Graphical Abstract
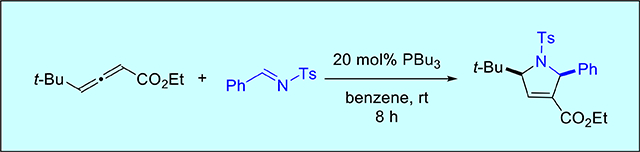
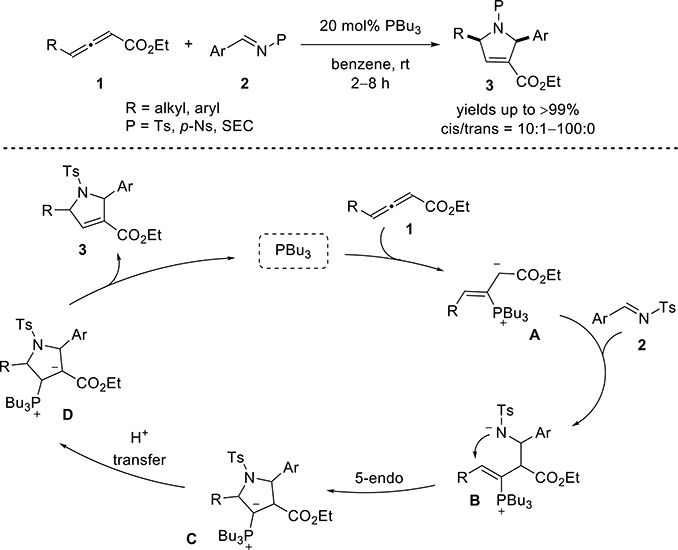
Nitrogen heterocycles are core structural units of many natural products and drugs.2 Among them, pyrrolines are particularly widespread in natural products and bioactive molecules.3 Since Lu’s pioneering discoveries, the phosphine-catalyzed [3 + 2] annulation of an imine and an aliéné is now one of the most attractive methods for the construction of a pyrroline.4 Our group5 and Shi’s6 have independently developed the phosphine-catalyzed allene-imine [3 + 2] annulation between γ-substituted allenoates with N-sulfonyl imines, broadening the scope of accessible pyrrolidine structures and, thereby, allowing preparation of several natural products and molecules of biological importance (vide infra) (Scheme 1).
Scheme 1.
Kwon’s synthesis of tetrasubstituted pyrrolines
Scheme 1 presents a potential mechanism for allene-imine [3 + 2] annulation. The zwitterionic intermediate A, generated from addition of tri-n-butylphosphine to the allenoate 1, adds to the imine 2 to form the phosphonium amide B. Intramolecular addition of the amide anion to the vinylphosphonium brings about the ylide C. Subsequent proton transfer results in the final intermediate D, which dissociates to the 3-pyrroline 3 and tri-n-butylphosphine. In recent years, many new phosphine-catalyzed [3 + 2] imine-allene annulations, and their synthetic applications, have been reported. These advances are the focus of this Addendum.
In 2005, Kwon and co-workers found that, in the presence of nucleophilic tri-n-butylphosphine, a variety of γ-substituted allenoates and N-sulfonylimines undergo [3 + 2] annulations to afford functionalized pyrrolines in excellent yields and with high diastereoselectivities (Scheme 1).5 Similar results were disclosed contemporarily by Shi and Zhao who used dimethylphenylphosphine as a catalyst for the [3 + 2] annulation of ethyl penta-2,3-dienoate (Scheme 2).6
Scheme 2.
Shi’s formation of tetrasubstituted pyrroline derivatives
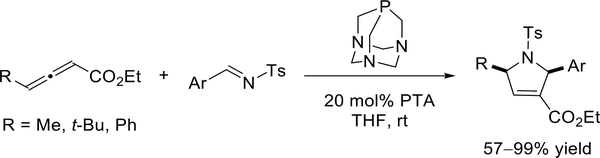
In 2007, He and co-workers demonstrated that the air-stable and readily available l,3,5-triaza-7-phosphaadamantane (PTA) is a practical and versatile nucleophilic phosphine catalyst for the [3 + 2] annulations of 4-substituted 2,3-butadienoates with N-tosylarylaldimines, delivering functionalized pyrrolines in good to excellent yields (Scheme 3)7
Scheme 3.
He’s PTA-catalyzed allene-imine [3 + 2] cycloaddition
In addition to N-sulfonylimines, He found that N-thiophosphorylimines (4) readily undergo Lu’s [3 + 2] annulation with ethyl allenoate or γ-substituted allenoates in the presence of triphenylphosphine (PPh3) or PTA, affording the corresponding N-(thio)phosphoryl 3-pyrrolines 5 in moderate to high yields and with good diastereoselectivities (Scheme 4)8 Ready deprotection of the thiophosphoryl group was achieved in MeOH under acidic conditions.
Scheme 4.
He’s [3 + 2] annulations of aliénés with N- thiophosphorylimines
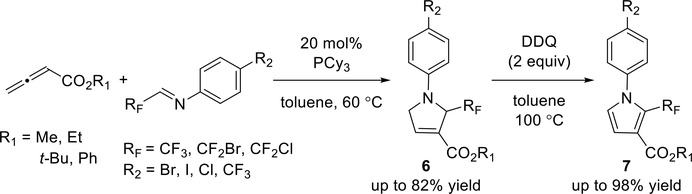
In 2017, Wu and co-workers reported a phosphine-catalyzed [3 + 2] annulation of N-aryl fluorinated imines with allenoates.9 Using 20 mol % of PCy3 as the catalyst in toluene at 60 °C, they prepared a series of fluorinated pyrrolines (6) in moderate yields (Scheme 5). They converted these product 3-pyrrolines to the fluorinated pyrroles 7 through dehydroaromatizations in the presence of 2,3-dichloro-5,6-dicyano-l,4-benzoquinone (DDQ) in high to excellent yields.
Scheme 5.
Wu’s phosphine-catalyzed [3 + 2] annulations of N-aryl fluorinated imines with allenoates
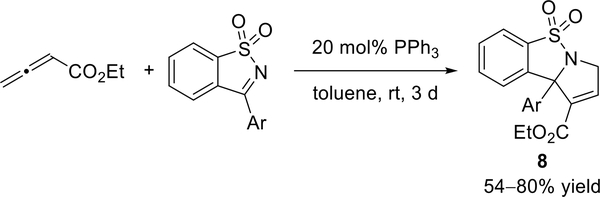
In 2012, Ye and co-workers described the use of cyclic imines for phosphine-catalyzed [3 + 2] annulations with ethyl allenoate to prepare functionalized sultam-fused pyrrolines (8) in moderate to good yields and with high regioselectivities (Scheme 6).10
Scheme 6.
Ye’s preparation of functionalized pyrrolines
Following Ye’s success at using cyclic imines to generate a class of functionalized dihydropyrroles, Guo11 and Wang12 independently disclosed the application of sulfamate-derived cyclic imines as [3 + 2] annulation partners (Scheme 7). In the presence of a catalytic amount of PPh3, their reactions afforded various functionalized cyclic sulfamidates (9), with a number of substitution patterns, in excellent yields.
Scheme 7.
Guo’s and Wang’s synthesis of functionalized cyclic sulfamidates
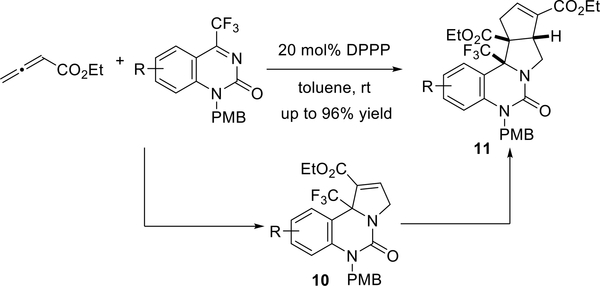
Subsequently, Ma and co-workers disclosed highly efficient 1,3- bis(diphenylphosphino)propane (DPPP)-catalyzed one-pot sequential [3 + 2]/[3 + 2] annulations of an allenoate with cyclic quinazolin-5-ones to provide N-fused polycyclic compounds (11, Scheme 8).13 The product of the first [3 + 2] annulation is an electron-deficient alkene, which prompts the second allene-alkene [3 + 2] annulation. This reaction is exceptionally regio- and diastereoselective.
Scheme 8.
Ma’s synthesis of functionalized polycyclic compounds
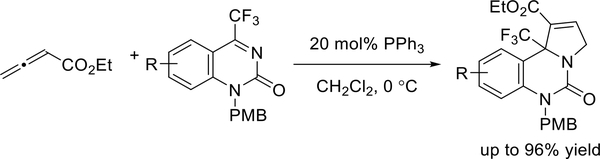
With further optimization, Ma and co-workers demonstrated the selective [3 + 2] annulations of cyclic quinazolin-5-ones (Scheme 9).14 Unlike the dual processes described in their previous report, here, in the presence of catalytic PPh3, only the allene-imine [3 + 2] annulation occurred, smoothly furnishing tricyclic dihydropyrrole derivatives in excellent yields.
Scheme 9.
Ma’s synthesis of functionalized dihydropyrroloquinazolin-5-ones
In 2011, Loh reported an elegant and highly efficient route toward functionalized 2-alkyl-substituted pyrrolines from N-sulfonyl alkylimines.15 N-Sulfonyl alkylimines can be difficult to handle because they readily decompose through hydrolysis, hampering their applicability in many phosphine-catalyzed reactions. In the presence of trimethylphosphine, Loh demonstrated that the in situ isomerization of 3-butynoates to 2,3- butadienoates and subsequent incorporation of N-sulfonyl alkylimines through [3 + 2] annulations afforded functionalized pyrrolines in good yields (Scheme 10).
Scheme 10.
Loh’s synthesis of functionalized 2-alkylpyrrolines
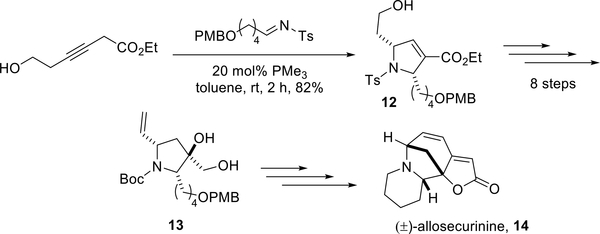
Applying this newly developed method for generating 2- alkyl-substituted pyrrolines, Loh completed a formal synthesis of (±)-allosecurinine featuring a step involving the formation of the 2,5-dialkylpyrroline 12 in 82% yield (Scheme 11). Eight subsequent steps furnished the known intermediate 13 in excellent yield. Kerr and co-workers had previous reported the total synthesis of (±)-allosecurinine 14 from the intermediate 13.16
Scheme 11.
Loh’s formal synthesis of (±)-allosecurinine
In 2011, Kinderman and co-workers reported the use of rare 2,3-dienenitriles 15 in Lu’s allene-imine [3 + 2] annulation, in place of the commonly employed 2,3-dienoates (Scheme 12).17 They prepared several pyrroline derivatives bearing a cyano group from these rare allenenitriles. Analogous to the reactivities of allenoates, the transformations of the 2,3-butadienenitriles were also catalyzed by PPh3, providing cyano-substituted pyrrolines (16) in good yields.
Scheme 12.
Kinderman’s preparation of cyanopyrrolines
Enantioselective Allene-Imine [3 + 2] Annulations Using Monofunctional Chiral Phosphines
Prior to the 2008 report by Jacobsen (vide infra), enantioselective allene-imine [3 + 2] annulations had been challenging when compared with their corresponding allene-alkene [3 + 2] annulations, with relatively few examples producing synthetically useful levels of enantioselectivity [i.e., enantiomeric excesses (ee’s) of greater than 90%]. In 2006, Gladysz employed the chiral rhenium-based phosphine P1 to catalyze Lu’s [3 + 2] annulations of ethyl 2,3-butadienoate with N-tosyl imines (Scheme 13).18 Using this catalyst, the desired pyrrolines were isolated in high yields and with moderate enantioselectivities.
Scheme 13.
Gladysz’s asymmetric [3 + 2] annulations using a rhenium-containing phosphine catalyst
Marinetti was among the forerunners in the area of chiral pyrroline construction, reporting numerous studies of various chiral phosphine catalysts and their abilities to render asymmetric induction.19 Among the many readily available chiral phosphines with axial and planar chirality that they tested, several induced the enantioselective formation of pyrrolines, albeit with low yields and moderate selectivities (Scheme 14). The chiral phosphines in Scheme 14 produced low to moderate yields of pyrrolines with minimal asymmetric induction. Preliminary screening revealed that several phosphine catalysts produced moderate levels of chiral induction, but the low product yields of these reactions suggested little synthetic utility.
Scheme 14.
Marinetti’s screening of chiral phosphines for [3 + 2] annulations giving pyrrolines
After an extensive search for the ideal catalyst for enantioselective formation of pyrrolines, Marinetti found that Gladiali’s phosphepine P6 functioned with moderate enantiocontrol (Scheme 15).20,21,22 They synthesized pyrrolines in moderate yields from substrates presenting various activating groups with a range of steric influence, albeit with moderate enantioselectivities.
Scheme 15.
Marinetti’s asymmetric [3 + 2] annulations forming pyrrolines
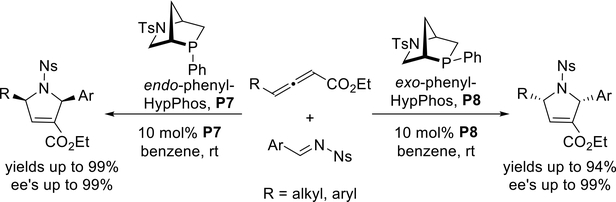
There exists only a single report of highly efficient asymmetric allene-imine [3 + 2] annulations using chiral phosphines that lack hydrogen-bonding motifs. In 2014, Kwon and co-workers developed two new pseudoenantiomeric [2.2.1] bicyclic phosphines—endo-phenyl-HypPhos (P7) and exo-phenyl-HypPhos (P8)—from naturally occurring trans-4-hydroxy-L-proline (Hyp), each in five steps. Both bridged bicyclic P-chiral phosphines are efficient at promoting [3 + 2] annulations of γ-substituted allenoates with activated imines, providing various chiral functionalized dihydropyrroles in excellent yields and with high ee’s (Scheme 16).23 This methodology has been applied in the synthesis of 1,2,3,5-substituted pyrroline derivatives, providing a geranylgeranyltransferase type I (GGTase-I) inhibitor that is viable for in vivo anticancer treatment.24 Both of the HypPhos ligands P7 and P8, along with eight other analogues, are at present available from Sigma-Aldrich.
Scheme 16.
Kwon’s synthesis of enantioenriched dihydropyrroles
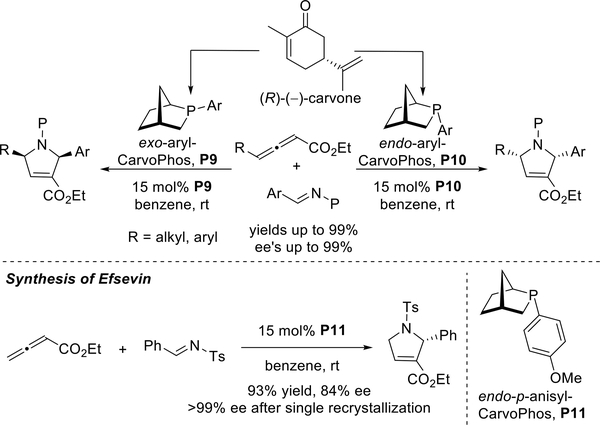
Although endo- and exo-phenyl-HypPhos behaved as pseudoenantiomers to form 1,2,3,5-tetrasubstituted pyrrolines in opposite enantiomeric forms, they both provided the (S)-l,2,3-trisubstituted pyrrolines when employing simple allenoates lacking a γ-substituent. In particular, endo-phenyl-HypPhos provided the (S)-l,2,3-trisubstituted pyrrolines with higher ee’s. To create chiral phosphines with the P-stereogenic 2-phosphabicyclo[2.2.1]heptane scaffold, very recently (in 2018) we adopted the natural terpenoid carvone as the starting materials. Hydrodealkenylation25 of the isopropenyl substituent of carvone allowed generation of both the exo- and endo-phenyl-CarvoPhos ligands P9 and P10.26 These novel P-stereogenic chiral phosphines P9 and P10 were applied to enantioselective [3 + 2] annulations of allenoates and imines to obtain a series of 1,2,3,5-tetrasubstituted pyrrolines in excellent yields and with high ee’s (Scheme 17). In particular, a biologically active small molecule, (R)-efsevin, was prepared in 93% yield and 84% ee.
Scheme 17.
Kwon’s synthesis of enantioenriched pyrrolines
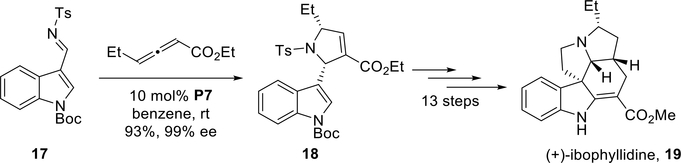
In 2012, the Kwon group highlighted the synthetic utility of the HypPhos-catalyzed allene-imine [3 + 2] annulation in the first enantioselective total synthesis of (+)-ibophyllidine.27 This achievement also marked the first nonformal total synthesis of a complex natural product employing a phosphine-catalyzed allene-imine [3 + 2] annulation. From the N-Boc-indole-3-imine 17, the key transformation—the asymmetric [3 + 2] annulation—was catalyzed by endo-phenyl-HypPhos (P7). The reaction afforded the pyrroline 18 in 93% yield and 99% ee (Scheme 18). When run on an approximately 30 g scale, the annulation proceeded in 94% yield and 97% ee. This powerful phosphine-catalyzed asymmetric [3 + 2] annulation set two of the stereocenters in ibophyllidine with excellent efficiency. The total synthesis of (+)-ibophyllidine 19 was completed in 13 steps from the enantiopure pyrroline 18.
Scheme 18.
Kwon’s enantioselective total synthesis of (+)-ibophyllidine
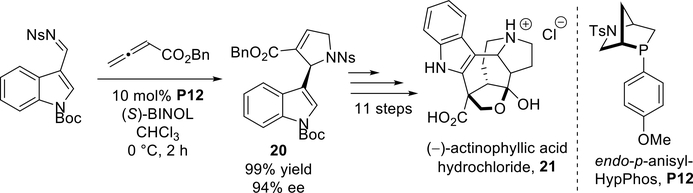
In 2016, Kwon et al. described another HypPhos-catalyzed [3 + 2] annulation of benzyl allenoate with an N-(o-nitrobenzenesulfonyl) (o-nosyl) imine to construct the pyrroline intermediate 20 in the total synthesis of the biologically active natural product (−)-actinophyllic acid hydrochloride (21, Scheme 19).28 The combination of endo-p-anisyl-HypPhos (P12) and (S)- BINOL facilitated the transformation to the cycloadduct in 99% yield and with 94% ee. Notably, the use of (R)-BINOL as an additive provided the same enantioselectivity as that of (S)-BINOL.
Scheme 19.
Kwon’s total synthesis of (−)-actinophyllic acid
Enantioselective allene-imine [3 + 2] annulations using multifunctional chiral phosphines
Early efforts toward the synthesis of enantiopure dihydropyrroles through the [3 + 2] annulations of allenoates with imines met with limited success. In 2008, Jacobsen and co-workers introduced the bifunctional thiourea phosphine catalyst P13 for the asymmetric [3 + 2] annulation of allenoates with diphenylphosphinoylimines (22).29 This protocol furnished a broad range of substituted dihydropyrroles (23) in excellent yield with very high enantioselectivities (Scheme 20). The addition of both water and triethylamine increased the rate of the reaction and suppressed undesired byproducts. The effect of adding water—increasing the reaction efficiency by facilitating proton transfer during the annulation—has been studied computationally.30
Scheme 20.
Jacobsen’s asymmetric synthesis of pyrrolines
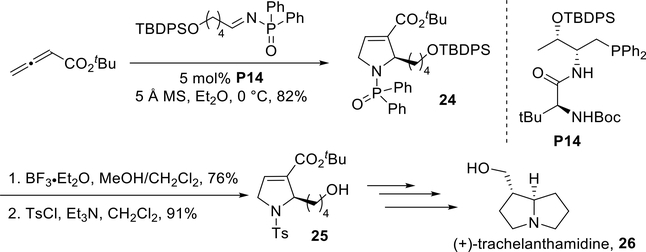
Lu reported, in 2012, an elegant process for asymmetric induction using the amino acid-derived chiral phosphine P14.31 Reminiscent of Jacobsen’s approach, they used sterically demanding diphenylphosphinoyl imines to achieve high enantiocontrol (Scheme 21). In addition to alkyl imines, aryl imines also afforded their functionalized pyrrolines with great efficiencies and ee’s. Using Jacobsen’s and Lu’s systems, both enantiomers of 2- pyrrolines can be accessed for synthetic applications.
Scheme 21.
Lu’s enantioselective synthesis of 2-alkyl- and 2-aryl- substituted pyrroline 3-carboxylates
In the same article, Lu and co-workers described the formal synthesis of the alkaloid natural product (+)-trachelanthamidine (26), with allene-imine [3 + 2] annulation as a key step, using the chiral phosphine catalyst P14 (Scheme 22). Several subsequent functional group manipulations were performed to remove the phosphoryl and silyl protecting groups. Sulfonylation of the free pyrroline amino group with p-tolylsu Ifonyl chloride rendered a formal synthesis of the target 25 toward (+)-trachelanthamidine (26). This short formal synthesis confirmed the applicability of this key [3 + 2] annulation in natural products synthesis.
Scheme 22.
Lu’s formal synthesis of (+)-trachelanthamidine
In 2013, the Guo group demonstrated the formation of functionalized tricyclic sulfamidates through reactions catalyzed by the L-isoleucine- derived bifunctional N-acylaminophosphine P1532 (Scheme 23). The transformations proceeded to give chiral sulfamate-fused dihydropyrroles in good yields and with moderate to excellent ee’s.
Scheme 23.
Guo’s asymmetric synthesis of functionalized tricyclic sulfamidates
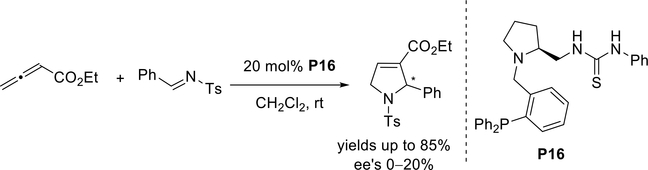
In 2014, Toffano and Vo-Thanh reported the synthesis of a chiral thiourea-phosphine organocatalyst derived from L-proline.33 The resulting annulation, however, yielded the dihydropyrrole in low ee when using the catalyst P16 (Scheme 24).
Scheme 24.
Toffano and Vo-Thanh’s use of a thiourea-phosphine organocatalyst in an asymmetric allene-imine [3 + 2] annulation
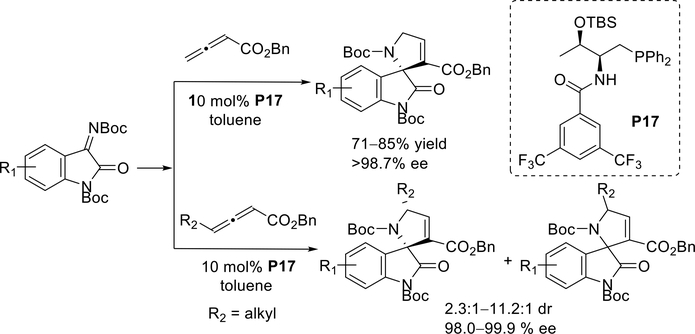
In 2016, the Lu group investigated the bifunctional chiral phosphine P17 as a catalyst for enantioselective [3 + 2] annulations of isatin-derived ketimines with both simple and γ-substituted allenoates (Scheme 25).34 In the presence of a catalytic amount of the bifunctional chiral phosphine P17, unique 3,2’-pyrrolidinyl spirooxindoles were synthesized in a highly enantioselective manner and in good yields.
Scheme 25.
Lu’s enantioselective phosphine-catalyzed [3 + 2] cycloadditions of allenoates with isatin-derived ketimines
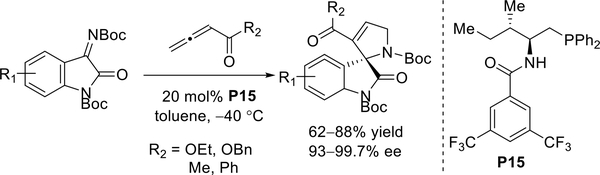
Kumar and co-workers performed similar transformations using Zhao’s N-acylaminophosphine P15 as the catalyst for the synthesis of 3,2’-dihydropyrrolyl spirooxindoles with high efficacies and with excellent enantioselectivities (Scheme 26).35
Scheme 26.
Kumar’s use of Zhao’s chiral phosphine catalyst in aliene-imine [3 + 2] annulations
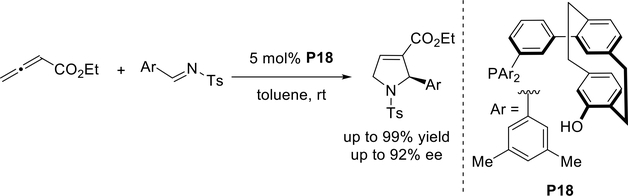
Very recently, Kitagaki and co-workers demonstrated that the planar-chiral [2.2]paracyclophane-based phosphine-phenol catalyst P18, which features a benzene ring spacer between the pseudo-ortho-substituted [2.2]paracyclophanol skeleton and the diarylphosphino group, is highly efficient at mediating enantioselective [3 + 2] annulations of allenoates and N-tosyl ¡mines (Scheme 27).36 This catalyst offered enantioselectivities as high as 92%—the highest reported to date for phosphine-catalyzed annulations of unsubstituted allenic esters with N-tosylaldimines.
Scheme 27.
Kitagaki’s planar-chiral [2.2]paracyclophane-based phosphine-phenol catalyst for allene-imine [3 + 2] annulations
In summary, phosphine-catalyzed [3 + 2] annulations of aliénés and imines can be efficient protocols for regio- and stereoselective syntheses, under mild conditions, of valuable substituted pyrrolines presenting a variety of functional groups. Such methods are intriguing because the seemingly innocuous phosphine acts, in most cases, as the catalyst with no need for additional ligands or additives. The broad substrate scope of phosphine-catalyzed allene-imine [3 + 2] annulations has been exploited in the total syntheses of important bioactive alkaloid natural products and medicinally relevant compounds. Such [3 + 2] annulations should find broader applications in the syntheses of other natural products, bioactive molecules, and functional materials.
Biography
 Ohyun Kwon, Professor of
Chemistry and Biochemistry at UCLA, received her B.S. and M.S. degrees from Seoul
National University in 1991 and 1993, respectively. After obtaining her Ph.D. from
Columbia University in 1998, and a postdoctoral stint at Harvard University, Kwon
began her independent career at UCLA in 2001. Her research involves the development
of phosphine-catalyzed reactions and their application to natural product synthesis
and chemical biology. She has played key roles in establishing phosphinocatalysis as
one of the main areas of organocatalysis, and is recognized as one of the leaders in
the field.
Ohyun Kwon, Professor of
Chemistry and Biochemistry at UCLA, received her B.S. and M.S. degrees from Seoul
National University in 1991 and 1993, respectively. After obtaining her Ph.D. from
Columbia University in 1998, and a postdoctoral stint at Harvard University, Kwon
began her independent career at UCLA in 2001. Her research involves the development
of phosphine-catalyzed reactions and their application to natural product synthesis
and chemical biology. She has played key roles in establishing phosphinocatalysis as
one of the main areas of organocatalysis, and is recognized as one of the leaders in
the field.
 Aslam C. Shaikh was born in 1989
in Ahmednagar (Maharashtra), India. He completed his M.Sc. (2012) in Chemistry from
HPT Arts and RYK Science College in Nashik. In 2013, he joined CSIR- National
Chemical Laboratory, Pune as a research assistant for Dr. Muthukrishnan. He obtained
his Ph.D. from CSIR-NCL, Pune in 2018, under the mentorship of Professor Nitin T.
Patil, having studied the synthesis of organic tluorophores through metal-catalyzed
difunctionalization of C-C multiple bonds. He is currently a Postdoctoral Associate
with Professor Ohyun Kwon at the University of California, Los Angeles, developing
new phosphine catalysts for asymmetric transformations.
Aslam C. Shaikh was born in 1989
in Ahmednagar (Maharashtra), India. He completed his M.Sc. (2012) in Chemistry from
HPT Arts and RYK Science College in Nashik. In 2013, he joined CSIR- National
Chemical Laboratory, Pune as a research assistant for Dr. Muthukrishnan. He obtained
his Ph.D. from CSIR-NCL, Pune in 2018, under the mentorship of Professor Nitin T.
Patil, having studied the synthesis of organic tluorophores through metal-catalyzed
difunctionalization of C-C multiple bonds. He is currently a Postdoctoral Associate
with Professor Ohyun Kwon at the University of California, Los Angeles, developing
new phosphine catalysts for asymmetric transformations.
References
- 1.Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095–1569, United States: ohyun@chem.ucla.edu Our research program is supported by the NIH (R01GM071779). [Google Scholar]
- 2.(a) Bràse S Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; RSC: Cambeidge, 2015; [Google Scholar]; (b) Attorusso E; Taglialatela-Scafati O Modern Alkaloids: Structure, Isolation, Synthesis and Biology; WileyVCEl: Weinheim, 2008. [Google Scholar]
- 3.For reviews, see:; (a) Fan EL; Peng J; Hamann MT; Flu J-F Client. Rev 2008, 108, 264–287; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Walsh CT; Garneau-Tsodikova S; Howard- Jones AR Nat. Prod. Rep 2006, 23, 517–531; [DOI] [PubMed] [Google Scholar]; (c) Felpin F-X; Lebreton J Eur. J. Org. Client 2003, 2003, 3693–3712; [Google Scholar]; (d) O’Hagan D Nat. Prod. Rep 2000, 17, 435–446; [DOI] [PubMed] [Google Scholar]; (e) Green MP; Prodger JC; Hayes C J. Tetrahedron Lett 2002, 43, 6609–6611. [Google Scholar]
- 4.(a) Xu Z; Lu X J Org. Client 1998, 63, 5031–5041; [Google Scholar]; (b) Xu Z; Lu X Tetrahedron Lett. 1997, 38, 3461–3464. [Google Scholar]
- 5.Zhu X-F; Henry CE; Kwon O Tetrahedron 2005, 61, 6276–6282 [Google Scholar]
- 6.Zhao G-L; Shi M J. Org. Client 2005, 70, 9975–9984. [DOI] [PubMed] [Google Scholar]
- 7.Tang X; Zhang B; He Z; Gao R; He Z Adv. Synth. Catal 2007, 349, 2007–2017. [Google Scholar]
- 8.Zhang B; Xu S; Wu G; He Z Tetrahedron 2008, 64, 9471–9479. [Google Scholar]
- 9.Zhu Z; Guo Y; Wang X; Wu F; Wu Y J. Fluorine Client 2017,195,102–107. [Google Scholar]
- 10.Chen X-Y; Lin R-C; Ye S Chem. Commun 2012,48,1317–1319. [DOI] [PubMed] [Google Scholar]
- 11.Yu H; Zhang L; Yang Z; Li Z; Zhao Y; Xiao Y; Guo H J. Org. Client 2013, 78, 8427–8436. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y-Q; Zhang Y; Dong H; Zhang J; Zhao J Eur. J. Org. Client 2013, 2013, 3764–3770. [Google Scholar]
- 13.Yang L-J; Wang S; Nie J; Li S; Ma J-A Org. Lett 2013,15, 5214–5217. [DOI] [PubMed] [Google Scholar]
- 14.Yang L-J; Li S; Wang S; Nie J; Ma J-A J. Org. Client 2014, 79, 3547–3558. [DOI] [PubMed] [Google Scholar]
- 15.Sampath M; Lee P-YB; Loh T-P Client. Sci 2011, 2, 1988–1991. [Google Scholar]
- 16.Leduc AB; Kerr MA Angeio. Chem., Int. Ed 2008,47, 7945–7948. [DOI] [PubMed] [Google Scholar]
- 17.Kinderman SS; van Maarseveen JH; Hiemstra H Synlett 2011,1693–1696. [Google Scholar]
- 18.Scherer A; Gladysz JA Tetrahedron Lett. 2006, 47, 6335–6337. [Google Scholar]
- 19.Jean L; Marinetti A Tetrahedron Lett. 2006,47, 2141–2145. [Google Scholar]
- 20.Fleury-Brégeot N; Jean L; Retailleau P; Marinetti A Tetrahedron 2007, 63,11920–11927. [Google Scholar]
- 21.Panossian A; Fleury-Brégeot N; Marinetti A Eur. J. Org. Chem 2008, 2008, 3826–3833. [Google Scholar]
- 22.Pinto N; Fleury-Brégeot N; Marinetti A Eur. J. Org. Chem 2009, 146–151. [Google Scholar]
- 23.Flenry CE; Xu Q; Fan YC; Martin TJ; Belding L; Dudding T; Kwon O J. Am. Chem. Soc 2014,136,11890–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Lu J; Chan L; Fiji, Fl DG; Dahl R; Kwon O; Tamanoi F Mol. Cancer Ther 2009, 8, 1218–1226; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zimonjic DB; Chan LN; Tripathi V; Lu J; Kwon O; Popescu NC; Lowy DR; Tamanoi F BMC Cancer 2013,13,198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smaligo AJ; Swain M; Quintana JC; Tan MF; Kim DA; Kwon O Science 2019, 364, 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smaligo AJ; Vardhineedi S; Kwon O ACS Catal. 2018, 8, 5188–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews IP; Kwon O Chem. Sei 2012, 3, 2510–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai L; Zhang K; Kwon O J. Am. Chem. Soc 2016,138, 3298–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y-Q; Jacobsen EN J. Am. Chem. Soc 2008,130, 5660–5661. [DOI] [PubMed] [Google Scholar]
- 30.(a) Xia Y; Liang Y; Chen Y; Wang M; Jiao L; Fluang F; Liu S; Li Y; Yu Z-X J. Am. Chem. Soc 2007, 129, 3470–3471; [DOI] [PubMed] [Google Scholar]; (b) Mercier E; Fonovic B; Flenry C; Kwon O; Dudding T Tetrahedron Lett. 2007, 48, 3617–3620. [Google Scholar]
- 31.Flan X; Zhong F; Wang Y; Lu Y Angew. Chem., Int. Ed 2012, 51, 767–770. [DOI] [PubMed] [Google Scholar]
- 32.Xiao FI.; Yang Y-Q; Liu W.; Zhao G Angew. Chem., Int. Ed. 2010, 49, 4467–4470. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T-H; Toffano M; Bournaud C; Vo-Thanh G Tetrahedron Lett. 2014, 55, 6377–6380. [Google Scholar]
- 34.Flan X; Chan W-L; Yao W; Wang Y; Lu Y Angew. Chem., Int. Ed 2016, 55, 6492–6496. [DOI] [PubMed] [Google Scholar]
- 35.Sankar MG; Garcia-Castro M; Golz C; Strohmann C; Kumar K RSC Adv 2016, 6, 56537–56543. [Google Scholar]
- 36.Kitagaki S; Nakamura K; Kawabata C; Ishikawa A; Takenaga N; Yoshida K Org. Biomol. Chem 2018,16,1770–1178. [DOI] [PubMed] [Google Scholar]