Abstract
Chlorinated paraffins (CPs) can be mixtures of nearly a half-million possible isomers. Despite the extensive use of CPs, their isomer composition and effects on the environment remain poorly understood. Here, we reveal the isomeric distributions of nine CP mixtures with single-chain lengths (C14/15) and varying degrees of chlorination. The molar distribution of CnH2n+2–mClm in each mixture was determined using high-resolution mass spectrometry (MS). Next, the mixtures were analyzed by applying both one-dimensional 1H, 13C and two-dimensional nuclear magnetic resonance (NMR) spectroscopy. Due to substantially overlapping signals in the experimental NMR spectra, direct assignment of individual isomers was not possible. As such, a new NMR spectral matching approach that used massive NMR databases predicted by a neural network algorithm to provide the top 100 most likely structural matches was developed. The top 100 isomers appear to be an adequate representation of the overall mixture. Their modeled physicochemical and toxicity parameters agree with previous experimental results. Chlorines are not evenly distributed in any of the CP mixtures and show a general preference at the third carbon. The approach described here can play a key role in understanding of complex isomeric mixtures such as CPs that cannot be resolved by MS alone.
Introduction
The molecular structure of organic pollutants determines their physicochemical properties and toxicities, which are essential in investigating pollutant fate, transport, and environmental impacts.1−3 Even small structural changes can greatly affect physicochemical properties. For instance, large variations in KOA were observed between isomers of hexachlorocyclohexane,4 resulting in different long-range transport pathways to the Arctic.5 As another example, the metabolism of polybrominated diphenyl ethers differs among congeners with different bromine substitution patterns.6 The specific molecular structure also determines the chemical activity. A recent study showed that halogen substitution neighboring the phenolic hydroxyl groups of bisphenol A analogues significantly enhanced their antimicrobial activity.7 Despite the importance of the molecular structure of organic pollutants, extracting this information can be a great challenge for substances of unknown or variable composition, complex reaction products, and biological materials (UVCBs).8 In particular, this challenge has existed for decades for the UVCBs known as chlorinated paraffins (CPs).9
CPs are high-production volume industrial products (>1 million metric tons/year) used as extreme pressure lubricant additives, flame retardants, and plasticizers.10 They are usually synthesized under free radical chlorination with low positional selectivity.9 Therefore, a typical CP product is an extremely complex mixture with potentially hundreds of thousands of possible isomers of polychlorinated n-alkanes (i.e., CnH2n+2–mClm),11 with manufacturers only specifying the carbon chain length range and degree of chlorination (% Cl, w/w). These specifications result in a rough classification of CP products: short-chain (SCCPs, C10–13), medium-chain (MCCPs, C14–17), and long-chain (LCCPs, C≥18) CP mixtures.12 Although all CPs have been identified as potentially hazardous substances since the early 1980s13,14 and appear to be ubiquitous environmental contaminants,15 SCCPs were only recently (2017)16 identified and globally regulated as persistent organic pollutants (POPs), while MCCPs and LCCPs are still being used extensively.
The complexity of CPs is a serious obstacle for their analysis, research, and regulation.9 Although column chromatography can resolve stereoisomers in a CP isomer standard,17,18 no chromatographic technique has so far isolated individual isomers in a typical CP product.19 In an optimal situation, CP isomers with a given number of carbons and chlorines can be resolved as a congener group (denoted as CnClm) using high-resolution mass spectrometry (HRMS).20 While mass spectrometry (MS) can provide valuable insights into the mass distribution of the mixture, the exact isomeric distribution can be impossible to elucidate and other spectroscopic methods, such as nuclear magnetic resonance spectroscopy (NMR), are required.21
NMR is a powerful and non-invasive tool capable of providing detailed structural information about even the most complicated systems. The chemical shifts of 1H and 13C nuclei in one-dimensional (1D) NMR provide an indication of their chemical environment and were used to identify the chlorine arrangement in substances such as pure CP isomers22−24 but showed extensive overlap for the CP mixtures.19,25 The peak capacity of a two-dimensional (2D) 1H–13C NMR spectrum is around 2 million, and the additional spectral dispersion versus 1D NMR helps reduce spectral overlap.26,27 Sprengel et al. used 2D heteronuclear single-quantum coherence spectroscopy (HSQC) to assign nine substructures to single-chain length CPs synthesized by free radical chlorination using sulfuryl chloride.19 However, the complexity and overlap of 2D NMR spectra made direct assignments of individual isomers impossible. Through the application of predicted 2D NMR to CPs, it is possible to profile the “spectral fingerprints” of different isomers and after databasing use the signatures for “best fit pattern matching” against the experimental 2D CP NMR. Such an approach was developed for identifying unknown organics in soil28 but has yet be used to investigate complex isomeric environmental contaminant mixtures.
Herein, we significantly improve our knowledge of CP composition from the congener group level to actual isomeric discrimination. In this manner, multidimensional NMR was applied and vast 1D and 2D NMR databases were predicted using neural networks, which were then matched against the experimental data to provide an understanding of the most likely components present in CP mixtures. Multiple single-chain length CP mixtures were analyzed using both an established MS method29 and the new NMR approach, resulting in identification of the isomers that are most likely to be present in each CP mixture. On the basis of the identified isomers, it became possible to predict the physicochemical properties and toxicities of the most likely CP mixture components.
Materials and Methods
Chemicals
Single-chain length CP mixtures were used to reduce the complexity from a range of chain lengths.19 Nine samples of CP mixtures were synthesized by Quimica del Cinca (Barcelona, Spain), a CP manufacturer, by chlorination of n-tetradecane and n-pentadecane. The mixtures were provided gratis by the Chlorinated Paraffin Industry Association (CPIA, Washington, DC). These consisted of five chlorinated n-tetradecanes (C14 CPs with degrees of chlorination of 39.75% Cl, 44.86% Cl, 50.07% Cl, 55.34% Cl, and 60.14% Cl) and four chlorinated n-pentadecanes (C15 CPs with degrees of chlorination degrees of 40.30% Cl, 44.80% Cl, 50.00% Cl, and 54.75% Cl). For detailed synthesis of CPs and sample preparation, see the Supporting Information.
MS Spectrometry
Congener groups in the C14/15 CP mixtures were measured using a direct injection dichloromethane-enhanced APCI-Q-Orbitrap MS30 instrument (Q-Exactive, Thermal-Fisher Scientific) operating in negative full scan mode (m/z 300–2000) with an MS resolution of 120000 fwhm (full width at half-maximum). The molar compositions of congener groups in each CP mixture were quantified on the basis of an algorithm developed previously.29 Each congener group was quantified on the basis of its own instrumental response factor, which thus reduces the level of response discrimination in CPs.31 The quantification performance was evaluated by the CP mixture’s degree of chlorination calculated on the basis of the congener group distribution.32,33 For detailed instrumental settings and response factor calculation, see the Supporting Information and Figure S1.
NMR Spectroscopy
The NMR measurements consist of (i) 1D 1H NMR, (ii) 1D 13C NMR, (iii) 2D 13C–1H HSQC, and (iv) 2D 1H–13C heteronuclear multiple-bond coherence (HMBC). All NMR measurements were performed on a Bruker Advance III HD spectrometer operating at a 1H frequency of 500.30 MHz fitted with a TCI Prodigy Cryoprobe. The assignment of the experimental NMR spectra was performed on the basis of matches against the predicted databases (1D 13C, HSQC) and then cross referenced for consistency using experimental HMBC J couplings.34 For detailed NMR settings and assignment information, see the Supporting Information.
Prediction and Matching of 13C NMR Spectra
All possible CP isomers in the form of SMILES codes for the congener groups identified as major components by MS [C14Cl1–10 and C15Cl1–7 (Figure 1e and Figure S3)] were calculated. After identical structures had been removed (i.e., mirror images that were not stereoisomers), 410000 unique chemical structures remained. The 13C NMR spectra were then predicted for all possible isomers using a neural network algorithm. These were arranged into one database containing the C14 CP isomers and one database containing the C15 CP isomers. Next, each 13C NMR spectrum of the chlorinated mixtures was quantitatively compared against the predicted databases using the ACD/Labs similarity search algorithm (Figure S2a). The top 1000 matches, as ranked by hit quality index (HQI, eq S1), were retained. For validation, experimental and predicted data (13C and 1H) were compared for three polychlorinated alkane isomers: 1,2,3-trichloropropane, 1,1,1,2,2,3,3-heptachloropropane, and pentaerythrityl tetrachloride (Figure S3). Further details are provided in the Supporting Information.
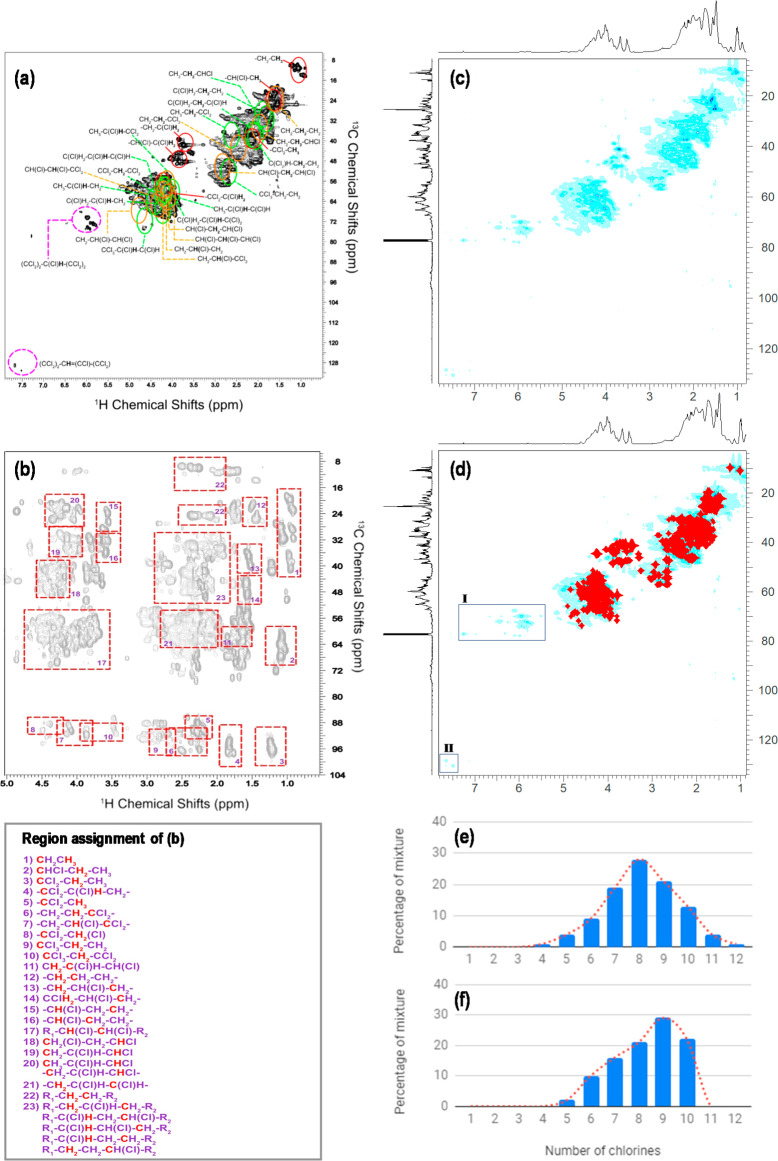
Figure 1.
2D NMR spectra and MS result of the C14 60.14% Cl CP mixture. (a) 1H–13C HSQC spectrum and assignment of all regions based on both manual assignment and matches from predict databases. (b) 2,3J HMBC 1H–13C spectrum and assignment of all regions. Comparison of (c) the HSQC spectrum and (d) the same HSQC spectrum with the top 100 isomer spectra overlaid. (e) Molar distribution of molecular formulas (i.e., congener groups) determined using MS. (f) Molar distribution of the top 100 isomers determined using NMR.
Prediction and Matching of 1H–13C HSQC Spectra
CP structures were further filtered on the basis of the 2D NMR HSQC spectra. The HSQC spectra were predicted for the top 1000 isomers using a neural network algorithm. For matching, each of the 1000 isomers was compared against the CP mixtures (Figure S2b), yielding the top 100 isomers based on HQI (eq S2). The 100 isomers of each C14/15 mixture were grouped on the basis of the molecular formulas to form the molar distribution in the mixture. The formula distributions identified independently by NMR and MS were compared on the basis of the coefficient of determination (R2), with an R2 of 1 indicating a perfect match and an R2 of 0.5 a threshold of match.35 Finally, physicochemical and toxicity parameters of the top 100 isomers were predicted using the Percepta Platform (ACD/Labs). Further details are provided in the Supporting Information.
Results and Discussion
Molar Composition of Congener Groups
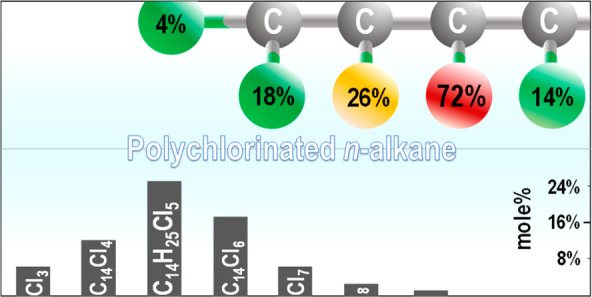
The MS results show that molar abundances of congener groups in each C14 or C15 CP mixture (Figure 1e and Figure S4) are the highest for congener groups with degrees of chlorination close to that of the mixture. For example, C14Cl4 (42.18% Cl) and C14Cl3 (35.25% Cl) are the most abundant congener groups in the C14 39.75% Cl mixture, and C15Cl6 (50.75% Cl) is the most abundant congener group in the C15 50.00% Cl mixture. This trend of congener group distribution in a CP mixture is consistent with previous studies.29,33 The calculated degrees of chlorination of the C14/15 CP mixtures agree with the manufacturer-reported values (r2 > 0.99) with the absolute deviation ranging from −0.22% Cl to 0.06% Cl (Table S1). In addition, C14Cl15 was found in the C14 60.14% Cl mixture. This confirms the presence of multi-chloro-substituted carbons in technical mixtures of CPs that have been reported in previous studies.19,25,29
Fragments of Functional Groups in CP Mixtures
The 1H NMR spectra of C14 or C15 CP mixtures downfield shift with an increasing degree of chlorination due to deshielding from the chlorines (Figure S5). Such a trend was also reported by Sprengel et al.19 However, the 1H NMR spectra provide little detailed molecular information due to a lack of dispersion. In contrast, the 13C NMR spectra provide better dispersion, allowing identification of discrete regions where major CP fragments occur, along with comparisons of their relative quantities (Table S2). With increasing degrees of chlorination, the quantities of [-CH2-] and methyl groups in the CP mixtures decrease, while the quantities of chlorine-substituted groups increase. 13C NMR also helped further confirm the presence of dichloro-substituted carbons, and a small quantity of trichloromethyl terminations36 and unsaturated compounds37,38 was also identified. However, 1D 13C NMR still shows considerable overlap of fragment regions.
The 2D HSQC spectra improve the assignment by increasing spectral dispersion and providing one-bond H–C correlations.19 Though spectral overlap is reduced in a 2D plot, due to the complex nature of the CPs, it is still highly complicated, and with assistance via pattern matching from the predicted databases (see below), the overlap from different functional moieties is extensive (Figure 1a). To support the HSQC assignments, HMBC was used for the analysis of CPs. Upon inclusion of all of the 2,3J1H–13C correlations (i.e., carbons within two or three bonds of the selected proton resonance), the assignment of major fragment regions is less ambiguous (Figure 1b). While spectral assignment can provide an overview of the major structural components that are present, to gain information about individual isomers and their distributions, spectral matching of vast predicted NMR databases is required.
Prediction and Matching of NMR Data
The total number of possible isomers for C14 and C15 CPs is more than 410000, showcasing the extreme complexity of CP mixtures. This number is ∼25 times larger than the number of 1627111 calculated on the basis of ref (39). This is because the latter assumed no more than one chlorine atom bound to any carbon atom, while our calculation allowed for carbon atoms to be substituted by up to two chlorines. The number of possible isomers increases by geometric progression with an increase in chain length39 and the number of substituted chlorines (Figure S6). Therefore, we limited the list of isomers within the ranges of C14Cl1–10 and C15Cl1–7.
It was not possible to generate 1H–13C HSQC spectra for all of the isomers, due to the computational expense of HSQC prediction. Instead, the 13C NMR spectra of all of the 410000 isomers were predicted, and the top 1000 isomers matching the experimental 13C NMR spectra were identified for each CP mixture (shown in Worksheets 10 and 11). Then 1H–13C HSQC spectra were predicted for the top 1000 isomers and compared with the spectra of all of the C14/15 CPs, resulting in the top 100 isomers (Worksheets 1–9).
2D HSQC experiments tend not to be fully quantitative.40 The peak search algorithm matches on the basis of patterns28 in the 2D data rather than intensity. However, as the peak picking used is done both computationally and then by eye, both approaches will always bias the most intense and well-defined peaks in the data. As such, the results from the 2D mixture searches will favor the most abundant components that best match the chemical shift patterns in the experimental data.
Top 100 Isomers
The overlaid spectrum (an example is shown in Figure 1d) of the top 100 isomers (Worksheet 5) adequately represents the dominant signals (i.e., major components) in the experimental NMR spectrum of the C14 60.14% Cl mixture (Figure 1c). Region I in the HSQC spectrum (Figure 1d) is not represented by the top 100 isomers, which arises from methine protons between two perchlorinated neighbors. While these compounds are included in the predicted databases, their very low abundance leads to negative discrimination by the 13C matching algorithm (see the Supporting Information). It is also noteworthy that the simulations exclude unsaturated isomers [that were at very low concentrations of <1% (Table S2)], which explains the lack of signal around 6 ppm (1H)/70 ppm (13C), where unsaturated compounds resonate (region II in Figure 1d).
The top 100 isomers of all of the C14/15 CP mixtures are generally comparable [median R2 = 0.83; range of 0.30–0.93 (Table S1)] with the molecular formulas identified independently by MS (Figure 1e,f and Figure S4); they are thus an adequate representation of the overall mixture, although biased toward the most abundant components in the mixtures. The low R2 (0.37) for the C14 55.34% Cl mixture might due to this bias. Moreover, C14Cl>10 and C15Cl>7 were included in the MS results but not in the NMR results. This results in the least comparable congener group distribution in this for C15 54.75% Cl (R2 = 0.30) as the MS data show that C15Cl>7 mixtures contribute in an amount of 33%. The degrees of chlorination calculated on the basis of the top 100 isomers show larger deviations (−6.99% Cl to 4.17% Cl) than the MS results but are comparable with the deviations in another study33 that quantified congener groups.
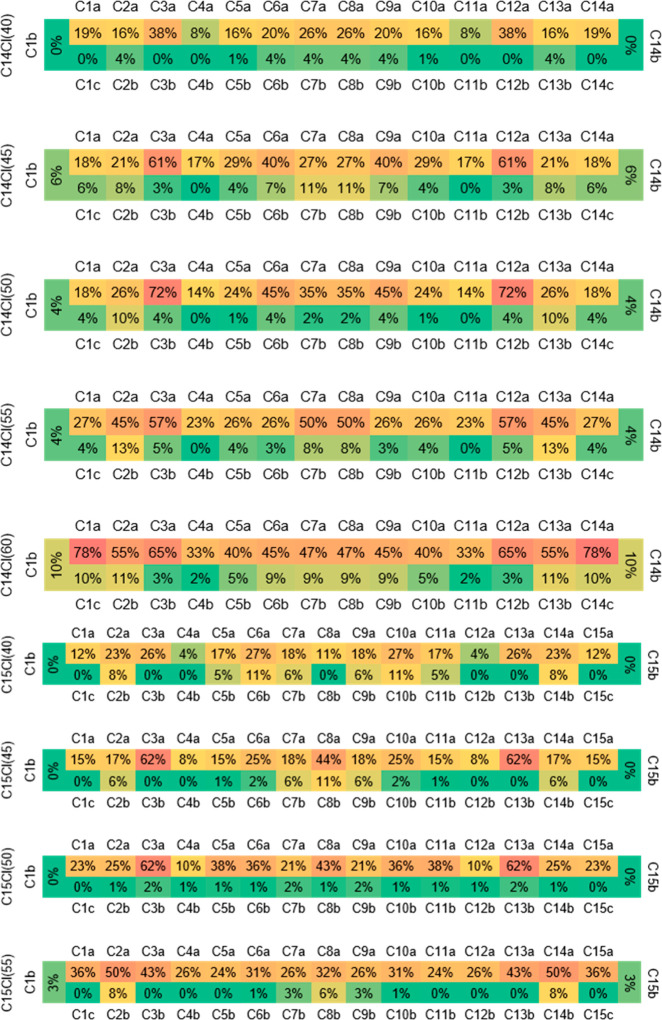
Positional Selectivity of Chlorine Substitution
The top 100 isomers of each CP mixture were depicted as a substitution heat map (Figure 2) of the most likely position of chlorine on the alkane. The maps were generated by summing the number of times a chlorine occurred at a specific position across the top 100 isomers for each mixture (for details, see the Supporting Information). The heat maps clearly show that chlorine substitution at each carbon is not statistically evenly distributed in any CP mixture. With C14 39.75% Cl (equivalent to C14H26.38Cl3.62) as an example, if chlorines were evenly distributed, all 30 hydrogens of the tetradecane would have an identical substitution percentage of ∼12%. Remarkably, the third carbon from an end of the chain, denoted as C(3), is the most reactive to chlorine in mixtures with low and intermediate degrees of chlorination (C14 39.75–55.34% Cl and C15 40.30–50.00% Cl). This may be explained by steric effects and the low bond energy at C(3). Gerson and Nyburg measured C–C bond lengths along n-tetracosane and n-hexacosane using X-ray crystallography; the C2–3 bond was the longest, while the C3–4 bond was the second longest.41 These two C–C bonds also showed the highest probability of breaking in the fragmentation experiment of n-tetradecane and n-nonane under electron impact.42 These studies suggest that C(3) is the most reactive position along the n-alkane under free radical chlorination. Meanwhile, C(4) in all CP mixtures (Figure 2) is least reactive to chlorine. Adjacent to a “hot spot” of chlorination [i.e., C(3)], C(4) may be influenced by both steric hindrance43 and the electron cloud of the chlorine on C(3). Despite being adjacent to C(3), C(2) has a weaker steric effect from the methyl group [i.e., C(1)] and thus shows a higher substitution percentage than C(4) in all of the CP mixtures. The steric hindrance from C(3) is lower for carbons farther from C(3) [e.g., C(5)], facilitating chlorination of these carbons.
Figure 2.
Heat maps showing chlorine substitution at each carbon as a percentage for the top 100 CP isomers in the database of nine samples. The name of a CP mixture, e.g., C14 39.75% Cl CPs, is shown as C14Cl(40). Note that stereoisomers are not considered. Therefore, both the mirrored carbons [e.g., C(1) and C(14) in a C14 CP mixture] and hydrogens a/b(/c) on a carbon atom are equivalent positions. The results are expressed as simple percentages, for example, for a specific position of the 10% displayed it means that in 10% of the top 100 isomers this position is substituted by chlorine.
Among different CP mixtures, the maximum substitution percentage at the central carbon(s) [i.e., C(7) and C(8) of C14 CPs and C(8) of C15 CPs] was found in CP mixtures with intermediate degrees of chlorination. Intermediately chlorinated CP isomers may tend to reach the lowest-energy fold,44,45 making the central carbon(s) experience the least steric hindrance. Chlorination is less preferable for primary carbons [i.e., C(1)] than secondary carbons,43 which can be seen in CP mixtures with the lowest level of chlorination (Figure 2). However, with an increase in the degree of chlorination, the chlorine substitution percentage grows at C(1), with the percentage at C(1) being the highest in highly chlorinated CP mixtures (C14 60.14% Cl). This may be because hydrogens on most secondary carbons are substituted with chlorine, which causes stronger steric hindrance compared with the primary carbon. Dichloro substitution is most likely at C(2) and the central carbon(s). The methyl group on C(2) has less steric hindrance to substitution of the second chlorine compared with the ethyl group on C(3).
Environmental Implications
The structures of CP isomers are crucial in predicting and elucidating physicochemical properties,45,46 environmental behaviors,47,48 and decomposition mechanisms49−51 of complex CP mixtures comprised of these isomers. In the work presented here, the predicted physicochemical and toxicity parameters of the top 100 isomers are summarized in the Supporting Information for each CP mixture. To compare our predictions with experimental data, we predicted parameters of each CP mixture (Table S3) on the basis of their isomeric composition, allowing comparison to experimentally available CP mixture data. The predicted LD50 parameters agree with previous findings that mammalian toxicity appears to decrease with chain length and increase with the degree of chlorination.52 However, caution should be used in interpreting these predicted data as they are based on only C14 and C15 CPs, while the chain length range of CPs can be from C10 to C30 and even wider. Meanwhile, CPs with 50–55% Cl showed the minimum experimental log KOW value among mixtures of the same chain length,46 which is consistent with our prediction (Table S3). Quantitatively, the experimental log KOW of C15 50.4% Cl was 6.65,46 while our prediction was 6.77 (C15 50.00% Cl), which is 1 order of magnitude closer than a prediction of 7.78 based on assumed isomer compositions45 (Table S4).
C14 and C15 CPs are major components of the commercial MCCP products.53 They have frequently been found to have the dominant chain lengths of CPs in wildlife,54−56 human,11,57,58 sediment,59,60 sewage sludge,61 and indoor dust samples62,63 worldwide. Our work addresses the urgent need for substantial knowledge of these mixtures and can extend to other chain lengths if individual mixtures are available.64 However, the geometric growth of the number of possible isomers with an increasing chain length and chlorines may pose a demanding challenge for NMR prediction and databasing. This requires more computing resources and/or techniques working in tandem, e.g., separation and concentration of CPs prior to the NMR analysis. More importantly, our work demonstrates the usefulness of a predicted and databased NMR matching framework for a better understanding of complex environmental isomeric mixtures. These techniques may not only play a key role in elucidating individual isomers in CPs but also potentially be extended to any complex organic mixtures.
Acknowledgments
The authors acknowledge Quimica del Cinca for synthesizing the nine samples of C14/15 chlorinated n-alkanes and thank Andrew Jaques of the Chlorinated Paraffins Industry Association for arranging for provision of the samples. The authors thank the Natural Sciences and Engineering Research Council of Canada (NSERC) [Strategic (STPGP 494273-16) and Discovery Programs (RGPIN-2019-04165)], the Canada Foundation for Innovation (CFI), the Ontario Ministry of Research and Innovation (MRI), and the Krembil Foundation for providing funding. A.J.S. thanks the Government of Ontario for an Early Researcher Award. B.Y. was supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS 2017-01276). D.C.G.M. was supported by the King Carl XVI Gustaf Professorship in Environmental Science at the Department of Environmental Science and Analytical Chemistry, Stockholm University (2018–19).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.0c00244.
Top 100 isomers (Excel Worksheets 1–9) and top 1000 isomers (Excel Worksheets 10 and 11) (XLSX)
Synthesis of CPs; MS experiment; NMR experiment; NMR data prediction, database creation, and spectral matching; comparison of NMR-MS results (Table S1); fragment quantities (Table S2); modeled physicochemical properties and toxicity parameters (Table S3); calculation of log KOW (Table S4); CnClm RF calculation (Figure S1); examples of spectral matching (Figure S2); NMR prediction validation (Figure S3); molar distributions (Figure S4); 1H NMR spectra (Figure S5); and numbers of possible isomers (Figure S6) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Selassie C.; Verma R. P.. History of Quantitative Structure–Activity Relationships. In Burger’s Medicinal Chemistry and Drug Discovery; Abraham D. J., Ed.; Wiley: Hoboken, NJ, 2010. [Google Scholar]
- Willett K. L.; Ulrich E. M.; Hites R. A. Differential Toxicity and Environmental Fates of Hexachlorocyclohexane Isomers. Environ. Sci. Technol. 1998, 32, 2197–2207. 10.1021/es9708530. [DOI] [Google Scholar]
- Sadik O. A.; Witt D. M. Peer reviewed: Monitoring endocrine-disrupting chemicals. Environ. Sci. Technol. 1999, 33, 368A–374A. 10.1021/es992961n. [DOI] [PubMed] [Google Scholar]
- Shoeib M.; Harner T. Using measured octanol-air partition coefficients to explain environmental partitioning of organochlorine pesticides. Environ. Toxicol. Chem. 2002, 21, 984–990. 10.1002/etc.5620210513. [DOI] [PubMed] [Google Scholar]
- Li Y. F.; Macdonald R. W.; Jantunen L. M. M.; Harner T.; Bidleman T. F.; Strachan W. M. J. The transport of β-hexachlorocyclohexane to the western Arctic Ocean: a contrast to α-HCH. Sci. Total Environ. 2002, 291, 229–246. 10.1016/S0048-9697(01)01104-4. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Letcher R. J.; Li J.; Baker J. E. Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio). Environ. Toxicol. Chem. 2004, 23, 1939–1946. 10.1897/03-462. [DOI] [PubMed] [Google Scholar]
- Ji F.; Wang C.; Wang H.; Liu G.; Chen B.; Hu L.; Jiang G.; Song M.; Liang Y. Tetrabromobisphenol A (TBBPA) exhibits specific antimicrobial activity against Gram-positive bacteria without detectable resistance. Chem. Commun. 2017, 53, 3512–3515. 10.1039/C7CC00613F. [DOI] [PubMed] [Google Scholar]
- Scialli A. R.; Guikema A. J. REACH and reproductive and developmental toxicology: still questions. Syst. Biol. Reprod. Med. 2012, 58, 63–69. 10.3109/19396368.2011.648301. [DOI] [PubMed] [Google Scholar]
- Tomy G. T.Analysis of Chlorinated Paraffins in Environmental Matrices: The Ultimate Challenge for the Analytical Chemist. In The handbook of environmental chemistry 10: Chlorinated paraffins; de Boer J., Ed.; Springer-Verlag: Berlin, 2010. [Google Scholar]
- De Boer J.; El-Sayed Ali T.; Fiedler H.; Legler J.; Muir D. C.; Nikiforov V. A.; Tomy G. T.; Tsunemi K. In The handbook of environmental chemistry 10: Chlorinated paraffins; de Boer J., Ed.; Springer-Verlag: Berlin, 2010. [Google Scholar]
- Zhou Y.; Yuan B.; Nyberg E.; Yin G.; Bignert A.; Glynn A.; Odland J. Ø.; Qiu Y.; Sun Y.; Wu Y.; Xiao Q.; Yin D.; Zhu Z.; Zhao J.; Bergman Å. Chlorinated Paraffins in Human Milk from Urban Sites in China, Sweden, and Norway. Environ. Sci. Technol. 2020, 54, 4356–4366. 10.1021/acs.est.9b06089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluge J.; Schinkel L.; Hungerbuhler K.; Cariou R.; Bogdal C. Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review. Environ. Sci. Technol. 2018, 52, 6743–6760. 10.1021/acs.est.7b06459. [DOI] [PubMed] [Google Scholar]
- Campbell I.; McConnell G. Chlorinated Paraffins and the Environment 0.1. Environmental Occurrence. Environ. Sci. Technol. 1980, 14, 1209–1214. 10.1021/es60170a001. [DOI] [Google Scholar]
- Jansson B.; Andersson R.; Asplund L.; Litzen K.; Nylund K.; Sellströom U.; Uvemo U.-B.; Wahlberg C.; Wideqvist U.; Odsjö T.; Olsson M. Chlorinated and brominated persistent organic compounds in biological samples from the environment. Environ. Toxicol. Chem. 1993, 12, 1163–1174. 10.1002/etc.5620120704. [DOI] [Google Scholar]
- van Mourik L. M.; Gaus C.; Leonards P. E.; de Boer J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155, 415–28. 10.1016/j.chemosphere.2016.04.037. [DOI] [PubMed] [Google Scholar]
- UNEP . UNEP/POPS/COP.8/SC-8/11. Listing of short-chain chlorinated paraffins. 2017. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-SC-8-11.English.pdf (accessed 2020-04-29).
- Frenzen G.; Sippel H.; Coelhan M. The relative configuration of a stereoisomer of 1, 2, 5, 6, 9, 10-hexachlorodecane. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1999, 55, IUC9800079–IUC9800079. 10.1107/S0108270199099965. [DOI] [Google Scholar]
- van Mourik L. M.; Lava R.; O’Brien J.; Leonards P. E. G.; de Boer J.; Ricci M. The underlying challenges that arise when analysing short-chain chlorinated paraffins in environmental matrices. J. Chromatogr. A 2020, 1610, 460550. 10.1016/j.chroma.2019.460550. [DOI] [PubMed] [Google Scholar]
- Sprengel J.; Wiedmaier-Czerny N.; Vetter W. Characterization of single chain length chlorinated paraffin mixtures with nuclear magnetic resonance spectroscopy (NMR). Chemosphere 2019, 228, 762–768. 10.1016/j.chemosphere.2019.04.094. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Muir D.; MacLeod M. Methods for trace analysis of short-, medium-, and long-chain chlorinated paraffins: Critical review and recommendations. Anal. Chim. Acta 2019, 1074, 16–32. 10.1016/j.aca.2019.02.051. [DOI] [PubMed] [Google Scholar]
- Fleming I.; Williams D. H.. Spectroscopic methods in organic chemistry; McGraw-Hill: London, 1966. [Google Scholar]
- Vasil’eva T. T.; Kruglova N. V.; Dostovalova V. I.; Freidlina R. K. Rearrangement of polychloroalkyl radicals with 1,4-, 1,5- and 1,6-migration of hydrogen atom. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1978, 27, 1629–1634. 10.1007/BF00925054. [DOI] [Google Scholar]
- Bessière J. M.; Boutevin B.; Taha M.; Vial F. 1H and 13C NMR study of vinyl chloride telomers with carbon tetrachloride. Org. Magn. Reson. 1984, 22, 792–793. 10.1002/mrc.1270221213. [DOI] [Google Scholar]
- Benedikt G. M. NMR Spectroscopy of Poly(Vinyl Chloride) Defects. 1H-NMR Analysis of the 1,2-Dichloroethyl End Group to the Triad Level. J. Macromol. Sci., Part A: Pure Appl.Chem. 1992, 29, 85–98. 10.1080/10101329208052154. [DOI] [Google Scholar]
- Hüttig J.Determination of the “new” problem group chloroparaffins in sediments by HRGC-LRMS; University of Basel, 2006. [Google Scholar]
- Hertkorn N.; Ruecker C.; Meringer M.; Gugisch R.; Frommberger M.; Perdue E. M.; Witt M.; Schmitt-Kopplin P. High-precision frequency measurements: indispensable tools at the core of the molecular-level analysis of complex systems. Anal. Bioanal. Chem. 2007, 389, 1311–1327. 10.1007/s00216-007-1577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher B. P.; Simpson A. J. Humic Substances in Soils: Are They Really Chemically Distinct?. Environ. Sci. Technol. 2006, 40, 4605–4611. 10.1021/es0608085. [DOI] [PubMed] [Google Scholar]
- Simpson A. J.; Lefebvre B.; Moser A.; Williams A.; Larin N.; Kvasha M.; Kingery W. L.; Kelleher B. Identifying residues in natural organic matter through spectral prediction and pattern matching of 2D NMR datasets. Magn. Reson. Chem. 2004, 42, 14–22. 10.1002/mrc.1308. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Bogdal C.; Berger U.; MacLeod M.; Gebbink W. A.; Alsberg T.; de Wit C. A. Quantifying Short-Chain Chlorinated Paraffin Congener Groups. Environ. Sci. Technol. 2017, 51, 10633–10641. 10.1021/acs.est.7b02269. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Tay J. H.; Papadopoulou E.; Haug L. S.; Padilla-Sánchez J. A.; de Wit C. A. Complex Mixtures of Chlorinated Paraffins Found in Hand Wipes of a Norwegian Cohort. Environ. Sci. Technol. Lett. 2020, 7, 198–205. 10.1021/acs.estlett.0c00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krätschmer K.; Schächtele A. Interlaboratory studies on chlorinated paraffins: Evaluation of different methods for food matrices. Chemosphere 2019, 234, 252–259. 10.1016/j.chemosphere.2019.06.022. [DOI] [PubMed] [Google Scholar]
- Zencak Z.; Reth M.; Oehme M. Dichloromethane-enhanced negative ion chemical ionization for the determination of polychlorinated n-alkanes. Anal. Chem. 2003, 75, 2487–92. 10.1021/ac034090c. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zhang H.; Zou L.; Wu P.; Yu Z.; Lu X.; Chen J. Quantification of Short-Chain Chlorinated Paraffins by Deuterodechlorination Combined with Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2016, 50, 3746–3753. 10.1021/acs.est.5b05115. [DOI] [PubMed] [Google Scholar]
- Soong R.; Pautler B. G.; Moser A.; Jenne A.; Lysak D. H.; Adamo A.; Simpson A. J. CASE (Computer-Assisted Structure Elucidation) Study for an Undergraduate Organic Chemistry Class. J. Chem. Educ. 2020, 97, 855–860. 10.1021/acs.jchemed.9b00498. [DOI] [Google Scholar]
- Bogdal C.; Alsberg T.; Diefenbacher P. S.; MacLeod M.; Berger U. Fast quantification of chlorinated paraffins in environmental samples by direct injection high-resolution mass spectrometry with pattern deconvolution. Anal. Chem. 2015, 87, 2852–60. 10.1021/ac504444d. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Alsberg T.; Bogdal C.; MacLeod M.; Berger U.; Gao W.; Wang Y.; de Wit C. A. Deconvolution of Soft Ionization Mass Spectra of Chlorinated Paraffins To Resolve Congener Groups. Anal. Chem. 2016, 88, 8980–8. 10.1021/acs.analchem.6b01172. [DOI] [PubMed] [Google Scholar]
- Schinkel L.; Lehner S.; Heeb N. V.; Marchand P.; Cariou R.; McNeill K.; Bogdal C. Dealing with strong mass interferences of chlorinated paraffins and their transformation products: An analytical guide. TrAC, Trends Anal. Chem. 2018, 106, 116–124. 10.1016/j.trac.2018.07.002. [DOI] [Google Scholar]
- Li T.; Gao S.; Ben Y.; Zhang H.; Kang Q.; Wan Y. Screening of Chlorinated Paraffins and Unsaturated Analogues in Commercial Mixtures: Confirmation of Their Occurrences in the Atmosphere. Environ. Sci. Technol. 2018, 52, 1862–1870. 10.1021/acs.est.7b04761. [DOI] [PubMed] [Google Scholar]
- Tomy G. T.; Stern G. A. Analysis of C-14-C-17 polychloro-n-alkanes in environmental matrixes by accelerated solvent extraction-nigh-resolution gas chromatography/electron capture negative ion high-resolution mass spectrometry. Anal. Chem. 1999, 71, 4860–4865. 10.1021/ac990458n. [DOI] [PubMed] [Google Scholar]
- Lane D.; Skinner T. E.; Gershenzon N. I.; Bermel W.; Soong R.; Dutta Majumdar R.; Liaghati Mobarhan Y.; Schmidt S.; Heumann H.; Monette M.; Simpson M. J.; Simpson A. J. Assessing the potential of quantitative 2D HSQC NMR in 13C enriched living organisms. J. Biomol. NMR 2019, 73, 31–42. 10.1007/s10858-018-0221-2. [DOI] [PubMed] [Google Scholar]
- Gerson A. R.; Nyburg S. Structures of two binary n-alkane solid solutions. Acta Crystallogr., Sect. B: Struct. Sci. 1994, 50, 252–256. 10.1107/S0108768193009504. [DOI] [Google Scholar]
- Lavanchy A.; Houriet R.; Gäumann T. The mass spectrometric fragmentation of n-alkanes. Org. Mass Spectrom. 1979, 14, 79–85. 10.1002/oms.1210140205. [DOI] [Google Scholar]
- Leffler J. E.; Grunwald E.. Rates and equilibria of organic reactions: As treated by statistical, thermodynamic and extrathermodynamic methods; Dover: New York, 2013. [Google Scholar]
- ChemTube3D. Polyvinyl Chloride Poly(chloroethene) PVC. https://www.chemtube3d.com/_pvcf/ (accessed 2020-02-13).
- Glüge J.; Bogdal C.; Scheringer M.; Buser A. M.; Hungerbuhler K. Calculation of Physicochemical Properties for Short- and Medium-Chain Chlorinated Paraffins. J. Phys. Chem. Ref. Data 2013, 42, 023103. 10.1063/1.4802693. [DOI] [Google Scholar]
- Hilger B.; Fromme H.; Volkel W.; Coelhan M. Effects of Chain Length, Chlorination Degree, and Structure on the Octanol-Water Partition Coefficients of Polychlorinated n-Alkanes. Environ. Sci. Technol. 2011, 45, 2842–2849. 10.1021/es103098b. [DOI] [PubMed] [Google Scholar]
- Fisk A. T.; Tomy G. T.; Cymbalisty C. D.; Muir D. C. G. Dietary accumulation and quantitative structure-activity relationships for depuration and biotransformation of short (C10), medium (C14), and long (C18) carbon-chain polychlorinated alkanes by juvenile rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2000, 19, 1508–1516. . [DOI] [Google Scholar]
- Diefenbacher P. S.; Bogdal C.; Gerecke A. C.; Glüge J.; Schmid P.; Scheringer M.; Hungerbühler K. Short-Chain Chlorinated Paraffins in Zurich, Switzerland—Atmospheric Concentrations and Emissions. Environ. Sci. Technol. 2015, 49, 9778–9786. 10.1021/acs.est.5b02153. [DOI] [PubMed] [Google Scholar]
- Xin S.; Gao W.; Wang Y.; Jiang G. Identification of the Released and Transformed Products during the Thermal Decomposition of a Highly Chlorinated Paraffin. Environ. Sci. Technol. 2018, 52, 10153–10162. 10.1021/acs.est.8b01729. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Pan W.; Lin Y.; Fu J.; Zhang A. Chlorination pattern effect on thermodynamic parameters and environmental degradability for C10-SCCPs: Quantum chemical calculation based on virtual combinational library. J. Environ. Sci. 2016, 39, 184–197. 10.1016/j.jes.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Li Y.; Hou X.; Chen W.; Liu J.; Zhou Q.; Schnoor J. L.; Jiang G. Carbon Chain Decomposition of Short Chain Chlorinated Paraffins Mediated by Pumpkin and Soybean Seedlings. Environ. Sci. Technol. 2019, 53, 6765–6772. 10.1021/acs.est.9b01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali T. E.; Legler J.. Overview of the Mammalian and Environmental Toxicity of Chlorinated Paraffins. In The handbook of environmental chemistry 10: Chlorinated paraffins; de Boer J., Ed.; Springer-Verlag: Berlin, 2010. [Google Scholar]
- U.S. Environmental Protection Agency. TSCA new chemicals review program standard review risk assessment on medium-chain chlorinated paraffins (PMN P-14-0683, P-14-0684). 2015. https://www.epa.gov/sites/production/files/2015-12/documents/standard_review_risk_assessment_p-14-683-684_qualice_docket.pdf (accessed 2020-04-29).
- Du X.; Yuan B.; Zhou Y.; Benskin J. P.; Qiu Y.; Yin G.; Zhao J. Short-, Medium-, and Long-Chain Chlorinated Paraffins in Wildlife from Paddy Fields in the Yangtze River Delta. Environ. Sci. Technol. 2018, 52, 1072–1080. 10.1021/acs.est.7b05595. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Vorkamp K.; Roos A. M.; Faxneld S.; Sonne C.; Garbus S. E.; Lind Y.; Eulaers I.; Hellström P.; Dietz R.; Persson S.; Bossi R.; de Wit C. A. Accumulation of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Marine and Terrestrial Animals from Scandinavia. Environ. Sci. Technol. 2019, 53, 3526–3537. 10.1021/acs.est.8b06518. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Benskin J. P.; Chen C.-E. L.; Bergman Å. Determination of Chlorinated Paraffins by Bromide-Anion Attachment Atmospheric-Pressure Chemical Ionization Mass Spectrometry. Environ. Sci. Technol. Lett. 2018, 5, 348–353. 10.1021/acs.estlett.8b00216. [DOI] [Google Scholar]
- van Mourik L. M.; Toms L.-M. L.; He C.; Banks A.; Hobson P.; Leonards P. E. G.; de Boer J.; Mueller J. F. Evaluating age and temporal trends of chlorinated paraffins in pooled serum collected from males in Australia between 2004 and 2015. Chemosphere 2020, 244, 125574. 10.1016/j.chemosphere.2019.125574. [DOI] [PubMed] [Google Scholar]
- Li T.; Wan Y.; Gao S.; Wang B.; Hu J. High-Throughput Determination and Characterization of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Human Blood. Environ. Sci. Technol. 2017, 51, 3346–3354. 10.1021/acs.est.6b05149. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Chang H.; Wang H.; Zhu Y.; Zhao X.; He Y.; Sun F.; Wu F. Spatial and Temporal Distributions of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Sediment Cores from Nine Lakes in China. Environ. Sci. Technol. 2019, 53, 9462–9471. 10.1021/acs.est.8b07296. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Brüchert V.; Sobek A.; de Wit C. A. Temporal Trends of C8–C36 Chlorinated Paraffins in Swedish Coastal Sediment Cores over the Past 80 Years. Environ. Sci. Technol. 2017, 51, 14199–14208. 10.1021/acs.est.7b04523. [DOI] [PubMed] [Google Scholar]
- Brandsma S. H.; van Mourik L.; O’Brien J. W.; Eaglesham G.; Leonards P. E.; de Boer J.; Gallen C.; Mueller J.; Gaus C.; Bogdal C. Medium-Chain Chlorinated Paraffins (CPs) Dominate in Australian Sewage Sludge. Environ. Sci. Technol. 2017, 51, 3364–3372. 10.1021/acs.est.6b05318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F.; Suzuki G.; Michinaka C.; Yuan B.; Takigami H.; de Wit C. A. Dioxin-like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada. Chemosphere 2017, 168, 1248–1256. 10.1016/j.chemosphere.2016.10.074. [DOI] [PubMed] [Google Scholar]
- Brits M.; de Boer J.; Rohwer E. R.; De Vos J.; Weiss J. M.; Brandsma S. H. Short-, medium-, and long-chain chlorinated paraffins in South African indoor dust and cat hair. Chemosphere 2020, 238, 124643. 10.1016/j.chemosphere.2019.124643. [DOI] [PubMed] [Google Scholar]
- Schinkel L.; Bogdal C.; Canonica E.; Cariou R.; Bleiner D.; McNeill K.; Heeb N. V. Analysis of Medium-Chain and Long-Chain Chlorinated Paraffins: The Urgent Need for More Specific Analytical Standards. Environ. Sci. Technol. Lett. 2018, 5, 708–717. 10.1021/acs.estlett.8b00537. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.