41524. doi: 10.1074/jbc.M110.178707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Goñi F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B., Pruchnicki A., Dickson D., Davies P. A neuronal antigen in the brains of alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Woodcock J., Griffin J.P., Behrman R.E. Development of novel combination therapies. N. Engl. J. Med. 2011;364:985–987. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Ma B., McElheny D., Parthasarathy S., Long F., Hoshi M., Nussinov R., Ishii Y. Aβ (1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015;22:499–505. doi: 10.1038/nsmb.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Yabuki C., Seubert P., Schenk D., Hori Y., Ohtsuki S., Terasaki T., Hashimoto T., Iwatsubo T. Aβ immunotherapy: intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J. Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K., Kfoury N., Jiang H., Mahan T., Ma S., Maloney S., Wozniak D., Diamond M., Holtzman D. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K., Jiang H., Mahan T.E., Maloney S.E., Wozniak D.F., Diamond M.I., Holtzman D.M. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann. Clin. Transl. Neurol. 2015;2:278–288. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Li S., Xu H., Walsh D.M., Selkoe D.J. Large soluble oligomers of amyloid β-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J. Neurosci. 2017;37:152–163. doi: 10.1523/JNEUROSCI.1698-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.C., Chiu M.J., Chen T.F., Chang H.L., Liu B.H., Yang S.Y. Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early-stage Alzheimer’s disease. J. Alzheimers Dis. 2018;61:1323–1332. doi: 10.3233/JAD-170810. [DOI] [PubMed] [Google Scholar]
- Yang T., Dang Y., Ostaszewski B., Mengel D., Steffen V., Rabe C., Bittner T., Walsh D.M., Selkoe D.J. Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann. Neurol. 2019;86:215–224. doi: 10.1002/ana.25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Cheng B., Li Y., Li X., Chen X., Zhang Y.W. Trem2 in alzheimer’s disease: Microglial survival and energy metabolism. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00395. Article 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.X. From induced fit to conformational selection: a continuum of binding mechanism controlled by the timescale of conformational transitions. Biophys. J. 2010;98:L15–L17. doi: 10.1016/j.bpj.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Blanco L.P., Smith D.R., Chapman M.R. In: Amyloid Proteins: Methods and Protocols. Sigurdsson E.M., Calero M., Gasset M., editors. Humana Press; Totowa, NJ: 2012. Bacterial amyloids; pp. 303–320. [Google Scholar]