ABSTRACT
Objective:
The aim of this study was to investigate the effect of repetitive peripheral magnetic stimulation (rPMS) on muscle atrophy prevention in the rectus femoris muscle (RF) of the paretic limb in acute stroke patients.
Methods:
Twelve acute stroke patients with a National Institute of Health Stroke Scale score >5 and a motor score of the paretic lower limb >2 at admission were divided into an intervention group (rPMS: mean age, 75±6.4 years) and a conventional care group (non-rPMS: mean age, 62±11.8 years). Baseline measurements were performed within 4 days of stroke onset. In the rPMS group, treatment was applied to the paretic thigh only for 2 weeks, 5 days a week, in addition to conventional care. The cross-sectional area (CSA) of the RF was assessed in both limbs using ultrasound at baseline and 2 weeks later. Data on patient characteristics were collected from the clinical records to assess correlations with the CSA rate of change.
Results:
Patients in the rPMS group were significantly older. Although the CSA of the RF did not change significantly on either side in the rPMS group, there was a significant decrease in the CSA on the paretic side in the non-rPMS group. However, no significant difference was observed in the CSA rate of change in the rPMS and non-rPMS groups. The CSA rate of change on the paretic side correlated negatively with age in the rPMS group.
Conclusions:
Our results suggest that rPMS prevents muscle atrophy more effectively in patients in their 60s than in patients more than 70 years old.
Keywords: aging, magnetic stimulation, muscle atrophy, stroke, ultrasonography
INTRODUCTION
Muscle atrophy reportedly appears within 10 days of stroke onset in stroke patients with hemiplegia.1) Muscle atrophy not only decreases a patient’s ability to perform activities of daily living (ADLs) but also causes a decline in the body’s glucose metabolism, resulting in a further risk for stroke.2,3,4) Furthermore, loss of muscle mass can contribute to the occurrence of osteoporosis and, consequently, increase the risk of paretic lower limb fracture.5,6) These studies have suggested that prevention of muscle atrophy from the acute phase of stroke is important not only for improving the ability to perform ADLs but also for preventing further diseases after discharge.
Although resistance training is reportedly an effective prevention strategy for muscle atrophy after stroke,7) it cannot be applied in patients with consciousness disturbance in the acute phase. Instead of resistance training, neuromuscular electrical stimulation (NMES) has been used to prevent muscle atrophy in patients with consciousness disturbance or hemiplegia. Several studies have investigated the preventive effect of NMES on muscle atrophy.8,9) These studies suggested that NMES is an effective method to prevent muscle atrophy in patients with hemiplegia. However, the discomfort associated with such stimulation is a disadvantage of NMES. Several studies that investigated the therapeutic effect of NMES reported that some patients had to discontinue the experiment because of the discomfort associated with NMES.10,11)
Recently, repetitive peripheral magnetic stimulation (rPMS) has been widely used as a method of stimulating skeletal muscle.12,13,14) Moreover, rPMS is associated with significantly less discomfort than that associated with NMES.15) The overload principle is applied to muscle strengthening generated by electrical stimulation,16) such as rPMS; that is, a strong contraction force is needed effectively to prevent muscle atrophy. Consequently, rPMS could be useful in preventing muscle atrophy because it can stimulate skeletal muscle with high intensity and reduced discomfort. Indeed, other studies have reported that the progress of muscle atrophy can be halted by using rPMS in rats with muscle atrophy induced by hindlimb suspension.17,18)
Nonetheless, the preventive effect of rPMS on muscle atrophy has not been clarified in stroke patients with hemiplegia. Therefore, we conducted a pilot study to investigate the preventive effects of rPMS on muscle atrophy in patients in the acute phase of hemiplegia.
MATERIALS AND METHODS
Subjects
This study enrolled stroke patients admitted to the Stroke Care Unit of Kawasaki Medical School Hospital between November 2015 and November 2017. Patients were included if they met the following criteria: (1) above 20 years of age, (2) first hemispheric stroke, (3) ability to walk independently before onset, (4) modified Rankin Scale less than 2 before admission, (5) National Institute of Health Stroke Scale (NIHSS) score greater than 5 and motor score of the paretic lower limb greater than 2. The exclusion criteria were as follows: (1) a contraindication according to the safety guidelines for magnetic stimulation,19) such as implanted metal, risk of epileptic seizure, and severe or recent heart disease, and (2) the inability to carry out the experiment because of severe dementia. We used block randomization (block size=2) to allocate patients into either the rPMS group or the non-rPMS group. For example, the first participant was randomly assigned to either group using a random number table. If the first participant was assigned to the rPMS group, the second one was automatically assigned to the non-rPMS group to keep the number of participants equal between the groups. Thereafter, the odd-numbered patients were randomly assigned to either group. In this way, we tried to manage any bias from the enrollment timing on the number of participants in the groups to prevent any potential influence of the stroke treatment strategy used in our hospital on the analysis of the outcomes. All patients underwent physical therapy, occupational therapy, and speech therapy as soon as possible after admission to the Stroke Care Unit.
This study received approval from the Ethics Committee of Kawasaki Medical School (No. 2206), and each patient or his/her authorized representatives provided written informed consent for study participation. This study was registered in the University Hospital Medical Information Network Center Clinical Trials Registry (UMIN000018750).
Experimental Procedure
The rectus femoris muscle (RF) of both legs of each patient was evaluated with ultrasound as a baseline measurement within 4 days of stroke onset. The patients in the rPMS group underwent rPMS 5 days per week in addition to conventional rehabilitation programs. In the rPMS group, the same evaluation was performed again a day after the completion of 10 rPMS interventions. The patients in the non-rPMS group underwent conventional rehabilitation programs. In the non-rPMS group, the same evaluation was performed 2 weeks after the baseline measurement. The purpose of this study was to investigate the preventive effect of rPMS on muscle atrophy in stroke patients with hemiplegia. A previous study reported that muscle atrophy appears within 10 days of stroke onset in patients with hemiplegia.1) Therefore, we considered that an intervention period of 10 days was the minimum-required period for this study. Furthermore, many patients are transferred from our hospital to another hospital when their general condition becomes stable. Consequently, we speculated that it would be difficult to continue the experiment for a longer period. Therefore, the intervention period was set at 2 weeks so that the experimental procedure could be completed in all patients. Rehabilitation programs that consisted of conventional approaches to improve the patient’s ability to perform ADLs were followed, with adjustment to match the condition of each patient.
Repetitive Peripheral Magnetic Stimulation
The rPMS interventions were performed once a day. A total of 10 interventions were conducted over 2 weeks, 5 days a week. The intervention was applied only to the paretic side using a repetitive peripheral magnetic stimulator (IFG Corp.; Pathleader). During the intervention, the patients were kept in the supine position or were seated in a wheelchair with 30° knee flexion. The patients were asked to keep their limbs completely relaxed during the intervention. For convenience and consistency, we chose the distal point at one-third of the distance between the anterior superior iliac spine and the center of the superior border of the patella as the stimulation site, as described in a previous study that reported the location of a motor point on the RF.20) The longitudinal axis of the stimulation coil was aligned with the longitudinal axis of the thigh, and the therapist held the coil by hand so that the coil was kept perpendicular to the stimulation site. The stimulation site was marked to ensure that the same site could be stimulated throughout the 2-week intervention period. The stimulation intensity was set at 0.9 Tesla, which was the maximum output of the stimulator; the stimulation frequency was set at 30 Hz. Visible muscle contraction of the RF was induced by these stimulation parameters in all patients in the rPMS group. The “on” time was set at 1.3 s, as the maximum continuous “on” time of the stimulator used in this study. One set included thirty stimulation repetitions, with a 3-s rest interval. In one daily session, we applied five sets of stimulation repetitions, with 2-min intervals between each set. This stimulation protocol was based on a previous study that confirmed the safe levels for the stimulation parameters,21) with additional attention paid to the avoidance of coil heating. A single intervention took approximately 20 min. The rPMS intervention was delivered to patients at a different time from the standard rehabilitation programs, and the schedule was different each day.
Measurement of the Cross-Sectional Area of the RF and the Subcutaneous Tissue Thickness
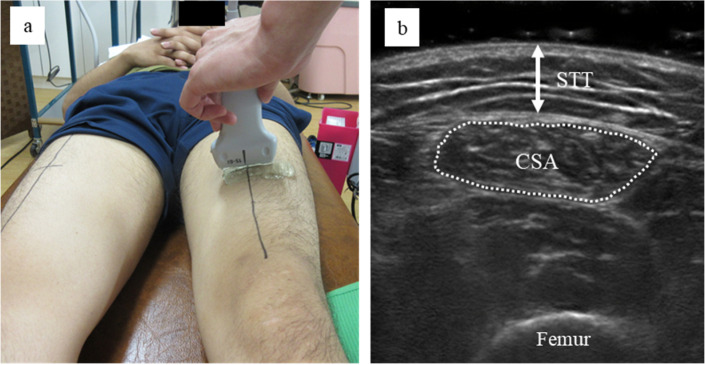
The cross-sectional area (CSA) of the RF, as an index of muscle mass, was measured on both sides in all patients at baseline and 2 weeks later. Furthermore, the subcutaneous tissue thickness (STT) was measured as an index of the distance from the surface of the anterior thigh to the RF in the paretic limb at baseline. Measurements were performed by the same experienced investigator who performed the rPMS intervention using B-mode ultrasonography with a 56-mm linear probe at 6−15 MHz (SonoSite Inc.; SonoSite M-Turbo). The measurement was performed at two-fifths of the distal point between the anterior superior iliac spine and the center of the superior border of the patella. During the ultrasound measurement, the patients were asked to keep their lower limb completely relaxed, with the knee extended in the supine position. The probe was positioned perpendicular to the longitudinal axis of the thigh, and cross-sectional images of the RF were obtained. An RF ultrasound measurement procedure similar to that used in the previous study was adopted.22) The measurement sites were marked to ensure that the same sites could be evaluated 2 weeks later. Three ultrasound images were generated from each leg at baseline and 2 weeks later. The CSA of the RF and the STT were calculated using ImageJ (Version 1.45, NIH, USA) for each image. The CSA of the RF was calculated with as much of the RF as possible on the image and without any surrounding fascia. In addition, the distance from the skin surface to the surface of the RF was measured at the center of the image as an index of the STT on the paretic side. Analysis of the ultrasound images using ImageJ was performed by an investigator who was blinded to the design of the present study and who did not perform the ultrasound measurements. Figure 1 shows the methods used to generate the ultrasound images and perform image analysis. The average CSA and STT values were calculated at baseline and 2 weeks later, and the CSA rate of change from baseline to 2 weeks later was also calculated.
Fig. 1.
The method for generating the ultrasound images, and a sample image of measurements of the cross-sectional area (CSA) and subcutaneous tissue thickness (STT) of the rectus femoris muscle. (A) Measurement setting of the ultrasound images. (B) The area surrounded by the broken line was defined as the CSA of the rectus femoris muscle for analysis using the software, and the arrow indicates the STT.
Patient Characteristics
Data on patient characteristics were collected from clinical records. These characteristics included patients’ age, sex, height, weight, body mass index, lesion location, NIHSS at admission, the number of days of physical therapy, the total units of physical therapy delivered until the measurement 2 weeks later, the number of days until the first physical therapy, the number of days until baseline measurement, the number of days until the start of rPMS, and the number of days until measurement 2 weeks later. Data on the peak C-reactive protein (CRP) levels as an index of inflammation during the experimental period and the lowest albumin level as an index of nutritional status during the experimental period were also collected. These measures were taken because inflammation and nutritional status are reported to be indicators of clinical outcomes in patients with acute stroke.23,24,25)
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). A Shapiro–Wilk test was first utilized to check the assumption of normality for all variables, and a parametric or non-parametric test was selected based on the results. Mann-Whitney U tests were used to determine whether there were differences in the lower limb NIHSS motor scores on the paretic side (NIHSS LL), the number of days until baseline measurement, and the CRP values between the two groups. Student’s t tests were used to compare other patient characteristics in the rPMS and non-rPMS groups. Paired t tests were used to assess the changes in the CSA within each group from baseline to 2 weeks later. The CSA rate of change and STT values were compared between the two groups using Student’s t tests. To assess the correlation between the CSA rate of change and the patient characteristics in each group, we used a Spearman’s rank correlation coefficient (following variables: NIHSS LL, the number of days until baseline measurement, and CRP value in the rPMS group) or Pearson’s product-moment correlation coefficient (other variables). Data are shown as mean ± standard deviation. A P-value of < 0.05 was considered statistically significant.
RESULTS
Table 1 shows the clinical characteristics of each patient. There was a significant difference in ages between the two groups. Patients in the rPMS group were significantly older (P < 0.05). There were no significant differences in any other variables.
Table 1. Characteristics of subjects in the rPMS and non-rPMS groups.
| rPMS | non-rPMS | |||||||||||||
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | Mean ± SD | 7 | 8 | 9 | 10 | 11 | 12 | Mean ± SD |
| Age (years) | 69 | 87 | 74 | 78 | 72 | 73 | 75.5 ± 6.4 | 71 | 77 | 53 | 59 | 46 | 69 | 62.5 ± 11.8 * |
| Sex | F | M | M | M | M | M | - | M | F | M | M | M | M | - |
| Height (cm) | 153.0 | 164.0 | 155.0 | 163.0 | 170.0 | 164.0 | 161.5 ± 6.3 | 158.6 | 148.0 | 170.0 | 165.0 | 154.5 | 162.0 | 159.7 ± 7.8 |
| Weight (kg) | 40.2 | 56.0 | 55.8 | 56.8 | 68.6 | 69.6 | 57.8 ± 10.7 | 53.2 | 40.7 | 89.6 | 66.3 | 73.8 | 74.8 | 66.4 ± 17.3 |
| BMI (kg/m2) | 17.2 | 20.8 | 23.2 | 21.4 | 23.7 | 25.9 | 22.0 ± 3.0 | 21.1 | 18.6 | 31.0 | 24.4 | 30.9 | 28.5 | 25.8 ± 5.2 |
| Lesion area | R thalamic hemorrhage |
R thalamic hemorrhage | R putamen hemorrhage | L MCA infarction | R MCA infarction | L MCA infarction | - | L MCA infarction | R thalamic hemorrhage | L putamen hemorrhage | R thalamic hemorrhage | R BAD | L corona radiata infarction | - |
| NIHSS | 8 | 10 | 14 | 7 | 12 | 12 | 10.5 ± 2.7 | 16 | 16 | 18 | 15 | 7 | 6 | 13.0 ± 5.1 |
| NIHSS LL | 2 | 2 | 4 | 3 | 2 | 2 | 2.5 ± 0.8 | 2 | 4 | 3 | 3 | 2 | 2 | 2.6 ± 0.8 |
| Total PT days | 12 | 16 | 12 | 11 | 11 | 15 | 12.8 ± 2.1 | 12 | 10 | 10 | 14 | 15 | 11 | 12.0 ± 2.1 |
| Total PT units | 25 | 29 | 29 | 14 | 20 | 36 | 25.5 ± 7.7 | 19 | 20 | 15 | 28 | 33 | 19 | 22.3 ± 6.7 |
| Days until first PT | 3 | 2 | 2 | 2 | 1 | 2 | 2.0 ± 0.6 | 2 | 3 | 3 | 2 | 4 | 2 | 2.7 ± 0.8 |
| Days until baseline measurement | 4 | 3 | 4 | 3 | 3 | 4 | 3.5 ± 0.5 | 3 | 3 | 4 | 3 | 4 | 2 | 3.1 ± 0.8 |
| Days until measurement 2 weeks later | 18 | 21 | 18 | 17 | 14 | 18 | 17.7 ± 2.3 | 17 | 17 | 19 | 18 | 20 | 18 | 18.2 ± 1.2 |
| Days until start of rPMS | 4 | 4 | 5 | 3 | 3 | 4 | 3.8 ± 0.8 | - | - | - | - | - | - | - |
| CRP peak value (mg/dL) |
0.22 | 0.24 | 0.06 | 15.65 | 0.18 | 7.06 | 3.09 ± 6.38 | 10.64 | 23.05 | 4.55 | 1.77 | No data | 0.44 | 8.09 ± 9.24 |
| ALB minimum value (g/dL) |
3.5 | 2.8 | 3.5 | 3.1 | 3.1 | 3.0 | 3.2 ± 0.3 | 1.9 | 2.4 | 3.6 | 2.9 | No data | 4.1 | 3.0 ± 0.9 |
SD, standard deviation; F, female; M, male; BMI, body mass index; R, right; L, left; MCA, middle cerebral artery; BAD, branch atheromatous disease; LL, lower limb motor score on the paretic side; PT, physical therapy; CRP, C-reactive protein; ALB, albumin.
CRP and ALB levels were not measured in Patient 11 during the experimental period.
*P < 0.05 vs. rPMS group.
Table 2 shows the STT and the CSA of the RF obtained from each patient before and after the intervention. On the non-paretic side, there were no significant differences in the mean CSA at baseline and 2 weeks later in either group. In the non-rPMS group, the mean CSA of the RF on the paretic side decreased significantly after 2 weeks (P < 0.01). In contrast, in the rPMS group, there was no significant difference in the mean CSA of the RF on the paretic side at baseline and 2 weeks later. However, a positive CSA change was observed in only two patients in the rPMS group, with other patients in the rPMS group showing rates of change comparable to those seen in the non-rPMS group (Table 2). Furthermore, the CSA rate of change on the paretic and non-paretic sides did not differ significantly in the two groups. The mean STT in the rPMS group and non-rPMS group were not significantly different.
Table 2. Cross-sectional area of the rectus femoris muscle and subcutaneous tissue thickness in the rPMS and non-rPMS groups.
| rPMS | non-rPMS | ||||||||||||||
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | Mean ± SD | 7 | 8 | 9 | 10 | 11 | 12 | Mean ± SD | |
| The CSA of RF in paretic limb (cm2) | Baseline | 3.50 | 4.40 | 4.61 | 2.46 | 6.23 | 5.14 | 4.39 ± 1.31 | 2.71 | 3.06 | 4.78 | 5.39 | 5.59 | 3.29 | 4.14 ± 1.26 |
| 2 weeks later | 3.52 | 3.51 | 4.25 | 2.22 | 6.72 | 4.69 | 4.15 ± 1.51 | 2.65 | 2.67 | 4.30 | 4.91 | 5.29 | 2.94 | 3.79 ± 1.19** | |
| Rate of change (%) | 1 | −20 | −8 | −10 | 8 | −9 | −6 ± 10 | −2 | −13 | −10 | −9 | −5 | −11 | −8 ± 4 | |
| The CSA of RF in non-paretic limb (cm2) |
Baseline | 3.68 | 4.60 | 4.32 | 2.23 | 7.24 | 5.31 | 4.56 ± 1.67 | 2.40 | 2.97 | 4.81 | 5.85 | 8.14 | 3.29 | 4.57 ± 2.16 |
| 2 weeks later | 3.42 | 4.13 | 4.16 | 2.03 | 6.17 | 5.40 | 4.22 ± 1.46 | 2.12 | 3.43 | 4.90 | 4.75 | 7.88 | 3.55 | 4.44 ± 1.97 | |
| Rate of change (%) | −7 | −10 | −4 | −9 | −15 | 2 | −7 ± 6 | −12 | 16 | 2 | −19 | −3 | 8 | −1 ± 13 | |
| STT of paretic limb (cm) |
Baseline | 0.60 | 1.26 | 0.47 | 1.02 | 0.83 | 0.96 | 0.86 ± 0.29 | 0.66 | 1.19 | 1.23 | 0.68 | 1.31 | 1.05 | 1.02 ± 0.28 |
**P < 0.01 vs. baseline.
Table 3 shows the correlation coefficients between the CSA rate of change on both sides and patient characteristics in each group. In the rPMS group, the CSA rate of change on the paretic side was negatively correlated with age and with the number of days until the measurement 2 weeks later. There was a positive correlation between the CSA rate of change on the non-paretic side in the rPMS group and the number of days until the baseline measurement. In the non-rPMS group, the CSA rate of change was not significantly correlated with any patient characteristic for either side.
Table 3. Correlation coefficients between the rate of change of the cross-sectional area and patient characteristics.
| Days until | ||||||||||||||||
| Age | Height | Weight | BMI | NIHSS | NIHSS LL | Total PT days | Total PT units | First PT | Baseline measurement | Measurement taken 2 weeks later | Start of rPMS | CRP | ALB | STT | ||
| rPMS | Paretic | −0.84* | 0.08 | 0.06 | −0.02 | 0.11 | −0.14 | −0.71 | −0.32 | −0.23 | 0.29 | −0.88* | −0.34 | −0.66 | 0.49 | −0.57 |
| Non-paretic | −0.23 | −0.42 | 0.07 | 0.35 | 0.30 | 0.27 | 0.40 | 0.73 | 0.43 | 0.88* | 0.36 | 0.61 | 0.24 | 0.18 | −0.22 | |
| Non-rPMS | Paretic | −0.28 | 0.03 | −0.00 | 0.06 | −0.07 | −0.71 | 0.58 | 0.32 | −0.01 | 0.25 | 0.13 | −0.17 | −0.53 | −0.43 | |
| Non-paretic | 0.37 | −0.43 | −0.14 | −0.04 | −0.17 | 0.38 | −0.68 | −0.42 | 0.32 | −0.17 | −0.09 | 0.48 | 0.31 | 0.73 | ||
*P < 0.05.
DISCUSSION
Our results showed that the CSA of the paretic limb RF decreased significantly in the non-rPMS group, but not in the rPMS group. However, there was no significant difference in the CSA rate of change between the two groups, and there was no obvious preventive effect of rPMS on muscle atrophy.
The severity of hemiparesis, immobilization, inflammation, and nutritional status have been reported as factors affecting the degree of muscle atrophy in stroke patients.23,24,25,26) As an index of these factors, we collected data on selected patient characteristics in this study. The results of statistical analyses showed that there was no significant difference between the two groups in patient characteristics, other than for age. Therefore, we considered that there was no difference in the influence of these factors, other than age, on the degree of muscle atrophy between the two groups. However, it is worth noting that, in this study, the non-rPMS group included significantly younger patients than the rPMS group. In a previous study that investigated the influence of aging on muscle atrophy after 2 weeks of immobilization in healthy men, the decline in quadriceps volume was larger in young men (mean age: 24.4 years) than in old men (mean age: 67.3 years).27) Although the differences in the mean age between the two groups in the current study was not as great as that in the previous study, we speculated that age did affect the degree of muscle atrophy. Furthermore, previous studies have reported that the decrease in muscle thickness was significantly larger on the paretic side than on the non-paretic side after 2 weeks of the acute phase.1,24,28) On the basis of these reports, the significant CSA decrease observed on the paretic side in the non-rPMS group was not surprising. Therefore, it remains unclear whether the effects of rPMS or the influence of patient age induced the lack of a significant CSA change in the paretic limb in the rPMS group. However, there was a significant negative correlation between the CSA rate of change in the paretic limb and age in the rPMS group. It has been reported that older individuals show an attenuated response to retraining after a few days of immobilization compared with younger individuals.27,29) Therefore, it is assumed that rPMS can prevent the reduction of muscle mass more effectively in young patients than in old patients.
There were some limitations to this study. The most important limitation was the small number of subjects, which may have affected the statistical strength of the results obtained. Moreover, we did not monitor the muscle contraction force induced by rPMS during the intervention. The muscle contraction force induced by rPMS decreases in inverse proportion to the distance between the surface of the stimulation coil and the motor point.30) It has been reported that, in healthy subjects, the motor points of the RF can vary by a standard deviation of 2.4 cm on the longitudinal axis of the thigh.20) Furthermore, among patients in the rPMS group, the highest STT was nearly three times the lowest STT. Therefore, it can be assumed that the muscle contraction force induced by rPMS likely differed among patients because of the influence of the location of the motor point and the variation in STT. Therefore, further randomized studies with a larger number of subjects and with monitoring of the muscle contraction force induced by rPMS are needed to overcome these limitations and to determine the preventive effects of rPMS on muscle atrophy.
CONCLUSION
In this study, we could not completely clarify the effects of rPMS on the prevention of muscle atrophy because of the possibility that age was a confounding factor influencing the degree of muscle atrophy. However, our results suggest that rPMS may prevent muscle atrophy more effectively in younger patients than in older patients.
ACKNOWLEDGMENTS
The authors would like to thank staff members of the Rehabilitation Center of Kawasaki Medical School Hospital. Gratitude is expressed to Mr. Junya Hirata for his contribution to data analysis. Special thanks go to Mr. Daisuke Kimura for his helpful advice concerning our work.
Footnotes
CONFLICTS OF INTEREST: There are no conflicts of interest.
REFERENCES
- 1.Nozoe M,Kanai M,Kubo H,Kitamura Y,Shimada S,Mase K: Changes in quadriceps muscle thickness in acute non-ambulatory stroke survivors. Top Stroke Rehabil 2016;23:8–14. 10.1179/1945511915Y.0000000002 [DOI] [PubMed] [Google Scholar]
- 2.Scherbakov N,Doehner W: Sarcopenia in stroke – facts and numbers on muscle loss accounting for disability after stroke. J Cachexia Sarcopenia Muscle 2011;2:5–8. 10.1007/s13539-011-0024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherbakov N,von Haehling S,Anker SD,Dirnagl U,Doehner W: Stroke induced sarcopenia: muscle wasting and disability after stroke. Int J Cardiol 2013;170:89–94. 10.1016/j.ijcard.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE,Sandee W,Algra A,Koudstaal PJ,Kappelle LJ,Dippel DW, Dutch TIA Trial Study Group: Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke 2006;37:1413–1417. 10.1161/01.STR.0000221766.73692.0b [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen L,Jacobsen BK: Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone 2001;28:655–659. 10.1016/S8756-3282(01)00434-3 [DOI] [PubMed] [Google Scholar]
- 6.Pang MY,Eng JJ,McKay HA,Dawson AS: Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int 2005;16:1769–1779. 10.1007/s00198-005-1925-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou J,Wang Z,Qu Q,Wang L: Resistance training improves hyperglycemia and dyslipidemia, highly prevalent among nonelderly, nondiabetic, chronically disabled stroke patients. Arch Phys Med Rehabil 2015;96:1291–1296. 10.1016/j.apmr.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 8.Hirose T,Shiozaki T,Shimizu K,Mouri T,Noguchi K,Ohnishi M,Shimazu T: The effect of electrical muscle stimulation on the prevention of disuse muscle atrophy in patients with consciousness disturbance in the intensive care unit. J Crit Care 2013;28:536.e1–536.e7. 10.1016/j.jcrc.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 9.Nozoe M,Kanai M,Kubo H,Takeuchi Y,Kobayashi M,Yamamoto M,Furuichi A,Yamazaki M,Shimada S,Mase K: Efficacy of neuromuscular electrical stimulation for preventing quadriceps muscle wasting in patients with moderate or severe acute stroke: a pilot study. NeuroRehabilitation 2017;41:143–149. 10.3233/NRE-171466 [DOI] [PubMed] [Google Scholar]
- 10.Chae J,Hart R: Comparison of discomfort associated with surface and percutaneous intramuscular electrical stimulation for persons with chronic hemiplegia. Am J Phys Med Rehabil 1998;77:516–522. 10.1097/00002060-199811000-00013 [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y,Ikuno K,Shomoto K: Comparison of the effect of sensory-level and conventional motor-level neuromuscular electrical stimulations on quadriceps strength after total knee arthroplasty: a prospective randomized single-blind trial. Arch Phys Med Rehabil 2017;98:2364–2370. 10.1016/j.apmr.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu LD,Schneider C: Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol Clin 2013;43:251–260. 10.1016/j.neucli.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu LD,Schneider C: Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiol Clin 2015;45:223–237. 10.1016/j.neucli.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Sakai K,Yasufuku Y,Kamo T,Ota E,Momosaki R: Repetitive peripheral magnetic stimulation for impairment and disability in people after stroke. Cochrane Database Syst Rev 2019;11:CD011968. 10.1002/14651858.CD011968.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han TR,Shin HI,Kim IS: Magnetic stimulation of the quadriceps femoris muscle: comparison of pain with electrical stimulation. Am J Phys Med Rehabil 2006;85:593–599. 10.1097/01.phm.0000223239.93539.fe [DOI] [PubMed] [Google Scholar]
- 16.Delitto A,Snyder-Mackler L: Two theories of muscle strength augmentation using percutaneous electrical stimulation. Phys Ther 1990;70:158–164. 10.1093/ptj/70.3.158 [DOI] [PubMed] [Google Scholar]
- 17.Sakuraba T,Shimada Y,Takahashi S,Matsunaga T,Itoi E,Kawatani M: The effect of magnetic stimulation on unloaded soleus muscle of rat: changes in myosin heavy chain mRNA isoforms. Biomed Res 2005;26:15–19. 10.2220/biomedres.26.15 [DOI] [PubMed] [Google Scholar]
- 18.Shimada Y,Sakuraba T,Matsunaga T,Misawa A,Kawatani M,Itoi E: Effects of therapeutic magnetic stimulation on acute muscle atrophy in rats after hindlimb suspension. Biomed Res 2006;27:23–27. 10.2220/biomedres.27.23 [DOI] [PubMed] [Google Scholar]
- 19.Rossi S,Hallett M,Rossini PM,Pascual-Leone A, Safety of TMS Consensus Group: Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botter A,Oprandi G,Lanfranco F,Allasia S,Maffiuletti NA,Minetto MA: Atlas of the muscle motor points for the lower limb: implications for electrical stimulation procedures and electrode positioning. Eur J Appl Physiol 2011;111:2461–2471. 10.1007/s00421-011-2093-y [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K,Hiraoka T,Tsubahara A,Ito T,Izumi SI,Yashima K,Iwachidou N: Considerations for safety of high-frequency repetitive peripheral magnetic stimulation of skeletal muscles in rats: assessment by histological analysis of muscles and biochemical blood tests. JJCRS 2015;6:56–63. [Google Scholar]
- 22.Seymour JM,Ward K,Sidhu PS,Puthucheary Z,Steier J,Jolley CJ,Rafferty G,Polkey MI,Moxham J: Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 2009;64:418–423. 10.1136/thx.2008.103986 [DOI] [PubMed] [Google Scholar]
- 23.Dávalos A,Ricart W,Gonzalez-Huix F,Soler S,Marrugat J,Molins A,Suñer R,Genís D: Effect of malnutrition after acute stroke on clinical outcome. Stroke 1996;27:1028–1032. 10.1161/01.STR.27.6.1028 [DOI] [PubMed] [Google Scholar]
- 24.Winbeck K,Poppert H,Etgen T,Conrad B,Sander D: Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke 2002;33:2459–2464. 10.1161/01.STR.0000029828.51413.82 [DOI] [PubMed] [Google Scholar]
- 25.Scherbakov N,Sandek A,Doehner W: Stroke-related sarcopenia: specific characteristics. J Am Med Dir Assoc 2015;16:272–276. 10.1016/j.jamda.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 26.Nozoe M,Kanai M,Kubo H,Kitamura Y,Yamamoto M,Furuichi A,Takashima S,Mase K,Shimada S: Changes in quadriceps muscle thickness, disease severity, nutritional status, and C-reactive protein after acute stroke. J Stroke Cerebrovasc Dis 2016;25:2470–2474. 10.1016/j.jstrokecerebrovasdis.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 27.Suetta C,Hvid LG,Justesen L,Christensen U,Neergaard K,Simonsen L,Ortenblad N,Magnusson SP,Kjaer M,Aagaard P: Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 2009;107:1172–1180. 10.1152/japplphysiol.00290.2009 [DOI] [PubMed] [Google Scholar]
- 28.Ryan AS,Dobrovolny CL,Smith GV,Silver KH,Macko RF: Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil 2002;83:1703–1707. 10.1053/apmr.2002.36399 [DOI] [PubMed] [Google Scholar]
- 29.Hvid LG,Suetta C,Nielsen JH,Jensen MM,Frandsen U,Ørtenblad N,Kjaer M,Aagaard P: Aging impairs the recovery in mechanical muscle function following 4 days of disuse. Exp Gerontol 2014;52:1–8. 10.1016/j.exger.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 30.Barker AT: An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol 1991;8:26–37. 10.1097/00004691-199101000-00005 [DOI] [PubMed] [Google Scholar]