Abstract
With the increased use of cancer immunotherapy, a number of immune-related adverse events (irAEs) are being identified. These irAEs can be compared with known autoimmune disorders in similar tissues, with important similarities and differences. Understanding the etiology of irAEs may bring to light concepts applicable to immune responses in cancer, autoimmunity, and infectious disease. This immunobiology is especially relevant to cancer patients with preexisting allogeneic transplants or autoimmune disease who are undergoing cancer immunotherapy. To address these facets of cancer immunotherapy, academic leaders from these various disciplines discussed current irAE basic and clinical research, irAE diagnosis and management, and the need for biomarkers and algorithms to identify individuals at risk for irAEs at a conference jointly sponsored by the National Cancer Institute, National Institute of Allergy and Infectious Diseases, and National Institute of Arthritis and Musculoskeletal and Skin Diseases in Bethesda, MD, on March 22–23, 2018. Mechanisms and models to characterize irAEs, standardize protocols, store biospecimens, and capture and analyze irAE data were also reviewed during the inaugural Cancer, Autoimmunity, and Immunology Conference. This summary highlights cancer immunotherapy–induced irAEs, the challenges ahead, and the opportunities for greater understanding of autoimmune conditions.
Keynote speakers Suzanne Topalian (Johns Hopkins University) and Jeffrey Bluestone (Parker Institute for Cancer Immunotherapy [PICI] and University of California–San Francisco) introduced each of the morning sessions of the 2-day conference that detailed the biology, diagnosis, treatment, management, and the implications of immune-related adverse events (irAEs) that accompany cancer immunotherapy. After an introduction to the recent history of cancer immunotherapy and the definition and recognition of irAEs by Anthony Fauci (National Institute of Allergy and Infectious Diseases [NIAID]), Dr. Topalian focused on irAEs that result from immune checkpoint blockade therapy. She described how T cells are activated by signal 1 (through the T cell Ag receptor) and signal 2 (through coreceptors). Checkpoint molecules expressed on the surface of T cells (such as PD-1 and CTLA-4) normally restrain the T cell activation response from becoming too long or out of control. Drugs such as nivolumab (anti–PD-1) and ipilimumab (anti-CTLA4) interfere with that control in a process commonly referred to as “releasing the brakes” on T cells. Dr. Topalian discussed how different immune checkpoint inhibitors have different mechanisms of action and different frequencies of associated irAEs. Studies of the etiology of irAEs could help elucidate mechanisms of antitumor activity, as irAE frequency tends to associate with higher antitumor response.
As noted by Dr. Bluestone, these irAEs may also provide deeper understanding of the basic immunological mechanisms underlying autoimmune conditions. The cross-talk among these specialties has enormous potential for reverse translation in the fields of cancer, autoimmunity, and immunology. Continued research into the basic biological responses to immune checkpoint inhibitors and other forms of immunotherapy is needed to further define the mechanisms of action, diagnosis, treatment, and management of distinct irAEs that are now being observed in clinical trials and standard oncology practice.
Murine models of irAEs
Although human irAEs are not completely reproducible in mice, a number of models have been developed that add to our knowledge of immunotherapy-induced irAEs. Keynote speaker Jeffrey Bluestone emphasized that in the future, we should attempt to predict which drug combinations will provide robust antitumor effects without irAEs, provided that well-characterized syngeneic mouse models can be developed. Further, it will be important for clinicians to choose the best combination for patients who may be predisposed to different subclinical autoimmune conditions and may develop particular irAEs. Different mouse models with distinct autoimmune tendencies can shed light on these issues. Wild-type mice can also be useful for immunotherapy research, as evidenced by the work of Patrizio Caturegli (Johns Hopkins University). His studies in wild-type mice revealed the expression of CTLA-4 in the pituitary, which provides the likely mechanistic basis for the hypophysitis that can occasionally develop in patients undergoing anti–CTLA-4 immunotherapy (1) (Fig. 1A). Mice with genetic deletion of CTLA-4, PD-1, and/or PD-L1 have revealed defects in T cell tolerance and display various degrees of autoimmunity. As explained by Javid Moslehi (Vanderbilt University), myocarditis occurs in CTLA-4−/− mice crossed with PD-L1−/− or PD-1−/− mice (2), suggesting that combination therapies may increase the risk for the development of myocardial inflammation (Fig. 1B). IFN-γ–induced PD-L1 expression on mouse heart endothelial cells and the enhanced specific-targeted CTL killing of these cells in the presence of anti–PD-L1 Abs was described by Andrew Lichtman (Harvard University). Mice with genetic tendencies toward autoimmunity (e.g., NOD mice that develop spontaneous type 1 diabetes) are also useful for immune checkpoint inhibitor studies. Jeffrey Bluestone and Kevan Herold (Yale University) described a rapid onset of type 1 diabetes in response to PD-1 or PD-L1, but not CTLA-4, blockade in NOD mice. Mechanisms of CD81 T cell destruction of normal tissues are currently being pursued in this model. Kristina Howard (Food and Drug Administration [FDA]) described a number of immunodeficient mouse strains with “humanized” immune systems that are being used to test the dosing of drugs in combination, dosing route, frequency, study duration, pharmacokinetics, and Fc receptor responses. Understanding the degree of humanization is important. For example, humanized mice retain mouse tissue that secretes murine cytokines and chemokines that may not be recognized by human immune cells. Each of these various mouse models can be examined for nontarget responses associated with bacteria or viruses that alter immune checkpoint inhibitor–induced responses and affect patients undergoing therapy. For example, Daniel Barber (NIAID) described the mechanisms underlying fatal T cell–mediated immunopathology during Mycobacterium tuberculosis infection in PD-1−/− mice. Barber et al. (3) later studied two patients with cancer who developed active tuberculosis during PD-1 blockade therapy for their cancers. Using longitudinal samples from one of the patients treated on a National Cancer Institute (NCI)–sponsored trial for Merkel cell cancer, the study team was able to show that the adverse event in this patient mirrored the previously published murine data (3). Retrieving the maximum amount of relevant information from these various mouse models and understanding their limitations is important in understanding the therapeutic implications from these preclinical efforts.
FIGURE 1.
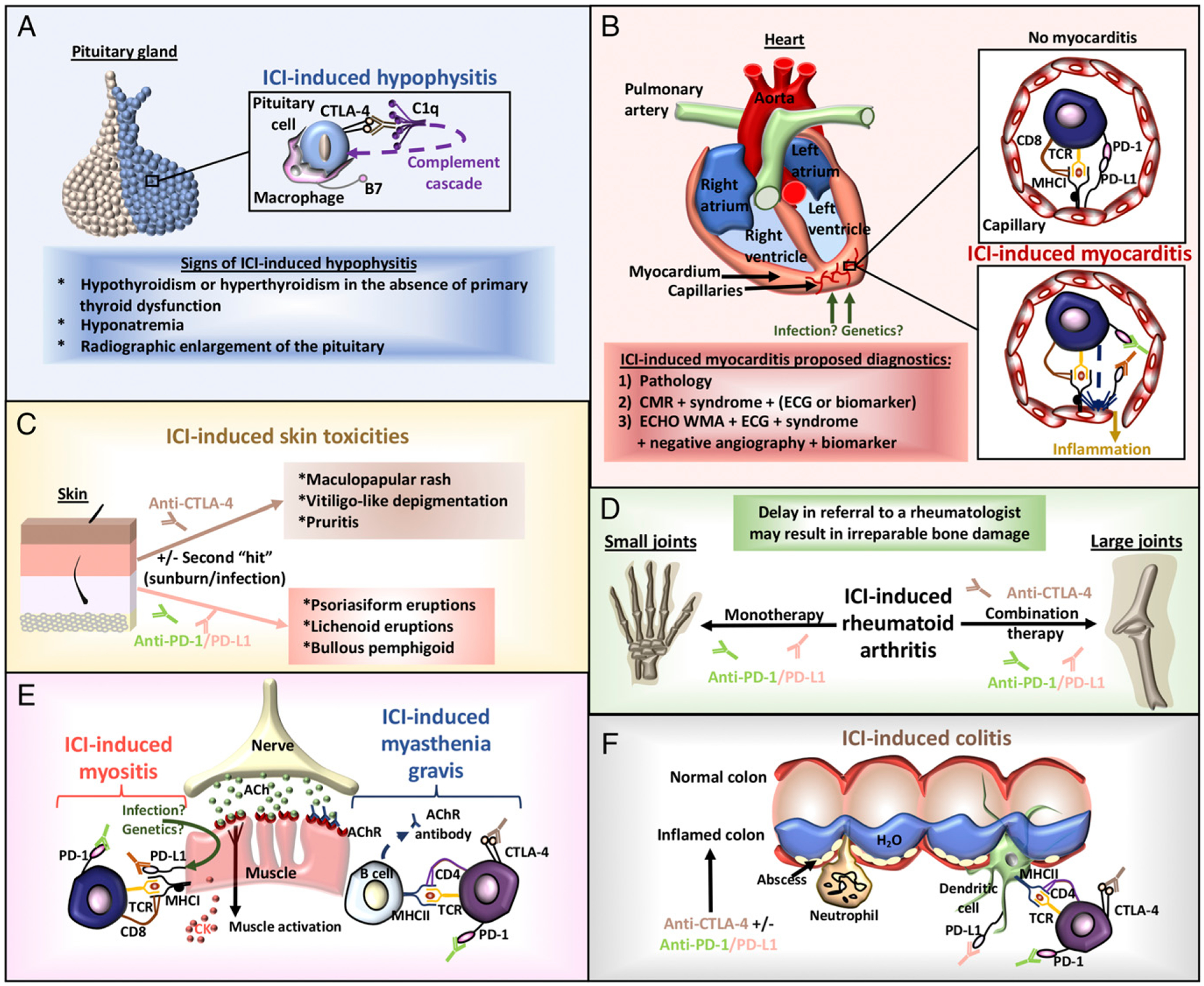
Identified immune checkpoint inhibitor (ICI)–induced toxicities. (A) ICI-induced hypophysitis. Ab blockade of pituitary cell CTLA-4 promotes the activation of the classical complement cascade (C1q) and recruitment of macrophages for targeted phagocytic engulfment of CTLA-4+ pituitary cells. Radiographic imaging and consultation with an endocrinologist can lead to early diagnosis and reduce treatment morbidity. (B) ICI-induced myocarditis. Infection or genetics may encourage endothelial PD-L1 expression that is bound by CD8 T cell PD-1. This form of tolerance is abrogated by Ab blockade of the PD-1 axis, resulting in CTL killing and inflammation. Diagnostics may include biopsy, cardiac magnetic resonance (CMR) imaging, electrocardiography (ECG), biomarkers, symptoms of the syndrome, negative angiography, or an echocardiogram of wall-motion abnormalities (ECHO WMA). (C) ICI-induced skin toxicities. The formation of irAEs in the skin may involve a second “hit” involving a sunburn or infection. Specific ICI therapies tend to develop distinct skin toxicities. (D) ICI-induced rheumatoid arthritis. Two major forms of arthritis are evolving secondary to PD-1/PD-L1 monotherapy and the CTLA-4 plus PD-1/PD-L1 combination. This irAE is poorly recognized in clinical trials, particularly when only small joints are affected. (E) ICI-induced myositis/myasthenia gravis. Unlike the characterized autoimmune disorders, myositis and myasthenia gravis can appear together subsequent to ICIs as identified by anti-acetylcholine (ACh) receptor (AChR) Abs and elevated creatine kinase (CK) levels. These irAEs may also occur concurrently with myocarditis. (F) ICI-induced colitis. ICI-induced gut toxicities physically appear more similar to colitis than inflammatory bowel disease, involve neutrophil and lymphocyte recruitment, and may occur more frequently with anti–CTLA-4 therapy.
Biological analysis of patient tumors and lymphocytes associated with irAEs
Collection and evaluation of clinical and biological information from patients are needed to understand the development of immune-related toxicity in specific organs. In identifying genetic signatures of immune cells, tumor cells, and stromal cells in the tumor microenvironment, researchers may effectively predict responders, nonresponders, and/or patient subsets susceptible to irAEs from patient biopsies. One approach, as described by both Emanual Maverakis (University of California–Davis) and Orit Rozenblatt-Rosen (Broad Institute), involves cross-comparing RNA sequencing data obtained from tumor samples from patients who have either responded or failed to respond to immune checkpoint blockade with bulk gene expression data from samples uploaded in The Cancer Genome Atlas. Researchers can also compare the gene signatures retrieved from autoimmune and cancer patients. David Hafler (Yale University) provided an example of melanoma patients with a BRAFV600K mutation that may develop myelin-autoreactive T cells subsequent to anti–CTLA-4 therapy, similar to T cells found in multiple sclerosis patients (4). Ignacio Sanz (Emory University) indicated that next-generation sequencing of TCRs and BCRs, as well as integrated transcriptome and epigenome differential and comparative analyses, may yield strategies for diagnostics and therapeutics. Systematic pipelines for profiling are being established in association with computational tools to better handle the data with the goal of generating a “tumor cell atlas,” similar to the international Immune Cell Atlas that is already in progress. To enable future efforts at immune profiling patients enrolled on NCI-sponsored clinical trials, the NCI is also funding a network of Cancer Immune Monitoring and Analysis Centers (CIMACs) and a Cancer Immunologic Data Commons (CIDC). As described by Sacha Gnjatic (Icahn School of Medicine at Mount Sinai), the CIMAC-CIDC network (https://cimac-network.org) is establishing multidisciplinary working groups to analyze tissue samples, blood, serum, and stool from both pre- and posttherapy specimens. The effort is focused mostly on improving therapeutic efficacy, but this approach can be easily adapted for the continued exploration of the biology of tumors and irAEs in patients to help identify and characterize biomarkers to prevent, diagnose, and treat irAEs.
irAE risk factors, diagnosis, and management
The number of irAEs occurring as the result of cancer immunotherapy is likely underestimated because of delayed onset, death, and a lack of adequate monitoring after treatment cessation. These irAEs may develop in virtually any tissue in the body (5) (Fig. 1). Although the identification and management of irAEs is primarily drawn from experience with similar autoimmune disorders, irAEs are distinct in their pathogenesis, suggesting that their treatments may also differ. Methods to identify risk factors and diagnose, treat, and manage irAEs are only beginning to be realized as experience accrues with time.
A variety of relevant risk factors are being explored in the development of irAEs. In the case of immune checkpoint inhibitor–induced skin toxicities, UV radiation may also be a factor, as described by Nicole LeBoeuf (Dana-Farber Cancer Institute). Pathogens as well as a genetic predisposition may stimulate the expression of PD-L1 on the surface of cells (Fig. 1). Kevan Herold (Yale University) stated that immune checkpoint inhibitor–induced, insulin-dependent diabetes can occur, with a family history of autoimmunity as a possible risk factor. Patients with organ transplants, previous chemotherapy/immunotherapy, or additional preexisting cardiac or autoimmune disease (Fig. 2A) may also have heightened risks for irAEs.
FIGURE 2.
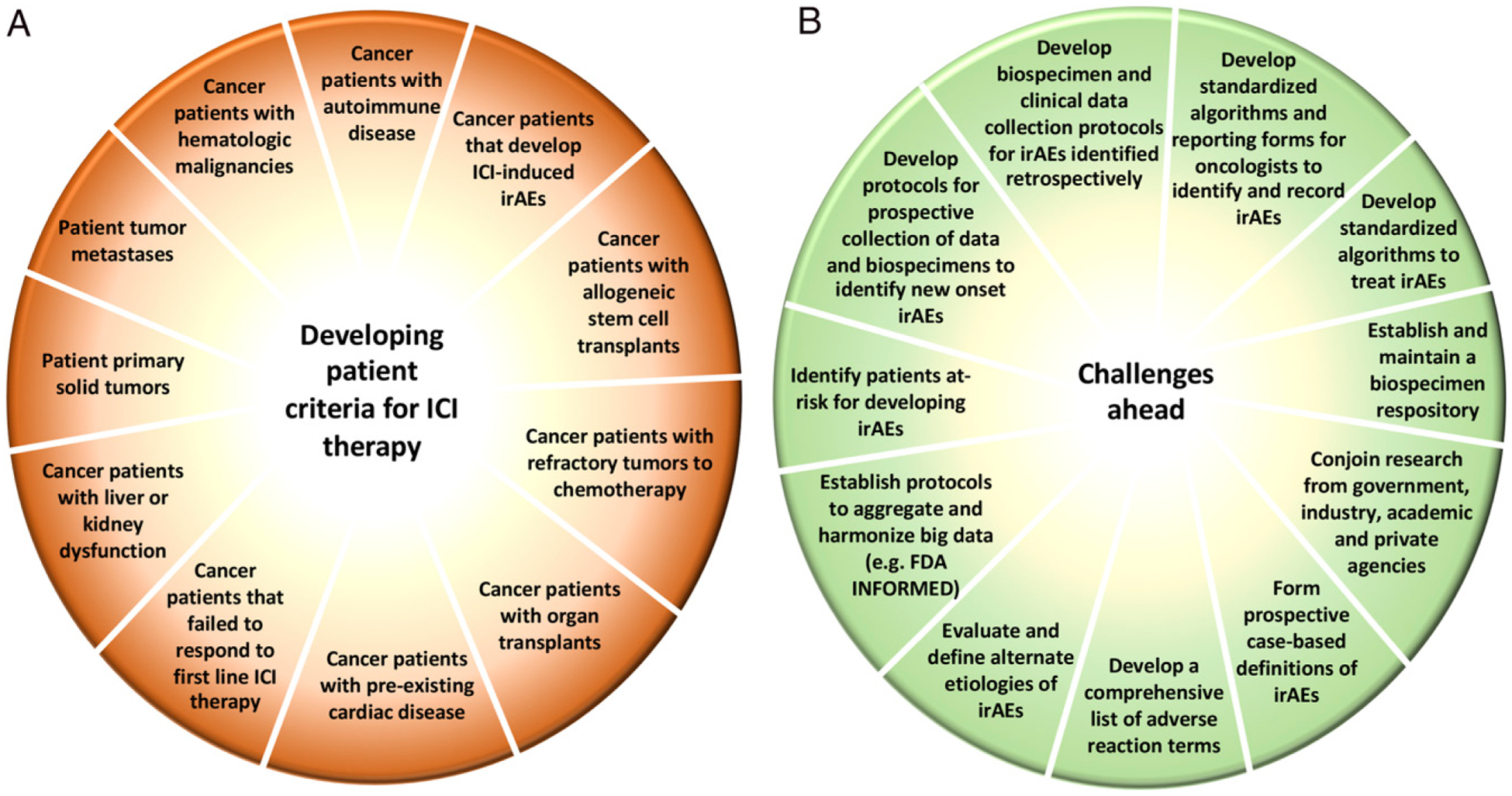
Capturing irAE data relevant to patient diversity. (A) The diversity of tumors and patient conditions is a significant factor in irAEs. The use of immune checkpoint inhibitors requires the development of criteria to manage potential irAEs or exclude certain patient subsets. (B) As patients continue to be treated with immune checkpoint inhibitors either in monotherapy or in combination therapies, the identification of irAEs is anticipated to grow. Managing the data from these trials will require the development of standardized definitions and protocols that can be broadly accepted across institutions. These are the challenges ahead.
Monica Girotra (Memorial Sloan Kettering Cancer Center), Clifton Bingham (Johns Hopkins University), and Javid Moslehi (Vanderbilt University) highlighted that the diagnosis of irAEs is complicated by a lack of comprehensive definitions to identify patients in need of referrals to specialists. This is due to a lack of standardized reporting, which can result in the underdiagnosis of conditions. Continued development of biomarkers and diagnostic techniques for irAEs will require strong multidisciplinary collaborations and rapid referrals to relevant specialists.
Immunosuppressive irAE management approaches are being drawn from experience with autoimmunity, other immune-based therapies, retrospective analyses, and prospective studies (Fig. 2B). The type of immunosuppression used should be determined in collaboration with specialists able to evaluate the state of their autoimmune disease (active or inactive), the degree of flares, and toxicities. Nirali Shah (NCI) described one example from the cell-based immunotherapy field that involves the treatment of cytokine release syndrome, most notably in patients who have been treated with CD19-targeting chimeric Ag receptor–T cell therapy. A significant minority of patients experienced life-threatening cytokine release syndrome, but the administration of anti–IL-6R Abs (tocilizumab) was shown early in trials to ablate this irAE while preserving the immune response to the tumor. Establishing similar algorithms to treat and potentially prevent the development of the more commonly observed irAEs is urgently needed. Retrospective analyses may also be useful for developing treatment parameters. Alexander Faje (Massachusetts General Hospital) described a recent study in melanoma patients with ipilimumab-induced hypophysitis. This study indicated that low-dose (≤7.5 mg of prednisone) compared with high-dose (>7.5 mg of prednisone) corticosteroid treatment may have benefits in terms of overall survival (6). Management of irAEs may persist long after cessation of active treatment with a particular immunotherapy regimen, in part because of prolonged activation of the offending T cell clones, inducing the irAE (7). In some cases (e.g., immune checkpoint inhibitor–induced rheumatoid arthritis), the irreparable damage will require lifelong care.
Using immunotherapy in patients with cancer in the setting of allogeneic transplants or preexisting autoimmunity
In the absence of additional treatment options, immune checkpoint inhibitor therapy is being considered for cancer patients with a prior history of allogeneic transplants or preexisting autoimmunity. Typically, these patients have been excluded from clinical trials, as the perceived risks of graft-versus-host disease (GVHD) or autoimmune disease flare were deemed to be too great. To address the treatment needs of these patients, the NCI is sponsoring several studies in this population with immune checkpoint inhibitor therapy. In metastatic melanoma and metastatic cutaneous squamous cell carcinoma patients who also had prior allogenic kidney transplants, Evan Lipson (Johns Hopkins University) described case reports of ipilimumab and pembrolizumab treatment, respectively, that resulted in durable partial responses for as long as 8 y and ongoing (8, 9). Although there is a significant risk of allograft rejection, the results suggest that immune checkpoint inhibitors can be highly effective against cancers arising in the context of immunosuppression. To selectively activate antitumor immunity and deter rejection, a prospective clinical trial (National Clinical Trial Identifier: NCT03816332) assessing nivolumab with a tacrolimus and prednisone immunosuppression regimen is being initiated in patients with advanced cancers and a preexisting renal allograft. The objectives are focused on safety, feasibility, and maintenance of the renal allograft. Correlative studies are planned to help characterize immunological changes in paired biopsies obtained pretreatment and on treatment; assess changes in donor-derived, cell-free DNA as an early marker for allograft rejection; and describe irAEs in these patients.
Treatment options for allogeneic hematopoietic stem cell transplantation (allo-HSCT) patients that experience subsequent relapse include salvage cytotoxic chemotherapy, withdrawal of immune suppression, donor lymphocyte infusion, and a repeated allo-HSCT. Jacqueline S. Garcia (Dana-Farber Cancer Institute) and Yvette Kasamon (FDA) described analyses of patients who were treated with immune checkpoint inhibitors following allo-HSCT for hematologic malignancies. Ipilimumab-induced durable clinical responses were noted in several patients with acute myelogenous leukemia who had previously relapsed following allo-HSCT. These occurred in association with GVHD and additional toxicities in a clinical trial sponsored by the NCI (10). In a retrospective analysis of 31 patients, durable complete responses with GVHD were also identified in patients with classical Hodgkin lymphoma treated with anti–PD-1 therapy following allo-HSCT and subsequent relapse (11). In another retrospective study, allo-HSCT after PD-1 blockade was also considered feasible but with toxicities such as hyperacute GVHD, hepatic veno-occlusive disease, and other irAEs, including transplant-related mortality (12). These patients therefore need to be followed closely for transplant-related complications to facilitate prompt interventions. The U.S. FDA has cited safety concerns about the administration of immune checkpoint inhibitors before and after allo-HSCT. Safety signals need to be further investigated to differentiate immune checkpoint inhibitor–induced toxicity from GVHD, which will require ongoing observational studies and long-term monitoring.
As noted, patients with preexisting autoimmunity are generally excluded from clinical trials because of concern over complications with the patients’ autoimmune diseases as described by Hussein Tawbi (MD Anderson Cancer Center). Consequently, the NCI is now initiating a prospective trial (National Clinical Trial Identifier: NCT03816345) exploring treatment options with immune checkpoint inhibitors for this category of patients in collaboration with multiple leading autoimmune disease experts who will serve as coinvestigators with the treating oncologists. The focus remains on conditions for which PD-1 and PD-L1 inhibitors have an approved indication. Retrospective efforts have suggested that benefits are possible with appropriate monitoring (13, 14). The initial disease cohorts will include patients with dermatomyositis, scleroderma, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, and multiple sclerosis. The aims are to establish safety and dose-limiting toxicities of treatments, accurately assess the kinetics of flares and the dynamics of other irAEs, assess antitumor response, and identify the mechanisms of toxicity associated with autoimmunity.
Management of irAE data and protocols
As the development and use of immunotherapies continue to expand, strategies to record, manage, and interpret an increasing amount of information are needed to maintain relevant standards in safety and labeling (Fig. 2B). Sean Khozin (FDA) discussed a multidisciplinary initiative to analyze adverse event reporting to the FDA, known as Information Exchange and Data Transformation (INFORMED) (15). The aggregation and harmonization of immune checkpoint clinical trials data submitted to the FDA are expected to better characterize the safety of these agents with respect to irAE incidence, risk factors, clinical presentation, treatment, and outcomes. These techniques can also be transferred to the evaluation of the patient experience by examining electronic health records. However, interpretation of these datasets is premised on the reporting terms used by investigators and clinicians, which can be quite variable in the reporting of irAEs. Marc Theoret (FDA) highlighted FDA efforts to generate a comprehensive list of adverse reaction terms, evaluations of alternate etiologies, and the use of immunosuppressive medications from retrospective and prospective studies to better design case-based definitions of irAEs.
The Alliance for Clinical Trials in Oncology, in collaboration with the NCI, is developing an irAE biorepository to help advance further translational research efforts relevant to irAEs. David Kozono (Dana-Farber Cancer Institute) discussed the Alliance-NCI irAE Biorepository, which is a centralized repository for acquiring and storing well-annotated biospecimens vital for translational studies and generating data relevant to the molecular pathogenesis and treatment of severe irAEs. This group aims to develop retrospective and prospective protocols for the collection of data and biospecimens from clinical trials and standard-of-care patients. Samantha Bucktrout (PICI) described PICI pilot grants that have included preclinical, prospective, and cross-site retrospective studies that generate data on immunotoxicity, tumor control, and immune responses.
Conclusions
The success of cancer immunotherapy has revealed untoward immune-related side effects that are anticipated to shed light on the complex mechanisms involved in immunity and disease. The use of mouse models and the extensive assessment of patient biospecimens and clinical data are expected to identify irAE risk factors and biomarkers that will be able to shed light on the potential pathogenesis of cognate autoimmune diseases. Understanding the various facets of the immune response will be pivotal in treating patients with a diversity of tumor types and preexisting conditions. Establishing irAE definitions and reporting standards will require multiple disciplines and collaborations between stakeholders to effectively understand, prevent, diagnose, and treat irAEs. This will be of utmost importance in recognizing irAEs that may arise as patients live longer and as combination therapies and new immunotherapies are investigated in clinical trials. These topics, additional irAEs, and their current status in clinical trials are subjects of the second annual Cancer, Autoimmunity, and Immunology Conference (Bethesda, MD, April 15–16, 2019).
Acknowledgments
We thank the conference speakers for providing time and insight: Orit Rozenblatt-Rosen (Broad Institute); Jacqueline S. Garcia, David Kozono, and Nicole LeBoeuf (Dana-Farber Cancer Institute); Kavita Dhodapkar and Ignacio Sanz (Emory University); Laleh Amiri-Kordestani, Kristina Howard, Yvette Kasamon, Sean Khozin, and Marc Theoret (FDA); Andrew Lichtman and Michael Dougan (Harvard University); Sacha Gnjatic (Icahn School of Medicine at Mount Sinai); Clifton Bingham, Patrizio Caturegli, Evan Lipson, Ami Shah, and Suzanne Topalian (Johns Hopkins University); Alexander Faje (Massachusetts General Hospital); Hussein Tawbi (MD Anderson Cancer Center); Monica Girotra (Memorial Sloan Kettering Cancer Center); Helen Chen, Jennifer Kanakry, Nirali Shah, and Ned Sharpless (NCI); Daniel Barber, Anthony S. Fauci, Ronald Germain, and Ethan Shevach (NIAID); Stephen I. Katz and Andy Mammen (National Institute of Arthritis and Musculoskeletal and Skin Diseases); Jennifer Blau and Maya Lodish (Eunice Kennedy Shriver National Institute of Child Health and Human Development); Jeffrey Bluestone (University of California–San Francisco and PICI); Samantha Bailey-Bucktrout (PICI); Emanual Maverakis (University of California–Davis); John Harris (University of Massachusetts–Worchester); Javid Moslehi (Vanderbilt University); Niroshana Anandasabapathy (Weill Cornell Medicine); and David Hafler and Kevan Herold (Yale University).
Abbreviations used in this article:
- allo-HSCT
allogeneic hematopoietic stem cell transplantation
- FDA
Food and Drug Administration
- GVHD
graft-versus-host disease
- irAE
immune-related adverse event
- NCI
National Cancer Institute
- NIAID
National Institute of Allergy and Infectious Diseases
- PICI
Parker Institute for Cancer Immunotherapy
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, and Lupi I. 2016. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am. J. Pathol 186: 3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtman AH 2013. The heart of the matter: protection of the myocardium from T cells. J. Autoimmun 45: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, Ashkin D, Cheng JH, Lundgren LM, Raabe VN, et al. 2019. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci. Transl. Med 11: eaat2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Nylander A, Ramanan S, Goods BA, Ponath G, Zabad R, Chiang VL, Vortmeyer AO, Hafler DA, and Pitt D. 2016. CNS demyelination and enhanced myelin-reactive responses after ipilimumab treatment. Neurology 86: 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June CH, Warshauer JT, and Bluestone JA. 2017. Is autoimmunity the Achilles’ heel of cancer immunotherapy? [Published erratum appears in 2017 Nat. Med. 23: 1004.] Nat. Med 23: 540–547. [DOI] [PubMed] [Google Scholar]
- 6.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, and Sullivan RJ. 2018. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124: 3706–3714. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. 2010. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipson EJ, Bodell MA, Kraus ES, and Sharfman WH. 2014. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J. Clin. Oncol 32: e69–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipson EJ, Bagnasco SM, Moore J Jr., Jang S, Patel MJ, Zachary AA, Pardoll DM, Taube JM, and Drake CG. 2016. Tumor regression and allograft rejection after administration of anti-PD-1. N. Engl. J. Med 374: 896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachireddy P, Hainz U, Rooney M, Pozdnyakova O, Aldridge J, Zhang W, Liao X, Hodi FS, O’Connell K, Haining WN, et al. 2014. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood 123: 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, Kamdar M, Kanate AS, Saad A, Mehta A, et al. 2017. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood 130: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merryman RW, Kim HT, Zinzani PL, Carlo-Stella C, Ansell SM, Perales MA, Avigdor A, Halwani AS, Houot R, Marchand T, et al. 2017. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 129: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, Guminski A, Puzanov I, Lawrence DP, Buchbinder EI, et al. 2016. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2: 234–240. [DOI] [PubMed] [Google Scholar]
- 14.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, et al. 2017. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol 28: 368–376. [DOI] [PubMed] [Google Scholar]
- 15.Khozin S, Pazdur R, and Shah A. 2018. INFORMED: an incubator at the US FDA for driving innovations in data science and agile technology. Nat. Rev. Drug Discov 17: 529–530. [DOI] [PubMed] [Google Scholar]


