Abstract
Coronavirus disease 19 (COVID-19) is a pandemic condition caused by the new coronavirus SARS-CoV-2. The typical symptoms are fever, cough, shortness of breath, evolving to a clinical picture of pneumonia and, ultimately, death. Nausea and diarrhea are equally frequent, suggesting viral infection or transmission via the gastrointestinal-enteric system. SARS-CoV-2 infects human cells by using angiotensin converting enzyme 2 (ACE2) as a receptor, which is cleaved by transmembrane proteases during host cells infection, thus reducing its activities. ACE2 is a relevant player in the renin-angiotensin system (RAS), counterbalancing the deleterious effects of angiotensin II. Furthermore, intestinal ACE2 functions as a chaperone for the aminoacid transporter B0AT1. It has been suggested that B0AT1/ACE2 complex in the intestinal epithelium regulates gut microbiota (GM) composition and function, with important repercussions on local and systemic immune responses against pathogenic agents, namely virus. Notably, productive infection of SARS-CoV-2 in ACE2+ mature human enterocytes and patients’ GM dysbiosis was recently demonstrated. This review outlines the evidence linking abnormal ACE2 functions with the poor outcomes (higher disease severity and mortality rate) in COVID-19 patients with pre-existing age-related comorbidities and addresses a possible role for GM dysbiosis. The article culminates with the therapeutics opportunities based on these pathways.
Keywords: COVID-19, SARS-CoV-2, ACE2, Gut microbiota dysbiosis, Poor outcomes, Age-related diseases
1. Introduction
Coronaviruses (CoVs) are a large family of virus phenotypically and genotypically diverse belonging to the subfamily Orthocoronavirinae of the Coronaviridae family (order Nidovirales) (Banerjee et al., 2019). Although enzootic infections in birds and mammals are more usual, coronaviruses have been infecting humans as well in the last decades (Schoeman and Fielding, 2019). The lethality of coronaviruses infection after crossing this species barrier is high, as demonstrated by the outbreak of severe acute respiratory syndrome (SARS) in 2002, followed by the Middle East respiratory syndrome (MERS) in 2012, originated by SARS‐CoV and MERS‐CoV, respectively (Coleman and Frieman, 2014; Zumla et al., 2015). In December 2019, cases of pneumonia of unknown cause were firstly reported in Wuhan, Hubei Province, China, which were later confirmed to be caused by the novel coronavirus SARS-CoV-2 (Lai et al., 2020; Zhou et al., 2020b). Evidence of human‐to‐human transmission was confirmed via respiratory droplets or direct contact with the infected patients, and this new coronavirus infection was further named COVID-19 (Lai et al., 2020; Li et al., 2020c) and recognized by World Health Organization as an ongoing coronavirus pandemic in march 11, 2020. The virus has spread quickly to almost all countries and already infected millions of people and caused hundreds of thousands of deaths. COVID-19 symptoms usually appear 2–14 days after viral exposure and usually include fever, cough, shortness of breath and pneumonia, among others. Severe cases often display respiratory, hepatic, gastrointestinal and neurological complications that ultimately culminate in hospitalization and death, with some differences in lethality when compared with the previous SARS‐CoV/MERS‐CoV infections (Meo et al., 2020; Siordia, 2020). Currently, there is no registered treatment or vaccine for SARS-CoV-2 infection and new solutions to halt or slow down virus replication and dissemination are an urgent need. Apart from the efforts to develop a vaccine, several therapeutic options are currently being evaluated, including drug repurposing of distinct anti-viral, immunomodulatory or anti-inflammatory agents, as well as drugs targeting a functional receptor for SARS-CoV-2 entry into human cells, the angiotensin converting enzyme 2 (ACE2) (Li et al., 2020b; Sanders et al., 2020; Shanmugaraj et al., 2020; Zhang and Liu, 2020).
ACE2, a homologue of ACE, has been described as a negative regulator of the Renin-Angiotensin System (RAS), alleviating the deleterious actions mediated by Ang II signaling through Ang II receptor type 1 (AT1R) (Kuba et al., 2013). This role is of extreme relevance in pathological conditions associated with RAS overactivation, including those related with cardiovascular, renal and pulmonary systems (Cole-Jeffrey et al., 2015). Besides, ACE2 also displays non-RAS-related roles linked with neutral amino acids transport and gut homeostasis; conditions of impaired ACE2 expression or function are potentially promoters of intestinal dysbiosis (Perlot and Penninger, 2013). This is aligned with the gastrointestinal (GI) symptoms, such as nausea and diarrhea, that have been reported in COVID-19 patients, suggesting an impact on gastrointestinal-enteric system (Kotfis and Skonieczna-Zydecka, 2020).
Gut microflora plays major functions for the host homeostasis, including metabolic effects, structural protection against pathobionts (namely preserving the intestinal barrier composition and permeability), as well as “education” of host immunity (Kumar Singh et al., 2019; Sekirov et al., 2010). Typically, the intestinal barrier prevents the translocation of harmful substances and microbes from the lumen to the bloodstream; however, deregulation of the intestinal milieu promotes gut dysbiosis, including increased gut barrier permeability and the passage of microbiota-derived inflammatory components that activate the innate immune system and promote a state of endotoxemia/inflammation (Fernandes et al., 2019). Gut microbiota (GM) composition and diversity is affected by many factors, including by ageing. An apparent age-related gut microbiota imbalance has been underscored in the last years, featured by an altered microbial diversity, a lower abundance of probiotic strains (e.g. Bifidobacteria) and a reduced number of species producing butyrate, a short chain fatty acid (SCFA) that plays important metabolic functions and has a major role in maintaining the integrity of intestinal epithelium (Mangiola et al., 2018). Accordingly, mounting evidences outline a key role for gut dysbiosis in age-related cardiovascular, metabolic and renal disorders (Abenavoli et al., 2019; Mafra et al., 2019; McAleer and Kolls, 2018; Sanchez-Rodriguez et al., 2020). Interestingly, poor outcomes of COVID-19 infection have been observed in elderly patients, particularly those with pre-existing cardiovascular, metabolic and renal disorders, in whom the disease severity and the mortality rate are considerably higher (Du et al., 2020; Li et al., 2020a; Mehra et al., 2020; Roncon et al., 2020; Shi et al., 2020; Siordia, 2020; Team, 2020; Wang et al., 2020c; Zhou et al., 2020a).
This review highlights the evidence pointing the ACE2 imbalance as a key player for the poor outcomes in the COVID-19 patients with those age-related preexisting comorbidities and addresses a possible role for gut microbiota dysbiosis in this interplay. The article culminates with the therapeutics opportunities based on the modulation of these pathways.
2. Trilogy of ACE2 roles: a unifying perspective
2.1. ACE2 as a SARS-CoV-2 receptor responsible for COVID-19
ACE2, an ubiquituous type I membrane-anchored glycoprotein enclosing 805 amino acids, displays monocarboxypeptidase catalytic activity with various substrates already identified. Full-length ACE2 consists of an N- terminal peptidase domain (PD) and a C-terminal collectrin-like domain (CLD) that ends with a single transmembrane helix and an intracellular tail (Guang et al., 2012; Yan et al., 2020). Furthermore, it is susceptible to cleavage between amino acids 716 and 741, with subsequent release of the catalytic active ectodomain, mainly due to TNF-α-converting enzyme (TACE, also known as ADAM-17).
ACE2 is a counter-regulator of ACE activity mainly by converting angiotensin I (Ang I) into a nonapeptide (Ang 1–9) and angiotensin II (Ang II) into a vasoprotective heptapeptide (Ang 1–7) (Patel et al., 2014). Notably, TACE overstimulation may culminate in RAS overactivation and both events have been reported in some cardiovascular inflammatory diseases, namely in heart failure and coronary artery disease (Gooz, 2010; Shen et al., 2018). Recently, ACE2 has garnered great interest on the basis of their peptidase-independent actions as a functional receptor of SARS coronaviruses, including the new SARS-CoV-2.
SARS-CoV-2 is a single-stranded RNA-enveloped virus provided with crown-like spike (S) glycoproteins on the outer surface that give the coronavirus its name. S glycoprotein is present as a trimer and displays two functional subunits: while S1 subunit encloses the receptor-binding domain (RBD) and is responsible for the recognition to the host ACE2 receptor, S2 subunit comprises the fusion machinery that mediates virus fusion in transmitting host cells (Astuti and Ysrafil, 2020). ACE2-spike interaction leads to endocytosis of virus particles and subsequent release of genomic material through internalization with ACE2.
Analogous to previously identified SARS-CoV, SARS-CoV-2 spikes need to be proteolytically activated for membrane fusion via irreversible conformational changes (Walls et al., 2020). Host proteases for S-protein priming, receptor binding and cell entry include type 2 transmembrane protease serine 2 (TMPRSS2) as well as cathepsin L, cathepsin B, trypsin, factor X, furin, and elastase (Hoffmann et al., 2020a; Shang et al., 2020; Wu et al., 2020a). Hence, a concerted action of receptor binding and S-protein proteolytic processing is required for SARS-CoV-2 entry into susceptible cells and great efforts have been made to fully disclose the underlying mechanisms towards effective pharmacological modulation. Notably, S-protein preactivation also comprises C-terminal ACE2 cleavage to enhance receptor-mediated endocytosis and S-protein-driven viral entry. Thus, the abrogation of membranar ACE2-mediated cytoprotection is a first event of SARS-CoV-2 infection.
Once inside the cell, SARS-CoV-2 activates the mechanisms of replication, namely by synthesizing viral polyproteins encoding for the replicase-transcriptase complex, followed by RNA synthesis by its RNA-dependent RNA polymerase, culminating in the synthesis of structural biomolecules required for virus assembly and subsequent release (Chen et al., 2020b; Shang et al., 2020; Wu et al., 2020a).
2.2. ACE2 as a RAS counterbalance in cardiovascular, renal, respiratory and GI systems
The RAS plays a relevant role in different body’ organs (such as the heart, kidney, and lung) and systems (namely circulatory and renal), through both local actions and systemic effects, including the regulation of blood pressure and electrolyte and liquid homeostasis (Lambert et al., 2010). RAS is a highly regulated system whose main functions are accomplished by several bioactive peptides originated from a cascade of proteases. Briefly, angiotensinogen is mainly produced and secreted by the liver and cleaved by renin (produced by the juxtaglomerular apparatus in the kidney) in the Ang I. This decapeptide can be then cleaved by ACE in the octapeptide Ang II, the main bioactive component of RAS, which exerts a variety of actions by linking with G-protein-coupled receptors Ang II receptor type 1 and type 2 (AT1R and AT2R, respectively). While Ang II signaling through AT1R is associated with vasoconstrictive, pro-hypertensive, pro-oxidant, pro-inflammatory, and pro-fibrotic effects on tissue, AT2R activation has opposing effects mediated by a vasodilatory cascade with participation of NO, cGMP, or bradykinin (Azushima et al., 2020). Over the last twenty years since its discovery, ACE2 has changed the paradigm of RAS in many aspects. ACE2 gene is expressed in many human tissues, including in the heart, kidney, liver, upper airways, lungs, gut, among others. ACE2 catalyzes the conversion of Ang II into Ang 1–7, a biologically active peptide that activates Mas receptor (MasR), its endogenous orphan receptor, promoting many opposite effects to those attributed to Ang II (Kuba et al., 2013; Santos et al., 2013). Thus, the ACE2/Ang 1–7/MasR axis over time was established as the physiological counter-regulator of Ang II in the RAS (Patel et al., 2016; Zhong et al., 2010). Apart from signaling through the MasR, Ang 1−7 can also act as an AT1R antagonist, further counteracting the ACE/Ang II/AT1R axis (Gironacci et al., 1999).
The role of this axis is of special relevance in conditions associated with RAS overactivation, which includes age-related cardiac, vascular, renal and respiratory diseases, among others (Cole-Jeffrey et al., 2015; Imai et al., 2010; Kuba et al., 2013). ACE2-protective effects can be ascribed by a combination of i) downregulation of ACE action due to degradation of its substrate (Ang I) to Ang 1–9, ii) reduction of Ang II-detrimental effects by promoting its degradation and iii) potentiation of cardioprotective actions of Ang 1–7 via MasR or by antagonizing AT1R. Beneficial effect of ACE2/Ang 1–7 have been described in several animal models, namely associated with anti-hypertensive properties, improvement of myocardial performance, cardiac remodeling, and increased survival in heart failure, ischemia/reperfusion injury and myocardial infarction (Wang et al., 2012; Zhong et al., 2010).
Regarding the kidney, ACE2 is expressed in the brush border of the proximal tubule epithelial cells, where it contributes to regulate blood pressure and salt and fluid homeostasis (Warner et al., 2005). Although ACE2 has been mostly associated with renoprotective effects in some animal models of nephropathy, including in diabetic kidney injury, namely by the reduction of Ang II levels and its pro-hypertensive and vasoconstrictor actions (Batlle et al., 2012; Wong et al., 2007), the renal injury induced by ACE2 and Ang 1−7 was paradoxically reported under other experimental conditions, including in diabetic nephropathy, deserving further clarification (Zimmerman and Burns, 2012).
In the lung, ACE2 seems to ameliorate pulmonary hypertension and pulmonary fibrosis (Ferreira et al., 2009; Li et al., 2008), in opposition to the effects reported for increased Ang II levels (Marshall et al., 2004). In addition, RAS activation has been associated with acute lung injury and acute respiratory distress syndrome (Marshall et al., 2002). Interestingly, recombinant ACE2 treatment was able to overcome the severe acute lung injury observed in ACE2 knock-out mice (Imai et al., 2005), suggesting a putative protection against lung injury.
Although the GI tract is one of the major players in fluid and electrolyte intake and excretion, the role of RAS in the GI system only recently was well characterized. There are now consistent preclinical evidences of the presence of all RAS components (including ACE2) throughout the GI tract required for autonomous regulation, supporting their involvement in GI physiology and pathophysiology (Garg et al., 2012; Spak et al., 2019). In fact, local RAS is involved in the regulation of glucose, amino acid, fluid and electrolyte absorption and secretion, blood flow, motility and inflammation, and could be a promising target for GI disorders, such as inflammatory bowel disease, among others (Garg et al., 2012).
Overall, suppression of ACE2-protective roles due to ACE2 depletion upon SARS-CoV-2 infection is very likely to uphold the poor outcomes observed in elderly COVID-19 positive patients, especially in those with pre-existing cardiovascular, metabolic and renal diseases.
2.3. ACE2 as a regulator of intestinal amino acid transport that impacts gut microbiota
It is now acknowledged that ACE2 also displays important non-RAS-related roles. A non-catalytic, yet functional role for ACE2 was first hypothesized upon the observation of a constitutive ACE2 expression within the luminal surface of differentiated small intestinal epithelial cells and colonic crypt cells (Harmer et al., 2002). ACE2 shares great homology (around 50 %) with collectrin, a type 1 transmembrane protein that regulates the transporter of neutral amino acids in the kidney (Zhang et al., 2001). Further studies confirmed that ACE2 functions as the chaperone for membrane trafficking of the aminoacid transporter B°AT1, which mediates the uptake of neutral amino acids into intestinal cells in a sodium-dependent manner, even in the absence of collectrin (Camargo et al., 2009; Kowalczuk et al., 2008). Studies with ACE2-KO animals showed reduced serum levels of neutral amino acids, namely tryptophan (Trp), together with downregulated expression of small intestinal antimicrobial peptides (AMPs) and impaired gut microbial composition, which was reestablished by Trp supplementation (Hashimoto et al., 2012). This was the first work linking the ACE2-mediated amino acid transport to gut microbial ecology. It is currently proposed that mTOR activation, trough nutrient sensing and/or through the Trp-nicotinamide pathway, regulates AMPs expression and subsequent gut microbiota composition (Hashimoto et al., 2012; Perlot and Penninger, 2013). Mounting evidences suggested beneficial effects of ACE2 on several cardiovascular conditions involving the use of AMPs (such as LL-37, defensins or PR-39) and/or impact on gut microbiota (Ikeda et al., 2001; Kougias et al., 2005; Zhao et al., 2012). The association between changes on Trp transport and intestinal microbiota composition and diversity has been also supported by both preclinical and clinical studies of malnutrition associated with Kwashiorkor. In fact, children from rural regions of Malawi, with a low protein diet, were unable to develop microbiota gene diversity in the adulthood (Smith et al., 2013). Furthermore, transplantation of their feces to gnotobiotic mice confirmed the association between malnutrition and intestinal disease, which was reinforced by studies using mice feed with protein or Trp-free diet that developed gut microbiota dysbiosis and colitis (Hashimoto et al., 2012).
3. Gut microbiota roles linking immunity, infection and cardiometabolic disease
3.1. Gut microbiota, host immune system and coronaviruses infection
After a virus invades the host, it is usually first recognized by antigen presenting cells (APCs) within host innate immune system. Several pattern recognition receptors (PRRs) located in distinct compartments of the host cells recognize viral structural components and/or intermediate products (e.g. nucleic acids, glycoproteins, dsRNA), collectively termed pathogen-associated molecular patterns (PAMPs), thereby triggering the production of immune system cell effectors (Astuti and Ysrafil, 2020). Toll-like receptors (TLRs), nucleotide binding oligomerization domain (NOD)-like receptors and/or cytoplasmatic RIG-like receptors (RLR) are some examples of PRRs expressed in innate immune cells (e.g. plasmacytoid dendritic cells) who serve as the primary viral sensors for the innate antiviral effector programs, such as the canonical type I interferon (Type I IFN) response. Downstream signaling cascades foster viral antigens phagocytosis by host macrophages and play a major role in orchestrating the development of the adaptive immune response to infection (Levy et al., 2011; Yi et al., 2020). In fact, cytokine microenvironment generated by APCs modulates Th1 type adaptive immunity. For instance, CD8+ T cells priming and activation by APCs accomplish virus-infected cells abrogation through distinct effector molecules (e.g. granzyme B, perforin). In addition, CD4+ T cells evoke a cytokine-mediated Th1-polarized cellular program in other immune cells (e.g. macrophages) and regulate regulate humoral immune responses towards virus-specific antibodies production. In short, a complex coordination between innate immune cells and lymphocytes is required to achieve a balanced immune response able to control systemic viral infection (Winkler and Thackray, 2019).
The mechanisms of how CoVs’ infection modulate host immune responses appear to follow similar traits (Prompetchara et al., 2020). Previous studies, especially on SARS-CoV and MERS-CoV infections, have shown an overreaction of the immune system and subsequent “cytokine storm”, featured by an exacerbated systemic inflammatory response and the massive release of TNFα, IL-1β, IL-2, IL-6, IFNα, IFNβ, IFNγ, and MCP-1 (Channappanavar and Perlman, 2017). These findings are in line with the high-levels of pro-inflammatory cytokines (e.g. IL-1, IL-2, IL-7, G-CSF, IP-10, MCP-1, MIP-1A and TNFα) observed in COVID-19 severe cases (Huang et al., 2020). Hence, the so-called “cytokine storm” is very likely to contribute to the poor outcomes and mortality rate of COVID-19 worldwide, as found in SASR-CoV and MERS-CoV infections. Strikingly, these conditions are often caused by multi-organ failure mainly in elderly patients with chronic comorbidities displaying a self-sustaining loop of immunosenescence and inflammaging (Franceschi et al., 2017; Fulop et al., 2017). Age-related cardiovascular, metabolic and renal diseases fit this unhelpful scenario (see Section 3). Briefly, adaptive changes in the immune system as a consequence of aging (immunosenescence) often parallels a scenario of low-grade inflammation (inflammaging) featured by high concentrations of acute phase reactants and pro-inflammatory cytokines (Fulop et al., 2016, 2017). Yet, in a given environment, deregulated immune responses to external factors may become particularly relevant, namely in the case of older individuals coping with infected pathogens (Shaw et al., 2013). Notably, this mutually maintained state between immunosenescence and inflammaging that ensues in the elderly is strongly modulated by changes in GM (Amsterdam and Ostrov, 2018; Biagi et al., 2012, 2013; Shaw et al., 2013).
Major advances in our understanding of the mutual interplay between host immune system and gut microbiota have been achieved in the last decade (Belkaid and Hand, 2014). It is now well established that commensal microbiota (GI tract, oral, respiratory, genitourinary, and dermal) plays a central role in assembling mammalian immune effectors for an optimal antiviral immune response (Chiu et al., 2017). Increasing evidences highlight that commensal gut microbiota-derived ligands, collectively termed microbe-associated molecular patterns (MAMPs), are able to engage PRRs towards a balanced tunning of pro- and anti-inflammatory responses arising from innate and adaptive host immunity (Chu and Mazmanian, 2013). Thus, an interconnected immune program with differential immunological reactions for pathogenic or commensal microorganisms-derived signals occurs through PRRs expressing cells, based on the ligand (PAMPs or MAMPs), cell type and/or receptors involved (Kawashima et al., 2018).
Although the underlying molecular pathways remain to be well established, there are distinct mechanisms through which gut microbiota shapes local and distal immune components during systemic viral infection. While viral antigens are recognized by endosomal and cytoplasmic PRRs, commensal gut microbiota helps to set a homeostatic type I IFN-dependent immune response at distal non-GI tract sites, a central component for virus control (Ma et al., 2018; Takeuchi and Akira, 2010). A second important topic relies on gut microbiota ability to regulate NF-kB and inflammasome-dependent release of pro-inflammatory cytokines (e.g. TNF-α, IL-6, IL-1β, and lL-18) which are crucial for the distal recruitment of immunocompetent cells (e.g. monocytes, granulocytes, dendritic cells) that circumscribe viral replication (Chen and Ichinohe, 2015). A tight coordination between adaptive immune responses and gut microbiota is also steadily highlighted. For instance, secondary lymphoid tissues development at distal non-GI tract sites and differentiation/maturation of T and B cells (including virus-specific effectors CD4+ and CD8+ T cells) are extensively shaped by commensal gut microbiota (Chen and Ichinohe, 2015). Moreover, gut microbial-derived metabolites are very likely to modulate systemic viral infection outcomes. Accordingly, a microbial-derived metabolite produced by the human-associated commensal gut bacteria Clostridium orbiscendens – desaminotyrosine – was able to protect mice from lethal Influenza Virus infection, presumably due to the vigorous priming and amplification of type I IFN-dependent antiviral immune response (Steed et al., 2017). Similarly, the impact of intestinal (and lung) commensal microorganisms on the fine tuning of host immunity upon distinct respiratory viruses infection is also well-known (Grayson et al., 2018; Kanmani et al., 2017; Wang et al., 2013). Notably, age-related alterations in gut microbiota composition and function deeply impairs local (gut) and distal immunity. For instance, it is known that the systemic priming of inflammasomes (a mechanism closely regulated by gut microbiota) increases with age (Fernandes et al., 2019). Hence, the threshold for full inflammasome activation by a “second hit” (for instance, a viral infection) might be reached easier with ageing, analogous to what has been reported in vascular and renal patients (Song et al., 2016; Wu et al., 2015). Taking into account that elderly patients often carry a silent “inflammaging” condition and an immunonescence phenotype, age-related gut dysbiosis may additionally support an imbalanced immune response and overreactive inflammatory phenotype when a new challenge is faced, as is the case of SARS-CoV-2 infection. By looking at the previous outbreak of SARS and MERS, the mechanisms of how SARS-CoV and MERS-CoV evade host immunity greatly depend on the inhibition of the canonical type I IFN antiviral response (Prompetchara et al., 2020). An imbalanced immune response towards an excessive inflammatory phenotype (“cytokine storm”) has harmful consequences to lungs and vital organ systems and seems to correlate with the poor outcomes in CoVs infections, including in elderly COVID-19 patients. Accordingly, new biomarkers directed towards a deep understanding of how age-related mechanisms modulate respiratory SARS-CoV-2 infectious disease may have long-term gain in COVID-19 pandemic, as recently highlighted (Fuellen et al., 2020).
SARS-CoV-2, as a member of the Betacoronavirus genus, potentially shares with SARS-CoV and MERS-CoV some immune evasion mechanisms and host immunological priming. As scrutinized beyond, a healthy gut microbiota may be a cardinal vantage for host immune system to thwart viral infections such as coronaviruses, namely on the basis of its impact on important host immune responses recruited upon CoVs infections. Noteworthy, GM dysbiosis was observed in hospitalized COVID-19 patients, featured by an imbalance of intestinal microflora diversity with decrease levels of probiotic bacteria (e.g. Lactobacillus and Bifidobacterium) (Xu et al., 2020), a higher relative abundance of opportunistic pathogens (e.g. Streptococcus, Rothia, Actinomyces) and a lower relative abundance of beneficial symbionts (Gu et al., 2020; Zuo et al., 2020). Notably, these shifts in GM composition persisted after respiratory symptoms resolution and were correlated with COVID-19 severity (Zuo et al., 2020). Altogether, these emerging observations pave the way for incoming studies centered on gut microbiota and mammalian host immune relationships upon SARS-CoV-2 infection which may bring new faithfull insights for COVID-19 management (He et al., 2020).
3.2. Gut microbiota dysbiosis and cardiovascular, metabolic and renal diseases
Mounting evidences coming from preclinical and clinical research strongly suggest that dysbiosis of intestinal microflora is involved in age-related cardiovascular, metabolic and renal diseases (McIntyre et al., 2011; Miele et al., 2015). Several studies showed a reduced diversity, resilience and impaired prevalence of Firmicutes to Bacterioidetes ratio (a marker of dysbiosis) in patients with obesity or type 2 diabetes mellitus (Burcelin, 2016; Lazar et al., 2019). Briefly, conditions that promote an impaired intestinal microbiota, such as hypercaloric diets, can cause i) deregulation of gut barrier integrity due to reduced tight junctions density and subsequent ii) translocation of bacteria fragments [namely lipopolysaccharide (LPS) and peptidoglycan (PG)] from the gut lumen to the bloodstream leading to endotoxemia, also referred as a low-grade inflammation state (Blandino et al., 2016; Gomes et al., 2017; McIntyre et al., 2011). These MAMPs, through TLRs signaling, namely TLR4, cause a pro-inflammatory and immune response, thereby affecting glucose and insulin metabolism and/or signaling (Blandino et al., 2016). This is aggravated by impaired production of SCFAs by symbiotic bacteria and reduced release of intestinal peptides such as peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), collectively promoting inflammation and insulin insensitivity in peripheral tissues as well as central satiety control impairment (Boulange et al., 2016). Concurrently, signals coming from the dysbiotic gut microbiota modulate immunometabolism by interfering with epithelial and immune cells, generating an immune-inflammatory milieu that favors the progression of diabetes and its complication including diabetic retinopathy and nephropathy (Fernandes et al., 2019).
Gut microbiota dysbiosis has been also associated with a diversity of age-related cardiac and vascular disorders, including hypertension, heart failure, myocardial infarction, stroke and coronary artery disease, among others (Busnelli et al., 2019; Sanchez-Rodriguez et al., 2020). Atherosclerosis, the background condition for several cardio and cerebrovascular disorders, is a chronic inflammatory disease that has been also associated with intestinal microbiota dysbiosis (Sanchez-Rodriguez et al., 2020). Globally, patients with cardiovascular disease have been associated with major changes on bacterial populations, including an increased prevalence of Firmicutes and reduced of Bacterioidetes (Jonsson and Backhed, 2017). In addition, experimental and clinical studies suggested that bacteria-derived endotoxins, namely LPS and PG, contributes to a chronic low-grade inflammation state, typical of atherosclerosis. Moreover, some populations of patients with increased risk for CVD presented seropositivity for some deleterious bacteria, namely Helicobacter pylori and Chlamydia pneumoniae (Jha et al., 2008). Overall, it is believed that bacteria-derived endotoxemia can contribute to CVD (Busnelli et al., 2019; Sanchez-Rodriguez et al., 2020).
Further evidences reinforce the idea that disbyotic intestinal microbiota recruits additional harmful mechanisms that may contribute to atherogenesis pathogenesis. In particular, the formation of trimethylamine-N-oxide (TMAO), a pro-atherogenic product of the oxidation of trimethylamine, which is a metabolite originated by microbiota-mediated degradation of dietary free choline, phosphatidylcholine and carnitine metabolism (Koeth et al., 2013; Ussher et al., 2013). Systemic TMAO has been described as a potent promoter of atherosclerosis by reducing cholesterol absorption in enterocytes and bile acids synthesis in hepatocytes, together with enhanced cholesterol uptake by macrophages and subsequent formation of foam cells in atherosclerotic lesions (Koeth et al., 2013). Several clinical studies showed a direct association between TMAO levels and the risk for CV events and mortality in distinct populations, including patients with age-related diseases, such as heart failure, coronary artery disease or acute coronary syndrome, which was recently reinforced by systemic reviews and meta-analysis (Heianza et al., 2017; Schiattarella et al., 2017).
Identical association of TMAO with CVD risk has been described in chronic kidney disease (CKD) patients, a disease with a marked inflammatory state and mortality mainly due to CV causes. In fact, increased TMAO levels were associated with augmented tubulointerstitial fibrosis, promotion of renal oxidative stress and inflammation in animal models (Sun et al., 2017), as well as with atherosclerotic disease and long-term mortality in kidney disease patients (Stubbs et al., 2016; Tang et al., 2015). In addition, several other metabolites formed upon degradation of food constituents by dysbiotic microbiota include uremic toxics, such as indoxyl sulfate (IS) and p-cresyl sulfate (PCS), end-products of amino acids (tryptophan, tyrosine and phenylalanine) fermentation, causing renal fibrosis and aggravation of kidney injury and dysfunction (Satoh et al., 2003). Serum levels of IS and PCS are negatively correlated with renal function and gradually increases with kidney disease severity (Huang et al., 2012; Lin et al., 2011). Changes in the gut microbiota composition have been also reported in CKD patients, even at earlier disease stages (Barrios et al., 2015). Inflammation and immune deregulation are important features of microbial dysbiosis in kidney disease (McIntyre et al., 2011). As a result of gut inflammation and of the leaky gut syndrome, LPS translocation stimulates immune system cells towards a massive production of pro-inflammatory cytokines which causes systemic inflammation (Anders et al., 2013; Ramezani and Raj, 2014). Collectively, evidences suggest a vicious cycle by which dysbiosis promotes chronic inflammation and contributes to kidney disease which, on the other hand, changes the gut milieu, thus modifying gut microbiome towards dysbiosis (Fernandes et al., 2019).
4. Crosstalk between gut microbiota dysbiosis, ACE2 and COVID-19
An association between gut microbiota dysbiosis and the poor outcomes in elderly COVID-19 patients, particularly in those with pre-existing cardiovascular, cardiometabolic and cardiorenal diseases, could be hypothesized based on two aspects: i) the above-mentioned linkages between age-related gut microbiota dybiosis and cardiometabolic, cardiorenal and inflammatory disease; ii) gut microbiota dysbiosis, inaccurate local/distal host immunity towards viral infection and RAS deregulation driven by SARS-CoV-2-induced ACE2 shedding. In fact, strong epidemiological and biochemical data coming from the COVID-19 pandemics shows that elderly people with pre-existing cardiovascular, metabolic, renal, and lung diseases (including hypertension, coronary disease, diabetes, CKD and respiratory syndromes) are at higher risk of severe disease and mortality when infected (Chen et al., 2020a; Guan et al., 2020; Li et al., 2020a; Mehra et al., 2020; Roncon et al., 2020; Wang et al., 2020c). It has been reported that the frequency of cardio‐cerebrovascular diseases, hypertension and diabetes in infected patients who received care in the intensive care unit (ICU) could be three‐, two‐, and two folds higher, respectively, than counterparts receiving non‐ICU care (Li et al., 2020a). The percentage of persons hospitalized, who further need intensive care, increases with age, which is in line with the increased mortality rate in the elderly (Du et al., 2020; Shi et al., 2020; Team, 2020; Zhou et al., 2020a). As scrutinized in Section 3, immunosenescence and inflammaging phenotypes are regular key features of aforesaid age-related conditions. Notably, an exacerbated inflammatory phenotype could be strengthened by an impaired RAS signaling due to the loss of ACE2-protective functions upon SARS-CoV-2 infection, further contributing to the systemic “cytokine storm” and tissue injury (Kuster et al., 2020; Wan et al., 2020; Wang et al., 2020b) (see Fig. 1 , right). In further support of this thesis, LPS-induced acute lung injury in mice was able to reduce the expression of ACE2, exacerbating inflammatory injury and causing upregulation of other components of the RAS, namely renin, Ang II, ACE, and AT1 receptors (Ye and Liu, 2020). Interestingly, increased Ang II levels has been shown to directly impact gut microbial composition and metabolomics in a sex-specific manner (Cheema and Pluznick, 2019).
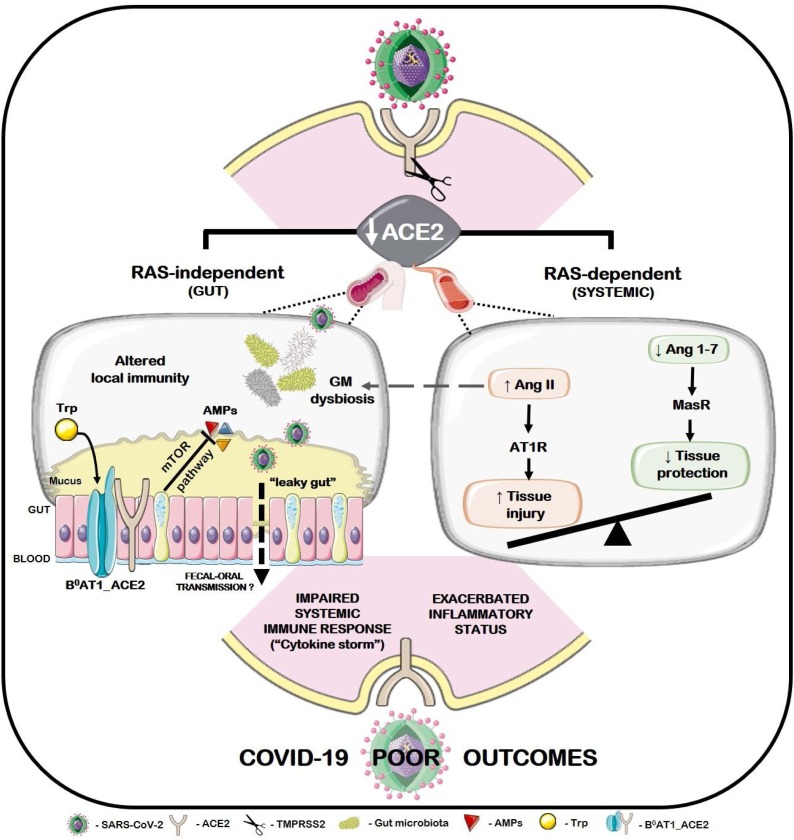
Fig. 1.
Putative association between ACE2 shedding after SARS-CoV-2 infection and the poor outcomes in elderly COVID-19 patients with pre-existing age-related cardiovascular, cardiometabolic and cardiorenal diseases. An imbalanced Ang II/Ang 1-7 ratio, due to the loss of ACE2 protective function, favors Ang II-AT1R signaling and subsequent vasoconstrictive, pro-hypertensive, pro-oxidant, pro-inflammatory and pro-fibrotic events (RAS-dependent pathway, right). Furthermore, ACE2 shedding may also dictate a dysbiotic gut condition (RAS-independent pathway, left). Briefly, loss of ACE2 integrity, a chaperone of intestinal B0AT1 carrier, may negatively impact neutral amino acids transport with subsequent mTOR-dependent AMPs disrupted synthesis, altered local immunity and gut microbiota dysbiosis. Concurrently, increased Ang II levels may also impair GM composition and function. Altered gut barrier permeability may add an extra-level of complexity to this scenario, allowing the translocation of local microbiota components to the bloodstream, including resident SARS-CoV-2 viral particles. Whether these events foster SARS-CoV-2 fecal-oral transmission deserves further studies. Nevertheless, such deleterious cascades may aggravate the pre-existing gut dysbiosis, systemic inflammation and impaired immune response in cardiovascular, cardiometabolic and cardiorenal patients, rendering them less suited to manage COVID-19 infection.
Besides, a RAS-independent cascade of events arising from reduced ACE2 function upon SARS-CoV-2 infection may also culminate in gut dysbiosis and increased susceptibility to inflammation, namely due to an impaired mTOR-mediated synthesis of AMPs (Perlot and Penninger, 2013) (see Fig. 1, left). Experimentally, gain or loss of ACE2 function have been associated with amelioration and worsening of leaky gut conditions, respectively (Duan et al., 2019; Hashimoto et al., 2012). Moreover, a RAS-independent ACE2 role on gut homeostasis (B°AT1/Trp transport) is concurrently reported. Gut microbiota dysbiosis, altered permeability of gut barrier and subsequent inefficient priming of local and systemic immunity are conditions that may be amplified due to the loss of ACE2 protective functions upon SARS-CoV-2 infection. Noteworthy, such events may be decisive in patients who previously display an immunosenescence, inflammaging and gut dysbiotic profile. Collectively, these observations stand for a putative involvement of gut dysbiosis within SARS-CoV-2 infection that may help to explain the poor outcomes of COVID-19 patients with pre-existing gut homeostasis derangements arising from age-related cardiovascular, renal and metabolic disorders. Accordingly, several recent evidences from COVID-19 patients highlight GI impairments (e.g diarrhea and other symptoms of GI discomfort) before the appearance of respiratory conditions (Kotfis and Skonieczna-Zydecka, 2020), similarly to what was reported in previous coronavirus outbreaks (Leung et al., 2003). Notably, abdominal pain was more frequent in ICU admitted patients than in individuals who did not required ICU assistance (Wang et al., 2020a). In previous CoVs outbreaks, the presence of viral RNA in feces was detected in a high percentage of patients with a more aggressive clinical course (Cheng et al., 2004). Similarly, SARS-CoV-2 was detected in feces from COVID-19 patients and fecal-oral transmission has been also suggested (Wu et al., 2020b). Collectively, the available evidences suggest that the GI tract of SARS-CoV-2 infected patients may act as a fertile ground for persistent viral replication, in line with previously reported for SARS-CoV. Strikingly, an elegant work of Zang et al. (2020) corroborates this assumption by unequivocally demonstrating that SARS-CoV-2 is able to infect ACE2+ mature enterocytes in human small intestinal enteroids, a process mediated by TMPRSS2 and TMPRSS4 proteases. Yet, SARS-CoV-2 was rapidly inactivated by simulated human colonic fluid and infectious viruses were not recovered from the stool specimens of COVID-19 patients (Zang et al., 2020). Hence, future studies aimed to address SARS-CoV-2 fecal-oral transmission are still warranted. Nevertheless, enteric coronaviruses infection potentially evoke gut-blood barrier disruption, leading to systemic spread of bacteria, endotoxins, and microbial metabolites, which may further compromise the host’s immune response to the infection and potentiate adverse outcomes, as detailed in Section 3 (Leung et al., 2003; Yeo et al., 2020). This storm may be coincident with disruption of the enteric ACE2 axis, cumulating in the dysfunction of several physiologic systems and, ultimately, septic shock (Chen et al., 2020a; Guan et al., 2020). Coherently, a gut-lung axis has been proposed in the onset of pulmonary hypertension associated with a phenotype of gut dysbiosis and leaky gut linked with overactivation of the ACE/Ang II/AT1R axis due to ACE2 loss (Kim et al., 2020). Host enterocytes continuous viral production and ACE2 depletion, as well as GM dysbiosis, may perpetuate a disrupted gut-lung axis, as previously suggested for SARS-CoV (Leung et al., 2003).
As above mentioned, a dysbiotic condition, viewed by decreased microbial diversity and richness, as well as impaired Firmicutes to Bacteroidetes ratio, have been reported in elderly people and in age-related cardiovascular, metabolic, renal and pulmonary conditions. Herein, it is hypothesized that pre-existing disorders displaying altered gut microbiome may worsen SARS-CoV-2 infection due to ACE2 integrity/functionality loss. This assumption is strengthen by evidences coming from the ACE2-/y-Akita mice who display gut barrier disruption and exacerbated diabetes-induced dysbiosis (Duan et al., 2019). In this sense, SARS-CoV-2 infection may promote a significant reduction of enteric ACE2 expression and subsequent leakage of gut barrier, altered microbiota composition and metabolomic and aggravated endotoxemia/inflammation. This possibility is supported by recent data showing that some patients with COVID-19 presented intestinal microbial dysbiosis (Xu et al., 2020). Yet, it will be important to address in future studies the putative relationship with concomitant drugs intake that are able to modulate GM composition, such as antibiotics.
5. ACE2-based and microbiota-directed therapeutic opportunities for COVID-19
The discover that ACE2 is the receptor for SARS-CoV-2 entry in human cells (Hoffmann et al., 2020b) has raised the possibility of ACE2 and/or other RAS modulators to potentially treat COVID-19 (Zhang et al., 2020a). The precise implications (benefits vs risks) of use ACE inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs) on COVID-19 patients, namely in patients with hypertension (Vaduganathan et al., 2020), have been intensely discussed during the last months and several clinical trials are currently ongoing (Table 1 ). On one hand, these drugs could theoretically provide benefits viewed by the reduction of the extent of experimentally induced lung injury in animal models under RAS inhibition (He et al., 2007); on the other hand, these drugs have been suggested to upregulate ACE2 expression, which could eventually aggravate the outcomes by enhancing viral entry into the host cells (Sanders et al., 2020). A study conducted with 18,472 patients tested for COVID-19 showed no association between ACEIs or ARBs use and COVID-19 test positivity (Mehta et al., 2020). ACEIs/ARBs exposure was not associated with a higher risk of COVID-19 infection, neither with a higher risk of having severe infection or mortality, but was associated with a lower risk of mortality compared to those on non-ACEIs/ARBs antihypertensive drugs (Zhang et al., 2020b). The study further supported the current professional society’s guidelines that discourage the discontinuation of ACEIs or ARBs in COVID-19 patients due to lower risk of mortality when therapy is maintained (Cannata et al., 2020). Identical conclusions were obtained in other relevant studies and publications (Fernandez-Ruiz, 2020; Reynolds et al., 2020), including in a multinational open science cohort study involving 1.1 million antihypertensive users with distinct anti-hypertensive drug classes, which concluded the absence of clinically significant increased risk of COVID-19 diagnosis or hospitalization with ACEs or ARBs use and a recommendation for not discontinuation or change of treatment to avoid COVID-19 (Morales et al., 2020).
Table 1.
Therapeutic opportunities under clinical evaluation for COVID-19 treatment targeting ACE2 or other RAS component (Clinicaltrial.gov).
| Therapeutic group(s)/ Drug(s) | Patients’ s conditions | Study type and nr participants* | Country | Trial ID and phase** |
|---|---|---|---|---|
| ACEIs/ ARBs |
COVID-19 patients with HT (stage 1−2) |
Obs. / Prosp. 10 participants |
Ukraine |
NCT04364984 Phase: NA |
| Hospitalized COVID-19 patients | Obs. 2574 participants |
Spain |
NCT04367883 Phase: NA |
|
| Hospitalized COVID-19 patients with HT |
Obs. / Retros. 275 participants |
China |
NCT04318301 Phase: NA |
|
| Hospitalized COVID-19 patients treated with ACEIs or ARBs | Obs./Retros. 5000 participants |
Italy |
NCT04318418 Phase: NA |
|
| COVID-19 patients | Obs./ Cross-Sec 2000 participants |
Italy (MC) |
NCT04331574 Phase 4 |
|
| Hospitalized COVID-19 patients Current use of ACEIs or ARBs |
Interv. / Par. Ass. 152 participants |
United States |
NCT04338009 Phase: NA |
|
| Hospitalized COVID-19 patients treated with ACEIs or ARBs | Interv. / Par. Ass. 554 participants |
France |
NCT04329195 Phase 3 |
|
| COVID-19 patients Current use of ACEIs or ARBs therapy for HT, DM, HF or CAD |
Interv. / Par. Ass. 208 participants |
25 locations (MC) |
NCT04353596 Phase 4 |
|
| Hospitalized COVID-19 patients Current use of ACEIs or ARBs |
Interv. / Par. Ass. 215 participants |
Denmark |
NCT04351581 Phase: NA |
|
| COVID-19 patients with HT, CAD, HF or DM | Obs. / Prosp. 226 participants |
Saudi Arabia |
NCT04357535 Phase: NA |
|
| Hospitalized COVID-19 patients Current use of ACEIs or ARBs |
Interv. / Par. Ass. 500 participants |
Brazil |
NCT04364893 Phase: NA |
|
| Hospitalized COVID-19 patients | Obs. / Retros 700 participants |
France |
NCT04374695 Phase: NA |
|
| COVID-19 patients | Obs. / Prosp. 504 participants |
China (MC) |
NCT04342702 Phase: NA |
|
| Synthetic AMD vs ACEIs or ARBs | COVID-19 patients using AMD or ACEIs or ARBs |
Obs. / Prosp. + Retros 6000000 participants |
France |
NCT04356417 Phase: NA |
| ACEIs | COVID-19 patients | Obs. / Retros 60 participants |
Turkey |
NCT04379310 Phase: NA |
| ACEI/ Captopril or enalapril |
COVID-19 patients | Interv. / Par.Ass. 60 participants |
Egypt |
NCT04345406 Phase 3 |
| ACEI/ Captopril |
Hospitalized COVID-19 patients with pneumonia | Interv. / Par. Ass. 230 participants |
France (MC) |
NCT04355429 Phase 2 |
| ACEI/ Ramipril |
Hospitalized COVID-19 patients | Interv. / Par. Ass. 560 participants |
Not Provided |
NCT04366050 Phase 2 |
| ARBs | IUC hospitalized COVID-19 patients | Obs. / Prosp. 100 participants |
France |
NCT04337190 Phase: NA |
| COVID-19 patients | Interv. / Par. Ass. 605 participants |
Australia |
NCT04394117 Phase 4 |
|
| ARB/ Candesartan |
COVID-19 patients with Hypertension |
Interv. / Par. Ass. 500 participants |
Austria (MC) |
NCT04351724 Phase 2/3 |
| ARB/ Losartan |
COVID-19 patients with HT | Interv. / Par. Ass. 200 participants |
United States (MC) |
NCT04340557 Phase 4 |
| COVID-19 patients with hypoxic respiratory failure | Interv. / Sin.Group 50 participants |
United States |
NCT04335123 Phase 1 |
|
| Hospitalized COVID-19 patients | Interv. / Par. Ass. 4000 participants |
United States (MC) |
NCT04328012 Phase 2/3 |
|
| Hospitalized COVID-19 patients age ≥ 40 years |
Interv. / Fact. Ass. 10000 participants |
Nigeria and Pakistan (MC) |
NCT04343001 Phase 3 |
|
| COVID-19 patients | Interv. / Fact. Ass. 500 participants |
United States |
NCT04349410 Phase 2/3 |
|
| COVID-19 patients | Interv. / Par. Ass. 580 participants |
United States (MC) |
NCT04311177 Phase 2 |
|
| Hospitalized COVID-19 patients | Interv. / Par. Ass. 200 participants |
United States (MC) |
NCT04312009 Phase 2 |
|
| COVID-19 patients with metastatic disease | Interv. / Par. Ass. 156 participants |
Brazil |
NCT04447235 Phase 2 |
|
| Hospitalized COVID-19 patients | Interv. / Par. Ass 20 Participants |
Mexico |
NCT04428268 Phase 2 |
|
| ARB/ Telmisartan |
Hospitalized COVID-19 patients | Interv. / Par. Ass. 1600 participants |
France |
NCT04359953 Phase 3 |
| COVID-19 patients with age ≥ 65 years |
Interv. / Par. Ass. 845 participants |
France (MC) |
NCT04356495 Phase 3 |
|
| Hospitalized COVID-19 patients | Interv. / Par. Ass. 40 participants |
United States |
NCT04360551 Phase 2 |
|
| COVID-19 patients | Interv. / Par. Ass. 400 participants |
Argentina |
NCT04355936 Phase 2 |
|
| ARB/ Valsartan |
Hospitalized COVID-19 patients | Interv. / Par. Ass. 651 participants |
Netherlands |
NCT04335786 Phase 4 |
| Angiotensin 1−7 | IUC hospitalized COVID-19 patients | Interv. / Par. Ass. 60 participants |
Not Provided |
NCT04332666 Phase 2/3 |
| Hospitalized COVID-19 patients | Interv. / Par. Ass. 100 participants |
No Provided |
NCT04401423 Phase 2 |
|
| Isotretinoin / Down-regulator of ACE2 receptors |
COVID-19 patients | Interv. / Par. Ass. 300 participants |
Not Provided |
NCT04361422 Phase 3 |
| Isotretinoin | IUC hospitalized COVID-19 patients with SRF | Interv. / Par. Ass. 1000 participants |
Egypt |
NCT04353180 Phase 2/3 |
| Isotretinoin + Tamoxifen | IUC hospitalized COVID-19 patients with SRF | Interv. / Seq. Ass. 160 participants |
Not Provided |
NCT04389580 Phase 2 |
| TMPRSS2 Inhibitor/ Nafamostat Mesilate | Hospitalized COVID-19 patients | Interv. / Par.Ass. 256 participants |
Not Provided |
NCT04352400 Phase 2/3 |
| TMPRSS2 Inhibitor/ Camostat Mesilate |
Hospitalized COVID-19 patients | Interv. / Par.Ass. 580 participants |
Denmark (MC) |
NCT04321096 Phase 1/2 |
| TMPRSS2 Inhibitor/ Bromhexine | Hospitalized COVID-19 patients | Interv. / Par.Ass. 90 participants |
Not Provided |
NCT04355026 Phase 4 |
| rhACE2/ APN01 |
Hospitalized COVID-19 patients | Interv. / Par.Ass. 200 participants |
Austria, Denmark and Germany (MC) |
NCT04335136 Phase 2 |
| rbACE2 | COVID-19 patients | Interv. / Par.Ass. 24 participants |
Not Provided |
NCT04375046 Phase 1 |
| rbACE2 + Isotretinoin | COVID-19 patients | Interv. / Par.Ass. 24 participants |
Not Provided |
NCT04382950 Phase 1 |
| Microparticles with ACE2 | COVID-19 patients | Obs. / Prosp. 175 participants |
Not Provided |
NCT04448743 Phase: NA |
ACE2, angiotensin converting enzyme 2; ACEIs, angiotensin-converting enzyme inhibitors; AMD, anti-malarial drugs; ARBs, angiotensin II receptor blockers; CAD, coronary artery disease; COVID-19, Coronavirus disease; Cross-Sec, Cross-Sectional; DM, diabetes mellitus; Fact. Ass., Factorial Assignment; HF, heart failure; HT, Hypertension; ICU, intensive care unit; Interv., interventional; MC, Multicentre; NA, not applicable; Obs., observational; Par. Ass., parallel assignment; Prosp., prospective; RAS, renin-angiotensin system; rbACE2, recombinant bacterial angiotensin-converting enzyme 2; Retros., retrospective study; rhACE2, recombinant human angiotensin-converting enzyme 2; SRF, severe respiratory failure; Seq. Ass., Sequential Assignment; Sin. Group., Single group assignment; TMPRSS2, Transmembrane Serine Protease 2.
Estimated number of total participants.
FDA definitions of clinical trial phases.
Other therapeutic approaches may rely on Ang 1–7 receptors agonists or recombinant human ACE2 (rhACE2) as replacement strategies, an attempt to maintain an adequate degree of RAS counter-regulation via the ACE2/Ang1–7/MasR axis that may be particularly useful in patients with pre-existing cardiovascular/metabolic/renal pathologies. Beneficial effects were observed in CVD and lung disease upon rhACE2 or AVE 0991 administration, an agonist of Ang 1–7 receptors (Patel et al., 2016). In fact, AVE 0991 showed cardiac, renal and pulmonary beneficial effects, while rhACE2 treatment was able to ameliorate the symptoms of CVD, kidney injury and acute lung injury in preclinical research (Imai et al., 2005; Jia, 2016; Zhong et al., 2010). Regarding acute lung injury of distinct etiologies, including the one induced by SARS-CoV, rhACE2 also promoted beneficial effects (Imai et al., 2005; Jia, 2016; Kuba et al., 2005). Due to the ability to scavenge circulating viral particles along with the putative beneficial effect arising from RAS counter-regulation, rhACE2 has been viewed as a promising therapeutic strategy for COVID-19 patients and is currently under clinical trials (Table 1).
It is worth to mention that previous studies with the rhACE2 APN01 (GSK2586881) have shown a safe profile both in healthy volunteers as well as in a small cohort of patients with acute respiratory distress syndrome (Haschke et al., 2013; Khan et al., 2017; Zhang and Baker, 2017). Overall, the supply of ACE2 soluble forms might exert a dual beneficial function within SARS-CoV-2 infection, by slowing the virus entry into host cells while ensuring the putative maintenance of ACE2 contra-regulatory functions on RAS, assisting in the protection from lung injury.
Another possibility to halt SARS-CoV-2 entry into host cells that deserves further attention is the inhibition of TMPRSS2 activity (Hoffmann et al., 2020a; Iwata-Yoshikawa et al., 2019). Camostat mesylate, a TMPRSS2 inhibitor, was tested in the past within SARS and MERS infections and has been reported as a possible effective option. In addition, blocking ACE2 with antibodies or small molecules/peptides can also be a promising strategy that deserves further research.
The possibility of consistent intestinal microbial dysbiosis in patients with COVID-19 is under evaluation (see Table 2 of ongoing clinical trials) and was already suggested by some studies (see Section 3.1). In this sense, therapeutic nutritional measures to equilibrate gastrointestinal function and microbiota could be important, including the use of prebiotics or probiotics in order to reduce the risk of secondary infection due to bacterial translocation. In addition, the use of bioengineered probiotic species has been envisaged as live vectors to deliver pharmacological agents. This approach was used with success in mice with diabetic retinopathy treated with the recombinant Lactobacillus paracasei expressing the secreted ACE2 in fusion with the non-toxic subunit B of cholera toxin (acts as a carrier to facilitate transmucosal transport) (Verma et al., 2019), and could be replicated using COVID-19 infection as the target conditions.
Table 2.
Clinical trials with COVID-19 patients for the study of gut microbiota (Clinicaltrial.gov).
| Intervention/Main aims | Patients’ s conditions | Study type and nr participants* | Country | Trial ID and phase** |
|---|---|---|---|---|
| Changes in fecal microbiota composition | Hospitalized COVID-19 patients | Obs. / Prosp. 170 participants | Hong Kong |
NCT04325919 Phase: NA |
| Changes in respiratory and fecal microbiota composition | COVID-19 patients admitted to the ICU | Obs. / Prosp. 30 participants |
Not Provided |
NCT04359706 Phase: NA |
| Changes in fecal microbiota composition | COVID-19 patients: self-isolated at home; in an isolated hospital and in the ICU of the hospital |
Obs. / Prosp. 60 participants |
Portugal (MC) |
NCT04355741 Phase: NA |
| Probiotic supplementation - SivoMixx | Hospitalized COVID-19 patients | Interv. / Par. Ass. 152 participants |
Italy |
NCT04366089 Phase 2 |
| Changes in fecal microbiota composition | Hospitalized COVID-19 patients | Obs. / Prosp. 2000 participants |
France |
NCT04332016 Phase: NA0 |
| Correlation of immune profiling with microbiome analysis | Hospitalized COVID-19 patients | Obs. / Prosp. 100 participants |
Not provided |
NCT04327570 Phase: NA |
| Probiotic supplementation | Hospitalized COVID-19 patients | Interv. / Par. Ass. 40 participants |
Spain |
NCT04390477 Phase: NA |
| Probiotic supplementation – SivoMixx + Azithromycin | Hospitalized COVID-19 patients | Obs. / Retros. 70 participants |
Italy |
NCT04368351 Phase: NA |
| Correlation of feces microbiome and clinical outcome for COVID-19 | COVID-19 patients | Obs. / Prosp. 150 participants |
Not provided |
NCT04359459 Phase: NA |
| Correlation between oral microbiome and COVID-19 infection status | Asymptomatic COVID-19 patients | Obs. / Prosp. 500 participants |
Not provided |
NCT04345510 Phase: NA |
| Supplementation with natural polyphenols-Tannins | Hospitalized COVID-19 patients | Interv. / Par. Ass. 140 participants |
Not provided |
NCT04403646 Phase: NA |
| Changes in fecal microbiota composition | COVID-19 patients | Obs. 250 participants |
United States |
NCT04359836 Phase: NA |
| Probiotic supplementation - Omnibiotic ® AAD | COVID-19 patients with pre-existing diarrhoea | Interv. / Par. Ass. 108 participants |
Austria |
NCT04420676 Phase: NA |
| Correlation of gut microbiome to disease | COVID-19 patients | Obs. 250 participants |
United States |
NCT04359836 Phase: NA |
COVID-19, Coronavirus disease; ICU, intensive care unit; Interv., interventional; MC, multicentre study; NA, not applicable; Obs., observational; Par. Ass., parallel assignment; Prosp., prospective.
Estimated number of total participants.
FDA definitions of clinical trial phases.
Among the range of therapeutic options that have been tested to treat patients with COVID-19, there are some that have been shown the ability to modulate the intestinal microbiota. Concerning drugs, the anti-malarial drugs chloroquine and hydroxychloroquine, the lipid-lowering drugs statins, together with immunomodulatory and anti-inflammatory agents such as some interferons and corticosteroids (including dexamethasone), have been shown to interfere with GM (Angelakis et al., 2014; Huang et al., 2015; Tschurtschenthaler et al., 2014; Zhao et al., 2020). Regarding nutraceutical interventions, several options able to modulate GM have been recently suggested as potentially useful in combating COVID-19 or its associated symptoms (Akour, 2020; Dhar and Mohanty, 2020; Zabetakis et al., 2020); among them, vitamins, selenium and zinc, flavonoids, omega‐3 polyunsaturated fatty acids and a panoply of herbs/compounds from traditional chinese medicine (Costantini et al., 2017; Feng et al., 2019; Kumar Singh et al., 2019; Steinert et al., 2020; Yang et al., 2020). Although the effects described for nutraceutical options are generically symbiotic, the same is not always true for drugs, some of which not only affect the microbiota composition and function but can also be affected by it, in interactions that can be symbiotic or dysbiotic. However, the effects on gut microbiota of these approaches have been mainly described in other pathological conditions (experimental or clinical), and there is still no relevant information on their influence in patients with COVID-19, deserving further elucidation.
Finally, a warning note related to this disease and intestinal microbiota dysbiosis. The existence of gastrointestinal symptoms in some patients affected by COVID-19, and the detection of SARS-CoV-2 in feces, which can persist positive even after the virus is eliminated from the respiratory tract, suggest the possibility of a fecal–oral route of transmission (Xiao et al., 2020). In this sense, an international group of experts in fecal microbiota transplantation and stool banking have suggested a rapid revision of recommendations in order to avoid contamination from donors, who should be carefully screened before donating (Ianiro et al., 2020).
6. Conclusions and future challenges
ACE2 has a trilogy of roles with clear relevance in COVID-19 pathophysiology with expected impact on both its outcome and management/treatment. ACE2 is the SARS-CoV-2 receptor in humans, which is cleaved by transmembrane proteases (e.g. TMPRSS2) upon viral infection. ACE2 signaling on MasR receptor displays an important RAS counter-regulatory activity of the pro-vasoconstrictor, pro-fibrotic, pro-oxidant and pro-inflammatory activities of Ang II via AT1R. When “neutralized” after viral infection, ACE2-protective effects on cardiovascular, renal and pulmonary systems are weakened, which is particularly critical in elderly individuals with aforesaid pre-existing age-related conditions, which have been described as the most severely affected upon SARS-CoV-2 infection. In addition, GI symptoms have also been described in a considerable percentage of infected patients, suggesting a gastrointestinal-enteric pathway. Gut microbiota dysbiosis and impaired intestinal-blood barrier (leaky gut) have been associated with the development of atherosclerosis and increased risk of CVD and mortality in cardiovascular patients, as well as in individuals with obesity, diabetes or chronic renal disease. Interestingly, ACE2 plays a major role in amino acid transport in the intestinal epithelium, a mechanism that is linked with the production of antimicrobial peptides, thus interfering with gut microflora equilibrium. In conditions of ACE2 shedding, as occurs after viral S-protein priming, gut microbiota dysbiosis might be favored, thus further contributing and eventually explaining the poor outcomes in elderly COVID-19 patients, especially in those with pre-existing age-related cardiovascular, metabolic and renal comorbidities, which present higher frequency of ICU admission (disease severity) and increased mortality rate.
Considering the crucial role of ACE2 on COVID-19 and the potential impact on the worsened outcome of some patients, several therapeutic approaches have been tested, which are mainly based on the following strategies: - soluble form of ACE2, such as rhACE2; - ACE2 blockers (anti-antibodies or small molecules/peptides); - TMPRSS2 inhibitors; - Ang 1–7 receptor agonists; - ACE inhibitors and/or ARBs. Some of these pharmacological strategies appear to show promising results and some of them are currently moving to clinical trials. In addition, manage the patients’ intestinal leakage and microbiota dysbiosis may be also relevant, namely using nutritional measures with prebiotic and/or probiotic activity, including bioengineered probiotic strategies able to deliver pharmacological agents.
The coming times will certainly uncover new data that will help to clarify this issue and, hopefully, effective solutions to solve this pandemic or other similar scenarios that the future might bring.
Funding
This work was supported by the European Regional Development Fund (FEDER), through Operational Programme for Competitiveness and Internationalisation COMPETE2020 (CENTRO-01-0145-FEDER-000012-HealthyAging2020) and by National funds via Portuguese Science and Technology Foundation (FCT): Strategic Projects UID/NEU/04539/2013, UID/NEU/04539/2019, UIDB/04539/2020 and UIDP/04539/2020 (CIBB), SFRH/BD/109017/2015 (PhD Fellowship) and PTDC/SAU-NUT/31712/2017, as well as by COMPETE-FEDER funds (POCI-01-0145-FEDER-007440 and POCI-01-0145-FEDER-031712).
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Abenavoli L., Scarpellini E., Colica C., Boccuto L., Salehi B., Sharifi-Rad J., Aiello V., Romano B., De Lorenzo A., Izzo A.A., Capasso R. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019:11. doi: 10.3390/nu11112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akour A. Probiotics and COVID-19: is there any link? Lett. Appl. Microbiol. 2020;10.1111/lam.13334 doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam D., Ostrov B.E. The impact of the microbiome on immunosenescence. Immunol. Invest. 2018;47:801–811. doi: 10.1080/08820139.2018.1537570. [DOI] [PubMed] [Google Scholar]
- Anders H.J., Andersen K., Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- Angelakis E., Million M., Kankoe S., Lagier J.C., Armougom F., Giorgi R., Raoult D. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 2014;58:3342–3347. doi: 10.1128/AAC.02437-14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azushima K., Morisawa N., Tamura K., Nishiyama A. Recent research advances in renin-angiotensin-aldosterone system receptors. Curr. Hypertens. Rep. 2020;22:22. doi: 10.1007/s11906-020-1028-6. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K. Bats and coronaviruses. Viruses. 2019:11. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios C., Beaumont M., Pallister T., Villar J., Goodrich J.K., Clark A., Pascual J., Ley R.E., Spector T.D., Bell J.T., Menni C. Gut-microbiota-metabolite axis in early renal function decline. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D., Wysocki J., Soler M.J., Ranganath K. Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2012;81:520–528. doi: 10.1038/ki.2011.381. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E., Candela M., Fairweather-Tait S., Franceschi C., Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr) 2012;34:247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E., Candela M., Turroni S., Garagnani P., Franceschi C., Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol. Res. 2013;69:11–20. doi: 10.1016/j.phrs.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Blandino G., Inturri R., Lazzara F., Di Rosa M., Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016;42:303–315. doi: 10.1016/j.diabet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Boulange C.L., Neves A.L., Chilloux J., Nicholson J.K., Dumas M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcelin R. Gut microbiota and immune crosstalk in metabolic disease. Mol. Metab. 2016;5:771–781. doi: 10.1016/j.molmet.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M., Manzini S., Chiesa G. The gut microbiota affects host pathophysiology as an endocrine organ: a focus on cardiovascular disease. Nutrients. 2019:12. doi: 10.3390/nu12010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A., Kuba K., Danilczyk U., Skovby F., Kleta R., Penninger J.M., Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannata F., Chiarito M., Reimers B., Azzolini E., Ferrante G., My I., Viggiani G., Panico C., Regazzoli D., Ciccarelli M., Voza A., Aghemo A., Li H., Wang Y., Condorelli G., Stefanini G.G. Continuation versus discontinuation of ACE inhibitors or angiotensin II receptor blockers in COVID-19: effects on blood pressure control and mortality. Eur. Heart J. Cardiovasc. Pharmacother. 2020;pvaa056 doi: 10.1093/ehjcvp/pvaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema M.U., Pluznick J.L. Gut microbiota plays a central role to modulate the plasma and fecal metabolomes in response to angiotensin II. Hypertension. 2019;74:184–193. doi: 10.1161/HYPERTENSIONAHA.119.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.Y., Ichinohe T. Response of host inflammasomes to viral infection. Trends Microbiol. 2015;23:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu L., Bazin T., Truchetet M.E., Schaeverbeke T., Delhaes L., Pradeu T. Protective microbiota: from localized to long-reaching Co-immunity. Front. Immunol. 2017;8:1678. doi: 10.3389/fimmu.2017.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Mazmanian S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Jeffrey C.T., Liu M., Katovich M.J., Raizada M.K., Shenoy V. ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J. Cardiovasc. Pharmacol. 2015;66:540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C.M., Frieman M.B. Coronaviruses: important emerging human pathogens. J. Virol. 2014;88:5209–5212. doi: 10.1128/JVI.03488-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini L., Molinari R., Farinon B., Merendino N. Impact of Omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017:18. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., Guo G.Y., Du J., Zheng C.L., Zhu Q., Hu M., Li X.Y., Peng P., Shi H.Z. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020:55. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Prasad R., Feng D., Beli E., Li Calzi S., Longhini A.L.F., Lamendella R., Floyd J.L., Dupont M., Noothi S.K., Sreejit G., Athmanathan B., Wright J., Jensen A.R., Oudit G.Y., Markel T.A., Nagareddy P.R., Obukhov A.G., Grant M.B. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ. Res. 2019;125:969–988. doi: 10.1161/CIRCRESAHA.119.315743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Fernandes R., Viana S.D., Nunes S., Reis F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:1876–1897. doi: 10.1016/j.bbadis.2018.09.032. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz I. RAAS inhibitors do not increase the risk of COVID-19. Nat. Rev. Cardiol. 2020;17:383. doi: 10.1038/s41569-020-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A.J., Shenoy V., Yamazato Y., Sriramula S., Francis J., Yuan L., Castellano R.K., Ostrov D.A., Oh S.P., Katovich M.J., Raizada M.K. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Vitale G., Capri M., Salvioli S. Inflammaging and’ Garb-aging’. Trends Endocrinol. Metab. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Fuellen G., Liesenfeld O., Kowald A., Barrantes I., Bastian M., Simm A., Jansen L., Tietz-Latza A., Quandt D., Franceschi C., Walter M. The preventive strategy for pandemics in the elderly is to collect in advance samples & data to counteract chronic inflammation (inflammaging) Ageing Res. Rev. 2020;62 doi: 10.1016/j.arr.2020.101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Dupuis G., Witkowski J.M., Larbi A. The role of immunosenescence in the development of age-related diseases. Rev. Invest. Clin. 2016;68:84–91. [PubMed] [Google Scholar]
- Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Angus P.W., Burrell L.M., Herath C., Gibson P.R., Lubel J.S. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2012;35:414–428. doi: 10.1111/j.1365-2036.2011.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironacci M.M., Coba M.P., Peña C. Angiotensin-(1–7) binds at the type 1 angiotensin II receptors in rat renal cortex. Regul. Pept. 1999;84:51–54. doi: 10.1016/s0167-0115(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Gomes J.M.G., Costa J.A., Alfenas R.C.G. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism. 2017;68:133–144. doi: 10.1016/j.metabol.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Gooz M. ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson M.H., Camarda L.E., Hussain S.A., Zemple S.J., Hayward M., Lam V., Hunter D.A., Santoro J.L., Rohlfing M., Cheung D.S., Salzman N.H. Intestinal microbiota disruption reduces regulatory t cells and increases respiratory viral infection mortality through increased IFNgamma production. Front. Immunol. 2018;9:1587. doi: 10.3389/fimmu.2018.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Chen Y., Wu Z., Chen Y., Gao H., Lv L., Guo F., Zhang X., Luo R., Huang C., Lu H., Zheng B., Zhang J., Yan R., Zhang H., Jiang H., Xu Q., Guo J., Gong Y., Tang L., Li L. Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clin. Infect. Dis. 2020;ciaa709 doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for, C Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(12):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang C., Phillips R.D., Jiang B., Milani F. Three key proteases--angiotensin-I-converting enzyme (ACE), ACE2 and renin–within and beyond the renin-angiotensin system. Arch. Cardiovasc. Dis. 2012;105:373–385. doi: 10.1016/j.acvd.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krahenbuhl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52:783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Han B., Mura M., Xia S., Wang S., Ma T., Liu M., Liu Z. Angiotensin-converting enzyme inhibitor captopril prevents oleic acid-induced severe acute lung injury in rats. Shock. 2007;28:106–111. doi: 10.1097/SHK.0b013e3180310f3a. [DOI] [PubMed] [Google Scholar]
- He Y., Wang J., Li F., Shi Y. Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front. Microbiol. 2020;11:1302. doi: 10.3389/fmicb.2020.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heianza Y., Ma W., Manson J.E., Rexrode K.M., Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. 2020. The Novel Coronavirus 2019 (2019-nCoV) Uses the SARS-coronavirus Receptor ACE2 and the Cellular Protease TMPRSS2 for Entry Into Target Cells. bioRxiv 2020.2001.2031.929042. [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280):e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.T., Shu K.H., Cheng C.H., Wu M.J., Yu T.M., Chuang Y.W., Chen C.H. Serum total p-cresol and indoxyl sulfate correlated with stage of chronic kidney disease in renal transplant recipients. Transplant. Proc. 2012;44:621–624. doi: 10.1016/j.transproceed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Huang E.Y., Inoue T., Leone V.A., Dalal S., Touw K., Wang Y., Musch M.W., Theriault B., Higuchi K., Donovan S., Gilbert J., Chang E.B. Using corticosteroids to reshape the gut microbiome: implications for inflammatory bowel diseases. Inflamm. Bowel Dis. 2015;21:963–972. doi: 10.1097/MIB.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiro G., Mullish B.H., Kelly C.R., Sokol H., Kassam Z., Ng S., Fischer M., Allegretti J.R., Masucci L., Zhang F., Keller J., Sanguinetti M., Costello S.P., Tilg H., Gasbarrini A., Cammarota G. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol. Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Young L.H., Scalia R., Ross C.R., Lefer A.M. PR-39, a proline/arginine-rich antimicrobial peptide, exerts cardioprotective effects in myocardial ischemia-reperfusion. Cardiovasc. Res. 2001;49:69–77. doi: 10.1016/s0008-6363(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Ohto-Nakanishi T., Penninger J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J. 2010;74:405–410. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J. Virol. 2019:93. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha H.C., Prasad J., Mittal A. High immunoglobulin A seropositivity for combined Chlamydia pneumoniae, Helicobacter pylori infection, and high-sensitivity C-reactive protein in coronary artery disease patients in India can serve as atherosclerotic marker. Heart Vessels. 2008;23:390–396. doi: 10.1007/s00380-008-1062-9. [DOI] [PubMed] [Google Scholar]
- Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Jonsson A.L., Backhed F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- Kanmani P., Clua P., Vizoso-Pinto M.G., Rodriguez C., Alvarez S., Melnikov V., Takahashi H., Kitazawa H., Villena J. Respiratory commensal bacteria corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front. Microbiol. 2017;8:1613. doi: 10.3389/fmicb.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T., Ikari N., Watanabe Y., Kubota Y., Yoshio S., Kanto T., Motohashi S., Shimojo N., Tsuji N.M. Double-stranded RNA derived from lactic acid bacteria augments Th1 immunity via interferon-beta from human dendritic cells. Front. Immunol. 2018;9:27. doi: 10.3389/fimmu.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Rigatto K., Gazzana M.B., Knorst M.M., Richards E.M., Pepine C.J., Raizada M.K. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75:1063–1071. doi: 10.1161/HYPERTENSIONAHA.119.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., Smith J.D., DiDonato J.A., Chen J., Li H., Wu G.D., Lewis J.D., Warrier M., Brown J.M., Krauss R.M., Tang W.H., Bushman F.D., Lusis A.J., Hazen S.L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Skonieczna-Zydecka K. COVID-19: gastrointestinal symptoms and potential sources of 2019-nCoV transmission. Anaesthesiol. Intensive Ther. 2020;40157 doi: 10.5114/ait.2020.93867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougias P., Chai H., Lin P.H., Yao Q., Lumsden A.B., Chen C. Defensins and cathelicidins: neutrophil peptides with roles in inflammation, hyperlipidemia and atherosclerosis. J. Cell. Mol. Med. 2005;9:3–10. doi: 10.1111/j.1582-4934.2005.tb00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczuk S., Broer A., Tietze N., Vanslambrouck J.M., Rasko J.E., Broer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J. 2008;22:2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ. J. 2013;77:301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- Kumar Singh A., Cabral C., Kumar R., Ganguly R., Kumar Rana H., Gupta A., Rosaria Lauro M., Carbone C., Reis F., Pandey A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. 2019:11. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P., Widmer A.F., Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020;41(19):1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Clarke N.E., Turner A.J. Not just angiotensinases: new roles for the angiotensin-converting enzymes. Cell. Mol. Life Sci. 2010;67:89–98. doi: 10.1007/s00018-009-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar V., Ditu L.M., Pircalabioru G.G., Picu A., Petcu L., Cucu N., Chifiriuc M.C. Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front. Nutr. 2019;6:21. doi: 10.3389/fnut.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.E., Marie I.J., Durbin J.E. Induction and function of type I and III interferon in response to viral infection. Curr. Opin. Virol. 2011;1:476–486. doi: 10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Molina-Molina M., Abdul-Hafez A., Uhal V., Xaubet A., Uhal B.D. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L178–185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.J., Chen H.H., Pan C.F., Chuang C.K., Wang T.J., Sun F.J., Wu C.J. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 2011;25:191–197. doi: 10.1002/jcla.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Ni G., Damania B. Innate sensing of DNA virus genomes. Annu. Rev. Virol. 2018;5:341–362. doi: 10.1146/annurev-virology-092917-043244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra D., Borges N., Alvarenga L., Esgalhado M., Cardozo L., Lindholm B., Stenvinkel P. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients. 2019:11. doi: 10.3390/nu11030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiola F., Nicoletti A., Gasbarrini A., Ponziani F.R. Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7404–7413. doi: 10.26355/eurrev_201811_16280. [DOI] [PubMed] [Google Scholar]
- Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J., Humphries S.E., Hill M.R., Laurent G.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- Marshall R.P., Gohlke P., Chambers R.C., Howell D.C., Bottoms S.E., Unger T., McAnulty R.J., Laurent G.J. Angiotensin II and the fibroproliferative response to acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L156–164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- McAleer J.P., Kolls J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018;48:39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C.W., Harrison L.E., Eldehni M.T., Jefferies H.J., Szeto C.C., John S.G., Sigrist M.K., Burton J.O., Hothi D., Korsheed S., Owen P.J., Lai K.B., Li P.K. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011;6:133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020;382(26):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., Carmona-Rubio A.E., Jacob M., Procop G.W., Harrington S., Milinovich A., Svensson L.G., Jehi L., Young J.B., Chung M.K. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;e201855 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., Alhowikan A.M., Al-Khlaiwi T., Meo I.M., Halepoto D.M., Iqbal M., Usmani A.M., Hajjar W., Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- Miele L., Giorgio V., Alberelli M.A., De Candia E., Gasbarrini A., Grieco A. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr. Cardiol. Rep. 2015;17:120. doi: 10.1007/s11886-015-0671-z. [DOI] [PubMed] [Google Scholar]
- Morales D.R., Conover M.M., You S.C., Pratt N., Kostka K., Duarte Salles T., Fernandez Bertolin S., Aragon M., DuVall S.L., Lynch K., Falconer T., van Bochove K., Sung C., Matheny M.E., Lambert C.G., Nyberg F., AlShammari T.M., Williams A.E., Park R.W., Weaver J., Sena A.G., Schuemie M.J., Rijnbeek P.R., Williams R.D., Lane J.C.E., Prats Uribe A., Zhang L., Areia C., Krumholz H., Prieto Alhambra D., Ryan P.B., Hripcsak G., Suchard M.A. 2020. Renin-angiotensin System Blockers and Susceptibility to COVID-19: a Multinational Open Science Cohort Study. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlot T., Penninger J.M. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rodriguez E., Egea-Zorrilla A., Plaza-Diaz J., Aragon-Vela J., Munoz-Quezada S., Tercedor-Sanchez L., Abadia-Molina F. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients. 2020:12. doi: 10.3390/nu12030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Santos R.A., Ferreira A.J., Verano-Braga T., Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J. Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- Satoh M., Hayashi H., Watanabe M., Ueda K., Yamato H., Yoshioka T., Motojima M. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp. Nephrol. 2003;95:e111–118. doi: 10.1159/000074327. [DOI] [PubMed] [Google Scholar]
- Schiattarella G.G., Sannino A., Toscano E., Giugliano G., Gargiulo G., Franzone A., Trimarco B., Esposito G., Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur. Heart J. 2017;38:2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Hu M., Fedak P.W.M., Oudit G.Y., Kassiri Z. Cell-specific functions of ADAM17 regulate the progression of thoracic aortic aneurysm. Circ. Res. 2018;123:372–388. doi: 10.1161/CIRCRESAHA.118.313181. [DOI] [PubMed] [Google Scholar]
- Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., Feng J., Yan S., Guan Y., Xu D., He G., Chen C., Xiong X., Liu L., Li H., Tao J., Peng Z., Wang W. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- Siordia J.A., Jr. Epidemiology and clinical features of COVID-19: a review of current literature. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J., Kau A.L., Rich S.S., Concannon P., Mychaleckyj J.C., Liu J., Houpt E., Li J.V., Holmes E., Nicholson J., Knights D., Ursell L.K., Knight R., Gordon J.I. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Ma Y., Bai X.Y., Chen X. The expression changes of inflammasomes in the aging rat kidneys. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:747–756. doi: 10.1093/gerona/glv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spak E., Hallersund P., Edebo A., Casselbrant A., Fandriks L. The human duodenal mucosa harbors all components for a local renin angiotensin system. Clin. Sci. (Lond.) 2019;133:971–982. doi: 10.1042/CS20180877. [DOI] [PubMed] [Google Scholar]
- Steed A.L., Christophi G.P., Kaiko G.E., Sun L., Goodwin V.M., Jain U., Esaulova E., Artyomov M.N., Morales D.J., Holtzman M.J., Boon A.C.M., Lenschow D.J., Stappenbeck T.S. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert R.E., Lee Y.K., Sybesma W. Vitamins for the gut microbiome. Trends Mol. Med. 2020;26:137–140. doi: 10.1016/j.molmed.2019.11.005. [DOI] [PubMed] [Google Scholar]
- Stubbs J.R., House J.A., Ocque A.J., Zhang S., Johnson C., Kimber C., Schmidt K., Gupta A., Wetmore J.B., Nolin T.D., Spertus J.A., Yu A.S. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016;27:305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Yin Z., Liu N., Bian X., Yu R., Su X., Zhang B., Wang Y. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem. Biophys. Res. Commun. 2017;493:964–970. doi: 10.1016/j.bbrc.2017.09.108. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tang W.H., Wang Z., Kennedy D.J., Wu Y., Buffa J.A., Agatisa-Boyle B., Li X.S., Levison B.S., Hazen S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team C.C.-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschurtschenthaler M., Wang J., Fricke C., Fritz T.M., Niederreiter L., Adolph T.E., Sarcevic E., Kunzel S., Offner F.A., Kalinke U., Baines J.F., Tilg H., Kaser A. Type I interferon signalling in the intestinal epithelium affects Paneth cells, microbial ecology and epithelial regeneration. Gut. 2014;63:1921–1931. doi: 10.1136/gutjnl-2013-305863. [DOI] [PubMed] [Google Scholar]
- Ussher J.R., Lopaschuk G.D., Arduini A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis. 2013;231:456–461. doi: 10.1016/j.atherosclerosis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-Aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Xu K., Du T., Zhu P., Liang Z., Liao S., Zhang J., Raizada M.K., Grant M.B., Li Q. Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol. Ther. Methods Clin. Dev. 2019;14:161–170. doi: 10.1016/j.omtm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–292):e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020:94. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Bodiga S., Das S.K., Lo J., Patel V., Oudit G.Y. Role of ACE2 in diastolic and systolic heart failure. Heart Fail. Rev. 2012;17:683–691. doi: 10.1007/s10741-011-9259-x. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li F., Sun R., Gao X., Wei H., Li L.J., Tian Z. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat. Commun. 2013;4:2106. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- Wang X., Fang X., Cai Z., Wu X., Gao X., Min J., Wang F. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020 doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J. Biol. Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- Winkler E.S., Thackray L.B. A long-distance relationship: the commensal gut microbiota and systemic viruses. Curr. Opin. Virol. 2019;37:44–51. doi: 10.1016/j.coviro.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.W., Oudit G.Y., Reich H., Kassiri Z., Zhou J., Liu Q.C., Backx P.H., Penninger J.M., Herzenberg A.M., Scholey J.W. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am. J. Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Hakimi M., Wortmann M., Zhang J., Bockler D., Dihlmann S. Gene expression of inflammasome components in peripheral blood mononuclear cells (PBMC) of vascular patients increases with age. Immun. Ageing. 2015;12:15. doi: 10.1186/s12979-015-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., Li J., Wang H., Yu L., Huang H., Qiu Y., Wei G., Fang Q., Zhou J., Sheng J., Liang T., Li L. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1):0. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.J., Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020:12. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020;113 doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020:12. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R., Gomez Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., Diamond M.S., Ciorba M.A., Whelan S.P.J., Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020:5. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21:305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wada J., Hida K., Tsuchiyama Y., Hiragushi K., Shikata K., Wang H., Lin S., Kanwar Y.S., Makino H. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J. Biol. Chem. 2001;276:17132–17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yu J., Pan L.Y., Jiang H.Y. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yan H., Yamashita S., Li W., Liu C., Chen Y., Zhou P., Chi Y., Wang S., Zhao B., Song L. Acute ST-segment elevation myocardial infarction is associated with decreased human antimicrobial peptide LL-37 and increased human neutrophil peptide-1 to 3 in plasma. J. Atheroscler. Thromb. 2012;19:357–368. doi: 10.5551/jat.10108. [DOI] [PubMed] [Google Scholar]
- Zhao H., Jiang X., Chu W. Shifts in the gut microbiota of mice in response to dexamethasone administration. Int. Microbiol. 2020;10.1007/s10123-020-00129-x doi: 10.1007/s10123-020-00129-x. [DOI] [PubMed] [Google Scholar]
- Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M., Loibner H., Wang X.H., Penninger J.M., Kassiri Z., Oudit G.Y. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122(717–728) doi: 10.1161/CIRCULATIONAHA.110.955369. 718 p following 728. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D., Burns K.D. Angiotensin-(1-7) in kidney disease: a review of the controversies. Clin. Sci. (Lond.) 2012;123:333–346. doi: 10.1042/CS20120111. [DOI] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A., Cheung C.P., Chen N., Lai C.K.C., Chen Z., Tso E.Y.K., Fung K.S.C., Chan V., Ling L., Joynt G., Hui D.S.C., Chan F.K.L., Chan P.K.S., Ng S.C. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.048. S0016-5085(20)34701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]