ABSTRACT
Objectives:
Diffusion tensor fractional anisotropy (FA) in the corticospinal tracts has been used to assess the long-term outcome in stroke patients. Patient age and the type of stroke may also affect outcomes. In this study, we investigated the associations of age, type of stroke, and FA in the ipsilesional and contralesional cerebral peduncles with stroke outcomes.
Methods:
This study involved 80 patients with stroke (40 hemorrhagic, 40 ischemic) that we had investigated previously. Diffusion tensor FA images were obtained between 14 and 21 days post-stroke. FA values in the ipsilesional and contralesional cerebral peduncles were extracted and their ratio (rFA) was calculated. Outcome was assessed using the Brunnstrom stage, the motor component of the Functional Independence Measure (FIM-motor) at discharge, and the length of stay until discharge from rehabilitation. Using forward stepwise multivariate regression, we assessed the associations of rFA, contralesional FA, age, and type of stroke with outcome measures.
Results:
rFA and contralesional FA were included in the final model for the Brunnstrom stage in the upper limbs. There was a strong association between hemorrhagic stroke and poorer lower extremity function. rFA, contralesional FA, and age were included in the final model for FIM-motor and length of stay. The effect of rFA on all outcome measures was stronger than that of contralesional FA. The effect of age on FIM-motor was as strong as that of rFA.
Conclusions:
Neural damage in the corticospinal tracts (indicated by rFA) had the strongest effect on outcome measures, whereas the level of disability (measured by FIM-motor) was associated with a broader range of factors, including age.
Keywords: correlation, hematoma, ischemia, outcome, prognosis
INTRODUCTION
The prediction of outcome is critically important when planning appropriate rehabilitation for stroke patients.1,2,3) Magnetic resonance imaging, transcranial magnetic stimulation, magnetoencephalography, and other modalities have been used for rehabilitation planning.4) A recent systematic review suggested that magnetic resonance diffusion tensor imaging (DTI) is potentially one of the most useful techniques to predict poststroke motor recovery.5) Fractional anisotropy (FA) is the most frequently used DTI-derived parameter,6) and several studies have found an association between decreased FA in the corticospinal tracts and poorer outcome.7,8,9,10)
A variety of factors have been suggested to affect stroke outcome. Some previous studies have identified age as the strongest predictor of stroke outcome,11,12) whereas others have indicated that the type of stroke (hemorrhagic or ischemic) is also important.13,14) Furthermore, recent studies have suggested a possible contribution of the neural integrity of the corticospinal tracts in the contralesional intact hemisphere.15,16) However, there is little evidence available on the relative contributions of these potential factors in predicting stroke outcome. Therefore, the aim of this study was to assess, by multivariate regression analysis, the effects of age, type of stroke, and neural integrity of the corticospinal tracts in the ipsilesional and contralesional hemispheres on the long-term outcome of stroke.
METHODS
Study Samples
The work presented here is an extension of earlier studies by our research group17,18,19) and is based on further analysis of previously reported data.19) The study population consisted of 80 stroke patients (40 with hemorrhagic lesions, 40 with ischemic lesions).17,18) Patient demographics (e.g., age, lesion site, severity of hemiparesis) have been reported elsewhere.17,18) The study protocol was approved by the Hyogo College of Medicine Ethics Committee (No. 2454).
Most patients were transferred to our hospital soon after the onset of symptoms. The diagnosis of stroke was confirmed on computed tomography and/or diffusion-weighted magnetic resonance images. All patients underwent physical, occupational, and speech therapy for up to 180 min daily, in line with the recommendations of the Japanese Guidelines for the Management of Stroke.20) The study population was limited to patients with first-ever stroke with hemorrhagic or ischemic supratentorial lesions who had been able to walk unaided and had been functionally independent in activities of daily living (ADL) before the stroke. This limitation was imposed to minimize the effects of variability in pre-stroke health status and lesion site. To minimize the effects of variability resulting from differences in the rehabilitation regimen, we used only data for patients who were transferred to our affiliated long-term rehabilitation facility (Nishinomiya Kyoritsu Rehabilitation Hospital) for at least 1 month of inpatient rehabilitation.
Acquisition of Diffusion Tensor Images
DTI was performed between 14 and 21 days post-admission using a 3.0-T magnetic resonance scanner (Trio; Siemens AG, Erlangen, Germany) with a 32-channel head coil. The DTI acquisition protocol has been described in detail elsewhere.21) Following this protocol, a single-shot echo-planar imaging sequence was used to obtain 1 non-diffusion-weighted image (b=0 s/mm2) and 12 images with noncollinear diffusion gradients (b=1000 s/mm2) for a total of 64 axial slices per patient (field of view, 230.4 mm × 230.4 mm; acquisition matrix, 128 × 128; gapless slice thickness, 3 mm; echo time, 83 ms; and repetition time, 7000 ms).
Outcome Measures
The functional status of the extremities was assessed using the Brunnstrom staging system (BRS),22) which is widely used by Japanese rehabilitation therapists.20) The BRS assesses stroke-related motor impairment (hemiparesis) of the upper and lower extremities. The recovery of affected extremities was evaluated using flexion and extension synergy patterns on a 6-point scale (1, very poor; 6, normal). BRS is widely used for functional evaluation of the lower extremity as well as the proximal (shoulder/elbow/forearm) and distal (hand/finger) components of the upper extremity, and its reliability and validity have been confirmed.23) Scores at discharge from the rehabilitation facility were entered into the analysis database.
We also obtained scores for the motor component of the Functional Independence Measure (FIM-motor).24) This measure comprises a battery of tests used to evaluate stroke patients during rehabilitation.20) It consists of the following 13 items, each graded on a 7-point scale (1, total dependence; 7, complete independence): eating, grooming, bathing, dressing the upper body, dressing the lower body, toileting, bladder management, bowel management, transfers to a bed/chair/wheelchair, transfers to a toilet, transfers to a tub/shower, walking/propelling a wheelchair, and using stairs. The total score for these items is frequently employed as an index of independence in ADL (scale range, 13–91). Patients were considered eligible for discharge from the rehabilitation hospital when there was no further increase in the FIM-motor score. The BRS stage and FIM-motor score were assessed at monthly intervals, and the data were collected at discharge from the facility. The length of hospital stay (LOS) was recorded in all cases.
Image Processing
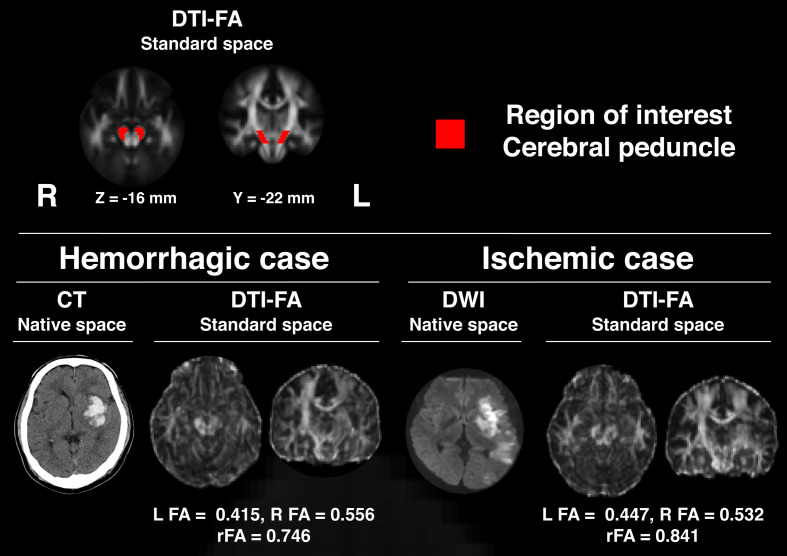
We processed all images using the FSL brain image analysis package (version 6.0.1, Oxford Centre for Functional MRI of the Brain, Oxford, UK).25) The methods used have been described elsewhere.21) In summary, the DTI data were initially corrected for motion and eddy current distortions via alignment of later images to the first image (b=0 s/mm2). Extracerebral regions were then removed from the images. Next, regional FA values for the brain were calculated to generate an FA brain map. This map was subsequently converted to a standard stereotaxic space. Regions of interest (ROIs) were set within the corticospinal tracts in the right and left cerebral peduncles (Fig. 1). These regions were selected because of the potential for magnetic resonance susceptibility effects of supratentorial stroke lesions to interfere with the validity of DTI data.26) The ROIs for the cerebral peduncles were set with reference to the International Consortium for Brain Mapping DTI-8127) (Fig. 1). FA values were calculated for the left and right ROIs with subsequent estimation of mean values for single voxels. The ratio of FA values between the ipsilesional and contralesional hemispheres (rFA) was calculated as the index of neural degeneration in the corticospinal tracts for each patient (Fig. 1).5,28)
Fig. 1.
Regions of interest in cerebral peduncles and examples of DTI-FA images from a patient with hemorrhagic stroke and a patient with ischemic stroke transformed into the standard space. CT, computed tomography; DWI, diffusion-weighted imaging.
Statistical Analysis
Data were analyzed by multivariate regression, and separate analyses were performed for the BRS subsets, FIM-motor, and LOS. rFA, contralesional FA, age, and type of stroke were set as explanatory variables in all analyses. A dummy variable for the type of stroke took the value of 1 for hemorrhagic stroke and 0 for ischemic stroke. The parameters included in the final regression models were identified by forward stepwise selection (P <0.1). Spearman’s correlation test was performed for all possible pairings of the four explanatory variables to identify any multicollinearity. All statistical analyses were performed using the JMP software package (SAS Institute Inc., Cary, NC, USA). A P-value <0.05 was considered statistically significant.
RESULTS
Patient demographics and clinical characteristics are shown in Table 1. The study population included 80 patients (40 with hemorrhagic stroke, 40 with ischemic stroke; 47 men, 33 women). The lesion was in the right hemisphere in 37 cases and in the left in 43. Patient demographics showed an almost normal distribution and were appropriate for regression analysis.
Table 1. Patient demographics and clinical characteristics.
| Type of stroke (hemorrhagic/ischemic) | 40/40 |
| Sex (male/female) | 47/33 |
| Hemisphere affected (right/left) | 37/43 |
| Age, years | 64.4 ± 12.4 |
| rFA | 0.880 ± 0.103 |
| Contralesional FA | 0.574 ± 0.031 |
| FIM-motor | 76.5 ± 8.2 |
| LOS, days | 140.9 ± 54.2 |
| BRS S/E/F | 4.1 ± 1.6 |
| BRS H/F | 3.9 ± 1.7 |
| BRS L/E | 4.6 ± 1.2 |
Data are shown as the mean and standard deviation or as the number, as appropriate.
S/E/F, shoulder, elbow, and forearm; H/F, hand and finger; L/E, lower extremity.
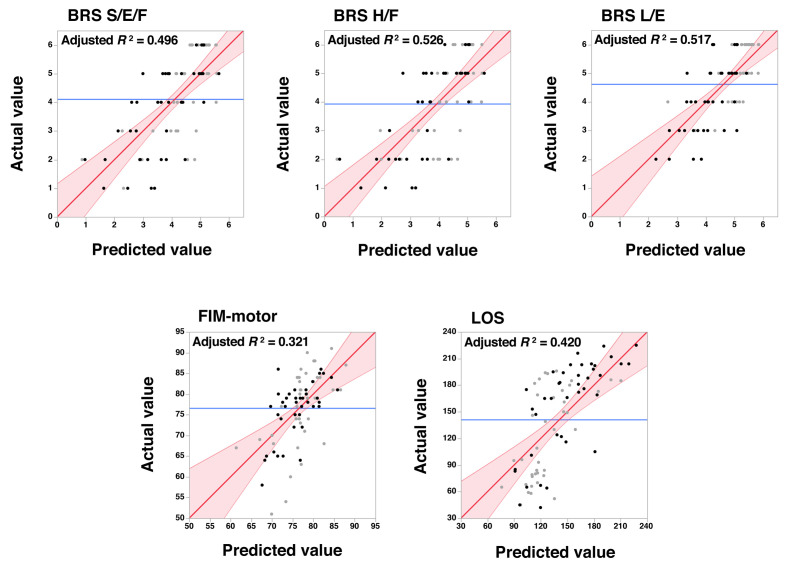
Table 2 shows the results of multivariate regression analyses for the BRS subsets. The rFA and contralesional FA values for the shoulder/elbow/forearm and hand/finger components were taken into the final models. The t-values indicated that more of the variability in BRS outcome data was accounted for by rFA than by the contralesional FA. When the type of stroke was added to rFA and contralesional FA in the final model, the BRS scores suggested a strong association between hemorrhagic stroke and poorer lower extremity function. Age was not included in the final model. The upper row in Fig. 2 shows the relationship between the observed and predicted values derived from the parameter estimates shown in Table 2.
Table 2. Results of multivariate regression analyses of BRS data.
| BRS S/E/F | BRS H/F | BRS L/E | ||||||||||
| Estimate | SE | t | P | Estimate | SE | t | P | Estimate | SE | t | P | |
| Age | - | - | - | - | - | - | - | - | - | - | - | - |
| rFA | 10.40 | 1.23 | 8.48 | <0.001 | 11.17 | 1.24 | 8.95 | <0.001 | 6.98 | 0.96 | 7.27 | <0.001 |
| Contralesional FA | 8.06 | 3.94 | 2.05 | 0.044 | 9.43 | 4.01 | 2.35 | 0.021 | 6.00 | 2.91 | 2.06 | <0.001 |
| Type of stroke | - | - | - | - | - | - | - | - | −0.47 | 0.20 | −2.39 | 0.019 |
| Intercept | −9.69 | 2.43 | −3.99 | <0.001 | −11.32 | 2.47 | −4.58 | <0.001 | −4.75 | 1.84 | −2.58 | 0.012 |
| Adjusted R2 | 0.496 | 0.526 | 0.517 | |||||||||
Dummy values were assigned for the type of stroke: 1, hemorrhagic; 0, ischemic.
SE, standard error.
Fig. 2.
Scatter plots showing the actual measured values (vertical axes) and the predicted values derived from the parameter estimates of multivariate regression analyses (see Tables 2 and 3). Black dots represent data from patients with hemorrhagic stroke and gray dots represent data from those with ischemic stroke. Red lines with a slope of 1 indicate a perfect fit, i.e., where the predicted and actual values are identical. Reddish areas indicate the 95% confidence intervals. Blue lines indicate the mean of the actual values obtained.
S/E/F, shoulder, elbow, and forearm; H/F, hand and finger; L/E, lower extremity.
Table 3 shows the results obtained by multivariate regression for FIM-motor and LOS. Unlike for BRS, age was included in the final models for both FIM-motor and LOS. The t-values obtained for FIM-motor indicated that age contributed as much as rFA to stroke outcome. The bottom row in Fig. 2 shows scatter plots of the observed and predicted values derived from the parameter estimates shown in Table 3.
Table 3. Results of multivariate regression analyses for FIM-motor and LOS.
| FIM-motor | LOS | |||||||
| Estimate | SE | t | P | Estimate | SE | t | P | |
| Age | −0.26 | 0.06 | −4.09 | <0.001 | 0.84 | 0.39 | 2.19 | 0.032 |
| rFA | 34.72 | 7.62 | 4.56 | <0.001 | −332.88 | 46.60 | −7.14 | <0.001 |
| Contralesional FA | 57.99 | 23.91 | 2.42 | 0.018 | −317.44 | 146.29 | −2.17 | 0.033 |
| Type of Stroke | - | - | - | - | - | - | - | - |
| Intercept | 29.22 | 15.14 | 1.93 | 0.057 | 562.01 | 92.59 | 6.07 | <0.001 |
| Adjusted R2 | 0.321 | 0.420 | ||||||
Dummy values were assigned for the type of stroke; 1, hemorrhagic; 0, ischemic.
Table 4 shows the correlations between the explanatory variables. Hemorrhage (dummy value, 1) was associated with a lower rFA (P=0.002), indicating that neural damage was more severe in patients with hemorrhagic stroke. Although not a statistically significant finding, patients with hemorrhagic stroke were younger than those with ischemic stroke (P=0.065). This result was consistent with the negative correlation between age and rFA (P=0.046).
Table 4. Correlations between explanatory variables.
| Age | rFA | Contralesional FA | |
| rFA | 0.224 (P=0.046) | - | - |
| Contralesional FA | −0.067 (P=0.555) | 0.085 (P=0.452) | - |
| Type of stroke | −0.208 (P=0.065) | −0.340 (P=0.002) | −0.055 (P=0.628) |
Dummy values are assigned for the type of stroke: 1, hemorrhagic; 0, ischemic.
DISCUSSION
Multivariate regression analyses of patients with supratentorial hemorrhagic or ischemic stroke in this study revealed that FA in the cerebral peduncles of the ipsilesional and contralesional hemispheres was associated with long-term outcome. Furthermore, age influenced the FIM-motor and LOS outcome data. However, the type of stroke had a minimal effect on outcome, except for the function of the lower extremities. These findings highlight the contributions of age and neural integrity in the corticospinal tracts to stroke outcome.
Previous studies have identified age as one of the most powerful predictors of stroke outcome.29,30) This study quantitively evaluated the impact of age in combination with neural integrity on outcome measures of BRS, FIM-motor, and LOS. For FIM-motor, multivariate regression analysis revealed that the impact of age was as robust as that of neural damage indexed by rFA. However, age had a minimal effect on outcome in terms of function in the extremities as assessed by the BRS. This discrepancy may reflect differences in the nature of the measures used, in that the BRS evaluates impairment, whereas FIM-motor assesses disability. Nevertheless, this finding suggests a direct relationship between impairment and neural damage, whereas disability reflects broader factors, including age.
The literature suggests that DTI-FA, an index of the integrity of neural fibers, can be used to predict the stroke outcome.4,9,31) Many previous studies have used the severity of neural damage in the ipsilesional hemisphere relative to that in the intact contralesional hemisphere, which is indexed by rFA.4,9,31) As in those studies, we found a strong correlation between rFA as an index of neural damage and stroke outcome (Tables 2 and 3). Correlation analyses of the explanatory variables indicated that hemorrhagic stroke was associated with a lower rFA (Table 4). However, multivariate regression analyses did not take the type of stroke into the final models, except for the lower extremity data. This observation suggests that hemorrhagic stroke is associated with more severe neural damage and symptoms.19) However, the neural damage indexed by rFA accounted for most of the variability in clinical severity. These findings confirm the usefulness of rFA in the cerebral peduncles for predicting outcome in stroke patients with hemorrhagic or ischemic lesions.4,9,19,31)
It has been suggested that a small number of corticospinal fibers project ipsilaterally and that they may contribute to motor recovery in patients with hemiparesis after stroke.32) As in previous studies,16,33) FA in the contralesional hemisphere was used in the final models for all multivariate regression analyses in the present study. This observation implies that better neural integrity within the ipsilateral corticospinal tracts is associated with a more favorable outcome.34) However, our results for parameter estimates (t-values) suggest that the contribution of FA in the contralesional hemisphere was relatively small (Tables 2 and 3). Accordingly, in terms of clinical significance, the role of ipsilateral (contralesional) corticospinal projections in motor recovery remains unclear.35)
Clinical severity in the initial phase of stroke is another important determinant of long-term outcome.3,36,37) We previously reported that the National Institutes of Health Stroke Scale score38) during the acute phase can predict the extent of FIM-motor recovery.39) The speed of recovery is also important for predicting stroke outcome.3,30) However, in this study, we could not systematically obtain data for initial clinical severity or for the speed of recovery because of the retrospective nature of the research. Nevertheless, the predictive accuracy of the models derived from multivariate regression analyses (adjusted R2) ranged from 0.321 to 0.526. Greater accuracy could be expected if the initial clinical severity and speed of recovery were included as explanatory variables. Further studies are needed to clarify this issue.
This study has several limitations. First, the outcome measures used were somewhat crude in that we sampled only BRS, FIM-motor, and LOS. However, patients with stroke have a variety of symptoms, including dysphagia, hemiparesis, and higher brain dysfunction (e.g., aphasia and hemispatial neglect), so our outcome measures might not have adequately accounted for other important symptoms. Second, we did not include a confirmatory analysis of the models derived from the multiple regression analyses. As a result of our stringent inclusion criteria, data for only 80 patients were used in the analysis. Therefore, the focus of the study was on model development. Future studies with larger numbers of patients are needed to assess the applicability of the models derived in the present study. However, given that the aim of this study was to assess the contributions of age, type of stroke, and FA in the ipsilesional and contralesional cerebral peduncles, we believe that our analysis was appropriate. Third, only patients who were functionally independent before stroke were enrolled in the study; such patients are likely to have a relatively good recovery. Moreover, we did not include patients with subarachnoid hemorrhage or those with brainstem or cerebellum lesions because their symptoms (altered consciousness, ataxia) are different from those in patients with supratentorial intramedullary lesions. Therefore, caution is needed when generalizing the present findings to the entire stroke population. Nevertheless, despite these limitations, the present study confirms that neural integrity within the corticospinal tracts and patient age are critical factors for predicting long-term stroke outcome.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS KAKENHI [JP16H03209]).
Footnotes
CONFLICTS OF INTEREST: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Veerbeek JM,Kwakkel G,van Wegen EE,Ket JC,Heymans MW: Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke 2011;42:1482–1488. 10.1161/STROKEAHA.110.604090 [DOI] [PubMed] [Google Scholar]
- 2.Stinear CM,Byblow WD,Ackerley SJ,Barber PA,Smith MC: Predicting recovery potential for individual stroke patients increases rehabilitation efficiency. Stroke 2017;48:1011–1019. 10.1161/STROKEAHA.116.015790 [DOI] [PubMed] [Google Scholar]
- 3.Stinear CM,Smith MC,Byblow WD: Prediction tools for stroke rehabilitation. Stroke 2019;50:3314–3322. 10.1161/STROKEAHA.119.025696 [DOI] [PubMed] [Google Scholar]
- 4.Boyd LA,Hayward KS,Ward NS,Stinear CM,Rosso C,Fisher RJ,Carter AR,Leff AP,Copland DA,Carey LM,Cohen LG,Basso DM,Maguire JM,Cramer SC: Biomarkers of stroke recovery: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017;12:480–493. 10.1177/1747493017714176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim B,Winstein C: Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabil Neural Repair 2017;31:3–24. 10.1177/1545968316662708 [DOI] [PubMed] [Google Scholar]
- 6.Puig J,Blasco G,Daunis-I-Estadella J,Thomalla G,Castellanos M,Figueras J,Remollo S,van Eendenburg C,Sánchez-González J,Serena J,Pedraza S: Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke 2013;44:2016–2018. 10.1161/STROKEAHA.111.000382 [DOI] [PubMed] [Google Scholar]
- 7.Lindenberg R,Renga V,Zhu LL,Betzler F,Alsop D,Schlaug G: Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010;74:280–287. 10.1212/WNL.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindenberg R,Zhu LL,Rüber T,Schlaug G: Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 2012;33:1040–1051. 10.1002/hbm.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moura LM,Luccas R,de Paiva JPQ,Amaro E Jr,Leemans A,Leite CDC,Otaduy MCG,Conforto AB: Diffusion tensor imaging biomarkers to predict motor outcomes in stroke: a narrative review. Front Neurol 2019;10:445. 10.3389/fneur.2019.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong WW,Fang Y,Chu WCW,Shi L,Tong KY: What kind of brain structural connectivity remodeling can relate to residual motor function after stroke? Front Neurol 2019;10:1111. 10.3389/fneur.2019.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scrutinio D,Lanzillo B,Guida P,Mastropasqua F,Monitillo V,Pusineri M,Formica R,Russo G,Guarnaschelli C,Ferretti C,Calabrese G: Development and validation of a predictive model for functional outcome after stroke rehabilitation. Stroke 2017;48:3308–3315. 10.1161/STROKEAHA.117.018058 [DOI] [PubMed] [Google Scholar]
- 12.de Ridder IR,Dijkland SA,Scheele M,den Hertog HM,Dirks M,Westendorp WF,Nederkoorn PJ,van de Beek D,Ribbers GM,Steyerberg EW,Lingsma HF,Dippel DW: Development and validation of the Dutch Stroke Score for predicting disability and functional outcome after ischemic stroke: a tool to support efficient discharge planning. Eur Stroke J 2018;3:165–173. 10.1177/2396987318754591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolucci S,Antonucci G,Grasso MG,Bragoni M,Coiro P,De Angelis D,Fusco FR,Morelli D,Venturiero V,Troisi E,Pratesi L: Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: a matched comparison. Stroke 2003;34:2861–2865. 10.1161/01.STR.0000102902.39759.D3 [DOI] [PubMed] [Google Scholar]
- 14.Douiri A,Grace J,Sarker SJ,Tilling K,McKevitt C,Wolfe CD,Rudd AG: Patient-specific prediction of functional recovery after stroke. Int J Stroke 2017;12:539–548. 10.1177/1747493017706241 [DOI] [PubMed] [Google Scholar]
- 15.Bradnam LV,Stinear CM,Barber PA,Byblow WD: Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex 2012;22:2662–2671. 10.1093/cercor/bhr344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen H,Alshikho MJ,Wang Y,Luo X,Zafonte R,Herbert MR,Wang QM: Correlation of fractional anisotropy with motor recovery in patients with stroke after postacute rehabilitation. Arch Phys Med Rehabil 2016;97:1487–1495. 10.1016/j.apmr.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama T,Marumoto K,Uchiyama Y,Miyake H,Domen K: Outcome assessment of hemiparesis due to intracerebral hemorrhage using diffusion tensor fractional anisotropy. J Stroke Cerebrovasc Dis 2015;24:881–889. 10.1016/j.jstrokecerebrovasdis.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 18.Koyama T,Domen K: Diffusion tensor fractional anisotropy in the superior longitudinal fasciculus correlates with functional independence measure cognition scores in patients with cerebral infarction. J Stroke Cerebrovasc Dis 2017;26:1704–1711. 10.1016/j.jstrokecerebrovasdis.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 19.Koyama T,Koumo M,Uchiyama Y,Domen K: Utility of fractional anisotropy in cerebral peduncle for stroke outcome prediction: comparison of hemorrhagic and ischemic strokes. J Stroke Cerebrovasc Dis 2018;27:878–885. 10.1016/j.jstrokecerebrovasdis.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 20.Shinohara Y,Yanagihara T,Abe K,Yoshimine T,Fujinaka T,Chuma T,Ochi F,Nagayama M,Ogawa A,Suzuki N,Katayama Y,Kimura A,Liu M,Eto F: VII. Rehabilitation. J Stroke Cerebrovasc Dis 2011;20(Suppl):S145–S180. 10.1016/j.jstrokecerebrovasdis.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 21.Koyama T,Tsuji M,Miyake H,Ohmura T,Domen K: Motor outcome for patients with acute intracerebral hemorrhage predicted using diffusion tensor imaging: an application of ordinal logistic modeling. J Stroke Cerebrovasc Dis 2012;21:704–711. 10.1016/j.jstrokecerebrovasdis.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 22.Brunnstrom S: Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 1966;46:357–375. 10.1093/ptj/46.4.357 [DOI] [PubMed] [Google Scholar]
- 23.Safaz İ,Ylmaz B,Yaşar E,Alaca R: Brunnstrom recovery stage and motricity index for the evaluation of upper extremity in stroke: analysis for correlation and responsiveness. Int J Rehabil Res 2009;32:228–231. 10.1097/MRR.0b013e32832a62ad [DOI] [PubMed] [Google Scholar]
- 24.Heinemann AW,Linacre JM,Wright BD,Hamilton BB,Granger C: Relationships between impairment and physical disability as measured by the functional independence measure. Arch Phys Med Rehabil 1993;74:566–573. 10.1016/0003-9993(93)90153-2 [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson M,Beckmann CF,Behrens TE,Woolrich MW,Smith SM: FSL. Neuroimage 2012;62:782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 26.Koyama T,Tsuji M,Nishimura H,Miyake H,Ohmura T,Domen K: Diffusion tensor imaging for intracerebral hemorrhage outcome prediction: comparison using data from the corona radiata/internal capsule and the cerebral peduncle. J Stroke Cerebrovasc Dis 2013;22:72–79. 10.1016/j.jstrokecerebrovasdis.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 27.Mori S,Oishi K,Jiang H,Jiang L,Li X,Akhter K,Hua K,Faria AV,Mahmood A,Woods R,Toga AW,Pike GB,Neto PR,Evans A,Zhang J,Huang H,Miller MI,van Zijl P,Mazziotta J: Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008;40:570–582. 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassidy JM,Tran G,Quinlan EB,Cramer SC: Neuroimaging identifies patients most likely to respond to a restorative stroke therapy. Stroke 2018;49:433–438. 10.1161/STROKEAHA.117.018844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte E,Marco E,Muniesa JM,Belmonte R,Aguilar JJ,Escalada F: Early detection of non-ambulatory survivors six months after stroke. NeuroRehabilitation 2010;26:317–323. 10.3233/NRE-2010-0568 [DOI] [PubMed] [Google Scholar]
- 30.Kwakkel G,Kollen BJ: Predicting activities after stroke: what is clinically relevant? Int J Stroke 2013;8:25–32. 10.1111/j.1747-4949.2012.00967.x [DOI] [PubMed] [Google Scholar]
- 31.Puig J,Blasco G,Schlaug G,Stinear CM,Daunis-i-Estadella P,Biarnes C,Figueras J,Serena J,Hernández-Pérez M,Alberich-Bayarri A,Castellanos M,Liebeskind DS,Demchuk AM,Menon BK,Thomalla G,Nael K,Wintermark M,Pedraza S: Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology 2017;59:343–351. 10.1007/s00234-017-1816-0 [DOI] [PubMed] [Google Scholar]
- 32.Ward NS,Newton JM,Swayne OB,Lee L,Thompson AJ,Greenwood RJ,Rothwell JC,Frackowiak RS: Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006;129:809–819. 10.1093/brain/awl002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaechter JD,Fricker ZP,Perdue KL,Helmer KG,Vangel MG,Greve DN,Makris N: Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 2009;30:3461–3474. 10.1002/hbm.20770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuka N,Miyashita K,Krieger DW,Naritomi H: Compensatory contribution of the contralateral pyramidal tract after stroke. Front Neurol Neurosci 2013;32:45–53. 10.1159/000348821 [DOI] [PubMed] [Google Scholar]
- 35.Alawieh A,Tomlinson S,Adkins D,Kautz S,Feng W: Preclinical and clinical evidence on ipsilateral corticospinal projections: implication for motor recovery. Transl Stroke Res 2017;8:529–540. 10.1007/s12975-017-0551-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doughty C,Wang J,Feng W,Hackney D,Pani E,Schlaug G: Detection and predictive value of fractional anisotropy changes of the corticospinal tract in the acute phase of a stroke. Stroke 2016;47:1520–1526. 10.1161/STROKEAHA.115.012088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connell LA,Smith MC,Byblow WD,Stinear CM: Implementing biomarkers to predict motor recovery after stroke. NeuroRehabilitation 2018;43:41–50. 10.3233/NRE-172395 [DOI] [PubMed] [Google Scholar]
- 38.Goldstein LB,Bertels C,Davis JN: Interrater reliability of the NIH stroke scale. Arch Neurol 1989;46:660–662. 10.1001/archneur.1989.00520420080026 [DOI] [PubMed] [Google Scholar]
- 39.Saito J,Koyama T,Domen K: Long-term outcomes of FIM motor items predicted from acute stage NIHSS of patients with middle cerebral artery infarct. Ann Rehabil Med 2018;42:670–681. 10.5535/arm.2018.42.5.670 [DOI] [PMC free article] [PubMed] [Google Scholar]