Abstract
Two-pore channels (TPCs) are cation-permeable channels located on endolysosomal membranes and important mediators of intracellular Ca2+ signalling. TPCs are involved in various pathophysiological processes, including cell growth and development, metabolism, and cancer progression. Most studies of TPCs have used TPC–/– cell or whole-animal models, or Ned-19, an indirect inhibitor. The TPC activation mechanism remains controversial, which has made it difficult to develop selective modulators. Recent studies of TPC structure and their interactomes are aiding the development of direct pharmacological modulators. This process is still in its infancy, but will facilitate future research and TPC targeting for therapeutical purposes. Here, we review the progress of current research into TPCs, including recent insights into their structures, functional roles, mechanisms of activation, and pharmacological modulators.
Keywords: two-pore channels, cell signalling, calcium, NAADP, endolysosomes, interactome
TPCs in Ca2+ Signalling
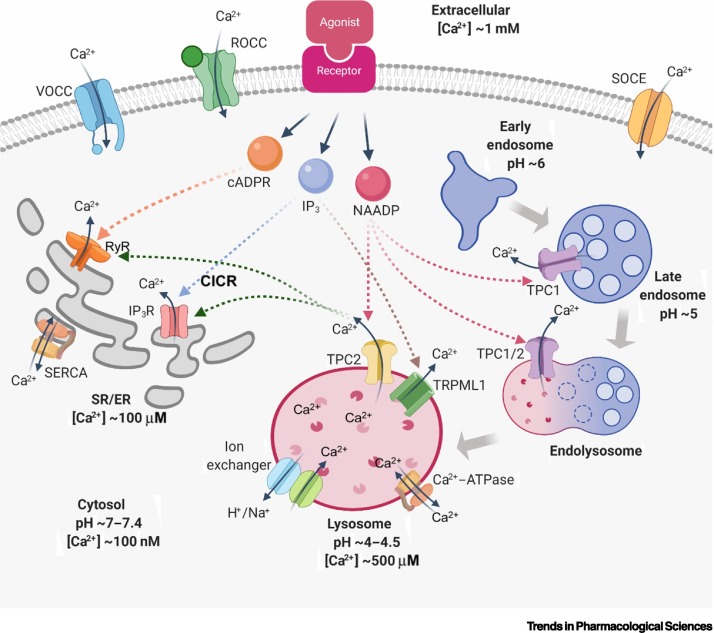
Changes in intracellular calcium (Ca2+) constitutes a key signalling mechanism that mediates diverse cellular functions [1]. One source of cytoplasmic Ca2+ is extracellular Ca2+, the entry of which into the cell is regulated by voltage-operated calcium channels (VOCCs), receptor-operated calcium channels (ROCCs), and storage-operated calcium entry (SOCE) (Figure 1 ) [2]. Another source is internal stores, which release Ca2+ when their receptors are activated by Ca2+-mobilising messengers, such as inositol 1,4,5-trisphosphate (IP3), cyclic adenosine diphosphate ribose (cADPR), and nicotinic acid adenine dinucleotide phosphate (NAADP). IP3 and cADPR activate IP3 receptors (IP3Rs) and ryanodine receptors (RyRs), respectively, both of which regulate Ca2+ release from the endo/sarcoplasmic reticulum (ER/SR); NAADP targets TPCs to mediate Ca2+ release from acidic endolysosomal organelles (Figure 1) [3,4]. NAADP is the most potent Ca2+-mobilising messenger, exerting its action even at low nanomolar concentrations [5]. NAADP-evoked Ca2+ signalling can further trigger Ca2+ release from the ER/SR, via Ca2+-induced Ca2+ release (CICR) [6]. TPCs have gained increasing attention as integral components of the NAADP-regulated Ca2+ channel system, although they can also act as sodium (Na+) channels regulated by phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] [7,8]. The exact activation mechanisms mediated by NAADP and PI(3,5)P2 remain controversial, adding further to the TPC enigma.
Figure 1.
Intracellular Ca2+ Homeostasis and Regulation Mechanisms.
Cytosolic Ca2+ is crucial in multiple cellular functions. Since extracellular [Ca2+] is significantly higher than intracellular [Ca2+], Ca2+ is released into the cytosol in several ways. Extracellular Ca2+ is transported across the cell membrane through voltage-operated calcium channels (VOCCs), receptor-operated calcium channels (ROCCs), and storage-operated calcium entry (SOCE). Intracellular Ca2+ stores are activated by second messengers when ligands bind to their plasma membrane receptor; these include inositol 1,4,5-trisphosphate (IP3), cyclic adenosine diphosphate ribose (cADPR), and nicotinic acid adenine dinucleotide phosphate (NAADP). Ca2+ in the endo/sarcoplasmic reticulum (ER/SR) is released through ryanodine receptors (RyRs) and inositol 1,4,5-triphosphate receptors (IP3Rs) triggered by cADPR and IP3, respectively. By contrast, acidic Ca2+ stores, which are also known as endolysosomal Ca2+ stores, are gated by two-pore channels (TPCs) and transient receptor potential, mucolipin subfamily 1 (TRPML1), triggered by NAADP and IP3, respectively. Ca2+ can be pumped into ER/SR by the action of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) against a concentration gradient. Notably, the Ca2+-induced Ca2+ release (CICR) phenomenon in SR/ER can be stimulated by TPC-released Ca2+ from acidic Ca2+ stores or by influx of extracellular Ca2+. In the transition from early endosomes to lysosomes, increasing acidity and [Ca2+] can be detected. Created withBioRender.com.
Structural Insights into TPCs
The animal TPC family comprises three subtypes: TPC1, TPC2 and TPC3. Only TPC1 and TPC2 are found in human and mouse cells, and differ in their pattern of distribution: TPC1 is found in a range of endolysosomal organelles, while TPC2 is distributed more predominantly in late endosomes and lysosomes (Figure 1) [9].
The past decade has witnessed remarkable progress in the development of detector technology and computational algorithms in structural biology study, facilitating the investigation of TPCs at an atomic level. Phylogenetic analysis of TPCs revealed that they share a common evolutionary origin with four-domain voltage-gated Ca2+ and Na+ channels (CaV and NaV), allowing the first predictions of their 3D conformations by homology modelling. This study also identified conserved residues at the channel pore region (see Glossary), suggesting that, similar to NaV and CaV, TPCs also exploit an asparagine-gated filter for cation selectivity [10].
In 2016, the 3D architecture of TPC1 from Arabidopsis thaliana (AtTPC1) was determined using X-ray crystallography, at a resolution of 3.3 Å and 2.87 Å [11,12]. This was the first structural determination of this homodimer, where each subunit comprises two homologous six-transmembrane (6-TM) domain helices. The two 6-TMs are domain swapped, so that the C-terminal of 6-TM 1 is connected with the N-terminal of 6-TM 2 by an EF hand motif [11,12]. However, the lack of critical glutamate and aspartate residues at the pore region makes AtTPC1 less selective to Ca2+ and Na+ compared with its mammalian counterpart [13] (see later).
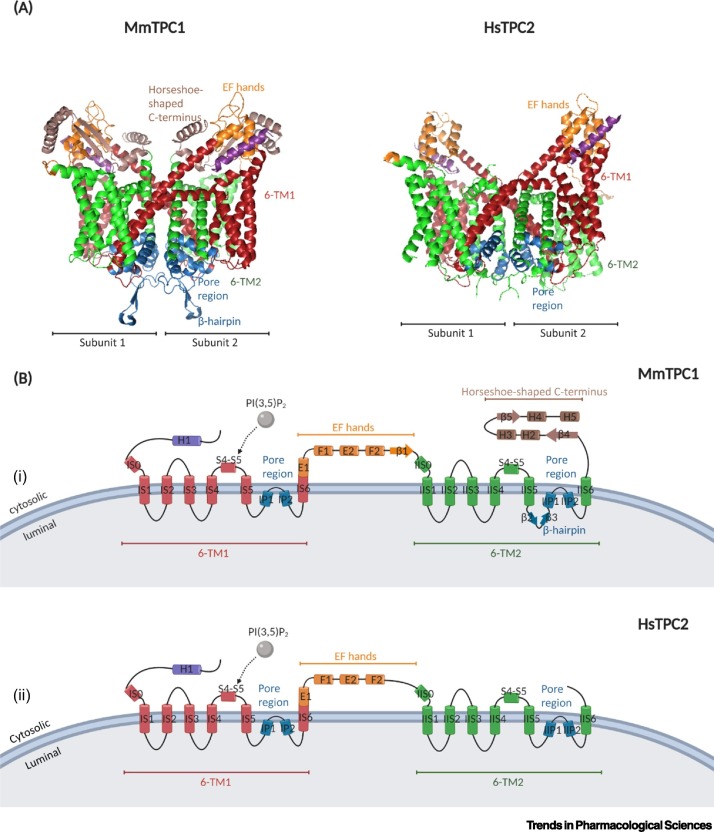
Recently, the 3D structures of mouse TPC1 (MmTPC1) and human TPC2 (HsTPC2) were determined with single-particle electron cryo-microscopy (cryo-EM) [14,15]. Despite the low sequence similarity with AtTPC1, the overall homodimer architecture is conserved between species. The first four helices in each domain (IS1–IS4 for subunit 1, and IIS1–IIS4 for subunit 2) are voltage-sensing domains (VSDs), while the fifth and sixth helices (IS5 and IS6 for subunit 1, and IIS5 and IIS6 for subunit 2) constitute the pore region (Figure 2 ). Interestingly, a β-hairpin structure and a prolonged (horseshoe-shaped) C-terminal were identified in MmTPC1 but not in HsTPC2 (Figure 2) [14,15]. The binding site of PI(3,5)P2 is located at 6-TM 1 for both MmTPC1 and HsTPC2, and this binding is sufficient to open the pore region of HsTPC2. In MmTPC1, the activation is both ligand and voltage dependent; IIS1–IIS4 from VSDs (but not IS1–IS4) are involved in sensing changes in membrane potential to open the channel [14]. VSDs in HsTPC2 are independent from the channel activation process [15]. Ion selectivity of both MmTPC1 and HsTPC2 is achieved by not only a ‘filter’, which is an asparagine-gated size sieve (in line with a previous prediction, as mentioned earlier [10]), but also a ‘gate’, which opens and closes while also restricting the size and charge of ions [14,15]. The involvement of certain amino acids is the key in this cation selection process, and can vary between subtypes and species (Table 1 ).
Figure 2.
An Overview of the Structure of Mouse Two-Pore Channel 1 (MmTPC1) and Human TPC2 (HsTPC2).
(A) Side view of the 3D structure of MmTPC1 [Protein Data Bank (PDB) ID: 6C96] and HsTPC2 (PDB ID: 6NQ1). Each channel contains two identical subunits. There are two six-transmembrane (6-TM) domains in each subunit (shown in red, named IS1–IS6 for subunit 1; and in green, named IIS1–IIS6 for subunit 2), connected by EF-hand motifs (in orange). MmTPC1 is distinguished from HsTPC2 in having a unique β-hairpin structure at the pore region (in blue) and a horseshoe shaped C terminus (in brown). (B) Topology and domain arrangement of a single subunit in MmTPC1 (i) and HsTPC2 (ii). Residues from both 6-TMs constitute the pore region. The binding site of phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] is located at the first 6-TM. Adapted from [14,15]. Created withBioRender.com.
Table 1.
Comparison of Key Amino Acid Residues that Contribute to Ion Selectivity between AtTPC1, MmTPC1, and HsTPC2
| TPC subtype | ‘Filter’ region amino acids | ‘Gate’ region amino acids | Ion selectivity | Refs |
|---|---|---|---|---|
| AtTPC1: Arabidopsis thaliana | Thr263, Thr264, Ser265, Asn266, and Asn267 from pore region of subunit 1; Val628, Met629, Gly630, Asn631, and Trp632 from pore region of subunit 2 | Leu301 and Tyr305 from IS6; Val668, Leu672, and Phe676 from IIS6 | Limited selectivity, selective to Ca2+, Na+ Ba2+, Li+, and K+ | [11,94] |
| MmTPC1: Mus musculus | Thr280, Ala281, and Asn282 from pore region of subunit 1; Val647, Asn648, and Asn649 from pore region of subunit 2 | Leu317, Phe321, and Asp322 from IS6; Val684, Leu688, and Glu689 from IIS6 | Highly selective to Na+, also selective to Ca2+ | [14] |
| HsTPC2: Homo sapiens | Thr271, Ala272, and Asn273 from pore region of subunit 1; Val652, Asn653, and Asn654 from pore region of subunit 2 | Thr308 and Tyr312 from IS6; Leu690 and Leu694 from IIS6 | Highly selective to Na+, also selective to Ca2+ | [15,94] |
Trends in Functional Studies of TPCs
Endolysosomal Ca2+ homeostasis and its underlying mechanisms and pathophysiological relevance were poorly investigated until the discovery of NAADP signalling and its link with TPCs. The use of genetically modified animal models further broadened knowledge in this area. TPCs have been shown to have important roles in various diseases and physiological conditions. Here, we briefly discuss the latest discoveries relating to their roles in growth, differentiation, development, pathophysiological processes in the pancreas, viral infection, and cardiac dysfunction.
Cell Growth, Differentiation, and Development
NAADP-mediated Ca2+ signalling via TPC1 has been found to regulate the acrosome reaction of mouse spermatozoa [16]. Similarly, knockdown of TPCs in sea star oocytes showed defected embryo development due to debilitated Ca2+ signalling [17]. Another study reported that TPC2 mediates osteoclastogenesis [18]. This finding is consistent with previous studies that identified a role for NAADP/TPC2-induced Ca2+ signals in neuronal and skeletal muscle differentiation [16,19,20]. Moreover, ADP-ribosyl cyclase 1-like (ARC1-like) was linked to NAADP-mediated Ca2+ release via TPC2 in zebrafish myogenesis [21]. However, TPC2 involvement in autophagy is debated [22., 23., 24., 25., 26.], which warrants further investigation to uncover the complex role and precise mechanisms underlying TPC2 action at different stages of the autophagic process and in diverse cellular contexts. A recent study found that cell proliferation was hindered by TPC2 gene silencing [24]. The critical role of NAADP/TPC/Ca2+ signalling in cellular differentiation and development and the role of TPC2 in cancer progression, from tumorigenesis to metastasis, has been reviewed extensively elsewhere [27,28]. Collectively, these studies highlight the critical role of NAADP/TPC/Ca2+ signalling in cellular differentiation, development, and associated pathophysiological processes, such as cancer.
Pathophysiological Processes in the Pancreas
TPC2-regulated Ca2+ release from acidic intracellular stores has important roles in both insulin and glucagon secretion by the endocrine pancreas. Studies have highlighted that intracellular Ca2+ signals regulate how adrenaline, a blood glucose-elevating hormone, works as a stimulus of glucagon secretion, and identified a link with the cAMP-protein kinase A (PKA) pathway [29]. cAMP has long been recognised as a key intracellular messenger in insulin release and reduced glucagon secretion because its cytoplasmic concentration regulates actions of many hormones [30]. In fact, cAMP is involved in the process by which Ca2+ triggers both glucagon and insulin secretion by α cells and β cells in pancreas islets [31]. A study used islets from TPC2-null mice or islets with pharmacologically inhibited TPC2 to test the participation of TPC2 in glucagon secretion and found that the stimulating function of adrenaline in glucagon secretion was dependent on TPC2-evoked Ca2+ mobilisation [29]. This research linking TPC2 to glucostasis enhanced the signalling map of cellular factors maintaining stable glucagon levels and identified TPC2 as a possible drug target in α cells for the treatment of diabetes.
In a whole-animal TPC-knockout (KO) mouse study, NAADP-evoked Ca2+, via TPCs, was shown to be positively correlated to glucose- and sulfonylurea-elicited membrane potential, intracellular Ca2+ signals, and insulin secretion [31]. However, another study found that pancreatic β cells (the predominant pancreatic cell type and the only source of circulating insulin [32]) isolated from β cell-specific TPC2-null mice did not show a significant change in Ca2+ dynamics evoked by nutrients or incretins. The authors suggested that TPC1 is sufficient to support the normal Ca2+ dynamics in the absence of TPC2 in this particular instance [33].
Moving onto the exocrine pancreas, a 1999 study revealed that NAADP selectively modulates cholecystokinin (CCK)-evoked Ca2+ release in pancreatic acinar cells; this was the first study to indicate NAADP as a potent Ca2+ modulator in mammalian cells, and also established the pancreatic acinar cell as a good model for studies of NAADP-evoked Ca2+ release [34]. Subsequent research showed that NAADP selectively modulates Ca2+ release from lysosome-related organelles in pancreatic acinar cells [35]. Under normal physiological conditions, Ca2+ released from the ER evokes the exocytosis of zymogens in pancreatic acinar cells, while NAADP releases small but detectable amounts of Ca2+ from endolysosomes, resulting from CICR from zymogen-containing granules and the ER, indicating that, in healthy pancreatic acinar cells, the NAADP-TPC-Ca2+ pathway is an important contributor to the regulation of zymogen secretion [36].
Viral Infection
Ebola virus (EBOV) infection causes symptoms that include fever, severe diarrhoea, vomiting, and massive internal and external bleeding, with a high mortality rate [37]. EBOV is endocytosed into host cells and subsequently transported through the endolysosomal system, where it binds to an intracellular glycoprotein receptor, Niemann-Pick C1 (NPC1) [38] and fuses with late lysosomes, following which viral RNA is released into the cytoplasm [39]. Therefore, blocking membrane fusion within the endolysosomal system could inhibit EBOV infection and, because the maturation and vesicle fusion of endosomes are regulated by the NAADP signalling pathway [40], this could be a way to inhibit EBOV infection. Indeed, living cell imaging showed that EBOV enters endolysosomes that contain NPC1 and TPC2 [39,41]. Moreover, virus trafficking and infection were attenuated by TPC KO, knockdown, and pharmacological inhibition [39].
TPCs also appear to have a role in the infectious cycle of another virus, Middle East Respiratory Syndrome coronavirus (MERS-CoV), where NAADP-released Ca2+ supports the activity of proprotein convertases, such as furin, which are required for MERS-CoV fusion activity into host cells and its cytoplasmic translocation (including internalisation, trafficking, and release of virus into cytoplasm) [42]. Indeed, knockdown of TPCs depressed MERS-CoV fusion with host cell membranes [43].
TPCs are involved in the life cycle of HIV; blocking TPC action by gene knockdown or pharmacological inhibition inhibits the release of HIV Tat protein from endolysosomes and transactivation of the viral long terminal repeat (LTR) gene promoter, which suppresses the efficiency of HIV replication in host cells [44]. Such findings indicate that pharmacological modulators of TPCs could have therapeutic value for the treatment of a range of viral infections.
Cardiac Dysfunction
In cardiac ischaemia, a reduced blood supply to the heart can result in shortage of oxygen and nutrients for cardiac myocytes. In addition, restoration of blood supply after ischaemia can also damage myocytes by inducing excessive oxidative stress, known as ischaemia-reperfusion injury (IRI); the involvement of TPCs in IRI was recently shown in TPC1-KO mice, which have alleviated IRI-induced cardiac infarction [45]. This was suggested to be due to decreased TPC1-induced CICR, because excessive Ca2+ can be detrimental during IRI by stimulating oxidative stress in mitochondria [45].
Apart from IRI, there is also evidence that links TPC2 with arrhythmia and cardiac hypertrophy. The NAADP-TPC2 pathway is involved in this process as a downstream effect of abnormal β-adrenoceptor stimulation [46]. This concept was also confirmed by an in vivo model, whereby TPC2-KO mice exhibited decreased proneness to both arrhythmia and hypertrophy [47].
TPC Activation
As stated earlier, NAADP can endogenously regulate Ca2+ release via TPCs [48]. Previous findings showing NAADP mediates endolysosomal Ca2+ release via TPCs have been contradictory, and it has been suggested that a separate unidentified NAADP-binding protein exists to accessorise TPC activation [8,15,49., 50., 51., 52., 53., 54.]. NAADP evokes endolysosomal cation release via TPC1 or TPC2 with distinctive isoform-specific ion selectivity [55]. Endolysosomal Ca2+ concentration and luminal pH regulate the sensitivity and reversibility of NAADP binding via TPC2 [49]. In a study utilising TPC1 overexpression, knockdown, and mutagenesis to investigate NAADP sensitivity, TPC1 was shown to have a vital role in exerting NAADP action [56]. Recently, it was shown that arginine residues in the first S4–S5 linker are required to trigger Ca2+ signalling upon NAADP binding to TPC1 [57]. An NAADP analogue [3-azido-5-azidomethylbenzoic acid attached to the amino group of 5-(3-aminopropyl)-NAADP] was recently synthesised and characterised, and warrants further investigation of its action on TPCs to elicit endolysosomal Ca2+ signalling [58].
While it is known that NAADP triggers Ca2+ release from endolysosomal organelles through TPCs [4], this thinking was challenged by a study that provided evidence that TPCs are Na+-selective channels activated by endolysosomal PI(3,5)P2 but not by NAADP [8]; instead endolysosomal Ca2+ was proposed to be triggered by PI(3,5)P2 through transient receptor potential, mucolipin subfamily 1 (TRPML1), a 6-TM-spanning endolysosomal nonselective cation channel in the TRP family, as shown in budding yeast [59].
TPC2 was also found to be regulated by Mg2+, with cytosolic Mg2+ inhibiting the TPC2 outward current while lysosomal Mg2+ inhibits both the outward and inward currents [7]. Two structurally and enzymatically similar protein kinases, P38 and c-Jun N-terminal kinases (JNK), were also shown to regulate TPC2 [60]. Interestingly, the changes in PI(3,5)P2, Mg2+, and in JNK and P38 activity all exert similar effects on NAADP-mediated Ca2+ release and TPC2 currents [4].
TPC Interactome
TPC interactomes have been classified into several functional groups, namely Ca2+ homeostasis, membrane trafficking, and organisation. The identification of NAADP-TPC interactomes is currently an area of great scientific interest. Since NAADP-evoked Ca2+ release from TPCs regulates diverse physiological functions, there is much interest in searching for TPC-associated proteins as potential therapeutic targets instead of directly targeting NAADP or TPCs, to enhance the specificity and efficacy of drug action and reduce adverse effects. Several studies have sought to identify proteins that specifically interact with TPCs [61., 62., 63., 64.]. A study that used proteomic analysis to define the TPC interactome found that Rab GTPases, which are regulators of cell pigmentation and endolysosomal trafficking dynamics, interact with TPC2 in frog oocytes and mammalian cells to support NAADP-evoked Ca2+ release [62].
NAADP has been shown to be involved in the pathway by which leucine-rich repeat kinase-2 (LRRK2) activates calcium-dependent protein kinase kinase-β (CaMKK-β)/AMP-activated protein kinase (AMPK), which has been linked to increased autophagosome formation in late-onset Parkinson’s disease (PD) [63]. LRRK2 regulates TPC2 action and this effect can be blocked by Ned-19, an NAADP antagonist, suggesting that LRRK2 acts between NAADP and TPC2 and, thus, could be investigated as a potential drug target for the treatment of PD [63].
The finding that mammalian target of rapamycin (mTOR) inhibits TPC2 has linked TPC2 to energy metabolism [64]. Further investigation of this link showed that Ca2+ release from lysosomes in pulmonary arterial myocytes is mediated by NAADP because cross-desensitisation was demonstrated between an mTOR inhibitor and NAADP [65]. Based on such findings, it has been suggested that the TPC channel detects nutrient status and remains open when nutrients are exhausted or when mTOR translocates to the lysosomal surface, where it can be activated and, therefore, is crucial in determining the sensitivity of endolysosomal resting membrane potential to Na+ and ATP, maintaining pH stability and amino acid homeostasis [61]. The findings also suggest NAADP as a potential therapeutic target for the treatment of idiopathic pulmonary hypertension due to its role in the mTOR signalling pathway [65].
For decades, soluble NSF attachment proteins receptors (SNAREs) have been recognised as the core machinery mediating membrane fusion [66]. As mentioned earlier, TPCs have been reported to be involved in the pathogenicity of EBOV by participating in the maturation and vesicle fusion of endosomes [39,40]. Related to this, a recent study demonstrated that TPC1 provides high local Ca2+ concentrations for SNARE-mediated vesicle fusion [67]. Using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) proteomic analysis, the same study revealed a direct interaction between TPC1 and syntaxins 7, 8, and 12 from the prominent group of SNARE proteins, which could explain how endosomal membrane fusion is triggered by local Ca2+ signals at the molecular level [67].
In addition, recent studies highlighted an interaction between TPCs and the Rab family, which regulates endolysosomal dynamics [62,68]. Notably, the interaction between TPC2 and Rabs, as well as their own activities, are essential in multiple cellular functions, including endolysosome proliferation, dysregulation of intracellular trafficking, and cellular pigmentation [62]. Intriguingly, in TPC2-null mice, low-density lipoprotein (LDL) or epidermal growth factor/epidermal growth factor receptor (EGF/EGFR) tended to accumulate in intracellular vesicles rather than being transported in the degradation pathway. This suggested that the deficiency of TPC2 debilitated the endolysosomal degradation pathway by disrupting vesicle trafficking and caused accumulation of cholesterol and cholesterol ester (CE), which eventually leads to nonalcoholic fatty liver disease (NAFLD) [68].
Pharmacological Modulators of TPCs
Given the roles of TPCs in various pathophysiological processes, including cell growth and development, metabolism, and cancer progression, an important goal will be to find effective pharmacological modulators to benefit both scientific research and drug discovery. This field of work is very preliminary, and only a few modulators are thought to exert their actions directly via TPCs. Here, we discuss these briefly and list them in Table 2 .
Table 2.
Summary of Existing Pharmacological Modulators that Act Directly or Indirectly on TPCs
| Name | Original use | Refs |
|---|---|---|
| Activators | ||
| Sphingosine | Endogenous bioactive lipid | [69] |
| Rapamycin | mTOR inhibitor | [65] |
| Torin-2 | ||
| Amitriptyline | Tricyclic antidepressant |
[71] |
| Clomipramine | ||
| Desipramine | ||
| Imipramine | ||
| Nortriptyline | ||
| Chlorpromazine | Tricyclic antipsychotic | |
| Triflupromazine | ||
| Riluzole | Glutamate receptor antagonist | |
| Inhibitors | ||
| Naringenin | Plant flavonoid | [77] |
| Diltiazem | L-type Ca2+ channel blocker | [39] |
| Nimodipine | ||
| Tetrandrine | ||
| Verapamil | ||
| Bepridil | [90] | |
| Ned-19 | N/A | [80] |
| Fluphenazine | Dopamine receptor antagonist | [90] |
| Pimozide | ||
| Prochlorperazine | ||
| Thioridazine | ||
| Trifluoperazine | ||
| Bazedoxifene | Selective estrogen receptor modulator | |
| Clomiphene | ||
| Raloxifene | ||
| Tamoxifen | ||
| Toremifene | ||
| Amodiaquine | Antimalaria reagent | |
| Piperacetazine | Antipsychotic prodrug | |
| Simvastatin | HMG-CoA reductase inhibitor | |
| TPC2-A1-N | N/A | [91] |
| TPC2-A1-P | ||
Sphingosine, Rapamycin, and Torin-2
Several endogenous molecules can modulate the action of TPCs per se, including sphingosine and rapamycin. Sphingosine stimulates the release of Ca2+ from intracellular organelles. It can evoke endolysosomal Ca2+ release via TPC1 and TPC2, which is also linked with Ca2+-derived autophagy [69]. Rapamycin is an mTOR inhibitor, by which it can indirectly induce endolysosomal Ca2+ release from TPC2 as an alternative route [65,70]. Similarly, torin-2, a synthesised mTOR inhibitor, evokes Ca2+ release from TPC2 in the same way as rapamycin [65].
Tricyclics
Electrophysiology-based high-throughput screening used to identify TPC agonists identified seven small molecules and classified them based on their structure as lysosomal Na+ channel voltage-dependent activators 1 (LyNa-VA1.x) and 2 (LyNa-VA2.x). Amitriptyline clomipramine, desipramine, imipramine, and nortriptyline were the five LyNa-VA1.x, and were categorised as tricyclic antidepressants (TCAs). Chlorpromazine and triflupromazine were the two LyNa-VA2.x identified, and were also recognised as tricyclic antipsychotics [71]. All seven identified molecules could act as agonist voltage-dependent gating modulators for both TPC1 and TPC2 [71]. Conversely, the study observed no significant activation of TPCs with carbamazepine (an anticonvulsant) and phenothiazine (an antipsychotic), which share a similar chemical structure with TCAs (the tricyclic ring). This distinct function could be attributed to the chemical structures of these compounds lacking the aliphatic chain, which might be responsible for TPC activation [71]. In addition, the study showed that riluzole, a drug approved by the US FDA in 1995 for the treatment of amyotrophic lateral sclerosis (ALS) [72] and classified as a glutamate receptor blocker, can act as a specific voltage-independent TPC2 agonist [71,72].
Naringenin and Tetrandrine
Another useful drug discovery strategy is to repurpose existing modulators of structurally similar ion channels to TPCs, one example being naringenin, a natural flavonoid found in grapefruit [73]. Naringenin has been shown to activate Ca2+-activated K+ (BKCa) channels [74], and inhibit the activity of TRPM3 and TRPP2 channels [73,75]. In addition, there was also evidence showing that naringenin antagonised the ability of vascular endothelial growth factor (VEGF) to mediate angiogenesis [76], which was considered to be linked to a functional role for TPC2 in this process [61]. Extending such findings, a recent study additionally demonstrated the inhibitory effect of naringenin on TPCs. In this study, naringenin significantly suppressed the VEGF- and NAADP-evoked Ca2+ response in endothelial cells, and curbed the formation of new blood vessels when the endothelial cells were transplanted into mice. Electrophysiology analysis also showed that naringenin can reversibly block the current conduction of both TPC1 and TPC2 [77]. A subsequent molecular docking study further predicted that naringenin can directly bind to TPC2, whereby the hydrophobic residues at the TPC2 pore region are involved in the binding, forming a physical barrier that impedes the passage of cations [78].
A similar approach used to search for TPC inhibitors to treat EBOV also yielded more TPC modulators. A selection of L-type Ca2+ channel blockers, including diltiazem, nimodipine, tetrandrine, and verapamil, block EBOV capsid release in macrophages; among these pharmacological agents, the plant alkaloid tetrandrine exhibited the highest potency (IC50 = 55 nM) [39]. Originating from a Chinese herbal remedy, tetrandrine also improved the survival rate of mice after EBOV injection [39]. A patch-clamp assay further demonstrated that tetrandrine could debilitate PI(3,5)P2-induced currents in endolysosomal vesicles mediated via both TPC1 and TPC2 [39]. However, so far, there is no evidence for a direct interaction between tetrandrine and TPCs. In addition to its effectiveness against EBOV, tetrandrine can also suppress the metastatic ability of murine cancer cells both in vitro and in vivo [79].
Ned-19
Ned-19, a selective membrane-permeant noncompetitive NAADP antagonist, was first identified by ligand-based virtual screening (LBVS) targeted against NAADP [80]. Ned-19 shows high similarity to NAADP in terms of its overall 3D architecture as well as in its electrostatic properties [80]. Recent research showed that the pharmacophore domain of Ned-19 is spread between the second VSD and the pore region on AtTPC1 [12]. However, increasing evidence has suggested that an accessory protein is required for NAADP-TPC binding [8,15,49., 50., 51., 52., 53., 54.], and it also appears that more than one binding site exists on this accessory protein for NAADP [81]. Therefore, Ned-19, a close analogue of NAADP, is likely to exert its inhibitory effect on TPCs in an indirect and complex manner, similar to NAADP itself. Nonetheless, the use of Ned-19 has, to a large extent, broadened our understanding of the roles of NAADP/TPCs in biological functions and diseases, especially over the past 5 years, in areas that include: cancer progression and metastasis [79,82], VEGF-induced angiogenesis [61], EBOV infection [39], proliferation and activation of T cells [83], autophagy of hepatocytes during liver injury [84], activation of endothelial progenitor cells [85], the noradrenaline-induced Ca2+ response in smooth muscle cells [86], and the growth of Plasmodium, the causative agent of malaria [87].
Modulators from Structure-based Virtual Screening
The recent availability of TPC1 and TPC2 3D structures [14,15] has enabled the use of structure-based virtual screening (SBVS) to search for effective TPC pharmacological modulators. Virtual screening was performed on a predicted mouse TPC2 structure that was generated based on the homology modelling of mouse TPC1 architecture [14], using a library containing ~1500 FDA-approved drugs [88]. By comparing the result with two previous high-throughput screenings of potential EBOV entry inhibitors [88,89], 14 drugs were shortlisted (Table 2), Interestingly, five of these (fluphenazine, pimozide, prochlorperazine, thioridazine, and trifluoperazine) were originally classified as dopamine receptor antagonists, while another five (bazedoxifene, clomiphene, raloxifene, tamoxifen, and toremifene) were selective estrogen modulators [88]. The remaining four drugs (amodiaquine, bepridil, piperacetazine ,and simvastatin) have various targets (Table 2). Subsequently, six of these chemicals (bepridil, clomiphene, fluphenazine, pimozide, raloxifene, and tamoxifen) were further tested, showing that they could selectively block NAADP-elicited Ca2+ responses in sea urchin egg homogenates, and delay TPC2 opening by decreasing both the average open time and the percentage of opened channels [90]. These chemicals also exhibited an inhibitory effect on the entry of EBOV-like particles into the cytoplasm, in a typical dose-response manner [90].
Novel Agonists of TPC2
A more recent study screened a chemical library by monitoring the Ca2+ response from TPC2, and identified two novel agonists of TPC2: TPC2-A1-N and TPC2-A1-P [91]. TPC2-A1-N was shown to evoke significant Ca2+ responses from TPC2, and this effect could be blocked by several of the aforementioned TPC blockers, including tetrandrine, raloxifene, and fluphenazine [39,90,91]. By contrast, the other agonist, TPC2-A1-P, only elicited a smaller and delayed Ca2+ response from TPC2 [91]. Interestingly, however, compared with TPC2-A1-N, TPC2-A1-P showed higher potency to induce Na+ mobilisation from TPC2 [91]. The binding sites on TPC2 of these two molecules were also shown to be different [91]. Neither a Ca2+ nor a Na+ response was observed when using TPC2-A1-N or TPC2-A1-P to treat TPC1 or TRPML [91], indicating that these two molecules have high selectivity to TPC2, which make them promising molecules for use in future scientific and clinical studies.
Concluding Remarks and Future Perspectives
In this review, we have outlined the pivotal role of TPCs in regulating intracellular Ca2+ signalling, by not only releasing Ca2+ from endolysosomes, but also triggering CICR from the ER/SR system. However, the mechanism of TPC activation remains unclear, since, while both NAADP and PI(3,5)P2 have been considered to be endogenous activators, the action of NAADP appears to be indirect. Evidence has shown that a NAADP-binding protein(s) may exist to facilitate this process. Further characterisation of the TPC interactome is necessary to identify the NAADP-binding protein. Genetic manipulation of interactome proteins would also be helpful to explore their roles in regulating TPC functions (see Outstanding Questions).
The past decade has witnessed the emerging importance of the NAADP/TPC signalling pathway as a mediator of a variety of pathophysiological processes, including in cell growth and development, pancreatic functions, and viral infections. Given this, it would be of interest to investigate whether this signalling pathway is also involved in infection by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); indeed, a recent study showed that TPC2 has a critical role in the mechanism of endocytosis by which SARS-CoV-2 gains entry to cells [92].
The roles of TPC1 and TPC2 may also differ, given that some of the TPC-associated pathophysiological functions are only linked to one specific isoform (see ‘Trends in Functional Studies of TPCs’). Advances in molecular biology have enabled cell type-specific conditional TPC1 and TPC2 KO cells and animals, allowing the study of the distinctive roles of TPC1 and TPC2. In addition, a recent report revealed that two variants of point mutations on human TPC2 are linked with alteration of hair pigmentation [93]. Therefore, our understanding of TPCs would also be broadened by studying data from national and international biobanks and investigating clinical symptoms or tissue samples of individuals identified with naturally occurring mutations on human TPC1 and TPC2 (see Outstanding Questions).
So far, most studies have used either gene knockdown/KO methods or pharmacological approaches to disrupt the functions of TPCs (see ‘Trends in Functional Studies of TPCs’). However, there are also drawbacks to these approaches: gene manipulation is suitable for studying animal and human cells in culture, and for the creation of animal models for laboratory research, but is less translational for the generation of new therapeutics. Ned-19, an analogue of NAADP, is not likely to act directly on TPCs; thus, one should always question the involvement of TPCs when using Ned-19. Several molecules have been identified as TPC activators or inhibitors (Table 2). These new pharmacological modulators of TPCs are making it possible to address important mechanistic questions about TPC action; for instance, a study that identified novel TPC2 agonists found that one of these (TPC2-A1-N) triggered a strong Ca2+ response and nonselective cation currents, while the other (TPC2-A1-P) induced Na+-selective currents but fewer Ca2+ signals [91]. These two properties are mirrored by NAADP and PI(3,5)P2, respectively. Additionally, these different agonist activities were mediated differentially by single-residue mutations on TPC2, and were associated with opposing changes in endolysosomal pH and exocytosis [91].
Nevertheless, this area has been poorly explored thus far and the majority of existing pharmacological modulators of TPCs lack target specificity (Table 2). Therefore, it is necessary to find novel pharmacological modulators of TPCs. The recent identification of the 3D structures of both mammalian TPC1 and TPC2 [14,15] as well as the search for NAADP-binding protein(s) (via interactome analysis) will undoubtedly promote the discovery of new TPC pharmacological modulators that may bind to the molecular moiety with high affinity and selectivity, and virtual screening could be a useful tool in this process. Furthermore, PI(3,5)P2 can also be utilised for LBVS to identify analogues with similar biochemical properties that will fit into its binding site in TPCs. Ultimately, the development of effective and selective pharmacological modulators of TPCs will be key to understanding and treating a range of endolysosomal-related diseases that are TPC regulated.
Outstanding Questions.
How does NAADP interact with TPCs and does this involve an NAADP-binding protein(s)?
How is the TPC interactome linked to TPC mechanisms of action and pathophysiological roles?
How do TPC1 and TPC2 differ in their respective pathophysiological roles and how is this linked to their distinctive endolysosomal locations and mechanisms of action?
How do naturally occurring mutations in TPC1 and TPC2 in humans affect susceptibility to particular diseases and human characteristics?
Given the recent studies on TPC structure and interactome, how can these help develop effective and selective pharmacological modulators for TPCs?
Alt-text: Outstanding Questions
Glossary
- Acrosome reaction
during fertilisation, when a sperm approaches an egg or secondary oocyte, it releases proteolytic enzymes to hydrolyse zona pellucida, a glycoprotein layer surrounding the plasma membrane of the egg or oocyte. This enables the sperm to penetrate the zona pellucida and to contact with the egg or oocyte. The proteolytic enzymes are stored in the acrosome, a membrane-bound organelle in the head of the sperm. Upon acrosome reaction, the acrosome fuses with the plasma membrane of the sperm to release the enzymes.
- Arrythmia
disorder in which the heartbeat is irregular, too slow, or too fast.
- Autophagy
physiological process that captures, degrades, and recycles proteins and organelles in lysosomes to maintain metabolism and cellular homeostasis.
- Cardiac hypertrophy
abnormal thickening of cardiac muscle, which alters the tension of ventricular wall. This can sometimes lead to heart failure.
- Channel pore region
substructure of ion channels through which ions can pass.
- EF hand motif
a helix-loop-helix structural domain found in biomacromolecules.
- Endocrine pancreas
also known as pancreatic islets, endocrine pancreas are regions on the pancreas that secrete hormones (including glucagon, insulin, somatostatin, and ghrelin) to circulation. By contrast, the exocrine pancreas releases pancreatic juice into the duodenum.
- Endolysosomes
organelle system that includes lysosomes; early, late, and recycling endosomes; and autophagosomes. It is also a storage source of calcium ions.
- Ligand-based virtual screening (LBVS)
virtual screening process that searches through chemical libraries for compounds that are structurally similar to a known active ligand. This can be useful when the 3D structure of the macromolecule is not available. The chemical libraries used are usually large in size (thousands to millions), allowing drug discovery on a wider scale and encouraging drug repurposing from one disease to another.
- Long terminal repeat (LTR)
repeated sequence found at the terminal of RNA of retroviruses. The LTR region can serve as a promoter after reverse transcription of viral RNA to DNA in host cells.
- Osteoclastogenesis
formation of new osteoclasts, a cell type that can dissolve and absorb bone tissue for bone maintenance, repairing, and remodelling.
- Pharmacophore
model of the steric and electrostatic features of a drug or ligand that are required to interact with a specific biomacromolecule target.
- Proprotein convertase
group of enzymes that modify and activate newly synthesised proteins from inactive forms.
- Structure-based virtual screening (SBVS)
virtual screening that uses computational power to dock chemical compounds from an established library onto macromolecules (e.g., TPCs) based on intermolecular interactions, and ranks these chemicals based on binding affinity.
- Syntaxin
member of the SNARE protein that is sufficient to drive the fusion of SNARE-containing vesicles with the plasma membrane.
- Tat protein
key transcription activator in HIV that significantly enhances the efficiency of transcription.
- Zymogen
inactive precursor of an enzyme.
References
- 1.Parrington J., Tunn R. Ca 2+ signals, NAADP and two-pore channels: role in cellular differentiation. Acta Physiol. 2014;211:285–296. doi: 10.1111/apha.12298. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J., et al. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Lee H.C., Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 4.Calcraft P.J., et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galione A. NAADP receptors. Cold Spring Harb. Perspect. Biol. 2019;11:a035071. doi: 10.1101/cshperspect.a035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis L.C., et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr. Biol. 2012;22:2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha A., et al. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruas M., et al. TPC1 has two variant isoforms, and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol. Cell. Biol. 2014;34:3981–3992. doi: 10.1128/MCB.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman T., et al. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014;7 doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J., et al. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kintzer A.F., Stroud R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:258–262. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S., et al. Two-pore channels enter the atomic era: structure of plant TPC revealed. Trends Biochem. Sci. 2016;41:475–477. doi: 10.1016/j.tibs.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.She J., et al. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556:130–134. doi: 10.1038/nature26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She J., et al. Structural mechanisms of phospholipid activation of the human TPC2 channel. eLife. 2019;8 doi: 10.7554/eLife.45222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arndt L., et al. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Mol. Biol. Cell. 2014;25:948–964. doi: 10.1091/mbc.E13-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos I., et al. Two-pore channels function in calcium regulation in sea star oocytes and embryos. Development. 2014;141:4598–4609. doi: 10.1242/dev.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notomi T., et al. Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J. Biol. Chem. 2017;292:20998–21010. doi: 10.1074/jbc.M117.780072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z.H., et al. Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0066077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aley P.K., et al. Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels. Proc. Natl. Acad. Sci. 2010;107:19927–19932. doi: 10.1073/pnas.1007381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelu J.J., et al. Characterization of ADP-ribosyl cyclase 1-like (ARC1-like) activity and NAADP signaling during slow muscle cell development in zebrafish embryos. Dev. Biol. 2019;445:211–225. doi: 10.1016/j.ydbio.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira G.J., et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondratskyi A., et al. Calcium-permeable ion channels in control of autophagy and cancer. Front. Physiol. 2013;4:272. doi: 10.3389/fphys.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun W., Yue J. TPC2 mediates autophagy progression and extracellular vesicle secretion in cancer cells. Exp. Cell Res. 2018;370:478–489. doi: 10.1016/j.yexcr.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Bootman M.D., et al. The regulation of autophagy by calcium signals: do we have a consensus? Cell Calcium. 2018;70:32–46. doi: 10.1016/j.ceca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 26.García-Rúa V., et al. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016;594:3061–3077. doi: 10.1113/JP271332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb S.E., et al. Role of two-pore channels in embryonic development and cellular differentiation. Cold Spring Harb. Perspect. Biol. 2020;12:a035170. doi: 10.1101/cshperspect.a035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alharbi A.F., Parrington J. Endolysosomal Ca2+ signaling in cancer: the role of TPC2, from tumorigenesis to metastasis. Front. Cell Dev. Biol. 2019;7:302. doi: 10.3389/fcell.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton A., et al. Adrenaline stimulates glucagon secretion by Tpc2-dependent Ca2+ mobilization from acidic stores in pancreatic α-cells. Diabetes. 2018;67:1128–1139. doi: 10.2337/db17-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butcher R.W., et al. The role of cyclic AMP in hormone actions. Adv. Enzym. Regul. 1968;6:357–389. doi: 10.1016/0065-2571(68)90023-x. [DOI] [PubMed] [Google Scholar]
- 31.Arredouani A., et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) and endolysosomal two-pore channels modulate membrane excitability and stimulus-secretion coupling in mouse pancreatic β cells. J. Biol. Chem. 2015;290:21376–21392. doi: 10.1074/jbc.M115.671248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutter G.A., et al. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015;466:203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 33.Cane M.C., et al. The two pore channel TPC2 is dispensable in pancreatic β-cells for normal Ca2+ dynamics and insulin secretion. Cell Calcium. 2016;59:32–40. doi: 10.1016/j.ceca.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancela J.M., et al. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki M., et al. Organelle selection determines agonist-specific Ca 2+ signals in pancreatic acinar and β cells. J. Biol. Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 36.Gerasimenko J.V., et al. Both RyRs and TPCs are required for NAADP-induced intracellular Ca2+ release. Cell Calcium. 2015;58:237–245. doi: 10.1016/j.ceca.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baize Emergence of Zaire Ebola virus disease in Guinea. N. Engl. J. Med. 2014;371:1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 38.Carette J.E., et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai Y., et al. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruas M., et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr. Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons J.A., et al. Ebolavirus glycoprotein directs fusion through NPC1 + endolysosomes. J. Virol. 2016;90:605–610. doi: 10.1128/JVI.01828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunaratne G.S., et al. NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium. 2018;75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan N., et al. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J. 2020;34:4147–4162. doi: 10.1096/fj.201902534R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson S.M., et al. Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc. Res. 2015;108:357–366. doi: 10.1093/cvr/cvv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gul R., et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-ribose (cADPR) mediate Ca2+ signaling in cardiac hypertrophy induced by β-adrenergic stimulation. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capel R.A., et al. Two-pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic β-adrenoceptor signaling in the heart. J. Biol. Chem. 2015;290:30087–30098. doi: 10.1074/jbc.M115.684076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feijóo-Bandín S., et al. Two-pore channels (TPCs): Novel voltage-gated ion channels with pleiotropic functions. Channels. 2017;11:20–33. doi: 10.1080/19336950.2016.1213929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitt S.J., et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J. Biol. Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruas M., et al. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin-Moshier Y., et al. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walseth T.F., et al. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J. Biol. Chem. 2012;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walseth T.F., et al. Nicotinic acid adenine dinucleotide 2-phosphate (NAADP) binding proteins in T-lymphocytes. Messenger. 2012;1:86–94. doi: 10.1166/msr.2012.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krogsaeter E.K., et al. The protein interaction networks of mucolipins and two-pore channels. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:1111–1123. doi: 10.1016/j.bbamcr.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitt S.J., et al. Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+ Sci. Signal. 2014;7 doi: 10.1126/scisignal.2004854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brailoiu E., et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel S., et al. NAADP-evoked Ca2+ signals through two-pore channel-1 require arginine residues in the first S4-S5 linker. Cell Calcium. 2017;68:1–4. doi: 10.1016/j.ceca.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asfaha T.Y., et al. The synthesis and characterization of a clickable-photoactive NAADP analog active in human cells. Cell Calcium. 2019;83:102060. doi: 10.1016/j.ceca.2019.102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong X.P., et al. PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson G.L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 61.Favia A., et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc. Natl. Acad. Sci. 2014;111:E4706–E4715. doi: 10.1073/pnas.1406029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin-Moshier Y., et al. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gómez-Suaga P., et al. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum. Mol. Genet. 2012;21:511–525. doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cang C., et al. mTOR regulates lysosomal ATP-sensitive two-pore Na+ channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogunbayo O.A., et al. mTORC1 controls lysosomal Ca 2+ release through the two-pore channel TPC2. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aao5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Söllner T., et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 67.Castonguay J., et al. The two-pore channel TPC1 is required for efficient protein processing through early and recycling endosomes. Sci. Rep. 2017;7:10038. doi: 10.1038/s41598-017-10607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grimm C., et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- 69.Höglinger D., et al. Intracellular sphingosine releases calcium from lysosomes. Elife. 2015;4 doi: 10.7554/eLife.10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang F.S., et al. A two-pore channel protein required for regulating mTORC1 activity on starvation. BMC Biol. 2020;18:1–16. doi: 10.1186/s12915-019-0735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X., et al. Agonist-specific voltage-dependent gating of lysosomal two-pore Na+ channels. eLife. 2019;8 doi: 10.7554/eLife.51423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dharmadasa T., Kiernan M.C. Riluzole, disease stage and survival in ALS. Lancet Neurol. 2018;17:385–386. doi: 10.1016/S1474-4422(18)30091-7. [DOI] [PubMed] [Google Scholar]
- 73.Straub I., et al. Citrus fruit and Fabacea secondary metabolites potently and selectively block TRPM3. Br. J. Pharmacol. 2013;168:1835–1850. doi: 10.1111/bph.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saponara S., et al. (+/-)-Naringenin as large conductance Ca(2+)-activated K+ (BKCa) channel opener in vascular smooth muscle cells. Br. J. Pharmacol. 2006;149:1013–1021. doi: 10.1038/sj.bjp.0706951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waheed A., et al. Naringenin inhibits the growth of Dictyostelium and MDCK-derived cysts in a TRPP2 (polycystin-2)-dependent manner. Br. J. Pharmacol. 2014;171:2659–2670. doi: 10.1111/bph.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Q., et al. Naringenin exerts anti-angiogenic effects in human endothelial cells: Involvement of ERRα/VEGF/KDR signaling pathway. Fitoterapia. 2016;111:78–86. doi: 10.1016/j.fitote.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 77.Pafumi I., et al. Naringenin impairs two-pore channel 2 activity and inhibits VEGF-induced angiogenesis. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-04974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benkerrou D., et al. A perspective on the modulation of plant and animal two pore channels (TPCs) by the flavonoid naringenin. Biophys. Chem. 2019;254:106246. doi: 10.1016/j.bpc.2019.106246. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen O.N.P., et al. Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res. 2017;77:1427–1438. doi: 10.1158/0008-5472.CAN-16-0852. [DOI] [PubMed] [Google Scholar]
- 80.Naylor E., et al. Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosen D., et al. Analogues of the nicotinic acid adenine dinucleotide phosphate (NAADP) antagonist Ned-19 indicate two binding sites on the NAADP receptor. J. Biol. Chem. 2009;284:34930–34934. doi: 10.1074/jbc.M109.016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Favia A., et al. NAADP-dependent Ca(2+) signaling controls melanoma progression, metastatic dissemination and neoangiogenesis. Sci. Rep. 2016;6 doi: 10.1038/srep18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali R.A., et al. Nicotinic acid adenine dinucleotide phosphate plays a critical role in naive and effector murine T cells but not natural regulatory T cells. J. Biol. Chem. 2016;291:4503–4522. doi: 10.1074/jbc.M115.681833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rah S.-Y., et al. NAADP-mediated Ca(2+) signaling promotes autophagy and protects against LPS-induced liver injury. FASEB J. 2017;31:3126–3137. doi: 10.1096/fj.201601290R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Nezza F., et al. Liposomes as a putative tool to investigate NAADP signaling in vasculogenesis. J. Cell. Biochem. 2017;118:3722–3729. doi: 10.1002/jcb.26019. [DOI] [PubMed] [Google Scholar]
- 86.Trufanov S.K., et al. The role of two-pore channels in norepinephrine-induced [Ca(2+)](i) rise in rat aortic smooth muscle cells and aorta contraction. Cells. 2019;8:1144. doi: 10.3390/cells8101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suárez-Cortés P., et al. Ned-19 inhibition of parasite growth and multiplication suggests a role for NAADP mediated signalling in the asexual development of Plasmodium falciparum. Malar. J. 2017;16 doi: 10.1186/s12936-017-2013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johansen L.M., et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 89.Kouznetsova J., et al. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microbes Infect. 2014;3 doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Penny C.J., et al. Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:1151–1161. doi: 10.1016/j.bbamcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerndt S., et al. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. Elife. 2020;9 doi: 10.7554/eLife.54712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ou X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chao Y.K., et al. TPC2 polymorphisms associated with a hair pigmentation phenotype in humans result in gain of channel function by independent mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E8595–E8602. doi: 10.1073/pnas.1705739114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo J., et al. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1009–1014. doi: 10.1073/pnas.1616191114. [DOI] [PMC free article] [PubMed] [Google Scholar]