ABSTRACT
Objective:
This study examined the risk factors in the early rehabilitation therapy of critically ill patients with weakness acquired in an intensive care unit and the impact of this therapy on walking independence after discharge from the hospital.
Method:
Of the 764 consecutive patients transported to the study facilities by ambulance, newly admitted to the intensive care unit (ICU), and treated with rehabilitation during hospitalization, 88 were included in this study after eliminating those who met a detailed list of exclusion criteria. To retrospectively examine the rate of walking independence and the effect of differing durations of rehabilitation activity, the study patients were divided into two groups: those with ICU-acquired weakness (AW) and those without ICU-acquired weakness (non-ICU-AW) on discharge from the ICU.
Results:
Analysis using the Kaplan–Meier estimator revealed that the non-ICU-AW group needed a markedly shorter period to achieve walking independence. In terms of the rehabilitation activities performed in the ICU, both in-bed exercises and the total duration of rehabilitation activity were significantly shorter in the ICU-AW group than in the non-ICU-AW group.
Conclusion:
The two groups were compared, and the amount of daily activity time significantly influenced the quality of patient outcome.
Keywords: activity time, early rehabilitation, ICU-AW, walking independence
INTRODUCTION
With the progress in life-saving and perioperative management techniques for critically ill patients in the intensive care unit (ICU), survival rates have markedly increased.1) However, because the majority of ICU patients are generally under management using mechanical ventilation and sedation, they have increased risks of functional disorders and impaired mobility as a result of disuse syndrome.2) These patients require long-term rehabilitation in some cases.3)
Muscle weakness and other functional disorders in ICU patients, such as ICU-acquired weakness (AW) and acquired delirium (AD), also influence their prognosis. Similarly, physical and mental dysfunction restricts their daily life and social activities. Such physical, mental, and social inactivity is termed “frailty”4) and is regarded as a negative outcome of ICU management.
Factors increasing the risks of ICU-AW and ICU-AD include a resting bedridden condition using mechanical ventilation, deep sedation, prolonged physical inactivity, and drug use.5) Early rehabilitation, mainly based on exercise therapy and/or early mobilization, is being promoted as a strategy to reduce these iatrogenic risks.6) In other countries, there have been several reports on the effectiveness and applicability of early rehabilitation, including its ability to improve prognosis.7) However, the intervention methods vary among these studies, and the influences of such variations on the therapeutic effects are yet to be clarified. Related differences in the functional prognosis, represented by walking independence, also remain unclear. Therefore, the present study examined the effectiveness of early rehabilitation of critically ill patients with ICU-AW and the relationship of this therapy with walking independence on discharge from the hospital.
METHODS
Study Facilities
The study was performed in cooperation with the ICUs of the National Hospital Organization Nagoya Medical Center, Kainan Hospital, and Ichinomiyanishi Hospital.
Study Design and Subjects
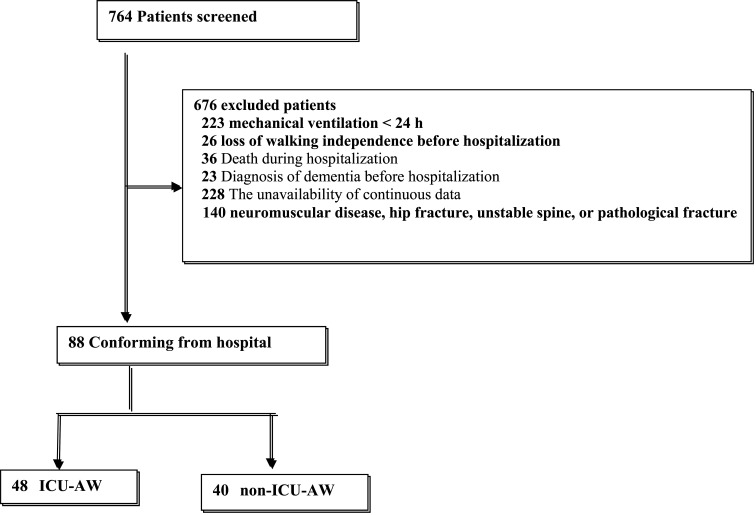
This retrospective observational study was conducted between April 1, 2015, and December 31, 2016. Of the 764 consecutive patients who had been newly admitted to the ICUs of the study facilities and treated with rehabilitation during hospitalization, 676 were excluded based on the following criteria: duration of mechanical ventilation <24 h, loss of walking independence before hospitalization, death during hospitalization, diagnosis of dementia before hospitalization, unavailability of continuous data, and difficulty in performing exercise due to nervous or orthopedic diseases. As a result, 88 patients were included in the study group (Fig. 1).
Fig. 1.
Flow chart of the patient selection process. ICU-AW: Intensive care unit-acquired weakness.
As performance measures, we set a threshold level of walking independence and also an expected level of walking independence with reference to previous studies.7,8,9) The required sample size was estimated on the basis of a threshold walking independence of 35% and an expected walking independence of 50%, with an 80% power level and a one-sided alpha value of 0.05, using the binomial test. With these parameters, the target sample size was determined to be at least 85 experimental subjects and 85 control subjects.
Study and Measurement Items
Based on the diagnostic criteria for ICU-AW developed by Stevens et al.,10) the patients were divided into two groups, depending on which set of the following characteristics applied:
1. Generalized weakness developing after the onset of critical illness;
2. Weakness is diffuse (involving both proximal and distal muscles), symmetric, flaccid, and generally spares cranial nerves;
3. Medical Research Council (MRC) sum-score <48 or mean MRC score <4 in all testable muscle groups noted on at least two occasions separated by 24 h;
4. Dependence on mechanical ventilation; and
5. Causes of weakness not related to the underlying critical illness have been excluded.
The minimum criteria used for diagnosing ICU-AW was either meeting criteria 1, 2, and 3 or 4 and 5.
The MRC sum-score is calculated by rating the muscle strength of the upper and lower limbs on a scale of 0–5 using the manual muscle test. The full score is 60.11) Target muscle groups were – upper limb: shoulder abductors, elbow flexors, and wrist extensors and lower limb: hip flexors, knee extensors, and ankle dorsiflexes.
Calculating the MRC sum-score is a simple evaluation method with a high level of inter-rater agreement; however, its reliability for patients being administered sedatives remains unclear.12) Participating institutions in this study each have sedative protocols, and sedated patients were not included when assessing MRC sum-scores.
Basic Information
As background factors, we examined each patient’s age, sex, height, weight, body mass index, score on the second version of the acute physiology and chronic health evaluation (APACHE II) instrument,13) admission category (cardiovascular, respiratory, abdominal/pelvic, surgery, and other), and length of stay in both the hospital and the ICU.
Primary Outcomes
The rate of walking independence and the number of days needed to achieve such independence were examined as the primary outcomes. The study followed each patient until discharge from the hospital. Walking independence was measured during hospitalization using a walking-related Barthel index (BI)14) score of 15 (i.e., being able to walk 45 m or further with or without an orthosis), as assessed by physical therapists and nurses.
Secondary Outcomes
This study also examined the time to first rehabilitation assessment (days), the duration of mechanical ventilation (days), rates of ICU-AD, discharge to home, functional status score for the ICU (FSS-ICU) on discharge from the ICU,15) and the BI score at hospital discharge. ICU-AD was assessed using two delirium screening scales: the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)16) and the Intensive Care Delirium Screening Checklist (ICDSC).17)
Rehabilitation Activity Time in the ICU
To quantify the amount of rehabilitation activity undertaken in the ICU, we measured the total number of activity days, total activity time, sum of the in- and out-of-bed exercise durations, and average daily activity time. This latter measure was calculated by adding the durations of all activities actually performed in the ICU and dividing by the duration of ICU stay (days); the duration (from start to finish) of each rehabilitation protocol activity actually performed was measured in seconds using a stopwatch. The time needed to prepare for rehabilitation and rest periods during exercise, assessment, and measurement were excluded from the activity time. Therefore, only the duration of the exercises actually performed was recorded.
During in-bed exercises, joint movements corresponding to “step I strength training: movements involving active muscle contraction, including free-weight exercise”18) or active movements and pretraining were executed. The subjective exercise intensity level when repeating limb movements was set at “easy” for each patient. In contrast, for resistance and assisted ergometer training, the subjective exercise intensity level was set at “relatively hard” in each case. Electrical muscle stimulation was recorded when muscle contraction was achieved. During out-of-bed exercise, sitting at the edge of the bed, standing, and walking were included in the activity time, whereas movements executed with full assistance were excluded. Unlike times recorded to calculate medical fees, our “durations of activities actually performed” did not include pulmonary rehabilitation or other related procedures.
Early Rehabilitation Protocol
Rehabilitation for the ICU patients in this study was performed through collaboration with ICU physical therapists, intensivists, and nurses on the basis of an early rehabilitation (mobilization) protocol.6) Rehabilitation started with passive limb range-of-motion training for a patient lying in bed and was gradually shifted to active movements to be independently executed by the patient. In addition, pulmonary physical therapy to improve/prevent pneumonia and atelectasis was performed. When a patient’s systemic condition had stabilized, mobilization, such as elevating the trunk on the bed, sitting, standing, and walking, was initiated. The feasibility of mobilization was confirmed at least once daily.
Regarding rehabilitation after discharge from the ICU, training to appropriately execute basic movements, such as walking, was performed only on weekdays, without a specific protocol.
This research was conducted with the approval of the institutional review board at the Nagoya Medical Center Hospital (IRB approval number 23). We followed a de-identification standard to protect the confidentiality of personal information. The study qualified for exemption status by the IRB because of its retrospective nature. Therefore, the need for patient consent to participate was waived. As part of the present study, we published our results on the homepage of the hospital, explaining our goals and methods, and offering any included patient the opportunity to be withdrawn.
Statistical Analyses
Background and course-related factors were compared between the ICU-AW group and the non-ICU-AW group. Continuous and ordinal variables were compared using the Mann–Whitney U test, whereas nominal variables were compared using the chi-square test. To examine factors associated with the development of ICU-AW on discharge from the ICU, logistic regression analysis based on the likelihood ratio was performed using the forward selection method, using items showing significant differences between the groups as explanatory variables. In the analysis, multicollinearity was examined by calculating Spearman’s rank correlation coefficient and Cramer’s contingency coefficient, adopting possible clinically significant variables when the absolute value of each correlation coefficient was 0.7 or higher. Finally, factors influencing walking independence were examined by analyzing the period needed to achieve walking independence using the Kaplan–Meier estimator. The log-rank test was also used for comparison between the groups. For statistical processing, JMP 13 was used with the significance level set at <5%.
RESULTS
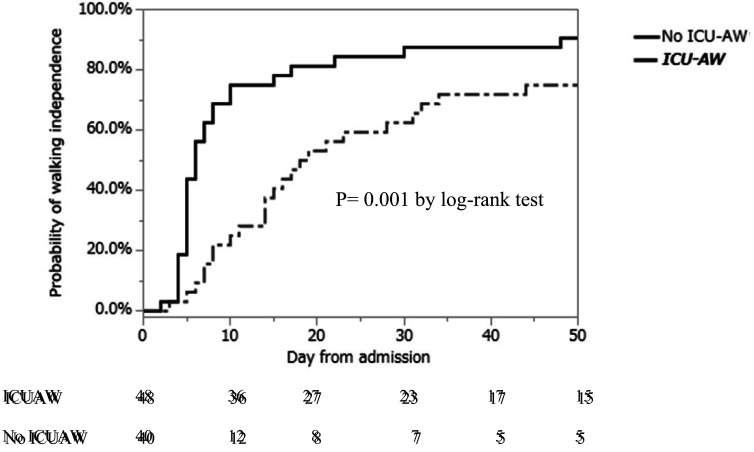
Among the background factors, a significant age difference was observed between the ICU-AW group and the non-ICU-AW group (P=0.015) (Table 1). In terms of the primary outcomes, there were no significant differences in the rates of walking independence between hospital admission and discharge between the groups (Table 2). In contrast, the time needed to achieve such independence varied significantly between the groups when compared using the log-rank test (P=0.001) (Fig. 2).
Table 1. Comparison of background factors.
| ICU-AW n= 48 |
Non-ICU-AW n= 40 |
P value | |
| Age (years) | 69 (58–78.5) | 65 (46.3–73) | 0.015 |
| Male sex, n (%) | 30 (61.2) | 28 (70.0) | 0.502 |
| Height (cm) | 160 (153–170) | 162.7(156.3–169.8) | 0.546 |
| Weight (kg) | 53 (48.2–66.4) | 59.9(53.4–70.6) | 0.273 |
| BMI | 22.1(19.7–24.5) | 22.8 (20.8–25.9) | 0.385 |
| APACHE II score | 24(17.5–32.0) | 20 (16.3–25.8) | 0.078 |
| Admission category | |||
| Cardiovascular, n (%) | 23 (46.9) | 20 (50.0) | 0.135 |
| Respiratory, n (%) | 8 (16.3) | 11 (27.5) | |
| Abdominal/pelvic surgery, n (%) | 6 (12.2) | 6 (15.0) | |
| Other, n (%) | 12 (16.9) | 3 (7.5) | |
| Hospital length of stay (days) | 38 (23–55.5) | 24 (17.3–31.8) | <0.0001 |
| ICU length of stay (days) | 6 (4–8) | 5 (4–7.8) | 0.946 |
Data are medians (25th–75th percentiles) or the number of patients.
BMI: body mass index. APACHE II: Acute Physiology and Chronic Health Evaluation.
Table 2. Comparison of clinical parameters.
| ICU-AW n= 48 |
Non-ICU-AW n= 40 |
P value | |
| Primary Outcome | |||
| Walking independence, n(%) | 33 (67.4) | 35 (87.5) | 0.078 |
| Secondary Outcome | |||
| Time to first rehabilitation assessment (day) | 2.3 (1.2–4.4) | 2.5 (1.4– 4.7) | 0.452 |
| Duration of mechanical ventilation (day) | 3 (1–6) | 2 (1–4) | 0.385 |
| ICU-AD, n (%) | 19 (38.8) | 3 (7.5) | <0.0001 |
| Discharge to home, n (%) | 27 (55.1) | 28 (70.0) | 0.031 |
| FSS-ICU at ICU discharge | 10 (4–17.5) | 22 (15.3–28) | <0.0001 |
| BI at hospital discharge | 60 (25–82.5) | 77.5 (65–90) | <0.0001 |
Data are medians (25th–75th percentiles) or the number of patients. ICU-AD: intensive care unit-acquired delirium.
FSS-ICU: Functional status score for the intensive care unit. BI: Barthel index.
Fig. 2.
Kaplan–Meier curves of walking independence for the ICU-AW group versus the non-ICU-AW group. Analysis using the log-rank test revealed significant differences between the ICU-AW and non-ICU-AW groups.
Analysis of the secondary outcomes revealed that the duration of hospitalization (P<0.0001) was significantly longer, the ICU-AD was higher, the FSS-ICU score on discharge from the ICU (P<0.0001) was lower, and the BI score on discharge from hospital (P<0.0001) was lower in the ICU-AW than in the non-ICU-AW group (Table 2). As for rehabilitation in the ICU, the in-bed exercise time (P <0.0001) and the total activity time (P <0.0001) were significantly shorter for the ICU-AW group (Table 3).
Table 3. Comparison of rehabilitation activity times while in the ICU .
| ICU-AW n= 48 | Non-ICU-AW n= 40 | P value | |
| Total number of activity days (day) | 5 (3–7) | 4 (3–7) | 0.705 |
| Total activity time (min) | 150 (96–285) | 184.5 (80.3–318.8) | 0.367 |
| In-bed exercise time (min) | 8.0 (6.4–13.5) | 25.1 (13.3–33.0) | <0.0001 |
| Out-of-bed exercise time (min) | 10.3 (5.2–15.7) | 10.1 (6.7–16.6) | 0.709 |
| Total daily activity time (min) | 18.3 (15–20.5) | 35.2 (28.5–41) | <0.0001 |
Data are medians (25th–75th percentiles)
Table 4 shows the results of multiple logistic regression analyses before matching. After exploring five explanatory variables, namely, age, APACHE II score, rate of ICU-AD, FSS-ICU score on discharge from the ICU, and total daily activity time in the ICU, only the total daily activity time in the ICU was selected (odds ratio: 0.777; 95% confidence interval: 0.69–0.851). The model’s kappa-squared value was significant, at P <0.01.
Table 4. Multivariate analysis of factors associated with ICU-AW (n=88).
| Odds ratio | 95% CI | P value | |
| Total daily activity time (min) | 0.777 | 0.690–0.851 | <0.0001 |
| Age (years) | 1.241 | 0.989–1.421 | 0.472 |
| APACHE II score | 0.987 | 0.895–1.090 | 0.796 |
| Rate of ICU-AD (n) | 2.047 | 0.182–32.832 | 0.577 |
| FSS-ICU score on discharge from the ICU | 0.989 | 0.938–1.063 | 0.088 |
Hosmer and Lemeshow, goodness-of-fit χ2 < 0.01, P = 0.52.
CI: confidence interval.
DISCUSSION
In the present study, patients were divided into two groups on the basis of the presence or absence of ICU-AW on discharge from the ICU. The associated factors and their influences on walking function were analyzed. The total daily activity time in the ICU was an independent risk factor for ICU-AW. Using the Kaplan–Meier estimator, it was found that the ICU-AW group needed a significantly longer period to achieve walking independence than the non-ICU-AW group did.
ICU-AW is a systemic muscle weakness that frequently develops in critically ill patients requiring intensive care.19) Moreover, ICU-AW is an independent risk factor for poor functional prognosis, indicating the necessity of developing effective methods for preventing and improving this condition.18) Furthermore, because the inactivity of skeletal muscles due to patients being bedridden is a leading cause of ICU-AW, early mobilization is expected to be a useful preventive measure against such weakness.20) However, for patients requiring acute care with unstable circulatory or respiratory conditions, early mobilization is frequently difficult because it may interfere with sedation or the systemic condition of the patient.
To examine the effectiveness of exercise therapy for critically ill patients in the ICU, Burtin et al.21) provided intervention for long-term ICU patients using 20 min/day of cycle ergometer training in addition to conventional exercise therapy. They reported that the intervention markedly improved the 6-min walking distance and lower limb muscle strength on discharge from the hospital. Similarly, Lida et al.22) examined critically ill patients requiring acute care, dividing them into the early (systemic inflammation from days 0 to 5) and late (inactivity after day 5) phases, and noted the necessity of considering appropriate exercise intensity levels in each phase.
In the present study, the total daily activity time in the ICU was shown to be an independent risk factor for ICU-AW. In addition, the in-bed exercise time was markedly longer in the non-ICU-AW group than in the ICU-AW group. This finding suggests the importance of extending muscle activity times at low exercise intensity levels for early-phase patients, in whom muscle proteolysis may be promoted as a result of hypermetabolism. Although only rehabilitation activities actually performed were measured in the present study, the durations of assessment/measurement, rest periods during exercise, and passive joint movements should also be measured in the actual clinical setting. Moreover, in the current medical fee calculation system, a single rehabilitation session (1 unit) lasting for 20 min is possibly insufficient to extend activity times in the ICU as a preventive measure against ICU-AW. It may be necessary to fundamentally reconsider the optimal duration of rehabilitation.
The duration of hospitalization was significantly longer and the FSS-ICU score on discharge from the ICU and the BI score on discharge from the hospital were lower in the ICU-AW group than in the non-ICU-AW group. Patients in the ICU-AW group also needed a markedly longer period to achieve walking independence.
Schweickert et al.7) reported that early exercise therapy for ICU patients was effective in improving their activities of daily living and walking distances on discharge from the hospital, while also reducing the prevalence of ICU-AD. When performing early rehabilitation for such patients, multi-professional collaboration facilitates changes of the content and timing of activities, consequently making earlier mobilization feasible. Furthermore, by extending activity times in the ICU and thereby preventing ICU-AW, it may be possible to improve a patient’s functional prognosis, represented by the period needed to achieve walking independence, and also to shorten the duration of hospitalization. The time to first rehabilitation assessment in the present study was not significantly different between the ICU-AW and non-ICU-AW groups. Compared with previous studies, the age of the participants in the present study was higher and the medical severity involved was greater. Therefore, it was initially suspected that differences in age and medical severity might have influenced the deviation of results from those of earlier studies.
The present work has some limitations. First, although the study was conducted in multiple facilities, the number of subjects and the study period were limited. Second, when measuring activity times, in- and out-of-bed exercises were separated, but the intensity level in each case was not clarified, making it difficult to generalize the results regarding the association with ICU-AW for all critically ill patients in the ICU. Third, the period needed to achieve walking independence was examined without evaluating detailed walking functions, such as the walking velocity and exercise tolerance, resulting in insufficient examination of the impact of extended activity times. Therefore, prospective studies involving larger numbers of subjects should be conducted in the future.
CONCLUSION
Analyses comparing the ICU-AW and non-ICU-AW groups indicated that the total daily activity time influenced the risk of ICU-AW. The ICU-AW group needed a markedly longer time to achieve walking independence than the non-ICU-AW group did. In terms of promoting early rehabilitation for critically ill patients in the ICU, it may be materially helpful to prevent muscular inactivity and to extend activity times to the greatest possible extent, while avoiding excessively high exercise intensities. This study demonstrated that increased activity levels in the ICU may help prevent ICU-AW, shorten the period needed to achieve walking independence, and shorten the duration of hospitalization.
Footnotes
CONFLICT OF INTEREST: The authors declare that there are no conflicts of interest.
REFERENCE
- 1.Pandharipande PP,Girard TD,Jackson JC,Morandi A,Thompson JL,Pun BT,Brummel NE,Hughes CG,Vasilevskis EE,Shintani AK,Moons KG,Geevarghese SK,Canonico A,Hopkins RO,Bernard GR,Dittus RS,Ely EW, BRAIN-ICU Study Investigators: Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306–1316. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmbhatt N,Murugan R,Milbrandt EB: Early mobilization improves functional outcomes in critically ill patients. Crit Care 2010;14:321. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowdy DW,Eid MP,Dennison CR,Mendez-Tellez PA,Herridge MS,Guallar E,Pronovost PJ,Needham DM: Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 2006;32:1115–1124. . [DOI] [PubMed] [Google Scholar]
- 4.Fried LP,Tangen CM,Walston J,Newman AB,Hirsch C,Gottdiener J,Seeman T,Tracy R,Kop WJ,Burke G,McBurnie MA, Cardiovascular Health Study Collaborative Research Group: Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 5.Schefold JC,Bierbrauer J,Weber-Carstens S: Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 2010;1:147–157. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kress JP,Pohlman AS,O’Connor MF,Hall JB: Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471–1477. . [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD,Pohlman MC,Pohlman AS,Nigos C,Pawlik AJ,Esbrook CL,Spears L,Miller M,Franczyk M,Deprizio D,Schmidt GA,Bowman A,Barr R,McCallister KE,Hall JB,Kress JP: Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe S,Morita Y,Suzuki S,Someya F: Association between early rehabilitation for mechanically ventilated ICU patients and their walking independence: a propensity score-matched analysis. J Phy Med Rehab. 2017;1:2–5. [Google Scholar]
- 9.Hodgson C,Bellomo R,Berney S,Bailey M,Buhr H,Denehy L,Harrold M,Higgins A,Presneill J,Saxena M,Skinner E,Young P,Webb S: Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-center, prospective cohort study. Crit Care 2015;19:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens RD,Marshall SA,Cornblath DR,Hoke A,Needham DM,de Jonghe B,Ali NA,Sharshar T: A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009;37(Suppl):S299–S308. . [DOI] [PubMed] [Google Scholar]
- 11.Guarneri B,Bertolini G,Latronico N: Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry 2008;79:838–841. . [DOI] [PubMed] [Google Scholar]
- 12.Herridge MS,Tansey CM,Matté A,Tomlinson G,Diaz-Granados N,Cooper A,Guest CB,Mazer CD,Mehta S,Stewart TE,Kudlow P,Cook D,Slutsky AS,Cheung AM, Canadian Critical Care Trials Group: Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–1304. . [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA,Draper EA,Wagner DP,Zimmerman JE: APACHEII: a severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 14.Katz PP: Measures of adult general functional status: The Barthel Index, Katz Index of Activities of Daily Living, Health Assessment Questionnaire (HAQ), MACTAR Patient Preference Disability Questionnaire, and Modified Health Assessment Questionnaire (MHAQ). Arthritis Care Res 2003;49:l5–l27. [Google Scholar]
- 15.Thrush A,Rozek M,Dekerlegand JL: The clinical utility of the functional status score for the intensive care unit (FSS-ICU) at a long-term acute care hospital: a prospective cohort study. Phys Ther 2012;92:1536–1545. . [DOI] [PubMed] [Google Scholar]
- 16.Ely EW,Margolin R,Francis J,May L,Truman B,Dittus R,Speroff T,Gautam S,Bernard GR,Inouye SK: Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001;29:1370–1379. . [DOI] [PubMed] [Google Scholar]
- 17.Bergeron N,Dubois MJ,Dumont M,Dial S,Skrobik Y: Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med 2001;27:859–864. . [DOI] [PubMed] [Google Scholar]
- 18.Piepoli MF,Conraads V,Corrà U,Dickstein K,Francis DP,Jaarsma T,McMurray J,Pieske B,Piotrowicz E,Schmid JP,Anker SD,Solal AC,Filippatos GS,Hoes AW,Gielen S,Giannuzzi P,Ponikowski PP: Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011;13:347–357. . [DOI] [PubMed] [Google Scholar]
- 19.Kress JP,Hall JB: ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014;370:1626–1635. . [DOI] [PubMed] [Google Scholar]
- 20.Brummel NE,Balas MC,Morandi A,Ferrante LE,Gill TM,Ely EW: Understanding and reducing disability in older adults following critical illness. Crit Care Med 2015;43:1265–1275. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burtin C,Clerckx B,Robbeets C,Ferdinande P,Langer D,Troosters T,Hermans G,Decramer M,Gosselink R: Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 2009;37:2499–2505. . [DOI] [PubMed] [Google Scholar]
- 22.Iida Y,Sakuma K: Skeletal muscle dysfunction in critical illness. Intech. 2017. : 142–168. [Google Scholar]