Abstract
IL-23 and IL-12, two structurally related heterodimeric cytokines sharing a common subunit, divergently promote T helper cell development and expansion. Both cytokines have been implicated in the pathogenesis of thyroid-associated ophthalmopathy (TAO), an autoimmune component of Graves’ disease. In TAO, CD34+ fibrocytes, putatively derived from bone marrow, can be identified in the orbit. There they masquerade as CD34+ orbital fibroblasts (CD34+ OF) and cohabitate with CD34− OF in a mixed fibroblast population (GD-OF). Slit2, a neural axon repellent, is expressed and released by CD34− OF and dampens the inflammatory phenotype of fibrocytes and CD34+ OF. Here we report that thyrotropin (TSH) and the pathogenic, GD-specific monoclonal autoantibody, M22, robustly induce IL-23 in human fibrocytes; however, IL-12 expression is essentially undetectable in these cells under basal conditions or following TSH-stimulation. In contrast, IL-12 is considerably more inducible in GD-OF, cells failing to express IL-23. This divergent expression and induction of cytokines appears to result from cell-type specific regulation of both gene transcription and mRNA stabilities. It appears that the JNK pathway activity divergently attenuates IL-23p19 expression while enhancing that of IL-12p35. The shift from IL-23p19 expression in fibrocytes to that of IL-23p35 in their derivative CD34+ OF results from the actions of Slit2. Thus, Slit2 might represent a molecular determinant of balance between IL-23 and IL-12, potentially governing immune responses in TAO.
Introduction
IL-23 and IL-12, both members of the IL-12 cytokine superfamily, are intimately involved in the inflammation associated with autoimmune diseases (1–3). These cytokines exert a polarizing influence on T helper cell (Th) development and expansion. IL-23 and IL-23 receptor (IL-23R) share common subunits with their respective IL-12 counterparts, namely p40 and Rβ1 (3,4). IL-23 comprises p19/p40 heterodimers while IL-12 consists of p35/p40 subunits (5). Prior to the identification of IL-23 as a distinct cytokine (6), the close functional relationships between these pathways resulted in an incorrect attribution of IL-23 activities to those of IL-12. In target cells, both cytokines activate Tyk2, Jak2, and STAT4 signaling (5,7–10). IL-23 promotes Th17 expansion and the generation of IL-17 and IL-22, actions mediated by STAT3, a critical transcription factor involved in Th17 differentiation (11,12). In contrast, IL-12 promotes Th1 polarization to interferon γ-producing cells while antagonizing Th17 production (13,14). Factors governing the relative expression of IL-23 and IL-12 have yet to be fully characterized.
Polymorphisms in IL-23R gene are associated with autoimmune diseases, including psoriasis and Crohn’s disease (15). Further, IL-23R represents a major susceptibility gene in thyroid associated ophthalmopathy (TAO), an autoimmune manifestation of Graves’ disease (GD) (16). Despite the central role for humoral immunity in GD, experimental evidence has suggested the dominance of Th1 in the disease (17). Very recent studies, however, demonstrate an increased frequency of circulating Th17 cells and elevated serum IL-17 levels in GD (18–20). Further, IL-17A can induce cytokines in orbital fibroblasts from patients with TAO (GD-OF), actions enhanced by CD40 ligand (CD154) (21). In aggregate, substantial evidence now supports involvement of both Th1 and Th17 responses in GD and TAO but the proximate molecular determinants promoting each are not understood. Whether IL-23 might play a role in TAO has not been explored previously.
CD45+CD34+ LSP+CXCR4+Collagen I+ fibrocytes were initially identified by Bucala and coworkers who characterized them as mediators of tissue activation, remodeling, and fibrosis (22). Fibrocytes are monocyte progenitor cells coming from the bone marrow that traffic to injured tissues as a consequence of chemokine signals (23). Besides their roles in wound repair and scar formation (24), fibrocytes possess phenotypic attributes suggesting their potential importance in immune function. They constitutively express MHC class II and efficiently present antigen, resulting in naïve T cell priming (25). Murine fibrocytes develop from CD11b+ monocytes (26), a process requiring activated CD4+ T cells. Fibrocytes express several inflammatory mediators, including cytokines, in response to signals emanating from their molecular and cellular niches (27).
Fibrocytes reside in orbits manifesting TAO (28) where they are identified as CD34+ CXCR4+Col I+ orbital fibroblasts (CD34+ OF) (29). While they are presumed to derive from circulating fibrocytes of bone marrow origin, based on studies performed in mice (22), this has yet to be proven experimentally in human beings. Within the orbit, CD34+ OF coexist with residential CD34− OF as a mixed fibroblast population, referred to collectively herein as GD-OF. In contrast, orbital fibroblasts from healthy orbital tissue are uniformly CD34− OF (28,29). CD34− OF have been shown recently to express Slit2 (30), an axon guidance glycoprotein playing critical roles in central nervous system development (31). The actions of Slit2 are mediated through its cognate receptor, roundabout 1 (32). Slit2 can inhibit the differentiation of fibrocytes and in so doing, retard tissue fibrosis (33). The molecule was recently found to suppress the inflammatory phenotype of CD34+ OF and fibrocytes, cells that do not express Slit2 (30). We proffer that CD34+ OF critically participate in the pathogenesis of TAO and that CD34− OF, by virtue of their release of Slit2, might modulate the disease-related activities of fibrocytes and their fibroblast derivatives within the orbit.
Fibrocytes express several proteins previously considered “thyroid-specific”, including the thyrotropin receptor (TSHR), a G protein-coupled membrane-spanning protein (34,35) which is cell surface-displayed at levels comparable to those on thyroid epithelial cells (28). TSHR expression by fibrocytes is dependent on the non-canonical transcription factor, autoimmune regulator protein (36). TSHR, the disease-specific, pathogenic autoantigen, is uniquely targeted in GD by activating autoantibodies, known as thyroid-stimulating immunoglobulins (TSI)(37). These antibodies underlie the hyperthyroidism frequently associated with GD but their role in TAO remains uncertain (38).
The capacity for human fibrocytes and CD34+ OF to express IL-23 has not been explored previously. Further, a potential mechanism through which differential regulation of IL-23 and IL-12 expression in the TAO orbit has not been described. Here we report a previously unidentified relationship between the actions of Slit2 and cytokine expression in fibrocytes and GD-OF that could determine T cell development. TSH and TSI robustly induce IL-23 in fibrocytes, resulting from transcriptional and post-transcriptional upregulation of IL-23p19. In contrast, expression and induction of IL-23 in fibrocyte-derivative CD34+ OF and in GD-OF is negligible but those cells express high levels of IL-12 in response to TSH. JNK pathway activation appears to downregulate IL-23p19 induction by TSH while enhancing the induction of IL-12p35. Likewise, rhSlit2 dramatically suppresses IL-23p19 induction in fibrocytes while enhancing that of IL-12p35. By virtue of their responses to TSH/TSI, fibrocytes and CD34+ OF may play distinct roles in pathogenesis of TAO. Further, Slit2 generated by CD34− OF could represent a determinant for whether IL-23 or IL-12 is produced by these cells.
Materials and Methods
Materials
We routinely check the reagents provided by outside parties for specificity. Fetal bovine serum (FBS, cat.# 16000–044), Dulbecco’s modified Eagle’s medium (DMEM, cat # 11965) containing 4.5 g/mL D-glucose and L-glutamine, IL-12 ELISA (cat.# KHC0121 and rhIL-23 (cat # PHC9324) were supplied by Life Technologies (Grand Island, NY). 5,6-dichlorobenzimidazole (DRB) came from Cayman (Ann Arbor, MI, cat # 10010302). bTSH (cat.# 609385) and JNK Inhibitor II (cat # SP600125) were from Calbiochem (La Jolla, CA,. M22, an activating anti-TSHR mAb was from Kronus (Star, ID, cat # M22–5c/00–690). rhSlit2 was from Sigma-Aldrich (St. Louis, MO, cat# SRP3155). IL-23 ELISA (cat # 88–7237) and anti-human IL-12p35 Ab (cat # BMS152) were from eBiosciences, San Diego, CA. Anti-hIL-23 Ab (cat # 3457–5N-500) was from Mabtech, Mariemont, OH. Anti-mouse CD34 IgG1- FITC (cat # 555822), anti-mouse IgG1 isotype control-PE (cat # 555749), and fixation and permeabilization solution (cat.# 554722) were from BD Biosciences, San Diego, CA. Accutase (cat # SCR005) and AKTi (cat.# 124011) were from Millipore, Billerica, MA. Human CD34+ nucleofection kit (cat # VPA-1003) was from Lonza, Allendale, NJ. siRNAs targeting Akt (cat #L-003000–00), tristetraprolin (cat # L-010789–01), Slit2 (cat #L-019853–00), JNK (cat #L-003514–00) and scramble siRNA (cat # D-001810–10) were from Dharmacon, Lafayette, CO. Anti-hIL-23p19 Ab was from Santa Cruz, Dallas, Tx (cat # sc-271279). Anti-phospho-Akt (ser 473), (cat # 4058) Ab, Anti-phospho-JNK (cat # 4668s) Ab and HRP-anti-rabbit IgG (cat # 7074S) were from Cell Signaling (Danvers, MA). HRP conjugated goat anti mouse IgG was from Dako, Carpinteria, CA (cat # P0447). JNK expression plasmid (cat # EX-ZO734-M02) and empty control vector (cat # EX-NEG-M02) were from GeneCopoeia, Rockville, MD.
GD-OF and fibrocyte isolation and cultivation
Human orbital tissue and peripheral venous blood were collected from study participants following their provision of written and verbal informed consent as approved by the Institutional Review Board of the University of Michigan Health System. GD-OF were cultivated from surgical waste obtained either from donors undergoing surgical decompression for severe TAO as previously described in detail (39). GD-OF comprise two distinct populations, one exhibiting a CD31−CD34+CXCR4+Col I+ TSHRhigh phenotype and the other displaying a CD31−CD34−CXCR4−Col I−TSHRlow phenotype. GD-OF were used for experiments between the 2nd and 11th passages, an interval in which they maintained stable phenotypes (39).
Fibrocytes were generated from peripheral blood mononuclear cells (PBMCs), either generously provided by the American Red Cross, from patients with GD or healthy individuals from an academic clinical practice. PBMCs were isolated by Ficoll Histopaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation and cultivated as originally described (22) with the exception that culture substratum was not coated with fibronectin, a modification we have employed for many years (27,28,30,40). Six-well plates were inoculated with 107 PBMCs and covered with DMEM containing 10% FBS. After 12–14 days in culture, adherent monolayers (<5% of the starting population) were rinsed and removed from substratum by mechanical scrapping following accutase treatment. Culture purity was confirmed routinely by FACS analysis to be >90% fibrocytes on the basis of their consistent CD31−CD45+CD34+CXCR4+Col I+ TSHRhigh phenotype. Cell viability was >90% by trypan blue exclusion.
Cell transfections
Small interfering RNA (siRNA) targeting TTP, AKT and Slit2 as well as a JNK expression plasmid were transfected into fibrocytes or GD-OF as described previously (39, 40). Briefly, Confluent cultures were washed twice with PBS, detached using Accutase and pelleted. Each cell pellet (1–5×106 cells per cuvette) was re-suspend with 100μl human CD34+ nucleofection solution at room temperature containing 3μg plasmid DNA or 300nM siRNA. Cell suspensions were transferred to an Amaxa nucleofector Ⅱ (Amaxa Biosystems, Gaithersburg, MD) instrument using the U-033 program. Transfected cells were then transferred to 6 well or 12 well plates and covered with DMEM supplemented with 10% FBS. After 3 d incubations, cultures were treated without or with bTSH for the times indicated in the figure legends. Media were subjected to ELISA and RNA was extracted from cell layers with an Aurum ™ Total RNA Mini Kit (cat.# 732–6820, Bio-Rad, Hercules, CA). Effectiveness of modifying levels of each target is provided in Fig. S1.
ELISA and Western blot analysis
Confluent cultures were shifted to DMEM with 1% FBS prior to addition of test compounds. Monolayers were untreated or received rhSlit2 (50 ng/mL) without or with bTSH (5 mU/mL) for the intervals indicated. Some cultures were transfected with control siRNA or targeting siRNA for Slit2 followed by treatment without or with bTSH for 6 h. Aliquots of media and solubilized cell layers were subjected to IL-23 and IL-12 ELISAs according to manufactures’ protocols. For Western blots, cell layers were solubilized in lysis buffer (Invitrogen) containing PMSF (1mM), boiled in double-strength Laemmli sample buffer (BIO-RAD) and subjected into SDS/PAGE. Proteins were transferred to PVDF membrane (Millipore) and probed with anti-IL-23p19 (1:1000 v/v), IL-12p35 (1:1000 v/v), and anti-p-Akt Abs (1:1000 v/v) overnight at 4 ºC. Washed membranes were probed with HRP-conjugated anti-rabbit IgG (1:2000) or anti-mouse IgG (1:1000) for 2 h at room temperature. ECL Plus reagent (Thermo Scientific) was used for signal generation.
RNA preparation and real-time PCR
Confluent cultures were treated with rhSlit2 (50 ng/mL), AKT inhibitor (AKTi, 1Μm) or transfected with control siRNA or targeting siRNA and then shifted to DMEM supplemented with 1% FBS for 16 h. Culture wells were treated with nothing, bTSH (5 mIU/mL), M22 (1 μg/mL) or the agents and treatment durations indicated in the figure legends. Cellular RNA was extracted. RNA (2 μg) was reverse-transcribed and subjected to real-time PCR using an Applied Biosystems instrument with a QuantiTect SybrGreen PCR kit (cat # 204143, Qiagen, Frederick, MD). The following primer sequences were synthesized by Life Technologies: GAPDH, forward, 5’-TTGCCATC-AATGACCCCTTCA-3’, reverse, 5’-CGCCCCACTTGATTTTGGA-3’; IL-23p19, forward, 5’-TCCACCAGGGTCTGATTTTT-3’ and reverse, 5’-TTTTGAAGCG-GAGAAGGAGA-3’; IL-12p35, forward, 5’- GATGGCCCTGTGCCTTAGTA-3’ and reverse, 5’-CCGGTTCTT-CAAGGGAGGAT-3’. Primers for tristetraprolin (TTP) (cat.# PPH17294C), Slit2(QT00007784) AKT (cat.#PPH00088B) and JNK (cat.# QT01149512 ) were from Qiagen, Germantown, MD. Values of samples were generated against a standard curve and normalized to respective GAPDH signals.
RNA Pol II ChIP transcription assay
Transcriptional assays were performed using the method of Wells and Farnham (41). Confluent fibrocyte cultures received diluent or rhSlit2 (50 ng/mL) for 7–9 d. GD-OF were transfected with control or Slit2-targeting siRNA and incubated for 3 d. Culture wells were rinsed and shifted to DMEM with 1% FBS for 16 h, treated without or with bTSH (5mU/mL) for 2 h. Recruitment of polymerase II to the IL-23p19 and IL-12p35 gene promoters was assayed using Pierce™ Agarose Chip Kit (Thermo Scientific, cat # 26156). Eluted fractions were reverse cross-linked and isolated DNA subjected to RT-time PCR using IL-23p19 and IL-12p35 primers (Qiagen, cat # GPH1003214 and cat. # GPH1009669, respectively) using GAPDH as the housekeeping gene. Values were generated according to the method protocols provided by the supplier. Studies were repeated at least 3 times.
mRNA stability assay
Stability of the relevant transcripts was assessed using techniques published previously by our group (30,42). Briefly, cell monolayers were shifted to serum-free medium for 16 h and then 5,6 dichlorobenzimidazole (20 μg/mL), an inhibitor of gene transcription (43), was added to all culture wells. After the respective treatments indicated in figure legends, cultures were incubated for graded intervals and cellular RNA was harvested at the times indicated. The transcripts were reverse transcribed and subjected to real-time RT-PCR.
Flow Cytometry and Cell Sorting
Characterization of fibrocytes and GD-OF was conducted using a BD LSR flow cytometer and FlowJo v10 software. In some studies, GD-OF were subjected to cytometric cell sorting with a FACSAria III (BD Biosciences) machine. Monolayers were stained for 30 min at 4°C with fluorescein isothiocynate-conjugated anti-mouse CD34 or its isotype control. Washed cells were sorted under sterile conditions with a GD-OF (parental), CD34+ OF, and CD34− OF were re-cultured for 48 h and then treated as indicated in the figure legends. Fibrocytes are routinely monitored for their display of CD45, CD34, CXCR4, TSHR, and collagen I.
Statistics
Statistical significance was determined with the two-tailed Student’s t test or by two-way analysis of variance (ANOVA).
Results
Divergent expression levels of IL-23p19 and IL-12p35 in GD-fibrocytes and GD-OF
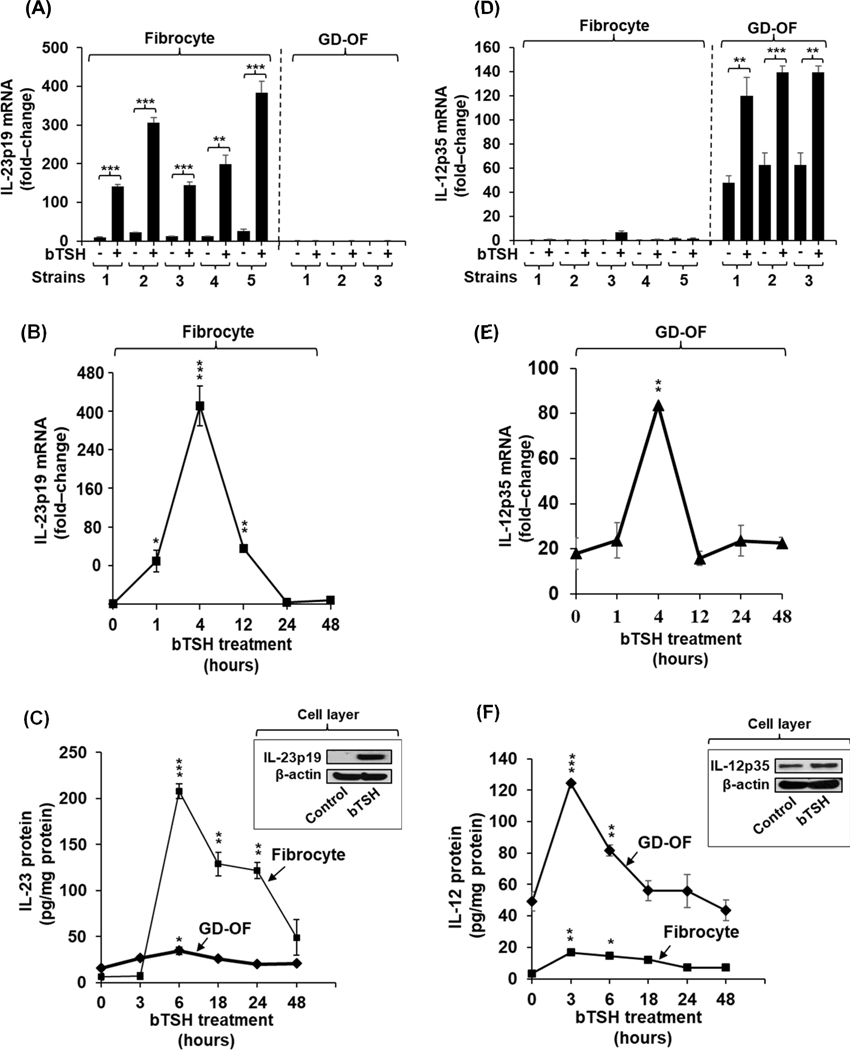
Basal levels of IL-23p19 expression are extremely low in all 5 strains of CD34+ GD-fibrocytes cultured from PBMCs and 3 GD-OF strains, each from a unique donor (Fig. 1A; GD-fibrocytes vs GD-OF, p= 0.01 by ANOVA). In contrast, bovine TSH (bTSH, 5 mIU/L) treatment for 6 h dramatically induces IL-23p19 mRNA in the GD-fibrocytes but expression in GD-OF remains below or at the level of detection (p< 0.001 vs GD-fibrocytes by ANOVA). bTSH-induced IL-23p19 mRNA levels are 50-fold above their respective baselines (p< 0.01 versus controls) among the GD-fibrocyte strains. Considerable variability exists in the individual strains concerning IL-23p19 mRNA expression, consistent with our previous findings regarding expression of other inflammatory genes in these cells (29,30,36). The effects of bTSH are time-dependent, peaking at 4 h and returning basal levels at 24 h (Fig. 1B). Induction of IL-23 and its release into the medium covering GD-fibrocytes is rapid, time-dependent, and peaks at 6 h (p<0.001 vs basal) (Fig. 1C). In contrast, IL-23 levels in medium covering cultured GD-OF are dramatically lower than those in fibrocyte cultures but also peak at 6 h. bTSH and M22, a monoclonal TSI (44), induce IL-23p19 mRNA in a concentration-dependent manner (Fig. S2A and S2B).
Fig. 1.
Divergent induction by bTSH of IL-23 and IL-12 in GD-fibrocytes versus GD-OF. (A,D) Expression levels of IL-23p19 and IL-12p35 mRNA under control (untreated) and bTSH-treated conditions in 5 strains of GD-fibrocytes and 3 strains of GD-OF, each from a different donor with GD. Medium covering confluent culture monolayers was replaced with DMEM supplemented with 1% FBS and containing nothing or bTSH (5 mIU/mL) for 6 h. Cell layers were harvested, cellular RNA was reverse transcribed, and subjected to quantitative real-time PCR for IL-23p19 and IL-12p35 mRNA. Values were normalized to their respective GAPDH levels and are expressed as the mean ± S.D of triplicate determinations from one representative experiment of three performed, **, p<0.01; ***, p<0.001 by ANOVA. (B and E) Fibrocyte cultures were treated with bTSH (5 mIU/mL) for the graded intervals indicated along the abscissa. IL-23p19 and IL-12p35 mRNA levels were quantified as in A and D and are expressed as mean ± SD. (C and F) Cultures were treated with bTSH for the graded intervals indicated. Media were collected and subjected to IL-23 and IL-12 ELISAs as described in “Methods”. Values were normalized to respective monolayer protein content. Data are expressed as mean ± SD of triplicate determinations. (Insets, C and F) Monolayer proteins from control and bTSH-treated cultures were subjected to Western blot analysis for IL-23p19 and IL-12p35 Blots were then re-probed with anti-β-actin.
In contrast to fibrocytes, the amplitude of bTSH-induced IL-12p35 mRNA in GD-OF is considerably higher than that of IL-23p19 (p<0.001) and significantly greater in GD-OF than in fibrocytes (p<0.001, Fig. 1D). Induction of IL-12p35 mRNA is also rapid, reaching a peak at 4 h and decaying to baseline by 12 h (Fig. 1E). Release of IL-12 into the medium is enhanced following exposure to bTSH, with near-maximal effects at 3 h and a return to baseline by 24–48 h (Fig. 1F). The identical, divergent pattern of expression and induction by bTSH of IL-23p19 and IL-12p35 was observed in fibrocytes (H-fibrocytes) and orbital fibroblasts (H-OF) cultivated from healthy donors (Fig. S3A, both p<0.001 by ANOVA).
Divergent induction patterns of IL-23p19 and IL-12p35 by bTSH in pure GD-OF subsets
Parental GD-OF cultures comprise a mixture of CD34+ OF and CD34− OF in ratios approximating 50:50 (28,40). Fibroblasts from healthy orbits (H-OF) are uniformly CD34− OF (28). The CD34+ OF subset putatively derives from circulating fibrocytes that have infiltrated the TAO orbit and transitioned to the fibroblast phenotype, an identification based on their array of cellular markers (45). To determine the expression and induction pattern of IL-23p19 and IL-12p35 in pure CD34+ OF and CD34− OF subsets, GD-OF were subjected to cytometric cell-sorting on the basis of cell-surface CD34 display and then interrogated. Both cytokine transcripts were expressed at extremely low basal levels in untreated cultures; however, bTSH induced IL-23p19 mRNA preferentially in pure CD34+ OF (Fig. S4A, p<0.01 versus either CD34− OF or parental GD-OF). In contrast, the amplitude of IL-12p35 mRNA induction is greater in CD34− OF compared to either GD-OF (Fig. S4B, p<0.05) or CD34+ OF (p<0.01). It would appear that the segregation of these fibroblast subsets drives the phenotypes of CD34+ OF and CD34− OF further apart and that a factor(s) emanating from CD34−OF suppresses IL-23p19 in CD34+OF.
Induction of IL-23p19 and IL-12p35 by bTSH results from enhanced gene transcription and prolonged mRNA stability
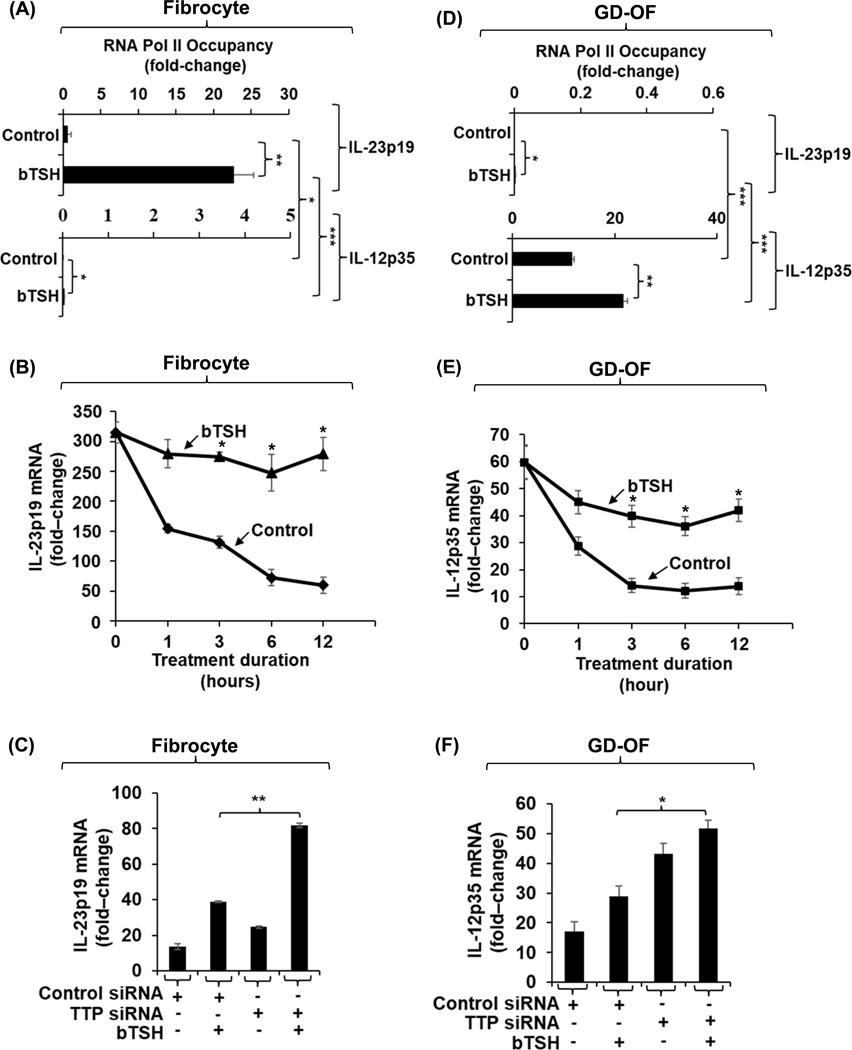
The molecular mechanisms mediating bTSH induction of IL-23p19 and IL-12p35 in fibrocytes and GD-OF were next explored. bTSH induced IL-23p19 gene transcription in fibrocytes dramatically above those observed at baseline (Fig. 2A, 20-fold above control (p<0.01) as assessed by an RNA Pol II ChIP transcription assay. Stability of IL-23p19 mRNA is also enhanced by bTSH in fibrocytes, resulting in transcript levels essentially unchanged from baseline at 12 h, the duration of the study (Fig. 2B). In contrast, IL-23 mRNA levels in untreated fibrocytes rapidly decay. The IL-12p35 gene transcription activity in fibrocytes, under both basal and bTSH-stimulated conditions is nil (Fig. 2A). The tandem zinc finger protein, TTP, is a key regulator of transcript destabilization through binding to AU-rich regions of target mRNAs (46). To determine whether TTP might represent an endogenous modulator of bTSH-induced IL-23p19 expression in fibrocytes, cultures were transfected with a specific TTP targeting siRNA. As the data in Fig. 2C demonstrate, down-regulating TTP expression enhances the induction of IL-23p19 mRNA in fibrocytes.
Fig. 2.
bTSH up-regulates IL-23p19 gene transcription and enhances IL-23p19 mRNA stability in fibrocytes while enhancing IL-12p35 gene transcription and mRNA stability in GD-OF. (A and D) Confluent monolayers of fibrocytes and GD-OF remained untreated or received bTSH (5 mIU/mL) for 2 h. Cultures were harvested and subjected to Pol II Chip transcription assay as described in “Methods”. (B and E) Confluent cultures were pre-treated with bTSH for 2 h. At time “0” all culture wells received 5,6-dichlorobenzimidazole (20 μg/mL) without or in the continued presence of bTSH. Monolayers were harvested at the times indicated along the abscissas, mRNA was reversed transcribed and subjected to real-time PCR for IL-23p19 and IL-12p35. (C and F) Monolayers were transfected with scrambled (control) siRNA (3μg) or tristetraprolin (TTP)-targeting siRNA (3μg) for 2– 3 d. They were then treated with nothing or bTSH for 6 h. Monolayers were harvested, cellular RNA, extracted, reverse transcribed, and subjected to RT-PCR for IL-23p19 and IL-12p35. Values were normalized to their respective GAPDH levels and are expressed as the mean ± SD of three independent determinations. Experiments were repeated three times. *, P<0.05; **, p<0.01; ***. P<0.001.
With regard to mechanisms involved in IL-23 and IL-12 expression in GD-OF and mechanisms involved in their induction by bTSH, both basal and bTSH-induced IL-12p35 gene transcription (Fig. 2D) is substantially greater than in fibrocytes. Further, bTSH enhances the stability of IL-12p35 mRNA over the 12 h duration of the study (Fig. 2E). In contrast to IL-12p35, IL-23p19 gene transcriptional activities (Fig. 2D) are dramatically lower in GD-OF. As with IL-23p19 in fibrocytes, knocking-down TTP with a specific targeting siRNA increases steady-state IL-12p35 mRNA levels in GD-OF (Fig. 2F). Thus, it would appear that bTSH induces both IL-23p19 and IL-12p35 expression through gene transcription and post-transcriptional mechanisms in fibrocytes and GD-OF, respectively. IL-23 predominates in fibrocytes whereas IL-12 levels are considerably higher in GD-OF as reflected by divergent gene transcription activities.
bTSH induction of IL-23p19 and IL-12p35 involves AKT and JNK signaling
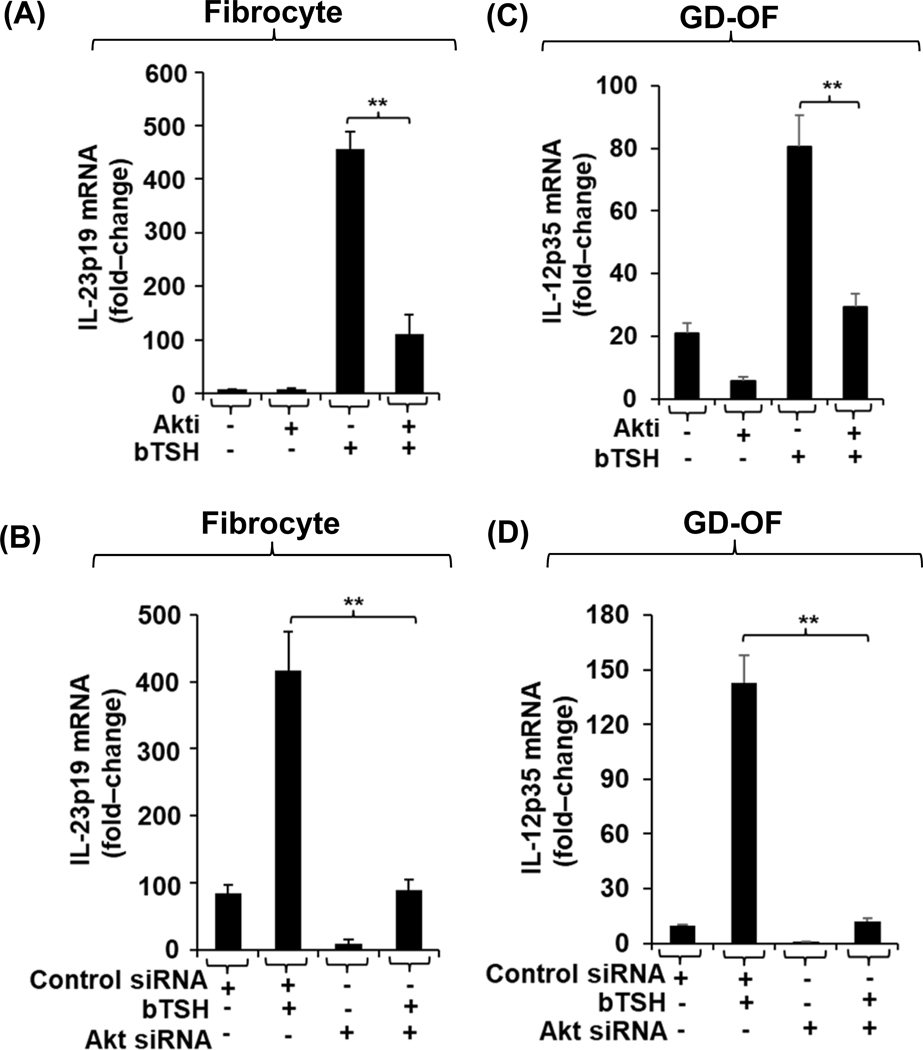
The signaling downstream from TSHR in the two cell-types was next assessed. Several gene targets in fibrocytes are activated through the AKT/mTOR pathway (47,48), thus potential involvement of this pathway was examined in the induction by bTSH of IL-23p19 and IL-12p35. AKTi (1 μM), a specific small molecule inhibitor, attenuates the bTSH-dependent phosphorylation of AKT in both fibrocytes (Fig. S1F, upper panel) and GD-OF (Fig. S1F, lower panel). The induction of both cytokine-encoding genes is similarly inhibited; IL-23p19 in fibrocytes (Fig.3A, p<0.01) and IL-12p35 in GD-OF (Fig. 3C, p<0.01). Knocking-down AKT with a specific targeting siRNA similarly attenuates these inductions (Fig. 3B and 3D, respectively, both p<0.01).
Fig. 3.
Induction by bTSH of IL-23p19 in fibrocytes and IL-12p35 in GD-OF is mediated through the Akt pathway. (A and C) Fibrocytes and GD-OFs were allowed to proliferate to confluence. They were pretreated with nothing or AKTi (1 μM) for 1 h, then treated without or with bTSH (5mIU/mL) for 6 h. (B, D) Cultures were transfected with control siRNA (3μg) or AKT-targeting siRNA (3μg), as described in “Methods”. Monolayers remained untreated or received bTSH for 6 h. Monolayers were harvested, RNA extracted, reverse transcribed, and subjected for real-time PCR for IL-23p19 or IL-12p35 as indicated. Values were normalized to their respective GAPDH levels and are expressed as mean ± SD, **, p<0.01 of three independent determinations.
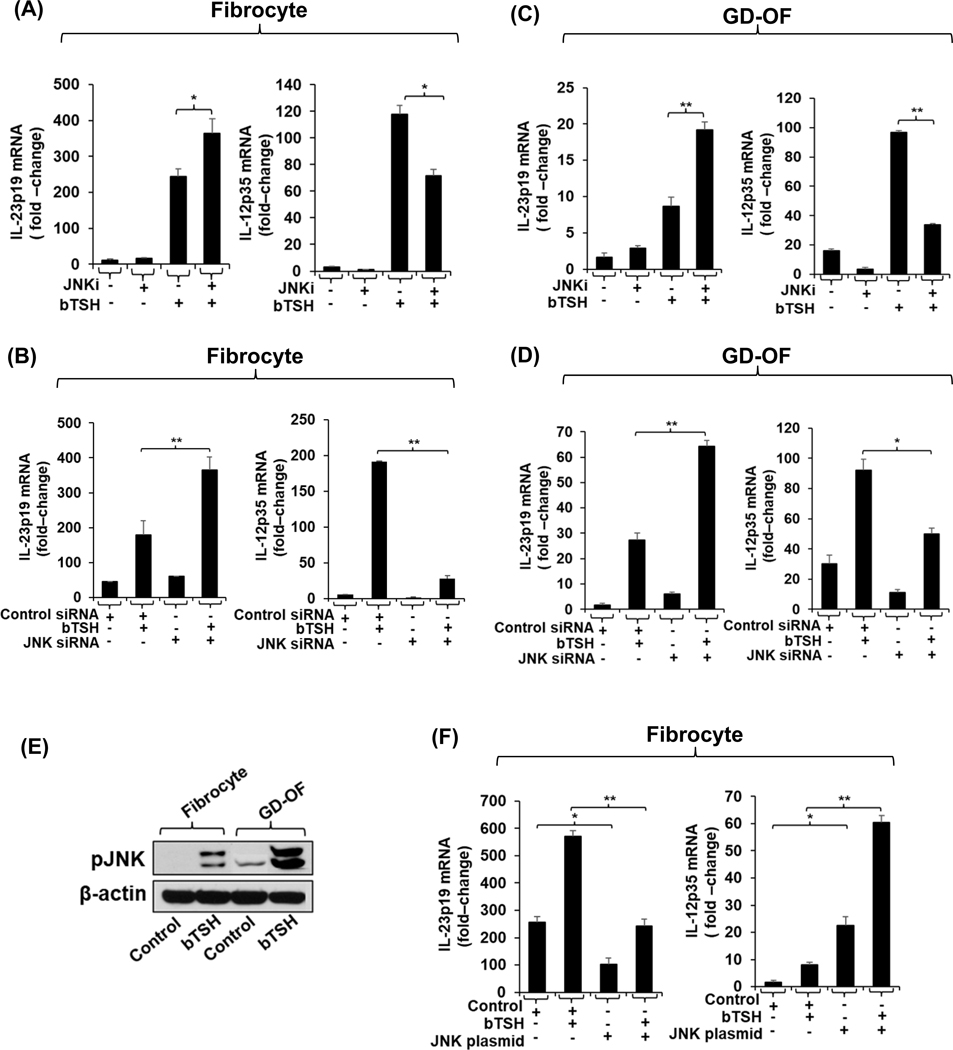
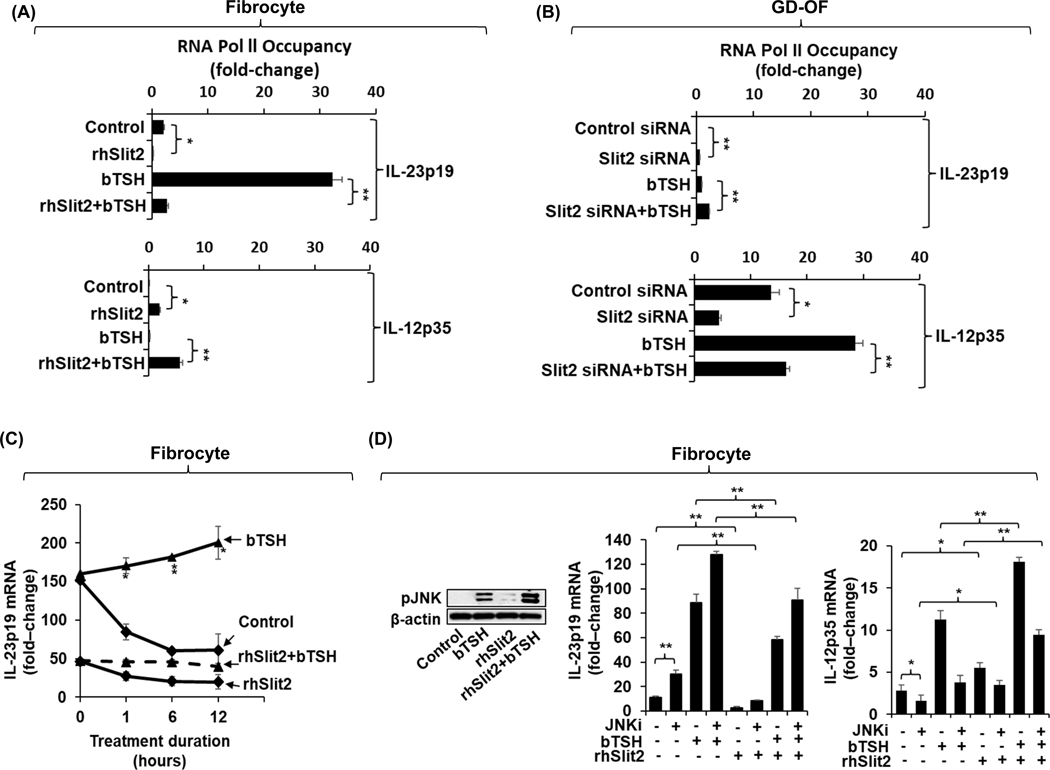
The JNK pathway has been shown previously to differentially alter the induction by Toxoplasma gondii of IL-23 and IL-12 in THP-1 cells (49). We thus have assessed the impact of bTSH on pJNK levels in fibrocytes and GD-OF. As the Western analysis in Fig. 4E shows, levels of pJNK are substantially higher in GD-OF than fibrocytes after 30 min of bTSH treatment (2.5-fold difference). In contrast to the up-regulatory signaling mediated through Akt to both IL-23p19 and IL-12p35, JNK phosphorylation/activation appears to be associated with a divergent pattern of gene expression for the two cytokines. Inhibiting pJNK activity provoked by bTSH results in an upregulation of IL-23p19 in fibrocytes (Fig. 4A) and in GD-OF (Fig. 4C) while this inhibition results in an attenuated expression of IL-12p35 in both fibrocytes (Fig. 4A) and GD-OF (Fig. 4C). Knocking-down JNK expression results in a similar pattern of enhancement and downregulation of bTSH-dependent IL-23p!9 and IL-12p35 expression, respectively as the effects of JNK inhibition in both cell types (Fig. 4B and 4D). Further, over-expressing JNK in fibrocytes results in attenuation of bTSH-induced IL-23p19 expression while substantially increasing that of IL-12p35 (Fig. 4F, both p<0.01 versus empty vector transfection).
Fig. 4.
JNK pathway exerts divergent impact on the induction by bTSH of IL-23p19 and IL-12p35 in fibrocytes and GD-OF. (A and C) Confluent cultures were pretreated with nothing or JNKi (50μM) for 18 h, and then without or with bTSH (5mIU/mL) for 6 h. (B, D) Cultures were transfected with JNK-targeting siRNA (3μg) or control siRNA (3μg). Transfected cells (B, D, and F) were left untreated or were incubated with bTSH for 6 h, RNA extracted, reverse transcribed, and subjected for real-time PCR for IL-23p19 or IL-12p35. (E) Fibrocytes and GD-OF were treated with nothing or bTSH (5mIU/mL) for 30 m. Cell layers were collected, proteins subjected to Western blot analysis for pJNK and re-probed with anti-β-actin. Densitometry values: Fibrocyte control, 7.3 AU, Fibrocyte bTSH 51 AU, GD-OF control 12.4 AU, GD-OF bTSH 159.4 AU (F) Fibrocytes were transfected with a JNK expression plasmid (3μg) or its negative control (3μg) for 2–3 d. Cells were harvested and incubated without or with bTSH for 6 h. Extracted RNA was reversed transcribed and subjected to RT-PCR for IL-23p19 and or IL-12p35 levels. Results were normalized to their respective GAPDH signals. Data are expressed as mean ± SD, *, P<0.05; **, p<0.01 of three independent determinations.
Slit2 appears responsible, at least in part, for the divergence between IL-23 and IL-12 expression in fibrocytes and GD-OF
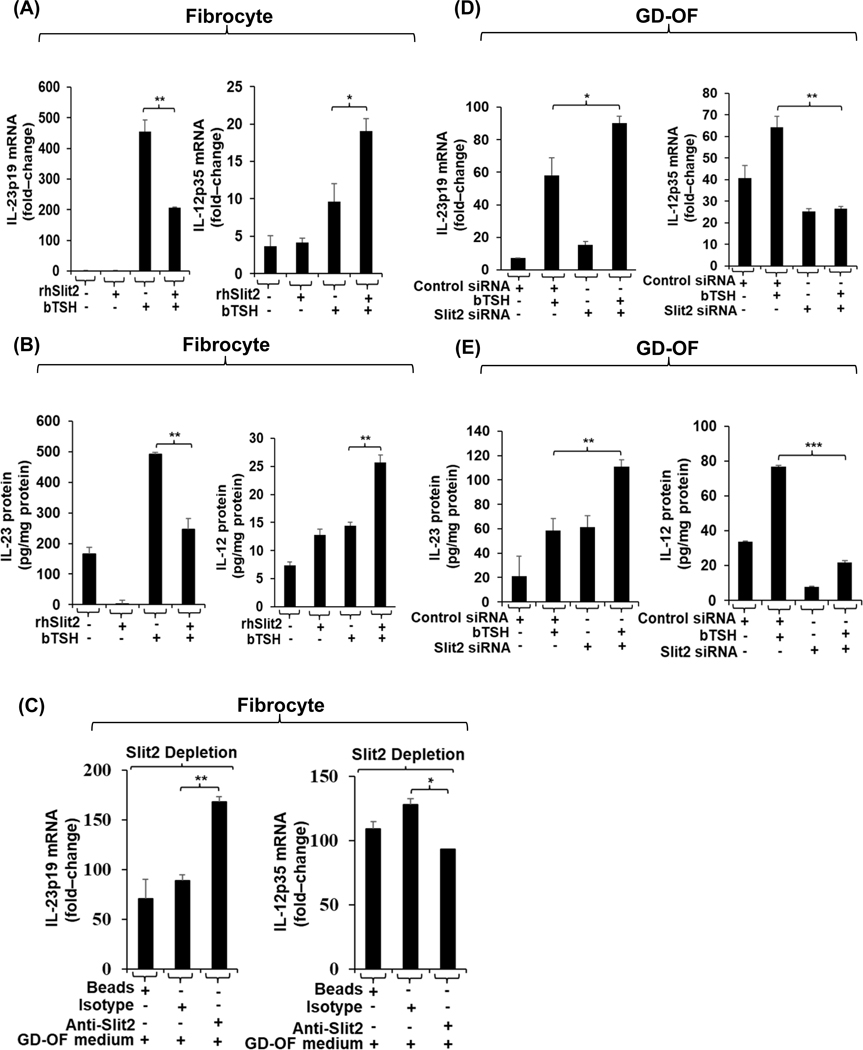
The molecular basis for cell-type-specific cytokine expression and induction by bTSH in fibrocytes and GD-OF was next explored. As apparent from the cell-sorting study (Fig. S4), when GD-OF subsets are separated on the basis of CD34 display, IL-23p19 expression in CD34+ OF is enhanced in the pure subset. We have reported recently that CD34− OF express and release Slit2 which has been shown previously to attenuate autoantigen expression in fibrocytes (30). Fibrocytes were treated with rhSlit2 (50 ng/mL) resulted in substantially attenuated IL-23p19 induction by bTSH (Fig. 1A, left panel; p<0.01 vs bTSH alone). In contrast, the induction of IL-12p35 is enhanced by rhSlit2 (Fig. 1A, right panel; p<0.05). The same pattern emerged with regard to IL-23 and IL-12 proteins released into the fibrocyte culture medium (Fig. 1B, left and right panels, respectively). Medium conditioned with CD34− OF as described in “Methods” was incubated with fibrocytes for 48 h. As the data in Fig. 1C demonstrate, IL-23p19 expression is considerably lower when compared to fibrocytes incubated in the same CD34− OF conditioned medium that had undergone Slit2 depletion (Fig. 1C, left panel). In contrast, Slit2 adsorption attenuates IL-12p35 expression in these fibrocytes (Fig. 1C, right panel). Knocking-down Slit2 in GD-OF similarly enhances IL-23p19 expression (Fig. 1D, left panel) while attenuating the induction of IL-12p35 mRNA (Fig. 1D, right panel). A similar pattern emerges in the release of IL-23 and IL-12 from GD-OF when Slit2 is knocked-down (Fig. 1E). To determine whether the effects of Slit2 on gene expression in fibrocytes were specific or generalized, its impact on prostaglandin E2 endoperoxide H synthase 1 expression was determined and found to be nil (control; 1.99 ± 0.39 fold-change; Slit2, 1.96 ± 0.065 fold-change).
Fig.5.
rhSlit2 attenuates bTSH-induced IL-23p19 while enhancing IL-12p35 induction in fibrocytes. Knocking-down Slit2 in GD-OF enhances IL-23p19 induction but attenuates the IL-12p35 response. (A and D) Fibrocytes and GD-OF were incubated with rhSlit2 (50 ng/ml) for 7–9 d, then received diluent or bTSH (5 mIU/mL) for 6 h. Cell layers were harvested, RNA extracted, reverse-transcribed and subjected to RT-PCR for the targets indicated. Values were normalized to their respective GAPDH signals. (B) Fibrocytes were treated as in A, media were harvested and subjected to IL-23 and IL-12 specific ELISAs. Values were normalized to respective cell layer protein content. (C) Fibrocytes were covered with GD-OF-conditioned medium that had been incubated for 48 h with uncoated beads or those coated with anti-Slit2 (100 μg) or isotype IgG (100μg), as described in “Methods”. Fibrocyte monolayers were processed as in (A). (D) Confluent GD-OF were transfected with scrambled (control) (3μg) siRNA or Slit2-targeting siRNA (3μg), were incubated for 3 d, and treated with diluent or bTSH for 6 h. They were then processed as in (A). (E) GD-OF were treated as in (D), media were collected and subjected to ELISAs for IL-23 and IL-12 as in (B). Data are expressed as the mean ± SD, *, p<0.05; **, p<0.01, ***, p<0.001 of three independent determinations.
Slit2 reduces IL-23p19 gene transcription activities while enhancing those activities of IL-12p35 in fibrocytes and GD-OF
To explore the mechanisms involved in the actions of Slit2 on cytokine expression, fibrocytes were treated with rhSlit2 (50 ng/mL) for 7 d while GD-OF were transfected with Slit2 siRNA. These cell layers were then subjected to ChIP Pol 2 gene transcription assays for IL-23p19 and IL-12pp35. IL-23p19 gene transcription in fibrocytes is significantly attenuated (Fig.6A, top panel, p<0.01) while IL-12p35 gene transcription is upregulated following treatment with rhSlit2 (Fig. 6A, bottom panel, p<0.01). In contrast Slit2-targeting siRNA transfection of GD-OF enhances IL-23p19 transcription (Fig. 6B, top panel, p<0.01) while reducing that of the IL-12p35 gene (Fig. 6B, lower panel, p<0.01). In contrast to the impact on gene transcription, Slit2 fails to alter the stabilities of either IL-23p19 or IL-12p35 mRNAs in fibrocytes or the stabilizing effects of bTSH (Fig. 6C). As the Western blot demonstrates, rhSlit2 enhances both the basal and bTSH-induced pJNK levels in fibrocytes as the Western blot demonstrates (Fig. 6D). That figure further demonstrates that JNKi enhances basal and TSH-induced IL-23p19 while attenuating IL-12p35 expression. In contrast, rhSlit2 enhances basal and TSH-induced IL-12p35 expression. Importantly, JNKi reduces the negative impact of Slit2 on IL-23p19 while attenuating its enhancing effects on IL-12p35 levels.
Fig. 6.
rhSlit2 attenuates bTSH induction of IL-23/p19 gene transcription while enhancing that of the IL-12p35 gene in fibrocytes. In contrast, knocking-down Slit2 in GD-OF enhances IL-23p19 gene transcription while attenuating that of the IL-12p35 gene. These effects are divergently affected by JNK inhibitor (JNKi). (A) Fibrocytes were incubated with nothing or rhSlit2 (50 ng/ml) for 7–9 d. (B) GD-OF monolayers were subjected to treatment with either control (3μg) siRNA or SLIT2-targeting siRNAs (3μg) for 3 d. (A, B), Cells were treated with nothing or bTSH (5 mIU/mL) for 2 h, harvested, and subjected to a Pol II Chip transcription assay as described in “Methods”. Values were normalized to respective monolayer protein content. (C) Confluent fibrocyte cultures treated without or with Slit2 for 7 d. were pre-treated with bTSH for 2 h. At time “0”, all cultures received 5,6-dichlorobenzimidazole (20 μg/mL) without or continued in the presence of bTSH. Monolayers were harvested at the times indicated along the abscissas, mRNA was reversed transcribed and subjected to real-time PCR for IL-23p19.. (D) Fibrocytes were pretreated without or with rhSlit2 (50 ng/ml) for 7 d, and were then incubated without or with bTSH (5mIU/mL) for 30 m. Cell layers were collected, proteins subjected to Western blot analysis for pJNK and then re-probed with anti-β-actin. Densitometric analysis: Control 5.4 AU, bTSH : 52.9 AU, rhSlit2 10.1 AU, bTSH plus rhSlit2 127.4 AU. rhSlit2 (50 ng/ml) treated fibrocyte cultures incubated with, nothing or JNKi (50μM) for 18 h, and then treated without or with bTSH (5mIU/mL) for 6 h. mRNA was reversed transcribed from the cell layers and subjected to real-time PCR for IL-23p19 and IL-12p35. Data are expressed as mean ± SD of triplicate determinations from a single experiment, representative of three studies performed. *, p<0.05; **, p<0.01
Discussion
Molecular mechanisms underlying IL-23 and IL-12 induction by bTSH in fibrocytes and GD-OF involve upregulating IL-23p19 and IL-12p35 gene transcriptional activities coordinated with enhancement of their respective mRNA stabilities (Fig. 2). Both genes have been found previously regulated at the levels of transcription (50–53) and mRNA stability (54,55). Rel-C (50), Rel-A (51), SMAD-3, and ATF-2 (52) can activate the IL-23p19 gene promoter in various cell-types. On the other hand, INFγ inhibits IL-23 expression by selectively targeting IL-23p19 mRNA stability (54). This is mediated through interactions between the 3’-untranslated region of the transcript and TTP (54). The critical role played by TTP in IL-23-dependent inflammation has been established in TTP−/− mice (55). The findings reported here suggest that substantial induction by bTSH of IL-23p19 and IL-12p35 in fibrocytes and GD-OF, respectively, occurs despite the modulatory effects of TTP (Figs. 2C and 2F). Knocking down TTP enhances levels of the respective cytokine mRNAs. This finding suggests that the zinc finger protein ordinarily exerts a suppressive influence on the production of both IL-23 and IL-12. Further studies will be required to identify the specific molecular effectors through which regulation of IL-23p19 and IL-12p35 by bTSH is mediated.
The divergence of IL-23p19 and IL-12p35 expression and induction by bTSH in fibrocytes and GD-OF (Fig. 1) is notable because GD-OF comprise fibrocyte-derived CD34+ OF (40). An interplay between this subset and CD34− OF appears to realign cytokine production, effects that are apparently mediated by Slit2 in repressing IL-23p19 while enhancing IL-12p35. Within the admixture of cells comprising GD-OF, Slit2 is expressed and released from CD34− OF (30), altering the cytokine production profile of CD34+ OF (Fig. S4). Those results suggest that CD34+ OF and CD34− OF exhibit distinct capacities for expressing IL-23 and IL-12. Slit2 reduces basal JNK phosphorylation and attenuates its upregulation by bTSH (Fig. 6D). In aggregate, they suggest that Slit2 helps determine which cytokine dominates the synthetic repertoire of these cells, thus potentially acting as a molecular fulcrum. The differences in patterns of IL-23 and IL-12 expression and induction in the two cell types and the impact on those cytokines exerted by Slit2 cannot be attributable to the genetic polymorphisms identified in the IL-23 and IL-12 genes associated with GD (56,57), since the identical relationships were observed in H-OF derived from healthy donors (Fig. S3).
The current findings suggest that both AKT and JNK pathways may play important roles in mediating the actions of bTSH on IL-23p19 and IL-12p35 expression in fibrocytes and GD-OF. Interfering with Akt activity either by inhibiting its activity or by knocking down its expression, attenuates the expression of both cytokines (Fig. 3). In contrast, inhibiting JNK (Fig. 4A and 4C) or knocking-down its expression (Fig. 4B and 4D) enhances IL-23p19 expression and its induction by bTSH in fibrocytes and GD-OF. Moreover, overexpression of JNK downregulates IL-23p19 while enhancing IL-12p35 expression in fibrocytes (Fig. 4F). Thus, the JNK pathway may play a pivotal role in determining the cell type-specific actions of bTSH exhibited on IL-23p19 and IL-12p35 induction. The JNK pathway also appears to influence the divergent effects of Slit2 on both IL-23p19 and IL-12p35 (Fig. 6D).
Our findings in aggregate raise important questions concerning whether factors existing outside the IL-12 superfamily might contribute substantially to the complex interplay between these cytokine family members (1). By virtue of their structural similarities, shared common subunits, and overlapping signaling pathways, these structurally related cytokines and their receptors exhibit remarkable promiscuity regarding actions on specific downstream targets. It is possible that Slit2 could influence the impact that each family member exerts within a particular molecular context. In addition, IL-12 and IL-23 are broadly considered to represent proinflammatory molecules while the remaining cytokines within the IL-12 family, namely IL-27 and IL-33, exert generally anti-inflammatory influences (58,59). Further studies will be necessary to determine whether Slit2 might also regulate the expression and/or actions of additional cytokines beyond the IL-12 family. In any event, the current findings suggest that Slit2 might help determine cytokine balance.
The biological roles ascribed initially to IL-23 involved its enhancement of development, expansion, and survival of IL-17-producing CD4+ cells (6). Subsequently, the cytokine was found to promote Th17 trafficking in experimental autoimmune encephalomyelitis (60). In the context of TAO, it is possible that fibrocytes activated through the TSHR signaling pathway may play an important role in recruiting Th17 cells to the orbit where they could participate in disease pathogenesis. The functional juxtaposition between IL-23 and IL-12 generating capacities of fibrocytes could reflect their determining Th1/Th17 balance in GD. Greater insights into those issues will necessarily depend on future studies conducted in vivo, ideally in a refined animal model of TAO.
Fibrocytes remain incompletely characterized and their already extensive array of biological functions has yet to be fully elucidated. The current findings, coupled with several recent reports, suggest that these cells represent a nexus between the regulatory TSH/TSHR pathway and immune surveillance (27,47,61). Fibrocytes appear to be particularly relevant to the development of TAO where they can be identified, based on their display of characteristic cell markers (45), as a discrete infiltrate of the orbit (28). We postulate that these CD34+ OF originate from the circulating pool of fibrocytes, predicated on studies conducted in mice where bone marrow-derived fibrocytes infiltrate a variety of tissues at sites of injury (22,23). Our recent findings and those reported here disclose that TSHR-activated fibrocytes express multiple cytokines with established, determinant roles in conditioning immune responses, T cell polarization, and expansion (27,47,61).
In GD, and especially in patients with intractable disease, peripheral T cells are skewed toward Th17 (18). However, the issue of whether GD is biased to the Th1, Th2, or Th17 paradigm remains contentious (17,19). Recent evidence implicates the IL-23/IL-17 pathway in thyroid autoimmunity, including GD and TAO (19,20,62). Further, the IL-17A pathway has been shown recently to activate pro-inflammatory and pro-fibrotic functions in GD-OF (63). Thus, the TSHR pathway might drive the cytokine milieu within orbital tissues through actions directed at CD34+ OF.
Current efforts in many laboratories and industry are focused on therapeutically targeting the IL-23/IL-17 pathway in chronic inflammatory diseases (64). Recently developed agents disrupting the actions of IL-23p19 as a potential treating psoriasis and inflammatory bowel disease. Those efforts have culminated in agents exhibiting clinical benefit and tolerable safety profiles (65,66). Our current findings may provide the mechanistic insight and rationale for specifically targeting this cytokine in severe, active TAO. Further, they may prove relevant to other disease processes where fibrocytes, activated perhaps through TSHR engagement, are potentially involved. These include the chronic low-level vascular inflammation and precocious atherosclerosis associated with primary hypothyroidism where serum TSH levels are typically elevated (67). In any event, we appear to have identified a heretofore unrecognized link between the TSHR pathway and Slit2 in determining the cytokine environment that might condition T cell development and cytokine expression in TAO.
Supplementary Material
Key Points.
Slit2, a neuron repellant, can determine fibrocyte cytokine expression repertoires
Slit2 attenuates IL-23 while enhancing IL-12
Divergent IL-23 and IL-12 is mediated through JNK
Acknowledgements
The authors are indebted to Ms. Linda Polonsky for her editorial comments and to Mrs. Leslie Bordine for her expert help in the preparation of this manuscript.
This work was supported in part by NIH grants EY008976, Autoimmune Center of Excellence AR088974, NEI Core grant EY007003, Bell Charitable Family Foundation and the RPB.
References
- 1.Vignali DA, and Kuchroo VK (2012) IL-12 family cytokines: immunological playmakers. Nat Immunol 13, 722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaffen SL, Jain R, Garg AV, and Cua DJ (2014) The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14, 585–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croxford AL, Kulig P, and Becher B. (2014) IL-12-and IL-23 in health and disease. Cytokine Growth Factor Rev 25, 415–421 [DOI] [PubMed] [Google Scholar]
- 4.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, and Moore KW (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. Journal of immunology (Baltimore, Md. : 1950) 168, 5699–5708 [DOI] [PubMed] [Google Scholar]
- 5.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, and O’Shea JJ (2004) Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev 202, 139–156 [DOI] [PubMed] [Google Scholar]
- 6.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, and Kastelein RA (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 [DOI] [PubMed] [Google Scholar]
- 7.Park WR, Park CS, Tomura M, Ahn HJ, Nakahira Y, Iwasaki M, Gao P, Abe R, Hamaoka T, and Fujiwara H. (2001) CD28 costimulation is required not only to induce IL-12 receptor but also to render janus kinases/STAT4 responsive to IL-12 stimulation in TCR-triggered T cells. Eur J Immunol 31, 1456–1464 [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto N, Nakahira M, Ahn HJ, Micallef M, Hamaoka T, Kurimoto M, and Fujiwara H. (2003) Differential requirements for JAK2 and TYK2 in T cell proliferation and IFN-gamma production induced by IL-12 alone or together with IL-18. Eur J Immunol 33, 243–251 [DOI] [PubMed] [Google Scholar]
- 9.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, Takada H, Hara T, Kawamura N, Ariga T, Kaneko H, Kondo N, Tsuge I, Yachie A, Sakiyama Y, Iwata T, Bessho F, Ohishi T, Joh K, Imai K, Kogawa K, Shinohara M, Fujieda M, Wakiguchi H, Pasic S, Abinun M, Ochs HD, Renner ED, Jansson A, Belohradsky BH, Metin A, Shimizu N, Mizutani S, Miyawaki T, Nonoyama S, and Karasuyama H. (2006) Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25, 745–755 [DOI] [PubMed] [Google Scholar]
- 10.Tokumasa N, Suto A, Kagami S, Furuta S, Hirose K, Watanabe N, Saito Y, Shimoda K, Iwamoto I, and Nakajima H. (2007) Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood 110, 553–560 [DOI] [PubMed] [Google Scholar]
- 11.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, and Becher B. (2007) IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. Journal of immunology (Baltimore, Md. : 1950) 179, 8098–8104 [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, and Littman DR (2007) IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8, 967–974 [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, and Murphy KM (1993) Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260, 547–549 [DOI] [PubMed] [Google Scholar]
- 14.Seder RA, Gazzinelli R, Sher A, and Paul WE (1993) Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A 90, 10188–10192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, Isobe K, and Hibi T. (2008) IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 57, 1682–1689 [DOI] [PubMed] [Google Scholar]
- 16.Huber AK, Jacobson EM, Jazdzewski K, Concepcion ES, and Tomer Y. (2008) Interleukin (IL)-23 receptor is a major susceptibility gene for Graves’ ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. The Journal of clinical endocrinology and metabolism 93, 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapoport B, and McLachlan SM (2014) Graves’ hyperthyroidism is antibody-mediated but is predominantly a Th1-type cytokine disease. The Journal of clinical endocrinology and metabolism 99, 4060–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanba T, Watanabe M, Inoue N, and Iwatani Y. (2009) Increases of the Th1/Th2 cell ratio in severe Hashimoto’s disease and in the proportion of Th17 cells in intractable Graves’ disease. Thyroid 19, 495–501 [DOI] [PubMed] [Google Scholar]
- 19.Peng D, Xu B, Wang Y, Guo H, and Jiang Y. (2013) A high frequency of circulating th22 and th17 cells in patients with new onset graves’ disease. PLoS One 8, e68446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SE, Yoon JS, Kim KH, and Lee SY (2012) Increased serum interleukin-17 in Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 250, 1521–1526 [DOI] [PubMed] [Google Scholar]
- 21.Fang S, Huang Y, Zhong S, Zhang Y, Liu X, Wang Y, Gu P, Zhou H, and Fan X. (2016) IL-17A Promotes RANTES Expression, But Not IL-16, in Orbital Fibroblasts Via CD40-CD40L Combination in Thyroid-Associated Ophthalmopathy. Invest Ophthalmol Vis Sci 57, 6123–6133 [DOI] [PubMed] [Google Scholar]
- 22.Bucala R, Spiegel LA, Chesney J, Hogan M, and Cerami A. (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1, 71–81 [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, and Strieter RM (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114, 438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesney J, Metz C, Stavitsky AB, Bacher M, and Bucala R. (1998) Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. Journal of immunology (Baltimore, Md. : 1950) 160, 419–425 [PubMed] [Google Scholar]
- 25.Chesney J, Bacher M, Bender A, and Bucala R. (1997) The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 94, 6307–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, and Mack M. (2009) CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A 106, 17892–17897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, Cockerham KP, Charara LK, Goncalves AC, Zhao SX, Ginter A, Lu Y, Smith TJ, and Douglas RS (2012) Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. The Journal of clinical endocrinology and metabolism 97, E740–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, and Smith TJ (2010) Increased generation of fibrocytes in thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism 95, 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith TJ (2015) TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol 11, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernando R, Grisolia ABD, Lu Y, Atkins S, and Smith TJ (2018) Slit2 Modulates the Inflammatory Phenotype of Orbit-Infiltrating Fibrocytes in Graves’ Disease. Journal of immunology (Baltimore, Md. : 1950) 200, 3942–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidd T, Bland KS, and Goodman CS (1999) Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785–794 [DOI] [PubMed] [Google Scholar]
- 32.Legg JA, Herbert JM, Clissold P, and Bicknell R. (2008) Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis 11, 13–21 [DOI] [PubMed] [Google Scholar]
- 33.Pilling D, Zheng Z, Vakil V, and Gomer RH (2014) Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci U S A 111, 18291–18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinau G, Neumann S, Gruters A, Krude H, and Biebermann H. (2013) Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr Rev 34, 691–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleinau G, and Krause G. (2009) Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev 30, 133–151 [DOI] [PubMed] [Google Scholar]
- 36.Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, and Smith TJ (2014) Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. The Journal of clinical endocrinology and metabolism 99, E1236–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams DD, Purves HD, and Sirett NE (1961) The response of hypophysectomized mice to injections of human serum containing long-acting thyroid stimulator. Endocrinology 68, 154–155 [DOI] [PubMed] [Google Scholar]
- 38.Smith TJ (2016) Rationale for therapeutic targeting insulin-like growth factor-1 receptor and bone marrow-derived fibrocytes in thyroid-associated ophthalmopathy. Expert Rev Ophthalmol 11, 77–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, and Sorisky A. (2002) Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism 87, 385–392 [DOI] [PubMed] [Google Scholar]
- 40.Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, and Smith TJ (2012) Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A 109, 7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells J, and Farnham PJ (2002) Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods (San Diego, Calif.) 26, 48–56 [DOI] [PubMed] [Google Scholar]
- 42.Tsui S, Fernando R, Chen B, and Smith TJ (2011) Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem 286, 24487–24499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dreyer C, and Hausen P. (1978) Inhibition of mammalian RNA polymerase by 5,6-dichlororibofuranosylbenzimidazole (DRB) and DRB triphosphate. Nucleic Acids Res 5, 3325–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Nunez Miguel R, Blundell TL, Furmaniak J, and Rees Smith B. (2004) Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid 14, 560–570 [DOI] [PubMed] [Google Scholar]
- 45.Pilling D, Fan T, Huang D, Kaul B, and Gomer RH (2009) Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 4, e7475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, and Blackshear PJ (1999) Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol 19, 4311–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raychaudhuri N, Fernando R, and Smith TJ (2013) Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One 8, e75100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, and Douglas RS (2014) Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. The Journal of clinical endocrinology and metabolism 99, E1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quan JH, Chu JQ, Kwon J, Choi IW, Ismail HA, Zhou W, Cha GH, Zhou Y, Yuk JM, Jo EK, and Lee YH (2015) Intracellular Networks of the PI3K/AKT and MAPK Pathways for Regulating Toxoplasma gondii-Induced IL-23 and IL-12 Production in Human THP-1 Cells. PLoS One 10, e0141550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carmody RJ, Ruan Q, Liou HC, and Chen YH (2007) Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. Journal of immunology (Baltimore, Md. : 1950) 178, 186–191 [DOI] [PubMed] [Google Scholar]
- 51.Mise-Omata S, Kuroda E, Niikura J, Yamashita U, Obata Y, and Doi TS (2007) A proximal kappaB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription. Journal of immunology (Baltimore, Md. : 1950) 179, 6596–6603 [DOI] [PubMed] [Google Scholar]
- 52.Al-Salleeh F, and Petro TM (2008) Promoter analysis reveals critical roles for SMAD-3 and ATF-2 in expression of IL-23 p19 in macrophages. Journal of immunology (Baltimore, Md. : 1950) 181, 4523–4533 [DOI] [PubMed] [Google Scholar]
- 53.Wen H, Dou Y, Hogaboam CM, and Kunkel SL (2008) Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood 111, 1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian X, Ning H, Zhang J, Hoft DF, Stumpo DJ, Blackshear PJ, and Liu J. (2011) Posttranscriptional regulation of IL-23 expression by IFN-gamma through tristetraprolin. Journal of immunology (Baltimore, Md. : 1950) 186, 6454–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molle C, Zhang T, Ysebrant de Lendonck L, Gueydan C, Andrianne M, Sherer F, Van Simaeys G, Blackshear PJ, Leo O, and Goriely S. (2013) Tristetraprolin regulation of interleukin 23 mRNA stability prevents a spontaneous inflammatory disease. J Exp Med 210, 1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu YH, Chen CC, Liao LL, Wan L, Tsai CH, and Tsai FJ (2012) Association of IL12B polymorphisms with susceptibility to Graves ophthalmopathy in a Taiwan Chinese population. Journal of biomedical science 19, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia H, Tao F, Liu C, Guo T, Zhu W, Wang S, Cui B, and Ning G. (2015) Both interleukin-23A polymorphism and serum interlukin-23 expression are associated with Graves’ disease risk. Cell Immunol 294, 39–43 [DOI] [PubMed] [Google Scholar]
- 58.Collison LW, and Vignali DA (2008) Interleukin-35: odd one out or part of the family? Immunol Rev 226, 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones LL, and Vignali DA (2011) Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res 51, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gyulveszi G, Haak S, and Becher B. (2009) IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur J Immunol 39, 1864–1869 [DOI] [PubMed] [Google Scholar]
- 61.Li B, and Smith TJ (2014) PI3K/AKT pathway mediates induction of IL-1RA by TSH in fibrocytes: modulation by PTEN. The Journal of clinical endocrinology and metabolism 99, 3363–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Figueroa-Vega N, Alfonso-Perez M, Benedicto I, Sanchez-Madrid F, Gonzalez-Amaro R, and Marazuela M. (2010) Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. The Journal of clinical endocrinology and metabolism 95, 953–962 [DOI] [PubMed] [Google Scholar]
- 63.Fang S, Huang Y, Wang S, Zhang Y, Luo X, Liu L, Zhong S, Liu X, Li D, Liang R, Miranda P, Gu P, Zhou H, Fan X, and Li B. (2016) IL-17A Exacerbates Fibrosis by Promoting the Proinflammatory and Profibrotic Function of Orbital Fibroblasts in TAO. The Journal of clinical endocrinology and metabolism 101, 2955–2965 [DOI] [PubMed] [Google Scholar]
- 64.Zwicky P, Unger S, and Becher B. (2019) Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. J Exp Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deepak P, and Sandborn WJ (2017) Ustekinumab and Anti-Interleukin-23 Agents in Crohn’s Disease. Gastroenterol Clin North Am 46, 603–626 [DOI] [PubMed] [Google Scholar]
- 66.Markham A. (2017) Guselkumab: First Global Approval. Drugs 77, 1487–1492 [DOI] [PubMed] [Google Scholar]
- 67.Duntas LH, and Wartofsky L. (2007) Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid 17, 1075–1084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.