To the Editor:
Most patients admitted to the ICU with a severe presentation of coronavirus disease (COVID-19) fulfill the acute respiratory distress syndrome (ARDS) criteria (1) and require invasive mechanical ventilation (2). In such patients, knowledge of respiratory mechanics and potential for lung recruitability may provide valuable information to guide adjustments in ventilator settings. Some authors have regularly reported from their clinical experience that the key feature of COVID-19 respiratory mechanics would be an uncommon association of severe hypoxemia and preserved respiratory system compliance, altogether with poor recruitability (3–5). However, a dramatic decrease in respiratory system compliance has also been reported in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related ARDS (6). Gattinoni and colleagues recently proposed to reconciliate these different observations, hypothesizing that the different phenotypes may result from interactions between the time course and severity of the disease and the patient’s ventilatory response, with an early L phenotype (low lung elastance, low recruitability) and a late H phenotype (high lung elastance, high recruitability) (5). However, physiological descriptions of COVID-19–associated ARDS and its comparison with non–COVID-19 classical ARDS remain scarce in the literature.
The aim of the present study is to describe the respiratory mechanics and lung recruitability of patients with COVID-19–associated ARDS, to compare it with that of non–COVID-19–associated ARDS, and to explore their possible relation with COVID-19 phenotypes.
Methods
This is an ancillary report of an ongoing prospective monocentric observational study on respiratory mechanics in patients with ARDS, conducted in the Henri Mondor University Hospital medical ICU, Créteil, France (Institutional Review Board 2018-A00867–48). Inclusion criteria were age >18 years and presence of ARDS according to the Berlin definition (7). Exclusion criteria were intubation for more than 24 hours prior to ICU admission. All consecutive patients with COVID-19 included in this study are reported here and compared with consecutive patients without COVID-19 who were previously enrolled. Written informed consent was waived owing to the observational nature of the study. The ventilator was set by the attending physician. During the first 48 hours of invasive mechanical ventilation, the ventilator’s settings were collected and the respiratory mechanics and lung recruitability were assessed once in supine position. Thus, airway and esophageal (when available) pressures were recorded during a 0.3-second end-inspiratory and a 1- to 2-second end-expiratory occlusion maneuver, at the positive end-expiratory pressure (PEEP) level previously set by the physician. The potential airway closure phenomenon was detected by measuring the airway opening pressure during a low flow (≤6 L/min) insufflation, as previously described (8). The potential for lung recruitment was assessed by the mean of the recruitment-to-inflation ratio (R/I ratio) computation, as previously detailed (8). By default, R/I ratio was assessed between 15 and 5 cm H2O of PEEP. However, in case of airway closure, the low PEEP was set above the airway opening pressure. Comparisons were made using nonparametric tests. A P < 0.05 was considered significant.
Results
Thirty consecutive patients with non–COVID-19–associated ARDS and 30 consecutive patients with COVID-19–associated ARDS were included in the report. Patients without COVID-19 were enrolled between January 17, 2019, and March 3, 2020, and those with COVID-19 were enrolled between March 11, 2020, and April 3, 2020. Five patients with COVID-19 and five without COVID-19 experienced prone position before inclusion in the study. Etiologies for non–COVID-19–associated ARDS were as follows: pneumonia (n = 27, of which 10 were related to respiratory viruses), pulmonary vasculitis (n = 2), and noncardiogenic shock (n = 1). A bacterial coinfection was documented in four patients with COVID-19 at the time of inclusion. Patients with and without COVID-19 did not differ significantly in age and ARDS severity, according to the PaO2/FiO2 ratio (Table 1). Patients with COVID-19 had a significantly higher body mass index (BMI).
Table 1.
Patients’ Characteristics, Respiratory Mechanics, and Recruitability
| COVID-19 (n = 30) | Non–COVID-19 (n = 30) | P Value | ||
|---|---|---|---|---|
| Age, yr | 58 (49–67) | 66 (52–73) | 0.15 | |
| Sex, M, n (%) | 26 (87) | 22 (73) | 0.19 | |
| Height, cm | 175 (167–178) | 170 (165–175) | 0.25 | |
| BMI, kg/m2 | 28 (24–31) | 22 (20–27) | <0.01 | |
| Noninvasive ventilatory support prior to intubation, n (%)* | 16 (53) | 10 (33) | 0.19 | |
| Duration of noninvasive ventilatory support, d | 1 (0–1.75) | 1 (0–2.25) | 0.77 | |
| FiO2 level, % | 70 (52–80) | 60 (40–80) | 0.55 | |
| PaO2/FiO2, mm Hg | 119 (97–163) | 136 (120–167) | 0.075 | |
| ARDS severity, n (%) | 0.22 | |||
| Moderate | 19 (63.3) | 24 (80) | ||
| Mild | 3 (10.7) | 4 (13.3) | ||
| Severe | 8 (26.7) | 2 (7.1) | ||
| Vt, ml/kg of PBW | 6.0 (5.9–6.7) | 6.3 (5.9–6.4) | 0.18 | |
| Respiratory rate, cycles/min | 28 (28–30) | 26 (25–30) | 0.03 | |
| PEEP, cm H2O | 10 (8–12) | 8 (8–10) | 0.33 | |
| Auto-PEEP, cm H2O | 1 (1–2) | 1 (1–2) | 0.2 | |
| Airway opening pressure ≥5 cm H2O†, n (%) | 12 (40) | 3 (11) | 0.01 | |
| R/I ratio† | 0.40 (0.23–0.50) | 0.20 (0.05–0.30) | 0.01 | |
| High recruitability, n (%) | 9 (30) | 4 (15) | 0.17 | |
| Pplat, cm H2O | 21 (20–24) | 20 (17–24) | 0.22 | |
| Driving pressure, cm H2O | 10 (8–12) | 9 (8–11) | 0.64 | |
| Rrs, cm H2O/L/s | 16 (14–19) | 16 (13–18) | 0.61 | |
| Crs, ml/cm H2O | 44 (35–51) | 42 (30–55) | 0.84 | |
| Patients with esophageal pressure, n | 19 | 29 | ||
| BMI, kg/m2 | 30 (26–32) | 22 (20–28) | <0.01 | |
| PaO2/FiO2, mm Hg | 111 (96–128) | 135 (120–159) | 0.02 | |
| Plend-insp, cm H2O‡ | 14 (14–18) | 14 (9–17) | 0.26 | |
| Plend-exp, cm H2O§ | 2 (0–4) | 0 (0–1) | 0.06 | |
| Ccw, ml/cm H2O | 144 (116–360) | 113 (92–150) | 0.06 | |
| Clung, ml/cm H2O | 59 (44–72) | 57 (47–90) | 0.81 | |
| El/Ers | 0.69 (0.63–0.89) | 0.64 (0.52–0.80) | 0.11 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; BMI = body mass index; Ccw = chest wall compliance; Clung = lung compliance; COVID-19 = coronavirus disease; Crs = respiratory system compliance; El = lung elastance; Ers = respiratory system elastance; PBW = predicted body weight; PEEP = positive end expiratory pressure; Plend-exp = transpulmonary pressure at end expiration; Plend-insp = transpulmonary pressure at end inspiration; Pplat = plateau pressure; R/I ratio = recruitment-to-inflation ratio (8); Rrs = respiratory system resistance.
Continuous variables are expressed as median (interquartile range). High recruitability denotes patients with R/I ratio ≥0.5. Auto-PEEP was computed as total PEEP minus applied PEEP. Bold values denote P values < 0.05.
Noninvasive ventilatory supports were continuous positive airway pressure (n = 13), noninvasive ventilation (n = 1), and high-flow nasal cannula (n = 2) for patients with COVID-19 and high-flow nasal cannula (n = 10) for those without COVID-19.
Not available in three patients without COVID-19.
Computed as follows: Plend-insp = Pplat × (El/Ers) (11).
Computed as follows: Plend-exp = PEEPt − Pesend-exp, where PEEPt is the total PEEP and Pesend-exp is the end expiratory esophageal pressure value.
Respiratory mechanics
Driving pressure as well as respiratory system compliance and resistance did not significantly differ between patients with and without COVID-19 (Table 1 and Figure 1A). These findings were similar in the subgroup of patients with esophageal pressure measurement (19/30 COVID-19 and 29/30 non–COVID-19). Especially, chest wall compliances of patients with and without COVID-19 were similarly preserved (Table 1). In patients with a PaO2/FiO2 ratio below 150 mm Hg (20 COVID-19 and 17 non–COVID-19), the respiratory system compliance was also similar between two groups (43 ml/cm H2O [interquartile range (IQR), 34–48] vs. 45 ml/cm H2O [IQR, 31–56], respectively; P = 0.68). Airway opening pressure and R/I ratio were available in all but three patients. Airway closure phenomenon (airway opening pressure ≥5 cm H2O) occurred more frequently in patients with COVID-19 as compared with their counterparts (12/30 [40%] vs. 3/27 [11%]; P = 0.01). The 12 patients with COVID-19 with airway closure phenomenon had a median airway opening pressure of 8 cm H2O (IQR, 5–10), whereas the 3 patients without COVID-19 with airway closure phenomenon had an airway opening pressure of 5, 5, and 9 cm H2O, respectively. There was a weak but significant correlation between the BMI and the airway opening pressure (Spearman’s ρ = 0.327; P = 0.017).
Figure 1.
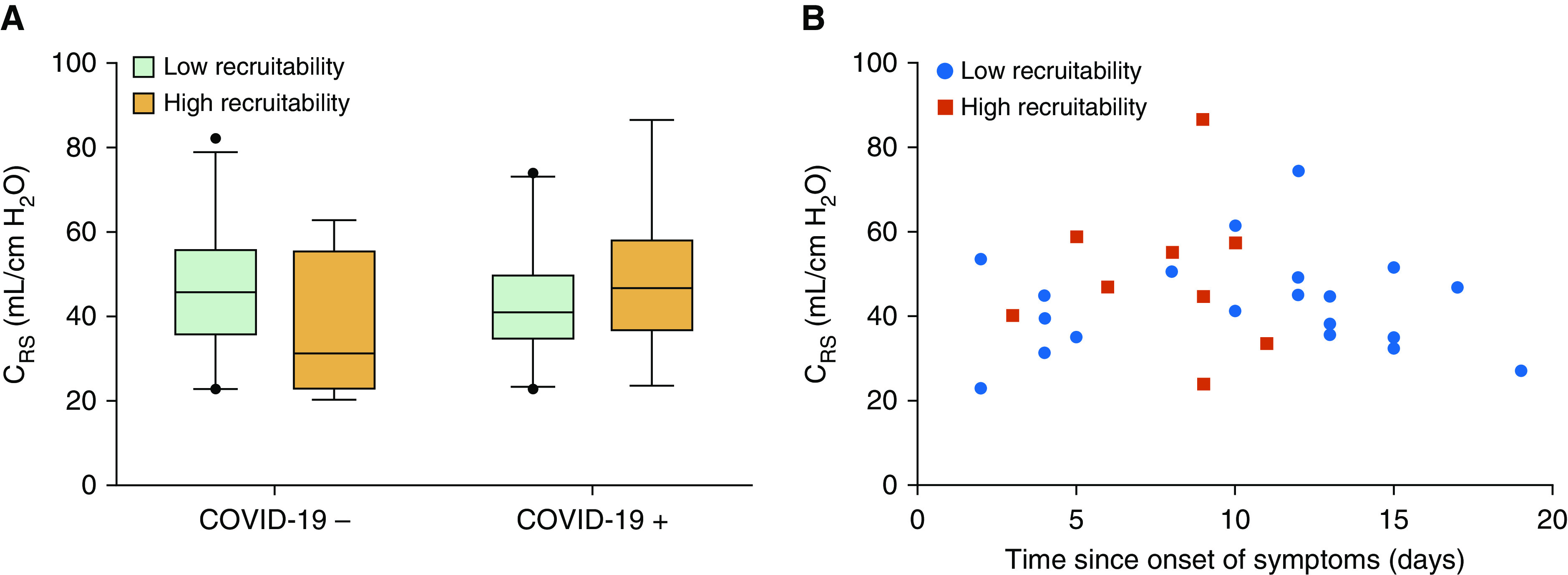
(A) Respiratory system compliance (Crs) according to both the coronavirus disease (COVID-19) status and the recruitability. High recruitability denotes a recruitment-to-inflation ratio ≥0.5. Conversely, low recruitability denotes a recruitment-to-inflation ratio <0.5. No significant difference was found between any subgroup. COVID-19− denotes patients without COVID-19; COVID-19+ denotes those with COVID-19. (B) Crs plotted against the time since onset of COVID-19 symptoms. No correlation was found between the Crs and duration of symptoms. Red squares represent patients with a recruitment-to-inflation ratio ≥0.5, and blue circles represent patients with a recruitment-to-inflation ratio <0.5.
Recruitability and COVID-19 phenotypes
Overall, the R/I ratio was significantly higher in patients with than in those without COVID-19 (Table 1). However, the difference in high potential for recruitability (as defined by an R/I ratio ≥0.5) (8) between patients with and without COVID-19 did not reach statistical significance (9/30 [30%] vs. 4/27 [15%]; P = 0.17).
In patients with COVID-19, the R/I ratio was significantly correlated with the PaO2/FiO2 ratio (Spearman’s ρ = −0.44; P = 0.001) but not with the respiratory system compliance (Spearman’s ρ = 0.29; P = 0.12; Figure 1A). The times since the onset of the first COVID-19 symptom and since the onset of dyspnea were not correlated with respiratory system compliance (Spearman’s ρ = −0.005 and 0.162; P = 0.98 and 0.39, respectively) (Figure 1B) or with the R/I ratio (Spearman’s ρ = −0.320 and −0.221; P = 0.09 and 0.24, respectively). No other correlation was found between the duration of the disease and any of the respiratory mechanics parameters assessed.
Subgroup analysis
A subgroup analysis focusing on moderate ARDS in patients with and without COVID-19 found similar results.
A comparison between patients with COVID-19 and the 27 patients without COVID-19 with pneumonia-related ARDS retrieved similar findings as well. As compared with the 10 patients without COVID-19 with viral pneumonia, those with COVID-19 had a significantly higher BMI (27.9 kg/m2 [IQR, 24.2–31.8] vs. 22.3 kg/m2 [IQR, 19.5–26.4]; P = 0.01) and a lower PaO2/FiO2 ratio (119 mm Hg [IQR, 97–163] vs. 146 mm Hg [IQR, 131–157]; P = 0.04) but with comparable respiratory mechanics (data not shown).
Discussion
The main findings of our prospective observational study were as follows: the respiratory mechanics of patients with COVID-19–related ARDS was heterogenous and as a global picture not much different from that of their non–COVID-19 counterparts; patients with COVID-19–related ARDS had a higher R/I ratio suggesting a higher recruitability; we could not formally identify specific COVID-19–related ARDS phenotypes using a raw assessment of relationship between respiratory mechanics, recruitability, hypoxemia severity, and time course of the disease.
Although some authors have described patients with COVID-19–related ARDS with intriguingly high compliance (3–5), others reported case series of patients with very low compliance (6). We found a higher R/I ratio in COVID-19–related ARDS, suggesting a higher recruitability. Gattinoni and colleagues proposed an integrative concept (5) hypothesizing a progressive transition from a phenotype characterized by low elastance, low lung weight, low recruitability, and low ventilation-to-perfusion ratio to a second phenotype characterized by high elastance, high lung weight, high right-to-left shunt, and high recruitability, the transition being mainly driven by the extent of the patient’s ventilatory response and its ability to promote patient self-inflicted lung injury (9). We could not retrieve such distinct phenotypes in our cohort, as no correlation was found between compliance, recruitability, or the time course of the disease. However, as we were not able to quantify the magnitude of the respiratory effort and the resulting negative pleural pressure swings prior to intubation, we could not assess the hypothesis of patient self-inflicted lung injury as the leading mechanism of respiratory mechanics impairment during COVID-19–related ARDS. In addition, as our study was monocentric with a small sample size, our results may not be generalizable to all patients with COVID-19–related ARDS. Nevertheless, the higher BMI in our patients with COVID-19 as compared with their non–COVID-19 counterparts is consistent with a previous report pointing out the high frequency of obesity in patients with COVID-19 requiring mechanical ventilation (10). This may explain, at least partly, the higher proportion of airway closure phenomenon in our cohort.
In conclusion, given the various associations of respiratory mechanics, hypoxemia severity, and lung recruitability in patients with COVID-19–related ARDS, our results advocate for the systematic assessment of respiratory mechanics and recruitability at the bedside to personalize ventilator settings in these patients. Larger cohort studies are warranted to scrutinize the phenotype(s) of COVID-19–related ARDS.
Supplementary Material
Footnotes
Author Contributions: A.-F.H.: data collection, data analysis, data interpretation, and writing. F.P.: data collection and data interpretation. S.T.: data collection, data analysis, and data interpretation. N.d.P. and K.R.: data interpretation. A.M.D. and G.C.: study design, data collection, data analysis, data interpretation, and writing. All authors helped to revise the draft of the manuscript. All authors read and approved the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202004-1226LE on June 1, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles [editorial] Am J Respir Crit Care Med. 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. [online ahead of print] 14 Apr 2020; DOI: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed]
- 6.Pan C, Chen L, Lu C, Zhang W, Xia J-A, Sklar MC, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome: a clinical trial. Am J Respir Crit Care Med. 2020;201:178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 9.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 10.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. Lille Intensive Care COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) doi: 10.1002/oby.22831. [online ahead of print] 9 Apr 2020; DOI: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med. 2017;5:285. doi: 10.21037/atm.2017.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


