Abstract
A number of studies have reported that decreased mitochondrial numbers are linked with neoplastic transformation and/or tumor progression, including resistance to apoptosis. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) is a multi-functional transcriptional coactivator that regulates the activities of multiple nuclear receptors and transcriptional factors involved in mitochondrial biogenesis. In this study, we observed that the number of mitochondria in sarcoma tissues, such as osteosarcoma and malignant fibrous histiocytoma, is significantly lower than that in normal muscle tissue or benign tumors and that increasing the number of mitochondria by PGC-1α overexpression induces mitochondrial apoptosis in human sarcoma cell lines. The findings suggest that decreased mitochondrial numbers may contribute to musculoskeletal tumor progression and that regulation of mitochondrial numbers by PGC-1α could be a potent therapeutic tool for human malignancies.
Electronic supplementary material
The online version of this article (doi:10.1038/srep03916) contains supplementary material, which is available to authorized users.
Subject terms: Cancer therapeutic resistance, Sarcoma
Introduction
Musculoskeletal malignancies are clinically aggressive and have high metastatic behavior in various organs. Although many chemotherapeutic protocols are used for human sarcomas, the current chemotherapeutic strategies for high-grade sarcomas have been ineffective and the prognoses of patients can be extremely poor because of local recurrence and distant metastases1. Therefore, new therapeutic strategies against high-grade sarcomas need to be established. We have previously reported several therapeutic strategies against high-grade sarcomas2,3,4.
Mitochondria are cytoplasmic organelles that play essential roles in cellular energy metabolism and programmed cell death5. Although the majority of mitochondrial proteins are synthesized by nuclear DNA (nDNA), mitochondria possess their own genome, called mitochondrial DNA (mtDNA)6. Thousands of mitochondria are found in each cell and the number of mitochondria per cell is known to vary with cell or tissue origin and to change under different internal or external microenvironments, such as hypoxia and stimulation by steroid hormones7,8. Quantitative changes in mitochondrial numbers have been observed in many cancers as a decrease in hepatocellular carcinoma, renal cell carcinoma, advanced gastric cancer and breast cancer9,10,11,12,13,14, or an increase in head and neck cancers, ovarian cancer and esophageal squamous cell carcinoma15,16,17. Changes in the reduction of mitochondrial numbers have been found to be associated with tumor progression and prognosis in patients with hepatocellular carcinoma and breast cancer9,10. It has been reported that a decrease in mitochondrial numbers is significantly associated with the development of metastasis in Ewing's sarcoma18. It is unclear whether mitochondrial numbers are decreased in other sarcomas.
Mitochondrial function is modulated by mitochondrial numbers and biogenesis19. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) is a multi-functional transcriptional coactivator that regulates the activities of multiple nuclear receptors and transcriptional factors involved in mitochondrial biogenesis, such as mitochondrial respiration and mtDNA replication and transcription20,21. In particular, PGC-1α regulates transcription of the gene coding mitochondrial transcription factor A (TFAM), a gene required for mitochondrial biogenesis, which mirrors the changing levels of mtDNA in the cell and plays a crucial role in mtDNA maintenance22. Recent studies have reported that PGC-1α expression decreases in various cancers23,24,25,26; and suggest that decreased mitochondrial biogenesis may contribute to tumorigenesis and/or tumor progression in human malignancies and that the regulation of mitochondrial numbers may induce antitumor effects on malignant tumor cells. Consistent with these hypotheses, it has been reported that 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, which is known to stimulate mitochondrial proliferation and increased expression of TFAM, inhibits cell growth and induces apoptosis in prostate cancer cells27. It is also reported that the introduction of normal epithelial mitochondria into human breast cancer cells inhibits cell proliferation and increases drug sensitivity through a reversal of glycolysis28. We have recently reported that transcutaneous application of carbon dioxide (CO2) increases mitochondrial biogenesis in skeletal muscle29 and that CO2 therapy induces mitochondrial apoptosis with mitochondrial proliferation via PGC-1α expression in human malignant fibrous histiocytoma (MFH)30.
Based on these previous findings, we hypothesized that mitochondrial numbers and PGC-1α expression might decrease in human sarcoma tissues and that increases in mitochondrial numbers by PGC-1α expression could induce antitumor effects on human sarcomas. In the current study, we examined mitochondrial numbers and expression of PGC-1α and TFAM in human musculoskeletal tumor tissues to verify mitochondrial biogenesis and investigated the effects of mitochondrial proliferation by PGC-1α on mitochondrial biogenesis and cellular apoptosis in human musculoskeletal tumor cell lines.
Results
Mitochondrial numbers significantly decrease in musculoskeletal malignant tumors
To investigate mitochondrial biogenesis in musculoskeletal tissues, we first evaluated mitochondrial numbers and the expression of mitochondria-related genes in human musculoskeletal tissue samples. We evaluated the number of mtDNA copies relative to mitochondrial numbers in human musculoskeletal tumors and normal muscle tissue by quantitative real-time PCR (qRT-PCR) and found that mtDNA copy numbers in musculoskeletal tumors, such as osteosarcomas and MFH, were significantly lower compared with normal muscle tissue (Table 1). In malignant tumors, mtDNA copy numbers were significantly lower than in benign schwannomas (Table 1). These results were corroborated by immunofluorescence staining of mitochondria (data was not shown). And, we have also evaluated sample tissues with electron microscopy to examine mitochondria size and shapes (Supplementry Fig. S1). Electron microscopy revealed that mitochondria was barely observed and were swollen with disrupted cristae in osteosarcoma and MFH tissues, but was extensively observed in normal muscle tissue. Consistent with mtDNA copy number analysis, qRT-PCR showed that mRNA expression of both PGC-1α and TFAM in musculoskeletal tumors were significantly lower compared with normal muscle tissue (Table 2 and 3). In musculoskeletal tumors, both PGC-1α and TFAM mRNA expression were significantly lower in malignant tumors compared with benign schwannomas (Table 2 and 3).
Table 1.
Relative mtDNA number in musculoskeletal tissues

Table 2.
Relative PGC-1α expression in musculoskeletal tissues

Table 3.
Relative TFAM expression in musculoskeletal tissues

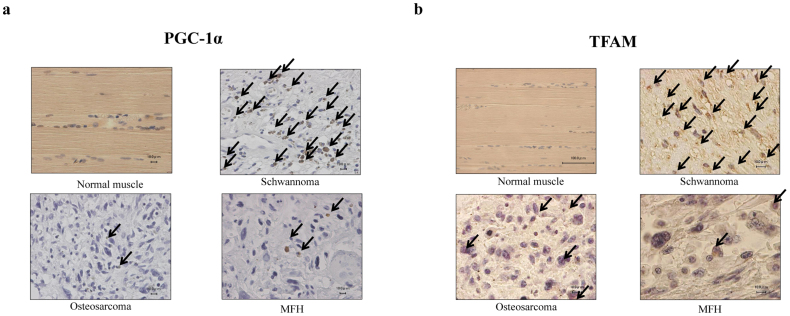
Immunohistochemical analysis revealed very little expression of either PGC-1α or TFAM in malignant tumor tissue, while strong expression was observed in normal muscle tissue (Fig. 1a and 1b).
Figure 1.
Imuunohistochemical staining for PGC-1α (a) and TFAM (b) in human musculoskeletal tumors and normal muscle tissue samples.
Arrows indicate PGC-1α (a) and TFAM (b) stained cells.
These results suggested that mitochondrial numbers and/or mitochondrial biogenesis via the PGC-1α/TFAM pathway may decrease in musculoskeletal malignancies and that decreased mitochondrial biogenesis may be associated with musculoskeletal tumor development.
Mitochondrial proliferation is induced by PGC-1α overexpression via the PGC-1α/TFAM pathway in human MFH cells
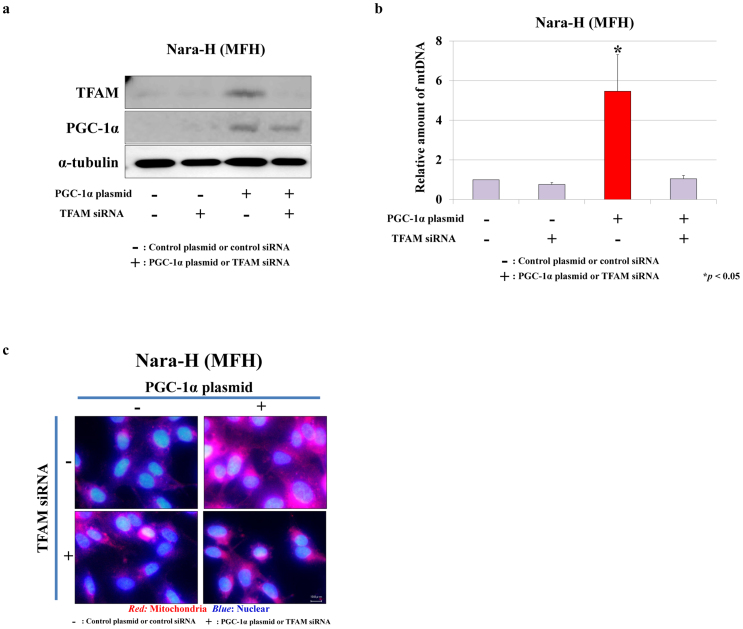
It has been reported that mitochondrial synthesis is stimulated by the PGC-1α/TFAM pathway in muscle tissue in vitro22; however, a relationship between mitochondrial numbers and the PGC-1α/TFAM pathway has not been reported in musculoskeletal malignancies. To elucidate the relationship between mitochondrial biogenesis and musculoskeletal tumorigenesis, we examined the direct effects of increased PGC-1α expression on mitochondrial biogenesis by co-transfection with PGC-1α plasmid and TFAM siRNA. Transfection with PGC-1α plasmid/control siRNA strongly increased the expression of both PGC-1α and TFAM, while TFAM siRNA strongly reduced PGC-1α-induced TFAM expression (Fig. 2a). The amount of mtDNA significantly increased with PGC-1α plasmid/control siRNA transfection (Fig. 2b, P < 0.05) and decreased with TFAM siRNA (Fig. 2b). The results were corroborated by immunofluorescence staining (Fig. 2c).
Figure 2.
Effects of PGC-1α plasmid and TFAM siRNA co-transfection on mitochondrial biogenesis in a human MFH cell line, Nara-H.
(a) Protein expression of PGC-1α and TFAM were evaluated by immunoblot analysis in Nara-H cells that were co-transfected with PGC-1α plasmid (or control plasmid) and TFAM siRNA (or control siRNA). (b) Relative mtDNA numbers in plasmid/siRNA transfected Nara-H cells were assessed by qRT-PCR. Data represent mean ± SE of at least three independent experiments (* P < 0.05). (c) Immunofluorescence staining of mitochondria was performed in plasmid/siRNA transfected cells. Bar = 10 μm.
Increased mitochondrial numbers by PGC-1α overexpression induces mitochondrial apoptosis in human sarcoma cells
We hypothesized that tumor progression can be caused by decreased mitochondrial biogenesis and that improving mitochondrial numbers might affect tumor cell growth. To test this hypothesis, we assessed apoptotic activity in human musculoskeletal cell lines after co-transfection with PGC-1α plasmid and TFAM siRNA.
In sarcoma cell lines, Nara-H, TNMY-1, KTHOS and MG-63, transfection with PGC-1α plasmid/control siRNA strongly increased the number of apoptotic cells, along with an increase in mitochondrial proliferation (Fig. 3a–d, Supplementary Fig. S2a–d). However, increased apoptotic cell numbers with mitochondrial proliferation were markedly suppressed by TFAM siRNA transfection (Fig. 3a–d, Supplementary Fig. S2a–d). Consistent with these results, immunofluorescence staining revealed that increased apoptotic cell numbers with mitochondrial proliferation were extensively observed in PGC-1α plasmid/control siRNA transfected sarcoma cells (Fig. 4a and b, Supplementary Fig. S3a and b). In addition, to evaluate the effect of mitochondrial proliferation on cellular apoptosis in normal cell, we examined apoptotic activity and mitochondrial proliferation in normal chondrocyte cell line (NHAC-kn) using same transfection. In a NHAC-kn cell, mitochondrial number was increased by PGC-1α plasmid/control siRNA transfection, however, there was no significant difference in the number of apoptotic cells among transfected cells (Supplementary Fig. S4a and b). Consistent with these results, immunofluorescence staining revealed that increased apoptotic cells with mitochondrial proliferation were not observed in PGC-1α plasmid/control siRNA transfected sarcoma cells (Supplementary Fig. S4c). These findings suggested that mitochondrial proliferation induced apoptosis might be specific to malignant tumors though one limitation is that normal cells used in this study are not of tumor origin.
Figure 3.
Mitochondrial apoptotic activity after PGC-1α plasmid and TFAM siRNA co-transfection in human musculoskeletal cell lines.
Apoptotic activity and mitochondrial numbers were assessed by flow cytometry in human MFH (Nara-H) (a) and human osteosarcoma (KTHOS) (b) cell lines. Results were normalized to control cell (control plasmid and control siRNA co-transfection) mean value. Data represent mean ± SE of at least three independent experiments (* P < 0.05). (c and d) Correlations between apoptotic activity and mitochondrial numbers in each transfected cell line were evaluated.
Figure 4.
Immunofluorescence staining of apoptotic nuclei (green) and mitochondria (red) in human musculoskeletal cell lines after PGC-1α plasmid and TFAM siRNA co-transfection.
(a) Nara-H, a human MFH cell line and (b) KTHOS, a human osteosarcoma cell line.
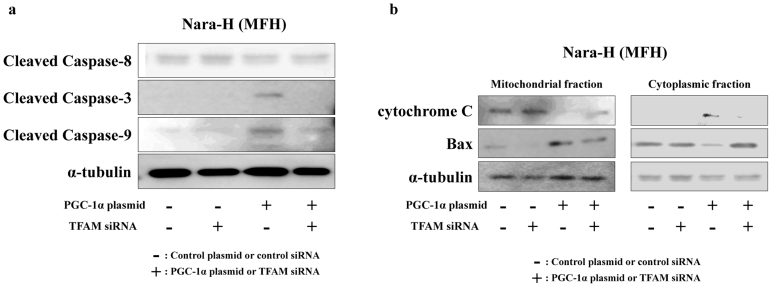
We also evaluated the expression of apoptosis-related proteins, such as caspases, cytochrome c and Bax, by immunoblot analyses in transfected MFH cells. In PGC-1α plasmid/control siRNA-transfected cells, cleavage of both caspase-3 and -9 strongly increased compared with other transfected cells, while the expression of cleaved caspase-8 did not increase (Fig. 5a). Cleaved forms of the three caspases did not change in TFAM siRNA-transfected cells (Fig. 5a).
Figure 5.
Western immunoblot analyses for apoptosis-related proteins in PGC-1α plasmid/TFAM siRNA transfected MFH cells.
(a) Expression of cleaved forms of caspase-3, -8 and -9. (b) Expression of cytochrome c and Bax were separately evaluated in mitochondrial and cytoplasmic fractions.
Protein expression of cytochrome c and Bax were separately evaluated in the mitochondrial and cytoplasmic fractions. We observed that, in PGC-1α plasmid/control siRNA-transfected cells, the expression of cytochrome c decreased in the mitochondrial fraction and increased in the cytoplasmic fraction (Fig. 5b). Conversely, Bax expression increased in the mitochondrial fraction and decreased in the cytoplasmic fraction (Fig. 5b). The results indicated that increasing mitochondrial numbers by PGC-1α overexpression could induce mitochondrial apoptosis in human sarcoma cells.
Discussion
Decreased mitochondrial numbers in renal cancer31, hepatocellular carcinoma12 and gastric cancer11 has been reported. Simonnet et al. reported that mitochondrial respiratory dysfunction through decreased mtDNA was observed in accordance with increased invasiveness of cancer cells32. Consistent with these reports, in the present study, we observed that mitochondrial numbers were significantly reduced in human sarcoma tissues compared with normal muscle tissue or benign musculoskeletal tumors. While it is generally accepted that there are differences between tissues in mtDNA content in mammalian tissues, the extent and entity of these differences are not clear. As far as we have examined previous studies, it was reported that there were differences of mitochondrial copy number in various normal tissues; however the differences were about 2 times at maximum or none (Supplemental Reference 1, 2). Then, we used normal muscles tissues as an alternative to various normal tissues in this study because it is difficult to obtain various normal tissue samples from human. Increased mitochondrial numbers have also been reported in head and neck cancers33, esophageal squamous cell carcinoma16 and endometrial adenocarcinoma34. These reports suggest that, on the basis of clonal expansion mechanisms, increases in mitochondrial copy numbers might be compensatory to allow damaged mitochondria to maintain function16,33. When cancer progresses to advanced stages because of excess damage, decompensation may be followed by decreases in mitochondrial copy numbers, resulting in impaired mitochondrial function and the Warburg effect35. Causes of decreases in mitochondrial numbers are not well known. mtDNA has a unique 1124 bp noncoding region, designated as a displacement D-loop, which is responsible for mtDNA transcription and replication36. Mutations in the D-loop region are very common in cancer cells37. Thus, alterations in the D-loop may interfere with sequences in promoter regions and may modify the binding affinities of inducers and/or modulators of mtDNA transcription and replication38. We hypothesized that if cancer progression is caused by decreased mitochondrial numbers, increasing the number of mitochondria might have antitumor effects in malignant tumors.
PGC-1α is a multi-functional transcriptional coactivator that regulates the activities of multiple nuclear receptors and transcriptional factors involved in mitochondrial biogenesis through TFAM20,21. It has recently been reported that PGC-1α expression decreases in certain cancers, such as breast23, colon24, hepatocellular25 and ovarian cancers26. These reports suggest that PGC-1α may be involved in the pathogenesis of various human malignancies, as well as the possible involvement of the PGC-1α/TFAM/mitochondria pathway in tumorigenesis. However, the role of PGC-1α and TFAM in musculoskeletal tumors has not been reported. In the present study, we evaluated the expression of PGC-1α and TFAM in human musculoskeletal tissue and observed that both PGC-1α and TFAM expression significantly decreased in human sarcoma tissue compared with normal muscle tissue or benign tumors. These results suggest that mitochondrial biogenesis through PGC-1α and TFAM may be reduced in musculoskeletal malignancies and that the PGC-1α/TFAM/mitochondria pathway may play crucial roles in musculoskeletal tumorigenesis or progression.
We examined the effects of PGC-1α overexpression in various musculoskeletal tumor cells to elucidate the role of the PGC-1α/TFAM/mitochondria pathway in musculoskeletal tumors. In this study, we observed that mitochondrial numbers increased with PGC-1α overexpression and that increasing mitochondrial numbers induced mitochondrial apoptosis in malignant musculoskeletal tumor cell lines. Consistent with our findings, Zhang et al. reported that PGC-1α expression in ovarian tumors was downregulated compared with normal ovaries and observed that overexpression of PGC-1α significantly and specifically induced cell apoptosis and decreased the Bcl-2/Bax expression ratio in a human ovarian carcinoma cell line26. The molecular mechanisms inducing apoptosis by PGC-1α remain unclear; however, the results of the current study indicate that PGC-1α overexpression may induce mitochondrial apoptosis via an improvement in the number of mitochondria.
It has been reported that mitochondria play important roles in apoptosis using mitochondrial DNA-deficient cells in the intrinsic apoptotic pathway39. In the extrinsic apoptotic pathway, Higuchi et al. reported that TNF-induced apoptosis was not observed in mitochondrial respiration-deficient cells, whereas apoptosis was induced in cells reconstituted with normal mtDNA40. It has also been reported that introduction of normal epithelial mitochondria into human cancer cells inhibited proliferation and increased drug sensitivity through a reversal of glycolysis28. These reports suggest that mitochondrial dysfunction may cause cells to become resistant to certain apoptotic pathways. The resistance of cancer cells to treatments is thought to be associated with flaws in their apoptotic programing. Therefore, successful elimination of cancer cells largely depends on the ability of anticancer treatments to stimulate suppressed apoptotic pathways. Although antitumor therapies targeting mitochondrial function have been reported41, studies on the regulation of mitochondrial proliferation as a therapeutic tool are limited. However, mitochondria could be a promising target for cancer treatment42. Increases in mitochondrial numbers after chemotherapeutic treatment, such as doxorubicin43, etoposide44,45 and taxol46, have been reported using various cancer cells. It has also been reported that mitochondrial amplification selectively increased sensitivity to doxorubicin in antiestrogen-resistant breast cancer cells47. We previously reported that transcutaneous CO2 therapy decreased tumor growth via induction of mitochondrial apoptosis with mitochondrial proliferation in musculoskeletal malignancies30. Taken together, these findings suggest that mitochondrial proliferation may precede nuclear apoptosis and may be an integral part of a cascade in apoptotic events30,43,45,46,47.
It has been recently reported that stimulation of mitochondrial activity and restoration of mechanisms of ATP generation characteristic of nonmalignant cells might be an efficient tool in anticancer therapy42. In particular, shifting cellular metabolism towards mitochondrial ATP production might overcome the glycolytic pathway42. Xu et al. reported that reversing glycolysis severely depleted ATP in cancer cells and lead to rapid dephosphorylation of the glycolysis–apoptosis integrating molecule bcl-2-associated death promoter, causing relocalization of Bax to mitochondria and massive cell death48. Although we did not examine whether ATP changes were induced by mitochondrial proliferation in our study, we speculate that one plausible explanation for the effect of mitochondrial proliferation on musculoskeletal malignant tumors is that mitochondrial proliferation shifts cellular metabolism from glycolysis to glucose oxidation, resulting in mitochondrial apoptosis being induced via the depletion of ATP in malignant musculoskeletal tumor cells. Taken together, the findings of the current study indicate that mitochondrial biogenesis via the PGC-1α/TFAM pathway may play crucial roles on apoptotic activity in musculoskeletal malignancies and that increasing mitochondrial numbers by PGC-1α overexpression could induce apoptosis.
In conclusion, we observed a decrease in mitochondrial biogenesis in human malignant musculoskeletal tumors and that increasing mitochondrial numbers by PGC-1α overexpression induced cell apoptosis in human sarcoma cell lines, but not in normal chondrocytes. To the best of our knowledge, this is the first report to observe a role of mitochondria in human musculoskeletal tumors and is also the first report focusing on mitochondria as a target for antitumor therapy. Although one limitation is that normal cells used in this study are not of tumor origin and further studies are required, our findings suggest that regulation of the PGC-1α/TFAM/mitochondria pathway may be a potent therapeutic target for human musculoskeletal malignancies.
Methods
Human musculoskeletal tissue samples and human musculoskeletal cell lines
Thirty-two musculoskeletal tumor tissue samples, including 14 osteosarcomas, eight MFH and 12 schwannomas and 10 normal muscle tissue samples were obtained by surgery at Kobe University Hospital in accordance with institutional guidelines. All patients participating in the study gave informed consent prior to surgery. All samples were immediately stored at −80°C until use.
Four human sarcoma cell lines, two osteosarcoma cell lines (KTHOS and MG-63) and two MFH cell lines (Nara-H and TNMY-1) and a normal human knee chondrocyte cell line (NHAC-kn) were used in in vitro studies. KTHOS and TNMY-1 were previously established at our laboratory49,50. The Nara-H cell line was obtained from ScienStuff Co. (Nara, Japan)51. MG-63 was purchased from the American Type Culture Collection. NHAC-kn was purchased from Cambrex (Charles City, IA, USA)52. Cells were grown in culture medium consisting of Dulbecco's modified Eagle's medium (Sigma-Aldrich Co., St Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 100 U/mL penicillin/streptomycin solution (Sigma-Aldrich). Cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
Evaluation of expression of mitochondria-related genes and mitochondrial numbers in musculoskeletal tissues
To examine mitochondrial biogenesis in musculoskeletal tissue samples, the relative amount of mtDNA copies and the expression of PGC-1α and TFAM were evaluated using qRT-PCR. Immunofluorescence staining was also performed to determine expression levels of mitochondria in musculoskeletal tissues.
Electron microscopy
Musculoskeletal tumor tissues and normal muscle, chondrocyte and osteocyte tissues was first fixed with 2.5% glutaraldehyde in 0.1 M PBS (pH 7.4) for 2 h at 4°C and then post-fixed in 1% osmium tetroxide, 0.1 M phosphate buffer (pH 7.4). After dehydration in ethanol and embedded in an Epon-Araldite mixture, thin sections were obtained with a Reichert-Jung Ultracut-E ultramicrotome and stained with lead citrate. Samples were examined with a JEM-EX electron microscope (JEOL, Tokyo, Japan).
In vitro experiments
To investigate the effects of mitochondrial proliferation on human sarcoma cells, we performed co-transfection of human musculoskeletal cell lines with a PGC-1α-expressing plasmid and a specific siRNA against TFAM. We used the pcDNA4 myc PGC-1 alpha (PGC-1α plasmid) (Addgene, Cambridge, MA, USA) for PGC-1α overexpression and a human TFAM-specific siRNA (TFAM siRNA) (Ambion Inc., Austin, TX, USA) for TFAM inhibition. pcDNA3 (Addgene) and a negative control siRNA (Ambion Inc.) were used as a control plasmid and a control siRNA, respectively. Briefly, 1 day before transfection, cells were seeded in 6-well culture plates in growth medium without antibiotics. Cells were then co-transfected with either PGC-1α plasmid or control plasmid and either TFAM siRNA or control siRNA using Lipofectamine2000 Transfection Reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). After co-transfection with the plasmid and siRNA, the relative amount of mtDNA copy numbers and the expression of mitochondria were evaluated by qRT-PCR and immunofluorescence staining. Expression of PGC-1α and TFAM in transfected cells were evaluated by immunoblot analysis. Mitochondrial proliferation and apoptotic activity were examined by immunoblot analysis, immunofluorescence staining and flow cytometry.
Quantitative real-time PCR
qRT-PCR was performed to analyze mRNA expression of PGC-1α and TFAM in human musculoskeletal tissues. Total RNA was extracted from tissue samples by selective binding to a silica-gel-based membrane using an RNeasy Mini Kit, following the manufacturer's protocol (QIAGEN, Valencia, CA, USA) and first strand cDNA were reverse transcribed. Real-time PCR was performed in a 20 μL reaction mixture using SYBR Green Master Mix Reagent (Applied Biosystems, Foster City, CA, USA) and an ABI prism 7500 sequence detection system (Applied Biosystems). PCR conditions were as follows: 1 cycle at 95°C for 10 minute followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Pre-designed primers specific for human PGC-1α, human TFAM and human β-actin were obtained from Invitrogen. Primer sequences were: human PGC-1α, 5′-GGC AGA AGG CAA TTG AAG AG-3′ (forward) and 5′-TCA AAA CGG TCC CTC AGT TC-3′ (reverse); human TFAM, 5′-CCG AGG TGG TTT TCA TCT GT-3′ (forward) and 5′-GCA TCT GGG TTC TGA GCT TT-3′ (reverse); human β-actin, 5′-GAT CAT TGC TCC TCC TGA GC-3′ (forward) and 5′-ACA TCT GCT GGA AGG TGG AC-3′ (reverse). Values were normalized against β-actin and relative expression of PGC-1α and TFAM were calculated using the delta-delta Ct method.
To evaluate mitochondrial numbers in human musculoskeletal tissues and cell lines, we examined the relative amount of mtDNA to nDNA. Genomic DNA was isolated from tissue samples and transfected cell lines using a GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich). The sequences of the primers designed to amplify a region corresponding to nn 16–408 of a D-loop of human mtDNA were: 5′-GCA GAT TTG GGT ACC ACC CAA GTA TTG ACT CAC CC-3′ (forward) and 5′-GCA TGG AGA GCT CCC GTG AGT GGT TAA TAG GGT GAT AG-3′ (reverse). The relative amount of mtDNA to nDNA was then calculated.
Immunofluorescence staining
Mitochondria in human musculoskeletal tissues were evaluated by immunofluorescence staining. Tissue samples were embedded in OCT compound (Sakura Finetek Co., Tokyo, Japan) and 10-μm thick sections were prepared on a cryostat and stored frozen at −80°C. Sections were incubated with anti-actin antibody (Sigma-Aldrich) diluted in PBS for 30 minutes at 37°C. After washing, sections were incubated with Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen) and MitoTracker Deep-Red FM (Invitrogen) in PBS for 30 minutes in a dark, humid chamber at 37°C. Nuclei were stained with DAPI. Fluorescence images were obtained using a BZ-8000 confocal microscope (Keyence, Osaka, Japan).
Immunofluorescence staining was also performed to verify the relationship between mitochondrial proliferation and cellular apoptosis in human sarcoma cells that were co-transfected with plasmid and siRNA. Staining was carried out using an APO-DIRECT Kit (BD Pharmingen, Franklin Lakes, NJ, USA) and MitoTracker Deep-Red FM (Invitrogen) according to the manufacturers' protocols. Briefly, cell lines co-transfected with plasmid and siRNA were fixed in 4% paraformaldehyde for 30 minutes at room temperature. Cells were incubated in prepared DNA Labeling Solution (APO-DIRECT Kit; BD Pharmingen) for 60 minutes and incubated in MitoTracker Deep-Red FM (Invitrogen) for 30 minutes. Nuclear staining was performed using propidium iodide. Stained cells were assessed using a BZ-8000 confocal microscope (Keyence).
Immunoblot analysis
Cell lysates were collected from transfected cells using a whole-cell lysis buffer (Mammalian Protein Extraction Reagent; Thermo Scientific, Rockford, IL, USA) supplemented with protease and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN, USA). Protein concentrations were quantified using Bradford Protein Assay Reagent (Bio-Rad, Richmond, CA, USA) and samples were processed using standard western immunoblotting procedures52. Membranes were incubated overnight at 4°C with primary antibodies in Can Get Signal Solution 1 (Toyobo Co., Ltd, Osaka, Japan). Following washing, membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase and exposed to an ECL Plus western blotting detection system reagent (GE Healthcare Bio-Sciences, Piscataway, NJ, USA). Signals were detected using a chemilumino analyzer LAS-3000 mini (Fujifilm, Tokyo, Japan).
Protein expression of cytochrome c and Bax were separately evaluated in mitochondrial and cytoplasmic fractions. The mitochondrial fraction from transfected cells was isolated using a Mitochondria Isolation Kit according to the manufacturer's protocol (Thermo Scientific). The expression of both cytochrome c and Bax were evaluated by immunoblot analyses.
Primary antibodies used in immunoblot analyses were: anti-human PGC-1α antibody (1:1000) (Cell Signaling Technology), anti-human TFAM antibody (1:1000) (Abnova), anti-human cleaved caspase-3 antibody (1:500) (Cell Signaling Technology), anti-human cleaved caspase-8 antibody (1:500) (Cell Signaling Technology), anti-human cleaved caspase-9 antibody (1:500) (Cell Signaling Technology), anti-human cytochrome c antibody (1:1000) (eBiosience Inc., San Diego, CA, USA), anti-human Bax antibody (1:1000) (Cell Signaling Technology) and anti-human α-tubulin antibody (1:10000) (Sigma-Aldrich).
Flow cytometric analysis
To investigate mitochondrial apoptosis in transfected cells, we assessed apoptotic activity and mitochondrial proliferation by flow cytometry. Briefly, transfected cells were collected and suspended in 1% paraformaldehyde in PBS, before being resuspended in ice cold ethanol at a concentration of 1 × 106 cells/ml. Each cell pellet was labeled using an APO-DIRECT Kit (BD Pharmingen) for apoptotic activity and MitoTracker Deep-Red FM (Invitrogen) for mitochondrial proliferation according to the manufacturers' protocols. Fluorescent intensity was analyzed using a FACS Calibur™ (BD Pharmingen).
Statistical analysis
Each experiment was performed independently at least three times. Data are presented as mean ± SE unless otherwise indicated. Statistical significance of differences between mean were evaluated by two-tailed Student's t-tests and by ANOVA with post hoc test to compare continuous values. A P value < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
We thank Minako Nagata, Maya Yasuda and Kyoko Tanaka for their expert technical assistance.
Author Contributions
Y.O., T.A., T.K., H.H., M.T., R.H., M.M. and M.K. participated in the study concept and study design. Y.O., T.A., T.K., T.U. and H.H. were responsible for manuscript drafting or manuscript revision regarding intellectual content. Y.O., T.A., T.K., T.U. and H.H. carried out the literature research. Y.O., T.A., T.K., H.H., M.T., R.H. and M.M. conducted experimental studies and participated in acquisition of data or data analysis/interpretation. Statistical analyses were undertaken by Y.O., T.A., T.K., H.H., M.T., R.H. and M.M. Y.O., T.A., T.K., H.H., M.T., R.H., M.M. and M.K. participated in manuscript revision. Y.O., T.A. and T.K. guarantee the integrity of the study. All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare no competing financial interests.
References
- LeDoussal V, et al. Prognostic factors for patients with localized primary malignant fibrous histiocytoma: a multicenter study of 216 patients with multivariate analysis. Cancer. 1996;77:1823–1830. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1823::AID-CNCR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Okada Y, et al. The effect of bevacizumab on tumour growth of malignant fibrous histiocytoma in an animal model. Anticancer. Res. 2010;30:3391–3395. [PubMed] [Google Scholar]
- Fukase N, et al. Protein kinase Cdelta in tumorigenesis of human malignant fibrous histiocytoma. Oncol. Rep. 2011;26:1221–1226. doi: 10.3892/or.2011.1415. [DOI] [PubMed] [Google Scholar]
- Onishi Y, et al. PKD1 negatively regulates cell invasion, migration and proliferation ability of human osteosarcoma. Int. J. Oncol. 2012;40:1839–1848. doi: 10.3892/ijo.2012.1400. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- Weitzel JM, Iwen KA, Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp. Physiol. 2003;88:121–128. doi: 10.1113/eph8802506. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M, Weibel ER, Fluck M. Response of skeletal muscle mitochondria to hypoxia. Exp. Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- Yamada S, et al. Correlation between copy number of mitochondrial DNA and clinico-pathologic parameters of hepatocellular carcinoma. Eur. J. Surg. Oncol. 2006;32:303–307. doi: 10.1016/j.ejso.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Yu M, et al. Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB. Life. 2007;59:450–457. doi: 10.1080/15216540701509955. [DOI] [PubMed] [Google Scholar]
- Wu CW, et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in gastric cancer. Genes. Chromosomes. Cancer. 2005;44:19–28. doi: 10.1002/gcc.20213. [DOI] [PubMed] [Google Scholar]
- Lee HC, et al. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat. Res. 2004;547:71–78. doi: 10.1016/j.mrfmmm.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Xing J, et al. Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J. Natl. Cancer. Inst. 2008;100:1104–1112. doi: 10.1093/jnci/djn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng LM, et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes. Chromosomes & Cancer. 2006;45:629–638. doi: 10.1002/gcc.20326. [DOI] [PubMed] [Google Scholar]
- Kim MM, et al. Mitochondrial DNA quantity increases with histopathologic grade in premalignant and malignant head and neck lesions. Clin. Cancer. Res. 2004;10:8512–8515. doi: 10.1158/1078-0432.CCR-04-0734. [DOI] [PubMed] [Google Scholar]
- Lin CS, et al. The role of mitochondrial DNA alterations in esophageal squamous cell carcinomas. J. Thorac. Cardiovasc. Surg. 2010;139:189–197 e184. doi: 10.1016/j.jtcvs.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu VW, Xue WC, Cheung AN, Ngan HY. Association of decreased mitochondrial DNA content with ovarian cancer progression. Br. J. Cancer. 2006;95:1087–1091. doi: 10.1038/sj.bjc.6603377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wan Y, Zou Q. Decreased copy number of mitochondrial DNA in Ewing's sarcoma. Clin. Chim. Acta. 2010;411:679–683. doi: 10.1016/j.cca.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell. Biol. 2005;37:822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol. Rep. 2004;12:483–488. [PubMed] [Google Scholar]
- Feilchenfeldt J, Brundler MA, Soravia C, Totsch M, Meier CA. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARgamma-coactivator 1 (PGC-1) Cancer. Lett. 2004;203:25–33. doi: 10.1016/j.canlet.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Ba Y, Zhang CN, Zhang Y, Zhang CY. Down-regulation of PGC-1alpha expression in human hepatocellular carcinoma. Zhonghua. Zhong. Liu. Za. Zhi. 2008;30:593–597. [PubMed] [Google Scholar]
- Zhang Y, et al. PGC-1alpha induces apoptosis in human epithelial ovarian cancer cells through a PPARgamma-dependent pathway. Cell. Res. 2007;17:363–373. doi: 10.1038/cr.2007.11. [DOI] [PubMed] [Google Scholar]
- Sauer H, Engel S, Milosevic N, Sharifpanah F, Wartenberg M. Activation of AMP-kinase by AICAR induces apoptosis of DU-145 prostate cancer cells through generation of reactive oxygen species and activation of c-Jun N-terminal kinase. Int. J. Oncol. 2012;40:501–508. doi: 10.3892/ijo.2011.1230. [DOI] [PubMed] [Google Scholar]
- Elliott RL, Jiang XP, Head JF. Mitochondria organelle transplantation: introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast. Cancer. Res. Treat. 2012;136:347–354. doi: 10.1007/s10549-012-2283-2. [DOI] [PubMed] [Google Scholar]
- Oe K, et al. The effect of transcutaneous application of carbon dioxide (CO2) on skeletal muscle. Biochem. Biophys. Res. Commun. 2011;407:148–152. doi: 10.1016/j.bbrc.2011.02.128. [DOI] [PubMed] [Google Scholar]
- Onishi Y, et al. Transcutaneous application of carbon dioxide (CO2) induces mitochondrial apoptosis in human malignant fibrous histiocytoma in vivo. Plos. One. 2012;7:e49189. doi: 10.1371/journal.pone.0049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvanayagam P, Rajaraman S. Detection of mitochondrial genome depletion by a novel cDNA in renal cell carcinoma. Lab. Invest. 1996;74:592–599. [PubMed] [Google Scholar]
- Simonnet H, et al. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759–768. doi: 10.1093/carcin/23.5.759. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice. Am. J. Physiol. Cell. Physiol. 2004;287:C790–796. doi: 10.1152/ajpcell.00425.2003. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. The increase of mitochondrial DNA content in endometrial adenocarcinoma cells: a quantitative study using laser-captured microdissected tissues. Gynecol. Oncol. 2005;98:104–110. doi: 10.1016/j.ygyno.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- Shadel GS. Expression and maintenance of mitochondrial DNA: new insights into human disease pathology. Am. J. Pathol. 2008;172:1445–1456. doi: 10.2353/ajpath.2008.071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JS, Huang P. Mitochondrial defects in cancer. Mol. Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DA. Replication and transcription of vertebrate mitochondrial DNA. Annu. Rev. Cell. Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS. Lett. 1999;454:173–176. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Aggarwal BB, Yeh ET. Activation of CPP32-like protease in tumor necrosis factor-induced apoptosis is dependent on mitochondrial function. J. Clin. Invest. 1997;99:1751–1758. doi: 10.1172/JCI119339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph SJ, Neuzil J. Mitochondria as targets for cancer therapy. Mol. Nutr. Food. Res. 2009;53:9–28. doi: 10.1002/mnfr.200800044. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends. Cell. Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Kluza J, et al. Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene. 2004;23:7018–7030. doi: 10.1038/sj.onc.1207936. [DOI] [PubMed] [Google Scholar]
- Reipert S, Berry J, Hughes MF, Hickman JA, Allen TD. Changes of mitochondrial mass in the hemopoietic stem cell line FDCP-mix after treatment with etoposide: a correlative study by multiparameter flow cytometry and confocal and electron microscopy. Exp. Cell. Res. 1995;221:281–288. doi: 10.1006/excr.1995.1376. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Gunter KK, Gunter TE. Bcl-2 prevents abnormal mitochondrial proliferation during etoposide-induced apoptosis. Exp. Cell. Res. 2003;289:275–281. doi: 10.1016/s0014-4827(03)00278-7. [DOI] [PubMed] [Google Scholar]
- Mancini M, et al. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J. Cell. Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skildum A, Dornfeld K, Wallace K. Mitochondrial amplification selectively increases doxorubicin sensitivity in breast cancer cells with acquired antiestrogen resistance. Breast. Cancer. Res. Treat. 2011;129:785–797. doi: 10.1007/s10549-010-1268-2. [DOI] [PubMed] [Google Scholar]
- Xu RH, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer. Res. 2005;65:613–621. [PubMed] [Google Scholar]
- Hitora T, et al. Establishment and characterization of a KIT-positive and stem cell factor-producing cell line, KTHOS, derived from human osteosarcoma. Pathol. Int. 2005;55:41–7. doi: 10.1111/j.1440-1827.2005.01787.x. [DOI] [PubMed] [Google Scholar]
- Nakatani T, et al. Establishment and characterization of cell line TNMY1 derived from human malignant fibrous histiocytoma. Pathol. Int. 2001;51:595–602. doi: 10.1046/j.1440-1827.2001.01253.x. [DOI] [PubMed] [Google Scholar]
- Kiyozuka Y, et al. Novel cell lines established from a human myxoid malignant fibrous histiocytoma arising in the uterus. Cancer. Genet. Cytogenet. 2001;127:7–15. doi: 10.1016/s0165-4608(00)00413-1. [DOI] [PubMed] [Google Scholar]
- Takayama K, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis. Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.