ABSTRACT
Background:
Magnetic resonance diffusion tensor imaging (DTI) is a new technique that evaluates neural fiber integrity within the brain. We conducted DTI in patients exhibiting aphasia during the acute stage post-infarct and investigated the neural tracts responsible by comparison with DTI data from age-matched controls.
Methods:
Fractional anisotropy (FA) maps were generated from diffusion tensor brain images obtained from aphasic patients 14−21 days following their first infarct. Tract-based spatial statistics (TBSS) analysis was then applied. In addition, regions of interest (ROIs) were set within the right and left arcuate fasciculus, and mean FA values were extracted from individual TBSS data. The ratios between FA values in the left and right hemispheres were compared with those of the control group.
Results:
The study examined 10 aphasic patients and 21 age-matched controls. Brain maps from TBSS analysis revealed significantly reduced FA in the left arcuate fasciculus of the patient group compared with that in the control group. Further ROI analyses confirmed significantly lower left/right arcuate fasciculus FA ratios in aphasic patients versus controls (median [range]: 0.955 [0.739−1.023] vs. 1.006 [0.982−1.088]; P = 0.0001 by Wilcoxon rank sum test).
Conclusions:
These results suggest that FA in the left arcuate fasciculus decreased in association with aphasia after cerebral infarct. Because patients in the acute stage have not yet experienced the neural recovery that occurs in the chronic stage, the findings indicate that the left arcuate fasciculus is a crucial neural structure in aphasia.
Keywords: assessment, language, outcome, prognosis, stroke
INTRODUCTION
Aphasia is a common symptom in the acute stage following cerebral infarct.1) Recent neuroimaging studies have revealed many aspects of the neuropathology of aphasia.2,3) Aphasia is often associated with lesions within language-related cortical regions such as Wernicke’s and Broca’s areas or within the neural fiber networks connecting these regions.4,5) However, the contributions of specific tracts remain unclear, in part because white matter tract disruption cannot be detected by classical neuroimaging modalities. In contrast to classical neuroimaging techniques, magnetic resonance diffusion tensor imaging (DTI) enables the assessment of neural fiber integrity in vivo.6) Consequently, DTI may be a useful tool for identifying the subcortical white matter structures responsible for aphasia.
Most recent DTI studies of aphasia caused by cerebral infarct have investigated patients in the chronic stage.7,8,9,10) However, patients with aphasia show various patterns of recovery during the months and years after stroke,11,12) partially resulting from compensation and/or neural plasticity among language-related and other brain areas.13,14,15) Accordingly, DTI studies of patients in the chronic phase may not be sufficient to fully describe the neuropathology of aphasia. In this study, we examined patients with aphasia caused by cerebral infarct in the acute stage by using DTI.16) To determine the lesions responsible, we applied tract-based spatial statistics (TBSS)17) in reference to age-matched control subjects.
METHODS
Patients and Controls
Patients admitted to Nishinomiya Kyoritsu Neurosurgical Hospital for cerebral infarct between April 2010 and September 2012 were entered into the analytical database. The patients were transferred to our hospital soon after stroke onset and were diagnosed as having cerebral infarct by diffusion-weighted magnetic resonance imaging (DWI). Details of the DWI acquisition sequence are published elsewhere.18) During hospitalization, patients underwent conservative treatments such as medication (e.g., anticoagulant or antiplatelet agents) and rehabilitation consisting of physical therapy, occupational therapy, and speech therapy for a combined daily total of up to 180 min.19) The diagnosis of aphasia was based on clinical observation in consultation with board-certificated neurologists, neurosurgeons, speech-language-hearing pathologists, and a physiatrist. Subtypes of aphasia (e.g., total, motor, sensory, conduction, and transcortical) were further diagnosed in reference to the patients’ abilities with respect to spontaneous speech, hearing/comprehension, naming, and repetition.20) Patients with aphasia sometimes exhibit coexisting limb apraxia.21) Because the neural deficits underlying these comorbidities could complicate analysis, we carefully observed patients during daily activities at the bedside. Patients who could not use a spoon and/or chopsticks for eating or a toothbrush and/or comb for grooming were regarded as having limb apraxia. Patients (or relatives when necessary) provided informed consent for the study, and the study protocol was approved by the hospital’s institutional review board.
To minimize the variability arising from differences in pre-onset health status and lesion site, the study population was limited to first-ever stroke patients with supratentorial cerebral infarct who were able to walk unaided and were functionally independent in daily activities before stroke.22) Because of MRI safety restrictions, patients with implanted metal items (e.g., artificial pacemakers) were excluded. Patients who subsequently required acute medical services (for recurrence of stroke, angina pectoris, or other comorbid conditions) were also excluded from our final analysis.
To characterize the abnormalities in patient DTI data, we sampled control data collected during our previous DTI studies.22,23) The details of the control group are reported elsewhere.23) In brief, the control group consisted of 21 outpatients (age > 60 years) with no history of neurological disorders who presented with chief complaints of headache, dizziness, or both, and whose symptoms were alleviated within 2 weeks without a conclusive diagnosis.
DTI Acquisition
DTI data were obtained at day 14–21 after admission using a 3-T MRI scanner (Trio; Siemens AG, Erlangen, Germany) with a 32-channel head coil. The timing of DTI acquisition was scheduled based on previous studies reporting that reliable DTI signal changes occur nearly 2 weeks after stroke.24,25) The acquisition parameters used were the single-shot echo-planar imaging sequence, an echo time of 83 ms, and a repetition time of 7000 ms. Other details on DTI acquisition were reported previously.23) In brief, the DTI protocol acquired 12 images with noncollinear diffusion gradients (b = 1000 s/mm2) and one nondiffusion-weighted image (b = 0 s/mm2). A total of 64 axial slices were obtained from each patient. The field of view was 230.4 mm × 230.4 mm, the acquisition matrix was 128 × 128, and the slice thickness was 3 mm with no gaps.
Image Processing
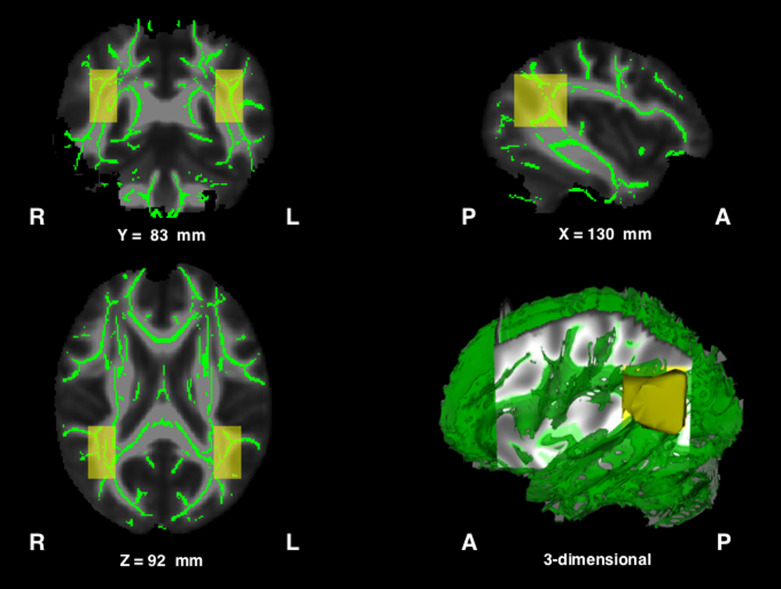
The brain image analysis package FSL was used for this study,26) and details of the image processing procedures were described in our previous publication.23) In brief, diffusion tensor images were corrected for motion and eddy current distortions, and extracerebral structures were excluded. Tensor diffusion and fractional anisotropy (FA) values were calculated for each patient. FA is often used as an index of neural fiber integrity in DTI studies.25) TBSS was performed to identify brain regions exhibiting neural fiber degeneration associated with cerebral infarct.17) TBSS facilitates the study of cerebral white matter integrity by using intrinsic anisotropic properties to project the FA of local tract structures onto a virtual skeleton, thereby providing an alignment-invariant tract representation of the median part of the tract. FA maps for each participant were registered into a standard brain template included in the FSL suite (FMRIB58_FA) using the nonlinear spatial transformation tool. A mean FA image was then compiled by averaging aligned FA maps from all participants in each group. To generate a mean FA skeleton representing the centers of all tracts common to the group, the map threshold was set for voxels showing FA values ≥ 0.2. Aligned FA data for each participant were projected onto the standard skeletonized FA image by searching the area around the skeleton in the direction perpendicular to each tract to find the highest local FA value, and assigning this value to the skeleton. A mean FA image was then compiled by averaging aligned FA maps from each participant (shown in Fig. 1).
Fig. 1.
Mean fractional anisotropy skeleton (green) and bilateral ROIs (yellow). The ROI in the left hemisphere ranged from −45 to −30 mm in the x-axis, −66 to −36 mm in the y-axis, and from 80 to 110 mm in the standard brain space. The ROI in the right hemisphere was set at the mirror coordinates of the left.
Voxel-wise spatial statistical analysis comparing patients with controls was conducted by permutation testing (n = 5000) using the FSL “randomize” program. Thresholding was carried out using threshold-free cluster enhancement, and clusters were assessed for significance at P < 0.01, fully corrected for multiple comparisons across space. To confirm that the areas of the voxels that exhibited a statistically significant FA decrease were consistent with individual brain images, the areas defined in the mean FA skeleton were inversely projected onto each individual skeletonized FA image defined in the standard brain space.
To quantitatively assess neural degeneration (FA decrease) within the brain, the skeletonized FA images of each patient were sampled using the region-of-interest (ROI) methodology.23) Previous DTI studies of aphasia caused by stroke reported that FA decreased in the left arcuate fasciculus and adjacent white matter fibers.5,27,28,29,30,31,32) In accordance with these previous findings, we set ROIs to include the arcuate fasciculus as defined in the skeletonized FA images (shown in Fig. 1).In each subject, the FA values from nonzero voxels were averaged within the right and left ROIs and the left/right FA ratio was then calculated. The obtained FA ratios from the patient group were statistically compared with those from the control group by the Wilcoxon rank sum test, with P < 0.01 considered significant. All statistical analyses were performed using the JMP software package (SAS Institute, Cary, NC, USA).
RESULTS
During the study period, we enrolled 259 first-ever cerebral infarct patients who were functionally independent pre-onset. Of these, we were able to acquire DTI data from 170 patients (this number was limited by MRI scanner availability). Of these, 11 patients showed aphasia with only very mild hemiparesis as indicated by a manual muscle-strength test score > 4 for both upper and lower extremities on the affected side. Of these 11 patients, 1 showed signs of apraxia (e.g., the inability to use a toothbrush and comb) in addition to aphasia. To focus on aphasia only, we excluded this subject from our final database. Consequently, we examined 10 subjects whose main clinical manifestation was aphasia. All 10 subjects were right-handed with infarct lesions in the left hemisphere.
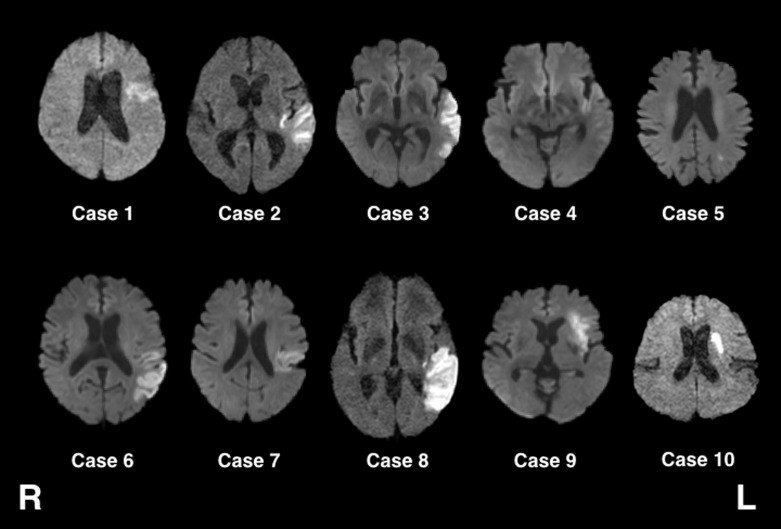
Table 1 presents the demographic data and results for both the patient and control groups. Although the patients were slightly younger than the controls, the difference was not statistically significant (t-test, P = 0.170). Figure 2 shows DWI brain images for all 10 patients. There was substantial variability in lesion location within the left hemisphere, with three patients exhibiting lesions in the frontal lobe and underlying white matter (cases 1, 9, and 10), and four with lesions in the temporal lobe (cases 2, 3, 4, and 8).
Table 1. Patient profiles.
| Case | Age (years) |
Sex | Group | Type of aphasia | Etiology | Left FA | Right FA | L/R FA Ratio |
| 1 | 70 | Female | Aphasia | Motor | Atherothrombotic | 0.455 | 0.462 | 0.985 |
| 2 | 75 | Male | Aphasia | Sensory | Cardioembolic | 0.377 | 0.437 | 0.862 |
| 3 | 76 | Male | Aphasia | Sensory | Cardioembolic | 0.409 | 0.454 | 0.900 |
| 4 | 65 | Male | Aphasia | Sensory | Cardioembolic | 0.433 | 0.453 | 0.955 |
| 5 | 80 | Male | Aphasia | Transcortical | Atherothrombotic | 0.422 | 0.431 | 0.979 |
| 6 | 67 | Male | Aphasia | Sensory | Cardioembolic | 0.461 | 0.483 | 0.955 |
| 7 | 65 | Male | Aphasia | Motor | Atherothrombotic | 0.425 | 0.461 | 0.922 |
| 8 | 70 | Female | Aphasia | Sensory | Cardioembolic | 0.324 | 0.438 | 0.739 |
| 9 | 70 | Male | Aphasia | Motor | Atherothrombotic | 0.469 | 0.483 | 0.971 |
| 10 | 62 | Male | Aphasia | Motor | Atherothrombotic | 0.409 | 0.399 | 1.023 |
| 11 | 61 | Female | Control | - | - | 0.467 | 0.465 | 1.003 |
| 12 | 72 | Female | Control | - | - | 0.456 | 0.461 | 0.991 |
| 13 | 80 | Female | Control | - | - | 0.488 | 0.486 | 1.004 |
| 14 | 64 | Female | Control | - | - | 0.465 | 0.458 | 1.014 |
| 15 | 77 | Male | Control | - | - | 0.436 | 0.437 | 0.998 |
| 16 | 67 | Male | Control | - | - | 0.491 | 0.452 | 1.088 |
| 17 | 64 | Female | Control | - | - | 0.493 | 0.474 | 1.042 |
| 18 | 78 | Female | Control | - | - | 0.443 | 0.443 | 1.002 |
| 19 | 79 | Female | Control | - | - | 0.458 | 0.432 | 1.061 |
| 20 | 86 | Male | Control | - | - | 0.449 | 0.448 | 1.002 |
| 21 | 80 | Female | Control | - | - | 0.464 | 0.461 | 1.006 |
| 22 | 82 | Male | Control | - | - | 0.494 | 0.497 | 0.994 |
| 23 | 75 | Female | Control | - | - | 0.492 | 0.460 | 1.068 |
| 24 | 79 | Female | Control | - | - | 0.479 | 0.462 | 1.037 |
| 25 | 61 | Male | Control | - | - | 0.530 | 0.512 | 1.036 |
| 26 | 73 | Female | Control | - | - | 0.428 | 0.435 | 0.984 |
| 27 | 70 | Male | Control | - | - | 0.460 | 0.461 | 0.997 |
| 28 | 64 | Female | Control | - | - | 0.512 | 0.495 | 1.035 |
| 29 | 79 | Male | Control | - | - | 0.472 | 0.468 | 1.009 |
| 30 | 84 | Female | Control | - | - | 0.445 | 0.453 | 0.982 |
| 31 | 76 | Male | Control | - | - | 0.488 | 0.466 | 1.045 |
FA, fractional anisotropy. Patient 4 underwent intravenous administration of tissue plasminogen activator.
Fig. 2.
Diffusion-weight MRI images of all 10 patients included in this study.
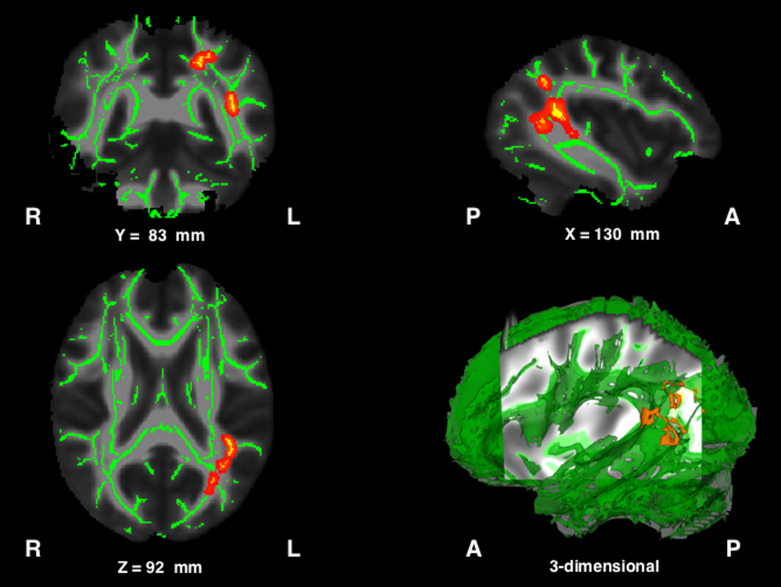
Figure 3 presents the results obtained from TBSS analyses. The images show voxels with significantly lower FA values in the left arcuate fasciculus and the posterior part of the left superior longitudinal tract. However, not all patients showed high-intensity signals (indicative of lesions) in these areas on DWI images (cases 1, 9, and 10 in Fig. 1).
Fig. 3.
Results obtained from tract-based spatial statistics. Red–yellow areas indicate voxels where FA values were significantly lower in patients than in controls. To aid visualization, regions showing significantly lower FA values (P < 0.01) were thickened using the “tbss_fill” command implemented in FSL.
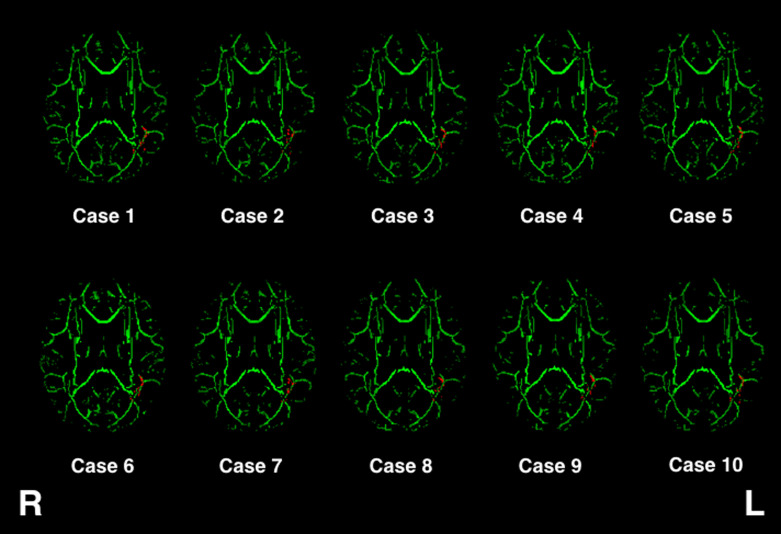
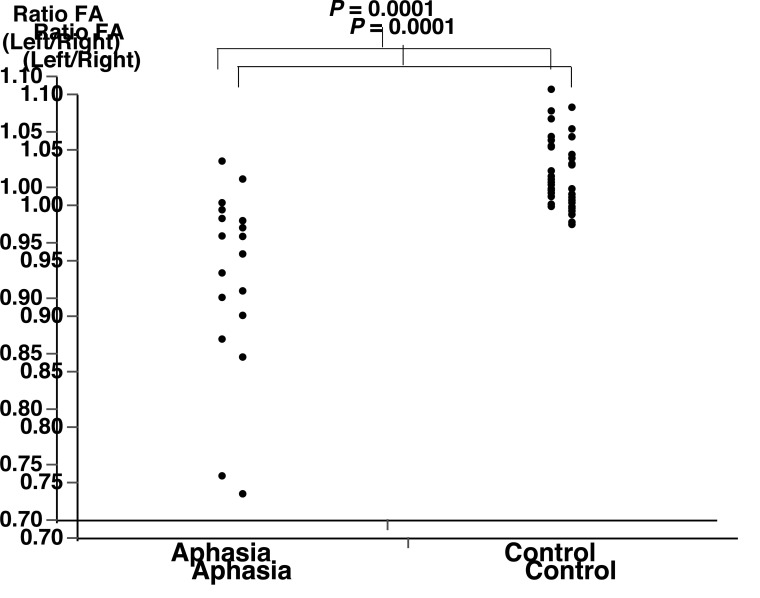
Figure 4 presents the skeletonized FA map projected onto the standard brain template for each subject. The areas with voxels of significantly decreased FA (shown in Fig. 3) were inversely projected onto each subject’s data, suggesting that TBSS was appropriately performed. In this study, we further assessed the FA decreases quantitatively by ROI measurements (Fig. 5). The control subjects showed larger FA values in the left hemisphere than in the right hemisphere (FA ratio > 1). In contrast, patients showed lower FA values in the left hemisphere than in the right hemisphere. The decrease in FA was statistically significant in the patient group compared with the control group (P = 0.0001).
Fig. 4.
FA skeleton of individual cases. The red–yellow areas from Fig. 3 (shown in red) were inversely projected onto individual images.
Fig. 5.
Scatter plots of the left/right FA ratio. An FA ratio > 1 indicates left dominance.
DISCUSSION
Neural structures related to language function are widely distributed throughout the brain, including the well-known Wernicke’s area for speech comprehension in the caudal temporal lobe and Broca’s area for speech production in the frontal lobe. Language comprehension and appropriate speech generation depend on the neural fiber networks connecting specialized motor and sensory cortical areas.33) Thus, evaluation of fiber integrity is critically important for the mechanistic study and diagnosis of aphasia. For this purpose, we used DTI for analysis of white matter tracts in patients with aphasia following cerebral infarct. In the analyses, TBSS detected significantly reduced FA values in the left arcuate fasciculus connecting Wernicke’s and Broca’s areas. Further ROI analysis of individual subjects confirmed that reduced FA in the left arcuate fasciculus was consistent across the aphasia patient group, implying that disruption of the arcuate fasciculus in the language-dominant hemisphere is a critical pathogenic mechanism for aphasia.
MRI-DWI, which assesses the motion of water molecules associated with cytotoxic edema, is the most powerful tool currently available to detect ischemic regions within the brain. In Japan, where MRI scanners are widely available, DWI is almost routinely applied in the emergency unit for suspected cerebral infarct. However, DWI does not allow the evaluation of neural fiber integrity. In addition, degeneration of neural fibers (Wallerian degeneration) often occurs during the weeks post-onset in areas separate from the original infarct. This delayed axon degeneration limits the diagnostic applicability of DWI for patients with higher brain dysfunctions such as aphasia associated with cerebral infarct.8) DTI may compensate for such drawbacks of DWI. Based on a methodology similar to that used in the present study, we previously conducted a series of studies demonstrating the diagnostic applicability of DTI for patients with hemiparesis.18,22) The present findings further suggest the diagnostic applicability of DTI for aphasia.30,34,35,36)
Previous DTI studies in normal subjects reported that fiber connection is greater in the left arcuate fasciculus than in the right,5,37) implying left brain dominance for language function in humans. To reevaluate this issue, we set ROIs (Fig. 1) in the left and right hemispheres and assessed the FA ratio; the findings were consistent with previous studies.5,37) In sharp contrast, aphasic patients lost the FA dominance of the left hemisphere (FA ratio < 1). The left/right FA ratio for the arcuate fasciculus may thus serve as a biomarker for the neural damage associated with aphasia32) in the early post-stroke stage.
The posterior part of the superior longitudinal fasciculus, which lies adjacent to the arcuate fasciculus, is also believed to be involved in language function.38,39) The present FA mapping findings showing reduced integrity in this pathway are consistent with this notion. In addition to the arcuate fasciculus and the superior longitudinal fasciculus, recent studies have implicated the uncinate fasciculus and the inferior longitudinal fasciculus in language function.40,41) Moreover, a recent case report found that a patient with severe damage to the left arcuate fasciculus exhibited good recovery from aphasia.42) Accordingly, the contribution of the arcuate fasciculus to language function should be interpreted in the context of a wider neural network including the aforementioned structures.
There are several limitations to the current study. Our sample was restricted to cerebral infarct patients. Although patients with intracerebral hemorrhage also often exhibit aphasia, we excluded such patients because DTI data obtained in the acute stage may be susceptible to artifacts from hemosiderin.43) In addition, the final number of patients was small (n = 10). In the preliminary stage of this study, we included patients with aphasia and other symptoms such as hemiparesis. However, the analyses became overly complex. To keep the analytical procedure simple, we focused on pure aphasic cases only. Despite the small sample size, significant differences were found between the patients and controls that were in agreement with previous studies. However, the sample size precluded analyses of clinical subclasses or subgroups stratified by aphasia severity.
ACKNOWLEDGEMENT
This study was partially supported by a Grant-in-Aid for Challenging Exploratory Research from the Japan Society for the Promotion of Science (JSPS KAKENHI [JP15K12590]).
This publication was supported by JSPS KAKENHI Grant Number JP16HP1002.
Footnotes
CONFLICT OF INTEREST: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Kauhanen ML,Korpelainen JT,Hiltunen P,Määttä R,Mononen H,Brusin E,Sotaniemi KA,Myllylä VV: Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis 2000;10:455–461. [DOI] [PubMed] [Google Scholar]
- 2.Meinzer M,Harnish S,Conway T,Crosson B: Recent developments in functional and structural imaging of aphasia recovery after stroke. Aphasiology 2011;25:271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smits M,Visch-Brink EG,van de Sandt-Koenderman ME,van der Lugt A: Advanced magnetic resonance neuroimaging of language function recovery after aphasic stroke: a technical review. Arch Phys Med Rehabil 2012;93(Suppl):S4–S14. [DOI] [PubMed] [Google Scholar]
- 4.Catani M,Jones DK,ffytche DH: Perisylvian language networks of the human brain. Ann Neurol 2005;57:8–16. [DOI] [PubMed] [Google Scholar]
- 5.Catani M,Mesulam M: The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 2008;44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bihan D,Mangin JF,Poupon C,Clark CA,Pappata S,Molko N,Chabriat H: Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–546. [DOI] [PubMed] [Google Scholar]
- 7.Breier JI,Hasan KM,Zhang W,Men D,Papanicolaou AC: Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol 2008;29:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonilha L,Rorden C,Fridriksson J: Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke 2014;45:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geva S,Correia MM,Warburton EA: Contributions of bilateral white matter to chronic aphasia symptoms as assessed by diffusion tensor MRI. Brain Lang 2015;150:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleichgerrcht E,Kocher M,Nesland T,Rorden C,Fridriksson J,Bonilha L: Preservation of structural brain network hubs is associated with less severe post-stroke aphasia. Restor Neurol Neurosci 2015;34:19–28. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen PM,Vinter K,Olsen TS: Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis 2004;17:35–43. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton RH,Chrysikou EG,Coslett B: Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang 2011;118:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y,Vikingstad EM,George KP,Johnson AF,Welch KM: Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke 1999;30:2331–2340. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez B,Cardebat D,Demonet JF,Joseph PA,Mazaux JM,Barat M,Allard M: Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke 2004;35:2171–2176. [DOI] [PubMed] [Google Scholar]
- 15.Szaflarski JP,Allendorfer JB,Banks C,Vannest J,Holland SK: Recovered vs. not-recovered from post-stroke aphasia: the contributions from the dominant and non-dominant hemispheres. Restor Neurol Neurosci 2013;31:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forkel SJ,Thiebaut de Schotten M,Dell’Acqua F,Kalra L,Murphy DG,Williams SC,Catani M: Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain 2014;137:2027–2039. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM,Jenkinson M,Johansen-Berg H,Rueckert D,Nichols TE,Mackay CE,Watkins KE,Ciccarelli O,Cader MZ,Matthews PM,Behrens TE: Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 18.Koyama T,Marumoto K,Miyake H,Domen K: Relationship between diffusion tensor fractional anisotropy and motor outcome in patients with hemiparesis after corona radiata infarct. J Stroke Cerebrovasc Dis 2013;22:1355–1360. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara Y,Yanagihara T,Abe K,Yoshimine T,Fujinaka T,Chuma T,Ochi F,Nagayama M,Ogawa A,Suzuki N,Katayama Y,Kimura A,Liu M,Eto F: VII. Rehabilitation. J Stroke Cerebrovasc Dis 2011;20(Suppl):S145–S180. [DOI] [PubMed] [Google Scholar]
- 20.Brust JC,Shafer SQ,Richter RW,Bruun B: Aphasia in acute stroke. Stroke 1976;7:167–174. [DOI] [PubMed] [Google Scholar]
- 21.Smania N,Aglioti SM,Girardi F,Tinazzi M,Fiaschi A,Cosentino A,Corato E: Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurology 2006;67:2050–2052. [DOI] [PubMed] [Google Scholar]
- 22.Koyama T,Marumoto K,Miyake H,Domen K: Relationship between diffusion tensor fractional anisotropy and long-term motor outcome in patients with hemiparesis after middle cerebral artery infarction. J Stroke Cerebrovasc Dis 2014;23:2397–2404. [DOI] [PubMed] [Google Scholar]
- 23.Koyama T,Marumoto K,Domen K,Miyake H: White matter characteristics of idiopathic normal pressure hydrocephalus: a diffusion tensor tract-based spatial statistic study. Neurol Med Chir (Tokyo) 2013;53:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomalla G,Glauche V,Weiller C,Röther J: Time course of Wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry 2005;76:266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C,Zhu C,Zhang Y,Chen H,Qin W,Wang M,Li K: A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage 2009;47:451–458. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M,Beckmann CF,Behrens TE,Woolrich MW,Smith SM: FSL. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 27.Hong JH,Kim SH,Ahn SH,Jang SH: The anatomical location of the arcuate fasciculus in the human brain: a diffusion tensor tractography study. Brain Res Bull 2009;80:52–55. [DOI] [PubMed] [Google Scholar]
- 28.Hosomi A,Nagakane Y,Yamada K,Kuriyama N,Mizuno T,Nishimura T,Nakagawa M: Assessment of arcuate fasciculus with diffusion-tensor tractography may predict the prognosis of aphasia in patients with left middle cerebral artery infarcts. Neuroradiology 2009;51:549–555. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH,Lee DG,You H,Son SM,Cho YW,Chang MC,Lee J,Jang SH: The clinical application of the arcuate fasciculus for stroke patients with aphasia: a diffusion tensor tractography study. NeuroRehabilitation 2011;29:305–310. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH,Jang SH: Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. AJNR Am J Neuroradiol 2013;34:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tak HJ,Jang SH: Relation between aphasia and arcuate fasciculus in chronic stroke patients. BMC Neurol 2014;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanova MV,Isaev DY,Dragoy OV,Akinina YS,Petrushevskiy AG,Fedina ON,Shklovsky VM,Dronkers NF: Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex 2016. [DOI] [PubMed] [Google Scholar]
- 33.Song X,Dornbos D 3rd,Lai Z,Zhang Y,Li T,Chen H,Yang Z: Diffusion tensor imaging and diffusion tensor imaging-fibre tractograph depict the mechanisms of Broca-like and Wernicke-like conduction aphasia. Neurol Res 2011;33:529–535. [DOI] [PubMed] [Google Scholar]
- 34.Wang J,Marchina S,Norton AC,Wan CY,Schlaug G: Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci 2013;7:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y,Wang C,Zhao X,Chen H,Han Z,Wang Y: Diffusion tensor imaging depicting damage to the arcuate fasciculus in patients with conduction aphasia: a study of the Wernicke-Geschwind model. Neurol Res 2010;32:775–778. [DOI] [PubMed] [Google Scholar]
- 36.Sommer M,Koch MA,Paulus W,Weiller C,Büchel C: Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet 2002;360:380–383. [DOI] [PubMed] [Google Scholar]
- 37.Glasser MF,Rilling JK: DTI tractography of the human brain’s language pathways. Cereb Cortex 2008;18:2471–2482. [DOI] [PubMed] [Google Scholar]
- 38.Davtian M,Ulmer JL,Mueller WM,Gaggl W,Mulane MP,Krouwer HG: The superior longitudinal fasciculus and speech arrest. J Comput Assist Tomogr 2008;32:410–414. [DOI] [PubMed] [Google Scholar]
- 39.Cloutman LL,Binney RJ,Morris DM,Parker GJ,Lambon Ralph MA: Using in vivo probabilistic tractography to reveal two segregated dorsal ‘language-cognitive’ pathways in the human brain. Brain Lang 2013;127:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinno R,Ohta S,Muragaki Y,Maruyama T,Sakai KL: Differential reorganization of three syntax-related networks induced by a left frontal glioma. Brain 2014;137:1193–1212. [DOI] [PubMed] [Google Scholar]
- 41.Smits M,Jiskoot LC,Papma JM: White matter tracts of speech and language. Semin Ultrasound CT MR 2014;35:504–516. [DOI] [PubMed] [Google Scholar]
- 42.Kwon HG,Jang SH: Excellent recovery of aphasia in a patient with complete injury of the arcuate fasciculus in the dominant hemisphere. NeuroRehabilitation 2011;29:401–404. [DOI] [PubMed] [Google Scholar]
- 43.Koyama T,Tsuji M,Nishimura H,Miyake H,Ohmura T,Domen K: Diffusion tensor imaging for intracerebral hemorrhage outcome prediction: comparison using data from the corona radiata/internal capsule and the cerebral peduncle. J Stroke Cerebrovasc Dis 2013;22:72–79. [DOI] [PubMed] [Google Scholar]