Abstract
While the ICD-DSM paradigm has been a major advance in clinical psychiatry, its usefulness for biological psychiatry is debated. By defining consensus-based disorders rather than empirically driven phenotypes, consensus classifications were not an implementation of the biomedical paradigm. In the field of endogenous psychoses, the Wernicke-Kleist-Leonhard (WKL) pathway has optimized the descriptions of 35 major phenotypes using common medical heuristics on lifelong diachronic observations. Regarding their construct validity, WKL phenotypes have good reliability and predictive and face validity. WKL phenotypes come with remarkable evidence for differential validity on age of onset, familiality, pregnancy complications, precipitating factors, and treatment response. Most impressive is the replicated separation of high- and low-familiality phenotypes. Created in the purest tradition of the biomedical paradigm, the WKL phenotypes deserve to be contrasted as credible alternatives with other approaches currently under discussion.
Keywords: schizophrenia, deep phenotyping, catatonia, supersensitivity psychosis, endogenous psychosis, phenotype, periodic catatonia, cataphasia, affect laden paraphrenia, cycloid psychoses, hebephrenia, system schizophrenia, epistemology, bipolar, unipolar, Wernicke, Kleist, Leonhard, classification
Abstract
Mettre la traduction ES
Abstract
Mettre la traduction FR
Introduction
The field of endogenous psychoses is the one for which the hypothesis of “brain diseases” is the most likely in psychiatry. The past 40 years’ exclusive use of International Classification of Diseases (ICD) – Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnoses, 1 although successful in the field of clinical psychiatry as an applied science, did not allow significant progress in biological psychiatry as a basic science. Two postulates of consensus classifications might have made them unsuitable for this task. First, consensus criteria could not be changed, ruling out any attempt to optimize the descriptions. Second, the atheoretical stance negated any etiological or pathophysiological hypothesis, 2 eg, making no distinction between endogenous and neurotic depressions such as bereavement. 3
The traditional biomedical paradigm starts from phenotypes rather than consensus-based disorders . Embracing the naturalistic framework , 4 it posits that a disease is a natural entity defined by an etio-pathophysiological model which accounts for the phenotype. 5 The model is given at the biological level, assuming a single and rare cause of major effect due to selection pressure. The typical correlation-experimental 2-step process is the theory-of-proof of the biomedical paradigm that validates the model, turning it into a disease . The major strength of this approach stems from this model validity or validity per se which translational research converts into the magic triplet of applied medicine: diagnosis, diagnostic test, and treatment. 4
The limited construct validity of ICD-DSM disorders, even for schizophrenia, 6 and the recurrent failures to validate any biological model that could account for them, raised doubt about the suitability of the biomedical paradigm in basic psychiatry. 7 The leading proposals now turn towards dimensional approaches, which come with a major paradigmatic framework shift with the adoption of normativistic assumptions. 8,9 Here a disease is defined as a pathological deviance , ie, a mere deviation from the norm, which makes the implicit hypothesis of multiple and frequent causes of very small effects. 4 These are typically referred to as risk factors or modifiers in medicine rather than diseases, and translate into much less efficient interventions. 4
Yet, consensus classifications never claimed to be fair implementations of the biomedical paradigm. They were mainly designed for clinical use and not for basic research. Hence, their lack of success in field does not rule out the relevance of the naturalistic framework in psychoses. Indeed, at least one research program, referred to as the Wernicke-Kleist-Leonhard (WKL) pathway, 10 was able to define clear-cut phenotypes. This paper gives an overview of the principles that guided their optimization, 11 and reviews the evidence supporting their construct validity. Validity per se will be only considered for periodic catatonia which currently has the most supported biological model. The terminology has been slightly changed relative to previous publications to adapt to current clinical psychiatry and neuroscience (see Appendix 1).
Appendix 1
Epistemological framework and methods
Major heuristics that guided the empirical elaboration of the phenotypes
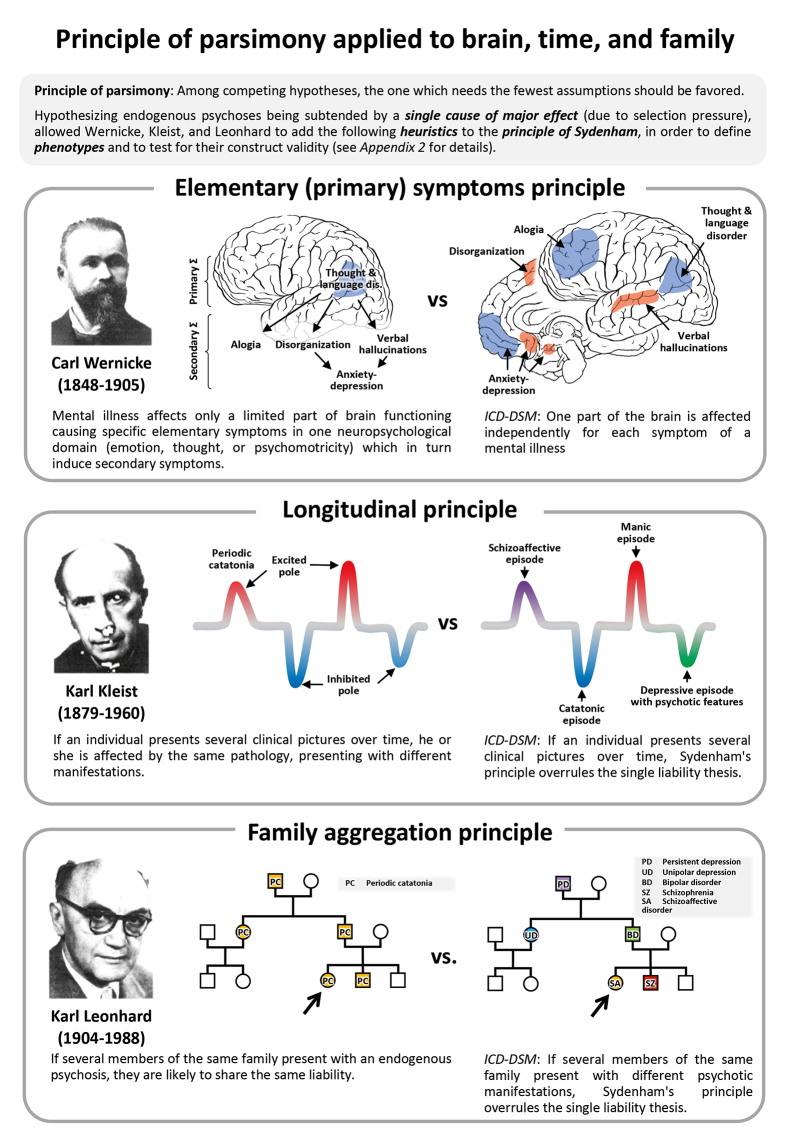
The naturalistic assumptions state that, due to selection pressure, disabling phenotypes are accounted for by a single and rare cause of major effect. Hence, they are categorical in nature and liable to the principle of parsimony . 4 A phenotype is a “typical” set of observable characteristics shared by a group of patients 12 which includes the clinical presentation , ie, the set of reported symptoms and clinical signs collected from the patient’s examination, but also the course of the symptoms , ie, how they appear, which ones persist, which ones disappear, or whether they completely change from one clinical picture to its opposite (bipolarity). Finally, typical contextual elements might also enrich the description. The WKL School empirically optimized their phenotype descriptions by sorting patients according to their long-term catamnestic observations following heuristics stemming from the principle of parsimony: symptom- complex, longitudinal and family-aggregation principles (Box 1;Appendix 2).
Box 1.
Appendix 2
Validity assessment of WKL phenotypes
The construct validity of a phenotype encompasses many different properties. Firstly, to comply with the logical positivist’s call for objectivity, a phenotype must be reliable, 13 and this reliability is assessed by inter-rater reproducibility. Secondly, the fulfillment of the naturalistic assumptions behind the concept of a phenotype could be supported by its predictive, face, differential, and taxonomic validities. Test-retest reproducibility will not only be considered here as a measure of predictive validity 14 but also of face validity , ie, how closely patients match the “typical” definition and to what extent it accounts for all of the patients’ manifestations. 15 Indeed, it shows that phenotype descriptions are either comprehensive enough to include all possible clinical pictures or focus on an unchanging symptom-complex for the diagnosis to remain lifelong stable. Hence it avoids resorting to comorbidities other than behavioral complications, eg, drug abuse. Differential validity looks for the selective associations of a phenotype with external validators through head-to-head comparisons. These can be any clinical, contextual, or biological features that are not part of the original description, 16 eg, age of onset, familiality, gender difference, treatment response, any biological parameter etc. Finally, taxonomic validity appraises the fulfilment of the categorical structure of the phenotypes through taxometric analyses. 17
Validity per se demands a biological causal model accounting for a phenotype. The model validity is assessed through a two-step process acknowledged as the “ theory of proof ” 18 in medicine: the demonstration of a strong correlation with the biological cause and the outbreak or the alleviation of the phenotype with the experimental manipulation of the cause. It has mainly been investigated in periodic catatonia.
Overview of WKL classification
The field of endogenous psychoses
The WKL classification is limited to the field of endogenous psychoses. Psychosis does not have the same meaning here as in the DSM or the ICD . It is not restricted to hallucinations or delusions, but stands for a wide range of specific emotional, cognitive, and behavioral disturbances, supposed to be mainly accounted for by some qualitative disturbances of one cerebral process, ie, naturalistic assumption. It is opposed to the old concept of “neuroses” which putatively results from nature-and-nurture-interactions, eg, the maladaptive response of a given personality coping with a specific life event. These are complex diseases mixing trait risk factors (addition of multiple causes of very small effect, ie, normativistic) interacting with other factors, ie, synergistic assumptions. 4
The endogenous feature refers to Kraepelin’s, Jaspers’, or Birnbaum’s tripartite system in which psychiatric symptoms could be organic (exogenous, ie, secondary to a medical condition including substance-related), reactive (neurotic) or endogenous. 19 Endogeneity assumes a single cause, which yet remains to be discovered.
While the ICD and the DSM distinguish exogenous disorders, the endogenous - neurotic distinction, which could be rephrased as simple vs complex diseases, has completely disappeared due to the endorsement of the atheoretic principle. 13 Consequently, on the psychotic side, psychotic post-traumatic stress disorder, psychotic body dysmorphic disorder, or stress-related brief psychotic reactions, as observed in borderline personality disorder, are not endogenous psychoses. Yet the largest differences lie on the affective side. Reactive, eg, bereavement, and neurotic depressions, which probably account for most major depressive disorders, 20 are not part of the endogenous psychoses in the WKL perspective. It is worth reminding the endogenous-neurotic distinction has been repetitively supported by taxometric analyses of depressive disorders. 21
While most WKL phenotypes are within the scope of affective and psychotic ICD or DSM disorders, there are some exceptions, eg, some system schizophrenias might be diagnosed in the autistic spectrum or in cluster A personality disorders.
Basic features and relationship with consensus classifications
The WKL school defines 35 phenotypes, accounting for about 90% of endogenous psychoses 22 ( Table I ). To achieve this, descriptions do not focus on what phenotypes have in common, but rather in what aspects they differ from one another. For instance, positive symptoms might occur in many phenotypes and hence are not helpful per se. Moreover, in contrast to ICD/DSM , symptoms have no meaning by themselves but only as part of a specific symptom-complex organized according to the primary-secondary principle ( Box 1, Appendix 2 ).
Table I. Overview of the WKL phenotypes (inspired by ref 97). Only the 35 major forms are displayed; the 36 minor forms are two by two combinations of system schizophrenias. See for the consensus on the English translation.
| Course | Family | Neuropsychological domains | Polarity | |||
| Affect | Thought | Psychomotricity | ||||
| Relapsing- remitting | Phasic affective psychoses | Pure depressions (D) | Pure euphorias (E) | Mono- polar | ||
| Agitated D | Unproductive E | |||||
| Hypochondriacal D | Hypochondriacal E | |||||
| Self-torturing D | Exalted E | |||||
| Suspicious D | Confabulatory E | |||||
| Non-participatory D | Non-participatory E | |||||
| Pure mania | ||||||
| Pure melancholia | ||||||
| Manic-depressive illness | Bipolar | |||||
| Cycloid psychoses | Anxiety-happiness psychosis | Excited- inhibited confusion psychosis | Hyperkinetic- akinetic motility psychosis | |||
| Progressive relapsing | Non-system schizophrenias | Affect-laden paraphrenia | Cataphasia | Periodic catatonia | ||
| Progressive | System schizophrenias | (System) hebephrenias (H) | System paraphrenias (P) | System catatonias (C) | Mono- morphic | |
| Foolish H | Hypochondriacal P | Parakinetic C | ||||
| Eccentric H | Voice- hearing P | Pseudo- compulsive C | ||||
| Shallow H | Incoherent P | Proskinetic C | ||||
| Autistic H | Fantastic P | Negativistic C | ||||
| Combined H n=6 | Confabulatory P | Short-circuit- speech C | ||||
| Expansive P | Absentminded C | |||||
| Combined P n=15 | Combined C n=15 |
Phenotypes are grouped into five families 22,23 according to their course, mono- or bipolarity, and their primary affected neuropsychological domains: affect, thought, and psychomotricity ( Table I ). There are one monopolar, three bipolar, and one monomorphic families, gathering not 1 but 12 monopolar affective phenotypes and not 1 but 7 bipolar phenotypes. According to the WKL perspective, the term “ schizophrenia ” only applies to phenotypes with residual symptoms which encompasses one bipolar and the monomorphic families.
The WKL classification is strikingly different from consensus ones. While ICD-10 and DSM-IV have a concordance of λ=0.86 with one another, WKL clearly gathers patients differently since its concordance is only of λ=0.4 with ICD-10 and of 0.56 with DSM-IV . 24
Reliability of WKL phenotypes
On average, WKL phenotypes are highly reliable with 97% of inter-rater diagnostic consistency when performed by expert raters, giving an average kappa value of 0.82 to 0.93. 25,26 In comparison, consensus disorders have kappa values of 0.84 for schizophrenia, 0.71 to 0.83 for bipolar disorder, and 0.22 for schizoaffective disorder. 27
Test-retest reproducibility, prognostic and face validity
In prospective studies, the test-retest reproducibility at 15 years, follow-up was 93% and ranged from 76% to 93% at 33 years follow-up. 28,29 This stands well even in comparison to the much broader ICD diagnosis of schizophrenia which remains consistent in 90% of the patients in retrospective chart review after a follow-up of 25 years. 30
Differential validity of the main phenotypes
Monopolar affective phenotypes with purely relapsing-remitting course
Pure melancholia and pure mania
Pure melancholia and pure mania are monopolar affective phenotypes. 31 The term “ monopolar ” is used here rather than “unipolar” to emphasize the differences between the original WKL concept and consensus classifications. Monopolarity implies symptomatic stability both within and between the episodes, ie, monomorphy , as well as the absence of mixed or incomplete states (see manic-depressive illness). Hence, monopolarity applies also to the manic pole. The independence of the pure mania phenotype has been replicated in the Zurich cohort. 32 While grounded in the affect, pure mania and pure melancholia characteristically also affect the other domains, eg, drive, speed of thought, and psychomotricity.
The prevalence of pure melancholia is many times higher, accounting for up to 10% of endogenous psychoses, whereas pure mania is below 1%. The course is purely relapsing-remitting with an average of 12 months for an episode of melancholia. 33 Symptoms typically respond to usual antidepressant or antimanic therapeutics. Both phenotypes have little inheritance with 3% of affected first-degree relatives which significantly differs from manic-depressive illness (22% to 36%). 34,35
Pure depressions and pure euphorias
These are also relapsing-remitting monopolar phenotypes, ie, monomorphic without mixed or incomplete states (see manic-depressive illness, MDI). 31 The five pure depressions and the five rare pure euphorias are characterized by specific disturbances of distinct emotional systems within the affective domain sparing thought, drive, and psychomotricity. They often go along with characteristic delusions or hallucinations: delusional guilt in self-torturing depression, persecutory ideas in suspicious depression and unpleasant bodily sensations in hypochondriacal depression. These may be ICD -diagnosed as depression with psychotic features or schizoaffective disorders. These phenotypes only account for 4% of inpatients with endogenous psychoses. 33 In contrast to pure melancholia, their episodes typically last years with progressive beginnings and endings 36 and they are less responsive to therapeutics. 36,37 They also have a low familiality when compared with MDI (3% vs 22% to 36%). 34,35
Bipolar phenotypes with purely relapsing-remitting course
In the WKL sense, bipolarity is not limited to affective disorders but extends to schizophrenia-like psychoses as well. Only manic-depressive illness belongs to the affective disorders in the narrower sense.
Manic-depressive illness
MDI 31 is the most frequent bipolar phenotype, accounting for 19% of patients with endogenous psychoses. 35 Even though the ICD/DSM’s concept of bipolar disorder stems from the WKL-MDI one, there are major differences. Episodes have distinctive clinical features allowing MDI to be diagnosed even in patients having depressive recurrences only, in most cases from the first episode. 38 Affective episodes are characterized by their polymorphic manifestations and the mixed or incomplete features, both being currently rediscovered under the emerging concept of bipolar depression. 39 Clinical manifestations are qualified as polymorphic because they change within and between episodes. The span of MDI’s clinical presentations is so large that it can mimic any monopolar or cycloid picture, yet generally not in a stable way. The trigger for these phenotypical changes can be endogenous, but these patients are also highly reactive to external events. For instance, patients can be talkative and lively during the interview, showing no outer manifestation of depression, while apathy and suffering can come back as soon as they walk out of the office. Such mood reactivity can also be observed in neurotic forms, but then of lesser magnitude. Mixed states are defined as the co-occurrence of both the manic and depressive pole among the different domains: affect, thought, and psychomotricity. This can be seen for instance in the combination of inhibited affect (sadness), excited thinking process (racing thoughts), and excited psychomotricity (agitation). 38 Incomplete states are an extension of the former concept, meaning that aside from being excited and inhibited, a single domain can also be completely unaffected. For instance, affect and psychomotricity might be inhibited while the speed of thought might be normal.
On average, MDI episodes are of shorter duration than monopolar ones, ie, 6 months on average for depressive episodes. 33 Acute onset, sudden cessation; or rapid switches are common. This phenotype shows more frequent relapses than monopolar phenotypes and this tendency tend to increase with aging. 33
The hereditary burden of MDI is significantly higher than for monopolar affective phenotypes and cycloid psychoses, with 22% to 36% of affected first-degree relatives. 34,35,40 There are two reasons for the familiality of MDI to exceed the one of ICD/DSM bipolar affective disorder (9%). 41 Firstly, as the MDI diagnosis can be made early, even if the clinical presentation is purely depressive, most intra-familial incongruencies vanish as nearly all of the (pseudo-)unipolar patients are diagnosed as MDI. 42 Secondly, ICD/DSM bipolar disorder subsumes some cycloid psychoses which have low familiality.
Cycloid psychoses
Cycloid psychoses are bipolar phenotypes of purely relapsing-remitting course. They have more intense psychotic manifestations, and are hence routinely diagnosed by ICD/DSM as schizoaffective or schizophrenic disorders. There are three different cycloid psychoses corresponding to the predominantly affected domain within which they quantitatively oscillate between opposite extremes. These are referred to as “poles,” organized into three axes:
• Hyperkinetic-akinetic motility psychosis in the psychomotor domain
• Anxiety-happiness psychosis in the emotional domain
• Excited-inhibited confusion psychosis in the thought domain.
Cycloid psychoses represent 20% of all endogenous psychoses. 35 Their clinical manifestations are highly polymorphic , due to rapid changes in the intensity and even in the polarity of the manifestations within the same episode. Importantly however, the opposite poles always manifest successively and never at the same time.
The ICD-10 diagnosis of “acute and transient psychotic disorders” or ATPD (F23), was designed to embody these phenotypes together with the “ bouffées délirantes aiguës des dégénérés ” 43 or BDA (acute delusional outburst of the degenerates). 44 Yet, studies have found that ATPD only overlaps with the BDA and cycloid diagnoses in half of the cases. 44,45 Furthermore, cycloid psychoses are defined as lifelong phenotypes, while BDA and ATPD are only defined as episodes. Hence the latter diagnoses are instable on follow-up: a third of initial BDA switches to schizophrenia or schizoaffective disorder after 10 years, 46 while it happens in half of initial ATPD after 5 years. 47
Cycloid episodes usually last between 1 to 3 months, and have acute onset and ending in up to two thirds of the cases. 33 Yet, these two features are neither sensitive nor specific enough to be used as diagnostic criteria. 48 The relapsing-remitting course means that, in the interepisode interval, patients develop full insight about their illness and do not present significant residual symptoms whatever the number of recurrences. 48,49 Cycloid psychoses might be related to minimal brain damage. Unspecific MRI abnormalities are more frequent relative to non-system schizophrenias, eg, enlarged ventricles, white matter hyperintensity, or small cortical defects. 50‑52 These might be acquired early: mothers of cycloid patients report significantly more infections of the upper airway during the first trimester of pregnancy, childbirth complications are more frequent and seasonality of birth is larger in cycloid phenotypes relative to controls and non-system schizophrenias. 52‑54 Conversely, the heritability of these phenotypes is low, with only 5% of affected first-degree relatives, indistinguishable from controls and significantly lower than in MDI, cataphasia, and periodic catatonia. 34,35,40,55
Patients affected by cycloid psychoses are more vulnerable to precipitating factors: stress, sleep disorders, cannabis, etc. Women are especially sensitive to estrogen drop: 88% of episodes start in the luteal phase, which is significantly higher than for any other phenotype. 56 Accordingly, cycloid phenotypes account for 60% of postpartum psychoses, with motility psychosis accounting for 36% on its own. 57
Antipsychotics shorten the episodes but should be used with caution in motility psychosis, which is especially at risk for neuroleptic malignant syndrome. 58 They are also effective in relapse prevention, bearing in mind that these patients are especially sensitive to their side effects. The maintenance of too-high doses of first-generation antipsychotics after remission favors post-psychotic depression and abulia, so that otherwise fully remitted cycloid patients might appear to suffer from residual schizophrenia. 59 Yet, once maintained for more than a month, the rapid discontinuation of antipsychotics increases the risk of relapse to a point that was unknown in the pre-neuroleptic era, 59,60 raising the hypothesis that most these relapses might be induced dopamine supersensitivity psychosis. 59,60 Mood stabilizers not only help as an add-on treatment in the acute phase, but might also be considered as viable alternatives to antipsychotics in the maintenance phase considering their decent relapse prevention. 48
Phenotypes with build-up of residual symptoms: the schizophrenias
In the WKL perspective, the term “ schizophrenia ” carries a prognostic value as these phenotypes progress toward a persistent residual state of which abulia is a frequent, though not characteristic, feature. WKL schizophrenias have phenotype-specific residual symptoms.
“System” and “nonsystem” schizophrenias have nothing to do with the concept of “delusion systematization,” ie, the logical organization of delusional ideas. Here, “ system ” must be understood analogously to the involvement of a specific biological function as in organic medicine, ie, system diseases. Regarding brain diseases, these systems are functional networks, eg, the pyramidal system is the one that degenerates in amyotrophic lateral sclerosis. Multiple systems can be affected, as in multiple-system atrophy, which combines the degeneration of extrapyramidal, cerebellar, and vegetative systems. Due to their clear-cut and life-long monomorphic residual symptoms, system schizophrenias are qualified as such because they are supposed to be accounted for by the impairment of such specific functional networks, whereas non-system schizophrenias are polymorphic , bipolar , and putatively involve many “systems.”
Non-system schizophrenias
There are three non-system schizophrenias characterized by a predominantly affected domain within which they can express both poles. In contrast to cycloid psychoses, changes are not purely quantitative, but also qualitative , with symptoms from both poles occurring together. Because of their bipolarity, they show a broad, yet specific, clinical spectrum. They mostly run a progressive-relapsing course and develop a characteristic set of residual symptoms of increasing severity. All have a specific heredity burden, without crossed liability . Interestingly, domain-specific attenuated symptoms have been reported in nonpsychotic relatives, especially in obligate carriers. 61 As a whole, nonsystem schizophrenias respond much better to antipsychotics 62,63 and to the addition of mood stabilizers 37 compared with system schizophrenias. However, treatments mostly improve acute manifestations but have virtually no effect on residual symptoms.
Affect-laden paraphrenia is a schizophrenic bipolar phenotype of the affective domain. It only accounts for 5% of endogenous psychoses but for 10% of ICD/DSM psychotic disorders. 33,35 Its various clinical presentations have been independently described by many authors under various names around the world. 46,64-66,46,67-69 The WKL school subsumed them under the same phenotype because individuals could change from one clinical picture to the other and because relatives could display one of the other clinical pictures ( Box 1 ). 70 The core of affect-laden paraphrenia is a paranoid mood which encompasses the strong mistrust, irritability and hostility of one pole blended with the feeling of self-importance of the other pole. This specific affective state leads to more or less systematized delusions of persecution and grandiosity often accompanied by multimodal hallucinations. 71 The residual picture is the irritated reference syndrome , which is a delusional construction about intentions of specific others regarding oneself. Besides the pathological affect underpinning the delusions, emotional responses dampen over time. A feature that repeatedly impressed many authors was the contrast between the judgment errors , up to the acceptance of fantastic ideas, with a generally well-organized thought process which is constant out of the episode. 46,64,69 The course is mostly progressive-relapsing. Over 10 to 30 years, patients develop increasingly pervasive reference ideas of more and more fantastic coloring. Yet they remain able to adapt to the interviewer in superficially denying their delusions.
Antipsychotics help in blunting the affective pressure that ensues, but also fuels the delusions, yet never allowing the patients to fully distance themselves from their ideas (84% of responders). 62,63 The median age of onset is 36 years, but is highly variable explaining late-onset cases. The phenotype has an autosomal recessive inheritance pattern with 12% of affected siblings vs only 2% of affected parents. 34,35 There is also a significantly larger number of patients born from consanguineous weddings relative to other schizophrenias and cycloid psychoses (3.3% vs 1%). 56,61
Cataphasia (schizophasia) is a bipolar phenotype mainly affecting thoughts and language. It accounts for about 8% of endogenous psychoses and its estimated prevalence is about 0.1 to 0.2% in Germany. 72 Its excited pole was first described by Kraepelin under the label “ schizophasia .” The observation of multiplex families allowed to relate this clinical picture to its counter-pole dominated by thought inhibition. 73 The core of the phenotype is a specific thought and language disorganization with incoherence and logical derailment coming with syntactic and semantic errors, eg, paragrammatism, paraphasias, and neologisms. These core symptoms need to be specifically investigated, especially in the residual phase. As everyday concrete thinking is less affected, they frequently remain discreet in ordinary conversations and behavior. The thought and language test , a standardized WKL examination procedure that challenges abstract thinking, greatly sensitizes the detection of cataphasic features. 72,74,75 Language errors must be appraised in the context of patients’ skills, so are hence harder to ascertain in non-native speakers; in such cases they may be secured by long-term follow-up re-examination. As the disease progresses, nonspecific fluctuating persecutory ideas might remain but are secondary to the core residuum which impairs patients’ understanding, leading to misinterpretations in close similarity with residual Wernicke’s aphasias. 76 During episodes, patients exhibit a variety of affective and psychotic symptoms, that are frequently in the foreground.
Although the episodes respond to antipsychotics (up to 78% using first-generation drugs), 62,63 the specific symptoms are treatment-resistant. The association of thought disorganization with emotional turmoil make cataphasic patients particularly at risk for suicidal behavior (52% of patients) and deaths by suicide (18% of patients). 72 The phenotype shows familial aggregation, with 15% to 25% of affected first-degree relatives, on top of which 12% of non-psychotic first-degree relatives also show milder forms of the typical thought and language disorganization. 34,35,72 A genetic locus has recently been found for cataphasia on Chr11p, the strongest association being found with a gene coding for cathepsin-D, a lysosomal protease which mutations can cause neurodegenerative storage disorder (Roth et al, unpublished material).
In accordance with their residual thought and language impairments, cataphasic patients have a specific dysfunction of their temporoparietal junctions bilaterally; these are hypoactive and functionally disconnected. 77 This fits with multimodal imaging results showing that the same cortices, together with their underlying white matter, were hypo-myelinated and had an increased iron content (Foucher et al, unpublished material). Considering that the latter could likely result from microglial activation, these findings are in line with those reported in cathepsin-D deficits, 78 suggesting a neurodegenerative model for cataphasia.
Periodic catatonia is roughly as common as cataphasia, accounting for about 7% of patients suffering from endogenous psychoses. Despite its name, WKL’s periodic catatonia should not be viewed as the mere recurrence of IDC/DSM catatonic episodes. 79 Beyond mere global bipolar quantitative motility changes, the core of this phenotype is a specific disorganization of psychomotor functions, ie, mostly affecting expressive and reactive movements. The qualitative changes manifests as the mixing of both akinesia and hyperkinesia but to different body parts, eg, rigid hypokinesia of the upper limb together with facial restlessness. Other qualitative anomalies are parakinesias that alter simple movements, making them appear stiff and/or jerky, or distort expressive movements, especially the mimics, going as far as grimacing. 71 These are currently rediscovered under the name of spontaneous dyskinesias. 80 However, parakinesias have a wider spectrum and distinctive features that allow them to be differentiated from tardive dyskinesias. 81 The residual state includes the persistence of these characteristic psychomotor anomalies together with abulia, while residual psychotic symptoms are rare. The social or occupational impairment is highly variable (GAF =57±19 after an average of 13 years of progression). 77,82
This phenotype is responsive to antipsychotics (60% of responders with first-generation drugs), 62,63 but also sensitive to their extrapyramidal side effects, hence its large response increment after switching to clozapine. 37,83 It further benefits from benzodiazepines 84 and electroconvulsive therapy which are inefficient in system catatonias. 85 Yet all therapeutic efforts can only help coping with exacerbations but fail to improve the specific residual symptoms.
At the etiological level , several studies have confirmed the high heritability of periodic catatonia, with 21% to 26% of affected first-degree relatives, 34,35,83 which is significantly larger than for system catatonias (4%). 25 Considering the extended phenotype, ie, including nonpsychotic relatives with only psychomotor signs, the percentage raises to 32% to 41% of affected first-degree relatives. 86,87 Transmission is autosomal dominant with incomplete penetrance and anticipation. Two genome-wide linkage studies found a major susceptibility locus on Chr15q accounting for about two thirds of the pedigrees (OMIM 605419). 88,89 This has recently been supported by an association peaking in an intergenic region between CGNL1 and GCOM1 (Gawlik et al, unpublished material), the latter being implicated in an NMDA-dependent neuroprotection pathway that might be especially important for GABAergic interneurons. 90 Yet periodic catatonia is likely to by genetically heterogeneous: other pedigrees matched on other loci, eg, Chr21q13-ter. 89 The unity of the phenotype might be better explained at the pathophysiological level . Based on previous literature, especially on the independent replication of its specific left premotor hyperactivity 91,77 when compared with other psychoses, periodic catatonia is currently modeled as an acquired deficit of intra-cortical inhibition possibly ensuing the degeneration of GABA interneurons. As a first validation step, the strength of the correlation between left premotor hyperactivity and the phenotype was prospectively tested in individual patients by comparing a new group of periodic catatonias to other psychoses, including system catatonias. The association was found to be both sensitive (98%) and specific (88%), making the case for this functional imaging measure to be a viable biomarker. 92 As interventional validation step, personalized rTMS was used to correct left premotor inhibition deficit. Not only did the improvement of residual symptoms resulted in substantial functional gains, but was also specific for premotor targets (vs prefrontal and parietal ones) and for periodic catatonia (vs system catatonias). 93
System schizophrenias
System schizophrenias account for 21% of inpatients with endogenous psychoses. 33 They have an insidiously progressive course resembling that of slow encephalitis. They begin with a process phase of 1 to 5 years, in which unspecific dysthymic and psychotic manifestations can accompany the growth of a distinct symptom-complex, presumably due to the deterioration of a specific system. After processual symptoms vanish, the residual clinical picture will remain unchanged up to the end of the patient’s life, ie, monomorphic. 94 Phenotypes are ordered according to the domain to which belongs the affected system:
• The four phenotypes of hebephrenias share a specific disturbance of judgement emotions which leads to affective flattening and loss of initiative. Judgement emotions are the one needed to evaluate non-concrete and non-present issues such as the course of life
• There are six major phenotypes of system paraphrenias having specific combinations of hallucinatory and delusional features
• System catatonias consist of six varieties of definite qualitative psychomotor impairment.
The three subfamilies have different age of onset: 24 for system catatonias and 23 for hebephrenias, but 36 for system paraphrenias. 33 No significant hereditary burden has been reported. The percentage of 2% to 4% of affected first-degree relatives is not significantly different from what is seen in controls but significantly different from periodic catatonia. 25,34,35,83 In system catatonias, 34% of the mothers report an infection of the upper airways during the second trimester of pregnancy which significantly differs from periodic catatonia (8%). 95 Neuroimaging reveals significantly more cortical atrophy in system schizophrenias than in non-system phenotypes. 96,97 Finally, contrary to other phenotypes, interventions have little or no effectiveness: antipsychotics (1% to 40% of responders to first-generation antipsychotics), 62,63 no advantage for clozapine, 37,83 ECT, mood stabilizers, or antidepressants. 37
Conclusion
In accordance with the biomedical paradigm, the WKL School has empirically optimized the descriptions of putatively natural phenotypes inspired by neuroscience and based on common medical heuristics. They are reliable, they have good predictive validity and differential validity regarding gender ratio, age of onset, familiality (without crossed-heritability), pregnancy complications, and response to treatment. Only their taxonomic validity deserves to be further evaluated. While the biological model for cataphasia remain to be tested, the one for periodic catatonia has already been supported by correlational and interventional evidence.
Despite their elaboration in the purest tradition of the biomedical paradigm, yet diverging from dominant paradigms, these phenotypes received poor attention from basic researchers (see also Appendix 2 ). On the other hand, clinicians value them for their long-term stability and their prognostic and therapeutic relevance. We hope that this review will contribute to revive the interest of the psychosis research community for this research program which deserves to be confronted with others in an adversarial collaborative way. 4
Acknowledgments
The authors have no financial disclosures to declare. We dedicate this paper to the memory of Prof Gerald Stöber (03/03/1961 – 08/06/2017). We would like to express our gratitude to him for his enthusiastic teaching and his inspiring and tireless research work in the purest biomedical tradition
Contributor Information
Jack R. Foucher, ICube - CNRS UMR 7357, neurophysiology, FMTS, University of Strasbourg, France ; CEMNIS - Noninvasive Neuromodulation Center, University Hospital Strasbourg, France..
Micha Gawlik, Department of Psychiatry and Psychotherapy, University of Würzburg, Würzburg, Germany..
Julian N. Roth, Department of Psychiatry and Psychotherapy, University of Würzburg, Würzburg, Germany..
Clément de Crespin de Billy, ICube - CNRS UMR 7357, neurophysiology, FMTS, University of Strasbourg, France; CEMNIS - Noninvasive Neuromodulation Center, University Hospital Strasbourg, France..
Ludovic C. Jeanjean, IICube - CNRS UMR 7357, neurophysiology, FMTS, University of Strasbourg, France; CEMNIS - Noninvasive Neuromodulation Center, University Hospital Strasbourg, France..
Alexandre Obrecht, ICube - CNRS UMR 7357, neurophysiology, FMTS, University of Strasbourg, France; Pôle de Psychiatrie, Santé Mentale et Addictologie, University Hospital Strasbourg, France..
Olivier Mainberger, ICube - CNRS UMR 7357, neurophysiology, FMTS, University of Strasbourg, France. CEMNIS - Noninvasive Neuromodulation Center, University Hospital Strasbourg, France..
Julie M. E. Clauss, Pôle de Psychiatrie, Santé Mentale et Addictologie, University Hospital Strasbourg, France. SAGE – CNRS UMR 7363, FMTS, University of Strasbourg, France..
Julien Elowe, Department of Psychiatry, Prangins Psychiatric Hospital (CHUV), Route de Benex, Prangins, Switzerland;.
Sébastien Weibel, IPôle de Psychiatrie, Santé Mentale et Addictologie, University Hospital Strasbourg, France; Physiopathologie et Psychopathologie Cognitive de la Schizophrénie – INSERM 1114, FMTS, University of Strasbourg, France..
Benoit Schorr, Pôle de Psychiatrie, Santé Mentale et Addictologie, University Hospital Strasbourg, France; Physiopathologie et Psychopathologie Cognitive de la Schizophrénie – INSERM 1114, FMTS, University of Strasbourg, France..
Marcelo Cetkovich, Institute of Translational and Cognitive Neuroscience (INCyT), INECO Foundation, Favaloro University, Buenos Aires, Argentina; National Scientific and Technical Research Council (CONICET), Buenos Aires, Argentina..
Carlos Morra, ICube - CNRS UMR 7357, neurophysiology, FMTS, University of StInstitute of Translational and Cognitive Neuroscience (INCyT), INECO Foundation, Favaloro University, Buenos Aires, Argentina; National Scientific and Technical Research Council (CONICET), Sanatorio Morra, Córdoba, Argentina..
Federico Rebok, “Servicio de Emergencia”, Acute Inpatient Unit, Moyano Neuropsychiatric Hospital, Buenos Aires, Argentina..
Thomas A. Ban, International Network for the History of Neuropsychopharmacology (INHN), Córdoba, Argentina..
Barbara Bollmann, Klinik für Psychiatrie, Psychotherapie und Psychosomatik, Berlin, Germany..
Mathilde M. Roser, Department of Psychiatry, Mondor Hospital France, Creteil, France..
Markus S. Hanke, Universitäre psychiatrische Dienste Bern, Spiez, Switzerland..
Burkhard E. Jabs, Klinik für Psychiatrie and Psychotherapie, Städtisches Klinikum Dresden, Dresden, Germany..
Ernst J. Franzek, Yes We Can Clinics, Department of Research and Development, Eindhoven, The Netherlands..
Fabrice Berna, Department of Psychiatry and Psychotherapy, University of Würzburg, Würzburg, Germany; Department of Psychiatry, Prangins Psychiatric Hospital (CHUV), Route de Benex, Prangins, Switzerland..
Bruno Pfuhlmann, IKlinik für Psychiatrie and Psychotherapie, Städtisches Klinikum Dresden, Dresden, Germany..
REFERENCES
- 1.Kupfer DJ, First MB, Regier DA, Kupfer DJ, First MB, Regier DA. Washington, DC: American Psychiatric Association; 2002. Introduction A Research Agenda for DSM-V pp. xviii–xix. [Google Scholar]
- 2.Foucher J-R, Bennouna Greene V. La CIM et le DSM ou l’impossible validation : pourquoi le ver est dans le fruit. Ann Médico-psychologiques, Rev Psychiatr. 2010;168(8):609–615. [Google Scholar]
- 3.Friedman RA. Grief, depression, and the DSM-5. N Engl J Med. 2012;366(20):1855–1857. doi: 10.1056/NEJMp1201794. [DOI] [PubMed] [Google Scholar]
- 4.Foucher JR, et al Bases épistémologiques de la recherche sur les psychoses. Quelle solution pour le choc des cadres paradigmatiques ? Ann Med Psychol. 2019 doi: 10.1016/j.amp.2018.
10.022. [DOI] [Google Scholar]
- 5.Boorse C. Health as a theoretical concept. Philos Sci. 1977;44(4):542–573. [Google Scholar]
- 6.Tandon R. The nosology of schizophrenia. Psychiatr Clin North Am. 2012;35(3):557–569. doi: 10.1016/j.psc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Heckers S. Making progress in schizophrenia research. Schizophr Bull. 2007;34(4):591–594. doi: 10.1093/schbul/sbn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King LS. What is disease? Philos Sci. 1954;21(3):193–203. [Google Scholar]
- 9.Wakefield JC. The concept of mental disorder on the boundary between biological facts and social values. Am Psychol. 1992;47(3):373388. doi: 10.1037//0003-066x.47.3.373. [DOI] [PubMed] [Google Scholar]
- 10.Shorter E. New York, NY: Oxford University Press; 2005. Wernicke-Kleist-Leonhard pathway A Historical Dictionary of Psychiatry pp. 300–303. [Google Scholar]
- 11.Ungvari GS. The Wernicke-Kleist-Leonhard school of psychiatry. Biol Psychiatry. 1993;34(11):749–752. doi: 10.1016/0006-3223(93)90062-i. [DOI] [PubMed] [Google Scholar]
- 12.Dawkins R. Oxford, UK: Oxford University Press; 1989. The Extended Phenotype [Google Scholar]
- 13.Hempel CG, Zubin J. New York, NY: Grune and Stratton; 1961. Introduction to problems of taxonomy Field Studies in the Mental Disorders pp. 3–22. [Google Scholar]
- 14.Fulford KWM, Thornton T, Graham G, Fulford KWM, Thornton T, Graham G. Oxford, UK: Oxford University Press; 2006. Natural classifications, realism, and psychiatric science Oxford Textbook of Philosophy and Psychiatry pp. 316–383. [Google Scholar]
- 15.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1(1):9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126(7):107–111. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- 17.Beauchaine TP. A brief taxometrics primer. J Clin Child Adolesc Psychol. 2007;36(4):654–676. doi: 10.1080/15374410701662840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glymour C. Cambridge, MA: The MIT Press; 2015. Thinking Things through: An Introduction to Philosophical Issues and Achievements [Google Scholar]
- 19.Häfner H. Descriptive psychopathology, phenomenology, and the legacy of Karl Jaspers. Dialogues Clin Neurosci. 2015;17(1):19–29. doi: 10.31887/DCNS.2015.17.1/hhaefner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shorter E. The doctrine of the two depressions in historical perspective. Acta Psychiatr Scand. 2007;115(s433):5–13. doi: 10.1111/j.1600-0447.2007.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruscio J, Brown TA, Meron Ruscio A. A Taxometric investigation of DSM-IV major depression in a large outpatient sample. Assessment. 2009;16(2):127–144. doi: 10.1177/1073191108330065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonhard K. Vienna, Austria: Springer Vienna; 1999. Classification of Endogenous Psychoses and Their Differentiated Etiology [Google Scholar]
- 23.Leonhard K. Stuttgart, Germany: Thieme; 2003. Aufteilung Der Endogenen Psychosen Und Ihre Differenzierte Ätiologie : 54 Tabellen [Google Scholar]
- 24.Peralta V, Goldberg X, Ribeiro M, Sanchez-Torres AM, Fañanás L, Cuesta MJ. Familiality of psychotic disorders: A polynosologic study in multiplex families. Schizophr Bull. 2016;42(4):975–983. doi: 10.1093/schbul/sbv192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckmann H, Franzek E, Stöber G. Genetic heterogeneity in catatonic schizophrenia: A family study. Am J Med Genet. 1996;67(3):289–300. doi: 10.1002/(SICI)1096-8628(19960531)67:3<289::AID-AJMG5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Pfuhlmann B, Franzek E, Stöber G, Cetkovich-Bakmas M, Beckmann H. On interrater reliability for Leonhard’s classification of endogenous psychoses. Psychopathology. 1997;30(2):100–105. doi: 10.1159/000285036. [DOI] [PubMed] [Google Scholar]
- 27.Maj M, Pirozzi R, Formicola AM, Bartoli L, Bucci P. Reliability and Validity of the DSM-IV Diagnostic Category of Schizoaffective Disorder: Preliminary Data 2000 doi: 10.1016/s0165-0327(99)00059-2. [DOI] [PubMed] [Google Scholar]
- 28.von Trostorff S, Leonhard K. Catamnesis of endogenous psychoses according to the differential diagnostic method of Karl Leonhard. Psychopathology. 1990;23(4-6):259–262. doi: 10.1159/000284669. [DOI] [PubMed] [Google Scholar]
- 29.Pethő B, Tolna J, Tusnády G, et al The predictive validity of the Leonhardean classification of endogenous psychoses. Eur Arch Psychiatry Clin Neurosci. 2008;258(6):324–334. doi: 10.1007/s00406-007-0799-y. [DOI] [PubMed] [Google Scholar]
- 30.Marneros A, Deister A, Rohde A. Stability of diagnoses in affective, schizoaffective and schizophrenic disorders. Cross-sectional versus longitudinal diagnosis. Eur Arch Psychiatry Clin Neurosci. 1991;241(3):187–192. doi: 10.1007/BF02219720. [DOI] [PubMed] [Google Scholar]
- 31.Leonhard K. Vienna, Austria: Springer Vienna; 1999. Clinical pictures of phasic psychoses (without cycloid psychoses) Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 6–60. [Google Scholar]
- 32.Angst J, Grobler C. Unipolar mania: a necessary diagnostic concept. Eur Arch Psychiatry Clin Neurosci. 2015;265(4):273–280. doi: 10.1007/s00406-015-0577-1. [DOI] [PubMed] [Google Scholar]
- 33.Leonhard K. Vienna, Austria: Springer Vienna; 1999. Age of onset, sex incidence, course Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 250–274. [Google Scholar]
- 34.Leonhard K. Vienna, Austria: Springer Vienna; 1999. Etiology of Endogenous Psychoses Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 278–329. [Google Scholar]
- 35.Leonhard K, Seidel K, Neumärker K-J, Schulze HAF. Vienna, Austria: Springer Vienna; 1986. Lassen sich die Schizophrenien klinisch und ätiologisch trennen? Zur Klassifikation Endogener Psychosen pp. 26–42. [Google Scholar]
- 36.Kutcher M, Ban TA, Fjetland OK, Morey LC, Beckmann H, Neumärker K-J. Berlin, Germany: Ullstein-Mosby; 1995. Leonhard’s classification of unipolar depression - a comparison with other classifications Endogenous Psychoses: Leonhard’s Impact on Modern Psychiatry pp. 203–207. [Google Scholar]
- 37.Ban TA. Clinical pharmacology and Leonhard’s classification of endogenous psychoses. Psychopathology. 1990;23(4-6):331–338. doi: 10.1159/000284677. [DOI] [PubMed] [Google Scholar]
- 38.Pfuhlmann B. Dresden, Germany: Steinkopff Verlag; 2003. Leonhards Konzeption der manisch-depressiven Erkrankung Familienbefunde Bei Bipolaren Phasischen Psychosen Und „atypischen“ Psychosen pp. 15–17. [Google Scholar]
- 39.Ghaemi SN, Saggese J, Goodwin FK, El-Mallakh RS, Ghaemi SN. Washington, DC: American Psychiatric Pub; 2006. Diagnosis of bipolar depression Bipolar Depression: A Comprehensive Guide pp. 3–36. [Google Scholar]
- 40.Pfuhlmann B. Heidelberg, Germany: Steinkopff Verlag; 2003. Familienbefunde bei bipolaren phasischen Psychosen und „atypischen“ Psychosen Familienbefunde Bei Zykloiden Psychosen Und Manisch-Depressiver Erkrankung pp. 39–47. [Google Scholar]
- 41.Wilde A, Chan H-N, Rahman B, et al A meta-analysis of the risk of major affective disorder in relatives of individuals affected by major depressive disorder or bipolar disorder. J Affect Disord. 2014;158:37–47. doi: 10.1016/j.jad.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet Part C Semin Med Genet. 2003;123C(1):48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 43.Legrain PM. Paris, France: Asselin; 1886. Le Délire Chez Les Dégénérés [Google Scholar]
- 44.Pillmann F, Haring A, Balzuweit S, Blöink R, Marneros A. Concordance of acute and transient psychoses and cycloid psychoses. Psychopathology. 34(6):305–311. doi: 10.1159/000049329. [DOI] [PubMed] [Google Scholar]
- 45.Pillmann F, Haring A, Balzuweit S, Blöink R, Marneros A. Bouffée délirante and ICD-10 acute and transient psychoses: a comparative study. Aust N Z J Psychiatry. 2003;37(3):327–333. doi: 10.1046/j.1440-1614.2003.01184.x. [DOI] [PubMed] [Google Scholar]
- 46.Ey H, Bernard P, Brisset C, Garrabé J, Guelfi J-D. Paris, France: Masson; 1960. Manuel de Psychiatrie [Google Scholar]
- 47.Marneros A, Pillmann F, Marneros A, Pillmann F. Cambridge, UK: Cambridge University Press; The long-term outcome Acute and Transient Psychoses pp. 143–156. [Google Scholar]
- 48.Perris C. A study of cycloid psychoses. Acta Psychiatr Scand Suppl. 1974;253:1–77. [PubMed] [Google Scholar]
- 49.Beckmann H, Fritze J, Lanczik M. Prognostic validity of the cycloid psychoses: A prospective follow-up study. Psychopathology. 1990;23(4-6):205–211. doi: 10.1159/000284662. [DOI] [PubMed] [Google Scholar]
- 50.Becker T, Stöber G, Lanczik M, Hofmann E, Franzek E, Beckmann H, Neumärker K-J. Berlin, Germany: Ullstein-Mosby; 1995. Cranial computed tomography and differentiated psychopathology: are there patterns of abnormal CT findings? Endogenous Psychoses: Leonhard’s Impact on Modern Psychiatry pp. 230–234. [Google Scholar]
- 51.Franzek E, Becker T, Hofmann E, Flöhl W, Stöber G, Beckmann H. Is computerized tomography ventricular abnormality related to cycloid psychosis? Biol Psychiatry. 1996;40(12):1255–1266. doi: 10.1016/0006-3223(95)00623-0. [DOI] [PubMed] [Google Scholar]
- 52.Supprian T, Rückert S, Bendszus M, Hofmann E, Franzek E, Franzek E, Ungvari GS, Rüther E, Beckmann H. Würzburg, Germany: International Wernicke-Kleist-Leonhard society; 2000. Cranial computed tomography parameters in endogenous psychoses: A prospective study Progress in Differentiated Psychopathology pp. 199–205. [Google Scholar]
- 53.Beckmann H, Franzek E. Deficit of birthrates in winter and spring months in distinct subgroups of mainly genetically determined schizophrenia. Psychopathology. 1992;25(2):57–64. doi: 10.1159/000284754. [DOI] [PubMed] [Google Scholar]
- 54.Lange V, Beckmann H, Neumärker K-J. Berlin, Germany: Ullstein-Mosby; 1995. Contribution of human genetics to the Leonhardian classification of endogenous psychoses Endogenous Psychoses: Leonhard’s Impact on Modern Psychiatry pp. 133–141. [Google Scholar]
- 55.Pfuhlmann B, Jabs B, Althaus G, et al Cycloid psychoses are not part of a bipolar affective spectrum: results of a controlled family study. J Affect Disord. 2004;83(1):11–19. doi: 10.1016/j.jad.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Althaus G, Pfuhlmann B, Franzek E, Franzek E, Ungvari GS, Rüther E, Beckmann H. Würzburg, Germany: International Wernicke-Kleist-Leonhard society; 2000. Is the premenstrual exacerbation of psychotic symptoms specific to schizophrenia Progress in Differentiated Psychopathology pp. 206–212. [Google Scholar]
- 57.Pfuhlmann B, Franzek E, Beckmann H, Stöber G. Long-term course and outcome of severe postpartum psychiatric disorders. Psychopathology. 1999;32(4):192–202. doi: 10.1159/000029090. [DOI] [PubMed] [Google Scholar]
- 58.Franzek E, Franzek E, Ungvari GS. Hong Kong: Würzburg; 1997. Cycloid psychosis, neuroleptic malignant syndrome and “lethal” catatonia Recent Advances in Leonhardian Nosology pp. 25–35. [Google Scholar]
- 59.Albert E, Seidel K, Neumärker K-J, Schulze HAF. Vienna: Springer Vienna; 1986. Über den Einfluß von neuroleptischer Langzeitmedikation auf den Verlauf von phasischen und remittierenden Unterformen endogener Psychosen Zur Klassifikation Endogener Psychosen pp. 97–107. [PubMed] [Google Scholar]
- 60.Leonhard K. Vienna, Austria: Springer Vienna; 1999. Introduction Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 1–5. [Google Scholar]
- 61.Trostorff S, Seidel K, Neumärker K-J, Schulze HAF. Vienna, Austria: Springer Vienna; 1986. Rezessiver Erbgang bei Affektvoller Paraphrenie (Das Vorkommen von Verwandtenehen) Zur Klassifikation Endogener Psychosen pp. 108–115. [Google Scholar]
- 62.Fish F. The influence of the tranquilizers on the leonhard schizophrenic syndromes. Encephale. 1964;53(Suppl):245–259. [PubMed] [Google Scholar]
- 63.Astrup C. The effects of ataraxic drugs on schizophrenic subgroups related to experimental findings. Acta Psychiatr Scand Suppl. 1959;34(136):388–393. doi: 10.1111/j.1600-0447.1959.tb07826.x. [DOI] [PubMed] [Google Scholar]
- 64.Magnan V. Paris, France: Lecrosnier et Babé; 1890. Le Délire Chronique à Évolution Systématique [Google Scholar]
- 65.de Clérambault GG. Les délires passionnels. Érotomanie, Revendication, Jalousie. Bull la Société Clin Médecine Ment. 1921:61. [Google Scholar]
- 66.Kraepelin E. Leipzig, Germany: Johann Abrosius Barth; 1915. Psychiatrie : Ein Lehrbuch Für Studierende Und Ärzte. Achte, Vollständig Umgearbeitete Auflage. IV Band. Klinische Psychiatrie. III. Teil [Google Scholar]
- 67.Roth M, Morrissey JD. Problems in the diagnosis and classification of mental disorder in old age; with a study of case material. J Ment Sci. 1952;98(410):66–80. doi: 10.1192/bjp.98.410.66. [DOI] [PubMed] [Google Scholar]
- 68.Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschritte Neurolo Psychiatr. 1943;15:259–290. [Google Scholar]
- 69.Volavka J. Late-onset schizophrenia: A review. Compr Psychiatry. 1985;26(2):148–156. doi: 10.1016/0010-440x(85)90035-5. [DOI] [PubMed] [Google Scholar]
- 70.Leonhard K. Biological fundation of affective paraphrenia on the basis of clinicial investigations. World Congress of Biological Psychiatry [Google Scholar]
- 71.Leonhard K. Vienna, Austria: Springer Vienna; 1999. The Unsystematic Schizophrenias Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 82–112. [Google Scholar]
- 72.Jabs BE. Würzburg, Germany: 2005. Untersuchungen zur Nosologie der Kataphasie: ein Beitrag zur Differenzierung von Psychosen mit formalen Denkstörungen Post-doctoral thesis. [Google Scholar]
- 73.Leonhard K. Die Spielbereite der unsystematischen Schizophrenien, besonders der Kataphasie. Arch Psychiatr Nervenkr. 1961;202:513–526. doi: 10.1007/BF00342112. [DOI] [PubMed] [Google Scholar]
- 74.Binder FA. Formale Denkstörungen in der Normalbevölkerung: Prävalenz und Vergleich zu Angehörigen vonPatienten mit Kataphasie 2009 (Doctoral thesis) Available at: [Google Scholar]
- 75.Mainberger O. Validation du test psychique expérimental opérationnalisé pour le diagnostic de cataphasie 2015 (Doctoral thesis) Available at: [Google Scholar]
- 76.Hinzen W, Rosselló J. The linguistics of schizophrenia: thought disturbance as language pathology across positive symptoms. Front Psychol. 2015;6:971. doi: 10.3389/fpsyg.2015.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foucher JR, Zhang YF, Roser M, et al A double dissociation between two psychotic phenotypes: Periodic catatonia and cataphasia. Prog Neuro-Psychopharmacology Biol Psychiatry. 2018;86:363–369. doi: 10.1016/j.pnpbp.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Ketterer S, Gomez-Auli A, Hillebrand LE, Petrera A, Ketscher A, Reinheckel T. Inherited diseases caused by mutations in cathepsin protease genes. FEBS J. 2017;284(10):1437–1454. doi: 10.1111/febs.13980. [DOI] [PubMed] [Google Scholar]
- 79.Gjessing LR. A review of periodic catatonia. Biol Psychiatry. 1974;8(1):23–45. [PubMed] [Google Scholar]
- 80.Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med. 2009;39(07):1065. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- 81.Leonhard K. Vienna, Austria: Springer Vienna; 1999. Periodic catatonia Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 104–112. [Google Scholar]
- 82.Stöber G. Genetic predisposition and environmental causes in periodic and systematic catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;25(suppl):I21–4. doi: 10.1007/pl00014196. [DOI] [PubMed] [Google Scholar]
- 83.Astrup C. Oslo, Sweden: Universitetsforlaget; 1979. The Chronic Schizophrenias [Google Scholar]
- 84.Ungvari GS, Leung CM, Wong MK, Lau J. Benzodiazepines in the treatment of catatonic syndrome. Acta Psychiatr Scand. 1994;89(4):285–288. doi: 10.1111/j.1600-0447.1994.tb01515.x. [DOI] [PubMed] [Google Scholar]
- 85.Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142(4):393–398. doi: 10.1007/s002130050904. [DOI] [PubMed] [Google Scholar]
- 86.Leonhard K. Ein dominanter und ein rezessiver erbgang bei zwei verschiedenen formen von schizophrenie. Nervenarzt. 1975;46(5):242–248. [PubMed] [Google Scholar]
- 87.Krüger S, Bräunig P, Beckmann H, Neumärker K-J. Berlin, Germany: Ullstein-Mosby; 1995. Studies on course outcome and genetic loading of periodic catatonia Endogenous Psychoses: Leonhard’s Impact on Modern Psychiatry pp. 181–185. [Google Scholar]
- 88.Stöber G, Saar K, Rüschendorf F, et al Splitting schizophrenia: periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet. 2000;67(5):1201–1207. doi: 10.1016/s0002-9297(07)62950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stöber G, Seelow D, Rüschendorf F, Ekici A, Beckmann H, Reis A. Periodic catatonia: confirmation of linkage to chromosome 15 and further evidence for genetic heterogeneity. Hum Genet. 2002;111(4-5):323–330. doi: 10.1007/s00439-002-0805-4. [DOI] [PubMed] [Google Scholar]
- 90.Roginski RS, Lau CW, Santoiemma PP, et al The human GCOM1 complex gene interacts with the NMDA receptor and internexin-alpha. Gene. 2018;648:42–53. doi: 10.1016/j.gene.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walther S, Schäppi L, Federspiel A, et al Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43(5):972–981. doi: 10.1093/schbul/sbw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foucher JR, de Billy C, Jeanjean L, et al A brain imaging-based diagnostic biomarker for periodic catatonia: Preliminary evidence using a Bayesian approach. Neuropsychobiology. 2019 Sep 10 doi: 10.1159/000501830. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 93.Foucher JR, de Billy C, Mainberger O, Schorr B, Clauss J, Berna F. Personalized rTMS improves chronic and treatment resistant catatonias – A proof of concept study. 3rd European Conference on Brain Stimulation in Psychiatry “From Mechanisms to Medicine” [Google Scholar]
- 94.Leonhard K. Vienna, Austria: Springer Vienna; 1999. The systematic schizophrenias Classification of Endogenous Psychoses and Their Differentiated Etiology pp. 113–247. [Google Scholar]
- 95.Stöber G, Franzek E, Beckmann H, Schmidtke A. Exposure to prenatal infections, genetics and the risk of systematic and periodic catatonia. J Neural Transm. 2002;109(5-6):921–929. doi: 10.1007/s007020200075. [DOI] [PubMed] [Google Scholar]
- 96.Sallet PC, Elkis H, Alves TM, et al Rightward cerebral asymmetry in subtypes of schizophrenia according to Leonhard’s classification and to DSM-IV: a structural MRI study. Psychiatry Res - Neuroimaging. 2003;123(1):65–79. doi: 10.1016/s0925-4927(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 97.Strik W. Cycloid psychoses, a taste of schizophrenia with the evolution of bipolar disorder: heredity, neuro-developmental load, imaging and electrophysiological findings. ICOSR Workshop 31.03.2009 : Towards a Valid Classification of Endogenous Psychosis, Time to Pay Attention to the Wernicke-Kleist-Leonhard School [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.