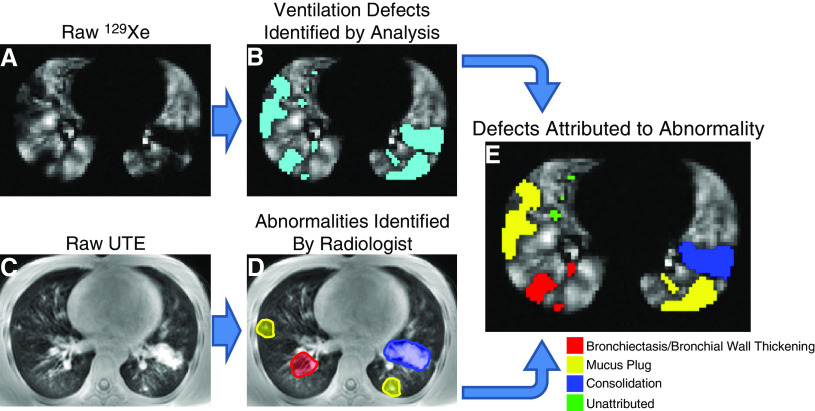
To the Editor:
Cystic fibrosis (CF) is a genetic disorder that exhibits a number of different structural pulmonary abnormalities such as mucus plugs (MP), bronchiectasis (BR), bronchial wall (BW) thickening, and consolidations (CNs), each of which contribute to abnormal ventilation via regional obstruction. Although structural imaging methods (generally X-ray computed tomography [CT]) can depict regional structural pathologies, the precise extent to which these structural abnormalities contribute to lung function decline is not well understood. Here, we demonstrate that hyperpolarized (HP) gas magnetic resonance imaging (MRI) can be combined with ultrashort echo (UTE) MRI (a radiation-free alternative to CT [1–3]) to quantify the relationships between individual regional pathologies and regional ventilation (4–6). The aim of this work was to quantify the size and extent of regional ventilation defects in CF using HP 129Xe MRI and to associate these with the presence of specific structural abnormalities identified by UTE MRI: BR, BW thickening, MP, ground-glass opacities, and CN. We hypothesized that low-ventilation regions could be attributed to spatially matched pathologies seen in UTE images.
Methods
A total of five healthy control subjects (mean ± SD age, 10.8 ± 3.9 yr) and 22 clinically stable patients with CF (age, 14.5 ± 10.6 yr) were imaged under an Institutional Review Board–approved protocol, with a Food and Drug Administration Investigational New Drug (123577) for 129Xe; informed consent was obtained from adult subjects or parents, and age-appropriate assent from pediatric subjects (demographic data in Table 1). Spirometry was obtained in each subject before imaging according to American Thoracic Society and European Respiratory Society guidelines. Subjects were imaged on a Philips 3T Achieva (Philips Healthcare) by coaching subjects to FRC before inhalation of a breath of HP 129Xe (one-sixth TLC, based on height and sex [7]) for ventilation imaging via a gradient-echo sequence (two-dimensional multislice, TR/TE = 8/4 ms; voxel size = 3 × 3 × 15 mm3; readouts = 96–196; phase encodes = 50–64; FA = 9–12°; and slices = 9–15). UTE magnetic resonance images were acquired during resting breathing, gated to FRC, with a 32-channel proton cardiac phased-array coil (radial three-dimensional stack-of-stars sequence, 40,000 projections, TE = 0.2 ms, TR = 4.8–6.2 ms, FA = 5°, voxel size = 1.19–1.45 mm2 in-plane resolution, slice thickness = 4 mm, and bandwidth ≈ 1.8 kHz). 129Xe signal intensity was normalized to the whole-lung HP gas signal mean and lung voxels with 129Xe signal <60% of the whole-lung signal were identified as defects (8, 9). The percentage of an individual’s lung volume identified as defect is quantified as the subject’s ventilation defect percentage (VDP). UTE magnetic resonance images were visually analyzed independently by two radiologists (A.S. and A.B.) for regions of structural abnormality: BR, BW thickening, MP, CN, and ground-glass opacities. The identified abnormalities were then visually matched to corresponding regions in the HP 129Xe MRI to associate the identified structural abnormalities with corresponding ventilation defects (Figure 1). Radiologists also assigned an integer quality score between 1 and 5 (low and high, respectively) to each subject’s UTE dataset, which were averaged to provide an overall image quality score.
Table 1.
Individual Subject Demographic Data and Spirometry/VDP Results
| Subject Number | Age (yr) | Sex | Height (cm) | CF Genotype | ppFEV1 (%) | VDP (%) | |
|---|---|---|---|---|---|---|---|
| 1 |
16 | F | 169 | Control | 110 | 0.8 | |
| 2 |
12 | M | 160 | Control | 92 | 1.0 | |
| 3 |
12 | M | 141 | Control | 103 | 1.5 | |
| 4 |
6 | F | 116 | Control | 95 | 2.0 | |
| 5 |
6 | M | 116.4 | ΔΔF508 | 100 | 2.3 | |
| 6 |
6 | M | 112 | ΔΔF508 | 99 | 2.7 | |
| 7 |
8 | M | 136 | Control | 91 | 2.8 | |
| 8 |
7 | M | 109.9 | ΔΔF508 | 116 | 3.9 | |
| 9 |
6 | F | 105.2 | ΔΔF508 | 97 | 5.4 | |
| 10 |
11 | F | 149.9 | ΔΔF508 | 119 | 6.1 | |
| 11 |
6 | M | 121 | ΔF508/R560T | 81 | 8.6 | |
| 12 |
11 | F | 147 | ΔΔF508 | 86 | 8.8 | |
| 13 |
11 | F | 152 | ΔΔF508 | 89 | 9.0 | |
| 14 |
13 | M | 146 | 3120+1G>A/3120+1G>A | 78 | 9.2 | |
| 15 |
12 | M | 158.6 | ΔF508/W1282X | 89 | 9.4 | |
| 16 |
46 | M | 165 | ΔΔF508 | 83 | 15.9 | |
| 17 |
6 | M | 119 | ΔΔF508 | 74 | 16.5 | |
| 18 |
11 | F | 144.1 | ΔΔF508 | 87 | 18.1 | |
| 19 |
9 | F | 132.1 | ΔΔF508 | 101 | 19.8 | |
| 20 |
8 | M | 120.7 | ΔΔF508 | 88 | 19.9 | |
| 21 |
16 | F | 153 | 3849/849+10kbC>T | 61 | 21.4 | |
| 22 |
11 | M | 142 | F508del/R1066H | 102 | 22.8 | |
| 23 |
16 | F | 159 | ΔΔF508 | 72 | 23.9 | |
| 24 |
19 | F | 163 | ΔΔF508 | 66 | 25.6 | |
| 25 |
26 | F | 157 | ΔΔF508 | 78 | 26.8 | |
| 26 |
26 | M | 175 | F508del/G551D | 39 | 33.8 | |
| 27 | 37 | F | 151.5 | F508del/S945L | 38 | 44.0 | |
Definition of abbreviations: CF = cystic fibrosis; ppFEV1 = percent predicted FEV1; VDP = ventilation defect percentage.
Figure 1.
Example of image analysis pipeline shown in a patient with cystic fibrosis (subject 22). (A and C) 129Xe and ultrashort echo (UTE) magnetic resonance images are collected in the same imaging session, respectively. (B and D) Ventilation defects are quantified in the 129Xe magnetic resonance imaging (B, defects colored cyan) and abnormalities are independently identified within the UTE by trained readers (D). (E) 129Xe defects are separately associated with proximal or adjacent structural abnormalities for quantitative analysis.
Results
Table 1 shows individual results for FEV1 and VDP. Average FEV1 was 98.2% ± 8.1% for controls and 83.8% ± 20.6% for subjects with CF (P = 0.02). Average VDP was 1.6% ± 0.8% for control subjects and 16.1% ± 10.8% for patients with CF (P < 10−5). Of the total calculated 129Xe defect volume across all subjects, only 50.6% was associated with a spatially matched structural abnormality from UTE MRI. Of the calculated 129Xe defect volumes that were associated to structural abnormalities, 76.8% were associated with BR and/or BW thickening and 76.6% were associated with MP, with significant overlap in attribution (only 16.2% and 17.9% exclusively associated with BR/BW and MP, respectively). Individual subject counts of structural abnormalities correlated strongly with VDPs (Pearson r = 0.81; P < 10−6) but only moderately with FEV1 (r = −0.68; P = 0.0001), as expected. Pearson correlation was r = −0.78 (P < 10−5) between subject VDP and FEV1.
Discussion
This work presents the first direct quantification of 129Xe ventilation impairment associated with specific regional structural abnormalities in CF lung disease. BR and MP were responsible for the vast majority of structurally attributed defects, but the large quantity of defects that could not be associated with structural abnormalities underscores the sensitivity of 129Xe MRI to mild obstruction. The lower resolution inherent to UTE MRI (here, ∼1.2 mm) may have resulted in undetectable small structural abnormalities that would have been detected with high-resolution CT (0.4 mm). X-ray CT remains the gold standard for structural imaging, especially with respect to quantitative assessment of airways and reduced lung parenchymal density on expiration, associated with air trapping. However, this further highlights 129Xe MRI as a sensitive technique for evaluating and quantifying regional lung function, particularly in cases in which structural imaging alone yields inconclusive results and in mild disease, in which functional declines are subclinical. This combination of MRI techniques may be beneficial in the future to aid clinical decision-making, particularly in evaluation of individual patient outcomes, in which repeated testing is useful, and as a biomarker for upcoming clinical trials. We find that 129Xe MRI often shows ventilation impairment even in the absence of identifiable structural abnormalities, and that within defect regions, 129Xe signal is greater if no structural abnormality could be regionally matched from structural imaging. This highlights 129Xe imaging as a sensitive tool for pulmonary research and may be clinically useful in the evaluation and/or management of patients with CF in early stages of lung function decline.
Supplementary Material
Footnotes
Supported by NIH grants T32 HL007752, R01 HL131012, and R44HL123299.
Author Contributions: Concept and design: R.P.T., L.L.W., D.J.R., N.H., A.S., A.B., J.P.C., Z.I.C., and J.C.W. Data acquisition: R.P.T., L.L.W., D.J.R., N.H., Z.I.C., and J.C.W. Image analysis: R.P.T. and J.C.W. Radiological interpretation: A.S. and A.B. Interpretation of results: R.P.T., L.L.W., D.J.R., N.H., A.S., A.B., J.P.C., Z.I.C., and J.C.W. All authors contributed to the intellectual content of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202001-0031LE on April 3, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Roach DJ, Crémillieux Y, Serai SD, Thomen RP, Wang H, Zou Y, et al. Morphological and quantitative evaluation of emphysema in chronic obstructive pulmonary disease patients: a comparative study of MRI with CT. J Magn Reson Imaging. 2016;44:1656–1663. doi: 10.1002/jmri.25309. [DOI] [PubMed] [Google Scholar]
- 2.Dournes G, Grodzki D, Macey J, Girodet PO, Fayon M, Chateil JF, et al. Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology. 2015;276:258–265. doi: 10.1148/radiol.15141655. [DOI] [PubMed] [Google Scholar]
- 3.Higano NS, Fleck RJ, Spielberg DR, Walkup LL, Hahn AD, Thomen RP, et al. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging. 2017;46:992–1000. doi: 10.1002/jmri.25643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wielpütz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189:956–965. doi: 10.1164/rccm.201309-1659OC. [DOI] [PubMed] [Google Scholar]
- 5.Roach DJ, Crémillieux Y, Fleck RJ, Brody AS, Serai SD, Szczesniak RD, et al. Ultrashort echo-time magnetic resonance imaging is a sensitive method for the evaluation of early cystic fibrosis lung disease. Ann Am Thorac Soc. 2016;13:1923–1931. doi: 10.1513/AnnalsATS.201603-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahl M, Wielpütz MO, Graeber SY, Joachim C, Sommerburg O, Kauczor HU, et al. Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med. 2017;195:349–359. doi: 10.1164/rccm.201604-0893OC. [DOI] [PubMed] [Google Scholar]
- 7.Zapletal A, Paul T, Samánek M. Significance of contemporary methods of lung function testing for the detection of airway obstruction in children and adolescents (author’s translation) [in German] Z Erkr Atmungsorgane. 1977;149:343–371. [PubMed] [Google Scholar]
- 8.Thomen RP, Sheshadri A, Quirk JD, Kozlowski J, Ellison HD, Szczesniak RD, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 2015;274:250–259. doi: 10.1148/radiol.14140080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros. 2017;16:275–282. doi: 10.1016/j.jcf.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.