Introduction:
There has been a rapid increase in the utilization of MRI in rectal cancer staging in the USA [1], essentially replacing endorectal ultrasound and mimicking the trend in Europe seen in the 1990s and 2000s. There is a heightened awareness of the importance of staging colorectal cancer in view of the recently reported increasing incidence of rectal cancer and young onset colorectal cancer [2]. This has led to vast new amounts of research and educational activities regarding rectal cancer at major conferences [3] and at academic teaching hospitals [4] with a slow, but steady proliferation of knowledge into the community and private sector. Accompanying this trend, there is a demand, and recognized need, for greater precision and clarification of confusing, misunderstood and poorly understood concepts, facts, statements and nomenclature (see Lexicon, this issue). As such, this Review, part evidence-based and part expert opinion, will attempt to elucidate and clarify several concepts the authors have encountered in 25 years of imaging rectal cancer, focusing on MRI.
1. “Circumferential Resection Margin” (preferably referred to as margin between tumor and Mesorectal Fascia [MRF]).
There is confusion and misunderstanding regarding the significance of the proximity of tumor to the mesorectal fascia; the so-called “CRM” distance. Although CRM is used as short-hand in reports and in common parlance (instead of “MRF”), an increasing number of radiologists are aware that the distinction is a pragmatic one [5]; the CRM is actually that which the surgeon creates when attempting to do total mesorectal excision (TME) surgery, and may, if done perfectly, proceed along the “golden plane” of the visible mesorectal fascia, but may also be imperfect and breach the MRF to include some mesorectal fat (and appear as a “waist” at pathology) [6], or less commonly may include additional true pelvic fat exterior to the MRF. The MRF is an anatomic structure delimiting the mesorectal envelope and containing the rectum, fat, nodes, nerves and vascular structures. The misunderstanding relates to the radiologic measurement of the proximity of tumor to the MRF. There have been differing descriptions of how to perform this, leading to confusion and mixed messages. Some sources indicate that this distance is to be measured from a malignant node or tumor-bearing vessel or tumor deposit (N1c) to the MRF [7]. Other sources indicate that this distance was intended to represent the distance from the primary tumor (growing from the rectal wall in T3 tumors or higher) to the MRF [8]. This confusion can be easily clarified once certain risk factors can be understood from prior landmark studies. There are two different explanations that shed light on why the distance from any tumor-bearing structure to the MRF is important, albeit for different reasons. The first stems from the landmark study of Dr. Phillip Quirke from the Royal College of Pathologists, published in Lancet 1986, in which it was shown that there were vastly different recurrence rates between superficial and deep T3 lesions [9]. The risk of local recurrence in tumors involving the CRM at histopathology is much higher with deeper tumors. The death rate is 3 times higher if involved and there is only a 15% 5-year survival. This study established that primary tumors involving the CRM are a poor prognostic feature. The MERCURY study further established that for primary tumors there is a close correlation between MRI measured tumor distance and histopathologic tumor distance [10]. In the treatment paradigms of some countries (e.g. UK and other parts of Europe) a “good, bad and ugly” approach began to take hold that included more aggressive treatment of tumors close to or involving the CRM at MRI based on this new imaging biomarker [11]. Essentially this is now a validated imaging biomarker used at baseline to determine treatment. For this reason, this measurement must be included in MRI reports from the very first evaluation to help plan neoadjuvant treatment in its many iterations including systemic-dose chemotherapy, chemoradiotherapy, both (so called total neoadjuvant therapy), radiotherapy only (short or long course) or no neoadjuvant treatment at all. The issue of other structures near the CRM that may contain tumor is an interesting one for which we have very little data about comparative risk for poor outcomes. Naagetal I. et al. [12] retrospectively studied specimens from the Dutch TME trial and found that tumor-bearing lymph nodes involving the CRM did NOT confer any added risk for increased local recurrence compared with cases in which no primary tumor involved the CRM. Furthermore, Birbeck et al. also showed that in addition to an added local recurrence rate of only 0.55% for LN (equivalent to no added risk as shown by Nagtegaal), the added rate of LR for tumor within a blood vessel was 20% and for discontiguous tumor (N1c) was 31% compared to 42% for primary tumor when these scenarios are compared with the risk of local recurrence when there is a clear margin, which their series was 10% [13]. As such, what is felt to possibly represent a malignant lymph node involving the CRM should not be measured as the “CRM” (or distance to the MRF at MRI) since this does not correlate with increased risk for poor outcomes and should not be used to determine aggressiveness of neoadjuvant therapy. In a study of 296 patients with rectal cancer by Shihab et al. “involvement of the CRM exclusively by a metastatic lymph node [was] rare,” and none of these patients presented a CRM infiltrated by a positive lymph node at histopathologic analysis. Thus, the authors concluded that it is necessary to be careful when recommending neoadjuvant treatment based solely on the presence of a suspicious lymph node close to the mesorectal fascia by MRI, because, regardless of the nodal status determined by MRI, it is uncommon that mesorectal lymph nodes are the only factor responsible for a positive CRM at histopathologic analysis [14]. More data are needed to determine the significance of the distance between EMVI or tumor deposits and the MRF. Intuitively, tumor confined within a macroscopic MR-visible vessel (EMVI) might be of less concern since it would be contained inside a wall. This may be the reason why tumor-containing nodes at the MRF show no greater risk for local recurrence, since there is a node capsule containing tumor. However, such speculation remains to be proven. So, what then is the importance of potentially involved lymph nodes, tumor deposits or EMVI at the CRM? The importance is one of surgical planning. It is of value to mention these on a preoperative MRI to alert the surgeon that the structure is near the mesorectal fascia (the intended CRM) so that they may pay attention to this region and avoid cutting through the structure potentially spilling tumor cells. It is not an issue of risk stratification at baseline MRI when planning for neoadjuvant treatment. On another note, given the poor accuracy for predicting positive LN by MRI; imagine the extent of overtreatment that might occur if LN were routinely used to determine the distance to the MRF. It would be very unsafe indeed.
2. “Anal verge”
This term is used frequently in rectal cancer discussions. There may be different working definitions used by surgeons, radiologists and pathologists and it may help to discuss with the clinical team which definition they are using in your practice group. The word “verge” literally means edge or border or margin (middle English). As medical students we were probably first made aware of the term in histology. The anal verge was defined as the junction of the hair-bearing, keratinizing skin with the non-hair-bearing, non-keratinizing skin [15]. Measurements using this definition will correspond to the bottom of the external anal sphincter (EAS) where it inserts on the skin and will proceed cephalad to the bottom of the tumor [16]. Relevant to proctoscopy/sigmoidoscopy, there may be a different location of the anal verge. In a patient undergoing endoscopic evaluation under anesthesia including skeletal muscle paralytic agents, the EAS is relaxed, and consequently, the first verge, or margin encountered by the scope/endoscopist is the tonically contracted internal anal sphincter muscle (IAS) in its resting state. For this reason, some surgeons consider this to be the “anal verge.” The IAS terminates cephalad to the EAS separated by the intersphincteric groove (asterisk, Figure 1). Typically, this height difference is only on the order of 1-cm or less as was shown in a recent study comparing MRI to rigid sigmoidoscopy [17]. However, the measurement observed by the operator to the tumor is marked from this level and described as the “distance to the tumor from the anal verge.” As such, there may be a 1-cm or so discrepancy in measurements between surgeons/endoscopists and radiologists who use the EAS, well seen at MRI, when they perform their measurements on the sagittal images. Even in an un-sedated patient, when the gluteal folds are manually gently separated to insert the endoscope, the external sphincter may be stretched apart such that the contact of the “verge” with the scope, used for measurements, is actually closer to the bottom of the IAS. Again, there is no great clinical significance except possibly for tumors at the anorectal junction after treatment for planning of sphincter preservation. Because of these different definitions, we suggest the terms, “histologic anal verge” and “surgical anal verge”, as pragmatic and more precise monikers. When choosing how to measure on MRI, a brief discussion with the surgeons may clear up any confusion.
Figure 1.
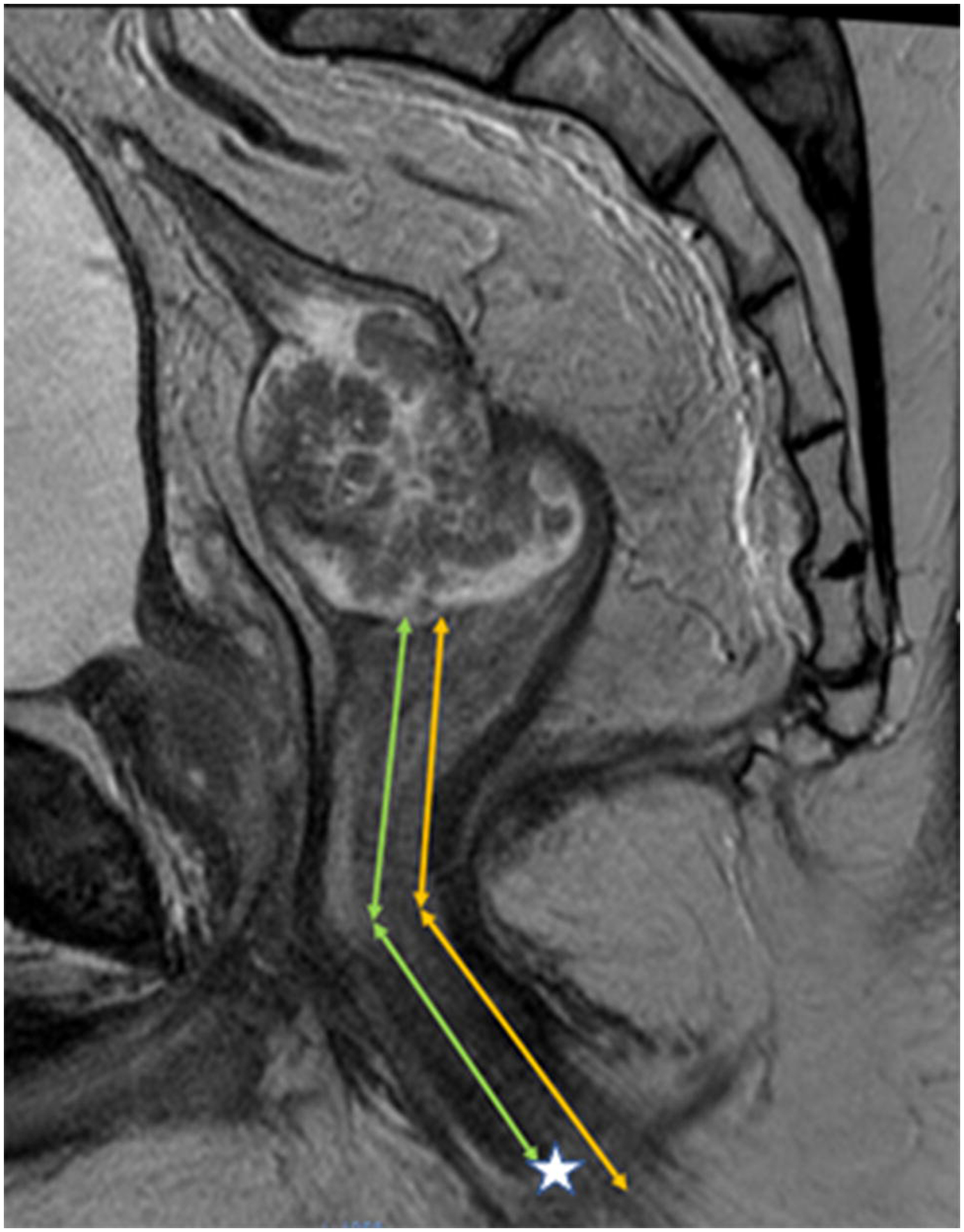
Sagittal T2 weighted MRI of the pelvis in 42-year-old male with a polypoid rectal adenocarcinoma. Measurement of height of tumor from lower border of external anal sphincter [EAS] (corresponding to histologic anal verge), yellow lines. Measurement of height of tumor from bottom of internal anal sphincter [IAS] (often corresponding to surgical anal verge due to anesthesia induced skeletal muscle relaxation or forcible manual separation of EAS at time of sigmoidoscopy. Difference in these distances is only about 1-cm and represents the intersphincteric notch (asterisk).
3. Anal canal and tumor staging
There are 3 potentially misunderstood factors amongst radiologists regarding the anal canal: (i) anatomy; (ii) AJCC staging; (iii) TNM applicability. b. (i) The anal canal is a part of the rectum. The rectum is 15 cm in length divided into 3 parts evenly. The anal canal length varies from 2–5 cm. Therefore, a portion of the lower 1/3 of the rectum is within the anal canal. Within the anal canal, there is both rectal mucosa (columnar) and anal mucosa (squamous) of varying degrees and merging at the dentate line or transition zone. It is incorrect to refer to a tumor “at 5-cm” if it is located 5-cm above the anorectal ring, (also known as the anorectal junction). This tumor location is 5 cm plus the anal canal length [18]; (ii) Staging of tumors in the anal canal must correspond with the histology of the tumor. “Rectal cancer” (adenocarcinoma) and “anal cancer” (squamous cell carcinoma), are treated very differently, the former with neoadjuvant chemoradiotherapy followed by surgery, and the latter by definitive chemoradiotherapy without surgery [19]. Thus, for the radiologist, it is important to know the histology, particularly when vague clinical information is supplied such as “anal tumor,” or “anal cancer.” For example, adenocarcinoma and squamous carcinoma are unfortunately, colloquially and imprecisely referred to on occasion, by some clinicians as “anal cancer;” (iii) A scenario leading to confusion occurs when a rectal adenocarcinoma is partly or wholly located within the anal canal. TNM staging does not apply and, if used, could be misunderstood or misconstrued by the treating oncologists. The TNM system is driven by outcomes stratification and it is not clear if the same anatomic thresholds apply in the anal region as in mid and upper rectal tumors. One may be tempted to simply use the histologic layers of the bowel wall and apply the TNM system, but these anatomic divisions are not proven to be applicable in this location. The internal anal sphincter (IAS) is the anatomic continuation of the inner circular muscle fibers of the rectal muscularis propria. The outer longitudinal fibers of the muscularis propria continue caudally into the fat-containing intersphincteric space (ISS) as the conjoined longitudinal muscle (CLM), though infrequently discussed. The outermost layer of the anal canal/sphincter apparatus is composed of the external anal sphincter (EAS). The ISS is a direct continuation of the mesorectal fat and is nicely depicted on properly angulated (parallel to the anal canal) coronal MRI. Several classification schemes have attempted to address low rectal cancer including that by Shihab O. et al. in 33 patients [20] and that by Bamba Y. et al in 66 patients [21]. In the Shihab study the nomenclature is “Level” 1–4, while the Bamba study describes the involvement of the CLM by MRI as a key landmark. Given the lack of consensus of which system to use between TNM, Shihab, or Bamba, there is growing consensus by professional societies to use descriptive prose to inform the surgeon of the anatomic plane involved by tumor to properly inform surgical treatment. Thus, tumor “involves the IAS”, “involves the ISS” or “involves the EAS,” albeit cumbersome, is becoming the accepted staging terminology for low rectal tumors extending into or solely involving the anal canal. The key point in surgical planning here relates to the ability of surgeons - using modern surgical technique - to resect a portion of the upper IAS by dissecting into the ISS if it is clear of tumor and thus offering the patient “sphincter preservation” without a permanent colostomy. The anastomosis is thus known as a ‘coloanal’ anastomosis and may be stapled or hand-sewn [22]. Another key point is that rectal adenocarcinoma tumors within 3-cm of the anal verge will have the radiation field extended to include the inguinal nodes since they now become “at risk.”
4. Lateral pelvic sidewall lymph nodes or “Lateral Lymph Nodes” (LLN) (Figure 2).
FIGURE 2.
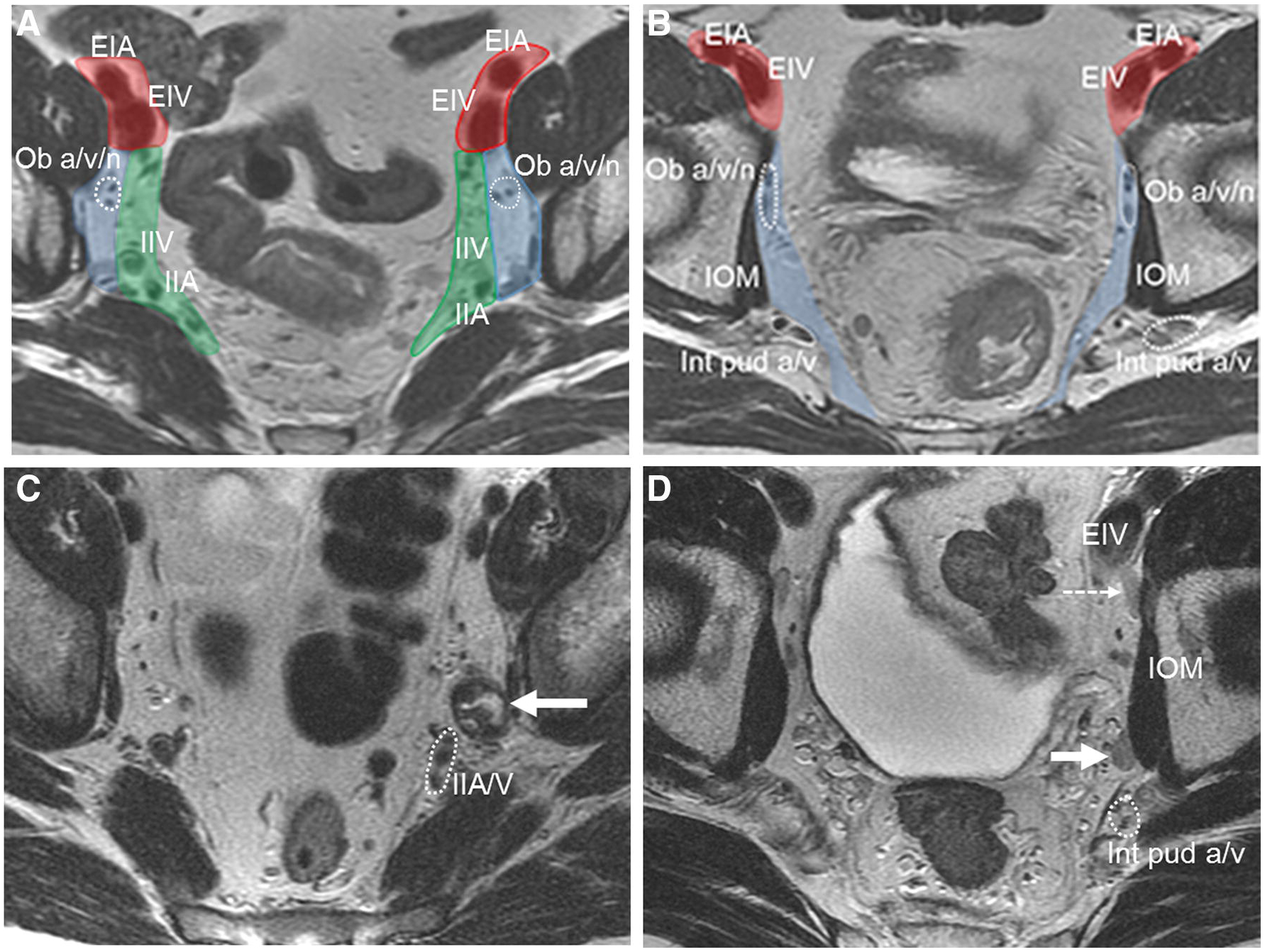
Axial T2 weighted elvic MRI at multiple levels. Anatomical classification of lateral lymph nodes in 2 different middle-aged male patients with rectal cancer (primary lesions not shown). A (proximal nodes) and B (distal nodes): Red-shaded areas are external iliac nodes, blue shaded areas are obturator nodes and green shaded areas are internal iliac nodes. Division between obturator and internal iliac represented by vertical plane through axis of internal iliac vessel. Lateral to this are obturator nodes .EIA = external iliac artery, EIV = external iliac vein, ob a/v/n = obturator artery//nerve, IOM = internal obturator muscle, int pud a/v = internal pudendal artery/vein, IIV = internal iliac vein, IIA = internal iliac artery. C and D, malignant pelvic sidewall nodes: Enlarged heterogeneous obturator nodes on the left in C, (solid arrow). Enlarged minimally heterogeneous left obturator node in D not removed initially leading to pelvic sidewall recurrence 9 months following surgery (solid arrow). Clinically unimportant posterior external iliac elongated lymph nodes (dashed arrow). IOM = internal obturator muscle. Int pud a/v = internal pudendal artery and vein 9enclosed in dotted circle), EIV = external iliac vein. IIA/V = internal iliac artery and vein.
The confusion regarding lateral lymph nodes is related to what criteria to use to suggest tumor-bearing nodes and; what is the importance of posterior external iliac chain nodes (PEI).Much has been written about mesorectal lymph nodes and how to interpret them at baseline MRI and after treatment. These nodes are removed in a standard total mesorectal excision (TME) as part of the specimen. Mesorectal nodes have undergone several node-for-node radiologic-pathologic validation studies [23, 24] using MRI and allowing some conclusions to be drawn based on a robust reference standard. Less has been written in the Western literature regarding LLN, which include the internal iliac, obturator and external iliac lymph nodes. These nodes are not part of a TME resection. For many years these nodes have been routinely removed by Japanese surgeons based on literature claiming lower rates of local recurrence [25]. Western surgeons have practiced a policy of selective resection of LLN after CRT, but based on ill-defined, non-standardized criteria. This selective policy is based on the much higher risk of damage to autonomic nerves with resultant bladder and sexual dysfunction that can occur with LLN dissection. The largest clinico-radiologic study has just been published by the LLN consortium, consisting of 1200 patients [26]. In part, lymph node criteria can be defined from this study based on size criteria. In patients with locally advanced rectal cancer 0–8 cm from the anal verge undergoing neoadjuvant treatment followed by surgery, local recurrence rates were higher in patients that had LLN of 7mm or greater, irrespective of size after treatment or resection status. This offers a guideline to interpret MRI. In another meeting abstract of a subset cohort of the above paper, 245 patients with 845 lateral lymph nodes on MRI [27], from patients with tumors at all levels; 5mm or greater LLN size was found to correlate with lower disease-free survival (DFS). In addition to size criteria, more rounded nodes and those with heterogeneous signal intensity; features also important in mesorectal nodes, conferred worse outcomes. Of note, the elongated nodes in the posterior (caudal) external iliac region (dashed arrow, Figure 2D) were found to be of little or no clinical significance and radiologists can effectively ignore these [27]. While further data on the size of involved nodes post-treatment is forthcoming (from the LLN Consortium and one of the authors’ [MJG] institutions), one small Japanese study indicates that 5mm at post-treatment MRI is a useful size threshold that surgeons could use to decide whether to remove nodes [28].
5. T4b tumors – tumors that invade organs and “surrounding structures”
The confusion here is the lack of precise definition of “other structures.” b. Due to this confusion, two oncologic practices or even two clinicians within the same practice, describing the same anatomically invaded structure might “stage” the lesion as a deep T3 while others may assign a T4 category. It is probably accepted that a tumor that has grown into the sacrum or ischial spine, is either more biologically aggressive and/or has been present longer. Also, we can likely intuitively agree to (and have practiced) the following assignments; invasion of fat in the pelvis outside the mesorectum, invasion of bone, invasion of skeletal muscle (obturator internus, piriformis, levator ani muscles [puborectalis, pubococcygeus and iliococcygeus] and ischiococcygeus), invasion of the ureter, invasion of the sacrospinous/sacrotuberous ligaments and invasion of the sciatic or sacral nerves or any named vessel outside the mesorectum is equivalent to T4b. Indeed, invasion of another loop of intestine low in the pelvis; either small or large bowel, separate from the primary site is also T4b. None of these “other structures” are (solid) organs, but must be defined as T4b, if arbitrarily for the sake of clarity and precision. Excluded from T4b would be the internal anal sphincter, intersphincteric space and anterior peritoneal reflection (T4a). A complete rectal MRI report will mention every structure that is invaded (or broadly abutted) so as to assist the surgeon in surgical planning.
6. Mucinous tumor
Mucinous tumors of the rectum, defined histologically as those containing 50% or more of stromal mucin, have a worse prognosis than similarly T-staged nonmucinous tumors [29–32]. Numerous controversies and pitfalls exist regarding mucinous tumors including; clinical diagnosis, imaging detection, nomenclature and response evaluation.
MRI has a diagnostic advantage over biopsy since biopsies are typically random and the sample obtained may not represent the whole tumor. In contradistinction, MRI depicts the whole tumor and can more broadly depict and quantitate the mucin content. While no personalized treatment yet exists for mucinous tumors, more accurate recognition of mucinous tumors could suggest a need for more intensified treatment or even more immediate surgery. Mucinous tumors are those with high T2WI signal in the stroma of the tumor. It is incorrect to call a tumor that secretes a large amount of mucin into the lumen, “mucinous.” Another diagnostic pitfall the authors have encountered, is underestimation of mucinous tumor extension beyond the wall of the bowel. The T2WI signal intensity of mucin may be exactly isointense to surrounding fat and thus go undetected (Figure 3). For that reason, sequences that are typically not needed during a standard rectal MRI [33, 34], such as fat saturation, contrast-enhanced sequences or T1 sequences may be of use in mucinous tumors. In our practice [MJG], we still use IV Gadolinium, and, in for mucinous, contrast enhancement has proven especially useful. DWI-MRI can also depict mucin, and since DWI-MRI has become standard in rectal MRI, gadolinium-enhanced and fat-saturated sequences are likely no longer needed to detect extensive mucinous tumors.
FIGURE 3.
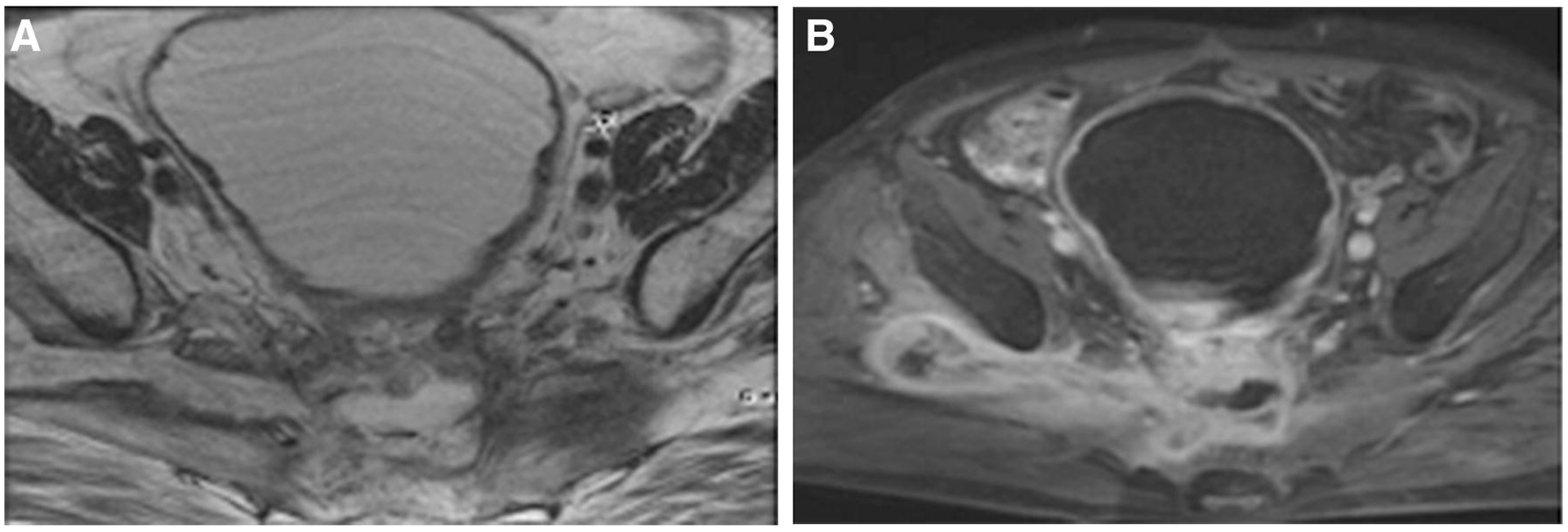
Pelvic MRI in middle-aged female with recurrent mucinous rectal adenocarcinoma. A: Axial T2 weighted image. High T2 signal intensity mass in mid pelvis and asymmetric appearance of piriformis muscles. More normal appearing on the left. High T2 weighted soft tissue along right sciatic notch seems to represent fat due to its iso-intensity. B: Fat -saturated T1 weighted contrast-enhanced image. Note enhancement around central mass and tumor extending out of sciatic notch, now more conspicuous.
Another highly confusing area is the mucinous or colloid degeneration that may occur in a nonmucinous tumor after treatment. These tumors are not generally referred to as mucinous, but rather as those with ‘mucin’ or ‘colloid’ change or degeneration in response to treatment. For radiologists; the presence of T2-bright mucin anywhere in a tumor or tumor bed after treatment, whether originally present or, as above, in response to treatment is confusing. MRI cannot distinguish whether the high T2 signal is due to acellular (thus benign) or cellular (thus malignant) mucin [29–32, 35, 36]. Interpretation of post-treatment MRI with mucin degeneration has become more important of late, with the emergence of non-operative management or watch and wait treatment approaches, because the surgeon wants to know if the MRI shows residual tumor before they would be willing to avoid defer an operation [37–41]. Further research is critical to overcome this diagnostic challenge and ensure proper treatment.
7. Rectal filling/enema
There is disagreement among expert radiologists as to whether to distend a patient’s rectum with fluid or gel or whether to use a cleansing enema prior to MRI scanning. Some evidence exists both in favor of, and opposed to, filling the rectum with the intent of better tumor visualization. It is an inarguable principle of GI radiology that in the hollow GI tract, better luminal distension allows for greater conspicuity, higher detection -and possibly more accurate staging of masses and wall thickening. As such, this principle was extrapolated early on in the approach to imaging the rectum. However, when distance to the mesorectal fascia (“CRM”) was identified as an important imaging biomarker, at least one study hypothesized that rectal distension might then be contraindicated because it might compress the mesorectal fat and alter the true distance between the tumor and the MRF. Such findings were proven in a small study [42], however the authors used 100mL of air rather than 60mL of fluid or gel. In fact, there was even fear that the lymph nodes near the tumor would be compressed thus obscuring their detection. This approach became “lore” for about a decade. With greater experience and the widespread use of MRI in the USA and other parts of the world, further research into this topic produced conflicting results. Several groups recently performed high quality studies showing that filling with typical quantities of fluid (30–60 mL) did not alter the measured distance from the tumor to the MRF, refuting earlier reports [43]. In addition, one study looked at T-stage accuracy and found no advantage using rectal filling. In fact, this same study did find a potential disadvantage with rectal filling not previously anticipated, and one this author has encountered, albeit rarely. Elevation of the rectum with filling (? overfilling) may spuriously push the tumor higher into the pelvis and farther away from the anorectal junction. This might in theory falsely depict a “safe” distal margin at imaging. Given lack of a clear advantage with rectal filling and given the potential to alter measurements, the ESGAR guidelines recommending against routine use of rectal filling seem to be appropriate [33, 44]. One aspect that may not have been adequately addressed however is tumor conspicuity, such as in small T1/T2 tumors. As radiologists gain more experience reading rectal MRI, smaller and smaller tumors will probably be recognized. Nonetheless, there will likely always be very subtle tumors or tortuous, redundant, collapsed rectums or fecal residue which will make tumor identification very difficult. However, either IV contrast, or DWI, or rectal filling, in the opinion of the authors, can be used to obviate this problem. The simplest strategy from a workflow perspective may be to use high quality DWI, since IV contrast and rectal filling require extra time, risk and discomfort and are not recommended by either the ESGAR or SAR guidelines [33, 34].
8. Gadolinium-based contrast agent (GBCA) use for rectal MRI
The use of GBCA in the evaluation of rectal cancer is not recommended by either the ESGAR or the SAR guidelines [33, 34]. The issue is controversial because GBCA is otherwise so commonly used in oncologic pelvic MRI, that it is presumably more effort to remove these enhanced sequences from a practice’s “pelvic MRI” protocol than to simply continue routine practice. Added to that is the excellent safety profile of GBCA, and consistent third-party reimbursement for its use. In the author’s experience as the radiologic principal investigator of the recently closed PROSPECT study [45] , with hundreds of enrolling sites across the USA including academia and smaller community hospitals, at least 65% of practices are routinely using GBCA in spite of guidelines (unpublished data). In addition to older reviews which found no advantage in the use of GBCA for staging or re-staging rectal cancer [46], one of the authors [MJG] has investigated the use of GBCA in the following clinical scenarios; T-staging and tumor location with respect to the sphincter and the peritoneal reflection at baseline and re-staging after treatment [47]; Added value of GBCA in detecting T4b organ invasion [48]; Ability of dynamic contrast-enhanced (DCE) MRI to detect complete pathological response [49–51] and the added value of DCE-MRI in recurrent rectal cancer [52]. To summarize these articles; advantages with the use of GBCA were minimal and included (i.) more accurate identification of tumors in the anorectal junction (potentially overcome by digital rectal examination and proctoscopy), (ii.) an ability to determine pathologic complete response (only) if an anti-angiogenic agent is used in treatment (no longer standard of care), and (iii.) an ability to determine margin-free resectability of recurrent tumor using pharmacokinetic threshold values. However, the number of patients was small and the scenarios less common, and the overall message from these papers was that there is no convincingly strong advantage to using GBCA in rectal cancer MRI. Our group [MJG] has drastically reduced the use of GBCA but has not omitted it altogether. An issue of tumor conspicuity still exists. Some tumors are very small either at baseline or post-treatment. Their T2 signal may be indistinct from the normal rectal wall. In this scenario, we have anecdotally found GBCA to increase the conspicuity of the tumor. In our practice rectal filling is not used, and we have chosen to maintain the use of GBCA for recognition of small tumors at baseline. The use of rectal filling might overcome the poor conspicuity of smaller tumors. In addition, the use of diffusion weighted imaging, more routine now, may also overcome the poor conspicuity of smaller tumors. As such, we believe that either GBCA or rectal filling or DWI may be used to assist in the recognition of smaller tumors. There is also some early work suggesting that quantitative and semi-quantitative DCE analysis may prognosticate tumors at baseline, but multicenter validation is required before this approach can be recommended as a standard practice [53].
9. mrTRG/post-treatment scar
Response of rectal cancer to preoperative treatment, usually a combination of chemotherapy and radiation, is assessed in the surgical specimen at pathology to determine the relative amount of viable tumor compared with fibrosis, inflammation and necrosis. This is typically done using a categorical scale of tumor response grade (TRG) 0–4 or 1–5 and can used various published systems, e.g. Mandard [54], Dworak [55], Rodel [56]. There are also 3-point scales ranging from no response to complete response such as AJCC [57]. Some centers, such as our own [MJG], use a continuous percentage scale in the actual report which can then be collapsed into 3 categories, or simply reported as percentage “treatment effect” [58]. The controversy here involves the attempt to mimic this categorization 1:1 with MRI, since MRI can depict fibrosis as T2-weighted signal intensity decrease (tissue darkening). mrTRG (magnetic resonance Tumor Regression Grade) has arisen as such a system and undergone several studies revealing some success as a biomarker for treatment response [59]. The authors feel there are three limitations that potentially arise from attempts to use mrTRG widely in practice: (i) reproducibility, (ii) limitations of T2 signal intensity for complete response and (iii) dependency on treatment type. The laudable efforts of the Royal Marsden group to widely train radiologists in the use of mrTRG [59] could in theory be a model for dissemination of all new knowledge to reduce interobserver variability amongst radiologists, however, the practicality of this would seem limited. Outside of the few European centers involved in the MERCURY study [10] and a few sites visited by the group from the Royal Marsden in Europe and the USA and Canada, expertise in the use of mrTRG is limited. There can be no question that in experienced groups, this has emerged as a useful imaging biomarker for response [60], but its ability to be more widely disseminated into the larger community is in question. In a recently published article comparing the potential added value of dynamic contrast-enhanced imaging (DCE-MRI) to T2WI, DWI and endoscopy for determination of complete clinical response, which indicated no advantage of DCE-MRI, one expert radiologist with more than 20 years of experience with rectal MRI assessed the value of mrTRG compared with qualitative T2WI, qualitative DWI, DCE and clinical factors for complete clinical response assessment and found mrTRG to be inferior to all methods with an AUC 0.60 in comparison with clinical exam (AUC 0.72), T2WI and DWI-MRI (AUC 0.67) and T2WI plus DWI-MRI plus DCE-MRI (0.72) (unpublished data) [51]. To be fair, this expert does not use mrTRG in his practice and has never had formal group training by the Royal Marsden group as described in their publications [60, 61].
Currently, because of the mismatch between radiologic response assessment amongst European and US centers, the mrTRG scale is being prospectively assessed as a correlative objective in the US TNT trial (NCT02921256) by 3 experienced radiologists, one of whom has been formally trained by Dr. Gina Brown. The ongoing TRIGGER trial (NCT02704520) [62] will also prospectively assess mrTRG as an imaging biomarker. The second potential limitation to the widespread dissemination of mrTRG as a reliable assessment tool is that it essentially is assessing T2W signal intensity which is less robust as a biological tumor indicator than DWI, according to both a metanalysis and landmark single center study comparing DWI head-to-head with T2. DWI-MRI, therefore, while suffering from insensitivity to small amounts of microscopic tumor, has impressive specificity such that the presence of signal in the tumor bed effectively excludes complete clinical/pathological response [63, 64]. A third and final challenge for mrTRG, despite the results reported in a recent combined study by Sclafani et al. [60], is the relative paucity of fibrosis in response to chemotherapy treatment alone compared with radiotherapy. This has been the anecdotal experience of the authors who have a vast experience with patients treated with induction chemotherapy and receive MRI immediately (post FOLOX and pre-CRT) [65]. In addition, there is direct clinical evidence in support of histologically less reactive fibrosis to chemotherapy compared with radiotherapy [66]. Whether it will be mrTRG or another metric, the authors look forward to the identification and validation of a sorely needed method for reliably predicting complete response in patients, now that viable nonoperative treatment pathways exist [38, 40, 41].
| Controversy | Recommendations |
|---|---|
| Do I measure from node/deposit/EMVI to mesorectal fascia (MRF) or from the primary tumor? | The distance from the primary tumor to the MRF prognosticates risk and can be used for planning treatment. Involved nodes at the MRF confer no added risk [12]. Tumor deposits and EMVI near the MRF add some risk in small studies [13] and there is no universal policy to intensify neoadjvuant therapy compared with primary tumor threatening the margin. The issue becomes more one of surgical safety. Therefore, in addition to the measurement of primary tumor to MRF, a distance from a node/EMVI or deposit that is close to the MRF should be mentioned for surgical planning to allow careful dissection on the MRF and avoid cutting through this structure. |
| Where is the anal verge (AV)? | A discussion with the surgeons should take place. Ascertain whether they want the measurement from the anatomic AV (bottom of the EAS) or the “surgical” AV which often is the bottom of the IAS due to anesthesia- induced relaxation of the EAS or manual separation of the EAS when placing the scope. |
| How should I stage low rectal adenocarcinoma? | No accepted standard exists w/r/t T-categorization. To help surgical planning, tumor should be described as tumor “involves the IAS”, “involves the ISS” or “involves the EAS”. No T-stage should be applied. |
| What constitutes suspicious lateral pelvic side-wall lymph nodes | Those posterior to the external iliac vessels distally (at about the acetabulum and caudally are often elongated and can be ignored [27]. All rounded, heterogenous nodes measuring >7mm pre-treatment and >5mm on post treatment MRI should be included in the report as potentially involved [26, 28]. |
| What is included in Stage T4b? | AJCC refers to “other structures but does not define them. They can include muscles, pelvic fat outside of the mesorectum, nerves, ureters and blood vessels |
| Should I evaluate mucinous tumors with the routine protocol? | Because the mucin can be isointense to fat on T2, it can be useful to add an optional T2W FS or post contrast T1W FS images to fully visualize the extent of mucin invasion into surrounding fat/tissues. |
| Shall I use rectal filling? | This is based on personal preference but is not recommended by guidelines. For smaller tumors or if less familiar with reading rectal MRI, filling may help locate the tumor. If there is over-filling however, it can increase tumor distances from the anorectal junction. |
| IV contrast | Not recommended. May be useful in mucinous tumors |
| mrTRG | Validated response measure after XRT. May not work after chemotherapy only |
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Marc J. Gollub, Professor of Radiology, Director- Section of CT- Department of Radiology-Memorial Sloan Kettering Cancer Center. 1275 York Avenue, NY, NY 10065, U.S.A
Chandana Lall, University of Florida. Chair-Department of Radiology, Chief-Division of Abdominal and Body Imaging, Program Director- Abdominal Imaging Fellowship. Jacksonville, PA 32209, FL..
Neeraj Lalwani, Associate Professor Department of Radiology, Section of Abdominal Imaging Wake Forest University and Baptist Medical Center. Winston Salem, PA 27103, NC..
Michael Rosenthal, Assistant Professor of Radiology-Harvard Medical School, Attending Radiologist-Brigham and Women’s Hospital, Senior Physician- Dana-Farber Cancer Institute. Boston, PA 02215, MA..
References
- 1.Weiser M, Paper about treatment changes and survival and MRI usage. Pending, 2019.
- 2.Siegel RL, et al. , Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst, 2017. 109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al. K.D.e., Rectal Cancer Hands On Workshop. 2018.
- 4.al. G.M.e., Rectal Cancer Workshop. 2018.
- 5.Glimelius B, et al. , Mesorectal fascia instead of circumferential resection margin in preoperative staging of rectal cancer. J Clin Oncol, 2011. 29(16): p. 2142–3. [DOI] [PubMed] [Google Scholar]
- 6.Orsini RG, et al. , The modern anatomical surgical approach to localised rectal cancer. EJC Suppl, 2013. 11(2): p. 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nougaret S, et al. , The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology, 2013. 268(2): p. 330–44. [DOI] [PubMed] [Google Scholar]
- 8.Horvat N, Petkovska I, and Gollub MJ, MR Imaging of Rectal Cancer. Radiol Clin North Am, 2018. 56(5): p. 751–774. [DOI] [PubMed] [Google Scholar]
- 9.Quirke P, et al. , Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet, 1986. 2(8514): p. 996–9. [DOI] [PubMed] [Google Scholar]
- 10.Group MS, Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology, 2007. 243(1): p. 132–9. [DOI] [PubMed] [Google Scholar]
- 11.Glimelius LBB, The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncologica, 2008. 47( 1): p. 5–8. [DOI] [PubMed] [Google Scholar]
- 12.Nagtegaal ID, et al. , Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol, 2002. 26(3): p. 350–7. [DOI] [PubMed] [Google Scholar]
- 13.Birbeck KF, et al. , Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg, 2002. 235(4): p. 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shihab OC, et al. , Magnetic resonance imaging-detected lymph nodes close to the mesorectal fascia are rarely a cause of margin involvement after total mesorectal excision. Br J Surg, 2010. 97(9): p. 1431–6. [DOI] [PubMed] [Google Scholar]
- 15.Stedman TL, Stedman’s medical dictionary. 28th ed 2006, Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- 16.Netter FH, Atlas of human anatomy. Sixth edition ed. 2014, Philadelphia, PA: Saunders/Elsevier. [Google Scholar]
- 17.Chung E, et al. , Accuracy of pelvic MRI in measuring tumor height in rectal cancer patients with or without preoperative chemoradiotherapy. Eur J Surg Oncol, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Dujovny N, Quiros RM, and Saclarides TJ, Anorectal anatomy and embryology. Surg Oncol Clin N Am, 2004. 13(2): p. 277–93. [DOI] [PubMed] [Google Scholar]
- 19.Nigro ND, et al. , Combined therapy for cancer of the anal canal. Dis Colon Rectum, 1981. 24(2): p. 73–5. [DOI] [PubMed] [Google Scholar]
- 20.Shihab OC, et al. , Can a novel MRI staging system for low rectal cancer aid surgical planning? Dis Colon Rectum, 2011. 54(10): p. 1260–4. [DOI] [PubMed] [Google Scholar]
- 21.Bamba Y, Itabashi M, and Kameoka S, Preoperative evaluation of the depth of anal canal invasion in very low rectal cancer by magnetic resonance imaging and surgical indications for intersphincteric resection. Surg Today, 2012. 42(4): p. 328–33. [DOI] [PubMed] [Google Scholar]
- 22.Scala D, et al. , Laparoscopic intersphincteric resection: indications and results. Updates Surg, 2016. 68(1): p. 85–91. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, et al. , High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol, 2004. 52(1): p. 78–83. [DOI] [PubMed] [Google Scholar]
- 24.Brown G, et al. , Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology, 2003. 227(2): p. 371–7. [DOI] [PubMed] [Google Scholar]
- 25.Fujita S, et al. , Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg, 2017. 266(2): p. 201–207. [DOI] [PubMed] [Google Scholar]
- 26.Ogura A, et al. , Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol, 2018: p. JCO1800032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazic I, P.S., Konishi T, Campbell N, Garcia-Aguilar J, Gollub MJ., Clinical Impact of Lateral Lymph Nodes at Pelvic MRI in Patients with Rectal Cancer . ECR2019 Accepted abstract, 2018. [Google Scholar]
- 28.Oh HK, et al. , Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol, 2014. 21(7): p. 2280–7. [DOI] [PubMed] [Google Scholar]
- 29.Chand M, et al. , Mucinous carcinoma of the rectum: a distinct clinicopathological entity. Tech Coloproctol, 2014. 18(4): p. 335–44. [DOI] [PubMed] [Google Scholar]
- 30.Hugen N, et al. , Modern Treatment of Rectal Cancer Closes the Gap Between Common Adenocarcinoma and Mucinous Carcinoma. Ann Surg Oncol, 2015. 22(8): p. 2669–76. [DOI] [PubMed] [Google Scholar]
- 31.Oberholzer K, et al. , Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys, 2012. 82(2): p. 842–8. [DOI] [PubMed] [Google Scholar]
- 32.Yu SK, et al. , Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer, 2014. 50(5): p. 920–7. [DOI] [PubMed] [Google Scholar]
- 33.Beets-Tan RGH, et al. , Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gollub MJ, et al. , Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY), 2018. [DOI] [PubMed] [Google Scholar]
- 35.Barbaro B, et al. , The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. Br J Radiol, 2017. 90(1069): p. 20150836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MJ, et al. , Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr, 2003. 27(1): p. 48–55. [DOI] [PubMed] [Google Scholar]
- 37.Beets GL, et al. , A new paradigm for rectal cancer: Organ preservation: Introducing the International Watch & Wait Database (IWWD). Eur J Surg Oncol, 2015. 41(12): p. 1562–4. [DOI] [PubMed] [Google Scholar]
- 38.Habr-Gama A, et al. , Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys, 2014. 88(4): p. 822–8. [DOI] [PubMed] [Google Scholar]
- 39.Habr-Gama A, et al. , Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg, 2004. 240(4): p. 711–7; discussion 717–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Valk MJM, et al. , Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet, 2018. 391(10139): p. 2537–2545. [DOI] [PubMed] [Google Scholar]
- 41.al J.J.S.e., A Watch and Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slater A, et al. , Distance between the rectal wall and mesorectal fascia measured by MRI: Effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol, 2006. 61(1): p. 65–70. [DOI] [PubMed] [Google Scholar]
- 43.Ye F, et al. , JOURNAL CLUB: Preoperative MRI Evaluation of Primary Rectal Cancer: Intrasubject Comparison With and Without Rectal Distention. AJR Am J Roentgenol, 2016. 207(1): p. 32–9. [DOI] [PubMed] [Google Scholar]
- 44.Stijns RC, et al. , The influence of endorectal filling on rectal cancer staging with MRI. Br J Radiol, 2018. 91(1089): p. 20180205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrag D, et al. , Challenges and solutions in the design and execution of the PROSPECT Phase II/III neoadjuvant rectal cancer trial (NCCTG N1048/Alliance). Clin Trials, 2019: p. 1740774518824539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vliegen RF, et al. , Rectal cancer: MR imaging in local staging--is gadolinium-based contrast material helpful? Radiology, 2005. 234(1): p. 179–88. [DOI] [PubMed] [Google Scholar]
- 47.Corines MJ, et al. , Gadolinium-Based Contrast Agent During Pelvic MRI: Contribution to Patient Management in Rectal Cancer. Dis Colon Rectum, 2018. 61(2): p. 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gollub MJ, et al. , Does gadolinium-based contrast material improve diagnostic accuracy of local invasion in rectal cancer MRI? A multireader study. AJR Am J Roentgenol, 2015. 204(2): p. W160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gollub MJ, et al. , Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol, 2012. 22(4): p. 821–31. [DOI] [PubMed] [Google Scholar]
- 50.Gollub MJ, et al. , Limited accuracy of DCE-MRI in identification of pathological complete responders after chemoradiotherapy treatment for rectal cancer. Eur Radiol, 2017. 27(4): p. 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gollub MJ, et al. , Value of adding dynamic contrast-enhanced MRI visual assessment to conventional MRI and clinical assessment in the diagnosis of complete tumour response to chemoradiotherapy for rectal cancer. Eur Radiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gollub MJ, et al. , Prognostic aspects of DCE-MRI in recurrent rectal cancer. Eur Radiol, 2013. 23(12): p. 3336–44. [DOI] [PubMed] [Google Scholar]
- 53.Dijkhoff RAP, et al. , Value of DCE-MRI for staging and response evaluation in rectal cancer: A systematic review. Eur J Radiol, 2017. 95: p. 155–168. [DOI] [PubMed] [Google Scholar]
- 54.Mandard AM, et al. , Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer, 1994. 73(11): p. 2680–6. [DOI] [PubMed] [Google Scholar]
- 55.Dworak O, Keilholz L, and Hoffmann A, Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis, 1997. 12(1): p. 19–23. [DOI] [PubMed] [Google Scholar]
- 56.Rodel C, et al. , Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol, 2005. 23(34): p. 8688–96. [DOI] [PubMed] [Google Scholar]
- 57.Lee SA, et al. , Impact of the new AJCC staging system and adjuvant treatment in rectal cancer. Cancer Res Treat, 2004. 36(2): p. 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trakarnsanga A, et al. , Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst, 2014. 106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel UB, et al. , Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol, 2011. 29(28): p. 3753–60. [DOI] [PubMed] [Google Scholar]
- 60.Sclafani F, et al. , Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer, 2017. 117(10): p. 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel UB, et al. , Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol, 2012. 19(9): p. 2842–52. [DOI] [PubMed] [Google Scholar]
- 62.Battersby NJ, et al. , A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials, 2017. 18(1): p. 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Paardt MP, et al. , Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology, 2013. 269(1): p. 101–12. [DOI] [PubMed] [Google Scholar]
- 64.Maas M, et al. , Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol, 2015. 22(12): p. 3873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gollub M, Pelvic MRI after Induction Chemotherapy and Before Long-Course Chemoradiation Therapy for Rectal Cancer: What are the Imaging Findings? European Radiology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakuyama N, et al. , Histological differences between preoperative chemoradiotherapy and chemotherapy for rectal cancer: a clinicopathological study. Pathol Int, 2016. 66(5): p. 273–80. [DOI] [PubMed] [Google Scholar]


