Highlights
-
•
BPA results shown in publications from independent labs were repeated in a guideline study.
-
•
BPA had effects at 2.5 ug/kg/day in the brain, prostate, urinary tract, ovary, mammary gland, heart.
-
•
In mammary gland, prostate stem cell numbers, ovary a non-monotonic BPA dose-response was observed.
-
•
2.5 ug/kg/day should be the new LOAEL for BPA.
Keywords: CLARITY-BPA, Guideline study, Bisphenol A, EDC, Endocrine disruptor, GLP, Systemic effects
Abstract
“Consortium Linking Academic and Regulatory Insights on BPA Toxicity” (CLARITY-BPA) was a comprehensive “industry-standard” Good Laboratory Practice (GLP)-compliant 2-year chronic exposure study of bisphenol A (BPA) toxicity that was supplemented by hypothesis-driven independent investigator-initiated studies. The investigator-initiated studies were focused on integrating disease-associated, molecular, and physiological endpoints previously found by academic scientists into an industry standard guideline-compliant toxicity study. Thus, the goal of this collaboration was to provide a more comprehensive dataset upon which to base safety standards and to determine whether industry-standard tests are as sensitive and predictive as molecular and disease-associated endpoints. The goal of this report is to integrate the findings from the investigator-initiated studies into a comprehensive overview of the observed impacts of BPA across the multiple organs and systems analyzed. For each organ system, we provide the rationale for the study, an overview of methodology, and summarize major findings. We then compare the results of the CLARITY-BPA studies across organ systems with the results of previous peer-reviewed studies from independent labs. Finally, we discuss potential influences that contributed to differences between studies. Developmental exposure to BPA can lead to adverse effects in multiple organs systems, including the brain, prostate gland, urinary tract, ovary, mammary gland, and heart. As published previously, many effects were at the lowest dose tested, 2.5μg/kg /day, and many of the responses were non-monotonic. Because the low dose of BPA affected endpoints in the same animals across organs evaluated in different labs, we conclude that these are biologically – and toxicologically – relevant.
1. Introduction
The Consortium Linking Regulatory and Academic Insights on the Toxicity of Bisphenol A (BPA), known as CLARITY-BPA, is a novel toxicity study designed to integrate the strength of a US Food and Drug Administration (FDA) industry-standard “guideline” study with investigator-initiated studies that focused on disease-associated and molecular endpoints. The hypothesis underlying this design was that the published results from the independent studies would be apparent in a guideline-compliant study and would thereby provide a stronger dataset for regulatory agencies as well as to test whether industry standard endpoints are sensitive, specific and predictive for agents that interfere with hormone systems. Details of the study design are published [1] and will only be briefly summarized here.
In CLARITY-BPA, pregnant NCTR-Sprague Dawley rats were gavaged daily with vehicle (carboxymethyl cellulose), ethinyl estradiol (EE; 0.05 or 0.5 μg/kg/day) as the positive control or BPA (2.5, 25, 250, 2500, or 25,000 μg/kg/day) from gestational day (GD) 6 to birth at the FDA National Center for Toxicological Research (NCTR) facility employing guideline protocols for toxicity testing. Offspring were gavaged with the same doses starting on postnatal day (PND) 1. Daily dosing was continued for 1–2 years (continuous-dose) or stopped at PND 21 (stop-dose). The Core guideline study included 50 rats/dose/sex/age. Rats were euthanized and blood and tissues isolated for analysis at 1 and 2 years. The independent labs that participated in the CLARITY-BPA study assessed a variety of endpoints related to disease/dysfunction and a variety of molecular endpoints. Endpoints were assessed at PND 1, 15, 21, 90, age 6 months, and age 1 year depending on study design. Importantly, all independent lab experiments were blinded to control and exposure groups, with data decoded only after completion of all studies.
Results of the Core guideline study were published in a single report [2], and results of several academic studies were published independently in peer-reviewed journals [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. Assessments of the overall study design [18,19] and a summary of available datasets from multiple CLARITY-BPA studies [20] are also published in peer-reviewed journals.
Importantly, there has not been a single publication to integrate the results from the independent studies. To address this critical gap, the present report represents a synthesis and integration of the results of 8 of the 14 independent studies by principal investigators willing to participate in an effort to coalesce the findings and assess patterns of BPA exposures across the various end organs examined in the same animal cohorts and, frequently, in the same animals. For each organ system, we provide the rationale for the study, overview of methodology, summary of major findings, and discussion of results of the CLARITY-BPA study in comparison to previous results from the independent labs along with plausible reasons for the differences. Additionally, we applied an integrative analysis approach by using r- and Circos-plots generated with the mixOmics [21] program to identify correlations in BPA responses across organs collected from the same or comparable individual rats within this same consortium study, thus expanding our findings to a systems biology level to reveal strong organismal relationships at three different timepoints: 21 days of age (weaning), 90–120 days of age (young adult) and 6 months of age (older adult). Additionally, relationships between investigator findings within this consortium study were tested at three different dosages: lowest dose (2.5 μg/kg/day), middle dose (250 μg/kg/day), and highest dose (2500 μg/kg/day). In this integrative correlation analyses section, we emphasize first those associations identified in males and females at the lowest dose and at 6 months of age, as evidence of such persistent or developmental origins of health and disease (DOHaD) effects would suggest that we need to rethink the lowest safe dose of BPA. Rosenfeld’s laboratory has used this mixOmics analyses approach in previous BPA studies to integrate various ‘omics and phenotypic data generated in her lab together [[22], [23], [24]]. However, to our knowledge, this is the first time such a program has been used to perform integrative correlation analyses with comprehensive datasets spanning different ages and dosages and generated in the laboratories of multiple investigators.
2. Independent studies
The CLARITY-BPA program consisted of 14 independent investigators. Table 1 shows a listing of all the independent CLARITY-BPA investigators and the endpoints assessed. Four investigators have not published their data, one investigator has a manuscript in preparation. Ten investigators have published their data and eight of those chose to be a part of this manuscript. A description of those studies and their results are described below.
Table 1.
Listing of Independent CLARITY-BPA investigators and Endpoints Assessed.
| Independent CLARITY-BPA Study Investigators | Endpoints Assessed |
|---|---|
| Ana Soto# | Mammary Gland Development/Cancer/Nature of the dose response curve |
| Gail Prins# | Prostate Cancer/Stem progenitor cell numbers |
| Shuk Mei Ho*** | Uterine Cancer |
| Frederick vom Saal# | Male Urogenital Abnormalities |
| Nestor Gonzalez-Cadavid* | Penile Function |
| Heather Patisaul # | Behavioral Changes/Brain Transcriptomics |
| Cheryl Rosenfeld# | Learning and Behavior/Neural DNA methylation and gene expression |
| Kim Boekelheide** | Testis Function/Sperm Counts |
| Jodi Flaws# | Ovarian Function/ Follicle Counts |
| Andrew Greenberg* | Diabetes |
| Nira Ben Jonathan* | Obesity |
| Norbert Kaminski** | Immune Function |
| R. Thomas Zoeller# | Thyroid Function |
| Scott Belcher# | Cardiovascular Function |
Indicates no CLARITY-BPA publication at time of writing of this manuscript.
Indicates CLARITY-BPA publications but not participating author.
Indicates CLARITY-BPA data published and included in this manuscript.
Indicates publication pending.
3. Gail Prins: BPA effects on the prostate
3.1. Introduction
The Prins lab has established that the developing prostate has heightened sensitivity to estrogenic exposure that can reprogram the gland to have elevated disease risk in adulthood [3,[25], [26], [27], [28]]. Together with Dr. Shuk-Mei Ho and using Sprague Dawley rats, we determined that while developmental exposure to BPA at environmentally relevant doses alone is not sufficient to drive prostate pathology, such early-life exposure reprograms the rat prostate epigenome and increases susceptibility to estrogen-driven carcinogenesis with aging [3,[29], [30], [31], [32], [33]]. In addition, using a humanized prostate model containing normal human prostate stem and progenitor cells, our lab found similar results wherein low-dose in vivo BPA exposure increases susceptibility to estrogen carcinogenicity, implicating direct relevance of the rodent model to human disease [3,30,[34], [35], [36]].
Most recently, a detailed dose-response study in Sprague Dawley rats (Zivic Miller Laboratories, Pittsburgh, PA) that included internal free BPA and BPA-glucuronide (BPA-G) dosimetry demonstrated a non-monotonic response to brief neonatal BPA exposures in a rat prostate lobe-specific manner [37]. Significantly more lateral lobe high-grade prostate intraepithelial neoplasia (PIN) lesions—the precursor to prostate cancer—as well as progression to adenocarcinoma were found in rats developmentally exposed to low-dose BPA (≤10 μg/kg/day) and given testosterone plus estradiol (T + E) implants in adulthood that doubled circulating estradiol (E2) levels. This finding is biologically relevant because E2 levels increase in aging men [38] and, together with testosterone, induce prostate cancer in rat and human epithelia [39] and accelerate prostate cancer progression [28,40,41]. Further, estrogenic activity is amplified in metastatic prostate cancer in humans [39,42].
A separate laboratory independently analyzed neonatal BPA exposures (both oral and subcutaneous depot) to Sprague Dawley rat pups with adult T + E treatments and similarly determined that low-dose BPA exposures increase susceptibility to estrogen-driven high-grade PIN in the dorsolateral lobe with aging [33,43]. Taken together, we propose that a combination of developmental BPA exposures with rising adult estrogen levels may augment prostate cancer risk.
3.2. Study goals
The goals of CLARITY-BPA studies on the prostate gland were to 1) examine whether developmental and/or chronic BPA exposures are sufficient to drive pathology in separate regions of the prostate gland in rats supplied by the FDA; 2) test the hypothesis that early-life BPA exposures increase susceptibility to later-life neoplasia and adenocarcinoma in response to elevated E2 levels, as occurs in aging men; and 3) assess whether chronic BPA exposures modify stem cell homeostasis within the dorsolateral prostate lobes.
3.3. Methodology
For Goal 1, NCTR Sprague Dawley rats (CLARITY-BPA study) were gavaged daily with vehicle, EE (0.5 μg/kg/day), or BPA (2.5, 25, 250, 2500, or 25,000 μg/kg/day) from GD 6 to 1 year (continuous-dose) or from GD 6 to PND 21 (stop-dose). For Goal 2, rats were gavaged daily from GD 6 to PND 21 (stop-dose) and were given T + E implants at PND 90 to drive carcinogenesis with aging. Prostates were collected at 1-year necropsy at FDA labs, coded, and shipped to the University of Illinois at Chicago, where they were processed and analyzed for histopathology by Dr. Maarten Bosland, who was blinded to treatments and controls. For Goal 3, NCTR Sprague Dawley rats were gavaged daily with vehicle, EE (0.5 μg/kg/day), or BPA (2.5, 25, or 250 μg/kg/day) from GD 6 to 6 months (continuous-dose), at which time prostates were removed and shipped on ice overnight to the Prins laboratory for stem cell isolation and culture. Dorsolateral lobe epithelial stem cells were isolated by direct prostasphere 3D culture and passaged three times to enhance stem cell purification. BPA was absent during this 3-week culture period. We measured spheroid numbers and size as well as gene expression by qRT-PCR to determine whether in vivo exposure to BPA altered stem cell self-renewal, progenitor cell proliferation, and lineage commitment.
3.4. Results
Prostate findings generated in this CLARITY-BPA study are published [3]. Developmental or continuous exposure to BPA alone at any dose did not produce prostate pathology that differed from vehicle controls, similar to findings reported in the Core studies. However, we confirmed our prior reports that developmental BPA exposure sensitizes the prostate to later-life E2-driven carcinogenesis, an apical adverse outcome (Fig. 1 A,B). Specifically, compared to vehicle controls, perinatal exposure of rats to BPA at low (2.5 μg/kg/day), medium (250 μg/kg/day), and high (25,000 μg/kg/day) doses resulted in more severe PIN lesions, shifting from low-grade PIN in controls to high-grade PIN with the highest severity score at the lowest tested BPA dose [3]. Notably, high-grade PIN in humans is a precursor to prostate cancer, while low-grade PIN is not considered clinically relevant. Importantly, the 2.5 μg/kg/day BPA exposure led to a four-fold increase in adenocarcinoma multiplicity in the dorsolateral prostate ducts, an effect not seen at higher BPA doses.
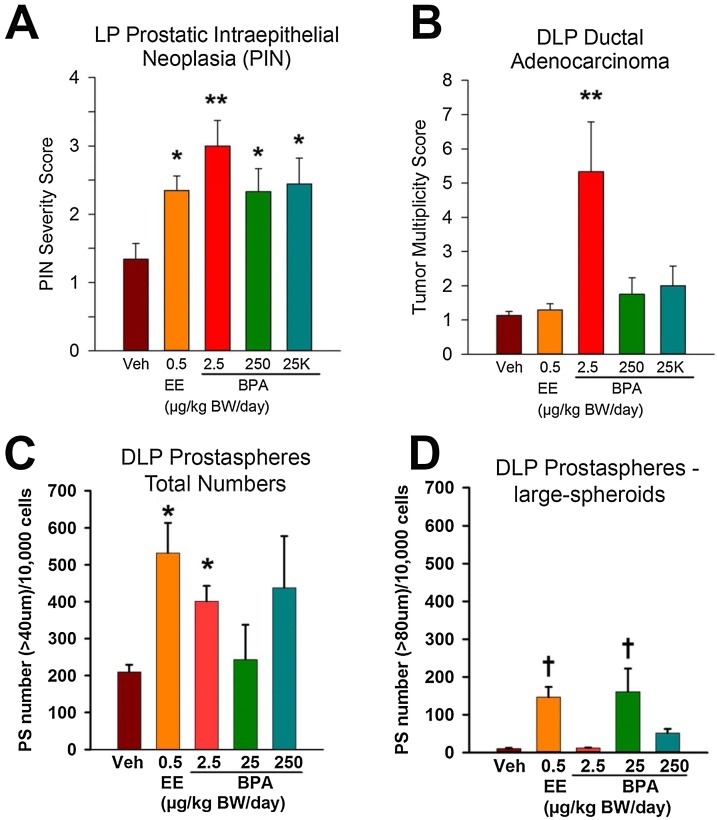
Fig. 1.
Dorsolateral prostate pathology and prostasphere numbers in rats treated with vehicle or increasing doses of BPA. A—B) Prostate pathology in 1-year-old rats treated with bisphenol A (BPA) during gestation to weaning (stop-dose) and given implants of testosterone + estradiol (T + E) at postnatal day 90 to elevate circulating estradiol levels. A) Severity scores of lateral lobe prostate (LP) intraepithelial neoplasia (PIN) lesions were significantly elevated in rats given ethinyl estradiol (EE) or 2.5, 250, or 25,000 μg/kg/day BPA during development as compared to vehicle controls. *P < 0.05, **P < 0.01 vs controls. B) Multiplicity of dorsolateral prostate (DLP) ductal adenocarcinoma was significantly increased in rats treated with 2.5 μg/kg/day BPA during early life as compared to vehicle controls. **P < 0.01 vs controls. CD—) Prostaspheres in 6-month old rats treated continuously with vehicle or BPA from gestational day 6 through time of tissue collection. Number of prostaspheres cultured from DLPs of rats exposed to BPA from gestation through 6 months of age. Daily exposure to EE or 2.5 μg/kg/day BPA doubled the spheroid numbers as compared to vehicle controls (ANOVA = 0.02; *P < 0.02 vs vehicle). Treatment with 250 μg BPA also doubled prostasphere numbers, but this was not significant due to high variance. D) Prostasphere sizes showed a significant increase in large spheroids (>80 μm) from DLPs exposed in vivo to EE or 25 μg/kg/day BPA as compared to vehicle controls (ANOVA < 0.01; †P < 0.01, *P < 0.02 vs vehicle). N for each group: vehicle=4; EE=5; 2.5 μg BPA=5; 25 μg BPA=3; and 250 μg BPA= 5.
Dose-specific responses to chronic BPA exposures were observed for stem and progenitor cells harvested from dorsolateral prostates at 6 months of age. In vivo continuous exposure to 2.5 μg/kg/day BPA doubled the total prostasphere number, reflecting increased stem cell numbers in adult prostates (Fig. 1C). Prostasphere size, a marker of progenitor cell proliferation in cultured spheroids, increased steeply in response to 25 μg BPA and to a lesser degree to 250 μg BPA compared to vehicle-treated controls (Fig. 1D). Tightly paralleling prostasphere size effects, exposure to EE or 25 μg/kg/day BPA significantly increased CK5, Sox2, and HoxB13 expression, while EE or 25 or 250 μg/kg/day BPA suppressed CK8, Trop2, and Tbx3 mRNA [3]. This indicates that chronic BPA exposure permanently modifies the lineage commitment of prostate stem cell progeny, increasing basal progenitors and suppressing luminal progenitor cells.
3.5. Discussion
Together, these results show that chronic low-dose BPA exposure alters adult prostate stem cell homeostasis in a dose-dependent manner, increasing stem cell numbers at the lowest dose and elevating progenitor cell proliferation, while also shifting lineage commitment to favor basal progenitor cells at 10- and 100-fold higher doses. The dose-specific responses observed over a 100-fold BPA range are likely due to differential engagement of estrogen receptor (ER) populations and membrane versus nuclear signaling pathways. Reprogramming of adult rat prostate stem cell homeostasis by chronic low-dose BPA may underpin an increased carcinogenic risk in the prostate with aging. Collectively, the results provide unbiased evidence that BPA exposures at human-relevant doses result in adverse effects on the rat prostate gland.
Overall, the CLARITY-BPA study on prostate endpoints confirmed previous studies that developmental exposure to BPA at environmentally relevant low doses markedly increases prostate cancer susceptibility to aging-related elevations in circulating estrogens. Further, low-dose BPA exposure alone was confirmed as sufficient to increase prostate stem cell numbers and reprogram the epithelial progenitor cell lineage. Nonetheless, there are also several differences between these results and previously reported findings using a similar model.
First, few ventral or dorsal lobe lesions were noted in the CLARITY-BPA study using NCTR-Sprague Dawley rats derived from >30 years of breeding at the FDA facility. This contrasts with our previous dose-response study using Zivic-Miller Sprague Dawley rats, where inverted U-shaped dose-response curves were observed in PIN severity in those prostate regions [33,37,44]. Further, the incidence of lateral lobe PIN and dorsolateral prostate ductal adenocarcinomas was not affected by perinatal BPA or EE exposure with adult T + E treatment in the present study, whereas our previous findings found elevated PIN and carcinoma incidence in the lateral lobe at 7 months and 1 year, respectively, in rats treated neonatally with 10 μg/kg/day BPA plus adult T + E [37,44]. These divergent findings likely result from multiple variations in experimental designs between studies, including differences in Sprague Dawley rat sub-strains, diet compositions, exposure periods (GD 6 to PND 21 vs PND 1, 3, and 5 used previously), and exposure routes (daily gavage vs subcutaneous oil depot used previously), as well as the lack of T + E tube replacement every 8 weeks in the present study as done previously. Notably, the chronic high incidence of lateral prostate inflammation found in all rats in the present studies, including 80 %–100 % penetrance in control rats and the elevated mortality in T + E treated rats, phenomena not observed in our prior work with Sprague Dawley rats, is possibly related to housing conditions and treatment protocols. Despite these divergent design details that may account for differences in histopathology findings, the overall conclusions regarding BPA effects on the prostate are consistent between studies.
The use of rats as a model for prostate cancer in CLARITY-BPA is also a confounding variable. Unlike men, who develop prostate adenocarcinoma at high rates with aging [45], most rat strains including Sprague Dawley do not spontaneously develop prostate cancer, highlighting fundamental biological differences in prostate carcinogenesis between the two species. The use of rats as a model for human prostate carcinogenesis requires either potent chemical carcinogens and/or extended exposure to natural sex steroids with high receptor affinity [46]. Of the compounds used to induce prostate cancer in rats, extended exposure to testosterone at physiological levels with two-fold elevated E2 is a physiologically relevant model because E2 levels rise in aging men. As such, developmental BPA exposure combined with adult T + E is the most relevant experimental regime for testing BPA effects on prostate carcinogenesis in a rat model, with potential for direct applicability to humans.
A major limitation of our prostate CLARITY-BPA studies was sample size, which was severely underpowered to detect statistical differences for all carcinogenic endpoints for the rats treated with T + E. Power calculations based on our prior studies were presented in our animal plan, showing the need for 18 animals per dose in the aging study. However, that number was only achieved in vehicle and EE control groups, whereas BPA groups had 4–11 animals per dose. The very low number of animals in the 25 and 2500 μg/kg/day BPA groups given adult T + E (n = 4) resulted in non-normality of data distributions, which prevented inclusion in statistical analysis. Low numbers for the remaining doses may account for lack of significance in several endpoints. The underpowering of this study at NCTR labs defies the FDA’s own recommendations of 50 animals/group for carcinogenicity studies [19,47] which was the number used for the parallel CLARITY-BPA Core study.
We had not previously examined stem cell homeostasis in the rat prostate gland, so the CLARITY-BPA dataset on that endpoint is novel. We have examined BPA effects previously on adult human prostate stem cells and human embryonic stem cells, and both of those studies observed similar stimulatory effects of BPA on stem and progenitor cell proliferation, among other endpoints [34,36].
4. Frederick S. vom Saal and William A. Ricke: BPA effects on the urethra and dorsal prostate
4.1. Introduction
The vom Saal and Ricke labs have demonstrated that low BPA doses alter the testes, epididymis, seminal vesicles, preputial glands, and prostate in male mice [[48], [49], [50]]. We have also found statistically significant effects of endogenous and exogenous estrogens on the urogenital sinus (UGS) of fetal mice and rats [48,[51], [52], [53], [54], [55]]. These studies involved examination of males during fetal life as well as later in adulthood to determine if there were long-term effects. We found that BPA increases estrogen receptor alpha gene expression (Esr1) in UGS mesenchyme in male mouse fetuses in both primary culture [53,54] and in vivo; this latter experiment suggested epigenetic mechanisms as we found changes in DNA methyltransferases [55]. BPA exposure during fetal life also caused an increase in androgen receptor gene expression in the fetal UG S mesenchyme, and subsequently, an increase in androgen receptor protein in adult male mouse prostate [48,56,57].
An indication of the very high sensitivity of the UGS to estrogens was shown in a study in which a 0.1 pg/mL increase in fetal serum estradiol (E2) administered via a Silastic capsule implanted in the mother significantly decreased the size of the urethral lumen based on 3D reconstructions [48]. It also significantly increased the number of prostatic glandular buds, size of the glands, and overall prostate size, particularly in the dorsal (colliculus) region of the UGS when mice are examined at birth using a 3D computer assisted reconstruction technique (Fig. 2 ); these effects persisted and enlarged prostates were found later in adulthood.
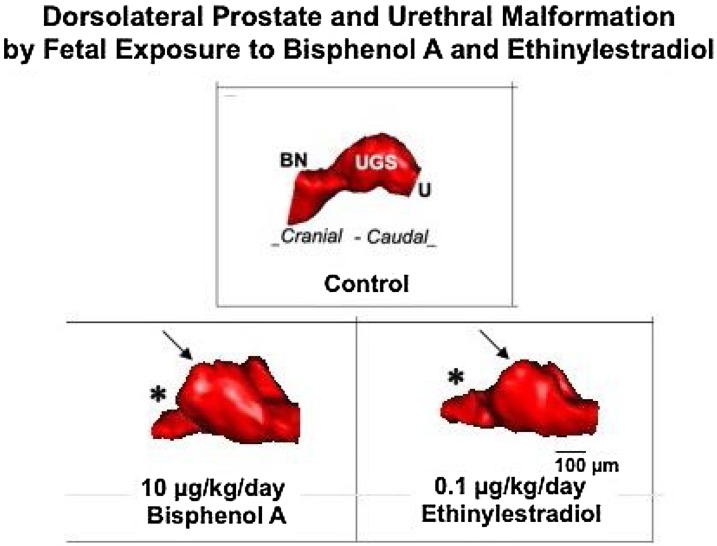
Fig. 2.
3D serial section reconstruction of the urogenital sinus (UGS) from gestation day (GD) 19 male CD-1 mice exposed to low doses of bisphenol A (BPA) and ethinyl estradiol (EE) from gestation day 14-18 via feeding the pregnant dam. UGS depicted for each treatment was closest to the group mean. There was a marked alteration in urethra shape, particularly at the junction of the bladder and urethra, which is constricted (*) in mice exposed to estrogenic chemicals compared to controls. In addition, the UGS region (prostatic sulcus or colliculus, arrow) is significantly enlarged by BPA compared to controls, based on data published in Timms et al. 2005 [59].
The malformation of the colliculus was associated with enlargement of the utriculus, which is the remnant of the portion of the Mullerian duct that differentiates into the cranial region of the vagina in a female and persists within the prostate tissue near the colliculus. The size of the utricle increases as a function of estrogenic chemicals present during the critical period of UGS differentiation, which occurs near the end of the first trimester in human pregnancy and shortly before birth in rats and mice [58]. In summary, malformation of the UGS, particularly in the collicular region, as well as decreased size of the urethra (Fig. 2) have been observed in all of our prior studies involving elevated estrogen in mice and rats exposed as fetuses via the pregnant dam. Consistent with several studies, high doses of estrogens have the opposite effect on the prostate, resulting in non-monotonic dose-response relationships [48,59].
The finding that increased estrogen decreases the size of the urethra led us to conduct additional studies in adult mice treated with testosterone (T) and E2 capsules to determine whether adverse effects of estrogens on the bladder-urethra were restricted to initial development of the UGS in males or continued throughout life. The estrogen treatment mimics the gradual increase in free serum estradiol in men as they age. Adult exposure to T + E2 induced obstructive voiding disorder, associated with droplet voiding pattern and an inability to exhibit sustained voids, bladder hypertrophy, diverticula, calculi, and eventual decompensation with hydronephrosis [60]. Administration of BPA and testosterone (BPA + T) produces similar results in adult male mice [50]. Taken together, these findings suggest that the male urogenital system is subject to disruption by estrogenic chemicals, including BPA, during both fetal and adult life.
4.2. Study goals
Our primary objective was to examine the impact of BPA and EE on development of the urethra and morphology of the UGS.
4.3. Methodology
We examined PND 1 male rats as described previously. [1]. Briefly, NCTR Sprague Dawley female rats were assigned to one of eight treatment groups: vehicle control; 2.5, 25, 250, 2500, or 25,000 μg/kg/day BPA; or 0.05 or 0.5 μg/kg/day EE. Starting at GD 6 and continuing until parturition, dams were administered chemicals daily by gavage. There were no naïve (not gavaged) controls included, although a pre-CLARITY study showed that gavage significantly alters fetal development [61].
To eliminate the possibility of litter effects, one pup per litter was randomly selected by FDA personnel and coded. We were thus blind to treatment group. From each pup, the UGS was removed, fixed, and sectioned. Serial tissue sections were imaged (distance between each image = 15 μM), and all images were imported into a three-dimensional reconstruction software program (BioVis3D; Montevideo, Uruguay). BioVis3D was used to trace the urethra and other structures in each image to render a 3D image. Immunohistochemistry was completed using 9 different antibodies focusing on the urethral epithelial area located within the widest part of the urethra and surrounding tissue.
4.4. Results
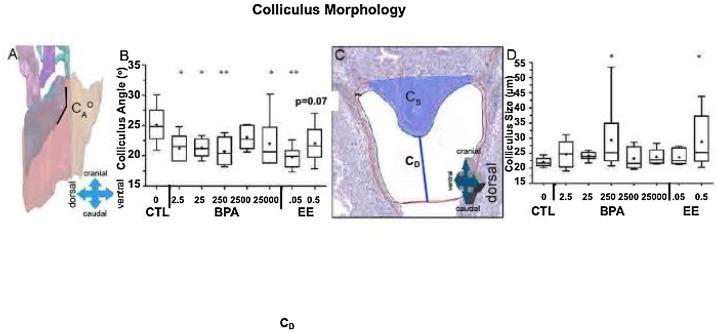
All results were analyzed relative to the body weight of the animal. We replicated our prior finding that the colliculus region of the UGS showed significant changes in structure and morphology (Fig. 3 A). The changes were observed at all BPA doses except the 2500 μg dose group, which only had 5 animals, all of which were selected by NCTR personnel from only one of the replicate breedings that occurred over 5 months (this is not standard procedure). The only significant effect at the highest dose of BPA (25,000 μg/kg/day) was on the shape of the colliculus, which is associated with the remnant of the Mullerian duct that forms the cranial region of the vagina in females. Unlike all other outcomes, there was a monotonic dose-response to estrogens (Fig. 3B). The low EE dose significantly differed from vehicle controls, while the higher EE dose only tended to differ from vehicle controls (P = 0.07). In addition, the 250 dose of BPA and 0.5 dose of EE resulted in a significantly enlarged colliculus (Fig. 3D).
Fig. 3.
Colliculus measurements. Colliculus angle (CAo) was defined as the angle the colliculus makes at the juncture with the cranial urethra (A) and analyzed by treatment (B). The colliculus size (CS), shaded blue was determined by measuring the colliculus distance between the lowest caudal point of the colliculus and the lowest caudal point drawn on the urethra and taking the reciprocal (C) and analyzed by treatment (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
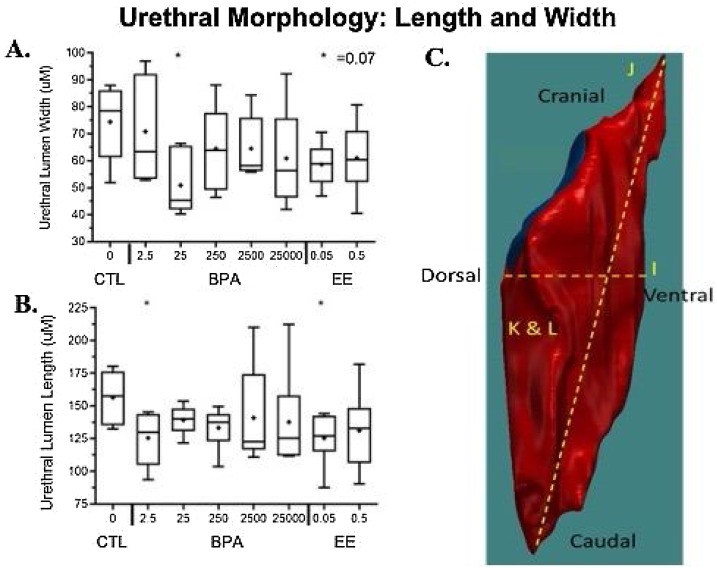
The second major finding was that BPA reduced the length and width of the urethra (Fig. 4 ). These effects occurred as a result of maternal exposure to low doses of BPA (2.5, 25 μg/kg/day). The 0.05 dose of EE also significantly reduced the length of the urethra.
Fig. 4.
C. Urethra 3D reconstruction using BioVis3D demonstrating how this software can isolate and quantify individual regions of the UGS to calculate length and width (μM), surface area (μM2), and volume (μM3) of the urethra. Shown is urethral distance measured straight across from the dorsal (most cranial point of urethra on most dorsal section) to ventral aspect: dorsal-ventral distance (I); cranial-caudal distance (J); urethral volume (K); and surface area (L). A. The width of the urethral lumen was significantly smaller in the BPA 25 and EE 0.05 groups relative to vehicle controls. B. the length of the urethra was significantly shorter in the BPA 2.5 and EE 0.05 groups relative to negative controls.
Collectively, these findings suggest that low-dose effects of BPA on the colliculus and urethra are via an estrogenic mode of action. An exception was our finding that males treated with 25, 250, or 2500 μg/kg/day BPA showed a significant decrease in thickness of the urothelium, which was not affected by either dose of EE. Immunohistochemistry results using 9 antibodies were highly variable, and there were no consistent histological localization changes associated with treatment of BPA or EE, in contradiction to prior results.
In contrast to our findings from studies with various strains of mice, BPA increased body weight at birth, but only in the low-dose BPA 2.5, 25 and 250 μg/kg/day groups. In addition, both doses of EE were significantly heavier than vehicle gavaged controls (Fig. 5 B). Adding a quadradic term in our statistical analysis revealed a significant non-monotonic effect of BPA on body weight at birth. Other similar findings that show an increase in body weight at birth due to fetal exposure to a low dose of BPA in rats has been reported [62].
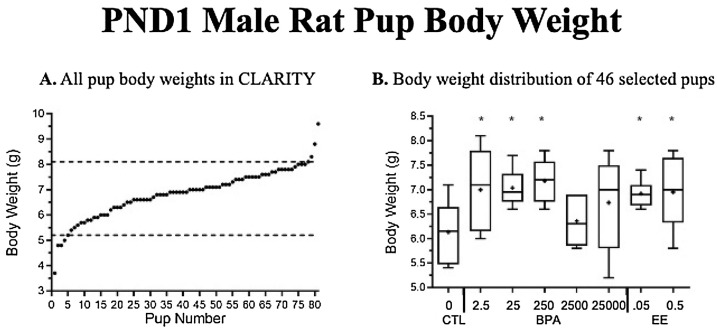
Fig. 5.
(A) Body weight data on postnatal day (PND) 1 from 81 male rats collected and weighed by the NCTR staff to study effects of BPA and EE on urethra and bladder morphology. (B) From 81 males, 46 met the weight range criteria of 5 – 8.1 g and were provided blind for our urethra study by staff at the NCTR (Fig. 5-B). There were 5 blocks of matings, but males exposed to 2500 μg/kg/day BPA were all selected from treatment block 2, which tended to be lighter than pups from other blocks.
4.5. Discussion
Variability among the collected pup body weights was higher than expected (Fig. 5A), which was perhaps due to chronic stress associated with daily gavage [63]. For example, rat pups selected for morphological and structural analyses of the PND1 urogenital system ranged in body mass from 3.7 to 9.6 g, a >250 % range in body mass. However, even with this and other limitations [19] we observed consistent findings that supported our prior studies of UGS malformations, decrease in the size of the urethra, and enlargement of the colliculus as a result of exposure to BPA and EE.
The effects we found to be significantly different from controls occurred at specific doses of BPA and EE, consistent with findings that through a variety of mechanisms, hormones, hormonal drugs, such as EE, and hormonally active chemicals, such as BPA, result in non-monotonic dose response curves, with results at one dose being different from results at other doses when there is as much as a 10-fold difference between doses [64]. The interesting exception was the one finding of a high (25,000 μg/kg/day) dose of BPA impacting the shape of the colliculus, which contains the remnant of the Mullerian duct. This is consistent with our prior finding that a high (200 μg/kg/day) oral dose of DES in mice blocked the development of prostate ducts but resulted in the lack of regression of the Mullerian ducts that would normally regress in male fetuses in response to Mullerian inhibiting hormone [59]. No other statistically significant difference was found between the highest dose of BPA and vehicle controls. Finally, a finding that was not previously examined was measurement of the thickness of the urothelium, which in this study was significantly decreased by BPA at 25, 250 and 2500 μg/kg/day doses, while neither dose of EE produced this effect, suggesting that unlike our other findings, this effect may not have been mediated by estrogenic mechanisms.
5. Ana Soto: effects of BPA on the mammary gland
5.1. Introduction
The Soto laboratory has conducted studies on the effects of perinatal BPA exposure at several endpoints spanning reproduction, neuroendocrine development, behavior, obesity, and mammary gland development and carcinogenesis [[65], [66], [67], [68], [69]]. In these studies, exposure started at GD 8 and most of them ended at PND 16. Additionally, even the lowest doses (25 ng/kg /day) had significant effects.
Regarding the mammary gland, developmental studies in mice reveal altered mammary gland morphogenesis (from GD 18 to 6 months of age), increased sensitivity to E2, and development of intraductal hyperplasia after 3 months of age [[70], [71], [72], [73], [74], [75], [76], [77], [78], [79]]. Additionally, BPA exposure alters mammary gland development in fetuses of non-human primates [74].
In Wistar [80] and Wistar-Furth [81] rat models, we found that BPA induces intraductal hyperplasia in the mammary gland and changes in the DNA methylome, which manifest at all ages studied. At 50 days of age, methylome and transcriptome results are consistent with histological findings (i.e., intraductal hyperplasia) [82]. Additionally, carcinomas in situ are found in BPA-treated animals [81]. Sprague Dawley rat model studies also reveal that, regardless of whether animals are exposed gestationally or gestationally and lactationally, preneoplastic lesions develop in BPA-exposed female offspring across all doses as early as PND 50. Further, mammary gland adenocarcinomas develop in BPA-exposed offspring by PND 90, and carcinomas are present in BPA-treated animals but absent in controls [70].
Both mouse and rat models indicate a developmental effect of BPA that predisposes the mammary gland to neoplastic development. The fact that carcinomas appear in rats exposed solely to BPA suggests that BPA is a complete mammary gland carcinogen [70]. Additionally, early developmental effects revealed by altered morphometric parameters indicate that the mammary gland is a sensitive BPA target, as effects are detected at doses as low as 25 ng/kg /day. This suggests that the mammary gland could be used as an endpoint to assess developmental toxicity and as an indicator of increased propensity to neoplastic development [72,83].
Several studies indicate that BPA is a mammary gland toxicant [72,83]. Further, several studies have found that BPA exposure at diverse developmental stages (fetal, neonatal, adult) increases the propensity of developing mammary cancer in rodent models [[84], [85], [86], [87]]. Diverse studies spanning different endpoints ranging from development of the ductal system of the fetal mammary gland to mammary carcinogenesis reveal a non-monotonic dose-response curve [73,85].
5.2. Study goals
Our overarching goal was to obtain morphological markers of altered mammary gland development that could be used as indicators of an increased propensity for cancer and to provide a quantitative assessment of mammary gland development. We explored the following hypotheses: (1) pre-pubertal mammary gland morphology at PND 21 is an excellent predictor of pathological outcomes that manifest during adulthood, based on data obtained independently in our laboratory (mostly in mouse models) and that of Dr. S. Fenton (National Toxicology Program) (mostly using rat models); (2) DNA methylation profiles and concomitant alterations of gene expression at PND 21 are predictors of pathological outcomes that manifest during adulthood, which was done in collaboration with Dr. Shioda, Mass General Hospital); (3) perinatal exposure to BPA induces abnormal post-pubertal/adult development of the mammary gland; and (4) BPA generates non-monotonic dose-response curves.
5.3. Methodology
We obtained mammary glands from CLARITY-BPA rats treated with vehicle control, BPA, or EE. Female rats were dosed with vehicle, EE (0.05 or 0.5 μg/kg/day), or BPA (2.5, 25, 250, 2500, or 25,000 μg/kg/day) from GD 6 to PND 21 (stop-dose) or until the end of the experiment (2 years; continuous-dose).
Assessing mammary gland morphogenesis: In contrast to mouse models there is a paucity of reports on the effect of fetal BPA exposure on rat mammary gland morphogenesis. This is in part due to the florid structure of the ductal tree that grows more conspicuously into the third dimension and makes quantitative assessment beyond weaning challenging. This feature of the rat mammary gland hinders the use of standard morphometric tools for the analysis of the rat mammary ductal system. Instead, conventional scoring methods are used. They are called semi-quantitative because they construct a score from qualitative and countable morphological features, such as terminal end buds; the higher the score the more developed the gland is.
We compared a semi-quantitative method (the scoring method using the criteria reported in Davis and Fenton [88] modified for the present study per Montévil et al., [89] with a quantitative method consisting of confocal microscopy, 3D reconstruction, and analysis using a software tool we developed within the CLARITY study [89]. The analysis included quantities such as the aspect ratio (length/width), the epithelial area, and the fractal dimension of the epithelium in 2D (the projection of its 3D image), an evaluation of the surface of the epithelium, of its volume, and of its 3D fractal dimension. Additionally, several plugins from ImageJ were also used to count 3D objects, assess 3D shapes and to skeletonize the epithelium. The skeletonized epithelium was used for counting the number of branches, measuring average branch length, etc.
We developed a plugin that reconstructed the mammary tree for analysis. This reconstruction was then used as the basis for evaluating characteristics such as branching (branching angles and the tortuosity of the branches), local duct thickness, etc. Overall our method assesses 91 structural features of mammary glands. All these measurements were performed in whole-mounted mammary glands, with the exception of methylome and transcriptome studies which were done on frozen tissues.
5.4. Results
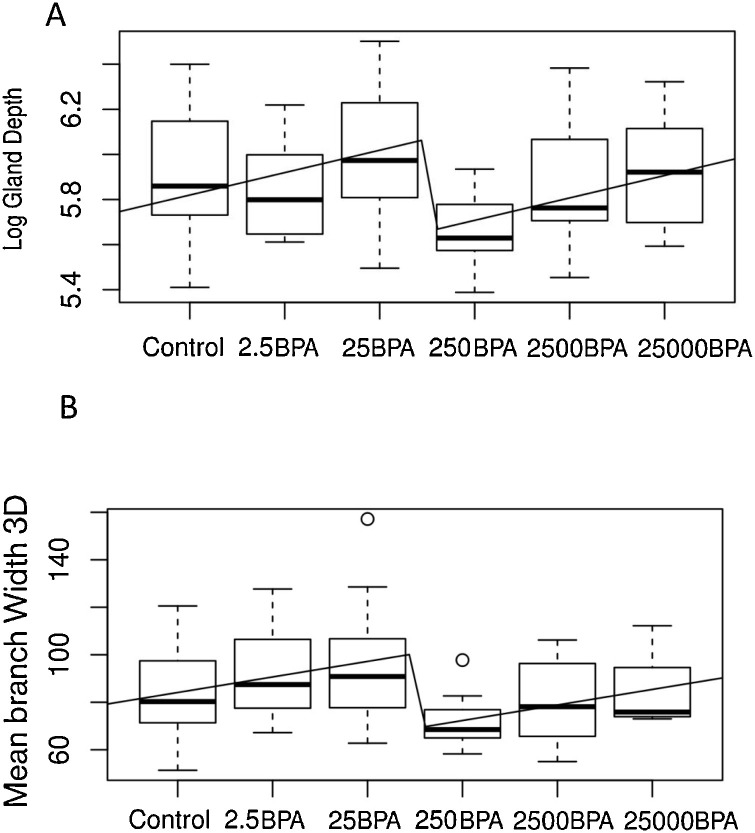
First and foremost, our computer-assisted unsupervised analysis that performs 91 distinct measurements demonstrated that the dose-response curve to BPA in PND 21 mammary glands is non-monotonic, with a breaking point between 25 and 250 μg/kg/day doses (Fig. 6 ).
Fig. 6.
Non-monotonic responses in (A) depth (measured in μm) of the epithelial gland compartment and (B) mean of average branch width (measured in μm) to bisphenol A (BPA) doses in postnatal day (PND) 21 animals. In both graph the x axis represents BPA doses in ug/kg/day. Non-linear regression illustrates a breaking point between 25 and 250 μg/kg/day doses. Graphs represent mean and standard deviation for each dose, and fit with a combination of linear and step functions. This pattern was observed for the majority of endpoints measured.
Similar to the analysis of PND 21 glands non-monotonic dose-response curve were observed in all quantitative studies (at PND 90 and 6 months) as well as semi-quantitative studies (at PND 21 and PND 90). These results show that various BPA effects are different from those of EE, while some are similar (Fig. 7 ). This is not surprising, since not all estrogenic substances produce the same effects [73,90,91]. Consistent with these data showing non-monotonic and more pronounced effects at low doses, the CLARITY-BPA Core study found a significant increase in mammary gland adenocarcinomas and adenomas with the lowest dose of BPA (2.5 μg/kg/day) when exposure stopped at PND 21.
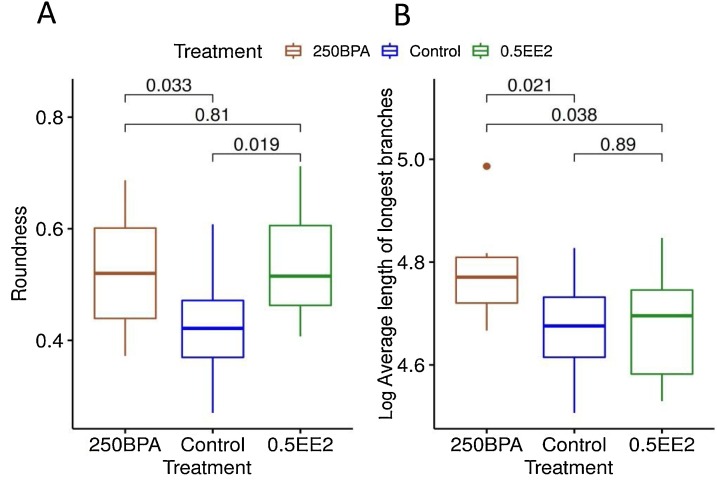
Fig. 7.
Box plots of (A) roundness (ratio between smallest and largest axes of gland) and (B) log of average length of longest branches (length > 75 μm) of postnatal day (PND) 21 animals treated with control, 250 μg BPA/kg/day, or 0.5 μg/kg/day EE (n = 8–10 animals per group). P-values correspond to pairwise t-test.
We compared automated quantitative measurements of the glands (method briefly described above) with the semiquantitative developmental scores for the PND 21 gland (assessed using the Davis and Fenton method) [88] and found correlations between this score and numerous morphological features. Highest correlations with the score were for 2D fractal dimension of the gland (CC: 0.88; P = 7.7e-27) and number of branches (CC: 0.86; P = 4.5 e-24).
Additionally, we also compared developmental scores with dimensions from principal component analysis (PCA). The scoring captured aspects of the two first dimensions of PCA (size and thickness of glands) and was not correlated to the third (length of ducts) or to any additional dimensions. This relationship between developmental score and PCA dimensions is meaningful because it corresponds to the directionality of developmental characteristics observed between control and 0.5 μg/kg/day EE-treated glands. Indeed, the developmental scoring criterion was optimized to detect effects resulting from EE exposure, the positive control for comparison with BPA-exposed mammary glands. Because the effects of EE and BPA were not similar in all studied endpoints, this comparison was insufficient to detect significant non-linear responses in ductal length and several other morphological features that were shown to be affected by other analyses. Nevertheless, semi-quantitative scoring did show a non-significant non-monotonic response in morphological development between glands exposed to 25 or 250 μg/kg/day BPA. BPA and EE resulted in different responses—while EE accelerated gland development, BPA led to abnormal development when assessed at PND 21.
Eight lesions were identified in whole-mounts and histological sections from eight PND 90 mammary glands across both continuous-dose and stop-dose treatment groups. No lesions manifested in vehicle-treated animals, and all lesions were diagnosed as benign or malignant, ranging from lobular hyperplasia, fibroadenoma, periductular fibrosis, or ductal epithelial necrosis with lymphocytic infiltration to ductal carcinoma in situ. We identified 33 total lesions in whole-mounts and excised 24 from 6-month-old mammary glands across both continuous-dose and stop-dose treatment groups. Three malignant tumors (adenocarcinomas) were classified from continuous-dose and stop-dose 0.5 μg EE-treated females, and the remaining lesions/benign tumors were found in vehicle and 2.5, 25, and 25,000 μg/kg/day BPA-treated females. Benign lesions included lobular or ductular alveolar dilatations (with and without secretions), periductular fibrosis (with and without lymphocytic infiltration), fibroadenomas, and adenomas. Notably, the number of animals per group in this experiment was ∼10, whereas our previous study (Acevedo et al.) had 27–35 animals per group. This could explain why there were neoplastic lesions in BPA groups (frequency: ∼1 per group) and none in controls.
5.5. Discussion
The most salient results of these experiments are that i) developmental exposure to BPA reveals a non-monotonic dose-response curve on mammary gland development at all ages examined, showing a break between the 25ug/kg/day and 250 ug/kg/day doses, ii) perinatal BPA exposure alters mammary gland development at all ages studied, and iii) PND21 mammary glands provide a very sensitive end point to assess developmental toxicity.
As noted above, our previous experiments in rats show neoplastic outcomes from developmental BPA exposures. Data reported in the CLARITY-BPA Core study agree with our previous work regarding neoplastic outcomes in the mammary gland, thus confirming our observation that BPA is a complete mammary gland carcinogen. The experiments detailed above were aimed at obtaining early morphological markers of altered mammary gland development that could be used as indicators of an increased propensity for cancer and to provide a quantitative assessment of mammary gland development. Our previous experience with effects of BPA on mammary gland development were done in mice, so the results described here cannot be directly compared to those mouse studies. Points assessed in previous rat experiments (intraductal hyperplasia, carcinoma in situ) were observed at PND 50 and 90 in Wistar-Furth rats [81] and in our Sprague Dawley model [70], although we did not examine PND 50 in the experiments described in this study.
Regarding transcriptome and methylome studies, most of our animals were co-housed with the highest-dosed animals (250,000 μg BPA), which has been shown to transmit detectable circulating BPA and BPA-G in some non BPA-exposed controls and thus raises the possibility of contamination [92,93]. Additionally, a majority of RNA samples had significantly damaged RNA with no detectable 18S and 28S peaks. This level of degradation precludes generation of RNA integrity numbers. We have previous evidence of significantly different gene expression patterns at PND 21 in Sprague Dawley rats exposed perinatally in our laboratory to 0, 2.5, or 250 μg BPA (not part of CLARITY-BPA). We are now comparing data of both experiments and hope to submit the paper by the end of the year. Our most salient result is non-monotonicity of the BPA effect.
There is little literature on the effect of BPA on rat mammary gland development, which generally parallels our far more extensive findings in mice. The comparison of experiments conducted in our lab with CLARITY-BPA studies regarding the transcriptome are likely to reveal in detail whether CLARITY-BPA data match our previous results regardless of protocol differences. In summary, there are no obvious discrepancies overall with our previous and present findings. The most important and novel finding of the present mammary gland study is the clear non-monotonicity of the BPA dose-response curve observed in PND21 mammary glands in a double-blinded experiment analyzed with a non-supervised computer-based technology involving 91 different measurements. The fact that non monotonic effects of similar shape and characteristics were also demonstrated using a simpler set of end points in PND90 and 6-month-old specimens is a promising finding regarding the feasibility of introducing these end points in toxicological studies. These results show the importance of applying statistical methods appropriate for non-monotonic responses. Linear models are a powerful tool to provide evidence of a causal relationship because they quantitatively relate the changes of a putative cause with the one of the effects. Hence, exhibiting a linear response provides empirical evidence of a causal relationship. However; this method does not apply to non-monotonic responses, which are common in endocrinology because the putative causes are involved in multilevel, complex regulatory processes resulting from the evolutionary history of hormone functions. In this complex context, an appropriate way to show the presence of causation is to demonstrate the prevalence of a specific non-monotonic pattern, here a breaking point between 25 μg/kg/day BPA and 250 μg/kg/day BPA. Hence, we conclude that the non-monotonic dose response curve (NMDRC) we documented reveals the presence of a causal relationship. Finally, given the extensive number of distinct measurements used in this study and the fact that BPA and EE2 do not induce identical changes, this study is informing us against dismissing end points that are not modified by ovarian estrogens or their analogues when testing the endocrine disrupting effects of “xenoestrogens”.
6. Jodi Flaws: effects of BPA on the ovary
6.1. Introduction
The Flaws laboratory has conducted several studies on the effects of BPA on ovarian function in mice. Collectively, those studies show that BPA may cause infertility in mice by destroying ovarian follicles, the key structures required to maintain fertility in females. Further, BPA may destroy mouse ovarian follicles by inhibiting their growth to the antral stage, which is required for normal ovulation and thus fertility [94,95]; Data also indicate that BPA may destroy mouse ovarian follicles by inducing follicle death via apoptosis, known as atresia [94]. Finally, BPA may cause infertility in mice by reducing the ability of the ovary to synthesize E2 levels [96,97]. These previous findings are published in several peer-reviewed journals [[94], [95], [96], [97], [98]] and review articles [99,100].
Several studies on the effects of BPA on the ovary and female reproductive outcomes indicate that BPA is an ovarian toxicant that significantly reduces fertility [[101], [102], [103], [104], [105]]. Further, some recent studies indicate that prenatal exposure to BPA causes adverse transgenerational effects on ovarian function and fertility in female offspring [103,104].
6.2. Study goals
We tested the hypothesis that BPA exposure inhibits ovarian follicle growth and induces atresia, leading to low E2 levels.
6.3. Methodology
We obtained ovaries and sera from rats treated by the FDA with vehicle control, BPA (2.5, 25, 250, 2500, or 25,000 μg/kg/day), or EE (0.05 or 0.5 μg/kg/day) from GD 6 until 1 year. Ovaries and sera were collected on PND 1, 21, and 90 and at 6 months and 1 year and shipped to the Flaws laboratory at the University of Illinois. Ovaries were histologically evaluated to determine the effect of BPA and EE on the numbers and health of primordial, primary, preantral, and antral follicles. We also measured E2 and progesterone levels in sera.
6.4. Results
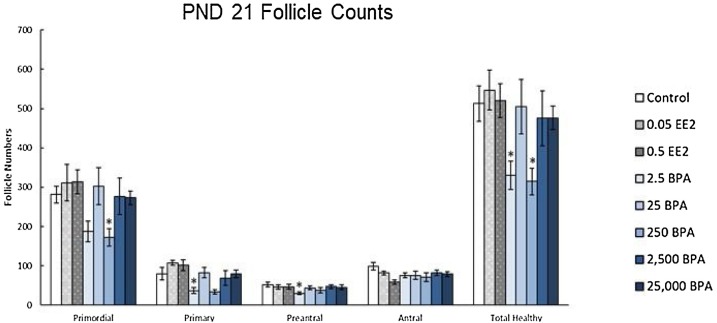
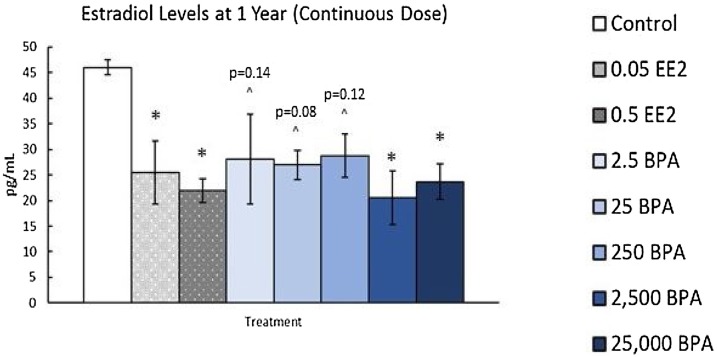
Exposure to BPA (2.5 and 250 μg/kg/day) decreased numbers of primordial, primary, preantral, and total healthy follicle numbers at PND 21 (Fig. 8 ). Exposure to EE (0.5 μg) decreased preantral follicles (PND 90, 6 months) and antral follicles (PND 21 and 6 months) and increased primary follicles (1 year) compared to controls. Additionally, both BPA (2500 and 25,000 μg/kg/day) and EE (0.05 and 0.5 μg/kg/day) exposure decreased E2 levels in animals dosed for 1 year (Fig. 9 ). Collectively, these results indicate that EE and BPA exposures at some doses and timepoints affect ovarian follicle numbers and sex steroid levels in rats [13].
Fig. 8.
Effects of EE and BPA on ovarian morphology at postnatal day (PND) 21. On PND 21, rats from each group were euthanized, and one ovary from each animal was fixed for histological evaluation of ovarian follicle types. Graph represents mean ± SEM of number of follicles. *Significant difference between control group and BPA or EE groups (n = 8–10; P ≤ 0.05).
Fig. 9.
Effects of continuous exposure to EE and BPA at 1 year. At 1 year, rats from each group were euthanized and serum was collected from the blood to measure sex steroid hormones. Graph represents means ± SEM of the amount of estradiol present in serum. *Significant difference between control group and BPA or EE groups (n = 3–9; P ≤ 0.05); ^P > 0.05.
6.5. Discussion
Our previous data indicate that BPA exposure increases atresia and reduces antral follicle growth in mice [94,96,98]. However, BPA exposure did not increase atresia or reduce antral follicle growth in the CLARITY-BPA study. We did observe some effects of BPA on ovarian follicles that were similar to studies in other species, such as lambs and mice. In our study, BPA exposure at 2.5 and 250 μg/kg/day decreased follicle numbers at PND 21. In a study of lambs, Rivera et al. showed that subcutaneous exposure to 50 μg BPA from PND 1–14 decreases the primordial follicle pool in PND 30 ovaries [106]. In a study of mice, prenatal exposure to 0.5 or 50 μg/kg/day BPA decreases the number of primordial follicles present in PND 4 ovaries [107]. These studies indicate that several species are susceptible to BPA-induced reductions in follicle numbers. Contrary to mouse and lamb studies, however, we did not examine follicle populations at PND 4 or 30. Thus, we may have missed effects of BPA on PND 4 and PND 30 ovaries. It is also possible that effects of BPA exposure on the rat ovary may occur at later timepoints than in the mouse or at earlier timepoints than in the lamb.
Similar to our study, other studies with rats show that exposure to BPA affects follicle numbers. One particular study found that BPA exposure (0.5 or 50 μg/kg/day from GD 9 to PND 21) decreases the number of primary follicles in female Wistar rats [108]. Another study found that exposure to BPA (3 μg) from GD 0 to PND 21 increases the number of primary, secondary, antral, and total follicles in the ovary compared to control Wistar rats [109]. Although the effects of BPA on specific follicle populations differed between our study and other rat studies, they collectively indicate that prenatal and prepubertal exposure to BPA can affect follicle numbers in the ovary. Any differences in the effects of BPA on specific follicle populations likely stem from different doses and timing of exposure.
Our previous studies indicate that BPA exposure significantly inhibits ovarian steroidogenesis, leading to reduced E2 levels [[95], [96], [97] 100], so we also expected that BPA exposure would significantly decrease sex steroid hormone levels in rats. Although some doses of BPA significantly decreased E2 levels, not all BPA doses affected E2 levels in rats. However, we may not have statistical power to observe significant effects of BPA exposure on hormone levels at some doses and timepoints. We initially planned to collect and analyze 10 serum samples from each treatment group and timepoint. However, cycling females were euthanized when predicted to be in estrus based on a vaginal smear from the previous day, and this method is not always successful in predicting estrous cyclicity on the collection day. Because it is important to measure hormone levels from animals on the same day of the estrous cycle, the sample size was <10 in some treatment groups used for hormone analysis. This could have reduced our statistical power to observe differences between treatment groups. In fact, we noticed that several BPA treatments reduced E2 levels compared to controls at 1 year, but this reduction was not always statistically significant (P > 0.05).
7. Heather Patisaul: behavioral endpoints
7.1. Introduction
More than a decade of work by the Patisaul laboratory and others using multiple rodent models has repeatedly shown that developmental exposure to BPA can alter the structure and sexual differentiation of many brain regions, including the anteroventral periventricular nucleus (AVPV), amygdala, medial preoptic area, and mediobasal hypothalamus, resulting in altered sexually dimorphic behaviors, particularly anxiety [[110], [111], [112], [113], [114], [115]]. Available human data corroborate animal data and link prenatal exposure to heightened risk of deleterious childhood behaviors including anxiety [[116], [117], [118], [119]].
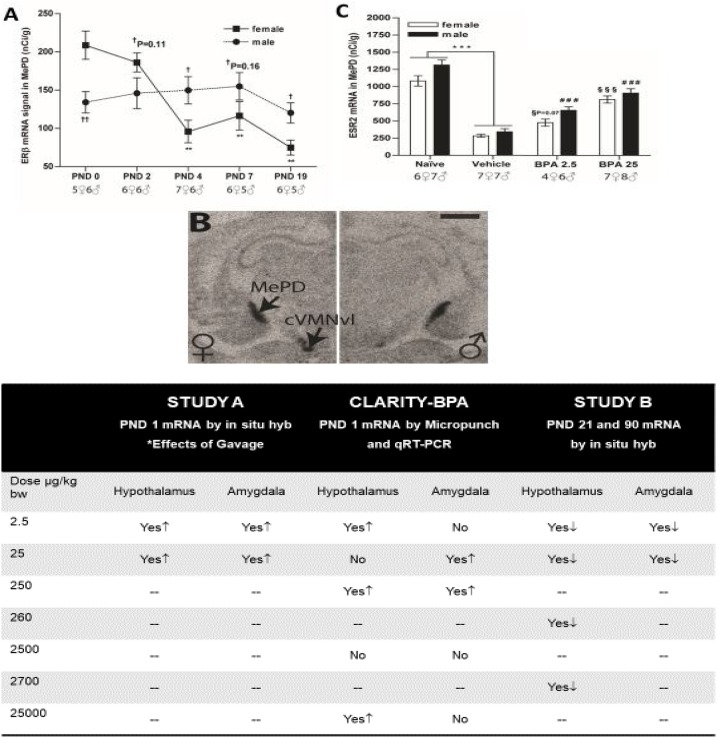
Further, our work shows that the AVPV is particularly sensitive, with perinatal BPA altering its physical size and its sex-specific dimorphism of dopaminergic and kisspeptin neurons, among other outcomes [111,120,121]. Of most relevance to CLARITY-BPA, work in multiple rat strains shows that developmental BPA exposure can alter expression of ERs in multiple brain regions, including the AVPV, amygdala, and surrounding structures, that coordinate reproductive and other sexually dimorphic behaviors [115,121]. Two of these studies were conducted in the same animal strain and in the same facility as CLARITY-BPA, as a prelude to CLARITY-BPA studies under nearly identical conditions and using a similar dose range. The results of these three studies are summarized in Fig. 10 .
Fig. 10.
Concordance of BPA-induced ER mRNA expression changes in the amygdala and hypothalamus across NCTR-based studies. A. Estrogen receptor beta (ESR2) expression in the medial amygdala (MePD) is sexually dimorphic at birth, with higher levels in females, but switches at approximately PND 4, demonstrating how expression can change across development. Significant differences in expression compared to PND 0 levels are represented by **p < 0.01; significant sex differences are represented by †<0.05. B. A representative autoradiogram depicting the sex‐specific expression of ESR2 in the MePD and the neighboring central portion of the ventrolateral region of the ventromedial nucleus (cVMNvl) on PND2. As the sex difference in MePD expression diminishes, the one in the cVMNvl remains pronounced demonstrating that sex differences in ER expression are age and region-specific. C. An example from Study 1 revealing how dramatically different ESR2 expression differed between the gavaged (vehicle) and naïve controls. BPA exposure elevated ESR2 expression in both sexes but not to the level of the naïve controls. ***p < 0.001; ###<0.001 compared to male vehicle controls; §§§<0.001 compared to female vehicle controls. The direction of ER (ESR1 and ESR2) expression changes by dose and study are summarized in Table 5 for the hypothalamus and amygdala. Images adapted and compiled from prior Patisaul publications [61,121,122].
The first of these studies (Study A [61]) was initiated by Sherry Ferguson and colleagues at NCTR and used two doses of BPA (2.5 and 25 μg/kg/day), two doses of EE (5 and 10 μg/kg/day), a vehicle control (carboxy methylcellulose, CMC), and, uniquely, a naïve control (which underwent the same handling as gavage without inserting the gavage needle). Exposure was entirely prenatal, with dams exposed from GD 6 through the day of birth. Heads of PND 1 offspring were rapidly frozen and shipped to the Patisaul lab for quantification of ERα and ERβ expression in the hypothalamus and amygdala via in situ hybridization.
As expected, BPA-related effects were region-, dose-, and sex-specific, with some known sex differences in ER expression eliminated at the lowest BPA dose of 2.5 μg/kg/day [61]. BPA- and EE-related effects were directionally similar, with exposure resulting in upregulation of ER (α or β) in most circumstances. The most striking result, however, was a substantial difference in ER levels between vehicle and naïve controls. ER levels were markedly lower in gavaged controls, particularly in the amygdala, a region integral to stress- and fear-related responses. ERβ expression was especially responsive.
These results suggest a suppressive effect of gavage on ER expression, a finding that has significant implications because gavage is traditionally the dosing method of choice for regulatory-compliant toxicity studies. Additionally, BPA and EE exposure-related increases in ER expression generally returned expression levels to a range typical of naïve animals. Thus, it was concluded that increased expression levels in exposed animals likely reflect an interaction of exposure and stress. Because naïve animals did not consume the vehicle, however, effect of the vehicle itself cannot be ruled out.
The second study conducted as a prelude to CLARITY-BPA (Study B [122]) was a 90-day subchronic study designed and carried out by NCTR and similar in scale to CLARITY-BPA [92,93]. The Patisaul laboratory’s portion of the project examined only females but included vehicle controls of both sexes to ensure known sex differences could be reliably detected and to establish the degree to which BPA and EE could “masculinize” the female brain. Four doses of BPA (2.5, 25, 260, and 2700 μg/kg/day) and two doses of EE (0.5 and 5 μg) were used. Because it was initiated before completion of the study described above, no naïve controls were included. Two exposure windows were used: GD 6 through PND 21, and GD 6 through PND 90. Offspring brains were isolated and frozen on PND 21 or PND 90 and analyzed for ER expression in the preoptic area via in situ hybridization, as done in the prior study.
Concordant with the PND 1 study, effects were region- and sex-specific. In this case, however, BPA exposure generally decreased ER expression, which is opposite of what was found on PND 1. This is not surprising given the dramatic age-dependent differences in baseline ER expression levels observed across the rodent brain [115,123,124]. As in the PND 1 study, ERβ appeared to be more sensitive. Effects of BPA and EE were, again, generally concordant in direction but not necessarily dose. Low-dose BPA effects were not always recapitulated by the lowest dose of EE, with similar results at higher doses. Many prior studies have shown that BPA and other endocrine disrupting chemical-related effects are not always linear [64,125], with some effects observable at low but not high doses for reasons that remain elusive. Whether these non-monotonic effects are reproducible in brain and other tissues was of interest in the CLARITY-BPA study.
7.2. Study goals
We hypothesized that BPA would alter sexually dimorphic and steroid hormone sensitive brain morphology, gene expression and behavior. We tested this hypothesis by 1) assessing brain transcriptomics in the hypothalamus, hippocampus and amygdala on PND 1; b) quantifying the size of multiple sexually dimorphic brain areas (the AVPV, sexually dimorphic nucleus (SDN), posterior dorsal portion of the medial amygdala (MePD), and locus coeruleus (LC)) in juveniles and; c) testing for behavioral changes in juveniles and adults.
7.3. Methodology
CLARITY-BPA studies used two groups of animals. The first group was for behavioral analyses by the Patisaul lab and the laboratory of Cheryl Rosenfeld from the University of Missouri, in collaboration with Sherry Ferguson and her research team at NCTR. Exposure spanned GD 6 to PND 21 and, given the laborious nature of the studies, included only a subset of dose groups available in the CLARITY-BPA study (vehicle; 2.5, 25, and 2500 μg/kg/day BPA; 0.5 μg/kg/day EE). Rats designated for this study were transferred from the main facility to a separate building at weaning to assess anxiety-related behaviors, exploratory behavior, and spatial navigation (Rosenfeld laboratory). One group of animals was tested as juveniles; another was tested as adults.
The brains of juvenile animals were collected and analyzed by unbiased stereology for evidence of abrogated volumetric sex differences in the AVPV, sexually dimorphic nucleus (SDN), posteriodorsal portion of the medial amygdala (MePD), and locus coeruleus (LC) [4].
The second group of CLARITY-BPA animals analyzed was similar to the first set of the two pre-CLARITY-BPA studies and, accordingly, ER expression on PND 1 was of primary interest. These rats were exposed prenatally to one of five doses of BPA (2.5, 25, 250, 2500, or 25,000 μg/kg/day), vehicle, or two doses of EE (0.05 or 0.5 μg/kg/day) and collected on PND 1. Pre-CLARITY-BPA studies used in situ hybridization, but while this technique allows exceptionally high anatomical resolution, it is limited to identification of only one or two genes per section at a time. Because identification of other previously unidentified gene pathways was also considered an important goal, CLARITY-BPA brains were analyzed by a combination of targeted and untargeted transcriptomics assays, with the hypothesis that exposure would alter ER expression levels and other targets in the ER signaling cascade. Three regions of interest (hypothalamus, hippocampus, and amygdala) were isolated by microdissection, and RNA was analyzed by RNA sequencing and qRT-PCR to obtain a richer picture of the genes impacted by exposure.
7.4. Results
Despite numerous prior studies showing robust and reproducible effects of developmental BPA exposure on anxiety and exploratory behaviors [9,12], effects in this case were subtle and sporadic. For example, in the open field test, which assesses exploratory behavior and anxiety, juveniles exposed to 2.5 and 25 μg/kg/day BPA had statistically significant effects at a few interval endpoints, such as time resting in the second five minutes of the test. However, overall evidence for BPA-related effects was minimal and inconsistent and thus not interpreted to be indicative of a biologically meaningful effect [12]. In unexposed controls, some anticipated sex differences were either not detected or the opposite of expected effects, leading to the conclusion that some behavioral sex differences may be uniquely different in the NCTR-Sprague Dawley strain compared to other Sprague Dawley strains [12], a finding not atypical for in-house rodent strains.
Vehicle controls showed expected volumetric sex differences (AVPV, SDN, and MePD) and no exposure eliminated those differences. Although one group has previously reported a volumetric sex difference in the LC that is sensitive to neonatal steroid manipulation, and another has reported sensitivity to developmental BPA exposure [126,127], neither phenomenon was observed in CLARITY-BPA animals. This dimorphism may be strain-specific and thus not a universally applicable endpoint for endocrine disruption.
As in prior studies, however, the AVPV was particularly sensitive to BPA. All doses of BPA enlarged the female AVPV, and a similar enlargement was observed in males at 25 and 2500 μg/kg/day doses. Because endogenous estrogen, via action of ERα, reduces (masculinizes) AVPV volume [128,129], the effect of BPA observed in CLARITY-BPA animals was consistent with anti-estrogenic activity. BPA also increased MePD volume, but only in the right MePD of males exposed to 2500 μg/kg/day BPA. The MePD has numerous structural and functional asymmetries, some of which are maintained by circulating androgens [130,131], thus an effect on only one side is biologically plausible. However, the functional significance of the BPA-related effect is not clear. The MePD integrates olfactory and pheromonal information with hormonal, social, and other cues to facilitate appropriate reproductive behaviors in adulthood [132].
In the PND 1 animals overall, the greatest number of differentially expressed genes were in the male hypothalamus and female amygdala [5,133]. In the hypothalamus, elevated ERα and ERβ expression was observed in both sexes at 2.5, 25, and 2500 μg BPA. In the hippocampus, the only evidence of ER disruption was heightened ERβ expression in males at the 25,000 μg/kg/day BPA dose. Similarly, only ERβ was altered in the amygdala, with expression levels non-monotonically heightened in both sexes. Pathway analysis in the amygdala of both sexes revealed enrichment for corticotropin releasing hormone signaling, an outcome concordant with extensive prior data suggesting BPA-related effects on anxiety and other stress-related behaviors. Similarly, gonadotropin releasing hormone (GnRH) signaling was also identified as a perturbed pathway, consistent with prior work by Patisaul and others showing BPA-related disruption of the AVPV and hypothalamic-pituitary-gonadal axis even at low doses.
Significantly, CLARITY-BPA transcriptome data were consistent with data obtained in the first study by the Patisaul laboratory in conjunction with NCTR showing heightened ER expression in BPA-exposed animals. This reproducibility is particularly remarkable because the two studies used different techniques. The CLARITY-BPA study used microisolated tissue containing the entire region of interest, which allowed assessment of the entire transcriptome but lacked the anatomical resolution of in situ hybridization. Nevertheless, both studies showed that prenatal BPA exposure disrupts neonatal ER expression in the hypothalamus and amygdala.
Transcriptomics analysis was also confirmatory for BPA-related effects in other hormone-sensitive pathways critical for sociosexual behaviors. Additional genes altered by BPA included oxytocin and GABA vesicular transporter (Slc32a1) in the hypothalamus, oxytocin in the hippocampus, and androgen receptor, oxytocin, and vasopressin receptors in the amygdala. Numerous genes involved in glutamate signaling were also disrupted in the amygdala. Further, disruption of oxytocin and vasopressin signaling has been identified previously by Patisaul and colleagues as sensitive to BPA exposure [112,134].
7.5. Discussion
The CLARITY-BPA studies and two preceding collaborative NCTR studies unequivocally show that ER expression in the rat brain is altered by developmental exposure to BPA at doses as low as 2.5 μg/kg/day. In neonates, ER expression is generally heightened, which likely sensitizes the brain to endogenous estrogen. This may explain why BPA is so often observed to be “estrogenic” in vivo, despite its limited binding affinity for ERs in vitro [135,136]. This is particularly significant given that the brain can synthesize its own estrogen and is thus not necessarily dependent on circulating levels [137,138]. Disruption of brain ER is one of the most consistently observed outcomes of developmental BPA exposure. Additionally, CLARITY-BPA studies provide further compelling evidence that developmental BPA exposure alters oxytocin- and vasopressin-related signaling pathways and AVPV volume.
Significantly, CLARITY-BPA gene expression data from the Rosenfeld lab with older animals are highly concordant. Using CLARITY-BPA rats that were tested on the Barnes maze to assess spatial navigation abilities and euthanized at 3 months of age, the Rosenfeld lab found evidence for disruption of hippocampal oxytocin and vasopressin gene expression in animals dosed at 2500 μg/kg/day BPA from GD 6 through PND 21 [6]. Hypothalamic ERα was downregulated in males exposed to BPA or 0.5 μg EE, while hypothalamic ERβ was only reduced in EE-exposed males. Directionally, these effects are consistent with those found by Patisaul’s team in PND 90 animals from the subchronic exposure pre-CLARITY-BPA study [122]. Collectively, these data are consistent with robust literature by Patisaul and others showing that BPA impacts estrogen, oxytocin, and vasopressin pathways throughout the brain [112,121,134,[139], [140], [141]].
8. Cheryl Rosenfeld and Shuk-Mei Ho: effects of BPA on neural DNA methylation, gene expression, and spatial navigational ability
8.1. Introduction
The Rosenfeld lab previously showed that developmental exposure to varying doses of BPA that are considered environmentally relevant affects spatial navigational learning and memory in polygynous deer mice (Peromyscus maniculatus bairdii) [142,143]. Male deer mice show enhanced spatial navigational ability compared to males of related Peromyscus spp. [144]. This behavior in deer mice is considered a sexually selected trait because it confers an advantage in locating females that are likely widely dispersed throughout the habitat [145]. However, male deer mice developmentally exposed to BPA show reduced spatial navigational learning and memory [142,143], suggesting that they would be at a disadvantage in locating potential female breeding partners. We also showed that even if they are able to locate potential reproductive partners, females prefer control males 2:1 over males with early BPA exposure [142]. Further, female deer mice developmentally exposed to BPA or EE show masculinized or improved spatial navigational learning and memory. These previous studies also show that the dietary exposure dose provided to dams results in similar serum concentrations identified in pregnant women unknowingly exposed to this chemical [143].
Follow-up studies in the related species of California mice (Peromyscus californicus), who are monogamous and biparental, show that developmental exposure to BPA and EE does not affect spatial navigational learning and memory in males or females. However, BPA reduces socio-communicative behaviors and affects biparental care [146]. Examination of the global transcriptomic profile in the hypothalamus revealed several genes that are differentially expressed in male and female California mice developmentally exposed to BPA [147]. Gene expression differences also persist in the hypothalamus of male and female California mice engaged in parental behaviors [148]. Such gene expression differences are likely due to DNA methylation and potentially other epigenetic changes [[149], [150], [151], [152]]. Our previous collective findings indicate that, in a rodent model, BPA can disrupt transcriptomic profiles in the hypothalamus that might be epigenetic in origin. Spatial navigational learning and memory can be affected by early exposure to BPA, especially in those species in which it is considered a sexually selected trait.
Other rodent and human studies suggest that males typically tend to exhibit enhanced spatial navigational learning and memory compared to females [[153], [154], [155]]. Several other rodent studies, including in rats, indicate that developmental exposure to BPA can affect spatial navigational learning and memory, with males typically being more vulnerable [[156], [157], [158], [159], [160], [161], [162]]. Boys developmentally exposed to a stronger estrogen, diethylstilbestrol (DES), during gestation also show impairments in this behavioral response compared to unexposed age-matched boys [163].
Past non-human primate models, rodent and zebrafish (Danio rerio) animal models, and in vitro cell culture studies strongly indicate BPA alters individual candidate genes in the hippocampus, hypothalamus, or isolated neurons from these and associated brain regions [115,147,[164], [165], [166], [167], [168], [169], [170]]. Some implicated genes and their protein products include ERα (Esr1 and transcript variants), ERβ (Esr2), DNA methyltransferases (Dnmt1, 3a, 3b), androgen receptor (Ar), brain derived neural factor (Bdnf), vasopression (Avp), and oxytocin receptor (Otr).a
8.2. Study goals
This study tested the following hypotheses. Study 1: Female and male rats developmentally exposed to BPA show later spatial navigational learning and memory impairments. Study 2: Developmental exposure of rats to BPA or EE induces behavior-relevant gene expression and DNA methylation changes in the hippocampus and hypothalamus at adulthood.
8.3. Methodology
In Study 1, pregnant NCTR-Sprague Dawley rats were orally dosed from GD 6 to parturition, and offspring were directly orally dosed until weaning (PND 21). Treatment groups included vehicle control, three BPA doses (2.5, 25, or 2500 μg/kg/day), and a 0.5 μg/kg/day EE reference estrogen dose. At adulthood, one animal/sex/litter was tested for 7 days in the Barnes maze. After completion of this and other behavioral tests, animals were humanely euthanized and measured for serum testosterone concentrations.
In Study 2, RNA and DNA were isolated from hypothalamus and hippocampus to examine expression of 10 genes (Dnmt1, Dnmt3a, Dnmt3b, Esr1, Esr2, Avp, Ar, Ot, Otr, and Bdnf) potentially affected by early-life BPA (2500 μg/kg/day) or 0.5 μg/kg/day EE exposure. Three genes (Bdnf, Dnmt3b, and Esr1) were examined for DNA methylation changes in their putative 5′ promoter regions. Molecular changes in the hippocampus were correlated to prior Barnes maze performance (measured in Study 1), including sniffing correct holes, distance traveled, and velocity.
8.4. Results
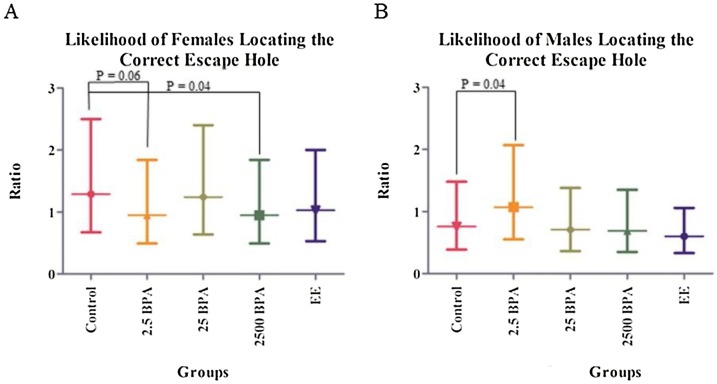
Study 1: The 2500 μg/kg/day BPA group sniffed more incorrect holes on day 7 than those in control, 2.5 μg/kg/day BPA, and EE groups. Notably, 2500 μg/kg/day BPA females were less likely than control females to locate the escape box in the allotted time (P = 0.04; Fig. 10A). Similarly, 2.5 μg/kg/day BPA females showed a trend for prolonged latency to locate the escape hole during the 5-minute time period. Paradoxically, 2.5 μg/kg/day BPA males showed improved latency to locate the correct escape hole relative to control males (P = 0.04; Fig. 10B). The significance of this finding remains uncertain. No differences in serum testosterone concentration were detected in any male or female treatment groups. These results suggest that developmental exposure of rats to BPA may disrupt aspects of spatial navigational learning and memory.
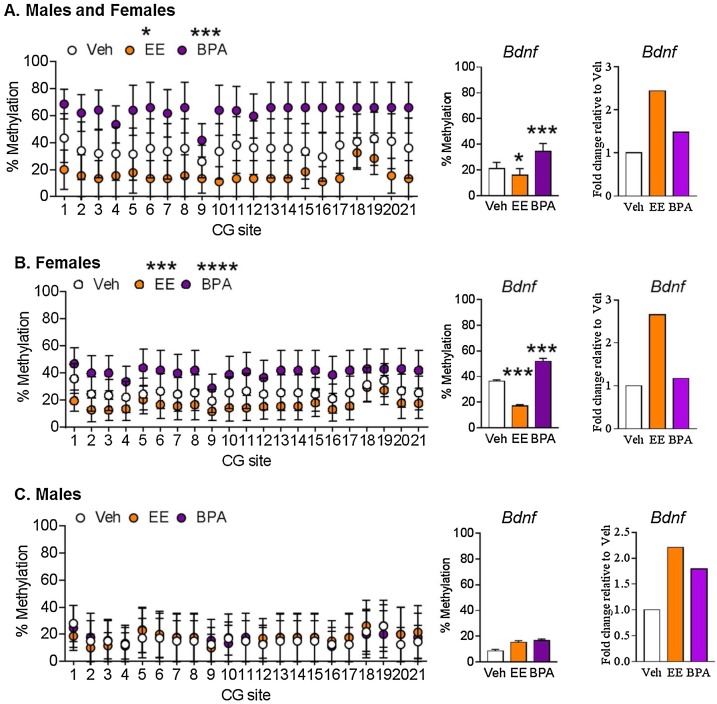
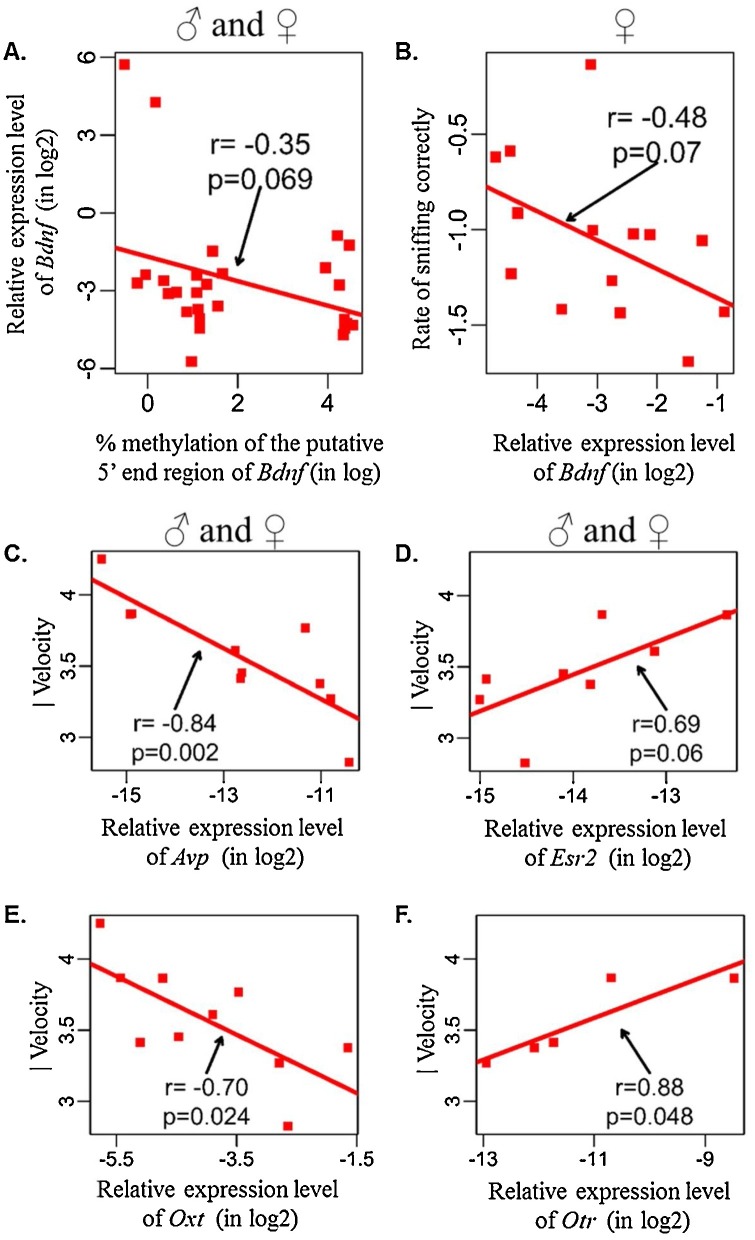
Study 2: Exposure to BPA and/or EE ablated normal profiles of sexually dimorphic gene expression/promoter DNA methylation that should have otherwise been observed in the hippocampus and hypothalamus. BPA exposure led to hypermethylation of the putative 5′ promoter region of hippocampal Bdnf, whereas in this same brain region, EE-exposure resulted in hypomethylation of Bdnf in female rats (Fig. 11 A–C). Bdnf methylation was weakly associated with Bdnf expression in hippocampi of male and of female rats (Fig. 12 A). Bdnf methylation tended to correlate with its gene expression pattern in female hippocampi. Hippocampal Bdnf expression in females showed a trending negative association with sniffing the correct hole in the Barnes maze (Fig. 12B). Hippocampal expression of Avp, Esr2, Oxt, and Otr were strongly and positively associated with velocity of control rats in the Barnes maze, but such correlations were absent in BPA- and EE-exposed rats (Fig. 12C–F). These findings suggest BPA exposure induces unique gene expression and epigenetic changes in hypothalamus and hippocampus of adult rats, with the latter brain region governing spatial learning and memory ability (Fig. 13 ).
Fig. 11.
Overall hazard ratio for (A) females and (B) males in each treatment group to locate the escape hole in a Barnes maze. Note that increasing ratio equates to shorter latency. For both graphs, upper, middle, and lower bars represent ratio of locating the correct escape hole at 95 % upper confidence limit, mean, and 95 % lower confidence limit, respectively, for each group. Hazard ratio was used to account for those individuals who did not locate the escape hole in the allotted time (5 min). Comparisons of the significant two-way interaction for treatment ∗ sex are shown. Fig. reproduced with permission from Hormones and Behavior.
Fig. 12.
Effect of early-life exposure to EE and BPA on methylation and expression of Bdnf in the adult rat hippocampus. (A) both males and females, (B) only females, and (C) only males with early-life exposure to vehicle control (white), 0.5 μg/kg/day EE (orange), or 2500 μg/kg/day BPA (violet). Data expressed as mean ± SEM. Each circle represents average percent methylation of each corresponding CG site from 6–7 individual colonies (N = 5 males and N = 5 females per group). For methylation analysis, *P < 0.05, ***P < 0.001, and ****P < 0.0001 vs vehicle by two-way ANOVA and Tukey’s multiple test comparison. For gene expression analysis, ***P < 0.001 vs vehicle by one-way ANOVA and Tukey test. Gene expression levels are expressed as gene expression level in treatment group relative to control group using the 2−ΔΔCT method. Reproduced with permission from the Epigenetics.
Fig. 13.
Correlation analyses for hippocampal Bdnf expression. (A) Overall correlation between expression of Bdnf and percent promoter methylation of Bdnf in rats using all data points. (B) Overall correlation between expression of Bdnf and rate of sniffing the correct hole in the Barnes maze in female rats. Gene expression levels are expressed in log 2 values and as CT value of Rpl19 relative to target gene. Percentage methylation of the putative 5′ end region of Bdnf is in log value. Correlation between velocity in the Barnes maze and hippocampal expression of (C) Avp, (D) Esr2, (E) Oxt, and (F) Otr in control rats. Gene expression levels expressed in log 2 values and as CT value of Rpl19 relative to target gene. Percentage methylation of putative 5′ end region of Bdnf is in log value. Velocity is expressed as mean from seven observations. P < 0.05 was considered statistically significant. P < 0.05 was considered statistically significant. Only samples with detectable CT values were used in correlation analysis. Reproduced with permission from the Epigenetics.
8.5. Discussion
There are likely several explanations why the current CLARITY-BPA study results differed in relation to the effects of BPA on spatial learning and memory behaviors from our past studies with deer mice where developmental exposure to BPA had greater effects in males by impairing spatial learning and memory [142,143]. In contrast, data obtained from the CLARITY-BPA study suggests that females were more vulnerable to the effects of BPA as developmental exposure to this chemical caused spatial learning and memory deficits in females, whereas, developmental exposure to BPA either did not alter this behavior in males or inexplicably at the lowest dose tested (2.5 μg/kg/day BPA) enhanced this trait in males. The first is species—as detailed above, spatial navigational learning and memory is an important behavioral response for polygynous deer mice. It is not clear the extent that this behavior has undergone evolutionary selection in male or female NCTR-Sprague Dawley rats. The fact that the response was greater in female than male rats indicates it is not a male sexually selected trait in this species.
Another major reason for conflicting findings is the dose and route of exposure. In our previous studies, BPA was incorporated into the diet to replicate the primary and chronic route of exposure in most humans [171,172]. Additionally, this method is considered non-invasive and induces minimal stress to pregnant dams and neonates. Instead, CLARITY-BPA studies used oral gavage to dose pregnant dams and neonates. While this might replicate daily oral exposure in humans, it could also introduce a degree of stress.
While internal serum concentrations of BPA were not measured in the current studies, an analogous FDA study did measure such concentrations following similar exposure doses [92]. That previous study reported internal dosimetry data collected in the preceding NCTR 90-day subchronic BPA study, which used the same animal model, dosing regimen (i.e., daily oral gavage of dams from GD 6 through start of parturition, and direct dosing of pups from PND 1), and similar dosing formulations (0.3 % CMC as vehicle; BPA and EE doses that approached or matched the ones included in the current CLARITY-BPA study).
The BPA doses chosen for CLARITY-BPA were selected to provide low, middle, and upper levels of exposure and to be below the no-observed-adverse-effect level (NOAEL) of 5 mg/kg/day BPA as detailed previously [173,174]. However, it is not clear if these doses actually replicate internal circulating concentrations in pregnant women and those unknowingly exposed to the chemical. In our past studies, we also exposed dams to BPA-treated diets from 2 weeks before conception through lactation, as studies suggest that BPA can be transferred across the placenta and via milk [[175], [176], [177], [178]]. However, current studies directly exposed neonates to BPA via oral gavage, which could have stressed the animals. Other potential explanations to account for disparate results between the CLARITY-BPA and past studies include differences in animal husbandry, such as phytoestrogen contamination in the diet and BPA contamination from cages and other housing equipment, and age of individuals at the time of behavior and biomolecular assessments. Even so, the experiments done as part of the CLARITY-BPA project suggest that BPA can affect aspects of spatial learning and memory and lead to associated changes in DNA methylation and gene expression in the hippocampus and hypothalamus.
9. Scott Belcher: effects of BPA in the heart
9.1. Introduction
The Belcher lab previously reported the effects of BPA on contractile function in isolated myocytes and in the hearts of both rats and mice. Those studies demonstrate that very low concentrations of BPA and 17α-estradiol sex-specifically alter rapid estrogen signaling in females by mechanisms that involve activation of ERα and ERβ [179]. In those studies, BPA exposure caused abnormal Ca2+ handling, altered excitation–contraction coupling, and increased arrhythmias in females [180]. Additional in vivo studies demonstrate that BPA exposure sex-specifically alters collagen content, modifies the extracellular matrix of hearts of both males and females, alters fatty acid and glycolytic metabolism, and increases sensitivity of the female heart to ischemic damage [181].
Several additional studies demonstrate that BPA impacts the heart and cardiac function and indicate that the heart is a target for effects of BPA [[182], [183], [184]]. The finding that BPA, like endogenous estrogen, can impact cardiac function is not surprising—ERs are expressed in the heart of both males and females [185], but the impacts of BPA in the heart are often sex-specifically regulated and can differ in males and females [[179], [180], [181], [182], [183]]. The human relevance of these experimental studies is supported by numerous epidemiological studies and systematic reviews that support an association between higher BPA exposures and increased risk for cardiovascular disease, obesity, type 2 diabetes, insulin resistance, and hypertension in adults as well as obesity in children [[186], [187], [188], [189], [190], [191], [192], [193]].
9.2. Study goals
This study tested the central hypothesis that BPA has harmful disruptive effects on the heart, in a dose-dependent fashion (possibly non-monotonic), that result in cardiac pathology.
9.3. Methodology
Quantitative morphometry and histopathology analysis were performed on hearts isolated at PND 21 and 90 and at 6 months of the CLARITY-BPA study to determine the effects of BPA and EE. At each analyzed timepoint, animals were weighed and euthanized. Hearts were harvested and their weights were recorded at NCTR. Isolated hearts from vehicle (0.3 % CMC), BPA (2.5, 25, 250, 2500, or 25,000 μg/kg/day), and EE (0.05 or 0.5 μg/kg/day) groups were fixed for 24 h in 10 % formalin, post-fixed in fresh neutral buffered formalin for an additional 24 h, transferred to 70 % ethanol, and shipped to the Belcher laboratory at the University of Cincinnati. There, hearts were prepared, sectioned, and stained with: 1) hematoxylin and eosin for histological evaluation of tissue structure, cellular morphology, and pathology; or 2) Picrosirius Red to quantify total collagen. Total left ventricle (LV) area, diameter, wall thickness, and collagen staining were measured and calculated [181,194]. Cardiomyopathy, late stage cardiomyopathy (focal fibrosis), diffuse degeneration, and inflammatory infiltration phenotypes were each scored according to the Standardized System of Nomenclature and Diagnostic Criteria [195], with cardiomyopathy and LV pathology assessed using a standardized four-point severity scale [196,197].
9.4. Results
The largest observed effects in the study animals analyzed were due to treatment duration (stop-dose vs continuous-dose) and linked to increased stress resulting from daily gavage of offspring. Mean body weight of stop-dose control males at PND 90 was 9.5 % greater than in the continuously exposed control group. Observed differences in body weights were indicative of effects due to post-weaning dosing procedures and/or vehicle in continuously dosed animals. The sex-specific decreased weight found only in males was consistent with previous studies showing that prolonged postnatal stress in males can decrease weight gain over time, whereas body weight of female Sprague Dawley rats is resistant to the effects of stress [[198], [199], [200]].
Evidence of BPA or EE alterations on gross cardiac endpoints related to cardiac hypertrophy were limited. Alterations in LV wall thickness were not observed at any dose in either sex. A significant decrease in heart weight and heart weight normalized to body weight was observed in females exposed to 2.5 μg/kg/day BPA, consistent with our previous findings in mice [181]. In males, collagen accumulation was increased in the highest EE dose group at PND 21. In female hearts, decreased collagen content was observed in rats treated with 25,000 μg/kg/day BPA at PND 90 and 0.5 μg/kg/day EE at 6 months.
In the CLARITY-BPA study, abundant early progressive cardiomyopathy lesions were found in the hearts of most control and exposed animals analyzed at PND 21 [8]. Lesion incidence and severity were greater in control males than in females. In BPA- or EE-treated females at PND 21, cardiomyopathy incidence was increased compared to control females, with significant increases in severity detected in 2.5, 250, or 25,000 μg/kg/day BPA groups and in both EE groups. In a male exposed to 250 μg/kg/day BPA and female from each of the two lowest BPA dose groups (2.5 and 25 μg/kg/day), a diffuse degeneration phenotype involving much of the myocardium was also observed [8].
At PND 90 and 6 months, cardiomyopathy in both males and females was observed in all control samples from both stop-dose and continuous-dose arms of the CLARITY-BPA study [8]. At PND 90, the diffuse degeneration phenotype was again observed in males and females from continuous-dose (males: 25, 250, 25,000 μg/kg/day BPA and 0.5 μg/kg/day EE; females: 2.5, 25 μg/kg/day BPA and 0.05, 0.5 μg/kg/day EE) and stop-dose (males: 250, 25,000 μg kg/day BPA and 0.05, 0.5 μg/kg/day EE; females: 250 μg/kg/day BPA) groups. This degree of extensive cardiac pathology is an indication of exposure-related cardiotoxicity [197].
9.5. Discussion
BPA significantly decreased heart weight and heart weight normalized to body weight in females exposed to 2.5 μg/kg/day BPA, consistent with our previous findings in mice [181]. In female hearts, decreased collagen content was observed in rats treated with 25,000 μg/kg/day BPA at PND 90 and 0.5 μg/kg/day EE at 6 months. Cardiomyopathy incidence was increased compared to control females in 2.5, 250, or 25,000 μg/kg/day BPA groups and in both EE groups. At PND 90 and 6 months, cardiomyopathy in both males and females was observed in all control samples from both stop-dose and continuous-dose arms of the CLARITY-BPA study, precluding detection of BPA-specific effects.
The lack of overt morphology phenotypes in CLARITY-BPA or EE-exposed hearts was expected. Pathology associated with the majority of cardiac insults, including toxicants, typically becomes evident only after adverse cardiovascular events, such as cardiac ischemia or myocardial infarction [181,183]. For experimental studies with rodents, it is well-accepted that an intervention resulting in increased β-adrenergic stimulation, ischemic injury, or genetic manipulation is required to elevate cardiac fibrosis, hypertrophy, and phenotypes resulting in overt cardiac pathology [201]. Such manipulations were not possible in the CLARITY-BPA study and limit any interpretations resulting from negative data. Additionally, compared to control mice, the hearts of control NCTR-Sprague Dawley rats had relatively higher levels of collagen due to known species-specific differences in the proportions of myocytes and fibroblasts present in mouse and rat hearts [202].
Based on increased severity and incidence of cardiomyopathy lesions and the diffuse cardiac degeneration phenotypes observed, the NOAEL for BPA in the heart found in CLARITY-BPA study was <2.5ug/kg/day. Cardiac lesions were also observed more often, and at lower doses in females, findings that support previously reported sex difference for the adverse effects of BPA in the heart.
10. R. Thomas Zoeller: effects of BPA on the thyroid
10.1. Introduction
Previous studies indicated that BPA may interfere with thyroid hormone action. Moriyama et al. [203] reported that BPA is an indirect antagonist on the two major forms of thyroid hormone receptor (TR), TRα1 and TRβ1. Others have shown that BPA can interfere with thyroid hormone-dependent processes in frogs [204] and zebrafish [205].
The Zoeller lab reported that BPA exposure increases total serum thyroxine T4 in 2-week-old male and female rat pups [206], consistent with an inhibitory effect of BPA on TRβ2 in the pituitary that mediates negative feedback of T4 on thyroid stimulating hormone (TSH) [207]. However, BPA exposure increases hippocampal RC3/neurogranin mRNA in male pups, which is directly regulated by thyroid hormone through TRα1 [208,209], consistent with elevated serum T4 [209]. These data indicate that BPA may selectively antagonize TRβ2 compared to TRα, producing a hormonal profile similar to that of thyroid resistance syndrome [210].
However, literature about the potential action(s) of BPA on thyroid hormone signaling is complex. Lee et al. [211] reported that BPA could reduce expression of several genes in rat GH3 cells related to controlling thyroid hormone levels, but only at 10 μM. No effects of BPA were observed in FRTL-5 cells. Sheng et al. [212] reported that 10−9 M BPA could suppress T3-induced gene expression in CV-1 cells, but through a non-genomic mechanism. Kitamura et al. [213] reported that BPA essentially does not bind to mammalian TR. Likewise, Xu et al. [214] reported that BPA does not affect thyroid hormone signaling in perinatal rats, and Kobayashi et al. [215] reported that BPA exposure of rat dams from GD 6 to PND 20 does not affect serum T4 levels in offspring at 9 weeks of age. In humans, Park et al. [216] reported that BPA is negatively associated with serum TSH, an observation similar to that of Aung et al. [217]. These data indicate that the effect of BPA on thyroid hormone signaling is dependent upon the context, including the cell type, receptor isoform, and species.
Table 2 tabulates animal studies published to date that have evaluated the effect of BPA on thyroid hormone. These data present a complex picture, but variability in experimental designs suggests that timing of analysis may be a key factor.
Table 2.
Animal studies evaluating the effect of BPA on thyroid hormone Action.
| Author | Animal strain | Exposure period | Route of exposure and dose | Analyte | Time of assay | Sex | Finding |
|---|---|---|---|---|---|---|---|
| Zoeller et al. [206] | Sprague Dawley (Zivic-Miller) | G6-P21 | Wafer 0, 1, 10, 50 mg/kg daily | Total T4 | P4, P8, P15, P35 | No sex differences | ↑ total T4 on P15 only |
| Maternal only | |||||||
| Xu et al. [214] | Sprague Dawley Nippon Clea | G11-P21 | Drinking water (in 0.01 % EtOH) | Free T4 | P0, P7, P11, P21 | No sex differences | ↔ T4 on P0, ↑ T4 on P7, ↓T4 on P21. |
| Maternal only | 0, 0.1 and 50 mg/L | ||||||
| Ahmed et al. [218] | Wistar | P15- P30 | Gavage | Free T4* | P30 | Not reported | ↓ T4 on P30 |
| VACSERA | Pups | 0, 20, 40 μg/kg | |||||
| Fernandez et al. [219] | Sprague Dawley | P1-P10 | Subcutaneous injection | Total T4 | P90-120 | Females only | ↓ T4 in estrus females |
| IBYME colony | Pups only | Nominal dose 0, 5, 50, 500 μg | |||||
| Kobayashi et al. [215] | Crj:CD(SD) | G6-P20 | Gavage | Total T4 | 3 and 9 weeks | No sex difference | ↔ T4 |
| Charles River Japan | Maternal Only | 0, 4, 40, 400 mg/kg | |||||
| Delclos et al., [93] | NCTR Sprague Dawley rats | G6-P15 or P21 | Gavage 0, 0, 2.5, 8, 25, 80, 260, 840, 2700, 100000, 300,000 μg/kg-BW/day | T3, T4, TSH | PND15 | Male | ↑T3, ↑TSH |
| Ferguson et al. [220] | NCTR Sprague Dawley rats | G6-P21 | Gavage 0, 2.5, 5.0 μg/kg-BW/day | T4, T3 | P21 | No sex difference | ↔T4 |
| Viguie et al. [221] | 2–5-year-old Lacaune ewes | G28-G145 | Subcutaneous injection | Total and free T4 | Newborn cord blood | No sex difference | ↓ TT4 |
| Maternal only | 5 mg/kg | ↓ fT4 |
*Personal communication; ↑ increase; ↓ decrease; ↔ no change.
10.2. Study goals
This CLARITY-BPA study on the thyroid aimed to determine whether: 1) BPA exposure reduces serum thyroid hormone levels, and 2) effects on serum thyroid hormone levels affect specific thyroid-dependent endpoints in the developing brain.
10.3. Methodology
We received serum, brain, liver, pituitary, and heart tissues from all doses of BPA in the CLARITY-BPA animals on PND 15 as well as a separate set of controls and rats of the same age treated with the drug propylthiouracil (PTU). PTU was used because it is known to reduce serum T4 and this would allow us to ensure that our techniques were capable of measuring endpoints sensitive to thyroid hormone. We measured serum thyroid hormones and several thyroid-dependent endpoints in CLARITY-BPA and PTU-treated animals and did follow-up studies on PTU-treated animals.
10.4. Results
Our findings indicate that NCTR-Sprague Dawley rats are remarkably insensitive to low thyroid hormones for unknown reasons and are therefore not appropriate to study thyroid “disruption” in general. Specifically, while PTU treatment reduced serum T4 and increased serum TSH in a predicable manner, there were virtually no effects on endpoints of thyroid hormone action in the developing brain. This observation is not consistent with any other published study of low thyroid hormone on brain development. BPA did not affect serum thyroid hormones in these NCTR Sprague-Dawley rats, in contrast to all other studies using different strains of rats.
10.5. Discussion
It would be incredibly complex to unravel why CLARITY-BPA data were not consistent with our previous findings or published literature. At this stage, our best guess is that NCTR-Sprague Dawley rats simply do not respond to low thyroid hormone in a manner that has been published before.
11. Integrative correlation analysis for independent CLARITY-BPA studies
With the same individual male and female rats tested for multiple experiments that span various systems, the current studies provide a unique opportunity to integrate these interdependent datasets together to determine how developmental exposure to BPA affects not just one independent system but may induce systemic and inter-related effects. Moreover, using the same rats across study designs may permit us to gain insights into the biology of how various bodily systems relate and may influence each other, whether through hormonal or other regulatory factors.
To achieve these lofty goals, we used the mixOmics R package [21], as we have done in previous BPA studies performed in the Rosenfeld Laboratory [[22], [23], [24]]. In this case, we used the program to correlate data obtained by independent CLARITY-BPA investigators. Independent datasets obtained from the master Chemical Effects in Biological Systems (CEBS) database at NIEHS were matched based on individual animal ID, such that data from the same individual used across various study designs were linked together. However, not all investigators used the same sets of animals. Thus, to increase the amount of data integration that could be achieved, we also linked those results for animals from the same litter but that were used in different studies. Rats used in Patisaul’s and Rosenfeld’s behavioral studies were generated from different litters than those used by other CLARITY-BPA investigators. To be able to link the young adult ∼120 days of age behavioral studies with results from around this same time, animals used for these studies were pair-matched to similar counterparts at around this age based on the similar birth date and animal body weight. In the initial analyses, we considered effects of the low dose BPA (2.5 μg/kg) at 180 days of age, Set 8, where several investigators used the same set of animals. In follow-up analyses, we considered the effects of dose (2.5, 250, and 2500 μg/kg/day) and age (21 days of age- weaning, young adult- 90–120 days of age, and older adult 180 days of age), and the integrative analyses for these additional dose and ages where done by using the methods described above. Even so, there were still individual investigator data because of sample replicates and other issues that could not be integrated with the data from other investigators. Herein, we present the r plot results for females and males at 180 days of age who were developmentally exposed to BPA at 2.5 μg/kg/day. The r plots based on these other ages, doses, and sexes, which were used to generate Table 3, Table 4 and Supplementary Tables 1–4, are included in Supplementary File 1.
Table 3.
MixOmics data integration comparison at 2.5, 250 and 2500 μg/kg/day of BPA at PND180 in females.
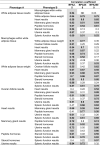 |
Table 4.
MixOmics data integration comparison at 2.5, 250 and 2500 μg/kg/day of BPA at PND180 in males.
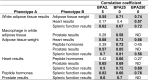 |
ND: Not determined.
We conducted sparse discriminant analysis with partial least square regression with function ‘block.splsda’. Circos plots were generated using the ‘circosPlot’ function, with correlations calculated as described by González et al. [222]. This analysis provides several diagrams to show data relationships. R plots shows overall correlation between collective categories, such as behavioral, cardiovascular, uterine, mammary, etc results. In contrast, the circos plots shows individual parameters measured within the broad categories, such as uterine assessments is one broad category and within this group, apoptosis and PCNA analyses were performed, relate to each other. The program developers recommend examining the circus plots at a correlation of at least 0.7 [21]. We tested several correlations spanning from 0.7 to 0.9 and found that 0.85 provided sufficient stringency and at the same time likely provided meaningful correlations.
For females at this age, integrative correlation analyses were performed for adipose tissue (measured by Dr. Ben-Jonathan’s group), ovarian follicle (Dr. Flaw’s group), heart (Dr. Belcher’s group), uterine (Dr. Ho’s group), splenic (Dr. Kaminski’s group), and mammary gland (Dr. Soto’s group) assessments. R-plot analyses revealed that several parameters significantly correlated with each other (Fig. 14 ). These data suggest that developmental exposure to BPA can simultaneously affect more than a single system, which could be due to direct targeting of the various organs and/or changes in one system lead to downstream affects in other organs. This type of data analysis, however, does not permit us to tease apart these possibilities, which may not be mutually exclusive, or determine directionality. Follow-up studies assessing the same organs at different time points might help to decipher how BPA-induced changes in one organ lead to downstream affects in other organs. This Fig. 14 does reveal adipose tissue fat weight and mammary gland histology showed strong correlation (r = 0.93). Other correlations were for adipose tissue weight and uterine proliferation (r = 0.84), ovarian follicle count and peptide hormones (r = 0.81), macrophages within adipose tissue and steroid hormones (r = 0.78), mammary gland results and peptide hormones (r = 0.72),mammary gland histology and uterine proliferation (r = 0.78), white adipose tissue weight and splenic function results (r = 0.87), heart results and splenic function results (r = 0.82), mammary gland results and splenic function results (r = 0.8), and uterine results and splenic function results (r = 0.84).
Fig. 14.
R plot correlations across 6-month-old female data for low-dose BPA exposure (2.5 μg/kg/day) and controls. This analysis compares white adipose tissue, ovarian follicle, heart, mammary gland, and uterine assessments with peptide and steroid hormone data, and splenic function results. Values with strong correlation (r ≥ 0.7) are highlighted. Representative PCA diagrams with corresponding r-values are also delineated. In the PCA diagrams, each individual replicate is represented with a circle, and the blue and orange circles represent those exposed to BPA or vehicle control, respectively. N = 6 for BPA and vehicle control, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Circos plot analyses revealed several positive correlations between individual parameters within these groups (Fig. 15 A). For example, serum E2 concentrations positively correlated with primordial follicles. Serum prolactin (PRL) concentrations positively correlated with primordial follicles and ovarian fat macrophages. Serum leptin concentrations positively correlated with select CD4+, CD25+/CD4+, and CD8+ T lymphocytes within the spleen. Mammary gland epithelial area positively correlated with CD62L cells within the spleen. CD62L, CD25+/CD4+, and other CD4+ splenic cells also positively associated with subcutaneous and gonadal fat pad weight. Apoptosis within the uterus positively correlated with heart luminal area and ovarian fat macrophages. Uterine weight positively correlated with mammary gland epithelial area and CD4+ and CD8+ T lymphocytes within the spleen. Serum leptin positively correlated with mammary gland fat pad area, subcutaneous fat pad weight, gonad adipocyte size, and ovarian fat macrophages. Ovarian fat macrophages positively correlated with heart weight and heart collagen area. Mammary gland epithelial area positively correlated with heart collagen area.
Fig. 15.
A) Positive and (B) negative circos plot correlations between white adipose tissue, ovarian follicle, heart, mammary gland, and uterine assessments with peptide and steroid hormone data in 6-month-old females exposed to low-dose BPA (2.5 μg/kg/day) or controls. Results for BPA-exposed females are indicated with a blue line outside of the circle; orange line indicates results for control females. Color of the line further from the circle indicates treatment group where these results are greater. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Several negative correlations were also evident for these females across categories (Fig. 15B). Serum E2 concentrations negatively correlated with subcutaneous fat pad weight, mammary gland fat pad area and coverage, uterine necropsy weight, and CD8+, CD4+, and CD25+/CD4+ T cells within the spleen. Total progesterone concentrations negatively associated with CD4+ cells within the spleen. Serum PRL was inversely associated with CD4+ and CD25+/CD4+ cells within the spleen. Mammary gland coverage negatively correlated with uterine apoptosis and heart luminal area. Preantral ovarian follicles negatively correlated with ovarian fat macrophages and subcutaneous fat pad weight. Primordial ovarian follicles inversely correlated with subcutaneous adipose size, gonadal fat weight, and CD+ and CD25+/CD4+ T lymphocytes within the spleen. Total healthy ovarian follicles negatively correlated with subcutaneous adipose tissue size and mammary gland weight. Total unhealthy ovarian follicles negatively associated with serum leptin concentrations.
As detailed above, we extended these analyses to examine the effects of dose and age for those datasets that could be integrated together with one of the above methods. We will first consider the effects of different dosages in female rats at 6 months of age. As shown in Table 3, which summarizes the R plots for these different dosages, several broad categories correlated strongly with each other at the lowest dosage of BPA (2.5 μg/kg/day), which is also shown in Fig. 14. This table though also reveals that several of these correlations were also evident at the middle (250 μg/kg/day) and highest dosage (2500 μg/kg/day). A few examples of such correlations that spanned all three dosages at 6 months of age include: white adipose tissue weight to heart, mammary gland peptide hormone steroid hormone results; ovarian follicle and mammary gland results; mammary gland to heart, peptide hormone, and steroid hormone results.
In tracing the correlations back to those present at 90−120 days of age (Supplementary Table 1). The correlations that extended all three dosages (2.5, 250, and 2500 μg/kg/day) were mammary gland histology and uterine results and peptide hormones and splenic function results. We next considered those correlations only observed at the low dose. For instance, mammary gland morphometrics correlated with periovarian adipose tissue qPCR (r = 0.53); mammary gland histology correlated with ovarian follicle results (r = 0.53) and uterine results (r = 0.57); ovarian follicle results correlated with peptide hormones (r = 0.6) and uterine results (r = 0.5); peptide hormones were associated with heart results (r = 0.52), behavior results (r = 0.86), and splenic function results (r = 0.88); periovarian and subcutaneous adipose tissue qPCR correlated with behavior results (r = 0.73 and 0.59, respectively); steroid hormones correlated strongly with uterine results but surprisingly not with the behavioral results (r = 0.60 and 0.21, respectively); heart and behavioral results correlated with splenic function results (r = 0.6 and 0.64, respectively).
In considering those correlations present in females at 21 days of age, the ones extend across all dosages relate to mammary gland changes (Supplementary Table 2).
For example, mammary gland morphometrics strongly correlated across all dosages with mammary gland histology and prolactin results. At the lowest and highest dosages of BPA, mammary gland morphometrics also correlated with ovarian follicle results (r = 0.55 and 0.66, respectively), and in turn ovarian follicle results were linked to uterine results at these two dosages (r = 0.76 and 0.64, respectively). At the low dose of BPA, mammary gland morphometrics and prolactin and steroid hormones were strongly correlated (r = 0.71 and 0.72, respectively). Prolactin (PRL) and steroid hormone results were strongly linked (r = 0.89), and brain and steroid function results were closely associated (r = 0.7).
For males at 6 months of age, integrative correlation analyses were performed for adipose tissue/peptide hormones (Dr. Ben-Jonathan’s group), heart (Dr. Belcher’s group), prostate (Dr. Prins’ group), and splenic function (Dr. Kaminsky’s group) measurements. R-plot analyses revealed correlations for adipose tissue weight and heart results (r = 0.68) (Fig. 16 ). Macrophages within adipose tissue correlated with prostate and urinary bladder weight (r = 0.5). Adipose tissue weight and splenic function results were strongly correlated (r = 0.7). Peptide hormones and splenic function results showed strong correlation (r = 0.82). Prostate to heart and prostate to splenic function results had some associations (r = and 0.65 and 0.6, respectively).
Fig. 16.
R plot correlations across 6-month-old male data for low-dose BPA exposure (2.5 μg/kg/day) and controls. This analysis compares white adipose tissue results, macrophages in white adipose tissue, adipose tissue weight, heart result, peptide hormones, prostate results, and splenic function results. Values with strong correlation (r ≥ 0.5) are highlighted. Representative PCA diagrams with corresponding r-values are also delineated. In the PCA diagrams, each individual replicate is represented with a circle, and the blue and orange circles represent those exposed to BPA or vehicle control, respectively. N = 7 for BPA and 6 for vehicle control, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Several positive correlations were evident in the circos plot (Fig. 17 A). Examples include heart luminal area positively correlated with prostate necropsy weight and subcutaneous adipose tissue size. PRL and adiponectin positively correlated with subcutaneous adipose tissue macrophages. Gonadal fat weight and subcutaneous (SQ) fat pad weight positively correlated with adipose tissue macrophages. Splenic NKT (CD4+) and CD25 + T lymphocytes positively correlated with SQ macrophages. Ventral prostate weight and gonadal fat weight positively correlated with splenic CD8+ cells.
Fig. 17.
(A) Positive and (B) negative circos plot correlations of white adipose tissue, heart, and prostate assessments with peptide hormone data in 6-month-old males exposed to low-dose BPA (2.5 μg/kg/day) or controls. Results for BPA-exposed males are indicated with a blue line outside the circle; orange line indicates results for control males. Color of the line further from the circle indicates treatment group where these results are greater. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Several negative correlations were evident for males at this age (Fig. 17B). For instance, PRL concentrations were inversely associated with heart wall length. Adiponectin was negatively associated with CD4+ and CD62L cells within the spleen. SQ fat pad weight was inversely linked to CD4+ T lymphocytes in the spleen. Total ventral prostate weight was negatively linked to gonadal fat macrophages. Prostate necropsy weight was in turn inversely correlated with splenic CD4+ T lymphocytes. Gonadal fat macrophages negatively associated with several splenic cells, including total NKT CD4+, CD25+/CD4+, CD33+, CD8+, and CD26L+/CD8+ T cells.
As with the female results, additional integrative correlation analyses were performed for males with the methods detailed above to examine relationships that spanned various dosages and ages. At 6 months of age, the only correlations that spanned all three dosages include adipose tissue weight strongly correlated with adipose tissue size, which is to be expected, and the heart results (Table 4). The surprising correlation between peptide hormones and prostate results evident at the lowest dosage (2.5 μg/kg/day) was absent at the other two dosages. At the middle dosage 250 μg/kg/day), adipose size, adipose tissue weight, and macrophages in adipose tissue correlated with prostate results (r = 0.51 to 0.85). Several other correlations were evident at this dosage for adipose tissue weight including to heart results and peptide hormones (r = 0.73 and 0.68, respectively). Finally, at this same dosage, heart and prostate results were strongly correlated (r = 0.69).
Several strong correlations were evident at 90–120 day old males exposed to all three doses of BPA (Supplementary Table 3). For instance, peptide hormones correlated strongly (r ≥ 0.6) at all three dosages for adipose tissue size and weight. Subcutaneous adipose tissue qPCR correlated with epididymal adipose tissue qPCR and macrophages in adipose tissue. qPCR for both types of adipose tissue correlated at all three dosages with splenic function results. Similar to female results at this age, strong correlations (r > 0.6) were evident at the low dosage for various adipose tissue measurements, peptide hormones, and behavioral results, but many of these were absent at the highest dosage tested. Interestingly, splenic function results showed robust correlation with behavioral results at the low and high dose of BPA (r = 0.75 and 0.78, respectively).
For males at 21 days of age, there were limited datasets that could be combined. Of those that could be integrated, the same dosages as above were not tested in all studies. As shown in Supplementary Table 4, heart and splenic function results correlated at the middle dosage but not the lowest dosage (r = 0.69 and 0.42, respectively). Brain and splenic function results correlated at the highest dosage but not the lowest dosage (r = 0.74 and 0. 34, respectively).
11.1. Discussion of MixOmics analyses
This integrative approach has assuredly provided important clues about how various systems relate to each other. However, the main limitation of this analysis is that it only reveals correlation and not causation, thus the potential sequential order of pathological changes cannot be determined. MixOmics diagrams reveal that in females exposed to low-dose BPA (2.5 μg/kg/day) considered safe by the FDA, there are strong inter-relationships among white adipose tissue, behavior, ovarian follicle, heart, mammary gland, and uterine assessments with peptide and steroid hormone measurements. In males, this same BPA dose results in strong correlations between white adipose tissue, heart, and prostate gland measurements and peptide hormones.
At all three doses of BPA, in both sexes, and multiple ages, clear associations between adipose tissue measurements, splenic function in terms of various white blood cells, and peptide hormones were evident. The circus plots (Fig. 15, Fig. 17) representing the low dose of BPA (2.5 μg/kg/day) in males and females further revealed that adipose tissue measurements, including macrophages within this tissue, were strongly linked to T lymphocyte populations in the spleen but the dynamics showed sex-dependent differences. For instance, in male at this low dosage an increased in gonadal fat macrophages was negatively associated with total NKT CD4+, CD25+/CD4+, CD33+, CD8+ cells, and CD26L+/CD8 T lymphocytes. Thus, the findings suggest that obesity may affect T lymphocyte cells, including regulatory T Cells (Treg- CD25+) and NK- natural killer cells, present to fight various infections, including viruses. As detailed above though this approach only reveals potential associations. However, it is clear from human studies that obese individuals have reductions in these key T lymphocyte lineages [[223], [224], [225], [226]]. Thus, the findings suggest that by acting as an obesogen [227], BPA may thereby also compromise immune function and render individuals susceptible to pathogens, such as COVID-19.
The findings broadly indicate that there is strong and potentially even unrecognized inter-connectedness between organ systems, and thus, by targeting even a single tissue or organ, BPA can lead to downstream and widespread pathological changes. Conceivably, BPA might also induce systemic effects on multiple tissues and organs and on hormone production. Notwithstanding, these integrative analyses reveal that the effects of low-dose BPA are not confined to a single tissue, organ, or system. Instead, complex changes occur in several systems in male and female rats developmentally exposed to BPA. By analyzing multiple ages, BPA doses, and results in males and females with this mixOmics analysis approach, we have identified key associations across body systems. This large-scale approach was possible because of the unique design of these consortium studies, in which several parameters were measured in individual male and female rats. Thus, this analysis makes it impossible to disregard effects at all three doses on health outcomes in this experiment. The fact that several associations between multiple organ systems in males and females were observed at the lowest dose tested, 2.5 μg BPA/kg/day provides convincing evidence that even this low dose, which is considered safe by most regulatory agencies, can lead to systemic health consequences.
At the outset of these studies in 2011, such integrative correlation analyses programs did not exist. Consequently, it was not envisioned at the time to use the same animals for all of the investigator studies to allow for such analyses to be done. Several of the animals were used across multiple studies and by using approaches detailed above, we were able to pair-match animals across studies to allow for these assessments to be done. Yet, there were still individual investigator results that could not be integrated with this mixOmics approach. The fact though that we were able to integrate several investigators findings together and come up with meaningful biological correlations is important, and we are not aware of a comparable study that has used such an approach to inter-relate multi-investigator data across a range of organ systems/biological endpoints, BPA doses, ages, and sexes.
12. Integrated discussion
Overall, many of the independent laboratories confirmed BPA responses in a variety of organ systems in CLARITY-BPA, including results in the brain, prostate, urinary tract, ovary, mammary gland, and heart (summarized in Table 5 ). BPA effects were not observed on thyroid endpoints or on sperm and immune system parameters, the latter two of which were previously published [10,11,228]. But the failure of the NCTR Sprague-Dawley rats to exhibit significant responses to low T4 is unique to this strain and should disqualify it as a model of thyroid disruption. The absence of any effect on sperm at any dose is problematic because the authors did not include either of the positive control (EE) dose groups which are needed to provide verification of system sensitivity [229]. Thus, the ‘no effect’ conclusions drawn by the authors are difficult to interpret because the sensitivity of their study to detect effects of BPA is unknown. All other independent investigators included examination of the positive control. CLARITY-BPA also assessed immune function although the published literature on the effects of BPA on the immune system is contradictory with little consensus as noted in [11]. In the CLARITY-BPA study which examined the effect of BPA on splenocytes 35/630 measurements were statistically different from controls. The most significant effect was the augmentation of lymphoproliferation in response to pokeweed mitogen stimulations in one-year old rats. This effect was also observed in the EE group. The other positive effects were not dose dependent. The authors did not examine the data for non-monotonic dose responses and concluded that the observed changes were unlikely to compromise immune competence in adults [10,11].
Table 5.
Comparison of outcomes from CLARITY-BPA with prior academic studies.
| Organ | Outcome in CLARITY-BPA | Outcome in prior studies | Comparison |
|---|---|---|---|
| Prostate | No prostate pathology (in unstimulated males). | No prostate pathology (in unstimulated males) | Similar to our prior studies, BPA alone was insufficient to induce prostate pathology. |
| Severity of PIN lesions in lateral prostate after adult T + E treatment (increased in 2.5, 250, 25,000 μg/kg/day BPA stop-dose); peaking at 2.5 μg BPA dose. No dorsal, ventral lobe effect. | Lateral lobe PIN severity increased after adult T + E at all BPA doses (neonatal 0.1 to 5000 μg). Dorsal and ventral lobe increased at 100 μg BPA. | CLARITY-BPA confirmed prior publications showing increased prostate carcinogenic susceptibility to elevated E2 in adulthood. | |
| Increased carcinoma multiplicity (4-fold) in dorsolateral prostate ducts at 1 year after adult T + E at 2.5 μg BPA (stop-dose). | Increased carcinoma incidence in lateral prostate at 1 year after adult T + E at neonatal 10 μg BPA dose. | CLARITY-BPA data confirm our prior studies that low-dose BPA exposure increases prostate cancer after secondary estrogen exposure. | |
| Increased prostate stem-like cells at 6 mo. for 2.5 μg BPA (continuous-dose); increased prostate progenitor cells at 25, 250 μg/kg/day BPA; altered lineage differentiation at 2.5, 25, 250 μg/kg/day BPA doses. | Human prostate stem-like and progenitor cell proliferation and gene expression shifts at 10 μM BPA exposure in vitro. | Consistent results between CLARITY-BPA and prior studies with human prostate stem-like and progenitor cells showing low-dose BPA increases cell numbers and reprograms gene expression. | |
| Urogenital sinus (male) | Size of the urethra altered by 2.5, 25, 250 μg/kg/day BPA. | Small urethra. | Same results in CLARITY-BPA as published previously for same endpoint examined. |
| Shape of the colliculus altered by 2.5, 25, 250 μg/kg/day BPA. | Enlarged dorsolateral prostate, colliculus and utricle. | ||
| Urothelium thickness reduced by 25, 250, 2500 μg/kg/day BPA. | Increase in androgen receptors in the urogenital sinus. | ||
| Enlarged bladder. | |||
| Hydronephrosis, obstructive voiding disorder. | |||
| Size of colliculus increased by 2.5, 25, 250 μg/kg/day BPA. | |||
| Ovary | Number of primary, preantral, and total healthy follicles decreased in 2.5 and 250 μg/kg/day BPA. | BPA exposure at 10 and 100 μg/mL inhibits antral follicle growth and increases atresia in vitro. | CLARITY-BPA data confirm prior studies indicating that BPA exposure interferes with folliculogenesis. |
| Serum estradiol levels decreased in 2500 and 25,000 μg/kg/day BPA. | BPA exposure at 10 and 100 μg/mL inhibits steroidogenesis, leading to low estradiol levels in vitro. | Consistent results between CLARITY-BPA and prior studies with mice and rats indicating that BPA exposure impairs steroidogenesis and causes low E2 levels. | |
| Neurobehavior –spatial learning | Escape tasks in Barnes maze, increased latency in 2500 μg/kg/day BPA females, and trend for increased latency in 2.5 μg/kg/day BPA females at PND 90; males exposed to 2.5 μg/kg/day BPA show trend for improved latency, but relevance of this finding is uncertain. | Male deer mice developmentally exposed to BPA through maternal diet (5 mg or 50 mg/kg feed weight) show reduced spatial navigational learning and memory. | CLARITY-BPA studies differ from our current findings that show males are more vulnerable to BPA-induced cognitive disruptions. |
| Exposure to 2500 μg/kg/day BPA disrupts patterns of sexually dimorphic gene expression/promoter. | Female deer mice exposed to BPA through the maternal diet (5 mg or 50 mg/kg feed weight) either show no affect or a trend for a masculinized response with improved latency. | Conflicting findings may be due to different rodent models (NCTR Sprague-Dawley rats vs. deer mice), route of exposure (oral gavage vs. dietary), dose and duration of BPA exposure, and other potential factors. | |
| Hippocampus of 2500/kg/day μg BPA female offspring have hypermethylated putative 5’ promoter regions of Bdnf. | |||
| Bdnf methylation weakly associated with Bdnf expression in hippocampi of female rats. | |||
| Neurobehavior –anxiety and juvenile anatomy | No systematic effects of BPA observed on any endpoint related to anxiety and exploration in juveniles or adults. | Expansive literature by us and others shows heightened anxiety in multiple species, including rats, mice, deer mice, and prairie voles. | In CLARITY-BPA, not all expected behavioral sex differences were observed in NCTR-Sprague Dawley rats, suggesting unique strain differences. |
| In juveniles, statistically significant effects of 2.5 and 25 μg/kg/day BPA identified on few endpoints in the interval open field analysis, but overall evidence for BPA-related effects minimal and inconsistent. | Changes in AVPV volume. | No effect of EE on behavior or AVPV volume in CLARITY-BPA raises some question about the sensitivity of this strain to the masculinizing influence of estrogen and/or efficacy of EE in the brain. | |
| Enlarged female AVPV at all BPA dose levels; male AVPV enlargement at 25 and 2500 μg/kg/day BPA doses. | Changes in AVPV neuron numbers, TH, and kisspeptin. | CLARITY-BPA results on AVPV and MePD volume are consistent with our prior work in rats showing BPA-related effects on AVPV volume and sexual differentiation and gene expression changes in the juvenile amygdala. | |
| Enlarged MePD (right side only) in males exposed to 2500 μg/kg/day BPA. | Disruption of gene expression in juvenile rat amygdala, including downregulation of Erβ, and sex‐specific disruption of Mc4r, Mc3r, and Tac2. | ||
| Disrupted ER expression in female juveniles of the same strain examined as part of the subchronic study that preceded CLARITY-BPA. | |||
| Neonatal neurodevelopment – PND 1 transcriptome | Sex and dose-specific effects on hypothalamic, hippocampal, and amygdalar transcriptome. | Disruption of hypothalamic PND 1 ER expression in the same strain following prenatal exposure. | Disruption of ER expression, particularly in the hypothalamus, is one of the most consistent findings across BPA literature. In every NCTR study we have worked on, including CLARITY-BPA, ER expression is disrupted at doses as low as 2.5 μg BPA. |
| Disruption of ER expression in hypothalamus, hippocampus, and amygdala. | Disruption of ERβ expression across postnatal rat brain. | We have also found evidence of disrupted oxytocin and vasopressin signaling in adult rats and prairie voles. | |
| Disruption of OT and AVP or its receptors in hippocampus, hypothalamus, and amygdala. | Interference with GABAergic and glutamatergic signaling has also been shown in several different capacities. | ||
| Disruption of genes and pathways related to GABA and glutamate signaling in the amygdala. | |||
| Heart | Decreased collagen in hearts at PND 90 and age 6 mo. | Altered rapid estrogen signaling in females that involves activation of ER. | Same results in CLARITY-BPA as published previously for same endpoint examined. |
| Myocardial degeneration in males and females at PND 21 and 90. | |||
| Female cardiomyopathy incidence and severity at PND 21 at BPA doses of 2.5, 250 and 25,000 μg/kg/day. | In vivo studies demonstrate that BPA exposure sex-specifically alters collagen content, modifies the extracellular matrix of hearts of both males and females, alters fatty acid and glycolytic metabolism, and increases sensitivity of the female heart to ischemic damage. | ||
| Increased sensitivity of heart to ischemic damage. | |||
| BPA significantly decreased heart weight and heart weight normalized to body weight in females exposed to 2.5 μg/kg/day BPA. | |||
| Thyroid | No effect on body weight. | Body weight of dams reduced in dose-dependent manner. | CLARITY-BPA is the only published study that does not report an effect of BPA on serum T4. |
| No effect on serum T4. | Serum T4 in pups increased on PND 15 but not on PND 8, or 35 (dosing stopped at weaning). | ||
| No effect on brain endpoints. | TSH not affected in pups. | ||
| PTU decreased serum T4 as expected. | RC3/neurogranin mRNA increased in dentate gyrus of PND 15 brain. | ||
| PTU did not affect brain endpoints known to be affected by thyroid hormone. | |||
| Mammary gland | Altered morphology of the mammary gland; non-monotonic dose-response curves to BPA with a breaking point between 25 and 250 μg/kg/day BPA doses. | Altered mammary gland morphology, non-monotonic dose response curves, intraductal hyperplasia and neoplasms, changes in methylome and transcriptome resulting from developmental BPA exposure, at doses as low as 25 ug/kg/day. Different effects of BPA and EE. | Morphological results expand our previous work demonstrating non-monotonic dose-responses to BPA exposure. In the Core study, significant increase of adenocarcinomas and adenomas in the lowest dose (2.5 μg) when exposure stopped at PND 21 is consistent with our early findings. |
| Although for some endpoints BPA and EE had similar results, for other endpoints the results were different and even opposite. | |||
| Data on methylome and transcriptome are highly suspect of contamination as a result of co-housing 53 of the 56 animals with those receiving the highest BPA dose. | |||
| Neoplastic results found in Core study, but this part of study had too few animals to measure incidence. |
Toxicity of BPA was observed across a range of doses that differed in the examined organs. Such organ/endpoint differences in sensitivity is expected due to the diversity of cell types, hormone receptor expression levels, vascularity, metabolism, and complexity of signaling pathways across organs. Nonetheless, patterns did emerge when findings across organ systems were integrated and examined together. In many instances, the greatest, and in some cases only, statistically significant effects were observed at the lowest exposure dose used in CLARITY-BPA (2.5 μg/kg/day), as delineated previously [20]. This demonstrates that 2.5 μg/kg-day is not a “No Adverse Effect Level” [79].
Toxicity evaluations for a compound require determination of the lowest observable adverse effect levels (LOAEL). A strength of the CLARITY-BPA Consortium approach is that we were able to conduct a novel mixOmics statistical analysis utilizing multiple data sets from CLARITY that identified statistically significant associations in 2.5 μg/kg/day outcomes reported by multiple investigators across multiple organ systems. This analytical approach provided clear, quantitative findings demonstrating that the lowest dose tested by CLARITY investigators (2.5 μg/kg/day) led to consistent statistically significant adverse effects that cannot be dismissed as occurring randomly and are “not biologically plausible. Our mixOmics statistical analysis thus provides convincing evidence that 2.5 μg/kg/day is the new oral LOAEL for BPA.
In several of these studies, a non-monotonic BPA dose-response was observed. For some organs such as the mammary gland, there was a break between 25 and 250 μg/kg/day doses in all datasets at all ages, which was analyzed by specific statistical methods appropriate for non-monotonicity. The fact that this break is present in all time points analyzed and in the vast majority of the specific measurements clearly indicate that the NMDRC is not spurious [17]: on the contrary, it shows that there is an underlying causal relationship.
For others a U-shaped or inverted U-shaped response (e.g., prostate stem cell numbers) was reported [230,231] or a W-shaped response (e.g. ovary [13]) was observed. Given the variety of these non-linear dose-responses, it is reasonable to question whether the results reflect true biological effects of BPA exposure, or are spurious and not toxicologically relevant. This can be addressed in two ways. First, this was expected because non-monotonicity is a hallmark feature of many endocrine responses and is well described for BPA [136,232]. For example, dose-specific responses across a 1000-fold BPA dose range could be due to differential engagement of multiple receptors that mediate BPA actions (e.g., ERα, ERβ, GPER, ERRs, AR, TR) through both genomic and non-genomic (i.e., membrane-initiated) pathways, each with differing threshold responses. It is well-known that increasing BPA doses (in this experiment, 10,000-fold from 2.5 to 25000 μg/kg/day) activate the expression of entirely different genes, as would be the case for any endogenous or exogenous hormone [233]. Additionally, during organogenesis and tissue repair different cell types with different receptors interact to form organs, increasing the complexity of interactions and thus the likelihood of non-monotonic dose-response curves [231]. But, perhaps more importantly, we applied a rigorous mixOmics approach [21], as done in previous BPA studies performed in the Rosenfeld Laboratory [[22], [23], [24]], to ascertain whether the low dose of BPA simultaneously affected several endpoints in different laboratories. As such, if low-dose effects in the same animals are identified across multiple systems, it seems unlikely that these findings are not important. This approach confirmed that the low dose effects of BPA were observed in many organs and tissues at once while shedding light on the interconnectedness of these low dose effects on the different organs and tissues. These results show the irreversible deviation from normal development that occurs after exposure to BPA.
It was also expected that the results of the CLARITY-BPA study would not exactly match the previously published findings of the investigator-initiated studies due to important differences in experimental designs. As highlighted in Table 6 many study design aspects in CLARITY-BPA, such as species (rat vs mouse), strain of rat model (NCTR-Sprague Dawley; not used in any independent labs), route of exposure (direct gavage including gavage in newborn animals not used in any independent labs), use of CMC vehicle (not used in any independent labs), and dosing throughout the lifetime (not typically done in any independent labs), are different from previously published investigator-initiated studies. These protocol differences can have major implications for the results including gavage [18,19,234]. Importantly, prior work conducted by the Patisaul lab in collaboration with NCTR under conditions similar to CLARITY-BPA produced strong evidence that maternal gavage can induce effects in the newborn offspring brain [61]. Gavage has been shown to induce stress [61,63], which can affect all studied endpoints.
Table 6.
Comparison of CLARITY-BPA study design with prior academic studies. (For interpretation of the references to colour in this table legend, the reader is referred to the web version of this article.)
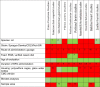 |
Red color indicates that aspect of the published independent studies was different from that of the CLARITY-BPA study design. For example, no published independent studies used the Sprague-Dawley/CD23/Nctr BR, the CMC vehicle.
Green color indicates that aspect of the published independent studies was similar to that of the CLARITY-BPA study design. For example, all published independent studies analyzed their results blinded, as did the CLARITY-BPA study.
The numbers explain some subtle differences between the published independent studies and the CLARITY-BPA study.
1. Mice and rats used.
2. No prior studies have used gavage, although many have used oral exposure routes.
3. Sprague Dawley from Zivic Miller.
4. Sprague Dawley, Holzman.
5. Small number of prior studies have examined rat mammary glands (Wistar/Furth and Sprague Dawley), but more data from the Soto lab has come from mouse models.
6. BPA administered via diet.
7. Diet used in some studies; other diets also used.
8. No studies dosed pups directly.
9. For studies in the Rosenfeld laboratory, females were exposed 2 weeks before gestation and throughout the postnatal period to replicate real-world exposure.
10. Polypropylene cages and glass water bottles.
11. Animals in the Rosenfeld laboratory were housed in polypropylene or polystyrene cages.
12. Polyethylene cages and water bottles.
13. Sample size in CLARITY-BPA less than requested.
14. Variable sample sizes, some larger than CLARITY-BPA.
Much has been written about how factors including animal model, diet, vehicle, housing conditions can produce variability across studies [142,143,145,146,235,236], and thus will not be exhaustively described here. Some specifically identified differences were the result of design issues of guideline studies per se, and some were due to situations that occurred during the study. These limitations include direct gavage of dams and pups, lack of a non-gavage control to assess possible effects of gavage, use of a block design in which each block did not contain the same controls and doses (an example is that all the 2500 μg/kg/day BPA animals in the PND1 urogenital sinus studies [15] which were selected from Block 2, abnormal variance in animal weights (see Fig. 5)), failure to euthanize on the same day of estrus, and inadequate steps to preserve RNA quality for analysis of the mammary gland studies. None of the independent researcher used gavage thus they did not use the CMC vehicle which has possible side effect of causing inflammation of the gut altering the gut microbiome [237]. Any extrinsic factor that alters gut microbiota can lead to pathophysiological changes in the host through various axes, including the microbiota-gut-brain axis [22,[238], [239], [240], [241]].
The large differences in animal weights within a group and the failure to euthanize animals on the same day of the estrous cycle can diminish the ability to detect significant effects on numerous sensitive endpoints. The decision to exclude a negative control group that was not gavaged is particularly surprising because in a pre-CLARITY study it was reported that the non-gavaged negative controls were significantly different from gavaged negative controls [61].
Importantly, animal numbers were severely limited for several independent studies, leaving them underpowered for appropriate statistical analyses despite uniform requests from the labs for appropriate numbers (see for example the Prins study [3]). This compromised data analysis for many endpoints (see Flaws and Prins above). Although CLARITY-BPA animals displayed phenotypes similar to those shown in prior academic studies, they were not always statistically significant while independent studies with appropriate power found significant effects.
Another important variable is the reported possibility of contamination of control and lowest-dose exposure animals due to co-housing of several cohorts with a high-dose group (250,000 μg/kg/day BPA) that was not used in common CLARITY-BPA studies. This was further complicated by lack of assessment of blood levels of free BPA and BPA-G across the lifespan at the FDA-NCTR facility to confirm or rule-out possible cross-contamination of animals. The FDA previously published the recommendation that no BPA study should be conducted without biomonitoring for the possibility of contamination of vehicle controls with BPA, which they reported to occur in the pre-CLARITY-BPA study [92].
Nonetheless, every independent study reported here, except for the thyroid study, showed effects of BPA on a variety of health-predictive or apical endpoints. All studies showed effects of BPA at the lowest dose, 2.5ug/kg/day. Taken together the findings presented here provide strong evidence that the previous BPA results in publications from independent labs could be repeated in, and effectively augment, a guideline study even with many significant differences in study design. In addition, mixOmics analysis of the data indicates that BPA’s effects in one tissue were correlated with effects in another tissue, providing strong evidence that the data are revealing true effects of a low dose of BPA in this guideline, GLP compliant study and that by targeting one organ system, BPA may induce systemic effects. While the underlying mechanisms accounting for the different dose-response shapes is unknown, this knowledge is not required to conclude that they are biologically and toxicologically relevant. Even if the dose-response shape were linear, the mechanism would not be known; thus, this requirement represents a fundamental bias in the interpretation of toxicological data. It is important, therefore, to focus on what is known rather than what is not known.
We agree, as recently published [18], that even in the light of the limitations of study design, that CLARITY-BPA was an important and powerful exercise. It demonstrated that independent scientists focusing on hypothesis-driven disease focused endpoints at relevant levels of biological organization and federal scientists (FDA, NTP) focusing on a guideline compliant study could work together to design and perform a complex experiment with endpoints ranging from epigenetic to organ to whole animal. Even with major study design differences across studies, the CLARITY-BPA study and related, previously published data, show that BPA affects multiple organ systems is reproducible, even in a guideline compliant study. Therefore, the current data from the investigator-initiated studies should be considered for regulatory purposes.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was supported by National Institutes of Health grants U01ES020835 (JAF), U01ES020888 (AMS), U01ES020908 (RTZ), U01ES020929 (CSR), R03ES023098 (SMB), U01ES020929 (HBP), U01ES020886 (GSP), U01ES20952 (FVS).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.reprotox.2020.05.014.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Heindel J.J., Newbold R.R., Bucher J.R., Camacho L., Delclos K.B., Lewis S.M., Vanlandingham M., Churchwell M.I., Twaddle N.C., McLellen M., Chidambaram M., Bryant M., Woodling K., Gamboa da Costa G., Ferguson S.A., Flaws J., Howard P.C., Walker N.J., Zoeller R.T., Fostel J., Favaro C., Schug T.T. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 2015;58:33–44. doi: 10.1016/j.reprotox.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho L., Lewis S.M., Vanlandingham M.M., Olson G.R., Davis K.J., Patton R.E., Twaddle N.C., Doerge D.R., Churchwell M.I., Bryant M.S., McLellen F.M., Woodling K.A., Felton R.P., Maisha M.P., Juliar B.E., Gamboa da Costa G., Delclos K.B. A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110728. [DOI] [PubMed] [Google Scholar]
- 3.Prins G.S., Hu W.-Y., Xie L., Shi G.-B., Hu D.-P., Birch L., Bosland M.C. Evaluation of bisphenol A (BPA) exposures on prostate stem cell homeostasis and prostate cancer risk in the NCTR-Sprague-Dawley rat: an NIEHS/FDA CLARITY-BPA consortium Study. Environ Health Persp. 2018;126(11):117001. doi: 10.1289/EHP3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arambula S.E., Fuchs J., Cao J., Patisaul H.B. Effects of perinatal bisphenol A exposure on the volume of sexually-dimorphic nuclei of juvenile rats: a CLARITY-BPA consortium study. Neurotoxicology. 2017;63:33–42. doi: 10.1016/j.neuro.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arambula S.E., Jima D., Patisaul H.B. Prenatal bisphenol A (BPA) exposure alters the transcriptome of the neonate rat amygdala in a sex-specific manner: a CLARITY-BPA consortium study. Neurotoxicology. 2018;65:207–220. doi: 10.1016/j.neuro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheong A., Johnson S.A., Howald E.C., Ellersieck M.R., Camacho L., Lewis S.M., Vanlandingham M.M., Ying J., Ho S.-M., Rosenfeld C.S. Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: a CLARITY-BPA consortium study. Epigenetics. 2018;13(7):704–720. doi: 10.1080/15592294.2018.1497388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dere E., Anderson L.M., Huse S.M., Spade D.J., McDonnell-Clark E., Madnick S.J., Hall S.J., Camacho L., Lewis S.M., Vanlandingham M.M., Boekelheide K. Effects of continuous bisphenol A exposure from early gestation on 90 day old rat testes function and sperm molecular profiles: a CLARITY-BPA consortium study. Toxicol. Appl. Pharmacol. 2018;347:1–9. doi: 10.1016/j.taap.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gear R., Kendziorski J.A., Belcher S.M. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: a CLARITY-BPA study. Toxicol. Lett. 2017;275:123–135. doi: 10.1016/j.toxlet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson S.A., Javurek A.B., Painter M.S., Ellersieck M.R., Welsh T.H., Jr., Camacho L., Lewis S.M., Vanlandingham M.M., Ferguson S.A., Rosenfeld C.S. Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: a CLARITY-BPA study. Horm. Behav. 2016;80:139–148. doi: 10.1016/j.yhbeh.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Bach A., Crawford R.B., Phadnis-Moghe A.S., Chen W., D’Ingillo S., Kovalova N., Suarez-Martinez J.E., Zhou J., Kaplan B.L.F., Kaminski N.E. CLARITY-BPA: effects of chronic bisphenol A exposure on the immune system: part 1 - quantification of the relative number and proportion of leukocyte populations in the spleen and thymus. Toxicology. 2018;396–397:46–53. doi: 10.1016/j.tox.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Bach A., Crawford R.B., Phadnis-Moghe A.S., Chen W., D’Ingillo S., Kovalova N., Suarez-Martinez J.E., Zhou J., Kaplan B.L.F., Kaminski N.E. CLARITY-BPA: effects of chronic bisphenol A exposure on the immune system: part 2 - characterization of lymphoproliferative and immune effector responses by splenic leukocytes. Toxicology. 2018;396–397:54–67. doi: 10.1016/j.tox.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebuli M.E., Camacho L., Adonay M.E., Reif D.M., Aylor D.L., Patisaul H.B. Impact of low-dose oral exposure to bisphenol A (BPA) on juvenile and adult rat exploratory and anxiety behavior: a CLARITY-BPA Consortium Study. Toxicol. Sci. 2015;148(2):341–354. doi: 10.1093/toxsci/kfv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S., Brehm E., Gao L., Rattan S., Ziv-Gal A., Flaws J.A. Bisphenol A exposure, ovarian follicle numbers, and female sex steroid hormone levels: results from a CLARITY-BPA study. Endocrinology. 2017;158(6):1727–1738. doi: 10.1210/en.2016-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal R., Zoeller R.T. CLARITY-BPA: bisphenol A or propylthiouracil on thyroid function and effects in the developing male and female rat brain. Endocrinology. 2019;160(8):1771–1785. doi: 10.1210/en.2019-00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchtmann K.S., Taylor J.A., Timms B.G., Stahlhut R.W., Ricke E.A., Ellersieck M.R., Vom Saal F.S., Ricke W.A. Fetal bisphenol A and ethinylestradiol exposure alters male rat urogenital tract morphology at birth: Confirmation of prior low-dose findings in CLARITY-BPA. Reprod. Toxicol. 2019;91:131–141. doi: 10.1016/j.reprotox.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belcher S.M., Cline J.M., Conley J., Groeters S., Jefferson W.N., Law M., Mackey E., Suen A.A., Williams C.J., Dixon D., Wolf J.C. Endocrine disruption and reproductive pathology. Toxicol. Pathol. 2019;47(8):1049–1071. doi: 10.1177/0192623319879903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montévil M., Acevedo N., Schaeberle C.M., Bharadwaj M., Fenton S.E., Soto A.M. A combined morphometric and statistical approach to assess nonmonotonicity in the developing mammary gland of rats in the CLARITY-BPA study. Environ. Health Perspect. 2020;128(5):057001. doi: 10.1289/EHP6301. Published online 2020 May 20, PMCID: PMC7263454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenberg L.N., Hunt P.A., Gore A.C. Endocrine disruptors and the future of toxicology testing — lessons from CLARITY–BPA. Nat. Rev. Endocrinol. 2019;15(6):366–374. doi: 10.1038/s41574-019-0173-y. [DOI] [PubMed] [Google Scholar]
- 19.vom Saal F.S. Flaws in design, execution and interpretation limit CLARITY-BPA’s value for risk assessments of bisphenol A. Basic Clin. Pharmacol. Toxicol. 2019;125(Suppl. 3):32–43. doi: 10.1111/bcpt.13195. [DOI] [PubMed] [Google Scholar]
- 20.Prins G.S., Patisaul H.B., Belcher S.M., Vandenberg L.N. CLARITY-BPA academic laboratory studies identify consistent low-dose bisphenol A effects on multiple organ systems. Basic Clin. Pharmacol. Toxicol. 2019;125(Suppl. 3):14–31. doi: 10.1111/bcpt.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohart F., Gautier B., Singh A., Le Cao K.A. mixOmics: an R package for’ omics feature selection and multiple data integration. PLoS Comput. Biol. 2017;13(11) doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall B.L., Liu Y., Farrington M.J., Mao J., Helferich W., Schenk A.K., Bivens N.J., Sarma S.J., Lei Z., Sumner L.W., Joshi T., Rosenfeld C.S. Early genistein exposure of California mice and gut microbiota-brain axis effects. J. Endocrinol. 2019;242(2):139–157. doi: 10.1530/JOE-19-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao J., Jain A., Denslow N.D., Nouri M.Z., Chen S., Wang T., Zhu N., Koh J., Sarma S.J., Sumner B.W., Lei Z., Sumner L.W., Bivens N.J., Roberts R.M., Tuteja G., Rosenfeld C.S. Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta-brain axis. Proc. Natl. Acad. Sci. U. S. A. 2020;117(9):4642–4652. doi: 10.1073/pnas.1919563117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler M.C., Long C.N., Kinkade J.A., Green M.T., Martin R.E., Marshall B.L., Willemse T.E., Schenk A.K., Mao J., Rosenfeld C.S. Endocrine disruption of gene expression and microRNA profiles in hippocampus and hypothalamus of California mice: Association of gene expression changes with behavioural outcomes. J. Neuroendocrinol. 2020 doi: 10.1111/jne.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prins G.S., Birch L., Couse J.F., Choi I., Katzenellenbogen B., Korach K.S. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61(16):6089–6097. [PubMed] [Google Scholar]
- 26.Huang L., Pu Y., Hu W.Y., Birch L., Luccio-Camelo D., Yamaguchi T., Prins G.S. The role of Wnt5a in prostate gland development. Develop Biol. 2009;328(2):188–199. doi: 10.1016/j.ydbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pu Y., Huang L., Prins G.S. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev. Biol. 2004;273(2):257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu W.Y., Shi G.B., Lam H.M., Hu D.P., Ho S.M., Madueke I.C., Kajdacsy-Balla A., Prins G.S. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology. 2011;152(6):2150–2163. doi: 10.1210/en.2010-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho S.M., Tang W.Y., Belmonte de Frausto J., Prins G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prins G.S., Huang L., Birch L., Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann. N. Y. Acad. Sci. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prins G.S., Ye S.H., Birch L., Ho S.M., Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod. Toxicol. 2011;31(1):1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang W.Y., Morey L.M., Cheung Y.Y., Birch L., Prins G.S., Ho S.M. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153(1):42–55. doi: 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong R.L., Wang Q., Trevino L.S., Bosland M.C., Chen J., Medvedovic M., Prins G.S., Kannan K., Ho S.M., Walker C.L. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10(2):127–134. doi: 10.1080/15592294.2015.1009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderon-Gierszal E.L., Prins G.S. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho S.M., Cheong A., Lam H.M., Hu W.Y., Shi G.B., Zhu X., Chen J., Zhang X., Medvedovic M., Leung Y.K., Prins G.S. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family noncoding RNAs via histone modification. Endocrinology. 2015;156(11):3984–3995. doi: 10.1210/en.2015-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prins G.S., Hu W.Y., Shi G.B., Hu D.P., Majumdar S., Li G., Huang K., Nelles J.L., Ho S.M., Walker C.L., Kajdacsy-Balla A., van Breemen R.B. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155(3):805–817. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prins G.S., Ye S.H., Birch L., Zhang X., Cheong A., Lin H., Calderon-Gierszal E., Groen J., Hu W.Y., Ho S.M., van Breemen R.B. Prostate cancer risk and DNA methylation signatures in aging rats following developmental BPA exposure: A dose-response analysis. Environ. Health Perspect. 2017;125(7) doi: 10.1289/EHP1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vermeulen A., Kaufman J.M., Goemaere S., van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5(2):98–102. [PubMed] [Google Scholar]
- 39.Bosland M.C., Ford H., Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17 beta or diethylstilbestrol. Carcinogenesis. 1995;16(6):1311–1317. doi: 10.1093/carcin/16.6.1311. [DOI] [PubMed] [Google Scholar]
- 40.Chakravarty D., Sboner A., Nair S.S., Giannopoulou E., Li R., Hennig S., Mosquera J.M., Pauwels J., Park K., Kossai M., MacDonald T.Y., Fontugne J., Erho N., Vergara I.A., Ghadessi M., Davicioni E., Jenkins R.B., Palanisamy N., Chen Z., Nakagawa S., Hirose T., Bander N.H., Beltran H., Fox A.H., Elemento O., Rubin M.A. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat. Commun. 2014;5:5383. doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Setlur S.R., Mertz K.D., Hoshida Y., Demichelis F., Lupien M., Perner S., Sboner A., Pawitan Y., Andrén O., Johnson L.A., Tang J., Adami H.O., Calza S., Chinnaiyan A.M., Rhodes D., Tomlins S., Fall K., Mucci L.A., Kantoff P.W., Stampfer M.J., Andersson S.O., Varenhorst E., Johansson J.E., Brown M., Golub T.R., Rubin M.A. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J. Natl. Cancer Inst. 2008;100(11):815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montgomery B., Nelson P.S., Vessella R., Kalhorn T., Hess D., Corey E. Estradiol suppresses tissue androgens and prostate cancer growth in castration resistant prostate cancer. BMC Cancer. 2010;10:244–251. doi: 10.1186/1471-2407-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q., Trevino L.S., Wong R.L., Medvedovic M., Chen J., Ho S.M., Shen J., Foulds C.E., Coarfa C., O’Malley B.W., Shilatifard A., Walker C.L. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol. Endocrinol. 2016;30(8):856–871. doi: 10.1210/me.2015-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheong A., Zhang X., Cheung Y.Y., Tang W.Y., Chen J., Ye S.H., Medvedovic M., Leung Y.K., Prins G.S., Ho S.M. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics. 2016;11(9):674–689. doi: 10.1080/15592294.2016.1208891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou C.K., Check D.P., Lortet-Tieulent J., Laversanne M., Jemal A., Ferlay J., Bray F., Cook M.B., D. S.S Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int. J. Cancer. 2016;138(6):1388–1400. doi: 10.1002/ijc.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosland M.C. Chemical and hormonal induction of prostate cancer in animal models. Urol. Oncol. 1996;2(4):103–110. doi: 10.1016/s1078-1439(97)82840-2. [DOI] [PubMed] [Google Scholar]
- 47.Aungst J., Twaroski M.L. In: Bisphenol A (CAS RN. 80-05-7): Review of Low Dose Studies Memorandum. U.F.a.D. Administration, editor. US Food and Drug Administration, US Food and Drug Administration; 2009. [Google Scholar]
- 48.vom Saal F.S., Timms B.G., Montano M.M., Palanza P., Thayer K.A., Nagel S.C., Dhar M.D., Ganjam V.K., Parmigiani S., Welshons W.V. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Nat Acad Sci. 1997;94(5):2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.vom Saal F.S., Cooke P.S., Buchanan D.L., Palanza P., Thayer K.A., Nagel S.C., Parmigiani S., Welshons W.V. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health. 1998;14(1–2):239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson T.M., Nguyen J.L., Leverson G.E., Taylor J.A., Vom Saal F.S., Wood R.W., Ricke W.A. Endocrine disruptor bisphenol A is implicated in urinary voiding dysfunction in male mice. Am. J. Physiol. Renal Physiol. 2018;315(5):F1208–f1216. doi: 10.1152/ajprenal.00582.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welshons W.V., Nagel S.C., Thayer K.A., Judy B.M., Vom Saal F.S. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol. Ind. Health. 1999;15(1–2):12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- 52.Thayer K.A., Ruhlen R.L., Howdeshell K.L., Buchanan D.L., Cooke P.S., Preziosi D., Welshons W.V., Haseman J., vom Saal F.S. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol. Hum. Reprod. 2001;16(5):988–996. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- 53.Richter C.A., Taylor J.A., Ruhlen R.L., Welshons W.V., Vom Saal F.S. Estradiol and Bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ. Health Perspect. 2007;115(6):902–908. doi: 10.1289/ehp.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor J.A., Richter C.A., Suzuki A., Watanabe H., Iguchi T., Coser K.R., Shioda T., vom Saal F.S. Dose-related estrogen effects on gene expression in fetal mouse prostate mesenchymal cells. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhandari R.K., Taylor J.A., Sommerfeld-Sager J., Tillitt D.E., Ricke W.A., Vom Saal F.S. Estrogen receptor 1 expression and methylation of Esr1 promoter in mouse fetal prostate mesenchymal cells induced by gestational exposure to bisphenol A or ethinylestradiol. Environ. Epigenet. 2019;5(3) doi: 10.1093/eep/dvz012. dvz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonneman D.J., Ganjam V.K., Welshons W.V., Vom Saal F.S. Intrauterine position effects on steroid metabolism and steroid receptors of reproductive organs in male mice. Biol. Reprod. 1992;47(5):723–729. doi: 10.1095/biolreprod47.5.723. [DOI] [PubMed] [Google Scholar]
- 57.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 2000;224(2):61–68. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 58.McLachlan J.A., Newbold R.R., Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975;190(4218):991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- 59.Timms B.G., Howdeshell K.L., Barton L., Bradley S., Richter C.A., vom Saal F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. U. S. A. 2005;102(19):7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicholson T.M., Ricke E.A., Marker P.C., Miano J.M., Mayer R.D., Timms B.G., vom Saal F.S., Wood R.W., Ricke W.A. Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 2012;153(11):5556–5565. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao J., Rebuli M.E., Rogers J., Todd K.L., Leyrer S.M., Ferguson S.A., Patisaul H.B. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 2013;133(1):157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somm E., Schwitzgebel V.M., Toulotte A., Cederroth C.R., Combescure C., Nef S., Aubert M.L., Huppi P.S. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 2009;117(10):1549–1555. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandenberg L.N., Welshons W.V., Vom Saal F.S., Toutain P.-L., Myers J.P. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ. Health. 2014;13(1):46. doi: 10.1186/1476-069X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandenberg L.N., Colborn T., Hayes T.B., Heindel J.J., Jacobs D.R., Jr., Lee D.H., Shioda T., Soto A.M., vom Saal F.S., Welshons W.V., Zoeller R.T., Myers J.P. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cabaton N.J., Canlet C., Wadia P.R., Tremblay-Franco M., Gautier R., Molina J., Sonnenschein C., Cravedi J.P., Rubin B.S., Soto A.M., Zalko D. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ. Health Persp. 2013;121:586–593. doi: 10.1289/ehp.1205588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cabaton N.J., Wadia P.R., Rubin B.S., Zalko D., Schaeberle C.M., Askenase M.H., Gadbois J.L., Tharp A.P., Whitt G.S., Sonnenschein C., Soto A.M. Perinatal exposure to environmentally relevant levels of bisphenol-A decreases fertility and fecundity in CD-1 mice. Environ. Health Persp. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markey C.M., Coombs M.A., Sonnenschein C., Soto A.M. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol. Dev. 2003;5:1–9. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 68.Rubin B.S., Paranjpe M., DaFonte T., Schaeberle C., Soto A.M., Obin M., Greenberg A.S. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: the addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod. Toxicol. 2017;68:130–144. doi: 10.1016/j.reprotox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zalko D., Soto A.M., Canlet C., Tremblay-Franco M., Jourdan F., Cabaton N.J. Bisphenol A exposure disrupts neurotransmitters through modulation of transaminase activity in the brain of rodents. Endocrinology. 2016;157:1736–1739. doi: 10.1210/en.2016-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Acevedo N., Davis B., Schaeberle C.M., Sonnenschein C., Soto A.M. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environ. Health Perspect. 2013;121(9):1040–1046. doi: 10.1289/ehp.1306734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markey C.M. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol. Reprod. 2001;65(4):1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 72.Soto A.M., Brisken C., Schaeberle C.M., Sonnenschein C. Does cancer start in the womb? Altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. J. Mammary Gland Biol. Neoplasia. 2013;18:199–208. doi: 10.1007/s10911-013-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Speroni L., Voutilainen M., Mikkola M.L., Klager S.A., Schaeberle C.M., Sonnenschein C., Soto A.M. New insights into fetal mammary gland morphogenesis: differential effects of natural and environmental estrogens. Sci. Rep. 2017;7:40806. doi: 10.1038/srep40806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tharp A.P., Maffini M.V., Hunt P.A., Vandevoort C.A., Sonnenschein C., Soto A.M. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc. Natl. Acad. Sci. U. S. A. 2012;109:8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandenberg L.N., Maffini M.V., Schaeberle C.M., Ucci A.A., Sonnenschein C., Rubin B.S., Soto A.M. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod. Toxicol. 2008;26:210–219. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandenberg L.N., Maffini M.V., Wadia P.R., Sonnenschein C., Rubin B.S., Soto A.M. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vandenberg L.N., Schaeberle C.M., Rubin B.S., Sonnenschein C., Soto A.M. The male mammary gland: a target for the xenoestrogen bisphenol A. Reprod. Toxicol. 2013;37:15–23. doi: 10.1016/j.reprotox.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wadia P.R., Vandenberg L.N., Schaeberle C.M., Rubin B.S., Sonnenschein C., Soto A.M. Perinatal bisphenol-A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ. Health Persp. 2007;115:592–598. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munoz-de-Toro M., Markey C.M., Wadia P.R., Luque E.H., Rubin B.S., Sonnenschein C., Soto A.M. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146(9):4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durando M., Kass L., Piva J., Sonnenschein C., Soto A.M., Luque E.H., Munoz de Toro M.M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ. Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray T.J., Maffini M.V., Ucci A.A., Sonnenschein C., Soto A.M. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod. Toxicol. 2007;23(3):383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhimolea E., Wadia P.R., Murray T.J., Settles M.L., Treitman J.D., Sonnenschein C., Shioda T., Soto A.M. Prenatal exposure to BPA alters the epigenome of the rat mammary gland and increases the propensity to neoplastic development. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0099800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paulose T., Speroni L., Sonnenschein C., Soto A.M. Estrogens in the wrong place at the wrong time: fetal BPA exposure and mammary cancer. Reprod.Toxicol. 2015;54:58–65. doi: 10.1016/j.reprotox.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jenkins S., Raghuraman N., Eltoum I., Carpenter M., Russo J., Lamartiniere C.A. Oral exposure to bisphenol a increases dimethylbenzanthracene-induced mammary cancer in rats. Environ. Health Perspect. 2009;117(6):910–915. doi: 10.1289/ehp.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jenkins S., Wang J., Eltoum I., Desmond R., Lamartiniere C.A. Chronic oral exposure to bisphenol A results in a non-monotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ. Health Persp. 2011;119:1604–1609. doi: 10.1289/ehp.1103850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Betancourt A.M., Eltoum I.A., Desmond R.A., Russo J., Lamartiniere C.A. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ. Health Persp. 2010;118:1614–1619. doi: 10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamartiniere C.A., Jenkins S., Betancourt A.M., Wang J., Russo J. Exposure to the endocrine disruptor bisphenol A alters susceptibility for mammary cancer. Horm. Mol. Biol. Clin. Investig. 2011;5:45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis B., Fenton S. The mammary gland. In: Haschek W.M., Rousseaux C.G., Wallig M.A., Ochoa R., Bolon B., editors. 3rd ed. vol. 3. Elsevier; New York, NY: 2013. pp. 2665–2694. (Haschek and Rousseaux’s Handbook of Toxicologic Pathology). [Google Scholar]
- 89.https://www.biorxiv.org/content/10.1101/783019v1.
- 90.Shioda T., Chesnes J., Coser K.R., Zou L., Hur J., Dean K.L., Sonnenschein C., Soto A.M., Isselbacher K.J. Importance of dosage standardization for interpreting transcriptomal signature profiles: evidence from studies of xenoestrogens. Proc. Natl. Acad. Sci. U. S. A. 2006;103(32):12033–12038. doi: 10.1073/pnas.0605341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wadia P.R., Cabaton N.J., Borrero M.D., Rubin B.S., Sonnenschein C., Shioda T., Soto A.M. Low-dose BPA exposure alters the mesenchymal and epithelial transcriptomes of the mouse fetal mammary gland. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Churchwell M.I., Camacho L., Vanlandingham M.M., Twaddle N.C., Sepehr E., Delclos K.B., Fisher J.W., Doerge D.R. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol. Sci. 2014;139(1):4–20. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delclos K.B., Camacho L., Lewis S.M., Vanlandingham M.M., Latendresse J.R., Olson G.R., Davis K.J., Patton R.E., Gamboa da Costa G., Woodling K.A., Bryant M.S., Chidambaram M., Trbojevich R., Juliar B.E., Felton R.P., Thorn B.T. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 2014;139(1):174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peretz J., Craig Z.R., Flaws J.A. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol. Reprod. 2012;87(3):63. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peretz J., Flaws J.A. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2013;271(2):249–256. doi: 10.1016/j.taap.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peretz J., Gupta R.K., Singh J., Hernandez-Ochoa I., Flaws J.A. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 2011;119(1):209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peretz J., Neese S.L., Flaws J.A. Mouse strain does not influence the overall effects of bisphenol a-induced toxicity in adult antral follicles. Biol. Reprod. 2013;89(5):108. doi: 10.1095/biolreprod.113.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ziv-Gal A., Craig Z.R., Wang W., Flaws J.A. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod. Toxicol. 2013;42:58–67. doi: 10.1016/j.reprotox.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ziv-Gal A., Flaws J.A. Evidence for bisphenol A-induced female infertility: a review (2007-2016) Fertil. Steril. 2016;106(4):827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peretz J., Vrooman L., Ricke W.A., Hunt P.A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H.S., Swan S.H., VandeVoort C.A., Flaws J.A. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ. Health Perspect. 2014;122(8):775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abi Salloum B., Steckler T.L., Herkimer C., Lee J.S., Padmanabhan V. Developmental programming: impact of prenatal exposure to bisphenol-A and methoxychlor on steroid feedbacks in sheep. Toxicol. Appl. Pharmacol. 2013;268(3):300–308. doi: 10.1016/j.taap.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adewale H.B., Jefferson W.N., Newbold R.R., Patisaul H.B. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol. Reprod. 2009;81(4):690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berger A., Ziv-Gal A., Cudiamat J., Wang W., Zhou C., Flaws J.A. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod. Toxicol. 2016;60:39–52. doi: 10.1016/j.reprotox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ziv-Gal A., Wang W., Zhou C., Flaws J.A. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 2015;284(3):354–362. doi: 10.1016/j.taap.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou C., Wang W., Peretz J., Flaws J.A. Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Reprod. Toxicol. 2015;57:87–99. doi: 10.1016/j.reprotox.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rivera O.E., Varayoud J., Rodriguez H.A., Munoz-de-Toro M., Luque E.H. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod. Toxicol. 2011;32(3):304–312. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 107.Wang W., Hafner K.S., Flaws J.A. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol. Appl. Pharmacol. 2014;276(2):157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Santamaria C., Durando M., Munoz de Toro M., Luque E.H., Rodriguez H.A. Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. J. Steroid Biochem. Mol. Biol. 2016;158:220–230. doi: 10.1016/j.jsbmb.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 109.Gamez J.M., Penalba R., Cardoso N., Bernasconi P.S., Carbone S., Ponzo O., Pandolfi M., Scacchi P., Reynoso R. Exposure to a low dose of bisphenol A impairs pituitary-ovarian axis in prepubertal rats: effects on early folliculogenesis. Environ. Toxicol. Pharmacol. 2015;39(1):9–15. doi: 10.1016/j.etap.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 110.McCaffrey K.A., Jones B., Mabrey N., Weiss B., Swan S.H., Patisaul H.B. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. 2013;36:55–62. doi: 10.1016/j.neuro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patisaul H.B., Fortino A.E., Polston E.K. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006;28(1):111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 112.Patisaul H.B., Sullivan A.W., Radford M.E., Walker D.M., Adewale H.B., Winnik B., Coughlin J.L., Buckley B., Gore A.C. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolstenholme J.T., Rissman E.F., Connelly J.J. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 2011;59(3):296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nesan D., Sewell L.C., Kurrasch D.M. Opening the black box of endocrine disruption of brain development: lessons from the characterization of bisphenol A. Horm. Behav. 2018;101:50–58. doi: 10.1016/j.yhbeh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Cao J., Joyner L., Mickens J.A., Leyrer S.M., Patisaul H.B. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction. 2014;147(4):537–554. doi: 10.1530/REP-13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Braun J.M., Kalkbrenner A.E., Calafat A.M., Yolton K., Ye X., Dietrich K.N., Lanphear B.P. Impact of early-life bisphenol a exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harley K.G., Gunier R.B., Kogut K., Johnson C., Bradman A., Calafat A.M., Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perera F., Nolte E.L., Wang Y., Margolis A.E., Calafat A.M., Wang S., Garcia W., Hoepner L.A., Peterson B.S., Rauh V., Herbstman J. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10-12 years of age. Environ. Res. 2016;151:195–202. doi: 10.1016/j.envres.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perera F., Vishnevetsky J., Herbstman J.B., Calafat A.M., Xiong W., Rauh V., Wang S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Persp. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patisaul H.B. Effects of environmental endocrine disruptors and phytoestrogens on the kisspeptin system. Adv. Exp. Med. Biol. 2013;784:455–479. doi: 10.1007/978-1-4614-6199-9_21. [DOI] [PubMed] [Google Scholar]
- 121.Cao J., Mickens J.A., McCaffrey K.A., Leyrer S.M., Patisaul H.B. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rebuli M.E., Cao J., Sluzas E., Delclos K.B., Camacho L., Lewis S.M., Vanlandingham M.M., Patisaul H.B. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol. Sci. 2014;140(1):190–203. doi: 10.1093/toxsci/kfu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao J., Patisaul H.B. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. J. Comp. Neurol. 2011;519(15):2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao J., Patisaul H.B. Sex specific expression of estrogen receptors alpha and beta and kiss1 in the postnatal rat amygdala. J. Comp. Neurol. 2013;521(2):465–478. doi: 10.1002/cne.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lagarde F., Beausoleil C., Belcher S.M., Belzunces L.P., Emond C., Guerbet M., Rousselle C. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health. 2015;14:13. doi: 10.1186/1476-069X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guillamon A., de Blas M.R., Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468(2):306–310. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- 127.Kubo K., Arai O., Ogata R., Omura M., Hori T., Aou S. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci. Lett. 2001;304(1–2):73–76. doi: 10.1016/s0304-3940(01)01760-8. [DOI] [PubMed] [Google Scholar]
- 128.McCarthy M.M. Estradiol and the developing brain. Physiol. Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simerly R.B. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 130.Morris J.A., Jordan C.L., King Z.A., Northcutt K.V., Breedlove S.M. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris J.A., Jordan C.L., Breedlove S.M. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J. Comp. Neurol. 2008;506(5):851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- 132.Cooke B.M., Chowanadisai W., Breedlove S.M. Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav. Brain Res. 2000;117(1–2):107–113. doi: 10.1016/s0166-4328(00)00301-6. [DOI] [PubMed] [Google Scholar]
- 133.Arambula S.E., Belcher S.M., Planchart A., Turner S.D., Patisaul H.B. Impact of low dose oral exposure to bisphenol A (BPA) on the neonatal rat hypothalamic and hippocampal transcriptome: a CLARITY-BPA Consortium Study. Endocrinology. 2016;157(10):3856–3872. doi: 10.1210/en.2016-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patisaul H.B. Endocrine disruption of vasopressin systems and related behaviors. Front. Endocrinol. 2017;8:134. doi: 10.3389/fendo.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matthews J.B., Twomey K., Zacharewski T.R. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem. Res. Toxicol. 2001;14(2):149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 136.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015;36(6):E1–e150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Balthazart J. Steroid metabolism in the brain: from bird watching to molecular biology, a personal journey. Horm. Behav. 2017;93:137–150. doi: 10.1016/j.yhbeh.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barakat R., Oakley O., Kim H., Jin J., Ko C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016;49(9):488–496. doi: 10.5483/BMBRep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adewale H.B., Todd K.L., Mickens J.A., Patisaul H.B. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32(1):38–49. doi: 10.1016/j.neuro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sullivan A.W., Beach E.C., Stetzik L.A., Perry A., D’Addezio A.S., Cushing B.S., Patisaul H.B. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster) Endocrinology. 2014;155(10):3867–3881. doi: 10.1210/en.2014-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolstenholme J.T., Edwards M., Shetty S.R., Gatewood J.D., Taylor J.A., Rissman E.F., Connelly J.J. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jasarevic E., Sieli P.T., Twellman E.E., Welsh T.H., Jr., Schachtman T.R., Roberts R.M., Geary D.C., Rosenfeld C.S. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. U. S. A. 2011;108(28):11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jasarevic E., Williams S.A., Vandas G.M., Ellersieck M.R., Liao C., Kannan K., Roberts R.M., Geary D.C., Rosenfeld C.S. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm. Behav. 2013;63:180–189. doi: 10.1016/j.yhbeh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jasarevic E., Williams S.A., Roberts R.M., Geary D.C., Rosenfeld C.S. Spatial navigation strategies in Peromyscus: a comparative study. Anim. Behav. 2012;84:1141–1149. doi: 10.1016/j.anbehav.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jasarevic E., Geary D.C., Rosenfeld C.S. Sexually selected traits: a fundamental framework for studies on behavioral epigenetics. ILAR J. 2012;53(3–4):253–269. doi: 10.1093/ilar.53.3-4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Williams S.A., Jasarevic E., Vandas G.M., Warzak D.A., Geary D.C., Ellersieck M.R., Roberts R.M., Rosenfeld C.S. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): a monogamous animal model. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johnson S.A., Spollen W.G., Manshack L.K., Bivens N.J., Givan S.A., Rosenfeld C.S. Hypothalamic transcriptomic alterations in male and female California mice (Peromyscus californicus) developmentally exposed to bisphenol A or ethinyl estradiol. Physiol. Rep. 2017;5(3) doi: 10.14814/phy2.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson S.A., Ellersieck M.R., Rosenfeld C.S. Hypothalamic gene expression changes in F1 California mice (Peromyscus californicus) parents developmentally exposed to bisphenol A or ethinyl estradiol. Heliyon. 2018;4(6) doi: 10.1016/j.heliyon.2018.e00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosenfeld C.S. Animal models to study environmental epigenetics. Biol. Reprod. 2010;82(3):473–488. doi: 10.1095/biolreprod.109.080952. [DOI] [PubMed] [Google Scholar]
- 150.Rosenfeld C.S., Trainor B.C. Environmental health factors and sexually dimorphic differences in behavioral disruptions. Curr. Environ. Health Rep. 2014;1:287–301. doi: 10.1007/s40572-014-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosenfeld C.S. Chapter 11 - Animal models of transgenerational epigenetic effects. In: Tollefsbol T., editor. Transgenerational Epigenetics. Academic Press; Oxford: 2014. pp. 123–145. [Google Scholar]
- 152.Rosenfeld C.S. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front. Neurosci. 2015;9:1–15. doi: 10.3389/fnins.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galea L.A., Kavaliers M., Ossenkopp K.P., Hampson E. Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Horm. Behav. 1995;29(1):106–125. doi: 10.1006/hbeh.1995.1008. [DOI] [PubMed] [Google Scholar]
- 154.Gaulin S.J., FitzGerald R.W., Wartell M.S. Sex differences in spatial ability and activity in two vole species (Microtus ochrogaster and M. pennsylvanicus) J. Comp. Psychol. 1990;104(1):88–93. doi: 10.1037/0735-7036.104.1.88. [DOI] [PubMed] [Google Scholar]
- 155.Gaulin S.J.C. Evolution of sex differences in spatial ability. Yearb. Phys. Anthropol. 1992;35:125–151. [Google Scholar]
- 156.Carr R., Bertasi F., Betancourt A., Bowers S., Gandy B.S., Ryan P., Willard S. Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. J. Toxicol. Environ. Health A. 2003;66(21):2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- 157.Diaz Weinstein S., Villafane J.J., Juliano N., Bowman R.E. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain Res. 2013;1529:56–65. doi: 10.1016/j.brainres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 158.Kim M.E., Park H.R., Gong E.J., Choi S.Y., Kim H.S., Lee J. Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem. Toxicol. 2011;49(12):3383–3389. doi: 10.1016/j.fct.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 159.Kumar D., Thakur M.K. Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin3 expression in male mouse brain. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Kuwahara R., Kawaguchi S., Kohara Y., Cui H., Yamashita K. Perinatal exposure to low-dose bisphenol A impairs spatial learning and memory in male rats. J. Pharmacol. Sci. 2013;123(2):132–139. doi: 10.1254/jphs.13093fp. [DOI] [PubMed] [Google Scholar]
- 161.Mhaouty-Kodja S., Belzunces L.P., Canivenc M.C., Schroeder H., Chevrier C., Pasquier E. Impairment of learning and memory performances induced by BPA: evidences from the literature of a MoA mediated through an ED. Mol. Cell. Endocrinol. 2018;475:54–73. doi: 10.1016/j.mce.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 162.Xu X.H., Zhang J., Wang Y.M., Ye Y.P., Luo Q.Q. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm. Behav. 2010;58(2):326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 163.Reinisch J.M., Sanders S.A. Effects of prenatal exposure to diethylstilbestrol (DES) on hemispheric laterality and spatial ability in human males. Horm. Behav. 1992;26(1):62–75. doi: 10.1016/0018-506x(92)90032-q. [DOI] [PubMed] [Google Scholar]
- 164.Chen F., Zhou L., Bai Y., Zhou R., Chen L. Sex differences in the adult HPA axis and affective behaviors are altered by perinatal exposure to a low dose of bisphenol A. Brain Res. 2014;1571:12–24. doi: 10.1016/j.brainres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 165.Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R.L., Perera F.P., Champagne F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U. S. A. 2013;110(24):9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ceccarelli I., Della Seta D., Fiorenzani P., Farabollini F., Aloisi A.M. Estrogenic chemicals at puberty change ERalpha in the hypothalamus of male and female rats. Neurotoxicol. Teratol. 2007;29(1):108–115. doi: 10.1016/j.ntt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 167.Fukushima A., Funabashi T., Kawaguchi M., Mitsushima D., Kimura F. Bisphenol A induces transforming growth factor-beta3 mRNA in the preoptic area: a cDNA expression array and Northern blot study. Neurosci. Lett. 2007;411(1):81–85. doi: 10.1016/j.neulet.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 168.Wolstenholme J.T., Taylor J.A., Shetty S.R., Edwards M., Connelly J.J., Rissman E.F. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kundakovic M., Gudsnuk K., Herbstman J.B., Tang D., Perera F.P., Champagne F.A. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. U. S. A. 2015;112(22):6807–6813. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Elsworth J.D., Jentsch J.D., Vandevoort C.A., Roth R.H., Redmond D.E., Jr., Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–120. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sieli P.T., Jasarevic E., Warzak D.A., Mao J., Ellersieck M.R., Liao C., Kannan K., Collet S.H., Toutain P.L., Vom Saal F.S., Rosenfeld C.S. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ. Health Perspect. 2011;119(9):1260–1265. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Galloway T., Cipelli R., Guralnick J., Ferrucci L., Bandinelli S., Corsi A.M., Money C., McCormack P., Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ. Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tyl R.W., Myers C.B., Marr M.C., Sloan C.S., Castillo N.P., Veselica M.M., Seely J.C., Dimond S.S., Van Miller J.P., Shiotsuka R.N., Beyer D., Hentges S.G., Waechter J.M., Jr. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008;104(2):362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 174.Tyl R.W., Myers C.B., Marr M.C., Thomas B.F., Keimowitz A.R., Brine D.R., Veselica M.M., Fail P.A., Chang T.Y., Seely J.C., Joiner R.L., Butala J.H., Dimond S.S., Cagen S.Z., Shiotsuka R.N., Stropp G.D., Waechter J.M. Three-Generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002;68(1):121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 175.Kurebayashi H., Nagatsuka S., Nemoto H., Noguchi H., Ohno Y. Disposition of low doses of 14C-bisphenol A in male, female, pregnant, fetal, and neonatal rats. Arch. Toxicol. 2005;79(5):243–252. doi: 10.1007/s00204-004-0628-2. [DOI] [PubMed] [Google Scholar]
- 176.Tateoka Y. Bisphenol A concentration in breast milk following consumption of a canned coffee drink. J. Hum. Lact. 2015;31(3):474–478. doi: 10.1177/0890334414563732. [DOI] [PubMed] [Google Scholar]
- 177.Deceuninck Y., Bichon E., Marchand P., Boquien C.Y., Legrand A., Boscher C., Antignac J.P., Le Bizec B. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015;407:2485–2497. doi: 10.1007/s00216-015-8469-9. [DOI] [PubMed] [Google Scholar]
- 178.Zimmers S.M., Browne E.P., O’Keefe P.W., Anderton D.L., Kramer L., Reckhow D.A., Arcaro K.F. Determination of free Bisphenol A (BPA) concentrations in breast milk of U.S. women using a sensitive LC/MS/MS method. Chemosphere. 2014;104:237–243. doi: 10.1016/j.chemosphere.2013.12.085. [DOI] [PubMed] [Google Scholar]
- 179.Belcher S.M., Chen Y., Yan S., Wang H.S. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153(2):712–720. doi: 10.1210/en.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yan S., Chen Y., Dong M., Song W., Belcher S.M., Wang H.S. Bisphenol A and 17beta-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Belcher S.M., Gear R.B., Kendig E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology. 2015;156(3):882–895. doi: 10.1210/en.2014-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Patel B.B., Raad M., Sebag I.A., Chalifour L.E. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol. Sci. 2013;133(1):174–185. doi: 10.1093/toxsci/kft026. [DOI] [PubMed] [Google Scholar]
- 183.Patel B.B., Kasneci A., Bolt A.M., Di Lalla V., Di Iorio M.R., Raad M., Mann K.K., Chalifour L.E. Chronic exposure to bisphenol A reduces successful cardiac remodeling after an experimental myocardial infarction in male C57bl/6n mice. Toxicol. Sci. 2015;146(1):101–115. doi: 10.1093/toxsci/kfv073. [DOI] [PubMed] [Google Scholar]
- 184.Ljunggren S.A., Iggland M., Rönn M., Lind L., Lind P.M., Karlsson H. Altered heart proteome in fructose-fed Fisher 344 rats exposed to bisphenol A. Toxicology. 2016;347–349:6–16. doi: 10.1016/j.tox.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 185.Grohe C., Kahlert S., Lobbert K., Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J. Endocrinol. 1998;156(2):R1–7. doi: 10.1677/joe.0.156r001. [DOI] [PubMed] [Google Scholar]
- 186.Rancière F., Lyons J.G., Loh V.H.Y., Botton J., Galloway T., Wang T., Shaw J.E., Magliano D.J. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ. Health. 2015;14(1):1–23. doi: 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shankar A., Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab. 2011;96(12):3822–3826. doi: 10.1210/jc.2011-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shankar A., Teppala S., Sabanayagam C. Urinary bisphenol A levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol. 2012;2012 doi: 10.5402/2012/965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Carwile J.L., Michels K.B. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ. Res. 2011;111(6):825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Khalil N., Chen A., Lee M. Endocrine disruptive compounds and cardio-metabolic risk factors in children. Curr Opinion Pharmacol. 2014;19(0):120–124. doi: 10.1016/j.coph.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 191.Lang I.A., Galloway T.S., Scarlett A., Henley W.E., Depledge M., Wallace R.B., Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 192.Melzer D., Rice N.E., Lewis C., Henley W.E., Galloway T.S. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Silver M.K., O’Neill M.S., Sowers M.R., Park S.K. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patisaul H.B., Roberts S.C., Mabrey N., McCaffrey K.A., Gear R.B., Braun J., Belcher S.M., Stapleton H.M. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol. 2013;27(2):124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rubin Z., Arceo R.J., Bishop S.P., Kerns W.D., Mesfin G.M., Sandusky G.E., Van Vleet J.F. STP/ARP/AFIP; Washington, DC: 2000. Nonpproliferative Lesions of the Heart and Vasculature in Rats, Guides for Toxicologic Pathology. [Google Scholar]
- 196.Jokinen M.P., Lieuallen W.G., Johnson C.L., Dunnick J., Nyska A. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: the national toxicology program experience. Cardiovasc. Toxicol. 2005;5(2):227–244. doi: 10.1385/ct:5:2:227. [DOI] [PubMed] [Google Scholar]
- 197.Jokinen M.P., Lieuallen W.G., Boyle M.C., Johnson C.L., Malarkey D.E., Nyska A. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicol. Pathol. 2011;39(5):850–860. doi: 10.1177/0192623311413788. [DOI] [PubMed] [Google Scholar]
- 198.Bassett J.R., Cairncross K.D. Morphological changes induced in rats following prolonged exposure to stress. Pharmacol. Biochem. Behav. 1975;3(3):411–420. doi: 10.1016/0091-3057(75)90049-0. [DOI] [PubMed] [Google Scholar]
- 199.Baker S., Rees S., Chebli M., LeMarec N., Godbout R., Huta V., Bielajew C. Effects of gestational stress: 2. Evaluation of male and female adult offspring. Brain Res. 2009;1302:194–204. doi: 10.1016/j.brainres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 200.Baker S., Chebli M., Rees S., LeMarec N., Godbout R., Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 201.Rai V., Sharma P., Agrawal S., Agrawal D.K. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. J. Supramol. Struct. Cell. Biochem. Suppl. 2017;424(1–2):123–145. doi: 10.1007/s11010-016-2849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Banerjee I., Fuseler J.W., Price R.L., Borg T.K., Baudino T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007;293(3):29. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 203.Moriyama K., Tagami T., Akamizu T., Usui T., Saijo M., Kanamoto N., Hataya Y., Shimatsu A., Kuzuya H., Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002;87(11):5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 204.Heimeier R.A., Das B., Buchholz D.R., Shi Y.B. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology. 2009;150(6):2964–2973. doi: 10.1210/en.2008-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Terrien X., Fini J.B., Demeneix B.A., Schramm K.W., Prunet P. Generation of fluorescent zebrafish to study endocrine disruption and potential crosstalk between thyroid hormone and corticosteroids. Aquat. Toxicol. 2011;105(1–2):13–20. doi: 10.1016/j.aquatox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 206.Zoeller R.T., Bansal R., Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146(2):607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 207.O’Shea P.J., Williams G.R. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J. Endocrinol. 2002;175(3):553–570. doi: 10.1677/joe.0.1750553. [DOI] [PubMed] [Google Scholar]
- 208.Guadano-Ferraz A., Escamez M.J., Morte B., Vargiu P., Bernal J. Transcriptional induction of RC3/neurogranin by thyroid hormone: differential neuronal sensitivity is not correlated with thyroid hormone receptor distribution in the brain. Brain Res. Mol. Brain Res. 1997;49(1–2):37–44. doi: 10.1016/s0169-328x(97)00119-8. [DOI] [PubMed] [Google Scholar]
- 209.Iniguez M.A., De Lecea L., Guadano-Ferraz A., Morte B., Gerendasy D., Sutcliffe J.G., Bernal J. Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology. 1996;137(3):1032–1041. doi: 10.1210/endo.137.3.8603571. [DOI] [PubMed] [Google Scholar]
- 210.Ortiga-Carvalho T.M., Sidhaye A.R., Wondisford F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014;10(10):582–591. doi: 10.1038/nrendo.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee S., Kim C., Youn H., Choi K. Thyroid hormone disrupting potentials of bisphenol A and its analogues - in vitro comparison study employing rat pituitary (GH3) and thyroid follicular (FRTL-5) cells. Toxicol. In Vitro. 2017;40:297–304. doi: 10.1016/j.tiv.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 212.Sheng Z.G., Tang Y., Liu Y.X., Yuan Y., Zhao B.Q., Chao X.J., Zhu B.Z. Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a nongenomic mechanism. Toxicol. Appl. Pharmacol. 2012;259(1):133–142. doi: 10.1016/j.taap.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 213.Kitamura S., Jinno N., Ohta S., Kuroki H., Fujimoto N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 2002;293(1):554–559. doi: 10.1016/S0006-291X(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 214.Xu X., Liu Y., Sadamatsu M., Tsutsumi S., Akaike M., Ushijima H., Kato N. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci. Res. 2007;58(2):149–155. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 215.Kobayashi K., Miyagawa M., Wang R.S., Suda M., Sekiguchi S., Honma T. Effects of in utero and lactational exposure to bisphenol A on thyroid status in F1 rat offspring. Ind. Health. 2005;43(4):685–690. doi: 10.2486/indhealth.43.685. [DOI] [PubMed] [Google Scholar]
- 216.Park C., Choi W., Hwang M., Lee Y., Kim S., Yu S., Lee I., Paek D., Choi K. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population - Korean National Environmental Health Survey (KoNEHS) 2012-2014. Sci. Total Environ. 2017;584–585:950–957. doi: 10.1016/j.scitotenv.2017.01.144. [DOI] [PubMed] [Google Scholar]
- 217.Aung M.T., Johns L.E., Ferguson K.K., Mukherjee B., McElrath T.F., Meeker J.D. Thyroid hormone parameters during pregnancy in relation to urinary bisphenol A concentrations: a repeated measures study. Environ. Int. 2017;104:33–40. doi: 10.1016/j.envint.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ahmed R.G., Walaa G.H., Asmaa F.S. Suppressive effects of neonatal bisphenol A on the neuroendocrine system. Toxicol. Ind. Health. 2018;34(6):397–407. doi: 10.1177/0748233718757082. [DOI] [PubMed] [Google Scholar]
- 219.Fernandez M.O., Bourguignon N.S., Arocena P., Rosa M., Libertun C., Lux-Lantos V. Neonatal exposure to bisphenol A alters the hypothalamic-pituitary-thyroid axis in female rats. Toxicol. Lett. 2018;285:81–86. doi: 10.1016/j.toxlet.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 220.Ferguson S.A., Law C.D., Jr., Abshire J.S. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 2011;124(1):149–160. doi: 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- 221.Viguie C., Collet S.H., Gayrard V., Picard-Hagen N., Puel S., Roques B.B., Toutain P.L., Lacroix M.Z. Maternal and fetal exposure to bisphenol a is associated with alterations of thyroid function in pregnant ewes and their newborn lambs. Endocrinology. 2013;154(1):521–528. doi: 10.1210/en.2012-1401. [DOI] [PubMed] [Google Scholar]
- 222.Gonzalez I., Cao K.A., Davis M.J., Dejean S. Visualising associations between paired’ omics’ data sets. BioData Min. 2012;5(1):19. doi: 10.1186/1756-0381-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Berger S., Ceccarini G., Scabia G., Barone I., Pelosini C., Ferrari F., Magno S., Dattilo A., Chiovato L., Vitti P., Santini F., Maffei M. Lipodystrophy and obesity are associated with decreased number of T cells with regulatory function and pro-inflammatory macrophage phenotype. Int. J. Obes. 2017;41(11):1676–1684. doi: 10.1038/ijo.2017.163. [DOI] [PubMed] [Google Scholar]
- 224.Lobo T.F., Borges C.M., Mattar R., Gomes C.P., de Angelo A.G.S., Pendeloski K.P.T., Daher S. Impaired Treg and NK cells profile in overweight women with gestational diabetes mellitus. Am. J. Reprod. Immunol. 2018;79(3) doi: 10.1111/aji.12810. [DOI] [PubMed] [Google Scholar]
- 225.Donma M., Karasu E., Ozdilek B., Turgut B., Topcu B., Nalbantoglu B., Donma O. CD4(+), CD25(+), FOXP3 (+) T regulatory cell levels in obese, asthmatic, asthmatic obese, and healthy children. inflammation. 2015;38(4):1473–1478. doi: 10.1007/s10753-015-0122-4. [DOI] [PubMed] [Google Scholar]
- 226.Wagner N.M., Brandhorst G., Czepluch F., Lankeit M., Eberle C., Herzberg S., Faustin V., Riggert J., Oellerich M., Hasenfuss G., Konstantinides S., Schafer K. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity. 2013;21(3):461–468. doi: 10.1002/oby.20087. [DOI] [PubMed] [Google Scholar]
- 227.Heindel J.J., Blumberg B. Environmental obesogens: mechanisms and controversies. Annu. Rev. Pharmacol. Toxicol. 2019;59:89–106. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dere E., Anderson L.M., Huse S.M., Spade D.J., McDonnell-Clark E., Madnick S.J., Hall S.J., Camacho L., Lewis S.M., Vanlandingham M.M., Boekelheide K. Effects of continuous bisphenol A exposure from early gestation on 90day old rat testes function and sperm molecular profiles: a CLARITY-BPA consortium study. Toxicol. Appl. Pharmacol. 2018;347:1–9. doi: 10.1016/j.taap.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.vom Saal F.S., Welshons W.V. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 230.Soto A.M., Lin T.M., Sakabe K., Olean N., Damassa D.A., Sonnenschein C. Varients of the human prostat LNCaPcell line as tools for study discrete components of the androgen-mediated proliferative response. Oncol. Res. 1995;7:545–558. [PubMed] [Google Scholar]
- 231.Vandenberg L.N., Wadia P.R., Schaeberle C.M., Rubin B.S.D., Sonnenschein C., Soto A.M. The mammary gland response to estradiol:monotonic at the cellular level, nonmonotonic at the tissue-level of organization? J. Steroid Biochem. Mol. Biol. 2006;101:263–274. doi: 10.1016/j.jsbmb.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 232.Villar-Pazos S., Martinez-Pinna J., Castellano-Muñoz M., Alonso-Magdalena P., Marroqui L., Quesada I., Gustafsson J.-A., Nadal A. Molecular mechanisms involved in the non-monotonic effect of bisphenol-a on ca2+ entry in mouse pancreatic β-cells. Sci. Rep. 2017;7(1):11770. doi: 10.1038/s41598-017-11995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Coser K.R., Chesnes J., Hur J., Ray S., Isselbacher K.J., Shioda T. Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. Proc. Natl. Acad. Sci. U. S. A. 2003;100(24):13994–13999. doi: 10.1073/pnas.2235866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Heindel J.J., vom Saal F.S. Meeting report: batch-to-batch variability in estrogenic activity in commercial animal diets--importance and approaches for laboratory animal research. Environ. Health Persp. 2008;116(3):389–393. doi: 10.1289/ehp.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Patisaul H.B., Fenton S.E., Aylor D. Animal models of endocrine disruption. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32(3):283–297. doi: 10.1016/j.beem.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Thigpen J.E., Setchell K.D., Kissling G.E., Locklear J., Caviness G.F., Whiteside T., Belcher S.M., Brown N.M., Collins B.J., Lih F.B., Tomer K.B., Padilla-Banks E., Camacho L., Adsit F.G., Grant M. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. J. Am. Assoc. Lab. Anim. Sci. 2013;52(2):130–141. [PMC free article] [PubMed] [Google Scholar]
- 237.Martino J.V., Van Limbergen J., Cahill L.E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front. Pediatr. 2017;5:96. doi: 10.3389/fped.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Javurek A.B., Spollen W.G., Johnson S.A., Bivens N.J., Bromert K.H., Givan S.A., Rosenfeld C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7(6):471–485. doi: 10.1080/19490976.2016.1234657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rosenfeld C.S. Microbiome disturbances and autism spectrum disorders. Drug Metab. Dispos. 2015;43(10):1557–1571. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- 240.Rosenfeld C.S. Gut dysbiosis in animals due to environmental chemical exposures. Front. Cell. Infect. Microbiol. 2017;7:396. doi: 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rosenfeld C.S. Effects of phytoestrogens on the developing brain, gut microbiota, and risk for neurobehavioral disorders. Front. Nutr. 2019;6:142. doi: 10.3389/fnut.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.