ABSTRACT
Objective:
Pressure injuries seriously impact the quality of life of patients and increase public and private healthcare costs. Electrical stimulation therapy is recommended for wound contraction, and some clinical studies have shown that the application of a monophasic pulsed microcurrent can help to reduce the treatment period. However, the optimal stimulus conditions are unclear. The purpose of this study was to investigate the effect of different frequencies of monophasic pulsed microcurrent stimulation on the number and viability of human dermal fibroblasts.
Methods:
Human dermal fibroblasts were electrically stimulated in vitro (intensity: 200 μA; frequency: 1, 2, 4, 8, 16, 32, and 64 Hz; duty factor: 50%) for 1 h three times every 24 h. Controls were unstimulated. Cell numbers and cell viability were assessed after each electrical stimulation session.
Results:
In the 1-, 2-, 4-, and 8-Hz groups, cell numbers were significantly higher than those in the control group, whereas electrical stimulation at 64 Hz resulted in a decrease in cell numbers at 24 h after the third treatment (p < 0.05). Cell viability was high in both the control and low-frequency stimulation groups, with no significant differences between groups.
Conclusion:
Application of 1–8 Hz monophasic pulsed microcurrent stimulation increased the number of human dermal fibroblasts in vitro, and is proposed as the optimal condition for accelerating the healing of pressure injuries.
Keywords: electrical stimulation, fibroblasts, frequency, increase of fibroblasts, wound healing
INTRODUCTION
The development of pressure injuries has negative effects on esthetics and the quality of life of patients.1) The prevalence of pressure injuries in a long-term care hospital was reported to be 3.85%, imposing a significant burden on healthcare costs.2) According to the National Pressure Ulcer Advisory Panel (NPUAP) staging system, a stage III pressure injury is one that has lost the full thickness of the skin and requires a long treatment course. The formation of granulation tissue and epithelialization are necessary for the healing of stage III pressure injuries3); therefore, strategies to promote the migration of fibroblasts to the wound site, and their subsequent proliferation and differentiation, can help to shorten the therapeutic period.
In 2014, the NPUAP, the European Pressure Ulcer Advisory Panel, and the Pan Pacific Pressure Injury Alliance recommended electrical stimulation (ES) to treat pressure injuries.4) Several clinical studies have reported that ES of pressure injuries promotes wound healing,5,6,7,8,9,10,11,12) and in a systematic review, Kawasaki et al.13) showed that ES therapy is an effective treatment method. ES can improve wound healing through increasing the migration, proliferation, and differentiation of fibroblasts as well as increasing the blood flow to the wound.14,15,16,17,18) However, the optimal stimulus parameters are currently unclear, and clinical studies have shown beneficial effects with a variety of ES conditions.
Current intensity and polarity are the most important factors for promoting the migration of fibroblasts. Therefore, the purpose of this study was to investigate the effects of low-frequency monophasic pulsed microcurrent stimulation on the number and survival of human dermal fibroblasts (HDFs) in vitro.
Our previous studies revealed that electrically stimulated HDFs started migrating toward the cathode in an intensity-dependent manner for currents of 100–200 μA.14,15) Therefore, an intensity of 200 μA was adopted in the present study. Few studies have investigated the effects of ES on cell proliferation, despite its importance in wound healing. Goldman and Pollack16) reported that ES at 10 Hz increased the proliferation of fibroblasts compared with the control (unstimulated cells) and with cells stimulated at 100 Hz. Moreover, ES at a frequency of 2 Hz promoted the healing of pressure injuries in our clinical study.12) Thus, the effect of low-frequency (< 10 Hz) ES on fibroblast proliferation is currently unclear, and we hypothesized that the frequency of monophasic pulsed microcurrents would likely influence cell proliferation. In the present study, we evaluated the effect of low-frequency ES on the number of HDFs and their viability in vitro.
MATERIALS AND METHODS
Cell Culture
Primary HDFs (CC-2511), derived from an adult Caucasian female donor, were obtained from Clonetics (San Diego, CA, USA) and were cultured in Dulbecco’s modified Eagle’s medium (Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (Nichirei, Tokyo, Japan) in a CO2 incubator at 37°C. HDFs obtained at passages seven to eight were used for the current experiments.
Electrical Stimulation
HDFs (100 × 103 cells) were seeded in 60-mm tissue culture dishes (Iwaki, Tokyo, Japan) and cultured for 24, 48, or 72 h. ES was delivered for 1 h because 1-h electrical stimulation therapy is usually adopted in clinical practice.12,19) HDFs were electrical-stimulated once every 24 h after cell-seeding until 96 h (Fig. 1, 2). After stimulation, the electrical charge was removed with an electrical circuit, as described in our previous study.14) Platinum electrodes were used to prevent ion toxicity. Monophasic pulsed ES was delivered in a CO2 incubator at 37°C with a frequency of 1, 2, 4, 8, 16, 32, or 64 Hz. The control group received no electrical stimulation. At a frequency greater than 100 Hz, the current intensity was not stable, and therefore 64 Hz was chosen as the maximum frequency in this study. The duty factor was set to 50%, and the pulse length depended on the frequency (Table 1). The current intensity was 200 μA, which was previously reported as the intensity that most strongly promoted the migration of HDFs to the cathode.15)
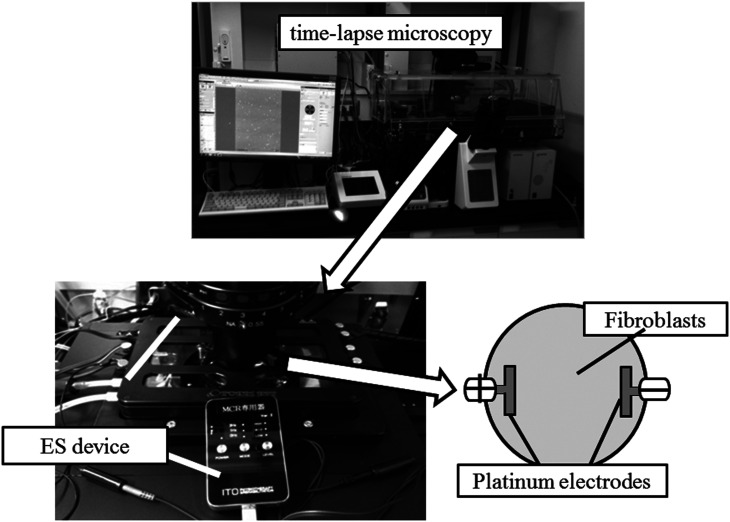
Fig. 1.
Experimental apparatus. Human dermal fibroblasts (HDFs) were observed with a time-lapse microscope until 24 h after the third electrical stimulation. Platinum electrodes were used to prevent ion toxicity.
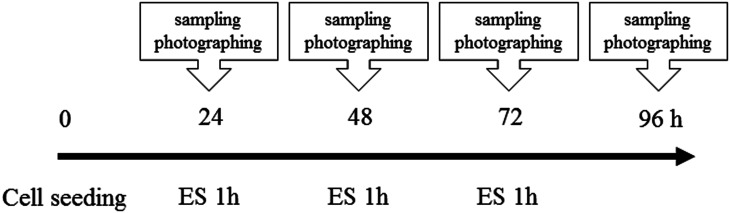
Fig. 2.
Protocol of electrical stimulation (ES) session. ES was conducted every 24 h after cell seeding until 96 h.
Table 1. Electrical stimulation conditions.
| Frequency | Pulse length | Intensity | Times |
| Control | No stimulation | 200 μA | 1 h |
| 1 Hz | 500 ms | ||
| 2 Hz | 250 ms | ||
| 4 Hz | 128 ms | ||
| 8 Hz | 64 ms | ||
| 16 Hz | 32 ms | ||
| 32 Hz | 16 ms | ||
| 64 Hz | 8 ms |
The duty factor was set to 50% and the pulse length depended on the frequency.
Cell Number and Viability
Cell toxicity as a result of ES was analyzed with a trypan blue exclusion test, and living and dead cells were counted with a hemocytometer at 24 h after the third stimulation. Furthermore, HDFs underwent ES at the frequencies that were found to most increase and most decrease the numbers of living cells. Cell numbers and cell viability were evaluated every 24 h until 96 h with one ES session per day at these two frequencies and for the control group. HDFs were observed under a time-lapse microscope (cellSens, OLYMPUS, Tokyo, Japan) using 100 × magnification at a central field in a 60-mm tissue culture dish. All experiments were repeated 7 times.
Statistical Analysis
All data are presented as the mean ± standard deviation. The Student t-test was used to determine the significance of differences between the control group and the ES groups. The significance level was set at P < 0.05.
RESULTS
Cell Numbers and Cell Viability at 24 h after the Third ES
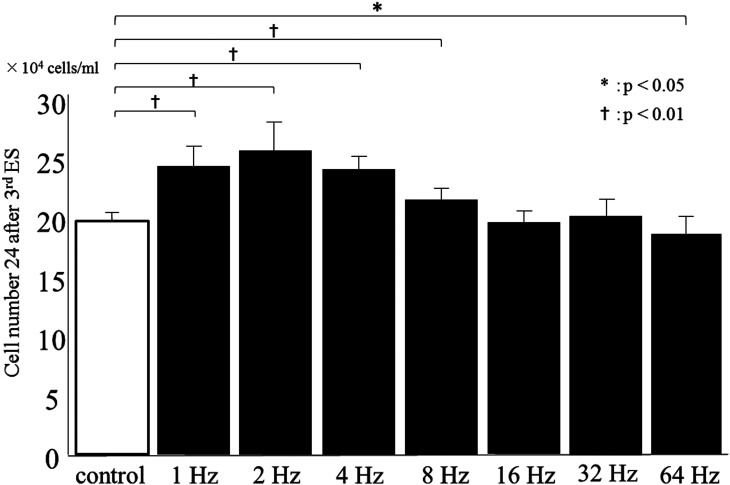
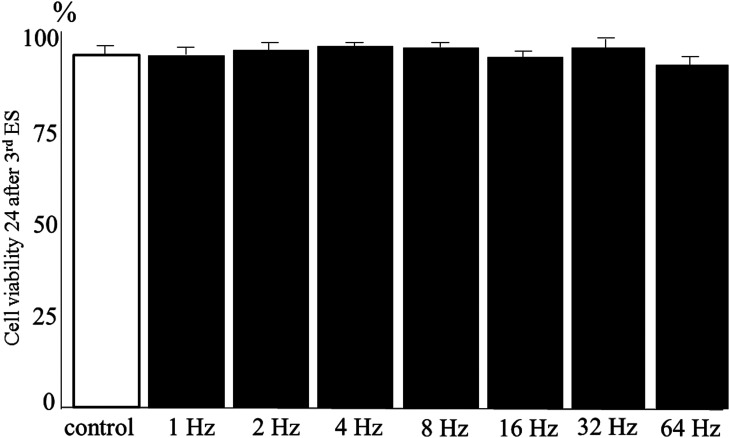
Figure 3 shows that cell numbers at 24 h after the third ES were significantly higher in the 1-, 2-, 4-, and 8-Hz groups compared with that in the control group (P < 0.01). The number of HDFs increased in a frequency-dependent manner up to 2 Hz and then decreased to the control level in the 16 Hz group. Moreover, the number of cells in the 64-Hz group was significantly lower than that of the control group (P < 0.05), suggesting that cell proliferation was suppressed by ES at 64 Hz. Cell viability for all groups was high (> 87%), with no significant differences between the control and ES groups (Fig. 4). Overall, ES at 2 Hz most strongly increased the number of living cells, whereas ES at 64 Hz resulted in a decrease in cell numbers compared to the control.
Fig. 3.
Effect of electrical stimulation (ES) on cell numbers. The number of living HDFs was most strongly increased by ES at 2 Hz. Data are expressed as mean ± SD. †P < 0.01 and *P < 0.05, Student t-test.
Fig. 4.
Cell viability at 24 h after the third ES delivered to HDFs at different frequencies.
Cell Numbers and Cell Viability over Time
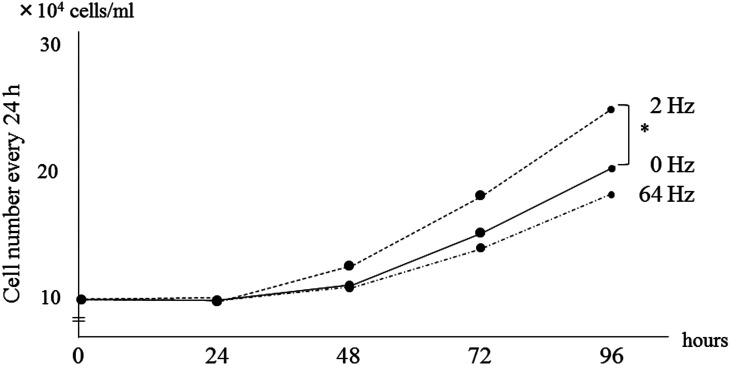
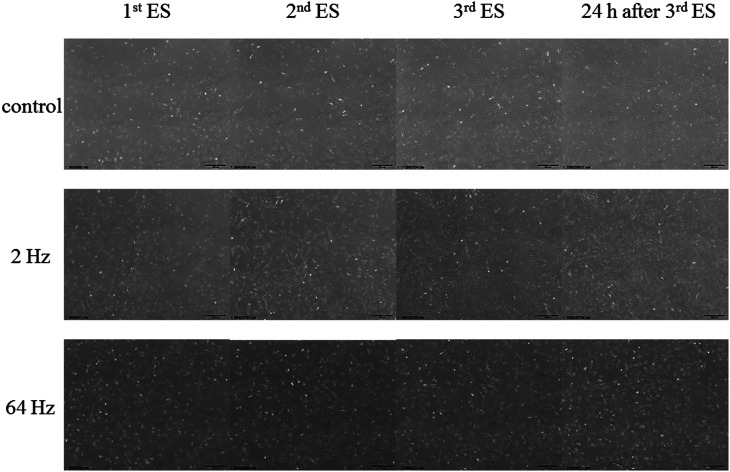
To detect changes in cell growth over time, cell numbers and cell viability in the control and in the 2-Hz and 64-Hz groups were observed every 24 h. There was no significant difference in cell numbers between the control and the 2- and 64-Hz groups after the first day of ES treatment. In the 2-Hz group, the HDFs proliferated after the first day of ES and cell numbers increased further after the second and third days of ES (Fig. 5). In contrast, the cell numbers in the 64-Hz group were lower than those of the control group at all time points. Cell viability was relatively high in the control and 2-Hz groups (91.4–91.9%); however, cell viability in the 64-Hz group slightly decreased over time (Table 2). Fig. 6 shows phase-contrast micrographs of HDFs observed until 96 h under the time-lapse microscopy and photos were taken every 24 h (Fig. 2). The cell density of HDFs stimulated at 2 Hz increased more than did the unstimulated group and the 64-Hz group. In particular, the cell density in the 2-Hz group had markedly increased at 24 h after the third day of ES compared to the other two groups. These data indicate that ES does not induce cell toxicity and that the frequency of ES influences the number of living cells.
Fig. 5.
Cell numbers and cell viability at 24 h after each ES treatment of HDFs in the 2-Hz and 64-Hz groups compared to the unstimulated control. *P < 0.05, Tukey-Kramer test.
Table 2. Cell viability in percent measured every 24 h.
| 24 h | 48 h | 72 h | 96 h | |
| Control | 91.7 | 91.8 | 91.6 | 90.4 |
| 2 Hz | 91.9 | 91.7 | 91.4 | 90.5 |
| 64 Hz | 91.7 | 90.8 | 90.3 | 89.7 |
Fig. 6.
Phase-contrast micrographs of HDFs at 24 h after each ES treatment at 2 Hz and 64 Hz as well as the unstimulated control.
DISCUSSION
The present study revealed that ES of 1, 2, 4, and 8 Hz increased the number of living HDF, and the cell viability was relatively high (87.1–91.8%) in all ES groups. The number of HDFs tended to increase at 24 h after the first, second, and third days of ES treatments in the 96-h experiments at 2 and 64 Hz. Furthermore, the increase in the number of HDFs observed at 24 h after the third ES was similar in the 1-, 2-, and 4-Hz groups. These results suggest that low-frequency ES promotes cell division. The cell numbers in the 8-Hz group increased the least compared with the control, whereas the number of HDFs in the 64-Hz group significantly decreased compared to control. There were no significant differences in cell viability between the control and ES groups. This result is in agreement with a previous study of neuronal precursor cells showing that ES of 50 Hz significantly inhibited cell proliferation compared with the 5- or 10-Hz groups.20) In addition, high-frequency and high-intensity ES suppressed the proliferation of mouse fibroblasts,21) and another study showed that the number of 100-Hz-stimulated cells was essentially unchanged compared with that of the unstimulated group.16) Therefore, the present study suggested that ES delivered at a frequency of more than 10 Hz may suppress the proliferation of HDFs, and ES of more than 64 Hz could substantially suppress cell proliferation. The increase in the number of living HDFs may depend on the pulse length as well as on the frequency of ES. However, the mechanism of the effects of ES on cell proliferation is currently unclear. Rouabhia et al.17) demonstrated that ES at 50–100 mV/mm enhanced the secretion by skin fibroblasts of fibroblast growth factor (FGF)-1 and FGF-2.17) FGFs are known to be important regulators of the growth of fibroblasts.22) In the present study, no effect on cell numbers was observed after the first ES; however, the 2-Hz group showed enhanced growth at 24 h after the third ES. Although we did not directly assess the expression of proliferation-related factors and the influence of ES on the cell cycle, the effects of ES on HDFs observed in the present study may be related to the stimulation of FGF-1/2 expression and promotion of cell cycle progression. To reveal the mechanism contributing to the observed increase in cell numbers, it will be necessary in future studies to investigate changes in the expression of these factors induced by ES.
Our previous clinical study also showed the effects of monophasic pulsed microcurrent stimulation on the healing of pressure injuries in seven patients.12) ES accelerated the healing rate by about ninefold (frequency: 2 Hz; intensity: 80 µA; pulse duration: 250 ms) with the cathode directly contacting the wound. Cell proliferation showed a significant influence on wound healing in a pressure injury model,23) and wound healing requires both the migration and proliferation of HDFs. Our study is the first to determine the optimal frequency for increasing the number of HDFs, and the results suggest that ES at 1–8 Hz with an intensity of 200 μA is the optimal condition to promote the healing of pressure injuries in clinical application, which should also help to safely accelerate healing and shorten the treatment period.
ACKNOWLEDGEMENTS
This study was partially supported by JSPS KAKENHI grant number 22500458.
This publication was supported by JSPS KAKENHI Grant Number JP16HP1002.
Footnotes
CONFLICT OF INTEREST: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Ohura T,Sanada H,Mino Y: Clinical activity-based cost effectiveness of traditional versus modern wound management in patients with pressure ulcers. Wounds 2004;16:157–163. [Google Scholar]
- 2.Sanada H,Miyachi Y,Ohura T,Moriguchi T,Tokunaga K,Shido K,Nakagami G: The Japanese pressure ulcer surveillance study: a retrospective cohort study to determine prevalence of pressure ulcers in Japanese hospitals. Wounds 2008;20:176–182. [PubMed] [Google Scholar]
- 3.Schreier T,Degen E,Baschong W: Fibroblast migration and proliferation during in vitro wound healing. A quantitative comparison between various growth factors and a low molecular weight blood dialysate used in the clinic to normalize impaired wound healing. Res Exp Med (Berl) 1993;193:195–205. [DOI] [PubMed] [Google Scholar]
- 4.National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Ulcer Advisory Panel (EPUAP) and Pan Pacific Pressure Injury Alliance (PPPIA): Prevention and treatment of pressure ulcers: clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel 2014;47. [Google Scholar]
- 5.Feedar JA,Kloth LC,Gentzkow GD: Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther 1991;71:639–649. [DOI] [PubMed] [Google Scholar]
- 6.Adegoke BO,Badmos KA: Acceleration of pressure ulcer healing in spinal cord injured patients using interrupted direct current. Afr J Med Med Sci 2001;30:195–197. [PubMed] [Google Scholar]
- 7.Houghton PE,Campbell KE,Fraser CH,Harris C,Keast DH,Potter PJ,Hayes KC,Woodbury MG: Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010;91:669–678. [DOI] [PubMed] [Google Scholar]
- 8.Recio AC,Felter CE,Schneider AC,McDonald JW: High-voltage electrical stimulation for the management of stage III and IV pressure ulcers among adults with spinal cord injury: demonstration of its utility for recalcitrant wounds below the level of injury. J Spinal Cord Med 2012;35:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin JW,Tooms RE,Mendius RA,Clifft JK,Vander Zwaag R,el-Zeky F: Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Phys Ther 1991;71:433–442, discussion 442–444. [DOI] [PubMed] [Google Scholar]
- 10.Kloth LC,Feedar JA: Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther 1988;68:503–508. [DOI] [PubMed] [Google Scholar]
- 11.Houghton PE,Kincaid CB,Lovell M,Campbell KE,Keast DH,Woodbury MG,Harris KA: Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther 2003;83:17–28. [PubMed] [Google Scholar]
- 12.Yoshikawa Y,Sugimoto M,Maeshige N,Uemura M,Takao A,Matsuda K,Terashi H: The promotional effect of low-intensity direct current stimulation with electrode placement of negative poles at wound site on pressure ulcer healing. J Jpn Phys Ther Assoc 2013;40:200–206 (in Japanese). [Google Scholar]
- 13.Kawasaki L,Mushahwar VK,Ho C,Dukelow SP,Chan LL,Chan KM: The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: a systematic review. Wound Repair Regen 2014;22:161–173. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto M,Maeshige N,Honda H,Yoshikawa Y,Uemura M,Yamamoto M,Terashi H: Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. J Wound Care 2012;21:5-10, discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 15.Uemura M,Maeshige N,Koga Y,Ishikawa-Aoyama M,Miyoshi M,Sugimoto M,Terashi H,Usami M: Monophasic pulsed 200-μA current promotes galvanotaxis with polarization of actin filament and integrin α2β1 in human dermal fibroblasts. Eplasty 2016;16:e6. [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman R,Pollack S: Electric fields and proliferation in a chronic wound model. Bioelectromagnetics 1996;17:450–457. [DOI] [PubMed] [Google Scholar]
- 17.Rouabhia M,Park H,Meng S,Derbali H,Zhang Z: Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One 2013;8:e71660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto E: Effects of electroacupuncture and transcutaneous electrical nerve stimulation on skin temperature in pressure ulcers. Jpn J PU 2013;15:99–104 (in Japanese). [Google Scholar]
- 19.Lim JH,McCullen SD,Piedrahita JA,Loboa EG,Olby NJ: Alternating current electric fields of varying frequencies: effects on proliferation and differentiation of porcine neural progenitor cells. Cell Reprogram 2013;15:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi T,Iwakawa N,Sugimoto M,Harada T: The promotional effect of electrotherapy on pressure ulcer healing. Jpn J Electrophysical Agents 2014;22:44–47 (in Japanese). [Google Scholar]
- 21.Dubey AK,Gupta SD,Basu B: Optimization of electrical stimulation parameters for enhanced cell proliferation on biomaterial surfaces. J Biomed Mater Res B Appl Biomater 2011;98B:18–29. [DOI] [PubMed] [Google Scholar]
- 22.Galzie Z,Kinsella AR,Smith JA: Fibroblast growth factors and their receptors. Biochem Cell Biol 1997;75:669–685. [PubMed] [Google Scholar]
- 23.Tandon N,Cimetta E,Villasante A,Kupferstein N,Southall MD,Fassih A,Xie J,Sun Y,Vunjak-Novakovic G: Galvanic microparticles increase migration of human dermal fibroblasts in a wound-healing model via reactive oxygen species pathway. Exp Cell Res 2014;320:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]