Abstract
Kidney transplant recipients who develop symptoms consistent with coronavirus disease 2019 (COVID-19) are bringing unique challenges to health care professionals. Telemedicine has surged dramatically since the pandemic in effort to maintain patient care and reduce the risk of COVID-19 exposure to patients, health care workers, and the public. Herein we present reports of 3 kidney transplant recipients with COVID-19 who were managed using telemedicine via synchronous video visits integrated with an electronic medical record system, from home to inpatient settings. We demonstrate how telemedicine helped assess, diagnose, triage, and treat patients with COVID-19 while avoiding a visit to an emergency department or outpatient clinic. While there is limited information about the duration of viral shedding for immunosuppressed patients, our findings underscore the importance of using telemedicine in the follow-up care for kidney transplant recipients with COVID-19 who have recovered from symptoms but might have persistently positive nucleic acid tests. Our experience emphasizes the opportunities of telemedicine in the management of kidney transplant recipients with COVID-19 and in the maintenance of uninterrupted follow-up care for such immunosuppressed patients with prolonged viral shedding. Telemedicine may help increase access to care for kidney transplant recipients during and beyond the pandemic as it offers a prompt, safe, and convenient platform in the delivery of care for these patients. Yet, to advance the practice of telemedicine in the field of kidney transplantation, barriers to increasing the widespread implementation of telemedicine should be removed, and research studies are needed to assess the effectiveness of telemedicine in the care of kidney transplant recipients.
Highlights
-
•
Telemedicine helped diagnose, triage, and manage transplant patients with coronavirus disease 2019 (COVID-19).
-
•
We proposed a practical workflow process for COVID-19 test and surveillance.
-
•
Telemedicine assisted patient care from home to inpatient settings, while avoiding emergency department or clinic visits.
-
•
Telemedicine facilitated care delivery for kidney transplant recipients with COVID-19.
-
•
Telemedicine provided uninterrupted follow-up care for immunosuppressed patients with prolonged COVID-19 shedding.
Kidney transplant recipients who develop symptoms consistent with coronavirus disease 2019 (COVID-19) are bringing unique challenges to health care professionals [1,2]. While the potential for both community and nosocomial spread of COVID-19 when such patients seek care is a major concern, proper diagnosis and management of these patients must be accommodated [[3], [4], [5], [6], [7]]. Telemedicine has emerged dramatically during the COVID-19 pandemic and allowed clinicians to offer the standard of care to their patients with COVID-19, but with heightened public health consciousness.
Telemedicine offers an immediate virtual patient-provider visit without increasing the risk of COVID-19 exposure to patients, health care workers, and the public. Telemedicine also helps to preserve the supply of personal protective equipment (PPE) by limiting the number of times providers need to don PPE to clinically evaluate patients [8]. Regulatory and financial burdens to the spread of telemedicine have been lifted temporarily in light of the COVID-19 pandemic [[9], [10], [11]]. In a national survey of US transplant centers, 98.4% limited in-person outpatient visits for solid organ transplant recipients, and 96.8% implemented telemedicine in response to the pandemic [12]. These adopted telemedicine initiatives leverage existing, underutilized telecommunications technology and nationally mandated electronic medical records [13].
Herein we present 3 kidney transplant recipients with COVID-19 who were managed using telemedicine via synchronous video visits integrated with an electronic medical records system, from home to inpatient settings.
Case Presentations
Patient 1
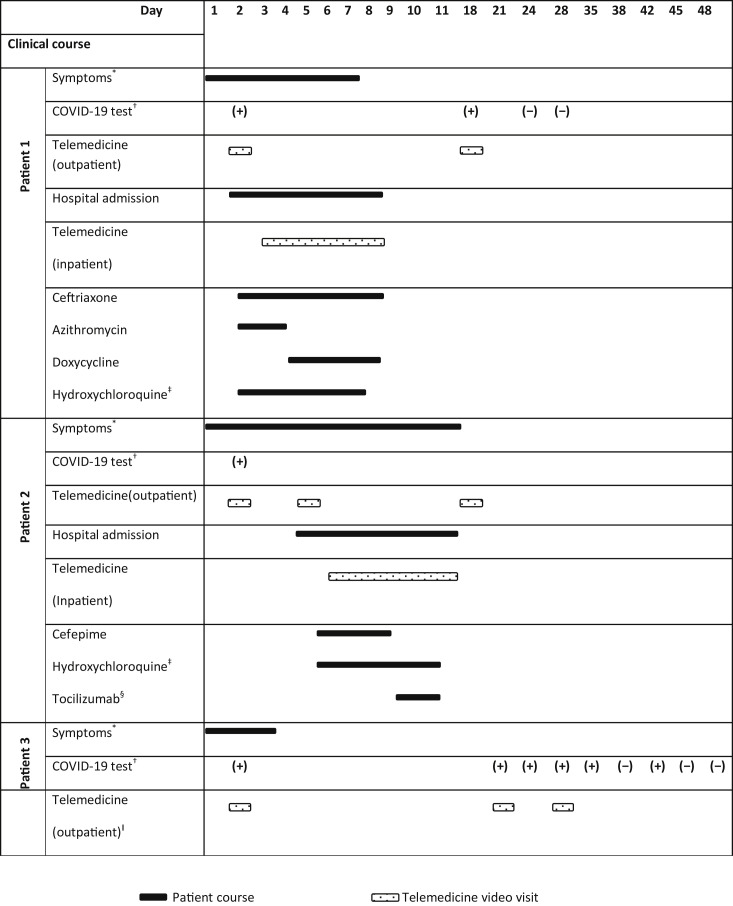
A 53-year-old man with a history of end-stage renal disease (ESRD) secondary to hypertensive nephrosclerosis underwent a living related kidney transplant in January 2012. He had induction therapy with rabbit antithymocyte globulin and was maintained on an immunosuppression regimen of prednisone 2.5 mg once daily and tacrolimus dosed to achieve target trough level of 4 to 6 ng/mL. He was off mycophenolate mofetil (MMF) owing to a history of BK viremia and skin cancer. He had stable allograft function with a baseline creatinine of 1.3 mg/dL. He called the transplant team to report dry cough, low-grade fever, chills, nausea and vomiting, watery diarrhea, and loss of the sense of smell. COVID-19 testing was performed in a designated drive-through area at our center on the same day (naso-oropharyngeal swab, nucleic acid test [NAT] was used to detect COVID-19 RNA by polymerase chain reaction). He was asked to self-quarantine while awaiting his test results, according to the Centers for Disease Control and Prevention guidelines [14]. On the following day, the COVID-19 test result was positive, and an immediate telemedicine video visit was conducted by a transplant nephrologist. During this virtual visit, the patient was found to have worsening shortness of breath and increased work of breathing. His home vital signs were notable for blood pressure of 130/82 mm Hg, heart rate of 88 beats per minute, oxygen saturation of 94% on room air using a patient home kit. Given stable vital signs but increased work of breathing, the decision was made to arrange for direct hospital admission to a dedicated COVID-19 inpatient unit, avoiding the emergency department route to reduce risk of exposure to patients, health care workers, and the public. A multidisciplinary team approach was initiated, including transplant infectious disease and nephrology, hospital medicine, and admission office. The infection control office provided special instructions to the patient facilitating safe transfer for hospital admission. His laboratory findings during hospitalization are summarized in Table 1 . He was started on ceftriaxone 1 g intravenous (IV) daily and azithromycin 500 mg by mouth daily (to treat potential bacterial superinfection) and hydroxychloroquine 400 mg by mouth twice daily for 1 day, then 400 mg/d on days 2, 3, 4, and 5. His electrocardiogram (ECG) showed QTc interval of 0.43 seconds. On day 3 of admission, he developed hypoxia and required 3 L of oxygen via nasal cannula; other vital signs remained within normal limits. Chest computed tomography (CT) scan findings were consistent with features for COVID-19 infection. Azithromycin was replaced with doxycycline 100 mg by mouth twice daily for concern of azithromycin-induced diarrhea. His oxygen requirements gradually improved, and eventually he was weaned off supplemental oxygen. On day 6 of admission, his symptoms resolved, and he was discharged home in stable condition with instructions to self-quarantine. During his hospital stay, he was followed by the transplant nephrology consult team via telemedicine video visits to reduce exposure risk and preserve PPE. Similarly, after discharge he was followed via telemedicine video visits. His COVID-19–related hospital course and treatment are summarized in Fig 1 .
Table 1.
Laboratory Test Results During Hospitalization for Patients 1 and 2 (Patient 3 Was Not Hospitalized)
| Hospital day | Patient 1 |
Patient 2 |
Reference Range (Unit) | ||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 6 | Day 1 | Day 4 | Day 7 | ||
| White blood cells | 4.58 | 4.73 | 4.39 | 4.37 | 3.88 | 4.69 | 4.50-11.0 (K/cu mm) |
| Absolute lymphocyte count | 0.31 | 0.24 | 0.42 | 0.22 | 0.20 | 0.37 | 1.10-4.80 (K/cu mm) |
| Hemoglobin | 12.0 | 11.6 | 12.3 | 12.6 | 11.4 | 12.0 | 13.9-16.3 (g/dL) |
| Platelets | 151 | 188 | 300 | 278 | 311 | 400 | 150-350 (K/cu mm) |
| Serum sodium∗ | 122 | 127 | 138 | 140 | 138 | 143 | 135-145 (mmol/L) |
| Serum potassium | 4.8 | 4.7 | 4.9 | 4.7 | 4.6 | 5.4 | 3.5-5.2 (mmol/L) |
| Serum calcium | 8.4 | 8.5 | 9.0 | 8.7 | 8.4 | 8.9 | 8.4-10.5 (mg/dL) |
| Serum bicarbonate | 20 | 20 | 23 | 26 | 25 | 24 | 21-31 (mmol/L) |
| Serum creatinine | 1.6 | 1.4 | 1.3 | 0.9 | 0.8 | 0.9 | 0.6-1.3 (mg/dL) |
| Urea nitrogen (serum) | 19 | 15 | 18 | 14 | 13 | 16 | 7-22 (mg/dL) |
| Albumin | 3.8 | 3.6 | 3.6 | 4.4 | 3.5 | 3.9 | 3.5-5.3 (g/dL) |
| Aspartate aminotransferase | 66 | - | 78 | 22 | 19 | 22 | 0-37 (U/L) |
| Alanine aminotransferase | 46 | - | 113 | 15 | 14 | 17 | 0-40 (U/L) |
| Alkaline phosphatase | 72 | - | 81 | 75 | 58 | 63 | 30-120 (U/L) |
| C-reactive protein | 6.8 | - | 1.9 | 6.1 | 6.2 | 0.7 | <0.5 (mg/dL) |
| Tacrolimus trough level | 5.1 | 7.0 | 5.7 | 7.0 | 6.6 | 6.3 | 5.0-15 (ng/mL) |
| D-Dimer | 0.63 | - | 0.51 | 0.27 | 0.28 | 0.24 | 0.00-0.49 (mg/L) |
| Lactate dehydrogenase | 307 | - | 289 | 182 | - | - | 118-273 (U/L) |
| Serum ferritin | 548 | - | 603 | 1103 | 990 | 1268 | 30-400 (ng/mL) |
| Interleukin 6 | 43.77 | - | - | 9.9 | - | - | <5 (pg/mL) |
Patient 1 had mild acute kidney injury and acute hyponatremia on admission; his spot urine sodium was 22 mmol/L, and serum osmolarity was 258 mosm/kg, suggestive of volume depletion. Hence, his home lisinopril was stopped, and he received Ringer’s lactate intravenous fluid replacement carefully.
Fig 1.
Summary of the clinical course of the 3 kidney transplant patients with coronavirus disease 2019 (COVID-19) who were managed via telemedicine. ∗Patient 1 had low-grade fever, dry cough, chills, nausea and vomiting, watery diarrhea, and loss of the sense of smell. During hospitalization, patient developed shortness of breath and hypoxia. Patient 2 had dry cough, chest tightness, and rhinorrhea, and subsequently developed high-grade fever and diarrhea. During hospitalization, patient developed shortness of breath and hypoxia. Patient 3 had mild headache, rhinorrhea, and fatigue; symptoms were improving gradually without specific treatment. †Naso-oropharyngeal swab, nucleic acid test (NAT) was used to detect COVID-19 RNA by polymerase chain reaction (PCR): (+) COVID-19 RNA detected, (−) COVID-19 RNA not detected. ‡Hydroxychloroquine 400 mg twice daily for the first day, then 400 mg once daily for days 2, 3, 4, and 5. §Tocilizumab 4 mg/kg per dose once daily for a total of 2 doses. ǁPatient was also followed by a transplant coordinator via telephone calls twice weekly.
Patient 2
A 56-year-old woman with a history of ESRD secondary to focal segmental glomerulosclerosis underwent a living unrelated kidney transplant in June 2019. She had induction therapy with rabbit antithymocyte globulin and was maintained on an immunosuppression regimen of prednisone 5 mg/d, tacrolimus dosed to achieve a target trough level of 5 to 8 ng/mL, and MMF 500 mg twice daily. She had stable allograft function with a baseline creatinine of 0.9 mg/dL. She called the transplant team to report dry cough, rhinorrhea, and chest tightness. The patient was asked to stop MMF. COVID-19 testing was done as mentioned in patient 1. On the following day, her COVID-19 test result was positive, and an immediate telemedicine video visit was conducted by a transplant nephrologist. During this virtual visit, she was found to have mild symptoms, and home vital signs were noted to be normal. Therefore, she was asked to continue self-quarantine and closely monitor her symptoms. On day 5 of the presentation, she reported not feeling well, with fever of 100.7°F and diarrhea. It was decided to proceed with a direct inpatient admission, with a similar multidisciplinary approach as was mentioned in patient 1. On admission, her blood pressure was 109/63 mm Hg, temperature was elevated at 101°F, heart rate was 94 beats per minute, and oxygen saturation was 99% on room air. Her laboratory findings during hospitalization are summarized in Table 1. She was started on cefepime 1 g IV every 8 hours (to treat potential urinary tract infection) and hydroxychloroquine 400 mg by mouth twice daily for 1 day, followed by 400 mg/d on days 2, 3, 4, and 5. Her ECG showed the QTc interval was 0.407 seconds. On the fourth day of admission, she developed hypoxia and required 4 L of oxygen via nasal cannula; other vitals remained stable. A chest x-ray showed left-sided lower lobe patchy opacity. She was treated with IV tocilizumab 4 mg/kg per dose for 2 doses on days 4 and 5 of admission. Her oxygen requirements gradually improved, and she eventually was weaned off supplemental oxygen. On the seventh day of admission, her symptoms resolved, and she was discharged home in stable condition with instructions to self-quarantine. During her hospitalization, she was followed by the transplant nephrology consult team via telemedicine video visits, as mentioned in the first case. Similarly, after discharge, she was followed via telemedicine video visits. Her COVID-19–related hospital course and treatment are summarized in Fig 1.
Patient 3
A 53-year-old woman with a history of ESRD and liver disease secondary to polycystic kidneys underwent a deceased donor simultaneous liver-kidney transplant in March 2014 and required bilateral native nephrectomies. She had induction therapy with basiliximab and was maintained on an immunosuppression regimen of prednisone 5 mg/d and tacrolimus dosed to achieve target trough level of 4 to 6 ng/mL. She had a history of cytomegalovirus viremia and colitis after her transplant and was off MMF. She had stable allograft function with a baseline creatinine of 1.0 mg/dL. The patient had a family gathering with her extended family members, and more than 20 family members were present. Several family members had mild symptoms with loss of smell and taste, and her husband had a low-grade fever. She called the transplant team to report mild headache, rhinorrhea, and fatigue. COVID-19 testing was done, as mentioned in the first case. On the following day, her COVID-19 test resulted as positive, and an immediate telemedicine video visit was conducted by a transplant nephrologist. During this virtual visit, she was found to have mild headache and no other symptoms. She reported home vital signs, which were noted to be normal. Thus, she was asked to continue to self-quarantine and closely monitor her symptoms. Her symptoms were improving gradually without specific treatment. She was followed as an outpatient by a transplant nephrologist via telemedicine video visits and a transplant coordinator via telephone calls. Her COVID-19–related outpatient course is summarized in Fig 1.
Discussion
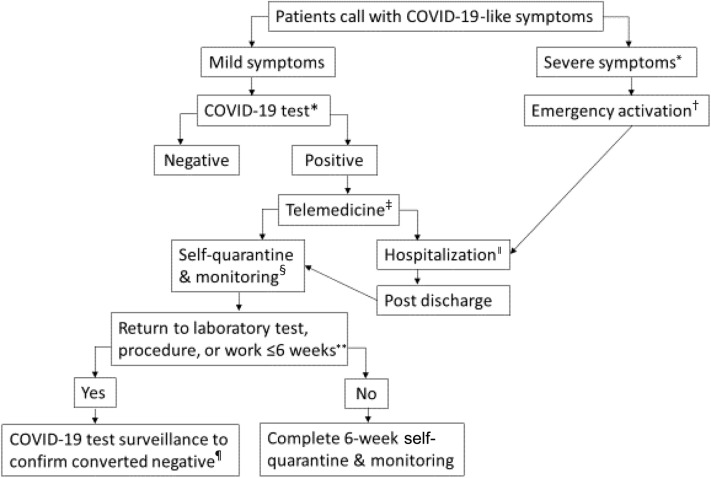
In these cases of kidney transplant recipients who presented with COVID-19 symptoms, telemedicine provided a safe and convenient approach to managing these patients in both home and inpatient settings. Telemedicine provided an immediate virtual patient-provider visit for clinical assessment, planning for COVID-19 testing, and safe management to reduce the risk of exposure. This approach allows for the rapid identification of those in need of hospitalization through an emergency department admission or an arranged direct admission, vs those who are able to remain home with self-quarantine and careful surveillance, as summarized in Fig 2 and Table 2 . Also, it facilitates uninterrupted follow-up care for patients during inpatient admission and outpatient monitoring. It is worth noting that the 3 patients expressed good experience and satisfaction.
Fig 2.
Practical workflow process for coronavirus disease 2019 (COVID-19) test and surveillance. ∗COVID-19 Test: Naso-oropharyngeal swab, nucleic acid test (NAT) to detect COVID-19 RNA by polymerase chain reaction (PCR), in a designated drive-through area. ★Severe symptoms: Severe shortness of breath, hypoxia, hypotension, acute chest pain, or confusion. †To call 911 for a transfer to hospital emergency department. ‡Telemedicine via a synchronous video visit for clinical assessment and management. ǁHospitalization through an emergency department admission or an arranged direct admission to COVID-19 inpatient unit. §To self-quarantine per Centers for Disease Control and Prevention (CDC) guidelines and to keep home log of vital signs twice daily in the first 2 weeks. Patients are instructed to seek immediate medical attention if symptoms worsening. To be followed-up by a transplant coordinator via telephone calls twice weekly and transplant nephrologist via telemedicine video visits close to two-week post COVID-19 diagnosis and thereafter as clinically determined. ∗∗Patient needs to return to laboratory test, procedure, or work that cannot be performed remotely and that will occur within 6 weeks of symptom onset. Decisions regarding the duration of self-quarantine are subject to change in consultation with infectious disease experts. ¶To repeat COVID-19 NAT at 2 weeks post–COVID-19 diagnosis twice weekly until it is converted negative on 2 consecutive occasions before returning to laboratory test, procedure, or work.
Table 2.
Document to Evaluate Severity and Progression of Coronavirus Disease 2019 During the Telemedicine Visits
| Time |
| Date of symptom onset |
| Date of positive COVID-19 test |
| Vital signs∗ |
| Temperature, blood pressure, pulse, and O2 saturation† |
| Symptoms |
| Fever (≥100.4°F [38°C]) |
| Cough |
| Dyspnea |
| Chest pain or pressure |
| Sore throat |
| Loss of smell and/or taste |
| Nausea/vomiting |
| Diarrhea |
| Abdominal pain |
| New headache |
| Rhinorrhea |
| Chills/rigors |
| Myalgias |
| Fatigue |
| Confusion |
Abbreviations: COVID-19, coronavirus disease 2019; O2, oxygen.
Vital signs reported by patient.
O2 saturation if patient has a home pulse oximetry device.
Our management of these patients is consistent with the emerging approach at other US transplant centers. Based on a recent national survey, nearly all in-person outpatient visits were suspended in response to the COIVD-19 crisis. Meanwhile, for those who would have been seen in an outpatient visit, telemedicine has emerged as the preferred option available to them [12]. This adoption of telemedicine on a national scale has been supported by the Centers for Medicare & Medicaid Services (CMS). Under the CMS 1135 Coronavirus waivers, Medicare will pay for office, hospital, and other visits furnished via telemedicine across the country [9]. Likewise, many states have issued licensure waiver with respect to telemedicine for out-of-state medical licensees [10].
Unlike immunocompetent patients, immunosuppressed patients may shed COVID-19 for longer periods of time, potentially increasing transmissibility [[15], [16], [17]]. This underscores the importance of telemedicine visits in reducing exposures from patients who have recovered from symptoms but might have persistently positive NATs. In our case reports, 2 patients who had follow-up COVID-19 tests remained positive at 18 and 21 days from onset of diagnosis. One converted to be negative at 24 and 28 days, whereas the other one was persistently positive by NAT at 24, 28, 35, and 42 days, although she had a negative test at 38 days, and eventually converted to be negative at 45 and 48 days. While there is limited information about the duration of viral shedding for immunosuppressed patients and it is not yet known whether protracted persistent NAT positivity reflects continued capacity for viral transmission, our case reports suggest longer periods of self-quarantine are appropriate for kidney transplant patients with COVID-19 [18,19]. For patients who need to return to laboratory test, procedure, or work that cannot be performed remotely and that will occur within 6 weeks of symptom onset, patients may undergo COVID-19 repeat test-based strategy as outpatients (Table 2) [20].
A key strength of telemedicine was the promptness and safety in the handling of these 3 patients with COVID-19. Patients called in with symptoms of COVID-19, an NAT rapidly confirmed that they were positive for COIVD-19, a telemedicine video visit was implemented, and a management plan was executed, all within 24 hours. Another important strength of telemedicine is the maintenance of continuity of care for transplant patients. Creating a systematic telemedicine schedule with the transplant clinician provides an expanded opportunity for patient questions, transmission of information, reassurance, and creating a sense that they are being cared for in a comprehensive way. This application of telemedicine should be generalizable to other solid organ transplant recipients. By extension, telemedicine may help increase access to live kidney donor transplant evaluation, especially for those who have financial challenges to come for an in-person visit or those who live a significant distance from a transplant center [[21], [22], [23]]. That said, we recognize the potential limitations of telemedicine. Access to a smart device or computer may not be available for every patient to connect via telemedicine video visits, although audio telephone visits are another option if video technology is not available [24]. Other barriers to telemedicine include out-of-state medical license requirements, reimbursement policies variations, and infrastructure support and personnel staffing need [25]. As such, cost-effectiveness and future reimbursements for telemedicine beyond the COVID-19 crisis remain poorly defined.
Conclusions
In these 3 reports of kidney transplant recipients, telemedicine helped assess, diagnose, triage, and treat patients with COVID-19 while avoiding an emergency department or outpatient clinic visit. Our experience emphasizes the opportunities of telemedicine in the safe management of kidney transplant patients with COVID-19 from home to inpatient settings and in the maintenance of uninterrupted follow-up care for such immunosuppressed patients with prolonged viral shedding. Telemedicine may help increase access to care for kidney transplant recipients during and beyond the pandemic. Yet, to increase the widespread implementation of telemedicine, existing regulatory and financial barriers should be removed permanently. Research studies are needed to advance the practice of telemedicine and assess its effectiveness in the care of kidney transplant recipients.
References
- 1.UNOS COVID-19 and solid organ transplant. https://unos.org/covid/ [accessed 12.04.20]
- 2.Michaels M.G., La Hoz R.M., Danziger-Isakov L., Blumberg E.A., Kumar D., Green M. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20:1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19): cases in U.S. Atlanta, GA: US Department of Health and Human Services. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [accessed 03.08.20]
- 4.Ning L., Liu L., Li W., Liu H., Wang J., Yao Z. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transplant. 2020;20:1864–1868. doi: 10.1111/ajt.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L., Xu X., Ma K., Yang J., Guan H., Chen S. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillen E., Pineiro G.J., Revuelta I., Rodriguez D., Bodro M., Moreno A. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandolfini I., Delsante M., Fiaccadori E., Zaza G., Manenti L., Delgi Antoni A. Delgi Antoni A, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20 doi: 10.1111/ajt.15891. 1941-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Shortage of personal protective equipment endangering health workers worldwide. https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide [accessed 03.08.20]
- 9.Centers for Medicare and Medicaid Services Medicare telemedicine health care provider fact sheet. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/1135-Waivers [accessed 03.08.20]
- 10.Federation of State Medical Boards States waiving telehealth licensure requirements. 2020. http://www.fsmb.org/advocacy/covid-19/ [accessed 03.08.20]
- 11.Center for Connected Health Policy.Telehealth coverage policies in the time of COVID-19. 2020. https://www.cchpca.org/resources/covid-19-telehealth-coverage-policies [accessed 03.08.20]
- 12.Boyarsky B.J., Chiang T.P.Y., Werbel W.A., Durand C.M., Avery R.K., Getsin S.N. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry J., Pylypchuk Y., Searcy T., Patel V. Adoption of electronic health record systems among US non-federal acute care hospitals: 2008-2015. 2016. https://www.healthit.gov/sites/default/files/briefs/2015_hospital_adoption_db_v17.pdf [accessed 4.15.20]
- 14.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19), if you are sick. 2020. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html [accessed 19.04.20]
- 15.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 16.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Characteristics of health care personnel with COVID-19 - United States, February 12–April 9, 2020. 2020. https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6915e6-H.pdf [accessed 15.04.20] [DOI] [PMC free article] [PubMed]
- 18.Centers for Disease Control and Prevention Ending home isolation for immunocompromised persons with COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ending-isolation.html [accessed 19.04.20]
- 19.Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection - challenges and implications [e-pub ahead of print]. N Engl J Med https://doi.org/10.1056/NEJMp2015897, accessed August 3, 2020. [DOI] [PubMed]
- 20.Centers for Medicare and Medicaid Services. SARS-CoV-2 testing strategy: considerations for non-healthcare workplaces. https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/testing-non-healthcare-workplaces.html [accessed 03.08.20].
- 21.Al Ammary F., Bowring M.G., Massie A.B., Yu S., Waldram M.W., Garonzik-Wang J. The changing landscape of live kidney donation in the United States from 2005 to 2017. Am J Transplant. 2019;19:2614–2621. doi: 10.1111/ajt.15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Ammary F., Thomas A.G., Massie A.B., Muzaale A.D., Shaffer A.A., Koons B. The landscape of international living kidney donation in the United States. Am J Transplant. 2019;19:2009–2019. doi: 10.1111/ajt.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Ammary F, Yu Y, Ferzola A, Motter JD, Massie AB, Yu S, et al. The first increase in live kidney donation in the United States in 15 years [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.16136, accessed August 3, 2020. [DOI] [PMC free article] [PubMed]
- 24.Kumar K., King E.A., Muzaale A.D., Konel J.M., Bramstedt K.A., Massie A.B. A smartphone app for increasing live organ donation. Am J Transplant. 2016;16:3548–3553. doi: 10.1111/ajt.13961. [DOI] [PubMed] [Google Scholar]
- 25.Concepcion B.P., Forbes R.C. The role of telemedicine in kidney transplantation: opportunities and challenges. Kidney360. 2020;1:420–423. doi: 10.34067/KID.0000332020. [DOI] [PMC free article] [PubMed] [Google Scholar]